Anesthesia for Endovascular Therapy for Stroke
Abstract
1. Introduction
1.1. Outcomes
Primary Outcome
2. Materials and Methods
2.1. Patients
2.2. Protocol for General Anesthesia
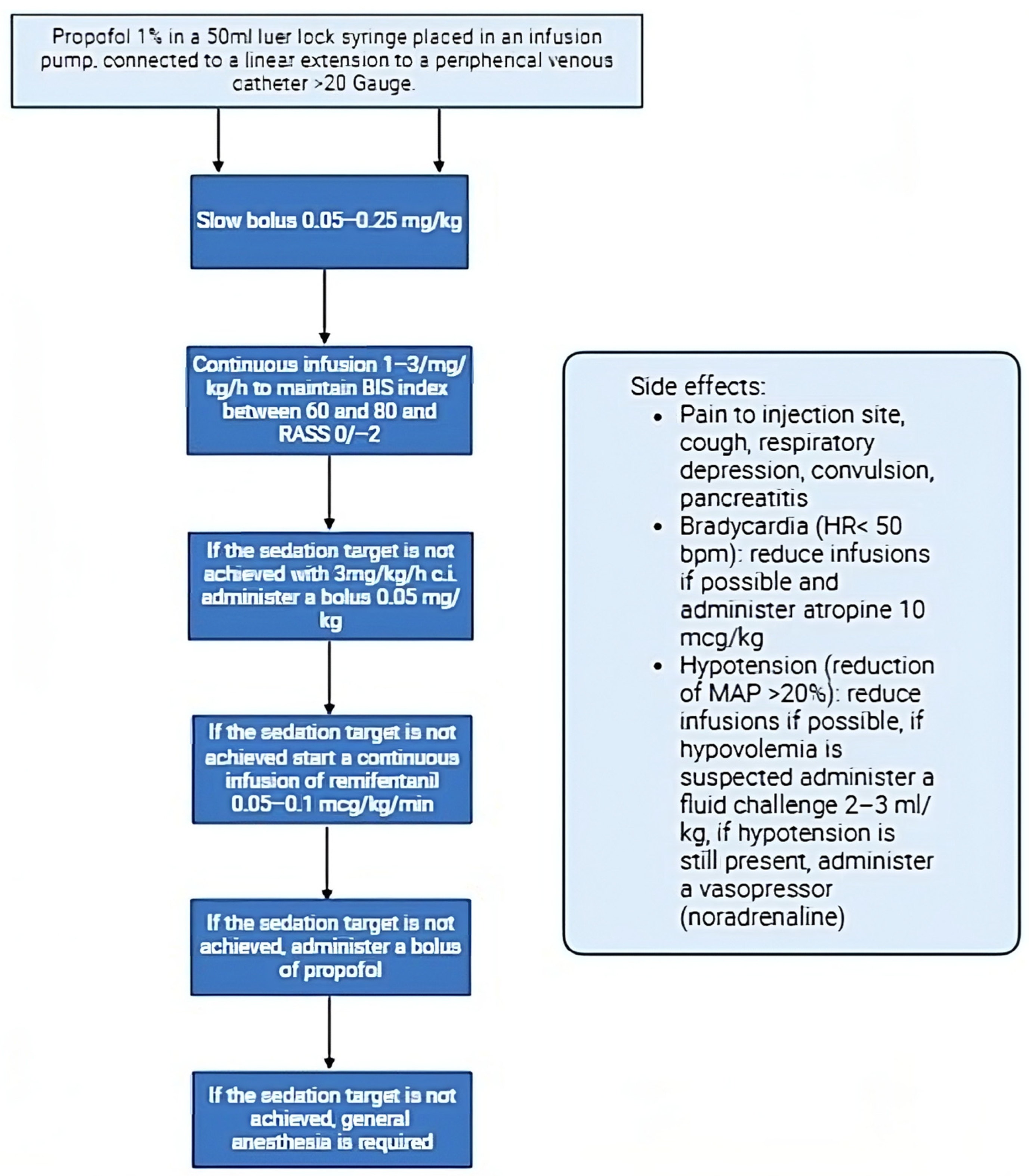
2.3. Protocol for Conscious Sedation
2.4. Protocol for Local Anesthesia
2.5. Study Design
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Strengths and Limitations
7. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Soto, A.; Guillén-Grima, F.; Morales, G.; Muñoz, S.; Aguinaga-Ontoso, I.; Fuentes-Aspe, R. Prevalence and incidence of ictus in Europe: Systematic review and meta-analysis. An. Sist. Sanit. Navar. 2022, 45, e0979. [Google Scholar] [CrossRef]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J. Neurointerv. Surg. 2019, 11, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, N.; Moreira, T.; Michel, P.; Steiner, T.; Jansen, O.; Cognard, C.; Mattle, H.P.; van Zwam, W.; Holmin, S.; Tatlisumak, T.; et al. Mechanical thrombectomy in acute ischemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int. J. Stroke 2016, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, S.; Bösel, J. Reply from Schönenberger et al. to the letter from Kofke and Sharma regarding “Sedation vs. Intubation for Endovascular Stroke TreAtment (SIESTA)—A randomized monocentric trial”. Int. J. Stroke 2016, 11, NP73. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, S.; Möhlenbruch, M.; Pfaff, J.; Mundiyanapurath, S.; Kieser, M.; Bendszus, M.; Hacke, W.; Bösel, J. Sedation vs. Intubation for Endovascular Stroke TreAtment (SIESTA)—A randomized monocentric trial. Int. J. Stroke 2015, 10, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, S.; Uhlmann, L.; Ungerer, M.; Pfaff, J.; Nagel, S.; Klose, C.; Bendszus, M.; Wick, W.; Ringleb, P.A.; Kieser, M.; et al. Association of Blood Pressure with Short- and Long-Term Functional Outcome after Stroke Thrombectomy: Post Hoc Analysis of the SIESTA Trial. Stroke 2018, 49, 1451–1456. [Google Scholar] [CrossRef]
- Wang, A.; Abramowicz, A.E. Role of anesthesia in endovascular stroke therapy. Curr. Opin. Anaesthesiol. 2017, 30, 563–569. [Google Scholar] [CrossRef]
- Schönenberger, S.; Uhlmann, L.; Hacke, W.; Schieber, S.; Mundiyanapurath, S.; Purrucker, J.C.; Nagel, S.; Klose, C.; Pfaff, J.; Bendszus, M.; et al. Effect of Conscious Sedation vs General Anesthesia on Early Neurological Improvement among Patients with Ischemic Stroke Undergoing Endovascular Thrombectomy: A Randomized Clinical Trial. JAMA 2016, 316, 1986–1996. [Google Scholar] [CrossRef]
- Pop, R.; Severac, F.; Happi Ngankou, E.; Harsan, O.; Martin, I.; Mihoc, D.; Manisor, M.; Simu, M.; Chibbaro, S.; Wolff, V.; et al. Local anesthesia versus general anesthesia during endovascular therapy for acute stroke: A propensity score analysis. J. NeuroInterv. Surg. 2021, 13, 207–211. [Google Scholar] [CrossRef]
- Kofke, W.A.; Sharma, D. SIESTA trial: Is GA a drug you get from the hospital pharmacy? Int. J. Stroke 2016, 11, NP70. [Google Scholar] [CrossRef]
- Koinis-Mitchell, D.; Boergers, J.; Yeo, A.J.; Molera, G.; Kopel, S.J.; McQuaid, E.L.; Chen, K.; Wolfson, A.R.; Chavez, L.; Jandasek, B.; et al. A Pilot Randomized Control Trial Demonstrating the Efficacy of the SIESTA Sleep Hygiene Intervention. Clin. Pediatr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Löwhagen Hendén, P.; Rentzos, A.; Karlsson, J.E.; Rosengren, L.; Leiram, B.; Sundeman, H.; Dunker, D.; Schnabel, K.; Wikholm, G.; Hellström, M.; et al. General Anesthesia versus Conscious Sedation for Endovascular Treatment of Acute Ischemic Stroke: The AnStroke Trial (Anesthesia During Stroke). Stroke 2017, 48, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Löwhagen Hendén, P.; Rentzos, A. Response by Löwhagen Hendén and Rentzos to Letter Regarding Article, “General Anesthesia versus Conscious Sedation for Endovascular Treatment of Acute Ischemic Stroke: The Anstroke Trial (Anesthesia During Stroke)”. Stroke 2017, 48, e265. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, C.Z.; Schönenberger, S.; Hendén, P.L.; Yoo, A.J.; Uhlmann, L.; Rentzos, A.; Bösel, J.; Valentin, J.; Rasmussen, M. Patients Requiring Conversion to General Anesthesia during Endovascular Therapy Have Worse Outcomes: A Post Hoc Analysis of Data from the SAGA Collaboration. AJNR Am. J. Neuroradiol. 2020, 41, 2298–2302. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, C.Z.; Sørensen, L.H.; Juul, N.; Johnsen, S.P.; Yoo, A.J.; Andersen, G.; Rasmussen, M. Anesthetic strategy during endovascular therapy: General anesthesia or conscious sedation? (GOLIATH—General or Local Anesthesia in Intra Arterial Therapy) A single-center randomized trial. Int. J. Stroke 2016, 11, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Butler, E.; Campbell, R.B.; Ho, J.; Barber, P.A. General Anesthesia Compared with Non-GA in Endovascular Thrombectomy for Ischemic Stroke: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Neurology 2023, 100, e1655–e1663. [Google Scholar] [CrossRef]
- Chabanne, R.; Geeraerts, T.; Begard, M.; Balança, B.; Rapido, F.; Degos, V.; Tavernier, B.; Molliex, S.; Velly, L.; Verdonk, F.; et al. Outcomes After Endovascular Therapy with Procedural Sedation vs General Anesthesia in Patients with Acute Ischemic Stroke: The AMETIS Randomized Clinical Trial. JAMA Neurol. 2023, 80, 474–483. [Google Scholar] [CrossRef]
- Liang, F.; Wu, Y.; Wang, X.; Yan, L.; Zhang, S.; Jian, M.; Liu, H.; Wang, A.; Wang, F.; Han, R. General Anesthesia vs Conscious Sedation for Endovascular Treatment in Patients with Posterior Circulation Acute Ischemic Stroke: An Exploratory Randomized Clinical Trial. JAMA Neurol. 2023, 80, 64–72. [Google Scholar] [CrossRef]
- Li, F.; Deshaies, E.M.; Singla, A.; Villwock, M.R.; Melnyk, V.; Gorji, R.; Yang, Z.J. Impact of anesthesia on mortality during endovascular clot removal for acute ischemic stroke. J. Neurosurg. Anesthesiol. 2014, 26, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kronsteiner, D.; Pfaff, J.A.R.; Schieber, S.; Bendszus, M.; Kieser, M.; Wick, W.; Möhlenbruch, M.A.; Ringleb, P.A.; Bösel, J.; et al. Emergency intubation during thrombectomy for acute ischemic stroke in patients under primary procedural sedation. Neurol. Res. Pract. 2021, 3, 27. [Google Scholar] [CrossRef]
- Flottmann, F.; Leischner, H.; Broocks, G.; Faizy, T.D.; Aigner, A.; Deb-Chatterji, M.; Thomalla, G.; Krauel, J.; Issleib, M.; Fiehler, J.; et al. Emergency Conversion to General Anesthesia Is a Tolerable Risk in Patients Undergoing Mechanical Thrombectomy. AJNR Am. J. Neuroradiol. 2020, 41, 122–127. [Google Scholar] [CrossRef]
- Brinjikji, W.; Murad, M.H.; Rabinstein, A.A.; Cloft, H.J.; Lanzino, G.; Kallmes, D.F. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: A systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2015, 36, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Romero Kräuchi, O.; Valencia, L.; Iturri, F.; Mariscal Ortega, A.; López Gómez, A.; Valero, R. National survey on perioperative anaesthetic management in the endovascular treatment of acute ischaemic stroke. Rev. Esp. Anestesiol. Reanim. 2018, 65, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Talke, P.O.; Sharma, D.; Heyer, E.J.; Bergese, S.D.; Blackham, K.A.; Stevens, R.D. Society for Neuroscience in Anesthesiology and Critical Care Expert consensus statement: Anesthetic management of endovascular treatment for acute ischemic stroke*: Endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J. Neurosurg. Anesthesiol. 2014, 26, 95–108. [Google Scholar] [CrossRef]
| Hospital Admission | Overall (58 Patients) | General Anesthesia (25.86%) | Conscious Sedation (46.55%) | Local Anesthesia (27.59%) |
|---|---|---|---|---|
| Comorbidities | ||||
| Cardiovascular | 53 (91.23%) | 13 (92.86%) | 25 (92.59%) | 14 (87.50%) |
| Respiratory | 13 (22.81%) | 5 (35.71%) | 5 (18.52%) | 3 (18.75%) |
| Kidney failure | 4 (7.02%) | 0 (0%) | 2 (7.41%) | 2 (12.50%) |
| Gastrointestinal | 15 (26.32%) | 3 (21.43%) | 6 (22.6%) | 6 (37.50%) |
| Neoplastic | 5 (8.77%) | 1 (7.14%) | 3 (11.11%) | 1 (6.25%) |
| Neurologic | 16 (28.07%) | 3 (21.43%) | 9 (33.3%) | 4 (25%) |
| Metabolic | 8 (14.04%) | 1 (7.14%) | 5 (18.52%) | 2 (12.50%) |
| Ischemic lesion site | ||||
| ACA | 1 (1.72%) | 0 (0) | 1 (3.70%) | 0 (0) |
| MCA | 36 (62.07%) | 6 (40%) | 18 (66.67%) | 12 (75%) |
| PCA, VA, BA | 7 (12.07%) | 6 (40%) | 0 (0) | 1 (6.25%) |
| ICA | 5 (8.62%) | 1 (6.67%) | 3 (11.11%) | 2 (12.50%) |
| ICA, MCA | 7 (12.07%) | 2 (13.33%) | 3 (11.11%) | 2 (12.50%) |
| MCA, ACA | 2 (3.45%) | 0 (0) | 2 (7.41%) | 0 (0) |
| Overall (58 Patients) | General Anesthesia (25.86%) | Conscious Sedation (46.55%) | Local Anesthesia (27.59%) | p Value | |
|---|---|---|---|---|---|
| Age | 71.22 ± 17.11 | 65.43 ± 17.45 | 74.89 ± 14.9 | 70.47 ± 20.65 | 0.227 |
| GCS Admission | 12.30 ± 2.36 | 12.15 ± 3.38 | 12.29 ± 1.91 | 12.43 ± 2.20 | 0.9513 |
| NIHSS Admission | 14.91 ± 6.74 | 15.50 ± 7.81 | 14.74 ± 6.15 | 14.68 ± 7.11 | 0.939 |
| MRS Admission | 0.21 ± 0.52 | 0.00 ± 0 | 0.40 ± 0.69 | 0.06 ± 0.25 | 0.023 |
| MEWS Admission | 1.40 ± 1.5 | 1.28 ± 1.13 | 1.44 ± 1.55 | 1.43 ± 1.86 | 0.948 |
| RASS | 0.00 ± 0.73 | −0.14 ± 1.23 | 0.07 ± 0.61 | 0.00 ± 0.0 | 0.675 |
| SOFA Admission | 2.26 ± 1.75 | 2.00 ± 1.75 | 2.59 ± 1.64 | 1.93 ± 1.98 | 0.4116 |
| Overall (58 Patients) | General Anesthesia (25.86%) | Conscious Sedation (46.55%) | Local Anesthesia (27.59%) | p Value | |
|---|---|---|---|---|---|
| Lactates after the procedure | 1.99 ± 3.06 | 2.23 ± 3.88 | 1.68 ± 1.01 | 1.35 ± 0.35 | 0.902 |
| GCS before the procedure | 12.16 ± 2.51 | 12.25 ± 2.70 | 11.81 ± 2.60 | 12.68 ± 2.27 | 0.549 |
| NIHSS before the procedure | 14.51 ± 6.63 | 16.55 ± 7.012 | 13.55 ± 6.12 | 14.56 ± 7.29 | 0.428 |
| MAP before the procedure | 102.33 ± 11.97 | 103.64 ± 12.69 | 100.53 ± 11.82 | 104.38 ± 11.89 | 0.411 |
| MAP during the procedure | 102.33 ± 11.97 | 96.09 ± 19.05 | 95.8 ± 15.76 | 99.52 ± 12.59 | 0.582 |
| MAP after the procedure | 89.44 ± 14.11 | 86.50 ± 16.58 | 90.01 ± 14.70 | 91.06 ± 10.99 | 0.657 |
| GCS after the procedure | 12.58 ± 2.6 | 11.50 ± 3.66 | 12.76 ± 2.26 | 12.81 ± 3.09 | 0.492 |
| SOFA after the procedure | 2.68 ± 2.13 | 3.42 ± 2.20 | 2.40 ± 1.81 | 2.50 ± 2.52 | 0.325 |
| NIHSS after 7 days | 10.04 ± 8.71 | 7.85 ± 4.59 | 11.62 ± 9.05 | 8.2 ± 8.53 | 0.374 |
| NIHSS at discharge | 7.61 ± 9.03 | 9.80 ± 9.16 | 8.38 ± 9.56 | 4.38 ± 7.48 | 0.301 |
| MRS at discharge | 3.26 ± 2.00 | 4.08 ± 1.72 | 3.19 ± 1.78 | 2.73 ± 2.43 | 0.215 |
| MRS after 3 months | 3.20 ± 2.25 | 4.75 ± 2.50 | 2.06 ± 2.02 | 3.5 ± 2.36 | 0.215 |
| Duration of the procedure | 95.59 ± 71.95 | 144.44 ± 94.97 | 86.16 ± 59.84 | 80.35 ± 66.31 | 0.072 |
| Survival rate | 52 (84.48%) | 13 (25%) | 25 (48.8%) | 14 (26.92%) | 0.824 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, A.; Vaccari, G.; Arturi, F.; Melegari, G.; Baroni, S. Anesthesia for Endovascular Therapy for Stroke. Neurol. Int. 2024, 16, 663-672. https://doi.org/10.3390/neurolint16030050
Gaspari A, Vaccari G, Arturi F, Melegari G, Baroni S. Anesthesia for Endovascular Therapy for Stroke. Neurology International. 2024; 16(3):663-672. https://doi.org/10.3390/neurolint16030050
Chicago/Turabian StyleGaspari, Arianna, Giulia Vaccari, Federica Arturi, Gabriele Melegari, and Stefano Baroni. 2024. "Anesthesia for Endovascular Therapy for Stroke" Neurology International 16, no. 3: 663-672. https://doi.org/10.3390/neurolint16030050
APA StyleGaspari, A., Vaccari, G., Arturi, F., Melegari, G., & Baroni, S. (2024). Anesthesia for Endovascular Therapy for Stroke. Neurology International, 16(3), 663-672. https://doi.org/10.3390/neurolint16030050






