Abstract
Tuber aestivum, commonly known as the summer truffle, is typically found in various parts of Europe where it grows naturally. However, its presence in Portugal was not confirmed until now. The first fruit bodies were collected in April 2024 at stone pine stands (Alenquer and Arruda dos Vinhos, Lisbon) and in June at holm oak stands (Salir, Faro). These specimens are characterized by hypogeous, subglobose, black ascomata with a peridium surface covered with pyramidal warts. Ascopores are subglobose-to-broadly ellipsoid, distinctively ornamented, usually 1–6 per asci. According to the results of the internal transcribed spacer (ITS) rDNA sequence analysis, these specimens form a well-supported group within the Aestivum clade, with T. aestivum being the closest phylogenetic taxon. This remarkable discovery opens up new opportunities for truffle exploitation in Portugal thanks to the summer truffle’s gastronomical value and high market prices.
1. Introduction
Recently, numerous new species of Tuber have been identified and recognized as valid species by the scientific community. It is difficult to assure how many Tuber species occur worldwide, since the Global Biodiversity Information Facility (GBIF) estimate that there are around 230 accepted species [1], but the Catalogue of Life only list 196 accepted species [2]. To estimate the number of Tuber species on the European continent, various sources were consulted and compared, based on material samples or preserved specimens’ records [1,2,3,4]. These sources suggest that 51 Tuber species have been found in Europe, namely T. aestivum (Wulfen) Spreng., T. albidum Fr., T. alcaracense Ant. Rodr. and Morte, T. anniae W. Colgan and Trappe, T. asa-foetida Lesp., T. bellonae Quél., T. borchii Vittad., T. brumale Vittad., T. cistophilum P. Alvarado, G. Moreno, Manjón, Gelpi and Jaime Muñoz, T. cryptobrumale Merényi, T. Varga and Bratek, T. davidlopezii Ant. Rodr., Morte and Muñ.-Moh., T. dryophilum Tul. and C. Tul., T. excavatum Vittad., T. ferrugineum Vittad., T. foetidum Vittad., T. fulgens Quél., T. gennadii (Chatin) Pat., T. gibbosum Harkn., T. himalayense B.C. Zhang and Minter, T. huidongense Y. Wang, T. lacunosum Mattir., T. levissimum Gilkey, T. lucentum Bordallo, T. lusitanicum Ant. Rodr. and Muñoz-Mohedano, T. macrosporum Vittad., T. maculatum Vittad., T. magentipunctatum Merényi, I. Nagy, Stielow and Bratek, T. magnatum Picco, T. malacodermum E. Fisch., T. malenconii Donadini, Riousset, G. Riousset and G. Chev., T. melanosporum Vittad., T. mesentericum Vittad., T. microsporum Vittad., T. mutabile Quél., T. nitidum Vittad., T. oligospermum (Tul. and C. Tul.) Trappe, T. panniferum Tul. and C. Tul., T. petrophilum Milenković, P. Jovan., Grebenc, Ivančević and Marković, T. pseudobrumale Y. Wang and Shu H. Li, T. pseudoexcavatum Y. Wang, G. Moreno, Riousset, Manjón and G. Riousset, T. puberulum Berk. and Broome, T. pulchrosporum Konstantinidis, Tsampazis, Slavova, Nakkas, Polemis, Fryssouli and Zervakis, T. rapaeodorum Tul. and C. Tul., T. regianum Montecchi and Lazzari, T. rufum Pollini, T. scleroneuron Berk. and Broome, T. scruposum R. Hesse, T. sphaerospermum (Malençon) P. Roux, Guy García and M.C. Roux, T. stramineum Ferry and Quél., T. suave Pacioni and M. Leonardi, T. vesicoperidium L. Fan.
According to GBIF, only eight Tuber species occurred in Portugal: T. borchii, T. excavatum, T. lacunosum, T. maculatum, T. oligospermum and T. puberulum, with less than 40 records in total. Although, T. gennadii [5], one of the most common truffles in Portugal, is not referred. In short, from all databases, there is no reference of the occurrence of T. aestivum in Portugal.
Tuber aestivum, commonly known as the summer truffle or Burgundy truffle, is highly prized worldwide for its distinctive aroma and flavor. Summer truffles have a milder, more delicate aroma compared to the more intense winter truffles, and its scent is often described as earthy, nutty, and slightly garlicky [6]. High demanded in gourmet and fine dining sectors, summer truffle can be used fresh or in infusions (oils, butter, cheeses) in a wide variety of prepared dishes. The economic value of summer truffle is influenced by its demand versus its availability, as well as by the ascocarps quality, size, and freshness. Fresh summer truffle market prices can vary widely between regions, with lower values in Spain (25 to 70 EUR per kg) and higher in Switzerland (200 to 600 EUR per kg) [7]. Additionally, summer truffles have medicinal properties, such as anti-angiogenic and anti-inflammatory activities [8]. In summary, T. aestivum holds significant gastronomical and economic value due to its unique flavor, culinary versatility, and medicinal properties. Although, not as costly as some other truffle varieties, it remains a luxury item that commands a premium price on the market.
T. aestivum belong to Tuberaceae (Pezizales, Ascomycota), a large group of ectomycorrhizal fungi growing in symbiosis with the roots of several vascular plant (Angiosperms: Betula spp., Carpinus spp., Castanea spp., Corylus avellana, Fagus spp., Populus spp., Quercus spp. and Tilia spp.; Gymnosperms: Abies spp., Cedrus spp., Picea spp. and Pinus spp.), fruiting abundantly at matures stands [9,10,11,12]. The T. aestivum fruit body is hypogeous, dark, globose with an average diameter of 7 cm and not easy to find without trained animals. The optimal soil conditions for its growth and development include specific ranges for soil pH (7.0 to 8.0), organic matter content (2 to 4%), and a C:N ratio (20:1 to 30:1) [12], mainly from loam to silt loam sandy–clayey soils [13]. Concerning climatic conditions, T. aestivum prefers mild temperatures (mean annual temperature between +7 °C and +11 °C (max. +18 °C) and moderate precipitation (annual precipitation between 700 mm and 900 mm (min. 400 mm)) [13,14].
T. aestivum natural occurrence exhibits a broad range distribution through Europe, Asia, and North Africa (Figure 1); however, its cultivation is wider and includes other continents. In Portugal, several edible mushrooms and truffles are collected for both personal consumption and retail sale. Mushroom and truffle hunters are typically rural individuals with a deep, intuitive knowledge gained from years of experience. They rely entirely on their expertise, without the aid of animals, like pigs or dogs, to assist in their mushroom search. It is important to note that many hypogeous sporocarps cannot be identified solely by observing the visual characteristics of the terrain or the presence of their host species, as they are buried more than 10 cm deep, leaving no visible trace of their presence. In such cases, the assistance of trained animals to detect them by their scents is necessary. Bearing this in mind, several mycological expeditions with trained dogs were performed in the last years, and many hypogeous fungi were collected (Santos-Silva, unpublished data), some of them novelties for Portugal. Although the authors had identified various Tuber species, both fructifications and mycelium, none of them were from the T. aestivum group. The main goal of the present study was to reveal, for the first time, the occurrence of the summer truffle in Portugal, and hypothesize about the reasons behind the late detection of this species in this territory. This finding, apart from its ecological importance, opens new opportunities to explore this delicacy in Portuguese gastronomy and can also become an economic asset for the country.
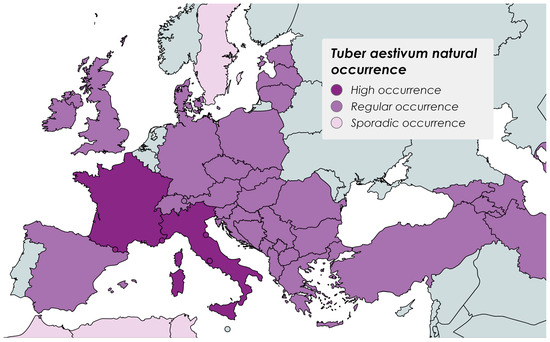
Figure 1.
Countries with Tuber aestivum natural occurrence. Data retrieved from [15,16,17,18,19,20,21,22,23,24], and created with mapchart.net.
2. Materials and Methods
2.1. Truffle Collection and Location
Truffles were collected between April and June 2024, with the assistance of trained dogs, at 3 different locations, in the center (Alenquer and Arruda dos Vinhos) and southern (Salir, Faro) Portugal. Putative tree hosts and organoleptic features of the collected truffles were registered. Fresh truffle specimens were photographed in the field and brought to the laboratory for morphological studies and molecular analysis. Small fragments of each specimen were frozen at −20 °C for DNA amplification, and the remaining were dried at 40 °C and stored in sealed plastic bags, labeled with collection details. The samples were deposited at the Évora University Herbarium (UEVH-FUNGI), Portugal.
2.2. Morphological Study
The external characteristics of ascocarp, including shape, color, and appearance, were recorded in detail from fresh specimens. The ascomata were then dissected, and the morphology of the peridium and gleba was described. Microscopic examination was performed using distilled water and KOH 5%. Spore dimensions were determined based on the measurements of randomly selected (≈100) mature spores, outside asci, mounted in distilled water. Asci and ascospores were examined using a Leica DM750 microscope equipped with a digital camera (Leica ICC50W, Wetzlar, Germany). For species-level determination, ascomata features were compared with descriptions provided by Leonardi et al. [10].
2.3. DNA Extraction, ITS Amplification and Sequencing
Genomic DNA was extracted from approximately 100 mg of fresh ascocarp tissue using the Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, Freiburg, Germany), following the manufacture’s protocol. The ribosomal internal transcribed spacer (ITS) region was amplified via a polymerase chain reaction (PCR) using standard primer pairs ITS4 and ITS5 primers [25]. Each PCR reaction was performed in a total volume of 25 μL, containing 1× DreamTaqTM Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), 7.5 pmol of each forward (ITS5) and reverse primers (ITS4), and approximately 20 ng of total DNA. The PCR program for ITS region amplification was as follows: initial denaturation at 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 s, 54 °C for 45 s, and 72 °C for 1 min, with a final extension at 72 °C for 5 min. PCR products were visualized on 1% agarose gels in 1× TAE buffer, stained with GreenSafe Premium (NZYtech Lda, Lisbon, Portugal), and the GeneRuler 100 bp Plus DNA ladder (Thermo Fisher Scientific, Waltham, MA, USA) was used as a molecular weight marker. The PCR products were purified using the DNA Clean & Concentrator kit (Zymo Research, Freiburg, Germany), and sequenced using the Sanger method with the ITS4 primer by Stab Vida (Lisbon, Portugal).
2.4. Phylogenetic Analyses
The sequence reads were analyzed and edited using BioEdit v.7.2.5 [26]. Trimmed sequences were deposited in the GenBank of the National Center for Biotechnology Information (NCBI) under accession numbers PP928456 to PP928462. For phylogenetic analysis, reference ITS sequences representing the closest relatives of the Tuber aestivum were selected, and the sequences of high nucleotide similarity were retrieved from the GenBank using the BLAST algorithm [27]. A total of 70 Tuber ITS rDNA sequences were used for phylogenetic analysis by including six new sequences of T. aestivum and 64 sequences from GenBank, which correspond to 22 Tuber taxa mainly belonging to Aestivum clade and of European distribution (Table 1). Choiromyces venosus (Fr.) Th. Fr. (JF300146, JF300147) and C. magnusii (Mattir.) Paol. (AH19770) were used as the outgroup.

Table 1.
Details of the ITS sequences used for the construction of the phylogenetic tree.
Sequence alignment was performed using the online version of the multiple-sequence alignment program MUSCLE 3.8.425 [28] and checked manually. The alignment comprised 1249 characters, with 550 parsimony-informative sites. This generated alignment was used for phylogenetic analyses and evolutionary model selection using IQ-TREE v 1.6.12 [29,30]. The best evolutionary model for the ITS marker, as selected by ModelFinder [31], was TNe + I + G4. Both maximum likelihood (ML) and Bayesian methods were used for phylogenetic analyses. The resulting tree is the consensus of 1000 replicates of two phylogenetic analyses; an ultra-fast maximum likelihood analysis [32] with 1000 bootstrap replicates, complemented with a Bayesian approximation branch support analysis [33]. The branch lengths of consensus tree were optimized by maximum likelihood on original alignment. The tree was visualized and edited using iTOL v 6.9.1 [34]. The Robinson–Foulds distance between the ML tree and the consensus tree of 2 indicates that no significant conflicts were found between the ML and Bayesian consensus tree topologies.
3. Results
3.1. Taxonomical Characterization
Morphological and anatomical features of the collected specimens agreed with those provided by Leonardi et al. [10] (Figure 2).

Figure 2.
Tuber aestivum specimens collected in Portugal. (A) Left to right: Giovanni Longo, Pina (dog) and Tanka Sapkota, at 1016 sample location site (https://www.nit.pt/wp-content/uploads/2024/06/49bdcfdd344747a0f30a145d5f6625b1-e1717409790694.jpg, accessed on 28 June 2024). (B) Left to right: Celeste Santos-Silva, Larissa Müller and Figo (dog) at 1022 sample location site (Algarve Truffle Group). (C) T. aestivum ascomata (Larissa Müller). (D). T. aestivum gleba (Celeste Santos-Silva). (E,F) T. aestivum asci and ascospores (Celeste Santos-Silva). Bars: (C,D) = 1 cm; (E,F). = 30 µm.
Family Tuberaceae Dumort.
Genus Tuber P. Micheli ex F.H. Wigg.
Tuber aestivum (Wulfen) Spreng.—Portugal, Arruda dos Vinhos (Lisboa), under Pinus pinea L. stand, 30-IV-2024, sample n. 1015, UEVH 2005855, GenBank PP928456; Portugal, Arruda dos Vinhos (Lisboa), under Pinus pinea L. stand, 20-V-2024, sample n. 1016, UEVH 2005856, GenBank PP928457 (Figure 2A); Portugal, Alenquer (Lisboa), under Pinus pinea L. stand, 26-V-2024, sample n. 1019, UEVH 2005859, GenBank PP928458; Portugal, Alenquer (Lisboa), under Pinus pinea L. stand, 26-V-2024, sample n. 1020, UEVH 2005860, GenBank PP928459; Portugal, Salir (Loulé, Faro), under Quercus rotundifolia Lam. stand, 09-VI-2024, sample n. 1022, UEVH 2005862, GenBank PP928460 (Figure 2B); Portugal, Salir (Loulé, Faro), under Quercus rotundifolia Lam. stand, 09-VI-2024, sample n. 1023, UEVH 2005863, GenBank PP928461; Portugal, Salir (Loulé, Faro), under Quercus rotundifolia Lam. stand, 09-VI-2024, sample n. 1024, UEVH 2005864, GenBank PP928462.
3.2. Phylogenetic Analyses
Phylogenetic analyses show that our specimens are assigned to the T. aestivum nest within the Aestivum clade, which is congruent with the morphological analyses (Figure 3). The consensus tree of Bayesian approximation and maximum likelihood resolves the T. aestivum/T. uncinatum as an independent, monophyletic and well-supported clade. This species appears as the sister clade of T. sinoaestivum. In addition, the ITS sequence of two Portuguese specimens, T. aestivum voucher UEVH 2005863 and T. aestivum voucher UEVH 2005864, had a 100% nucleotide similarity with the ITS sequence of the T. aestivum strain E17 from Italy, while the ITS sequence of T. aestivum voucher UEVH 2005859 and T. aestivum voucher UEVH 2005862 had a 100% nucleotide similarity with the T. aestivum strain E60 from Italy.
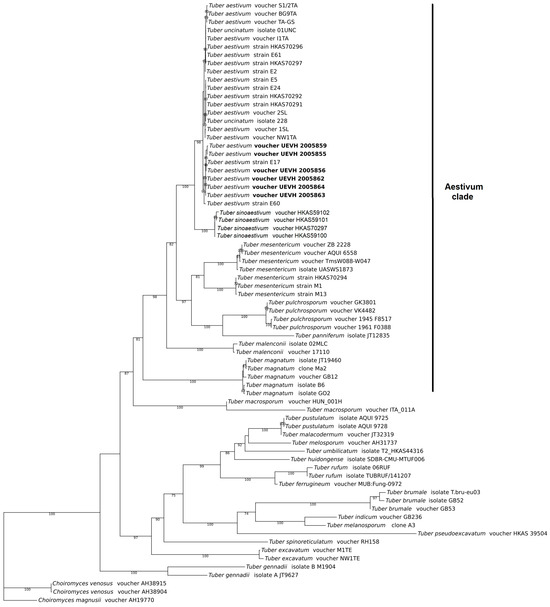
Figure 3.
Phylogenetic placement of Tuber aestivum specimens obtained in this study (bold) in the Aestivum clade. The consensus tree represents a Bayesian approximation with 1000 generations and a maximum likelihood analysis with 1000 bootstrap replicates. The tree is based on the ITS rDNA sequence alignment of 70 sequences assigned to 22 Tuber taxa. ITS rDNA sequences of Choiromyces venosus and C. magnusii were used as the outgroup. Bootstrap supports are shown at the nodes of the branches.
4. Discussion
The Aestivum clade comprises species associated with a wide range of host plants and is found in Europe, North Africa, and Asia [35], and most recently in America [1]. Notable species include T. aestivum Vittad. (the type species of this clade), T. panniferum Tul. and Tul., T. malenconii Donadini, Riousset, G. Riousset and G. Chev., and T. mesentericum Vittad., T. sinoaestivum Zhang and Liu from China, T. magnatum Picco and along with the recently described T. pulchrosporum Konstantinidis, Tsampazis, Slavova, Nakkas, Polemis, Fryssouli and Zervakis. Tuber aestivum (Wulfen) Spreng. (including T. uncinatum Chatin) and T. sinoaestivum J.P. Zhang and P.G. Liu specimens can be easily distinguished macroscopically by their blackish peridial surface with prominent pyramidal warts and ascospores bearing a complete reticulum. Both macro- and microscopical characteristics align with the T. aestivum descriptions provided by Leonardi et al. [10]. Additionally, molecular analysis revealed that the specimens collected in Portugal cluster with the T. aestivum specimens from other locations, confirming their identification as T. aestivum. Therefore, surprisingly, this is the first report of T. aestivum in mainland Portugal based on morphological and molecular analyses.
Several factors might explain why this is the first time the summer truffle has been reported in Portugal. One possible explanation is the use of trained dogs, which detect truffles underground without any surface indication. However, it is important to note that the initial signs of truffle presence were observed due to soil disturbance caused by wild boars, which unearthed some truffles. Another possible explanation is the exceptional weather conditions preceding the current fruiting season. Precipitation from September 2023 to May 2024 was above the climatological norm for six out of nine months (Figure 4). Notably, October 2024 was the fourth wettest month since 1941. Additionally, air temperatures during this period were higher than average. It is thus likely that the unusually warm and wet weather conditions likely contributed to the atypical fruiting of T. aestivum in 2024, which, in combination with animal behavior (wild boars), allowed for the detection of these species. Another possible explanation for the detection of summer truffle only in the present year could be related to shifts in habitat conditions or climate change, which might have made these regions suitable for the presence of this fungus and consequently its fruit body development.
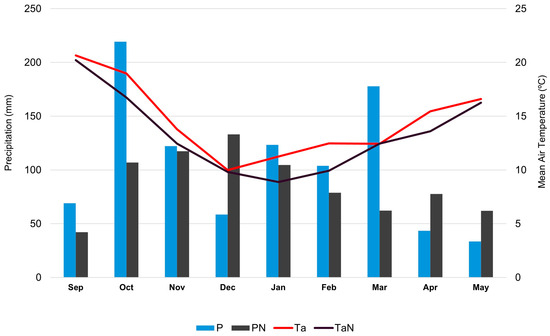
Figure 4.
Continental Portugal month precipitation (P) and the mean air temperature (Ta) from September 2023 to May 2024. Climatological standard normals (1941–2023) per month for precipitation (PN) and mean air temperature (TaN) (data from https://www.ipma.pt/pt/publicacoes/, accessed on 26 June 2024).
More studies are being conducted in areas where T. aestivum has been discovered to determine the biotic and abiotic soil conditions that may explain the appearance, persistence, and fruiting of summer truffles. These studies could also provide insights into other potential locations for these truffles. Targeted field trips with trained dogs will follow to investigate the presence of T. aestivum and identify its possible hosts. Pursuing this line of research can reveal new ecological relationships between truffles, their host trees, and other organisms, and deepen our understanding of the intricate web of life.
Author Contributions
C.S.-S.: conceptualization, specimen collection, morphological analysis, validation, investigation, resources, writing—draft and revision, funding acquisition; C.B.: molecular analyses, validation, investigation, resources, writing—draft and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financed by National Funds through FCT—Foundation for Science and Technology within the scope of project UIDB/05183/2020.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank to MED (https://doi.org/10.54499/UIDB/05183/2020 (accessed on 5 August 2024); https://doi.org/10.54499/UIDP/05183/2020 (accessed on 5 August 2024)) and to CHANGE (https://doi.org/10.54499/LA/P/0121/2020 (accessed on 5 August 2024)). We are grateful to Tanka Sapkota and to Larissa Möller, Nelli Eleonore dos Santos (Algarve Truffle Group (ATG)), Figo and Nala (Larissa and Nelli dogs). We sincerely thank Tânia Nobre for her help in refining the English language of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBIF.org. GBIF Occurrence. Available online: https://www.gbif.org/occurrence/download/0044310-240626123714530 (accessed on 1 August 2024).
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández-Robles, D.R.; Plata-Corredor, C.A.; Stjernegaard-Jeppesen, T.; Örn, A.; Vandepitte, L.; Hobern, D.; et al. Catalogue of Life (Version 2024-07-18) 2024; Catalogue of Life: Amsterdam, Netherlands, 2024. [Google Scholar] [CrossRef]
- Kirk, P. Index Fungorum. Available online: https://www.indexfungorum.org/names/names.asp (accessed on 1 August 2024).
- GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 1 August 2024).
- Santos-Silva, C.; Louro, R.; Raposo, J. Herdade de Margens do Sol Posto e do Sol Nascente; Universidade de Évora: Évora, Portugal, 2024; p. 37. ISBN 978-972-778-380-9. [Google Scholar]
- Mustafa, A.M.; Angeloni, S.; Nzekoue, F.K.; Abouelenein, D.; Sagratini, G.; Caprioli, G.; Torregiani, E. An Overview on Truffle Aroma and Main Volatile Compounds. Molecules 2020, 25, 5948. [Google Scholar] [CrossRef]
- Oliach, D.; Vidale, E.; Brenko, A.; Marois, O.; Andrighetto, N.; Stara, K.; Martínez de Aragón, J.; Colinas, C.; Bonet, J.A. Truffle Market Evolution: An Application of the Delphi Method. Forests 2021, 12, 1174. [Google Scholar] [CrossRef]
- Marathe, S.J.; Hamzi, W.; Bashein, A.M.; Deska, J.; Seppänen-Laakso, T.; Singhal, R.S.; Shamekh, S. Anti-angiogenic and anti-inflammatory activity of the summer truffle (Tuber aestivum Vittad.) extracts and a correlation with the chemical constituents identified therein. Food Res. Int. 2020, 137, 109699. [Google Scholar] [CrossRef]
- Chevalier, G.; Frochot, G. La Truffe de Bourgogne, Tuber Uncinatum Chatin; Petrarque: Levallois-Perret, France, 1997; p. 258. ISBN 9782911730016. [Google Scholar]
- Leonardi, M.; Iotti, M.; Mello, A.; Vizzini, A.; Paz-Conde, A.; Trappe, J.; Pacioni, G. Typification of the four most investigated and valuable truffles: Tuber aestivum Vittad., T. borchii Vittad., T. magnatum Picco and T. melanosporum Vittad. Cryptogam. Mycol. 2021, 42, 149–170. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Sierota, Z.; Palenzona, M. New tuber species found in Poland. Mycorrhiza 2008, 18, 223–226. [Google Scholar] [CrossRef]
- Piñuela, Y.; Alday, J.G.; Oliach, D.; Castaño, C.; Büntgen, U.; Egli, S.; Martínez Peña, F.; Dashevskaya, S.; Colinas, C.; Peter, M.; et al. Habitat is more important than climate for structuring soil fungal communities associated in truffle sites. Fungal Biol. 2024, 128, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, M.; Moreno-Arroyo, B.; Pulido, E.; Sánchez, M. Manual de Truficultura Andaluza; Consejería de Medio Ambiente: Junta de Andalucía, Spain, 2007; p. 176. ISBN 9788493519438. [Google Scholar]
- Stobbe, U.; Egli, S.; Tegel, W.; Peter, M.; Sproll, L.; Büntgen, U. Potential and limitations of Burgundy truffle cultivation. Appl. Microbiol. Biotechnol. 2013, 97, 5215–5224. [Google Scholar] [CrossRef] [PubMed]
- Bagi, I.; Fekete, A.O. Identification of Tuber aestivum habitats in the South Caucasus, Azerbaijan. In Proceedings of the 2nd Congress of the Tuber aestivum/uncinatum European Scientific Group, Juva, Finland, 20–22 August 2010; p. 27. [Google Scholar]
- Bratek, Z.; Merényi, Z.; Illyés, Z.; László, P.; Anton, A.; Papp, L.; Merkl, O.; Garay, J.; Viktor, J.; Brandt, S. Studies on the ecophysiology of Tuber aestivum populations in the Carpatho-Pannonian region. Acta Mycol. 2010, 47, 221–226. [Google Scholar]
- Dimitrova, E.; Gyosheva, M. Hypogeous ascomycetes in Bulgaria. Phytol. Balc. 2008, 14, 309–314. [Google Scholar]
- Global Biodiversity Information Facility GBIF. Species Search: Tuber aestivum. Available online: http://data.gbif.org/species/5258469/ (accessed on 28 June 2024).
- Jeandroz, S.; Murat, C.; Wang, Y.J.; Bonfante, P.; Le Tacon, F. Molecular phylogeny and historical biogeography of the genus Tuber the ‘true truffles’. J. Biogeogr. 2008, 35, 815–829. [Google Scholar] [CrossRef]
- Jurc, D.; Piltaver, A.; Ogris, N. Fungi in Slovenia: Species and distribution. In Studia Forestalia Slovenia; Biotechnical Faculty: Ljubljana, Slovenia, 2005; p. 407. Available online: https://api.semanticscholar.org/CorpusID:127819716 (accessed on 1 August 2023).
- Lucian, D.; Fekete, A.; Ionut, N.; Gheroghe, M. Tuber aestivum/uncinatum in Rumania, Sites, characteristics and harvesting periods. In Proceedings of the 4th Congress of the Tuber aestivum/uncinatum European Scientific Group, Gödöllö, Hungary, 28–30 September 2012; p. 15. [Google Scholar]
- Meiere, D.; Liepina, L.; Vimba, E. Truffles in Latvia: History and future perspectives. In Proceedings of the 2nd Congress of the Tuber aestivum/uncinatum European Scientific Group, Juva, Finland, 20–22 August 2010; p. 31. [Google Scholar]
- Milenkovic´, M.; Marjanovic´, Ž. Current results on Tuber spp. research in Yugoslavia. In Proceedings of the 5th International Congress “Science and Cultivation on Tuber and other edible hypogeus fungi”, Aix-en-Provence, France, 4–6 March 2020; pp. 4218–4225. [Google Scholar]
- Petrović, N.; Kosanić, M.; Ranković, B. The diversity of macromycetes in the territory of Batočina (Serbia). Kragujev. J. Sci. 2019, 41, 117–132. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for WINDOWS 95/98/ NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.-F.; Dessimoz, C.; Gascuel, O. Survey of Branch Support Methods Demonstrates Accuracy, Power, and Robustness of Fast Likelihood-based Approximation Schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Bonito, G.; Smith, M.E.; Nowak, M.; Healy, R.A.; Guevara, G.; Cázares, E.; Kinoshita, A.; Nouhra, E.R.; Domínguez, L.S.; Tedersoo, L.; et al. Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified Southern Hemisphere sister lineage. PLoS ONE 2023, 8, e52765. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).