Abstract
The tau protein is a microtubule-associated protein that promotes microtubule stabilization. The phosphorylation of the tau protein has been linked to its dissociation from microtubules. Here, we examined the relationship between neuronal depolarization activity and tau protein phosphorylation by employing model systems in culture as well as in vivo. The KCl-evoked depolarization of cultured neurons has often been used to investigate the effects of neuronal activity. We found dephosphorylation at AT8 sites (S202, T205), T212, AT180 sites (T231, S235), and S396 in KCl-simulated cultured neurons. We also found that the KCl-induced tau protein dephosphorylation increases the level of the tau protein fractionated with stable microtubules. In an in vivo experiment, we demonstrated that the exposure of mice to a new environment activates protein phosphatase 1 in the mouse hippocampus and induces tau protein dephosphorylation. We also found an increased amount of the tau protein in a stable microtubule fraction, suggesting that the dephosphorylation of the tau protein may lead to its increased microtubule association in vivo. These results suggest that the association of microtubules with tau proteins may be regulated by the tau protein phosphorylation status affected by neuronal electrical activity.
1. Introduction
The tau protein is a microtubule-associated protein that promotes microtubule stabilization [1,2]. Hyperphosphorylated and abnormally phosphorylated tau proteins form neurofibrillary tangles, observed in the brains of aged individuals and patients with Alzheimer’s disease, causing neuronal dysfunction [3]. Previous studies using in vitro models with purified tubulin and tau protein [4,5] or CHO cells expressing the tau protein [6,7] have shown that the binding of the tau protein with microtubules is reduced by tau protein phosphorylation. Thus, the phosphorylation of the tau protein has been associated with functional modifications in the physiology and pathology of the nervous system.
In studies using cultured neurons, KCl-induced depolarization is often used as a means of generating electrical excitation to mimic neuronal physiological activity [8,9,10,11,12,13,14,15]. Previous studies have shown that the depolarization of cultured neurons induced by KCl leads to tau protein dephosphorylation [16,17]. Previous reports have shown that phosphorylation at Ser214, Thr231, Ser262, and Ser356 reduces tau protein interactions with microtubules [6,18,19,20,21,22]. However, it is unknown whether KCl-induced dephosphorylation affects its association with microtubules. It is also unknown whether neuronal activity alters tau protein phosphorylation status. A recent study showed that dentate gyrus granule cells in the mouse hippocampus show a task-dependent small and transient depolarization of their membrane potential when an animal encounters a novel environment [23], suggesting that the cognition of a novel event causes neural depolarization. Here, we aimed to analyze the tau protein phosphorylation status and resultant events as a consequence of neuronal depolarization using models both in vitro as well as in vivo.
2. Materials and Methods
2.1. Animals
All animals were maintained in accordance with the guidelines of the National Center for Neurology and Psychiatry. The technical protocols for the animal experiments in this study were approved by the Committee on Ethical Issues in Animal Experiments of the National Center of Neurology and Psychiatry (approval number: 2023005, approval date: 8 February 2023). C57BL/6J mice and ICR mice were purchased from CLEA Japan (Tokyo, Japan).
For novel environment exposure experiments, C57BL/6J male mice (10–13 weeks old, 25–30 g, n = 51) were transferred to a novel environment consisting of a new cage, new bedding, and several plastic conical tubes (15 mL, 50 mL) for an indicated length of time (5 min–24 h) with free access to food and water and exposure to standard light–dark cycle (12 h–12 h).
2.2. Antibodies
For immunoblot analyses described in this work, we used the following primary antibodies: anti-acetylated tubulin (clone: 6-11B-1, ab24610, abcam, Cambridge, UK, mouse monoclonal, 0.1 μg/mL), anti-GAPDH (016-25523, Fujifilm, Tokyo, Japan, mouse monoclonal, 0.1 μg/mL), anti-phosphorylated tau protein (clone: AT8, #90206, Fujirebio, Gent, Belgium, mouse monoclonal, 0.2 μg/mL), anti-phosphorylated tau protein (Thr212) (44-740G, Thermo Fisher Scientific, Waltham, MA, USA, rabbit polyclonal), anti-phosphorylated tau protein (clone: AT270, #90207, Fujirebio, Gent, Belgium, mouse monoclonal, 0.2 μg/mL), anti-phosphorylated tau protein (clone: AT180, #MN1040, Thermo Fisher Scientific, USA, mouse monoclonal, 0.2 μg/mL), anti-phosphorylated tau protein (Ser396) (44-752G, Thermo Fisher Scientific, USA, rabbit polyclonal), anti-phosphorylated tau protein (Ser404) (clone: D2Z4G, #20194, Cell Signaling Technology, Danvers, MA, USA, rabbit monoclonal, 0.2 μg/mL), anti-Tau protein (clone: RTM38, Fujifilm, rat monoclonal, 0.5 μg/mL), and anti-Tubulin (clone: DM1A, T-9026, Sigma, St. Louis, MO, USA, mouse monoclonal, 0.1 μg/mL). We used the following secondary antibodies: anti-rabbit IgG HRP-linked antibody (#7074, Cell Signaling Technology, 1:5000), anti-mouse IgG HRP-linked antibody (#7076, Cell Signaling Technology, 1:5000), anti-rat IgG HRP-linked antibody (#7077, Cell Signaling Technology, 1:5000), and anti-goat IgG HRP-linked antibody (#705—35-003, Jackson Immunoresearch, West Grove, PA, USA, 1:5000).
2.3. Primary Cortical Neuronal Culture
Cerebral cortices were removed from day 15 embryonic mouse pups. Cells were dissociated with papain and seeded at a density of 5 × 105 cells/well onto 24-well plates coated with poly-L-lysine (Merck, Darmstadt, Germany) and laminin (Merck) in DMEM containing 10% FBS. From the second day in vitro, the cells were maintained in Neuro-medium (Miltenyi Biotec, Bergisch Gladbach, Germany) containing 2% Neuro-Brew-21 (Miltenyi Biotec) and 1 mM GlutaMAX (Thermo Fisher Scientific). The cells were cultured for 14 d and used for analyses. Upon the harvest of cells, culture plates were placed on ice prior to harvest to minimize the effects caused by unstable temperature during manipulation [24]. For the examination of phosphatase inhibitors, tautomycetin (for PP1, with lower affinity for PP2A) (#2305, Tocris Bioscience, Abingdon, UK) [25] and okadaic acid (for PP1 and PP2A) (#495604, Sigma, St. Louis, MO, USA) were added to the culture at a final concentration of 1 μM and 50 nM, respectively, immediately followed by KCl stimulation.
2.4. Biochemical Analysis of Hippocampus
The isolated hippocampus was homogenized with Tris-buffered saline (TBS) containing a protease inhibitor cocktail (#25955-11, Nacalai tesque, Kyoto, Japan) and a phosphatase inhibitor cocktail (#07575-51, Nacalai tesque), and subject to ultracentrifugation at 125,000× g, 4 °C, 20 min to obtain supernatant for immunoblot analysis.
2.5. Microtubule Fractionation
Microtubule fractionation was performed according to a previous report [26]. Briefly, harvested primary cultured neurons or isolated hippocampi were immediately homogenized in an ice-cold microtubule-stabilizing buffer [MSB: 0.1 M MES, pH 6.8; 10% glycerol; 1 mM MgSO4; 1 mM EGTA; 0.1 mM DTT; 0.5% Triton X-100; 10 μM Taxol; 2 μM GTP] containing a protease inhibitor cocktail (#25955-11, Nacalai tesque, Kyoto, Japan) and a phosphatase inhibitor cocktail (#07575-51, Nacalai tesque). After centrifugation at 2400× g for 5 min to remove the debris, the supernatants were centrifuged at 100,000× g using a TLA-55 rotor (Beckman Coulter, Inc., Brea, CA, USA) for 20 min to obtain pellet as the microtubules with microtubule-associated proteins (P2 fraction). The resultant supernatants (S2 fraction) were further centrifuged at 500,000× g using a TLA 100.3 rotor (Beckman) for 60 min to separate the insoluble protein complexes in the precipitation (P3 fraction) from soluble proteins in the supernatant (S3 fraction). All fractions were dissolved in the SDS-sample buffer and boiled for 5 min prior to immunoblot analysis.
2.6. Immunoblot Analyses
Proteins were separated on conventional 10% acrylamide gels, followed by transfer to PVDF membranes. The membranes were incubated with the indicated primary antibody overnight at 4 °C in TBS containing 0.05% Tween 20. After treatment with the horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature, the immunoblot signal was visualized using ImmunoStar Zeta or ImmunoStar LD reagents (Wako, Osaka, Japan) and captured with the LAS-4000 (Fujifilm, Tokyo, Japan) or FUSION (M&S Instruments, Osaka, Japan) for analysis using Image J software(1.53a).
2.7. Phosphatase Assay
The phosphatase assay was performed according to a previous report [27]. Hippocampal tissues were isolated from mice in their home cage and mice after exposure to the novel environment. Isolated tissues were homogenized with TBS containing a protease inhibitor cocktail (#25955-11, Nacalai tesque) and subject to ultracentrifugation at 125,000× g, 4 °C, 20 min. Aliquots of the resultant supernatants were incubated at 37 °C for the indicated time with/without phosphatase inhibitors, followed by termination of the reaction with the addition of the SDS-sample buffer. The protein phosphatase (PP) inhibitors used in this study were tautomycetin (for PP1, with lower affinity for PP2A) (#2305, Tocris Bioscience, Abingdon, UK) [27], fostriecin (for PP2A) (#344280, Sigma), cyclosporin A (for calcineurin) (#239835, Sigma), and sanguinarine chloride (for PP2C) (#2302, R&D Systems, Mineapolis, MN, USA).
2.8. Statistical Analyses
Statistical analyses were performed using Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). Results were expressed as the mean ± SEM. The significance of differences (* p < 0.05) between groups was examined using Student’s t-test and a one-way ANOVA followed by Dunnett’s multiple comparison test, or a one-way ANOVA followed by Sidak’s multiple comparison test, Dunnett’s multiple comparison test, or Tukey’s multiple comparison test.
3. Results
3.1. KCl Treatment Led to Tau Protein Dephosphorylation in Primary Cortical Neurons
KCl-induced depolarization has often been used as a model to investigate the effect of neuronal activity in culture [8,9,10,11,12,13,14,15]. To examine whether the phosphorylation status of the tau protein is affected by KCl-induced depolarization, we generated a mouse hippocampal neuron primary culture and performed KCl stimulation. As a KCl-induced subcellular Ca2+ level increase was reported to last for up to 4 h [16], we harvested neurons 4 h after the addition of 50 mM KCl to the culture and examined the levels of tau protein phosphorylation at several sites, including the AT270 site (T181), AT8 sites (S202, T205), T212, AT180 sites (T231, S235), S396, and S404. We found that phosphorylation at AT8 sites, T212, AT180 sites, and S396 was significantly decreased by KCl treatment, while phosphorylation at the AT270 site and S404 was unchanged (Figure 1A). The levels of total tau protein were comparable between the Ctrl and KCl-treated neurons (Figure 1A). Previous reports suggested that phosphorylated tau protein is dissociated from microtubules [4,5,6,7,18,20,22]. To investigate whether KCl-induced tau protein dephosphorylation affects its association with microtubules, we performed microtubule fractionation using primary cortical neurons [25]. We found that KCl treatment increased the tau protein in stable microtubule fractions (P2, indicated by the presence of acetylated tubulin) and decreased the tau protein in free tubulin fractions (S3) (Figure 1B). These results suggest that KCl treatment causes the dephosphorylation of tau proteins at some key residues to increase its binding to stable microtubules.
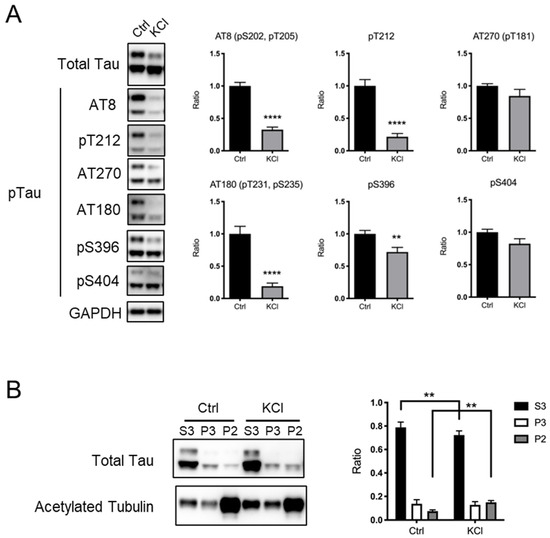
Figure 1.
KCl-induced neuronal depolarization in culture causes tau protein dephosphorylation, leading to its increased capacity to bind to microtubules. (A) Representative immunoblots (left) and the quantified expression levels normalized to the control (right) showing expression of phosphorylated tau protein. Statistical analysis was performed using Student’s t-test. The graph shows mean ± S.E.M. (Ctrl, n = 11; KCl, n = 10). (B) Results of microtubule fractionation. Representative immunoblots (left) and the quantified ratio in each fraction were shown (right). Statistical analysis was performed using two-way ANOVA followed by Sidak’s multiple comparison test. The graph shows mean ± S.E.M. (Ctrl, n = 5; KCl, n = 5). Ctrl, control; KCl, KCl treatment; Tau, tau protein; pTau, phosphorylated tau protein; ** indicates p < 0.01, **** indicates p < 0.0001.
3.2. Exposure to Novel Environment Decreases the Level of Phosphorylated Tau Protein
To investigate whether neuronal activity leads to tau protein dephosphorylation in vivo, we examined tau protein phosphorylation in the mouse cerebral cortex after exposure to a novel environment for various periods of time (from 5 min to 24 h). The AT8 sites, which are often hyperphosphorylated in brains with Alzheimer’s disease [28,29,30,31,32,33], are among the KCl-sensitive phosphorylation sites in this study (Figure 1) and the previous studies [16,17]. Therefore, we examined AT8 site phosphorylation status after novel environment exposure in mouse hippocampus. We found that the levels of phosphorylated tau protein at the AT8 sites were significantly reduced after new environment exposure for 5 min to 4 h (Figure 2A). The levels of total tau protein were comparable up to 24 h after exposure to a novel environment. These results suggest that neuronal depolarization caused by novel environment exposure may lead to tau protein dephosphorylation in vivo.
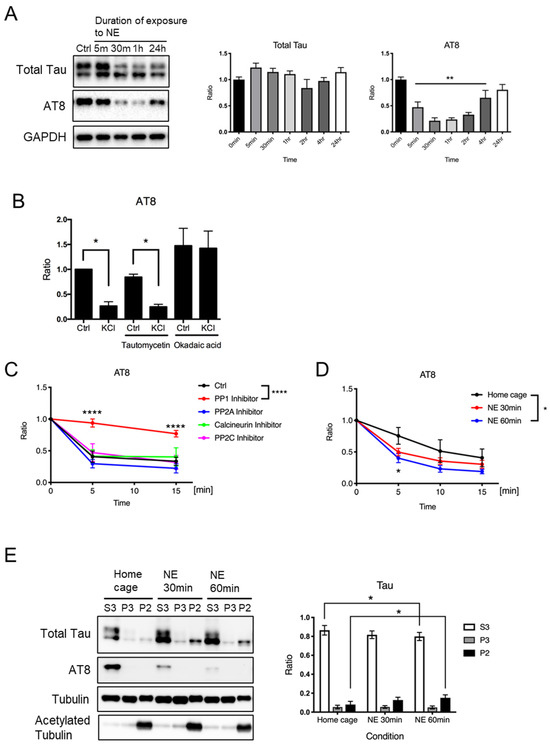
Figure 2.
Exposure to a novel environment causes neuronal tau protein dephosphorylation, leading to its binding to microtubules in vivo. (A) Representative immunoblots (left) and the quantified expression levels normalized to the control (right) showing expression levels of tau protein and phosphorylated tau protein at AT8 recognition sites in hippocampal tissues obtained from wild-type mouse brains at indicated time after novel environment exposure. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test. The values show mean ± S.E.M. (n = 6~11). (B) A bar graph showing phosphorylated tau expression levels after KCl-induced neuronal depolarization in the presence of indicated phosphatase inhibitors in cultured neurons detected by AT8 antibody using immunoblot analysis. Expression levels are shown relative to the level of unstimulated control. The values are shown as mean ± S.E.M. (* p < 0.05; n = 3). Statistical analysis was performed using one-way ANOVA followed by Sidak’s multiple comparisons test. (C,D) Phosphatase assay results. In (B), quantified levels of AT8 immunoreactivity were measured by immunoblot analysis in lysates from untreated wild-type mouse brain incubated at 37 °C for indicated time with 1 μM of inhibitors of each phosphatase, i.e., PP1 (tautomycetin), PP2A (fostriecin), Calcineurin (cyclosporin A), or PP2C (sanguinarine chloride), relative to the level at time zero, are shown. In (C), quantified levels of AT8 immunoreactivity were measured by immunoblot analysis in lysates from brains of wild-type mice exposed to a novel environment for 30 min or 60 min, incubated at 37 °C for the indicated time, relative to the level at time zero, are shown. The values are shown as mean ± S.E.M. (n = 3–7). Statistical analysis was performed using two-way ANOVA followed by Dunnet’s multiple comparison test. (E) Mice were exposed to a novel environment for the indicated time, and their hippocampi were subjected to microtubule fractionation. Representative immunoblots (left) and the quantified levels of AT8 immunoreactivity shown as ratios in each fraction (right) are shown. Statistical analysis was performed using two-way ANOVA followed by Tukey’s multiple comparison test. The values show mean ± S.E.M. (n = 6). Tau, tau protein; Ctrl, control; NE, new environment; * indicates p < 0.05, ** indicates p < 0.01, **** indicates p < 0.0001.
Previous reports showed that increased tau protein phosphorylation in physiological settings is caused by decreased phosphatase activity [26,34]. Based on these observations, we speculated that the dephosphorylation of tau proteins after neural activity might be caused by increased phosphatase activity. To examine this possibility, we added tautomycetin or okadaic acid (inhibitors for PP1/PP2A) to the neuronal culture and performed KCl stimulation. We found that the addition of the inhibitors may affect the AT8 site phosphorylation (Figure 2B), but we do not think we can conclude anything from this experiment because the inhibitors could also affect the activity of kinases that phosphorylate tau proteins, or modify signaling that mediates KCl-induced neuronal depolarization. To examine the role of phosphatase activity in regulating the phosphorylation status of tau proteins in response to neuronal activity more directly, we performed a phosphatase assay using brain tissues obtained from mice with or without novel environment exposure. Brain lysates were incubated at 37 °C with 1 μM of inhibitors for each phosphatase, i.e., protein PP1 (tautomycetin), PP2A (fostriecin), calcineurin (cyclosporin A), and PP2C (sanguinarine chloride), followed by the quantification of the level of AT8-positive tau protein. We confirmed a decrease in the level of AT8-positive tau protein in the absence of any inhibitor (Figure 2C, Ctrl). We found that the decline was significantly suppressed by the PP1 inhibitor tautomycetin, but not by any other inhibitors (Figure 2C). These results demonstrated that PP1 might be a potential phosphatase that dephosphorylates the AT8 sites. To investigate whether exposure to a novel environment induces an increase in phosphatase activity, we prepared brain lysates from mice in a home cage or mice after exposure to a novel environment for 30 min or 60 min and performed the phosphatase assay to evaluate PP1 activity by examining AT8 phosphorylation. We found that the lysates derived from mice exposed to a novel environment for 60 min showed significantly higher PP1 activity compared to lysates derived from mice in a home cage (Figure 2D). These results suggest that exposure to a novel environment may activate PP1.
3.3. Exposure to a Novel Environment Increases the Levels of Tau Protein Fractionated with Stable Microtubules
To examine whether novel environment exposure-induced tau protein dephosphorylation affects its function, we analyzed the association of tau proteins with microtubules using a microtubule fractionation assay using hippocampal tissues. We found that exposure to a novel environment for 60 min resulted in an increase in the level of tau protein fractionated into a stable microtubule fraction (P2) and a decrease in the level of tau protein fractionated into free tubulin fraction (S3) (Figure 2E). These results suggest that tau protein dephosphorylation caused by exposure to a novel environment may promote its binding to stable microtubules.
4. Discussion
Previous reports have shown that tau protein phosphorylation is elevated during different physiological processes, including development, hibernation, hypothermia, sleep, intermittent hypoxia, and brain ischemia [35,36,37,38,39,40,41,42,43,44,45]. However, the relationship between tau protein phosphorylation and neuronal activity is still unknown. To the best of our knowledge, our study is the first to report that exposure to a novel environment, a physiological stimulus to induce neuronal activity, leads to tau protein dephosphorylation and an increase in the level of tau proteins in stable microtubule fractions in vivo. We also found that PP1 activity is induced by novel environment exposure in mice. These results suggest that tau protein phosphorylation and its association with microtubules is regulated by neuronal electrical activity.
Similar to our current observations, previous reports have also demonstrated KCl treatment-dependent tau protein dephosphorylation [16,17], but the residues showing KCl treatment dependency in phosphorylation status seem to vary in different reports. Here, we found that the AT270 site (T181) and S404 are KCl-insensitive phosphorylation sites, while others reported that they are dephosphorylated by KCl treatment [17]. Since different experimental conditions were used in the reports, these data may suggest that the activity of kinases/phosphatases could be differentially regulated by varying degrees of depolarization. It is also possible that the substrate specificity of each kinase/phosphatase is not tightly determined and/or is affected by circumstantial conditions and post-translational modifications other than phosphorylation. Since the substrate specificity of PP1 is known to be affected by a number of different conditions, including interaction of other molecules and structural modifications [46,47,48], the PP1-dependent dephosphorylation status of tau proteins might also be affected by different experimental conditions.
We show here that neuronal depolarization leads to tau protein dephosphorylation, followed by an increase in its binding with microtubules in vitro and in vivo. We have no sufficient data here to extend the discussion on our current finding to human disease, so we do not think it is appropriate to talk about the putative influence of neuronal activity on tau protein aggregation at this point. While the results suggest the existence of mechanisms regulating tau protein phosphorylation in physiological conditions, further study is required to clarify whether the currently identified mechanism could be extended to that for pathological phosphorylation that causes aggregate formation in neurodegenerative disorders, including Alzheimer’s disease and post-ischemic brain neurodegeneration [45].
Author Contributions
Conceptualization, S.Y. and T.A.;. formal analysis, S.Y.; investigation, S.Y., M.S. and A.F.; writing—original draft preparation, S.Y. and T.A.; writing—review and editing, S.Y., S.W. and T.A.; supervision, S.W. and T.A.; project administration, T.A.; funding acquisition, S.Y. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Japan Society for the Promotion of Science (grant numbers: 19K07835, 23K06801) (S.Y.), Intramural Research Grant for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (grant numbers: 30-3; 30-5; 3-5) (T.A.), grants from Takeda Science Foundation (S.Y.) and Eli Lilly Japan (T.A.), and Daiichi-Sankyo Japan (T.A.).
Institutional Review Board Statement
The animal study protocol was approved by the Committee on Ethical Issues in Animal Experiments of the National Center of Neurology and Psychiatry (approval number: 2023005, approval date: 8 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are shown in the paper.
Acknowledgments
We thank Tadafumi Hashimoto and Tomohiro Kabuta (NCNP) for their technical support.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Pires, G.; McElligott, S.; Drusinsky, S.; Halliday, G.; Potier, M.C.; Wisniewski, T.; Drummond, E. Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer’s disease and not in other tauopathies. Acta Neuropathol. Commun. 2019, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef] [PubMed]
- Gustke, N.; Steiner, B.; Mandelkow, E.M.; Biernat, J.; Meyer, H.E.; Goedert, M.; Mandelkow, E. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 1992, 307, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Biernat, J.; Gustke, N.; Drewes, G.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: Distinction between PHF-like immunoreactivity and microtubule binding. Neuron 1993, 11, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Johnson, G.V. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 2003, 278, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Utton, M.; Gallo, J.M.; Miller, C.C. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 1996, 109, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Bading, H.; Ginty, D.D.; Greenberg, M.E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 1993, 260, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Arnold, F.J.; Bading, H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 1993, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Park, C.S.; Abbassi, N.R.; Tang, S.J. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J. Biol. Chem. 2006, 281, 18802–18815. [Google Scholar] [CrossRef]
- Zhou, X.P.; Wu, K.Y.; Liang, B.; Fu, X.Q.; Luo, Z.G. TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 17181–17186. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Neveu, P.; Kosik, K.S. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron 2009, 64, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Takemori, H.; Zhou, Y.; Xiong, Z.Q. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J. Neurosci. 2009, 29, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Matsubara, K.; Sakai, K.; Ito, M.; Ohno, K.; Ueda, M.; Yamamoto, A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015, 1613, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, Y.; Iwata, R.; Kimura, H.; Vanderhaeghen, P.; Yamamoto, N.; Sugo, N. Repetitive CREB-DNA interactions at gene loci predetermined by CBP induce activity-dependent gene expression in human cortical neurons. Cell Rep. 2024, 43, 113576. [Google Scholar] [CrossRef] [PubMed]
- Adamec, E.; Mercken, M.; Beermann, M.L.; Didier, M.; Nixon, R.A. Acute rise in the concentration of free cytoplasmic calcium leads to dephosphorylation of the microtubule-associated protein tau. Brain Res. 1997, 757, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.V.; Desjardins, A.; Leclerc, N. Tau secretion is correlated to an increase of Golgi dynamics. PLoS ONE 2017, 12, e0178288. [Google Scholar] [CrossRef] [PubMed]
- Bramblett, G.T.; Goedert, M.; Jakes, R.; Merrick, S.E.; Trojanowski, J.Q.; Lee, V.M. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Biernat, J.; von Bergen, M.; Mandelkow, E.; Mandelkow, E.M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 1999, 38, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.D.; Di Clerico, J.; Li, B.; Corbo, C.P.; Alaniz, M.E.; Grundke-Iqbal, I.; Iqbal, K. Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J. Biol. Chem. 2010, 285, 30851–30860. [Google Scholar] [CrossRef]
- Noble, W.; Hanger, D.P.; Miller, C.C.; Lovestone, S. The importance of tau phosphorylation for neurodegenerative diseases. Front. Neurol. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Haj-Yahya, M.; Gopinath, P.; Rajasekhar, K.; Mirbaha, H.; Diamond, M.I.; Lashuel, H.A. Site-Specific Hyperphosphorylation Inhibits, Rather than Promotes, Tau Fibrillization, Seeding Capacity, and Its Microtubule Binding. Angew. Chem. Int. Ed. Engl. 2020, 59, 4059–4067. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ocádiz, R.; Trippa, M.; Zhang, C.L.; Posani, L.; Cocco, S.; Monasson, R.; Schmidt-Hieber, C. A synaptic signal for novelty processing in the hippocampus. Nat. Commun. 2022, 13, 4122. [Google Scholar] [CrossRef] [PubMed]
- Canet, G.; Rocaboy, E.; Laliberté, F.; Boscher, E.; Guisle, I.; Diego-Diaz, S.; Fereydouni-Forouzandeh, P.; Whittington, R.A.; Hébert, S.S.; Pernet, V.; et al. Temperature-induced Artifacts in Tau Phosphorylation: Implications for Reliable Alzheimer’s Disease Research. Exp. Neurobiol. 2023, 32, 423–440. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Matsuura, N.; Ubukata, M.; Oikawa, H.; Shima, H.; Kikuchi, K. Tautomycetin is a novel and specific inhibitor of serine/threonine protein phosphatase type 1, PP1. Biochem Biophys Res Commun. 2001, 287, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Hagita, A.; Wada-Kakuda, S.; Nobuhara, M.; Kakuda, N.; Miyasaka, T. Quantitative fractionation of tissue microtubules with distinct biochemical properties reflecting their stability and lability. Biochem. Biophys. Res. Commun. 2021, 560, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Miyasaka, T.; Launey, T.; Chui, D.H.; Tanemura, K.; Sato, S.; Murayama, O.; Ishiguro, K.; Tatebayashi, Y.; Takashima, A. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: Implications for Alzheimer’s disease. J. Neurosci. 2004, 24, 2401–2411. [Google Scholar] [CrossRef]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta. 2005, 1739, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Plattner, F.; Angelo, M.; Giese, K.P. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006, 281, 25457–25465. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yamashita, S.; Fukuda, T.; Park, J.M.; Murayama, M.; Mizoroki, T.; Yoshiike, Y.; Sahara, N.; Takashima, A. Hyperphosphorylated tau in parahippocampal cortex impairs place learning in aged mice expressing wild-type human tau. EMBO J. 2007, 26, 5143–5152. [Google Scholar] [CrossRef]
- Verwer, R.W.; Sluiter, A.A.; Balesar, R.A.; Baayen, J.C.; Noske, D.P.; Dirven, C.M.; Wouda, J.; van Dam, A.M.; Lucassen, P.J.; Swaab, D.F. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007, 130, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shahpasand, K.; Uemura, I.; Saito, T.; Asano, T.; Hata, K.; Shibata, K.; Toyoshima, Y.; Hasegawa, M.; Hisanaga, S. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J. Neurosci. 2012, 32, 2430–2441. [Google Scholar] [CrossRef] [PubMed]
- Guisle, I.; Gratuze, M.; Petry, S.; Morin, F.; Keraudren, R.; Whittington, R.A.; Hébert, S.S.; Mongrain, V.; Planel, E. Circadian and sleep/wake-dependent variations in tau phosphorylation are driven by temperature. Sleep 2020, 43, zsz266. [Google Scholar] [CrossRef] [PubMed]
- Brion, J.P.; Smith, C.; Couck, A.M.; Gallo, J.M.; Anderton, B.H. Developmental changes in tau phosphorylation: Fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer’s disease. J. Neurochem. 1993, 61, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Stieler, J.; Strijkstra, A.M.; Hut, R.A.; Rüdiger, J.; Van der Zee, E.A.; Harkany, T.; Holzer, M.; Härtig, W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 2003, 23, 6972–6981. [Google Scholar] [CrossRef]
- Kenessey, A.; Yen, S.H. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993, 629, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Richter, K.E.; Nolan, C.E.; Finley, J.E.; Liu, L.; Wen, Y.; Krishnamurthy, P.; Herman, M.; Wang, L.; Schachter, J.B.; et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 2007, 27, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, X.; Drew, K.L.; Perry, G.; Smith, M.A.; Zhu, X. Physiological regulation of tau phosphorylation during hibernation. J. Neurochem. 2008, 105, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Run, X.; Liang, Z.; Li, Y.; Liu, F.; Liu, Y.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases. J. Neurochem. 2009, 108, 1480–1494. [Google Scholar] [CrossRef]
- Stieler, J.T.; Bullmann, T.; Kohl, F.; Tøien, Ø.; Brückner, M.K.; Härtig, W.; Barnes, B.M.; Arendt, T. The physiological link between metabolic rate depression and tau phosphorylation in mammalian hibernation. PLoS ONE 2011, 6, e14530. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, S.; Suzuki, S.; Yoshikawa, K.; Iida, K.; Hirata, A.; Suzuki, M.; Takashima, A.; Maruyama, K.; Hirasawa, A.; Awaji, T. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol. Brain 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Guisle, I.; Canet, G.; Pétry, S.; Fereydouni-Forouzandeh, P.; Morin, F.; Kérauden, R.; Whittington, R.A.; Calon, F.; Hébert, S.S.; Planel, E. Sauna-like conditions or menthol treatment reduce tau phosphorylation through mild hyperthermia. Neurobiol. Aging 2022, 113, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Czuczwar, S.J. Trans- and Cis-Phosphorylated Tau Protein: New Pieces of the Puzzle in the Development of Neurofibrillary Tangles in Post-Ischemic Brain Neurodegeneration of the Alzheimer’s Disease-like Type. Int. J. Mol. Sci. 2024, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Winder, D.G.; Sweatt, J.D. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2001, 2, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Taleski, G.; Sontag, E. The protein serine/threonine phosphatases PP2A, PP1 and calcineurin: A triple threat in the regulation of the neuronal cytoskeleton. Mol. Cell Neurosci. 2017, 84, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Aggen, J.B.; Nairn, A.C.; Chamberlin, R. Regulation of protein phosphatase-1. Chem. Biol. 2000, 7, R13–R23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).