Abstract
(1) Background: Influenza is a viral infection that has claimed many millions of lives over the past 100 years, and there is always a risk that a new influenza virus will emerge and cause another pandemic. One way to reduce such a potential new influenza virus will be heat inactivation. The question in this study is how much the heat sensitivities of previous influenza viruses differ. If they are very similar, it is expected that a new influenza virus can be inactivated with the same heat parameters as previous influenza viruses. (2) Methods: Through a literature search, published heat inactivation results are compiled and analyzed using Arrhenius models and regression equations for decimal reduction times for different temperatures and media determined. (3) Results: There are about 50 studies on heat inactivation of human and avian influenza viruses so far, showing large differences in heat sensitivity of influenza viruses in different media. However, within a single medium the differences between viruses are rather small. (4) Conclusions: At a temperature of 60 °C, previous influenza viruses can be reduced by 4 or more orders of magnitude within approximately 30 min in almost all media, and this is likely to be true for a potential new influenza virus. Further studies, especially on human influenza viruses, would be desirable.
1. Introduction
The coronavirus pandemic, which began back in 2019, is still ongoing in 2022, and the number of new COVID-19 (Coronavirus Disease 2019) infections worldwide is currently around 1 million per day [1]. Most of this transmission is airborne. Surfaces and other potential carriers seem to play a minor role [2,3,4].
Influenza is an infection that has many similarities with COVID. The source of severe respiratory illnesses in humans, which can occur in a pandemic, is also an enveloped RNA virus that can also be transmitted via air. Unlike coronaviruses, however, influenza viruses can remain infectious for prolonged periods on surfaces, in liquids, or on other fomites [5,6,7].
The triggers of human influenza infections are influenza viruses of types A and B, with type A appearing in the form of many subtypes that are distinguished on the basis of their surface proteins hemagglutinin (H1 to H18) and neuraminidase (N1 to N11). For example, the trigger of the largest influenza pandemic to date, the Spanish flu of 1918, with its estimated 50 million deaths, was an influenza A virus of the subtype H1N1 [8]. All subsequent influenza pandemics have also been caused by influenza A viruses [8]. Such a pandemic can be caused by humanity coming into contact for the first time with a new influenza A virus subtype for which no immunity exists in the population. Such a new subtype may appear, for example, when an influenza virus jumps from an animal to a human host. In the past, poultry and pigs have been particularly relevant in this regard, with water fowl considered the natural reservoir of influenza viruses [8,9].
In case of detected influenza infections in humans or animals—it is tried to stop the spread of the virus. For this purpose, it is necessary to inactivate influenza viruses on various fomites. These could be liquids like water, surfaces and animal foodstuffs.
Chemical disinfection and UV radiation are common and effective disinfection techniques, but they cannot always be applied or, in the case of UV radiation for example, may not reach the viruses. Heat inactivation is another well-known disinfection approach that also works in bulk materials. In this process, heat inactivates mainly the relevant viral proteins [10].
There are already some published studies that clearly demonstrate the effect of heat on influenza viruses [11,12,13,14,15,16,17]. However, so far, mostly only individual influenza A virus subtypes have been investigated for some contaminated media like phosphate buffered saline (PBS), animal food or filtering facepiece material [18,19,20,21,22]. It would be desirable to be able to make general statements on the temperature sensitivity of all influenza viruses in all relevant media. In case of the emergence of a new pandemic influenza virus, simple heat inactivation protocol suggestions would be already available.
Therefore, in the study presented here, published heat inactivation data for influenza viruses are collected and analyzed to estimate necessary heat application durations for 90% reduction, the so-called decimal reduction time or D value, for different temperatures and media. As a mathematical basis, it is assumed that virus inactivation follows at least approximately an exponential course:
c(t) is the concentration of non-inactivated viruses at time t and k(T) is the inactivation rate at temperature T (in Kelvin). In this representation, the reciprocal of the inactivation rate k(T) is equal to the necessary decimal reduction time D(T) for a 90% inactivation:
In order to compare virus data for different temperatures, a simple model is also applied for the temperature dependence of the inactivation rate, in which it is assumed that the inactivation rate depends exponentially on the temperature T:
with the temperature-independent parameters a and b. In this representation, the logarithm (base 10) of D(T) is a linear function of 1/T.
This approach is the so-called Arrhenius model, which was proposed by Hiatt 1964 [23], among others, and has been successfully applied for a variety of virus inactivation analyses, e.g. influenza viruses [12,24] and also many other viruses [25,26,27,28,29,30,31].
2. Materials and Methods
Pubmed and Google Scholar were searched for various combinations of the terms: “influenza”, “flu”, “heat”, “disinfection”, “inactivation”, “reduction” and “sterilization”. Matching publications were examined to determine if they could also be included in this study. In addition, the suitability of all recent publications that cited the previously found sources was reviewed.
When assessing the suitability of studies, only those that addressed the effect of heat of ≥40 °C were included. Studies involving lower temperatures or the simultaneous application of other potentially inactivating measures were not considered. Because high and low pH values can also have an inactivating effect, only studies with mean pH values between 5.5 and 8.5 were included.
The quantitative data required for this analysis were often not directly provided in the retrieved publications. In such cases, it was attempted to determine quantitative values from graphs or tables. For example, in tables of infected or dead chicken embryos, the values for EID50 (embryo infectious dose 50) or ELD50 (embryo lethal dose 50) were determined analogously to the procedure of Reed and Muench [32]. In some cases, the contaminated medium was not explicitly named. In that case, it was assumed that the medium in which the viruses were propagated was also used for the inactivation experiments.
The parameters for the Arrhenius model discussed above were then determined for each medium separately, using linear regression and D(T) was plotted as a linear function of 1/T in each case. With the help of the determined temperature dependence of D(T) for the different contaminated media, expected decimal reduction times for different potential inactivation temperatures are calculated at the end.
3. Results
In total, about 50 publications on heat inactivation were retrieved, the oldest of which is almost 75 years old [11]. The overview of all inactivation data found is given in Table 1. In all cases, influenza virus reduction by the application of heat was observed, but in some cases quantitative analysis was impossible. This was the case, for example, when the temperature changed over the observation period or a virus concentration was below the detection limit after heat application. Where possible, the inactivation duration D for 90% inactivation for the respective virus at the specified temperature is given in Table 1. The publication by Chu mentioned above [11] is the only evaluable study on heat inactivation of influenza B viruses. Otherwise, the results are exclusively for influenza A viruses.

Table 1.
Overview of published influenza virus thermal inactivation experiments with virus (sub-type), medium and determined decimal reduction time D(T). (* experiments were not included in the quantitative analysis. PBS: phosphate buffered saline, MEM: minimal essential medium, DMEM: Dulbecco’s Modified Eagle’s Medium, RH: relative humidity).
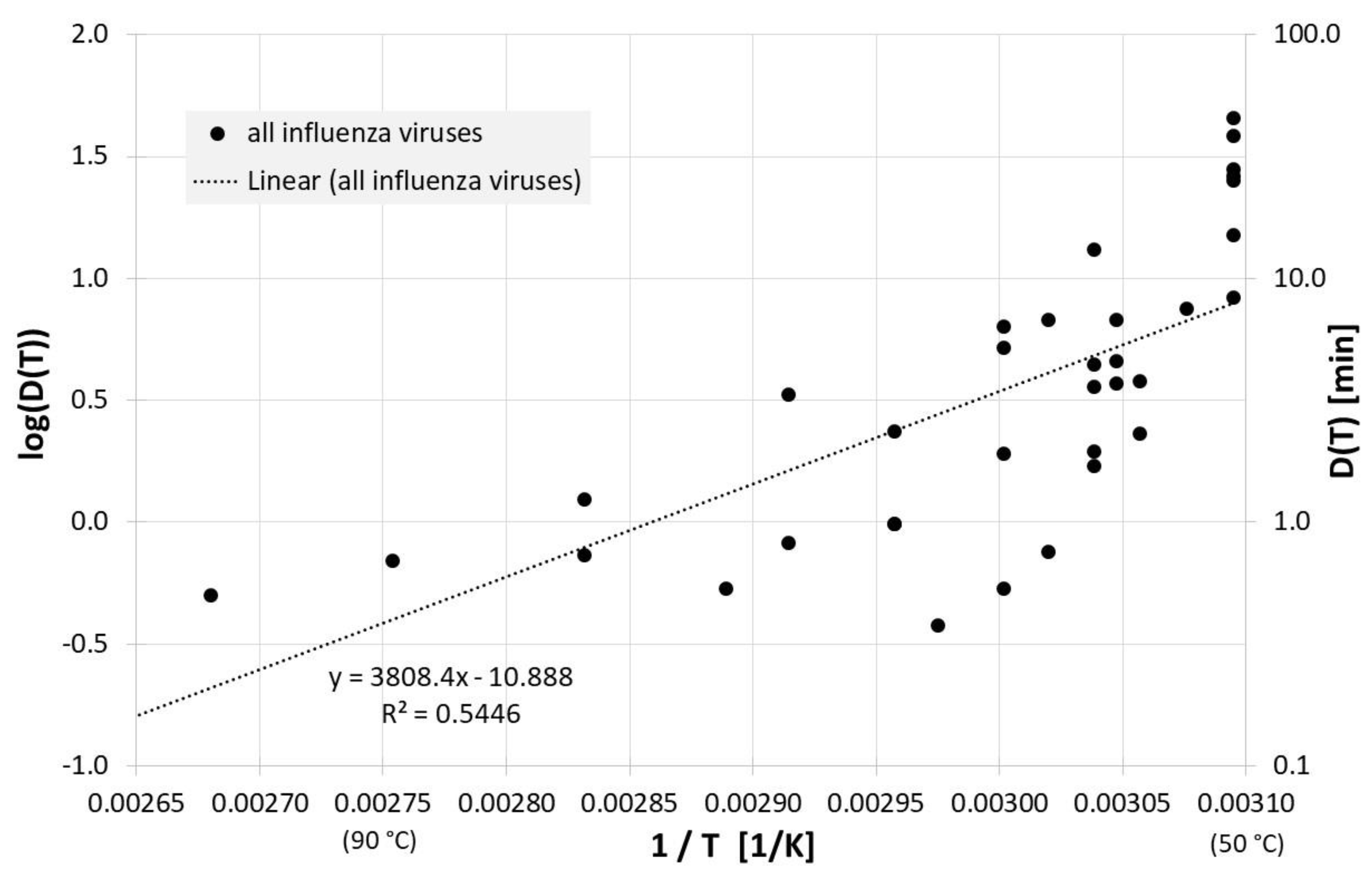
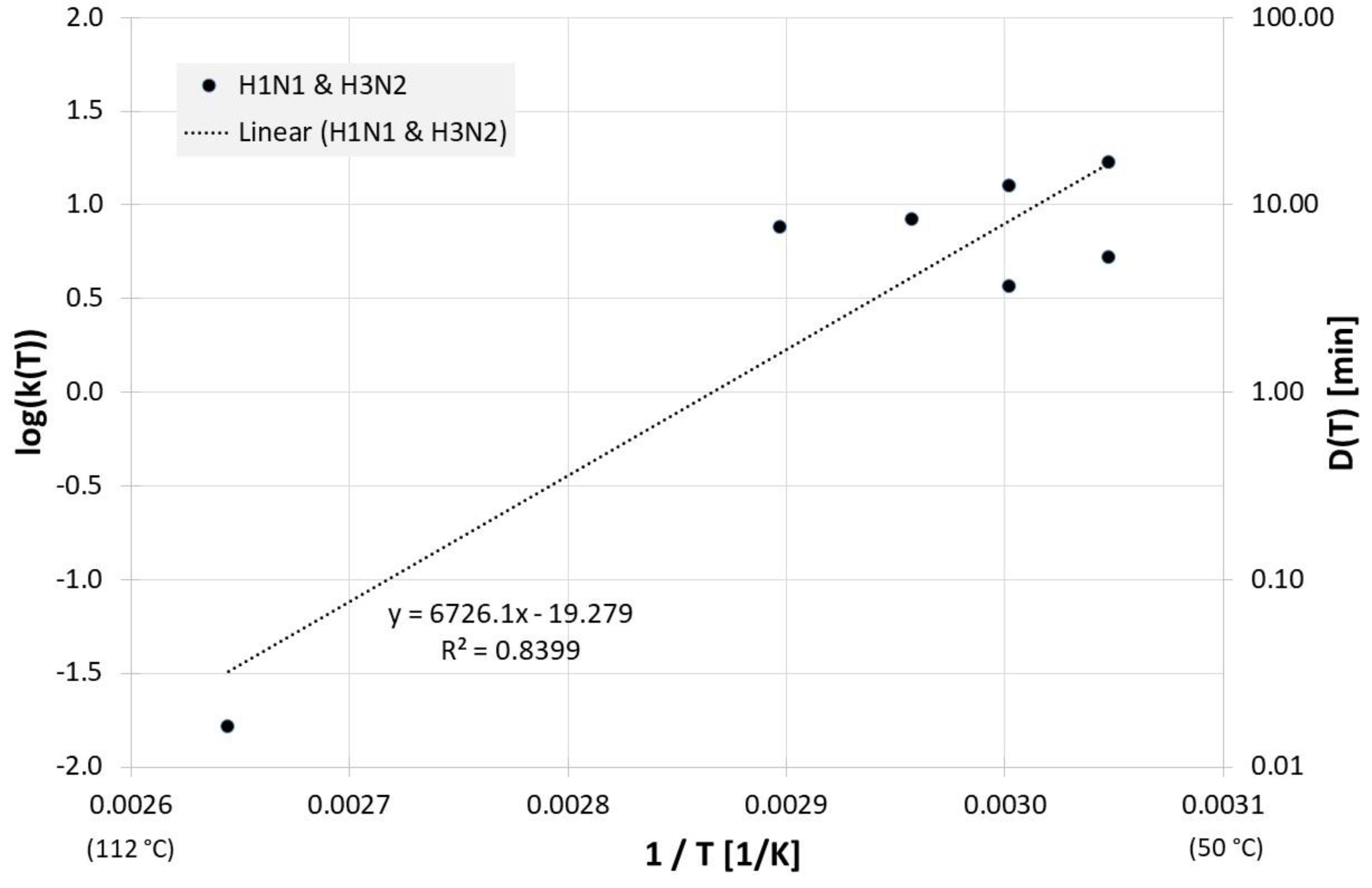
Figure 1 gives an overview of the correlation between 1/T and log(D(T)) for all influenza inactivation results in liquids except liquid animal foods or waste. Log(D(T)) = 0 indicates a decimal reduction time of 1 min, log(D(T)) = 1 is 10 min, and log(D(T)) = −1 represents 0.1 min. Also revealed is the result of a linear regression for D(T). The high scattering or deviation of the individual results from the regression curve is also represented in the relatively low square of the regression coefficient R2. For the corresponding Dlin regress(T) from linear regression holds:
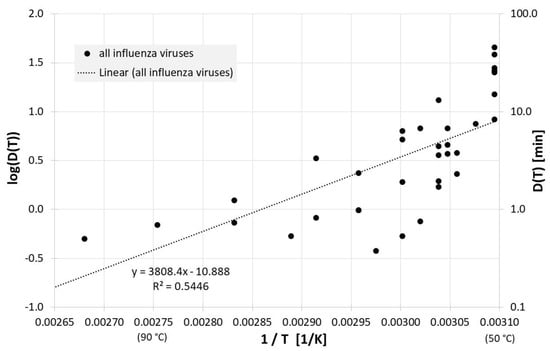
Figure 1.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in liquids other than animal foods or waste along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
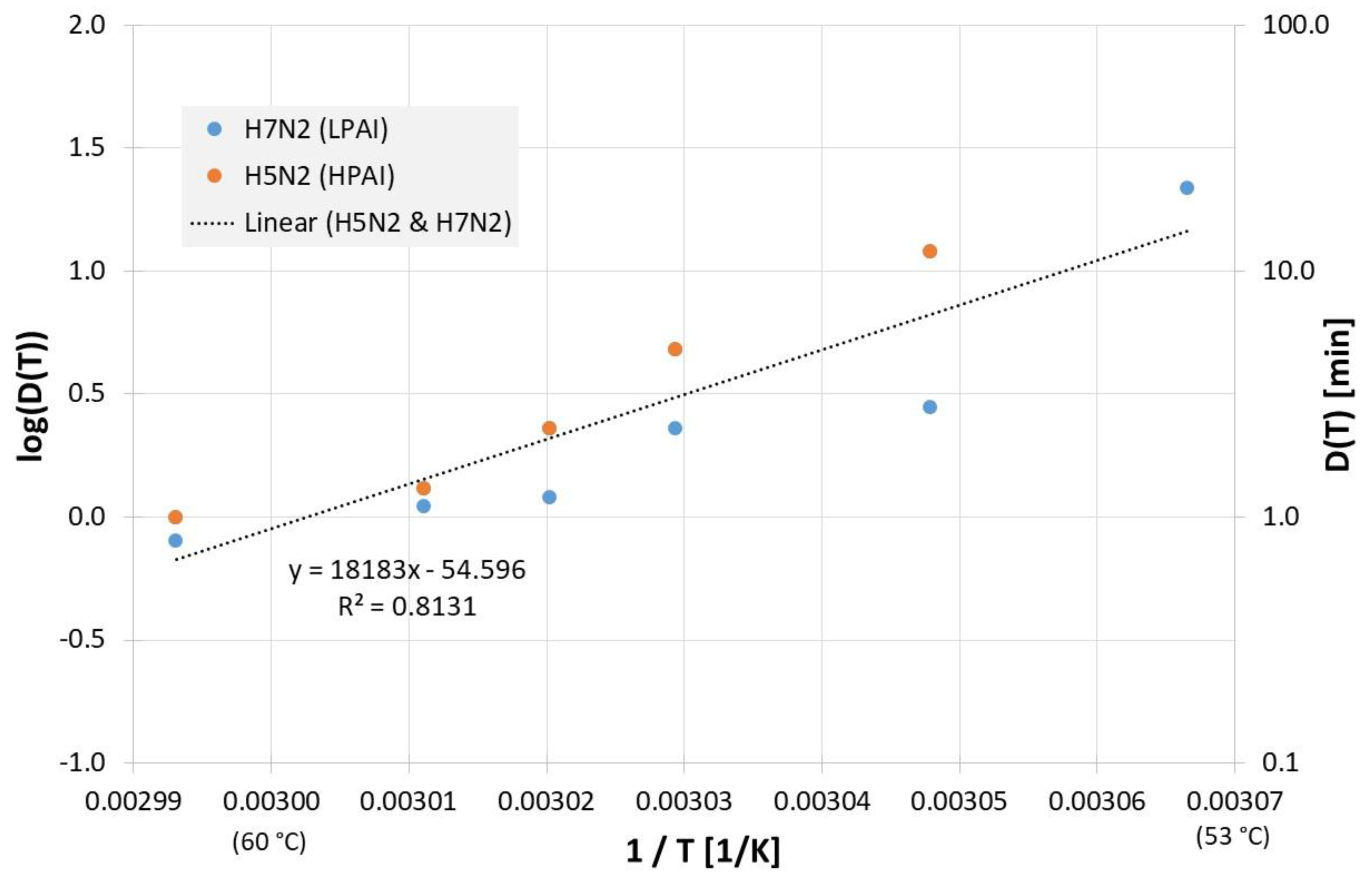
Due to the scatter of the data, the most often investigated liquids PBS (phosphate buffered saline), allantoic fluid and cell culture medium were also analyzed separately. Figure 2 reveals all results of evaluable inactivation experiments from Figure 1, which were performed in PBS. These are data from Chmielewski et al. [18] for one low pathogenic avian influenza virus subtype (LPAI) and one high pathogenic avian influenza virus subtype (HPAI). Within the limits of their scattering, the values for the two virus subtypes are relatively close to each other and, because of the small data base, no reliable conclusions can be drawn about differences between these viruses. For Dlin regress(T) the derived formula from the linear regression is:
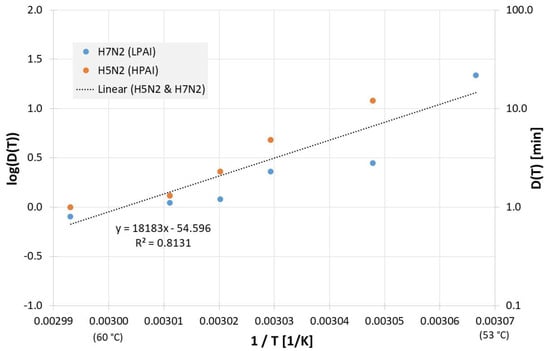
Figure 2.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in PBS along with the linear regression curve and for better understanding, D(T) is also given on the right Y-axis in a logarithmic scale. (LPAI: low pathogenic avian influenza, HPAI: high pathogenic avian influenza).
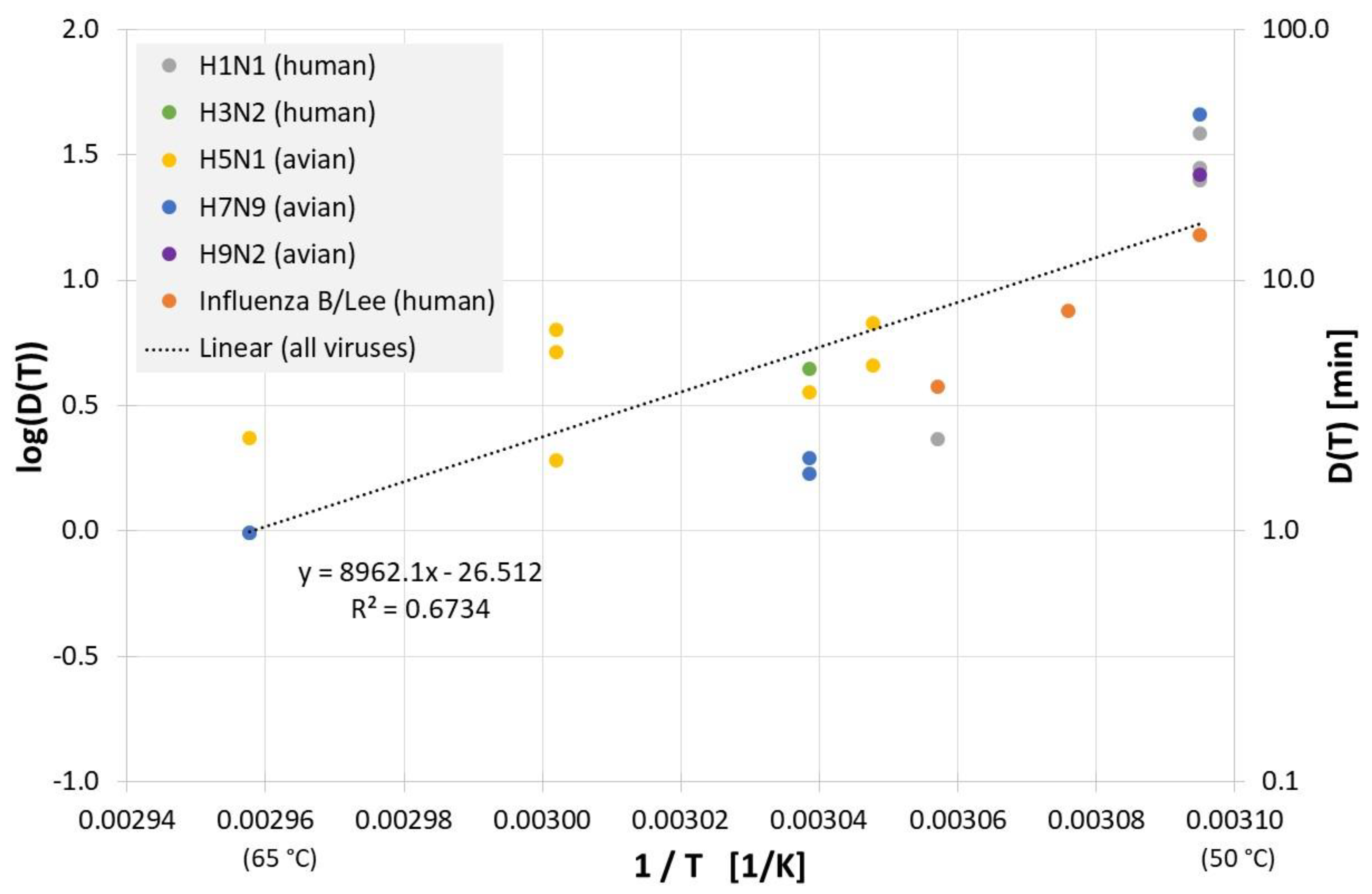
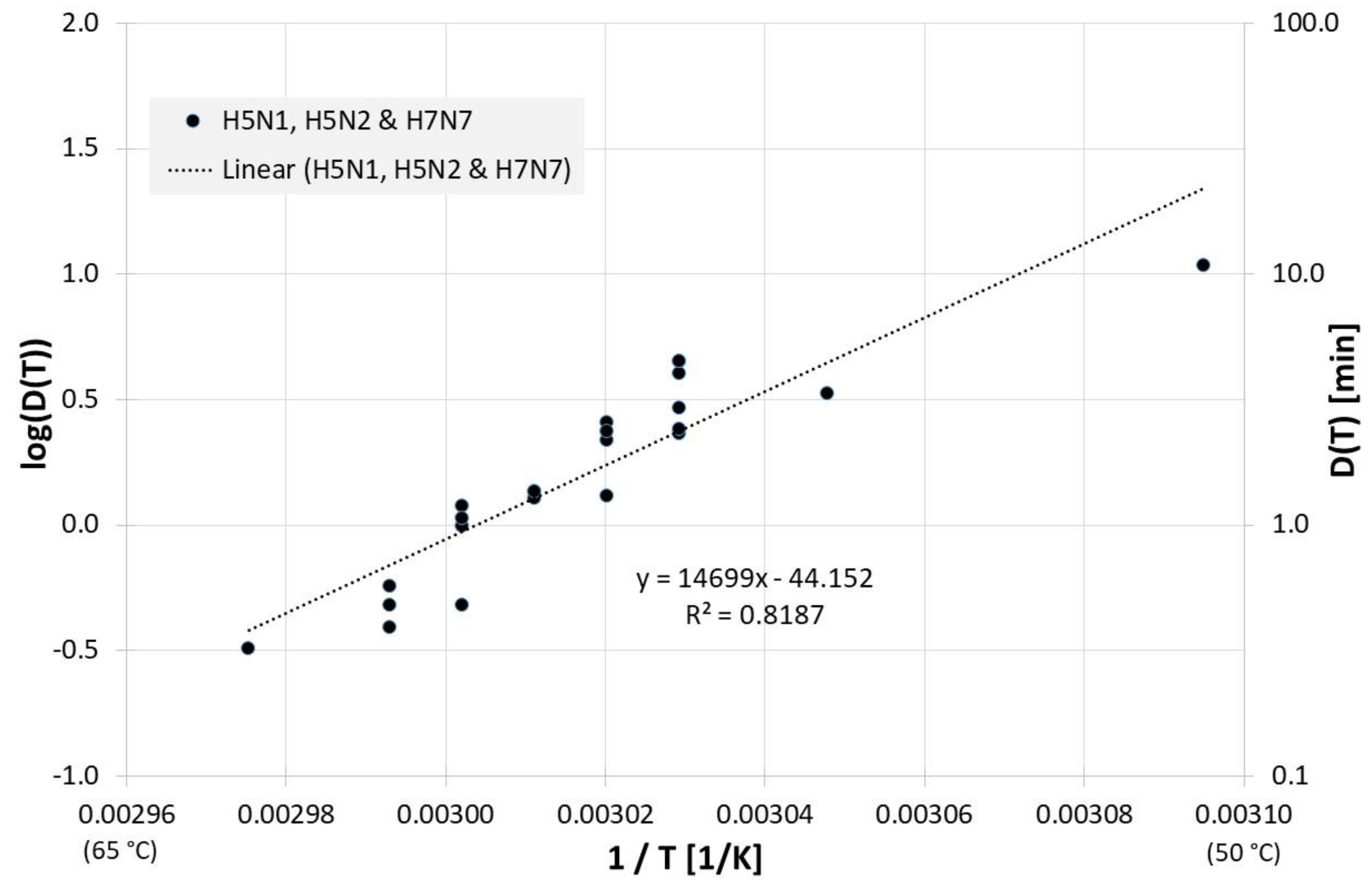
All results of evaluable inactivation experiments from Figure 1, which were performed in allantoic fluid are presented in Figure 3. Data exist for various influenza A virus subtypes and even for influenza B, but due to the small data base and the scatter of the individual values, no reliable conclusion can be drawn about differences in the temperature sensitivity of these influenza viruses. Based on data of all viruses in Figure 3 the decimal reduction time Dlin regress(T) is:
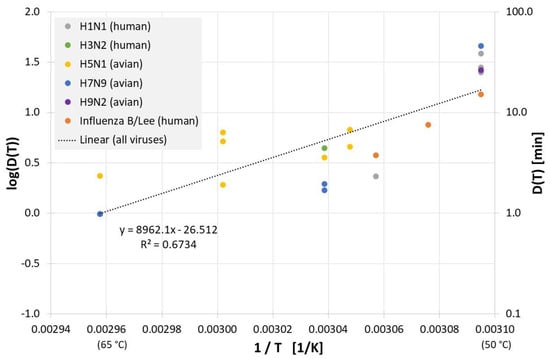
Figure 3.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in allantoic fluid along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
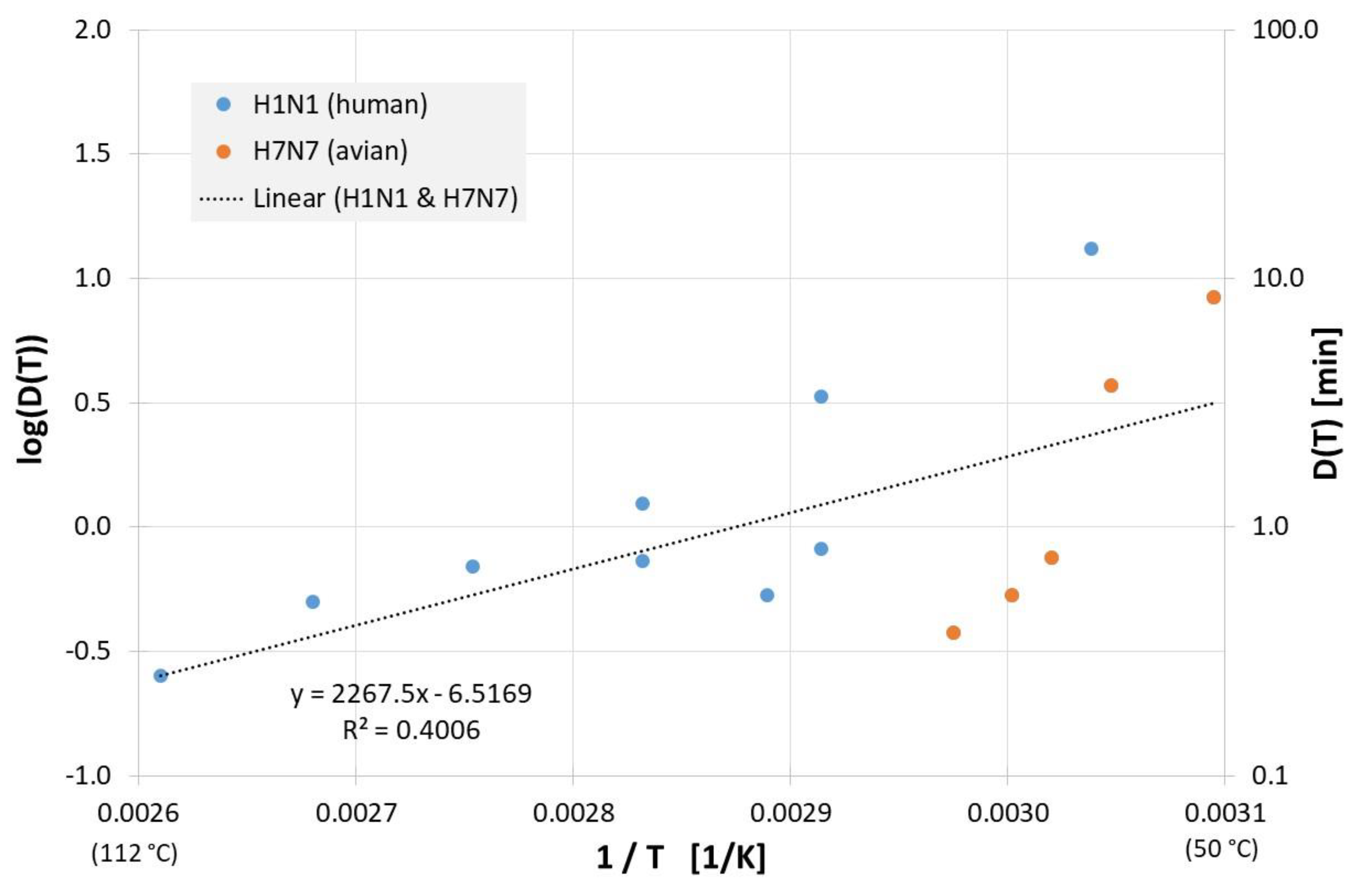
Figure 4 shows the results from evaluable inactivation experiments performed in cell culture media. Again, data exist for different influenza A virus subtypes (H1N1 and H7N7), but because the very limited database and different employed cell culture media, no meaningful conclusions can be reached about susceptibility differences between the different influenza viruses. Based on data of all viruses in Figure 4 the decimal reduction time Dlin regress(T) is:
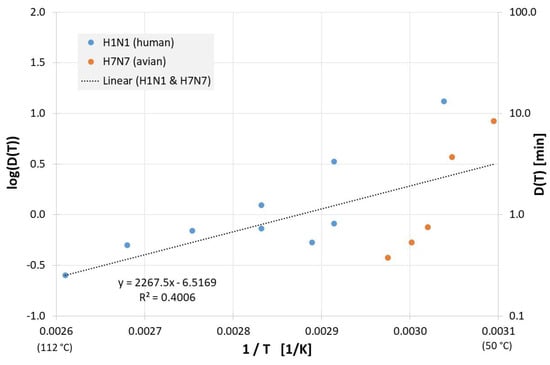
Figure 4.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in cell culture medium along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
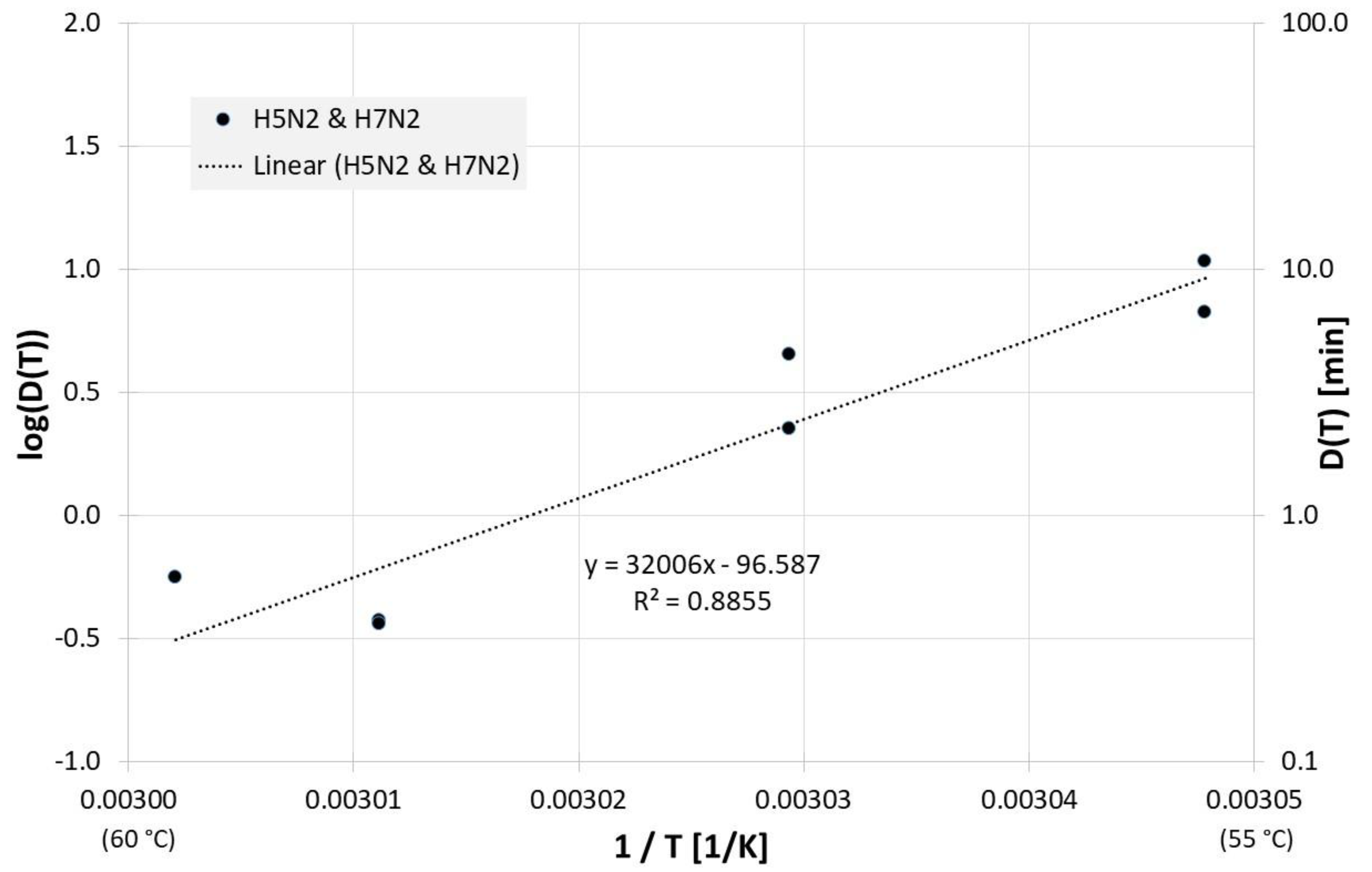
The correlation between 1/T and log(D(T)) for all influenza inactivation results on surfaces is presented in Figure 5. For the corresponding Dlin regress(T) from the linear regression is:
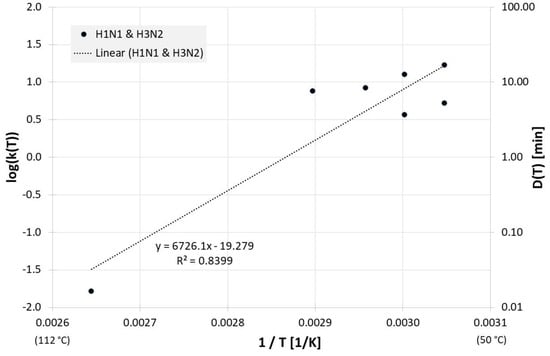
Figure 5.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data on surfaces along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
Many published studies deal with the inactivation of influenza viruses in products or waste from the poultry industry. For example, Figure 6 presents the correlation between 1/T and log(D(T)) for all influenza inactivation results in chicken meat. Figure 6 also includes the result of a linear regression for the decimal reduction time Dlin regress(T):
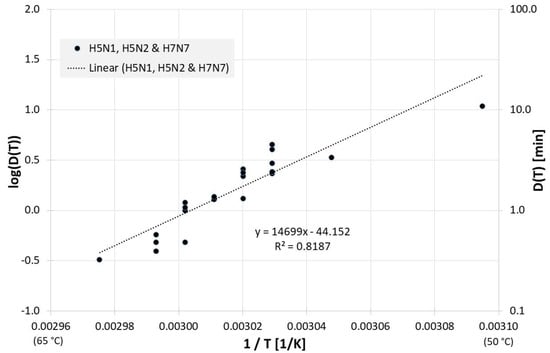
Figure 6.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in chicken meat along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
Figure 7 shows the corresponding correlation for homogenized whole egg and the decimal reduction time Dlin regress(T) is:
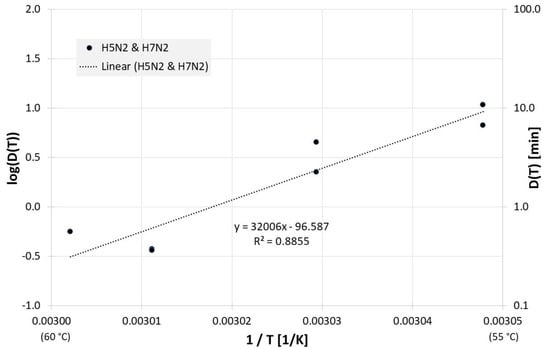
Figure 7.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in homogenized whole egg along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
The published inactivation data for dried egg white can be viewed in Figure 8. For the decimal reduction time Dlin regress(T) applies here:
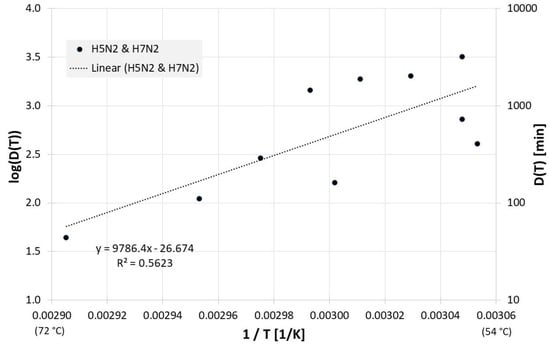
Figure 8.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in dried egg white along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
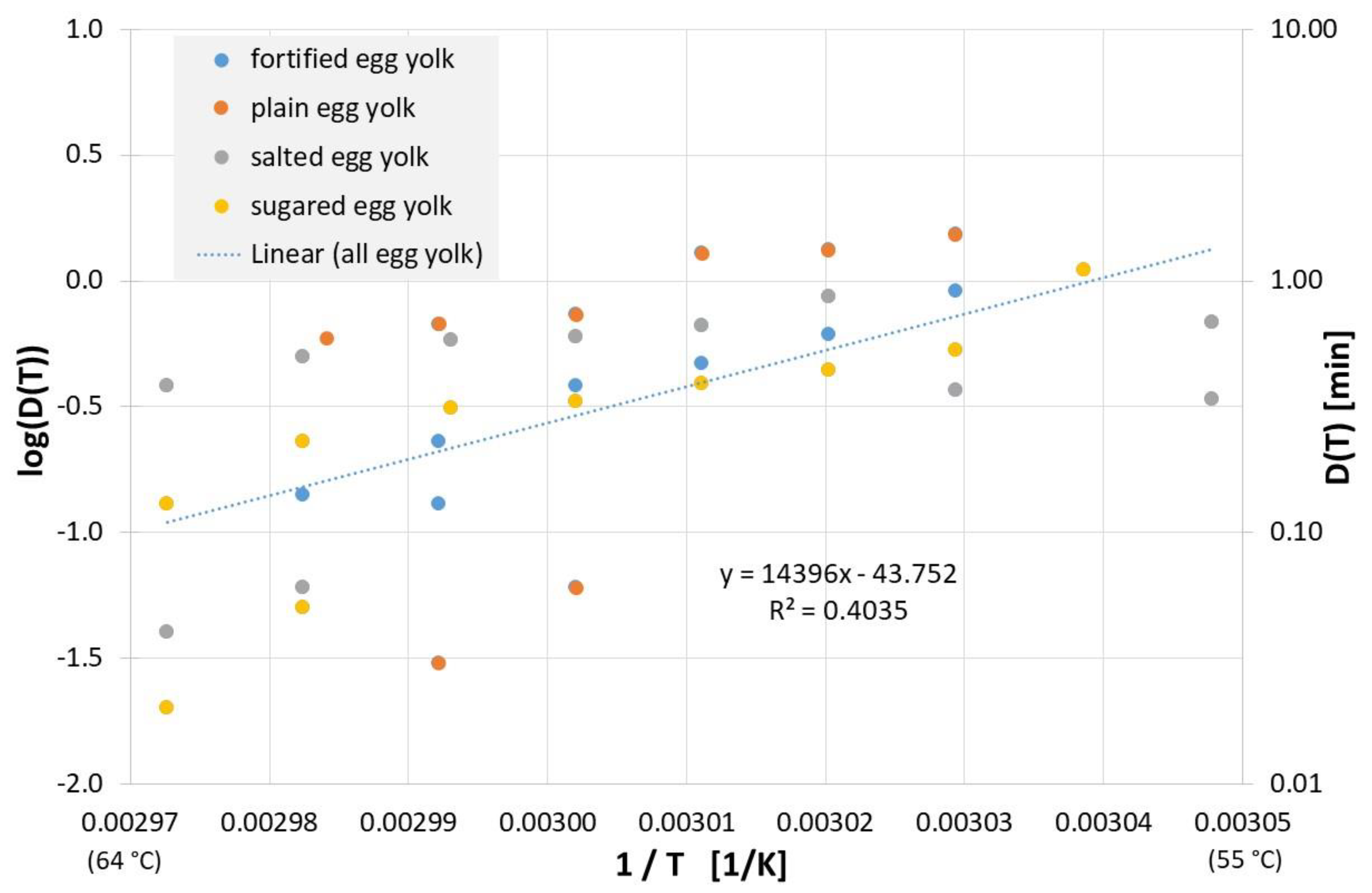
For plain, salted, sweetened, or fortified egg yolk, the inactivation results published to date can be found in Figure 9, and linear regression of all data yields the following equation for the decimal reduction time Dlin regress(T):
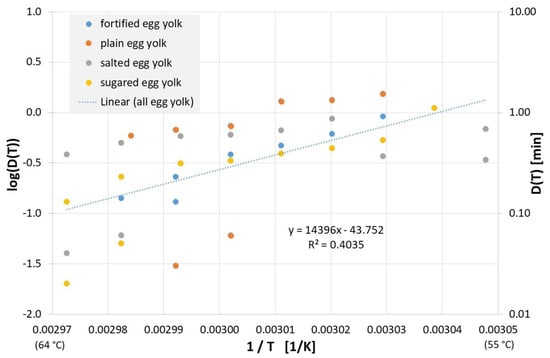
Figure 9.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in plain, sugared, salted and fortified egg yolk along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
Inactivation results also exist for fat-free eggs and egg replacements and are depicted in Figure 10, along with the regression line for the decimal reduction time Dlin regress(T):
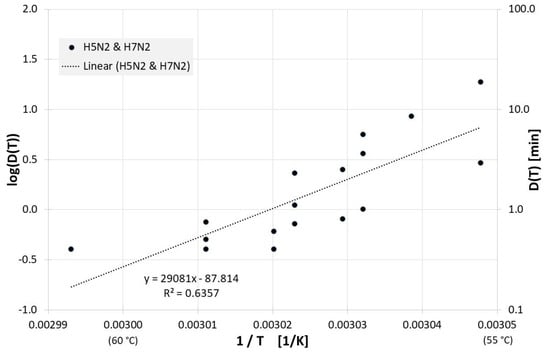
Figure 10.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in in fat free egg and egg substitutes along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
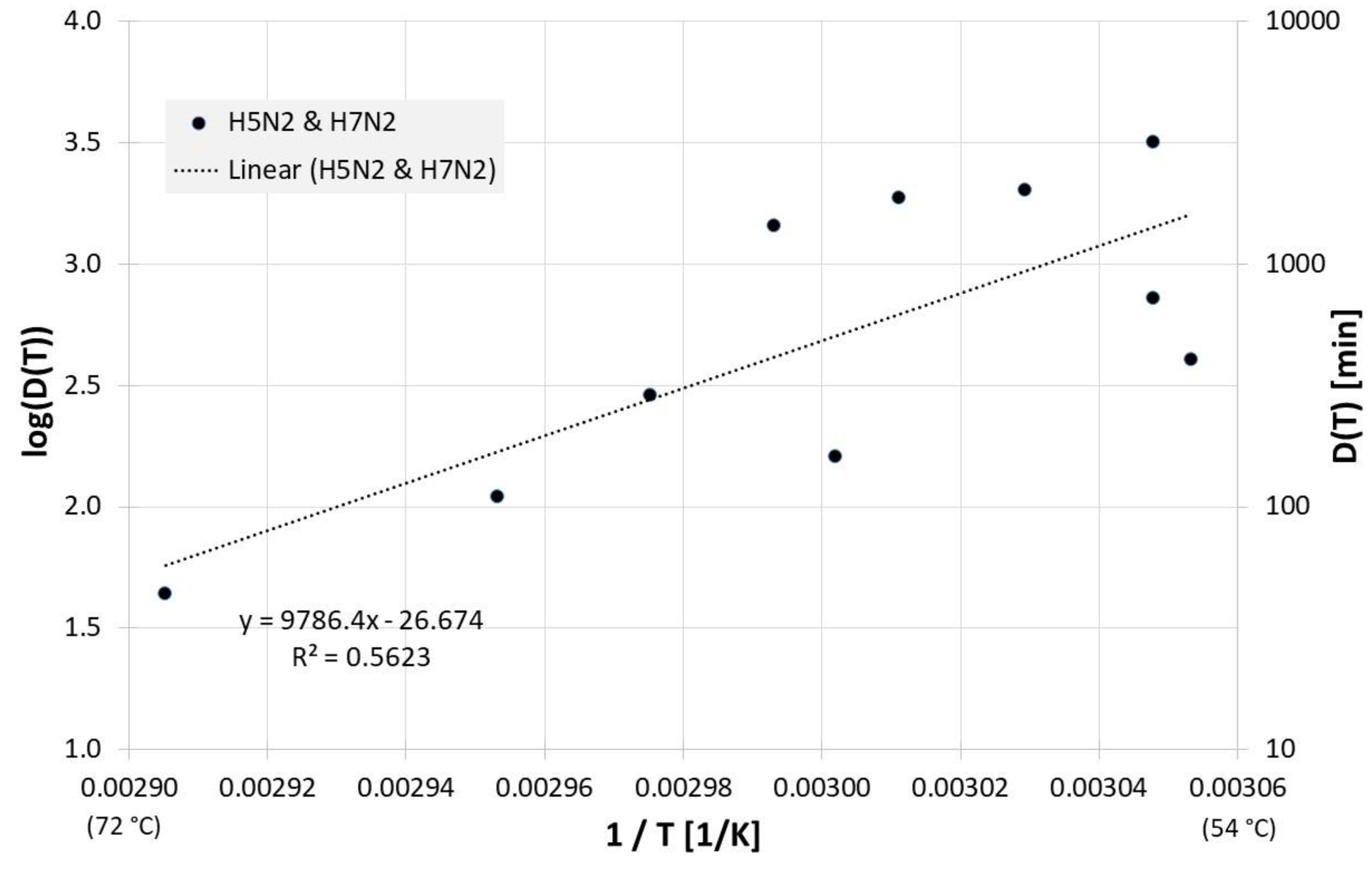
In the event of an influenza outbreak, waste products must also be contaminated. Investigation results for manure and litter can be found in Figure 11. For the decimal reduction time Dlin regress(T) applies:

Figure 11.
Correlation between 1/T and log(D(T)) for all published influenza virus thermal inactivation data in manure, litter and feces along with the linear regression curve. For better understanding, D(T) is also given on the right Y-axis in a logarithmic scale.
Applying the equations just determined for the decimal reduction times for the various contaminated media, the decimal reduction times for various temperatures were calculated and presented in Table 2.

Table 2.
Expected decimal reduction times in minutes for different temperatures and media, determined using the previously determined equations for decimal reduction times.
4. Discussion
Despite the seemingly large number of individual results, the scope of the data is scarce because the individual results differ not only in temperature, but also in the medium and virus subtype examined. This prevents a more comprehensive investigation for differences between virus subtypes or even for possible differences between avian and human influenza viruses, which could be caused by the different body temperatures with 37 °C for humans and 40–42 °C for birds [9,71].
The small number of available data is also evident, for example, in the investigations in liquid egg white (Figure 8) and compost. For each only 4 and 5 data points, respectively, are available for only two different temperatures. For some of the investigated media, R2 of the regression curve is only between 0.4 and 0.6, which means that only 40% to 60% of the observed variation of the measured values can be explained by the model (regression curve). It should be noted that the residual variations of 60–40% are probably at least partially due to statistical biological scatter. Additionally, some of the figures display results of different working groups with different equipment and laboratory procedures. E.g., there may be differences in the precision (and speed) with which these different groups were able to adjust and measure the temperature of their virus samples, which would also contribute to the data scattering and a lower R2.
Inactivation of influenza viruses at comparatively low temperatures starting at about 50 °C is nevertheless clearly evident, but there are major differences with respect to the contaminated media. In particular, in dried egg white, the virus is relatively stable even at high temperatures. For all other media examined, the decimal reduction time from 60 °C is less than 10 min. It should be mentioned that so far, there is no published investigation or even hypothesis on the reason for this obvious dependence of the influenza virus heat stability on the medium.
Antiviral measures officially require a virus reduction of at least 4 powers of ten [72,73]. Under the assumption made above of exponential virus inactivation, an inactivation time of 4 decimal reduction times is necessary for this. With the exception of the dried egg white, such a 99.99% reduction could be accomplished at 60 °C in approximately about half an hour or even faster in almost all media.
These estimates are based on the Arrhenius model and the limited available data. The latter is rather unexpected, since influenza is an infection that typically causes about 500,000 deaths annually [74] and even more in pandemics, up to the estimated 50 million deaths that fell victim to the Spanish flu [75,76]. That is significantly more victims than in the current coronavirus pandemic and yet there are not more studies. In fact, the last and only study of influenza B virus heat inactivation is even 75 years old and there is also only one quantitative investigation on heat inactivation of influenza A in PBS performed by Swayne and colleagues, who have even generated about half of all single results in this study.
Investigations that are more current are largely concerned with avian influenza viruses. The background does not always seem to be the threat influenza poses to humans, but at least also economic interests of the poultry industry. This is an understandable and comprehensible motivation, but especially against the background of the coronavirus pandemic and the experiences from the influenza pandemics of the last 100 years, one should not wait with further influenza inactivation studies until the next influenza pandemic arrives, which can come at any time [77].
5. Conclusions
It seems that even moderate temperatures around 60 °C, which are well below the 121 °C sterilization temperature commonly used in many areas, are sufficient for influenza virus inactivation within about half an hour. However, the differences in various contaminated media are very large and, at least in dried egg white, influenza viruses are very temperature stable.
Because of the limited data available, it is difficult to determine how large the differences in heat sensitivity are between different influenza virus (sub-)types, or whether there are also relatively heat insensitive influenza viruses and thus whether a future, emerging influenza virus may also be relatively heat stable. Further investigations—prior to the next influenza pandemic—seem reasonable.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the order of some references. This change does not affect the scientific content of the article.
References
- Coronavirus Resource Center. COVID-19 Dashboard: (Global Map). Available online: https://coronavirus.jhu.edu/map.html (accessed on 9 August 2022).
- Rahman, H.S.; Aziz, M.S.; Hussein, R.H.; Othman, H.H.; Salih Omer, S.H.; Khalid, E.S.; Abdulrahman, N.A.; Amin, K.; Abdullah, R. The transmission modes and sources of COVID-19: A systematic review. Int. J. Surg. Open 2020, 26, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020, 20, 892–893. [Google Scholar] [CrossRef]
- Weber, T.P.; Stilianakis, N.I. Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J. Infect. 2008, 57, 361–373. [Google Scholar] [CrossRef]
- Cowling, B.J.; Ip, D.K.M.; Fang, V.J.; Suntarattiwong, P.; Olsen, S.J.; Levy, J.; Uyeki, T.M.; Leung, G.M.; Malik Peiris, J.S.; Chotpitayasunondh, T.; et al. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013, 4, 1935. [Google Scholar] [CrossRef] [PubMed]
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7 (Suppl. 2), 42–51. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Hultin, J.V.; Morens, D.M. Discovery and characterization of the 1918 pandemic influenza virus in historical context. Antivir. Ther. 2007, 12, 581–591. [Google Scholar] [CrossRef]
- Donatelli, I.; Castrucci, M.R.; de Marco, M.A.; Delogu, M.; Webster, R.G. Human-Animal Interface: The Case for Influenza Interspecies Transmission. Adv. Exp. Med. Biol. 2017, 972, 17–33. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 3–20. [Google Scholar] [CrossRef]
- Chu, C.M. Inactivation of haemagglutinin and infectivity of influenza and Newcastle disease viruses by heat and by formalin. J. Hyg. 1948, 46, 247–251. [Google Scholar] [CrossRef] [PubMed]
- de Flora, S.; Badolati, G. Thermal inactivation of untreated and gamma-irradiated A2-Aichi-2-68 influenza virus. J. Gen. Virol. 1973, 20, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Ahl, R.; Böhm, R.; Strauch, D. Inactivation of viruses in liquid manure. Rev. Sci. Tech. 1995, 14, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.K.; Bae, J.E.; Kim, I.S. Inactivation of influenza A virus H1N1 by disinfection process. Am. J. Infect. Control 2010, 38, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Isbarn, S.; Buckow, R.; Himmelreich, A.; Lehmacher, A.; Heinz, V. Inactivation of avian influenza virus by heat and high hydrostatic pressure. J. Food Prot. 2007, 70, 667–673. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Viruses contained in droplets applied on warmed surface are rapidly inactivated. Microbes Environ. 2014, 29, 408–412. [Google Scholar] [CrossRef]
- Zou, S.; Guo, J.; Gao, R.; Dong, L.; Zhou, J.; Zhang, Y.; Dong, J.; Bo, H.; Qin, K.; Shu, Y. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol. J. 2013, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.; Day, M.; Spatz, S.; Yu, Q.; Gast, R.; Zsak, L.; Swayne, D. Thermal Inactivation of Avian Viral and Bacterial Pathogens in an Effluent Treatment System within a Biosafety Level 2 and 3 Enhanced Facility. Appl. Biosaf. 2011, 16, 206–217. [Google Scholar] [CrossRef]
- Chmielewski, R.A.; Beck, J.R.; Swayne, D.E. Evaluation of the U.S. Department of Agriculture’s egg pasteurization processes on the inactivation of high-pathogenicity avian influenza virus and velogenic Newcastle disease virus in processed egg products. J. Food Prot. 2013, 76, 640–645. [Google Scholar] [CrossRef]
- Chmielewski, R.A.; Beck, J.R.; Juneja, V.K.; Swayne, D.E. Inactivation of low pathogenicity notifiable avian influenza virus and lentogenic Newcastle disease virus following pasteurization in liquid egg products. LWT—Food Sci. Technol. 2013, 52, 27–30. [Google Scholar] [CrossRef]
- Swayne, D.E.; Beck, J.R. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathol. 2004, 33, 512–518. [Google Scholar] [CrossRef]
- Heimbuch, B.K.; Wallace, W.H.; Kinney, K.; Lumley, A.E.; Wu, C.-Y.; Woo, M.-H.; Wander, J.D. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control 2011, 39, e1–e9. [Google Scholar] [CrossRef]
- Hiatt, C.W. Kinetics of the inactivation of viruses. Bacteriol. Rev. 1964, 28, 150–163. [Google Scholar] [CrossRef]
- Lauffer, M.A.; Wheatley, M. Destruction and denaturation of influenza A virus. Arch. Biochem. Biophys. 1951, 32, 436–447. [Google Scholar] [CrossRef]
- Hahon, N.; Kozikowski, E. Thermal inactivation studies with variola virus. J. Bacteriol. 1961, 81, 609–613. [Google Scholar] [CrossRef]
- Turner, G.S.; Kaplan, C. Some properties of fixed rabies virus. J. Gen. Virol. 1967, 1, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Matsumoto, M. Heat inactivation of measles virus. Jpn. J. Microbiol. 1968, 12, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A.; Abuelzein, E.-T.M.E.; Azhar, E.I.; Al-Bar, H.M.S. Thermal inactivation of Alkhumra hemorrhagic fever virus. Arch. Virol. 2014, 159, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Rowell, C.E.R.; Dobrovolny, H.M. Energy Requirements for Loss of Viral Infectivity. Food Environ. Virol. 2020, 12, 281–294. [Google Scholar] [CrossRef]
- Hessling, M.; Hoenes, K.; Lingenfelder, C. Selection of parameters for thermal coronavirus inactivation—A data-based recommendation. GMS Hygiene and Infection Control; 15:Doc16/GMS Hygiene and Infection Control; 15:Doc16. GMS Hyg. Infect. Control 2020, 15. [Google Scholar] [CrossRef]
- Yap, T.F.; Liu, Z.; Shveda, R.A.; Preston, D.J. A predictive model of the temperature-dependent inactivation of coronaviruses. Appl. Phys. Lett. 2020, 117, 60601. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Siegert, R.; Braune, P. The pyrogens of myxoviruses II. Resistance of influenza a pyrogens to heat, ultraviolet, and chemical treatment. Virology 1964, 24, 218–224. [Google Scholar] [CrossRef]
- Abad, X.; Majó, N.; Rosell, R.; Busquets, N. Assay of Several Inactivation Steps on West Nile Virus and H7N1 Highly Pathogenic Avian Influenza Virus Suspensions. Biosafety 2012, 1. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, S.; Haque, E.; Rahman, M.; Uddin, A.; Khasruzzaman, A.K.M.; Sharif, M.; Rahman, R.; Amin, M.R.; Ali, M. Thermal and pH sensitivity of avian corona and influenza viruses: A model study for inactivation of SARS-CoV-2 (COVID-19) and other flu viruses. Int. Res. J. Med. Med. Sci. 2020, 8, 42–56. [Google Scholar] [CrossRef]
- Nian, Q.-G.; Jiang, T.; Zhang, Y.; Deng, Y.-Q.; Li, J.; Qin, E.-D.; Qin, C.-F. High thermostability of the newly emerged influenza A (H7N9) virus. J. Infect. 2016, 72, 393–394. [Google Scholar] [CrossRef]
- Ikizler, M.R.; Wright, P.F. Thermostabilization of egg grown influenza viruses. Vaccine 2002, 20, 1393–1399. [Google Scholar] [CrossRef]
- Gotlieb, T.; Hirst, G.K. The experimental production of combination forms of virus. Virology 1956, 2, 235–248. [Google Scholar] [CrossRef]
- Wanaratana, S.; Tantilertcharoen, R.; Sasipreeyajan, J.; Pakpinyo, S. The inactivation of avian influenza virus subtype H5N1 isolated from chickens in Thailand by chemical and physical treatments. Vet. Microbiol. 2010, 140, 43–48. [Google Scholar] [CrossRef]
- Lang, G.; Rouse, B.T.; Narayan, O.; Ferguson, A.E.; Connell, M.C. A new influenza virus infection in turkeys. I. Isolation and characterization of virus 6213. Can. Vet. J. 1968, 9, 22–29. [Google Scholar]
- Homme, P.J.; Easterday, B.C. Avian Influenza Virus Infections. I. Characteristics of Influenza A/Turkey/Wisconsin/1966 Virus. Avian Dis. 1970, 14, 66. [Google Scholar] [CrossRef]
- Scholtissek, C. Stability of infectious influenza A viruses at low pH and at elevated temperature. Vaccine 1985, 3, 215–218. [Google Scholar] [CrossRef]
- Tuladhar, E.; Bouwknegt, M.; Zwietering, M.H.; Koopmans, M.; Duizer, E. Thermal stability of structurally different viruses with proven or potential relevance to food safety. J. Appl. Microbiol. 2012, 112, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, J.; Strom, S.; Deason, H.; Vanmoorlehem, E.; Berube, N.; Hauta, S.; Fernando, C.; Hill, J.; Fonstad, T.; Gerdts, V. Time and temperature requirements for improved heat killing of pathogens in swine transport trailers. J. Swine Health Prod. 2021, 29, 19–28. [Google Scholar] [CrossRef]
- Kontarov, N.A.; Dolgova, E.I.; Pogarskaya, I.V.; Kontarova, E.O.; Yuminova, N.V. Kinetics of Influenza A/BANGKOK/1/1979(H3N2) Virus Thermal Inactivation in the Presence of Polyallylamine. Mosc. Univ. Biol. Sci. Bull. 2021, 76, 34–38. [Google Scholar] [CrossRef]
- Jonges, M.; Liu, W.M.; van der Vries, E.; Jacobi, R.; Pronk, I.; Boog, C.; Koopmans, M.; Meijer, A.; Soethout, E. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J. Clin. Microbiol. 2010, 48, 928–940. [Google Scholar] [CrossRef]
- Jeong, E.K.; Sung, H.M.; Kim, I.S. Inactivation and removal of influenza A virus H1N1 during the manufacture of plasma derivatives. Biologicals 2010, 38, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kreil, T.R.; Unger, U.; Orth, S.M.; Petutschnig, G.; Kistner, O.; Poelsler, G.; Berting, A. H5N1 influenza virus and the safety of plasma products. Transfusion 2007, 47, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Abubakar, M.; Hameed, S.; Hassan, S. Avian influenza virus (H5N1); effects of physico-chemical factors on its survival. Virol. J. 2009, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- King, D.J. Evaluation of Different Methods of Inactivation of Newcastle Disease Virus and Avian Influenza Virus in Egg Fluids and Serum. Avian Dis. 1991, 35, 505. [Google Scholar] [CrossRef]
- Khushi, M.; Das, P.; Yaqoob, T.; Riaz, A.; Manzoor, R. Effect of Physico-chemical factors on survival of Avian Influenza (H-7 type) Virus. Int. J. Agric. Biol. 2001, 2001, 416–418. [Google Scholar]
- McDevitt, J.; Rudnick, S.; First, M.; Spengler, J. Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Appl. Environ. Microbiol. 2010, 76, 3943–3947. [Google Scholar] [CrossRef]
- Marchesi, I.; Sala, A.; Frezza, G.; Paduano, S.; Turchi, S.; Bargellini, A.; Borella, P.; Cermelli, C. In vitro virucidal efficacy of a dry steam disinfection system against Human Coronavirus, Human Influenza Virus, and Echovirus. J. Occup. Environ. Hyg. 2021, 18, 541–546. [Google Scholar] [CrossRef]
- Rockey, N.; Arts, P.J.; Li, L.; Harrison, K.R.; Langenfeld, K.; Fitzsimmons, W.J.; Lauring, A.S.; Love, N.G.; Kaye, K.S.; Raskin, L.; et al. Humidity and Deposition Solution Play a Critical Role in Virus Inactivation by Heat Treatment of N95 Respirators. mSphere 2020, 5, e00588-20. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Arts, P.J.; Clack, H.L.; Fitzsimmons, W.J.; Gamba, M.; Harrison, K.R.; LeBar, W.; Lauring, A.S.; Li, L.; Roberts, W.W.; et al. Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital. Open Forum Infect. Dis. 2021, 8, ofaa610. [Google Scholar] [CrossRef]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 2012, 56, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Swayne, D.E. Thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. J. Food Prot. 2007, 70, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; King, D.J.; Swayne, D.E. Thermal inactivation of avian influenza and Newcastle disease viruses in chicken meat. J. Food Prot. 2008, 71, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Microassay for measuring thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. Int. J. Food Microbiol. 2006, 108, 268–271. [Google Scholar] [CrossRef]
- Thomas, C.; Swayne, D.E. Thermal inactivation of H5N2 high-pathogenicity avian influenza virus in dried egg white with 7.5% moisture. J. Food Prot. 2009, 72, 1997–2000. [Google Scholar] [CrossRef][Green Version]
- Chmielewski, R.A.; Beck, J.R.; Swayne, D.E. Thermal inactivation of avian influenza virus and Newcastle disease virus in a fat-free egg product. J. Food Prot. 2011, 74, 1161–1168. [Google Scholar] [CrossRef]
- Chumpolbanchorn, K.; Suemanotham, N.; Siripara, N.; Puyati, B.; Chaichoune, K. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian J. Trop. Med. Public Health 2006, 37, 102–105. [Google Scholar]
- Kurmi, B.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Dubey, S.C.; Kumar, M. Survivability of Highly Pathogenic Avian Influenza H5N1 Virus in Poultry Faeces at Different Temperatures. Indian J. Virol. 2013, 24, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.B.; Spackman, E. Thermal Inactivation of avian influenza virus in poultry litter as a method to decontaminate poultry houses. Prev. Vet. Med. 2017, 145, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kaoud, H.A.; Ismail, T.F.; Khalf, M.A. The effect of some physical and chemical agents on the infectivity of the highly pathogenic avian influenza virus in Egypt. Eur. J. Acad. Essays 2016, 2016, 267–271. [Google Scholar]
- Lu, H.; Castro, A.E.; Pennick, K.; Liu, J.; Yang, Q.; Dunn, P.; Weinstock, D.; Henzler, D. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 2003, 47, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Senne, D.A.; Panigrahy, B.; Morgan, R.L. Effect of composting poultry carcasses on survival of exotic avian viruses: Highly pathogenic avian influenza (HPAI) virus and adenovirus of egg drop syndrome-76. Avian Dis. 1994, 38, 733–737. [Google Scholar] [CrossRef]
- Guan, J.; Chan, M.; Grenier, C.; Wilkie, D.C.; Brooks, B.W.; Spencer, J.L. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real-time reverse transcriptase PCR. Avian Dis. 2009, 53, 26–33. [Google Scholar] [CrossRef]
- Elving, J.; Emmoth, E.; Albihin, A.; Vinneras, B.; Ottoson, J. Inactivation of avian flu and model virus in animal by-product composts. In Proceedings of the 2010 14th Ramiran International Conference Proceedings, Lisboa, Portugal, 13–15 September 2010. [Google Scholar]
- Elving, J.; Emmoth, E.; Albihn, A.; Vinnerås, B.; Ottoson, J. Composting for avian influenza virus elimination. Appl. Environ. Microbiol. 2012, 78, 3280–3285. [Google Scholar] [CrossRef]
- Fiszon, B.; Hannoun, C.; Garcia-Sastre, A.; Villar, E.; Cabezas, J.A. Comparison of biological and physical properties of human and animal A(H1N1) influenza viruses. Res. Virol. 1989, 140, 395–404. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Schwebke, I.; Blümel, J.; Eggers, M.; Glebe, D.; Rapp, I.; Sauerbrei, A.; Steinmann, E.; Steinmann, J.; Willkommen, H.; et al. Leitlinie der Deutschen Vereinigung zur Bekämpfung der Viruskrankheiten (DVV) e. V. und des Robert Koch-Instituts (RKI) zur Prüfung von chemischen Desinfektionsmitteln auf Wirksamkeit gegen Viren in der Humanmedizin: Fassung vom 1. Dezember 2014. Bundesgesundheitsblatt Gesundh. Gesundh. 2015, 58, 493–504. [Google Scholar] [CrossRef]
- DIN EN 14476:2019-10; Chemische Desinfektionsmittel und Antiseptika_—Quantitativer Suspensionsversuch zur Bestimmung der Viruziden Wirkung im Humanmedizinischen Bereich_—Prüfverfahren und Anforderungen (Phase_2, Stufe_1). Beuth Verlag GmbH: Berlin, Germany, 2019.
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Johnson, N.P.A.S.; Mueller, J. Updating the accounts: Global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002, 76, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).