Effects of Anti-CGRP Monoclonal Antibodies on Neurophysiological and Clinical Outcomes: A Combined Transcranial Magnetic Stimulation and Algometer Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Neurophysiological Parameters
2.2.1. Transcranial Magnetic Stimulation
- The resting motor threshold (RMT), from SP-TMS, is defined as the minimum stimulation intensity required to produce a peak-to-peak motor-evoked potential (MEP) amplitude of ≥50 μV in at least five of ten stimulations.
- A short-interval intracortical inhibition (SICI), from the PP-TMS session, evoked by delivering a subthreshold (80% RMT) Conditioning Stimulus (CS) followed by a suprathreshold (130% RMT) test stimulus (TS) at interstimulus intervals (ISIs) of 3 and 5 ms.
- Intracortical facilitation (ICF), from the PP-TMS session, with the same CS (80% RMT) and TS (130% RMT) at longer ISIs of 10 ms, 15 ms, and 20 ms.
- Eight MEPs were recorded from the SP-session, elicited by delivering a suprathreshold (130% RMT) TS.
2.2.2. Pressure Pain Threshold
2.3. Headache Parameters
2.4. Statistical Analysis
3. Results
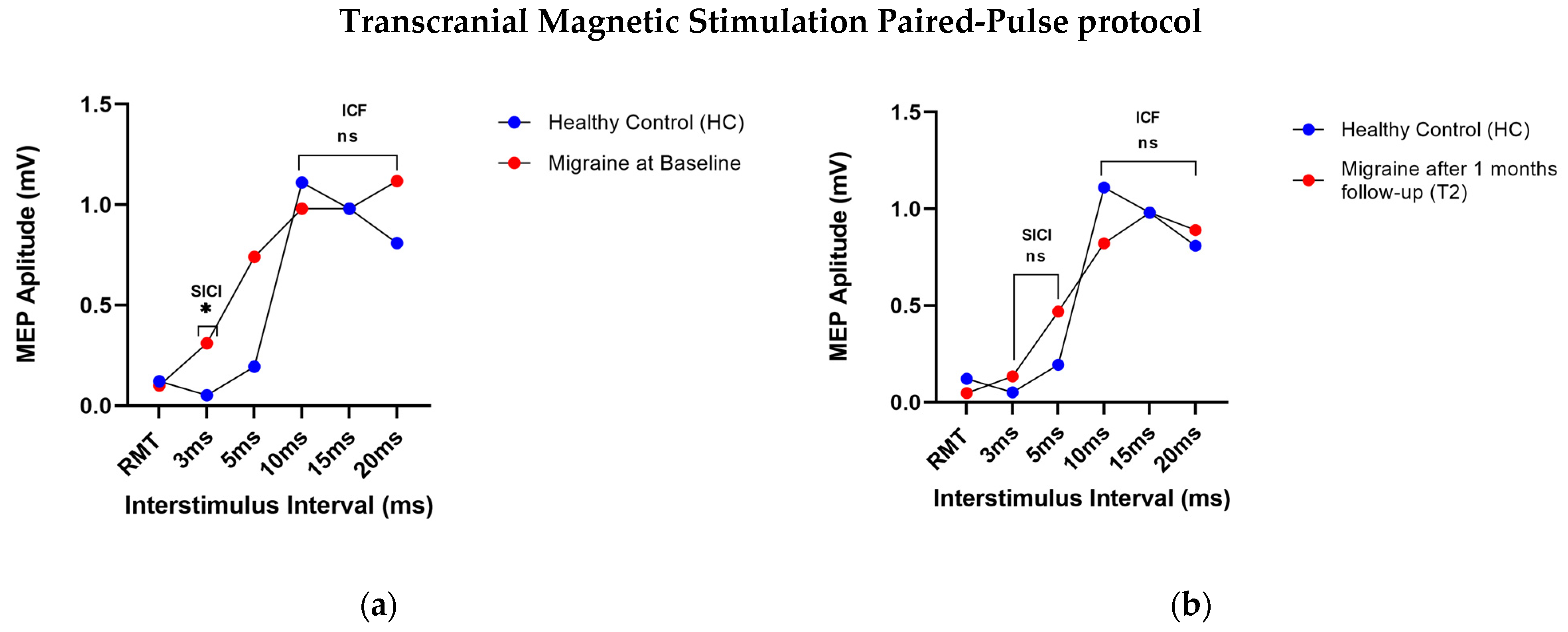
3.1. Transcranial Magnetic Stimulation
3.1.1. SP-Protocol: Resting Motor Threshold
| Gender | Age | Diagnosis | Previous Prophylactic Therapy | mAbs | MMDs | Duration | MDI | MIDAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t0 | t1 | t2 | t0 | t1 | t2 | t0 | t1 | ||||||
| Patients | ||||||||||||||||
| 1 | F | 46 | CM | Am; BoNTA; Tiz; Top | Er | 20 | 5 | 6 | 163 | 24 | 47 | 20 | 12 | 7 | 124 | 28 |
| 2 | F | 22 | CM | Am; BoNTA; Er; Flu; Prop; Top | Er | 20 | 19 | 16 | 526 | 123 | 104 | 5 | 4 | 5 | 126 | 52 |
| 3 | F | 62 | CM | Am; BoNTA; Flu; Prop; Top; Ven | Er | 20 | 1 | 2 | 518 | 6 | 14 | 12 | 2 | 1 | 114 | 4 |
| 4 | F | 61 | CM | Am; Flu; Prop; Top; VPA | Gal | 15 | 5 | 4 | 105 | 30 | 14 | 25 | 4 | 3 | 120 | 19 |
| 5 | M | 41 | HFEM | Am; BoNTA; Er; Flu; Prop; Top; VPA | Er | 10 | 9 | 5 | 52 | 49 | 45 | 12 | 15 | 14 | 64 | 31 |
| 6 | M | 42 | HFEM | Am; Flu; Top | Er | 14 | 8 | 9 | 42 | 40 | 48 | 14 | 7 | 6 | 84 | 25 |
| 7 | F | 65 | CM | Am; Preg | Er | 18 | 11 | 7 | 92 | 70 | 29 | 24 | 11 | 8 | 114 | 32 |
| 8 | F | 47 | HFEM | Am; Flu; Top | Fre | 12 | 10 | 8 | 141 | 87 | 80 | 9 | 6 | 4 | 61 | 30 |
| 9 | F | 33 | HFEM | Top | Er | 12 | 6 | 3 | 45 | 14 | 6 | 12 | 10 | 3 | 86 | 6 |
| 10 | M | 49 | CM | BoNTA; Met; Top;VPA | Fre | 19 | 7 | 4 | 85 | 29 | 11 | 30 | 9 | 9 | 131 | 7 |
| 11 | F | 34 | HFEM | Am; BoNTA; Top; VPA | Fre | 12 | 6 | 5 | 37 | 15 | 8 | 12 | 6 | 7 | 78 | 2 |
| Mean (SD) | 45 (±13) | 15 (±3) | 7 (±4) | 6 (±3) | 164 (±181) | 44 (±35) | 36 (±31) | 15 (±7) | 7 (±3) | 6 (±3) | 100 (±25) | 21 (±15) | ||||
| Median (IQR) | 46 (5.5–55) | 15 (12–19.5) | 7 (5.5–9.5) | 5 (4–7.5) | 92 (48.5–152) | 30 (19.5–59.5) | 29 (12.5–47.5) | 12 (12–22) | 7 (5–10.5) | 6 (3.5–7.5) | 114 (81–122) | 25 (6.5–30.5) | ||||
| Difference | ||||||||||||||||
| t0 vs. t1 | p ≤ 0.01 | p ≤ 0.05 | p = ns | p ≤ 0.0001 | ||||||||||||
| t0 vs. t2 | p ≤ 0.001 | p ≤ 0.01 | p ≤ 0.01 | - | ||||||||||||
| t1 vs. t2 | p = ns | p = ns | p = ns | - | ||||||||||||
3.1.2. PP-Protocol of TMS: Intracortical Inhibition and Intracortical Facilitation
3.2. Pressure Pain Threshold
3.3. Headache Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| anti-CGRP mAbs | Anti-Calcitonin Gene-Related Peptide monoclonal Antibodies |
| BoNTA | Onabotulinumtoxin-A |
| CGRP | Calcitonin Gene-Related Peptide |
| CSP | Cortical Silent Period |
| CM | Chronic Migraine |
| EM | Episodic Migraine |
| FDA | Food and Drug Administration |
| HF-EM | High-Frequency Episodic Migraine |
| ICHD-3 | International Classification of Headache Disorders, Third Edition |
| ISI | interstimulus interval |
| ICF | intracortical facilitation |
| LICI | long-interval intracortical inhibition |
| LF-EM | Low-Frequency Episodic Migraine |
| MOH | Medication Overuse Headache |
| MEP | motor-evoked potential |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| PPT | Pressure Pain Threshold |
| PP-TMS | Paired-Pulse Transcranial Magnetic Stimulation |
| RCT | Randomized Control Trial |
| RMT | resting motor threshold |
| SICI | short-interval intracortical inhibition |
| SP-TMS | Single-Pulse Transcranial Magnetic Stimulation |
| TMS | Transcranial Magnetic Stimulation |
References
- Di Cola, F.S.; Bolchini, M.; Caratozzolo, S.; Ceccardi, G.; Cortinovis, M.; Liberini, P.; Rao, R.; Padovani, A. Migraine Disability Improvement during Treatment with Galcanezumab in Patients with Chronic and High Frequency Episodic Migraine. Neurol. Int. 2023, 15, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Lorenzo, C.; Schoenen, J.; Pierelli, F. Habituation and sensitization in primary headaches. J. Headache Pain 2013, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Ishiyama, S. Neurite Damage in Patients with Migraine. Neurol. Int. 2024, 16, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Goto, M.; Ishiyama, S. Analysis of Migraine Pathophysiology by Magnetic Resonance Imaging. OBM Neurobiol. 2021, 6, 115. [Google Scholar] [CrossRef]
- Pleș, H.; Florian, I.-A.; Timis, T.-L.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Dumitrascu, D.-I.; Popa, A.A.; Bordeianu, A.; Ciurea, A.V. Migraine: Advances in the Pathogenesis and Treatment. Neurol. Int. 2023, 15, 1052–1105. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y. Migraine Pathophysiology Revisited: Proposal of a New Molecular Theory of Migraine Pathophysiology and Headache Diagnostic Criteria. Int. J. Mol. Sci. 2022, 23, 13002. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, S.; Shibata, Y.; Ayuzawa, S.; Matsushita, A.; Matsumura, A.; Ishikawa, E. The Modifying of Functional Connectivity Induced by Peripheral Nerve Field Stimulation using Electroacupuncture for Migraine: A Prospective Clinical Study. Pain Med. 2022, 23, 1560–1569. [Google Scholar] [CrossRef]
- Finocchi, C.; Di Antonio, S.; Castaldo, M.; Ponzano, M.; Bovis, F.; Villafañe, J.H.; Torelli, P.; Arendt-Nielsen, L. Increase pain sensitivity during the four phases of the migraine cycle in patients with episodic migraine. Neurol. Sci. 2022, 43, 5773–5775. [Google Scholar] [CrossRef]
- Di Antonio, S.; Arendt-Nielsen, L.; Ponzano, M.; Bovis, F.; Torelli, P.; Finocchi, C.; Castaldo, M. Trigeminocervical pain sensitivity during the migraine cycle depends on headache frequency. Neurol. Sci. 2023, 44, 4021–4032. [Google Scholar] [CrossRef]
- Brigo, F.; Storti, M.; Tezzon, F.; Manganotti, P.; Nardone, R. Primary visual cortex excitability in migraine: A systematic review with meta-analysis. Neurol. Sci. 2012, 34, 819–830. [Google Scholar] [CrossRef]
- Badawy, R.A.B.; Loetscher, T.; Macdonell, R.A.L.; Brodtmann, A. Cortical excitability and neurology: Insights into the pathophysiology. Funct. Neurol. 2012, 27, 131–145. [Google Scholar]
- Neverdahl, J.; Omland, P.; Uglem, M.; Engstrøm, M.; Sand, T. Reduced motor cortical inhibition in migraine: A blinded transcranial magnetic stimulation study. Clin. Neurophysiol. 2017, 128, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Granato, A.; Martini, M.; Stella, A.B.; Galmonte, A.; Murena, L.; Manganotti, P. Neurophysiological and Clinical Outcomes in Episodic Migraine Without Aura: A Cross-Sectional Study. J. Clin. Neurophysiol. 2024, 41, 388–395. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Vaegter, H.B.; Finocchietti, S.; Handberg, G.; Arendt-Nielsen, L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: A reliability study. Pain 2015, 156, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Granato, A.; Borgino, C.; Galmonte, A.; Manganotti, P. Instrumental assessment of physiotherapy and onabolulinumtoxin-A on cervical and headache parameters in chronic migraine. Neurol. Sci. 2021, 43, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Granato, A.; Ceschin, M.; Galmonte, A.; Manganotti, P. Algometer Assessment of Pressure Pain Threshold After Onabotulinumtoxin-A and Physical Therapy Treatments in Patients With Chronic Migraine: An Observational Study. Front. Pain Res. 2022, 3, 770397. [Google Scholar] [CrossRef]
- Edvinsson, L. The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine. Headache J. Head Face Pain 2017, 57, 47–55. [Google Scholar] [CrossRef]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache J. Head Face Pain 2019, 59, 659–681. [Google Scholar] [CrossRef]
- Benedicter, N.; Messlinger, K.; Vogler, B.; Mackenzie, K.D.; Stratton, J.; Friedrich, N.; Dux, M. Semi-Automated Recording of Facial Sensitivity in Rat Demonstrates Antinociceptive Effects of the Anti-CGRP Antibody Fremanezumab. Neurol. Int. 2023, 15, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef]
- Zhu, C.; Guan, J.B.; Xiao, H.; Luo, W.; Tong, R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine 2019, 98, e18483. [Google Scholar] [CrossRef]
- Dodick, D.W. CGRP ligand and receptor monoclonal antibodies for migraine prevention: Evidence review and clinical implications. Cephalalgia 2019, 39, 445–458. [Google Scholar] [CrossRef]
- Lambru, G.; Hill, B.; Murphy, M.; Tylova, I.; Andreou, A.P. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain 2020, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Delussi, M.; Gentile, E.; Ricci, K.; Quitadamo, S.G.; Libro, G. Effect of single dose Erenumab on cortical responses evoked by cutaneous a-delta fibers: A pilot study in migraine patients. Cephalalgia 2021, 41, 1004–1014. [Google Scholar] [CrossRef]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Peng, K.-P.; May, A. Quantitative sensory testing in migraine patients must be phase-specific. Pain 2018, 159, 2414–2416. [Google Scholar] [CrossRef]
- Delaruelle, Z.; on behalf of the European Headache Federation School of Advanced Studies (EHF-SAS); Ivanova, T.A.; Khan, S.; Negro, A.; Ornello, R.; Raffaelli, B.; Terrin, A.; Mitsikostas, D.D.; Reuter, U. Male and female sex hormones in primary headaches. J. Headache Pain 2018, 19, 117. [Google Scholar] [CrossRef]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Müller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef]
- Cosentino, G.; Di Marco, S.; Ferlisi, S.; Valentino, F.; Capitano, W.M.; Fierro, B.; Brighina, F. Intracortical facilitation within the migraine motor cortex depends on the stimulation intensity. A paired-pulse TMS study. J. Headache Pain 2018, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2020, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Petersen, M.W.; Svendsen, A.S.; Gazerani, P. Pressure pain thresholds assessed over temporalis, masseter, and frontalis muscles in healthy individuals, patients with tension-type headache, and those with migraine—A systematic review. Pain 2015, 156, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [PubMed]
- Manganotti, P.; Michelutti, M.; Furlanis, G.; Deodato, M.; Stella, A.B. Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin. Neurophysiol. 2023, 151, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; Allers, A.; May, A. Hypothalamus as a mediator of chronic migraine. Neurology 2017, 88, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Renzo, A.; Petolicchio, B.; Tinelli, E.; Di Lorenzo, C.; Serrao, M.; Calistri, V.; Tardioli, S.; Cartocci, G.; Parisi, V.; et al. Increased neural connectivity between the hypothalamus and cortical resting-state functional networks in chronic migraine. J. Neurol. 2019, 267, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Cesaris, F.; Ferrari, A.; Benemei, S.; Fattori, D.; Chiarugi, A. Effectiveness of anti-CGRP monoclonal antibodies on central symptoms of migraine. Cephalalgia 2022, 42, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Ashina, S.; Melo-Carrillo, A.; Bolo, N.R.; Borsook, D.; Burstein, R. Peripherally acting anti-CGRP monoclonal antibodies alter cortical gray matter thickness in migraine patients: A prospective cohort study. NeuroImage Clin. 2023, 40, 103531. [Google Scholar] [CrossRef]
- Cortese, F.; Pierelli, F.; Pauri, F.; Di Lorenzo, C.; Lepre, C.; Malavolta, G.; Merluzzo, C.; Parisi, V.; Ambrosini, A.; Serrao, M.; et al. Withdrawal from acute medication normalises short-term cortical synaptic potentiation in medication overuse headache. Neurol. Sci. 2019, 40, 963–969. [Google Scholar] [CrossRef]
- Chen, R.; Tam, A.; Bütefisch, C.; Corwell, B.; Ziemann, U.; Rothwell, J.C.; Cohen, L.G.; Rozand, V.; Senefeld, J.W.; Sundberg, C.W.; et al. Intracortical Inhibition and Facilitation in Different Representations of the Human Motor Cortex. J. Neurophysiol. 1998, 80, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Siniatchkin, M.; Kröner-Herwig, B.; Kocabiyik, E.; Rothenberger, A. Intracortical Inhibition and Facilitation in Migraine—A Transcranial Magnetic Stimulation Study. Headache J. Head Face Pain 2007, 47, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Barón, J.; Ruiz, M.; Palacios-Ceña, M.; Madeleine, P.; Guerrero, Á.L.; Arendt-Nielsen, L.; Fernández-De-Las-Peñas, C. Differences in Topographical Pressure Pain Sensitivity Maps of the Scalp Between Patients With Migraine and Healthy Controls. Headache J. Head Face Pain 2016, 57, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Melo-Carrillo, A.; Nir, R.-R.; Strassman, A.M.; Burstein, R. Non-Trigeminal Nociceptive Innervation of the Posterior Dura: Implications to Occipital Headache. J. Neurosci. 2019, 39, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, Y.; Xiong, H.; Hong, P. CGRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019, 9, e01215. [Google Scholar] [CrossRef]
- Alasad, Y.W.; Asha, M.Z. Monoclonal antibodies as a preventive therapy for migraine: A meta-analysis. Clin. Neurol. Neurosurg. 2020, 195, 105900. [Google Scholar] [CrossRef]
- Ziegeler, C.; Mehnert, J.; Asmussen, K.; May, A. Central effects of erenumab in migraine patients: An event-related functional imaging study. Neurology 2020, 95, e2794–e2802. [Google Scholar] [CrossRef]

| PP-TMS | M Mean (SD) Median (IQR) | HC Mean (SD) Median (IQR) | Differences t0 vs. t1 vs. t2 | Differences M vs. HC |
|---|---|---|---|---|
| 3 ms (SICI) | ||||
| t0 0.3 (SD ± 0.5) 0.1 (0.1–0.25) | 0.05 (SD ± 0.03) 0.05 (0.02–0.09) | t0 vs. t1: ns | U = 92; p = 0.04 * | |
| t1 0.2 (SD ± 0.2) 0.1 (0.35–0.25) | t0 vs. t2: p < 0.05 * | U = 86; p = 0.1 | ||
| t2 0.1 (SD ± 0.2) 0.04 (0.01–0.07) | t1 vs. t2: ns | U = 67; p = 0.6 | ||
| 5 ms (SICI) | ||||
| t0 0.6 (SD ± 1.4) 0.1 (0.06–0.3) | 0.1 (SD ± 0.1) 0.2 (0.01–0.3) | t0 vs. t1: ns | U = 62; p = 0.9 | |
| t1 0.4 (SD ± 0.5) 0.2 (0.1–0.5) | t0 vs. t2: ns | U = 72; p = 0.4 | ||
| t2 0.4 (SD ± 0.5) 0.3 (0.06–0.85) | t1 vs. t2: ns | U = 79; p = 0.2 | ||
| 10 ms (ICF) | ||||
| t0 0.9 (SD ± 1) 0.8 (0.1–1.3) | 1.1 (SD ± 1) 1 (0.25–1.4) | t0 vs. t1: ns | U = 73.5; p = 0.4 | |
| t1 1.2 (SD ± 1.1) 1 (0.5–1.9) | t0 vs. t2: ns | U = 67.5; p = 0.6 | ||
| t2 0.8 (SD ± 0.9) 0.5 (0.15–0.9) | t1 vs. t2: ns | U = 75.5; p = 0.3 | ||
| 15 ms (ICF) | ||||
| t0 0.9 (SD ± 1.2) 0.5 (0.14–1.15) | 0.9 (SD ± 0.9) 0.9 (0.2–1.4) | t0 vs. t1: ns | U = 72.5; p = 0.4 | |
| t1 1.6 (SD ± 1.2) 1.7 (0.75–2.2) | t0 vs. t2: ns | U = 82.5; p = 0.1 | ||
| t2 1 (SD ± 0.9) 0.5 (0.35–1.3) | t1 vs. t2: ns | U = 62.5; p = 0.9 | ||
| 20 ms (ICF) | ||||
| t0 1 (SD ± 1.2) 0.5 (0.19–1.3) | 0.8 (SD ± 0.6) 0.5 (0.25–1.4) | t0 vs. t1: ns | U = 61; p = 0.9 | |
| t11.4 (SD ± 1.2) 0.9 (0.55–1.9) | t0 vs. t2: ns | U = 76.5; p = 0.3 | ||
| t2 0.8 (SD ± 1) 0.3 (0.2–1.25) | t1 vs. t2: ns | U = 63.5; p = 0.8 | ||
| RMT | ||||
| t0 59.2 (SD ± 12.3) 62 (53.5–68) | 68.6 (SD ± 8.1) 70 (65–75) | t0 vs. t1: p < 0.05 * | U = 91; p = 0.04 * | |
| t1 66 (SD ± 12.3) 67 (60.5–74) | t0 vs. t2: ns | U = 69.5; p = 0.5 | ||
| t2 70 (SD ± 15.8) 70 (57–84.5) | t1 vs. t2: ns | U = 64.5; p = 0.8 |
| PPT | M Mean (SD) Median (IQR) | HC Mean (SD) Median (IQR) | Differences t0 vs. t1 vs. t2 | Differences M vs. HC |
|---|---|---|---|---|
| Temporalis left | t0 250.6 (SD ± 118) 346.9 (258–443.5) | 285.5 (SD ± 116) 260 (211–347.8) | t0 vs. t1: ns | U = 70; p = 0.5 |
| t1 285 (SD ± 138.8) 246.3 (209–325.6) | t0 vs. t2: ns | U = 65; p = 0.7 | ||
| t2 273.2 (SD ± 94.5) 237.1 (204.5–325.6) | t1 vs. t2: ns | U = 64; p = 0.8 | ||
| Temporalis right | t0 251.7 (SD ± 108.2) 319.4 (233.9–404) | 315.6 (SD ± 65.3) 328 (264.1–354.1) | t0 vs. t1: ns | U = 91; p = 0.04 * |
| t1 270 (SD ± 81.7) 268.5 (222.7–292) | t0 vs. t2: ns | U = 84; p = 0.1 | ||
| t2 255 (SD ± 75.8) 242.3 (202.2–291.7) | t1 vs. t2: ns | U = 89; p = 0.06 | ||
| Sub-occipitalis left | t0 257.2 (SD ± 109.8) 246.9 (195.3–334.4) | 563.7 (SD ± 668.5) 314.6 (295.4–475.6) | t0 vs. t1: p < 0.05 * | U = 91; p = 0.04 * |
| t1 345.3 (SD ± 77.1) 339.7 (279.3–377.2) | t0 vs. t2: p < 0.01 ** | U = 73; p = 0.4 | ||
| t2 341.2 (SD ± 96.7) 340.3 (285.2–379.2) | t1 vs. t2: ns | U = 69; p = 0.6 | ||
| Sub-occipitalis right | t0 241.4 (SD ± 109.5) 235.8 (172.4–310.8) | 318.1 (SD ± 64.6) 299.2 (283.7–344.9) | t0 vs. t1: ns | U = 91; p = 0.04 * |
| t1 309.2 (SD ± 84.2) 312.3 (249.9–379.5) | t0 vs. t2: ns | U = 65; p = 0.7 | ||
| t2 318.6 (SD ± 86.7) 326 (273.7–347.8) | t1 vs. t2: ns | U = 66; p = 0.7 | ||
| Masseter left | t0 214.7 (SD ± 93.4) 207.1 (159.7–234.5) | 257.9 (SD ± 41.5) 255.5 (232.6–266.6) | t0 vs. t1: ns | U = 91; p = 0.04 * |
| t1 219.9 (SD ± 63.4) 209 (192.7–243.7) | t0 vs. t2: ns | U = 91; p = 0.04 * | ||
| t2 232.9 (SD ± 86.7) 230.6 (182.2–249.9) | t1 vs. t2: ns | U = 89; p = 0.06 | ||
| Masseter right | t0 171.2 (SD ± 59.3) 170.3 (119.8–220.1) | 255.4 (SD ± 68.7) 235.2 (211–305.4) | t0 vs. t1: ns | U = 99; p = 0.01 * |
| t1 184.6 (SD ± 46.6) 180.3 (148.6–194.6) | t0 vs. t2: ns | U = 103; p = 0.004 * | ||
| t2 218.2 (SD ± 79.6) 209.1 (166.5–225.1) | t1 vs. t2: ns | U = 85; p = 0.1 | ||
| Trapezius left | t0 334.8 (SD ± 146.4) 346.9 (258–443.5) | 509.1 (SD ± 180.2) 471 (369.1–597) | t0 vs. t1: ns | U = 93; p = 0.03 * |
| t1 398.1 (SD ± 114.1) 369.5 (322.4–463.8) | t0 vs. t2: p < 0.05 * | U = 83; p = 0.1 | ||
| t2 423.11 (SD ± 145.7) 369.9 (324.9–509.4) | t1 vs. t2: ns | U = 78; p = 0.2 | ||
| Trapezius right | t0 321.7 (SD ± 125.2) 319.4 (233.9–404) | 522.8 (SD ± 171.9) 499.8 (409.9–600) | t0 vs. t1: p < 0.01 ** | U = 100; p = 0.008 * |
| t1 462.5 (SD ± 104.9) 479.5 (395.6–543.1) | t0 vs. t2: p < 0.01 ** | U = 70; p = 0.5 | ||
| t2 473.6 (SD ± 203.5) 454.7 (318.1–616.7) | t1 vs. t2: ns | U = 70; p = 0.5 | ||
| Procerus | t0 250.7 (SD ± 82.3) 246.9 (204.1–317.5) | 287.4 (SD ± 83.6) 285.5 (230.3–343.5) | t0 vs. t1: ns | U = 74; p = 0.4 |
| t1 308.7 (SD ± 75.4) 294 (275–338.1) | t0 vs. t2: ns | U = 68; p = 0.6 | ||
| t2 328 (SD ± 55.9) 310.3 (280.3–382.5) | t1 vs. t2: ns | U = 67; p = 0.6 | ||
| Tensor fasciae latae left | t0 490.8 (SD ± 238.5) 484.8 (339.4–563.1) | 667.7 (SD ± 182.3) 642.9 (574.9–768.7) | t0 vs. t1: ns | U = 92; p = 0.04 * |
| t1 560.2 (SD ± 217.6) 494.5 (409–731.1) | t0 vs. t2: ns | U = 79; p = 0.2 | ||
| t2 590.1 (SD ± 255.7) 510.2 (418.7–719.6) | t1 vs. t2: ns | U = 79; p = 0.2 | ||
| Tensor fasciae latae right | t0 486.6 (SD ± 235.2) 463.2 (259.3–651.4) | 1283.9 (SD ± 1891.8) 710.6 (584.5–917.6) | t0 vs. t1: ns | U = 95; p = 0.02 * |
| t1 558.2 (SD ± 235.5) 495.8 (401.4–730.4) | t0 vs. t2: ns | U = 89; p = 0.06 | ||
| t2 567.3 (SD ± 260.5) 658.5 (354.7–682) | t1 vs. t2: ns | U = 85; p = 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manganotti, P.; Deodato, M.; D’Acunto, L.; Biaduzzini, F.; Garascia, G.; Granato, A. Effects of Anti-CGRP Monoclonal Antibodies on Neurophysiological and Clinical Outcomes: A Combined Transcranial Magnetic Stimulation and Algometer Study. Neurol. Int. 2024, 16, 673-688. https://doi.org/10.3390/neurolint16040051
Manganotti P, Deodato M, D’Acunto L, Biaduzzini F, Garascia G, Granato A. Effects of Anti-CGRP Monoclonal Antibodies on Neurophysiological and Clinical Outcomes: A Combined Transcranial Magnetic Stimulation and Algometer Study. Neurology International. 2024; 16(4):673-688. https://doi.org/10.3390/neurolint16040051
Chicago/Turabian StyleManganotti, Paolo, Manuela Deodato, Laura D’Acunto, Francesco Biaduzzini, Gabriele Garascia, and Antonio Granato. 2024. "Effects of Anti-CGRP Monoclonal Antibodies on Neurophysiological and Clinical Outcomes: A Combined Transcranial Magnetic Stimulation and Algometer Study" Neurology International 16, no. 4: 673-688. https://doi.org/10.3390/neurolint16040051
APA StyleManganotti, P., Deodato, M., D’Acunto, L., Biaduzzini, F., Garascia, G., & Granato, A. (2024). Effects of Anti-CGRP Monoclonal Antibodies on Neurophysiological and Clinical Outcomes: A Combined Transcranial Magnetic Stimulation and Algometer Study. Neurology International, 16(4), 673-688. https://doi.org/10.3390/neurolint16040051





