Abstract
Several therapeutic agents for neurological disorders are usually not delivered to the brain owing to the presence of the blood–brain barrier (BBB), a special structure present in the central nervous system (CNS). Focused ultrasound (FUS) combined with microbubbles can reversibly and temporarily open the BBB, enabling the application of various therapeutic agents in patients with neurological disorders. In the past 20 years, many preclinical studies on drug delivery through FUS-mediated BBB opening have been conducted, and the use of this method in clinical applications has recently gained popularity. As the clinical application of FUS-mediated BBB opening expands, it is crucial to understand the molecular and cellular effects of FUS-induced microenvironmental changes in the brain so that the efficacy of treatment can be ensured, and new treatment strategies established. This review describes the latest research trends in FUS-mediated BBB opening, including the biological effects and applications in representative neurological disorders, and suggests future directions.
1. Introduction
Neurological disorders are medically defined as diseases that affect the brain and the nerves throughout the central and peripheral nervous systems. There are more than 600 neurological disorders, including degenerative diseases, such as Alzheimer’s or Parkinson’s; brain tumors; brain or spinal cord injury; convulsive disorders, such as epilepsy; and diseases of the blood vessels that supply the brain, such as stroke. However, the etiology of many central nervous system diseases has not yet been identified, and various new drugs are being developed accordingly. However, unlike other organs, the brain that oversees the central nervous system (CNS) is protected by a blood–brain barrier (BBB). The BBB separates the lumen of cerebral blood vessels from the brain parenchyma and selectively restricts permeation through tight junctions between vascular endothelial cells. Each of these tight junctions is composed of a protein complex of various transmembrane proteins, such as junctional adhesion molecules (JAM), occludin and claudin (Figure 1). Outside of the BBB, it forms a structure with astrocytes and pericytes. In particular, the astrocytic endfeet establish connections between neurons and blood flux and regulate the formation of the BBB []. The BBB regulates the homeostasis of the CNS by forming a special structure that prevents exogenous compounds and harmful or toxic substances from being delivered into the brain via the cerebral blood vessels. However, the BBB also limits the intra-brain delivery of various medications. Currently, many brain disease treatments are being developed, but 100% of the drugs with large molecules and 98% or more with small molecules cannot cross the BBB [].
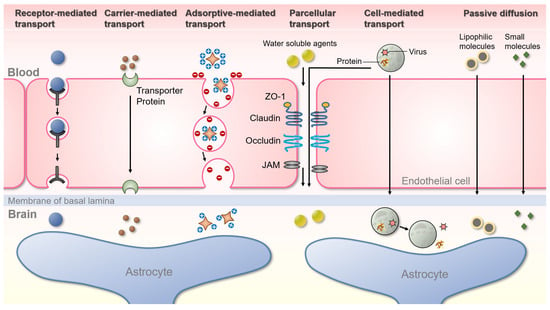
Figure 1.
Schematic representation of the various pathways for transport across the BBB. Receptor-mediated transport (Clathrin-mediated endocytosis): only receptor-specific substances are transported through this process, and cells take up metabolites, hormones, proteins, and in some cases, viruses by an internal invasion of the plasma membrane. Carrier-mediated transport is an energy-dependent pathway normally used by small hydrophilic molecules. Carrier membranes have specific receptors that recognize target molecules and pass through cells, and mainly amino acids, monosaccharides, and peptides are delivered in this process. Adsorptive-mediated transport: this is accomplished by the electrostatic interaction of negatively charged plasma membrane with oppositely charged ligands. Paracellular-mediated transport is a passive transport process across the epithelium through the intercellular space between endothelial cells, in which various tight junction proteins are intricately attached. Cell-mediated transcytosis: cells such as monocytes or macrophages migrate through the paracellular space or across the BBB by transcytosis into the brain to release specific proteins or viruses. Passive diffusion: most small molecules cross the BBB and reach the brain by passive diffusion.
Meanwhile, dysfunction of the BBB is a critical factor in various diseases such as epilepsy and stroke []. Reportedly, the BBB is broken down in neurodegenerative brain diseases such as Alzheimer’s disease (AD), but the role of this phenomenon is unclear []. In addition, malignancies damage the BBB through the formation of the blood–tumor barriers (BTBs) [], and although BTBs leak more than the BBB [], there remain limitations in drug delivery due to heterogeneous permeabilities and efflux transporters [,]. In order to pass through the luminal membrane of brain endothelial cells that consist of the BBB, a number of substances in the blood act on various metabolic enzymes or are actively released into the capillary lumen by embedded efflux transporters such as permeability-glycoprotein (Pgp). Pgp is a protein present in the plasma membrane of endothelial cells in the BBB and one of several efflux pumps. Pgp is overexpressed not only in the selectively permeable BTB, but also in the plasma membrane of tumor cells, which makes tumors cross-resistant to other anticancer drugs [,]. Therefore, a technique that selectively inhibits efflux transporters such as Pgp in the target region is needed. Tumor treatment strategies using FUS are highly important, given that FUS-mediated BBB opening not only affects vesicular transcellular transport, but also inhibits Pgp expression.
Several pathways can cross the BBB to maintain brain homeostasis. Representative pathways include paracellular, transcellular, carrier-mediated, receptor-mediated, adsorptive-mediated, and cell-mediated pathways [] (Figure 1). To date, numerous therapeutic strategies have been developed to overcome the BBB. The main method involves the transcellular lipophilic pathway, but many drugs are hydrophilic; therefore, the efficacy of this pathway is restricted. The method of changing the tight junction using mannitol, an osmotic diuretic, is an example of a paracellular pathway, and it is not useful enough to be applied clinically []. Recently, strategies to overcome the BBB through the development of carriers such as liposomes, nanoparticles, viruses, and exosomes, have been attempted. However, they face limitations in terms of safety and efficiency [].
An invasive surgical method, in which the skin is incised and the skull is opened to, has been used for a long time to treat brain diseases. However, minimally invasive or non-invasive surgical methods are being developed to avoid various risks associated with operating on a larger target area, such as functional damage to the brain and infection. Focused ultrasound (FUS) enables a superior penetration depth and spatial specificity without invasive surgical procedures or genetic modifications. Low-intensity FUS with microbubbles (MB) is a non-invasive technique that reversibly and temporarily opens the BBB [,]. Since contrast agents cannot pass through the BBB, FUS-induced BBB opening is usually confirmed using contrast-enhanced MRI (Figure 2) []. Although the mechanism underlying the effect of BBB opening using FUS has not been elucidated, it is generally thought that the physical oscillations caused by the MB affect the vascular endothelial cells and tissues (Figure 3). In one study, intravenous injection of MB followed by sonication of a specific area of the brain by FUS led to an acoustic cavitation phenomenon wherein the MB repeatedly contracted and expanded in the treated area []. The physical BBB opening returns to normal approximately 6 to 24 h after sonication []. In the past 20 years, drug delivery studies have been conducted for various diseases through FUS-mediated BBB opening. This review describes the latest research trends in FUS-mediated BBB opening.
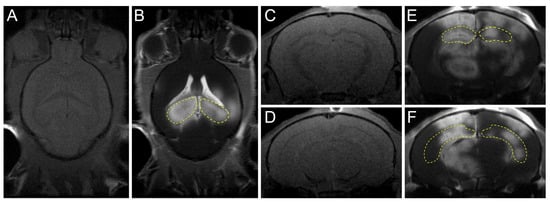
Figure 2.
Confirmation of FUS-induced BBB opening using MRI. The hippocampi (yellow dotted line) of mouse were targeted per sonication. (A,B) Transverse T1-weighted pre-/post-gadolinium MR images were taken to confirm the increased BBB permeability. (C–F) Coronal T1-weighted pre-/post-gadolinium MR images after FUS. (E,F) Coronal T1-weighted post-gadolinium MR images after FUS.
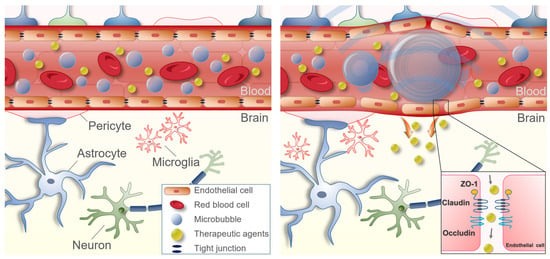
Figure 3.
Schematic representation of focused ultrasound-mediated focused blood–brain barrier opening. When FUS is sonicated in a specific area of the brain, an acoustic cavitation effect is induced, in which MB injected into the blood repeats contraction and expansion due to the pressure of FUS. The binding force between the vascular endothelial cells is loosened at this time. The loosened binding force lasts about 6 h, during which drugs can be delivered into the brain parenchyma.
2. Current Status of FUS-Mediated BBB Opening
2.1. Alzheimer’s Disease
The incidence of Alzheimer’s disease (AD), the most representative neurodegenerative brain disease, is steadily increasing as the aging population increases. However, only drugs that can alleviate and delay symptoms are currently being used, and no specific treatment methods or therapeutic agents [] have been developed yet. Over the past decades, several clinical trials have been conducted with various targets, focusing on these two clinical indications: amyloid beta plaques and neurofibrillary tau tangles []. However, all clinical trials have failed; only Aducanumab, which targets amyloid-β (Aβ) plaque removal, has shown a therapeutic effect, but it is controversial due to side effects [,]. Although the amyloid hypothesis remains controversial, since the accumulation of Aβ is a representative pathological hallmark of AD, numerous therapeutic studies targeting Aβ have been conducted.
The first preclinical study on FUS for AD aimed to deliver anti-Aβ antibodies targeting amyloid plaques into the brain by a BBB opening. Consequently, anti-Aβ antibodies bound to the Aβ plaques and rapidly reduced the plaque pathology []. Subsequently, research on delivering therapeutic agents through FUS-mediated BBB opening in patients with AD has gained attention [,,,,,,]. Interestingly, studies have reported that amyloid pathology [,,,,] and phosphorylated tau [,] are reduced only by FUS-induced BBB opening without specific drug delivery. Treatment delivery via FUS-mediated BBB opening also affected memory recovery in AD animal models [,,,,]. Research studies on various biological changes by FUS-mediated BBB opening are ongoing. However, for FUS to be a promising non-pharmacological treatment delivery method for AD, further research is needed on why amyloid is reduced and cognitive function is restored. FUS induces the activation of microglia and astrocytes, which may increase phagocytosis of the amyloid plaques [,,]. Recently, a study confirming the therapeutic effect in an AD mouse model (5×FAD) by combining FUS and Aducanumab was reported []. Aducanumab, a monoclonal antibody targeting fibril forms and beta-amyloid oligomer, has been proven effective since receiving FDA approval in 2021. However, due to side effects, debate continues as to whether or not it should be used.
In conclusion, combined treatment with FUS and Aducanumab reduced amyloid plaque levels, increased hippocampal neurogenesis, and restored cognitive function. Here, FUS activated phagocytic microglia and increased the number of astrocytes associated with amyloid plaques. This suggests that FUS can induce a reduction in amyloid plaques through phagocytosis. In addition, an RNA sequencing analysis showed that the combined treatment with FUS and Aducanumab upregulated neuroinflammation signaling, phagosome formation, reelin signaling, and CREB signaling []. The immunomodulatory effect of FUS, such as the activation of various innate immune cells, plays a vital role in reducing amyloid plaques []. Regarding the recovery of cognitive function by FUS, the increase in hippocampal neurogenesis [,,,] or synaptic plasticity [,] may play a role here, but further research is needed on this topic. We summarized the most relevant preclinical studies on FUS-mediated BBB opening in AD (Table 1).

Table 1.
Recent preclinical studies on focused ultrasound-mediated blood–brain barrier opening in Alzheimer’s disease.
2.2. Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative brain disease accompanied by motor dysfunction due to the loss of dopaminergic neurons. PD is neuropathologically characterized by proteinaceous inclusions called Lewy bodies []. Notably, as many studies have reported that α-synuclein plays a direct role in disease development, PD is classified as α-synucleinopathies []. Currently, there are no clear treatments to slow or alleviate the progression of neurodegenerative diseases such as PD. Treatment with glial-derived neurotrophic factor (GDNF) is considered appropriate for PD due to its neuroprotective and neurotrophic effects [,,]. The overexpression of neuroprotective genes that induce dopamine regeneration in activated neurons can delay disease progression []. The potential benefit of GDNF with regard to recovery and the functional improvement of dopaminergic neurons has been confirmed [,,]; however, one study was discontinued due to safety concerns in clinical trials []. Animal studies of GDNF gene delivery by FUS began in PD and have highlighted the possibility of effective gene therapy [,,].
Since neurturin has been found to have neuroprotective and neuro-regenerative effects on dopaminergic neurons [], the FUS-based delivery of neurturin has been studied to find an alternative to GDNF [,]. Recently, recombinant adeno-associated viral (rAAV) vectors have received much attention as a tool for gene delivery to the brain. The technology of delivering rAAV using FUS-mediated BBB permeability and expressing the delivered gene has already been examined []. Accordingly, recent studies on PD models using FUS mainly involve gene delivery using rAAV. While there are many studies on delaying disease symptoms by delivering various therapeutic agents using FUS, there is a lack of preclinical research studies on α-synuclein-based PD models. We summarized the most relevant preclinical studies on FUS-mediated BBB opening in PD (Table 2).

Table 2.
Recent preclinical studies on focused ultrasound-mediated blood–brain barrier opening in Parkinson’s disease.
2.3. Brain Tumor
Glioblastoma is the most aggressive brain tumor with a high recurrence rate and poor prognosis despite treatments such as resection, radiotherapy, and chemotherapy []. The blood–tumor barrier (BTB) is created by the often heterogeneous disruption of the BBB within the tumor due to aberrant angiogenic signaling. As the delivery of anticancer drugs is limited despite the irregular leakiness of the BTB, quantitative drug delivery through FUS-mediated BBB opening is required []. Many previous studies on drug delivery by FUS have involved patients with brain tumors. Doxorubicin is a chemotherapeutic agent that inhibits cell growth and induces apoptosis in malignant glioma cells; however, it is not commonly used because it cannot cross the BBB. In 2007, Treat et al. delivered doxorubicin to a tumor in the brain via FUS-mediated BBB opening, indicating that this drug could be a viable treatment option []. Until now, various therapeutic agents have been used to treat glioblastomas, and FUS-mediated BBB opening technology is being developed. In the early days of FUS research, unencapsulated drugs such as the common anticancer drug temozolomide (TMZ) [,], carmustine (BCNU) [], and immunostimulatory interleukin-12 (IL-12) [,] were mainly used.
Brain metastasis represents an important predictor of mortality for various non-brain cancers such as breast cancer. Like primary brain tumors, brain metastases do not have an intact BBB, but most therapeutics still have lower intra-tumoral bioavailability than non-brain tumors []. FUS studies have continued to treat metastatic brain tumors as well as primary brain tumors. In 2012, there was a study confirming the therapeutic effect by delivering Trastuzumab based on FUS-BBB opening in a breast cancer brain metastases model []. Additional research reported in 2016 demonstrated that the administration of trastuzumab and pertuzumab in a brain metastasis mouse model of breast cancer inhibited the growth of brain metastasis when used with FUS, compared to chemotherapy alone [].
Whether it is a primary brain tumor or a metastatic brain tumor, the critical factor in the tumor microenvironment is to what extent the anticancer drugs could be delivered into the target region. It has been reported that the delivery of chemotherapeutic agents with small molecular weights to the brain tumor microenvironment is approximately 3.9-fold higher under FUS-mediated BBB opening conditions []. This enhanced delivery rate has been shown to increase median survival by approximately 30% compared to chemotherapy alone.
However, efflux transporters such as Pgp are overexpressed in cancer cells and prevent the uptake of anticancer drugs into the cells, resulting in resistance to them. FUS-mediated BBB opening temporarily inhibits Pgp expression, thereby preventing drug efflux and interfering with functional components of the BBB []. Additional research is needed on efflux transporter inhibitors targeting cancer cells. In addition to unencapsulated drugs, studies have reported that tumors (metastatic breast cancer) can be effectively controlled by delivering natural killer cells under BBB opening []. Furthermore, studies on suppressing brain tumors by delivering patient-specific antibodies or complexes loaded on short-hairpin RNA-liposomes have also been previously reported []. Since then, several studies have been conducted to enhance the safety and efficiency of tumor treatment by delivering encapsulated therapeutics through the conjugation of existing drugs or genes with improved MB, virus, and nanoparticles [,,,]. As immunotherapy is a critical issue in neuro-oncology, additional research on immunotherapy using FUS-mediated BBB opening is expected to become more active in the future. We summarized the most relevant preclinical studies on FUS-mediated BBB opening in brain tumors (Table 3).

Table 3.
Recent preclinical studies on focused ultrasound-mediated blood–brain barrier opening in brain tumors.
3. Secondary Biological Effects
3.1. Neurogenesis
In 2014, Scarcelli et al. first reported that FUS-meditated BBB opening significantly increased the number of proliferating cells and newborn neurons in the dentate gyrus of the hippocampus []. Since then, FUS has been considered a therapeutic strategy to improve learning and memory in patients with neurological disorders such as AD, thus going beyond a tool for drug delivery. Neurogenesis is induced under conditions involving BBB opening within appropriate parameters, but not FUS stimulation without MB []. The fact that FUS-mediated BBB opening induces neurogenesis has been proven in many studies [,,]. In our previous study, adult hippocampal neurogenesis was induced after 18 days of FUS treatment, and BDNF and early growth response protein-1 were upregulated [].
In addition, studies have recently reported that the regulation of ERK signaling cascades is involved in neurogenesis after BBB opening. However, it is necessary to understand the specific mechanism underlying FUS-induced neurogenesis. FUS can be a non-pharmacological therapeutic strategy for treating neurodegenerative brain diseases in older patients or patients with AD who have decreased hippocampal neurogenesis [,].
3.2. Glymphatic System
The glymphatic system is a unique fluid transport system of perivascular channels formed by astroglial cells to facilitate the efficient clearance of soluble proteins and metabolites from the CNS []. Damage to the glymphatic system is closely related to several neurological diseases, such as AD, PD, stroke, and traumatic brain injury [,,,,]. Conversely, since improvements in an impaired glymphatic system can alleviate these diseases, attempts have been made to find ways to improve the glymphatic system [,,,].
FUS-mediated BBB opening without any drug delivery in the AD model reduced the amyloid pathology, improved cognitive function, and increased the phagocytosis of glial cells [,,]. Since the glymphatic system can promote the removal of pathological proteins such as amyloid plaques, it is necessary to study the amyloid plaque reduction effect of FUS-mediated BBB opening. According to related research results, FUS-meditated BBB opening increases brain-to-CSF Aβ drainage and induces glymphatic–lymphatic reduction in Aβ []. Previously, Meng et al. investigated the accumulation of MRI contrast agents in the draining vein and subarachnoid space after FUS-meditated BBB opening in the human brain []. Ye et al. also reported that FUS-mediated BBB opening could enhance glymphatic transportation in the brain [].
It is not confirmed whether the glymphatic system studied in rodents also exists in humans []. This is because there are no human studies that have characterized the flow of this system. In sum, since the activation of the glymphatic system through FUS can induce the clearance of various harmful proteins, such as Aβ or α-synuclein, specific additional studies on the glymphatic system are needed. In addition, the cerebral blood flow and lymphatic systems are structurally and functionally different between humans and rodents. Therefore, research involving the visualization of the blood flow or glymphatic–lymphatic system needs to be conducted in mammals, starting with primates.
3.3. Inflammatory Response
One of the secondary effects beginning within hours after FUS-mediated BBB opening is inflammatory responses. Inflammatory responses are biological responses to harmful stimuli and act as a defense mechanism involving immune cells, blood vessels, and inflammatory mediators. Microglia are one of the basic innate immune cells in the brain. Studies have reported that microglia activation occurs 1, 6, and 24 h after FUS-mediated BBB opening [,]. Further, as mentioned in Section 2.1 Alzheimer’s Disease, a study reported that FUS and aducanumab combined treatment in an AD mouse model reduced amyloid plaques. At this time, increased microglia and astrocytes were suggested to reduce plaques through the phagocytosis effect []. Similarly, studies were also reported confirming that the immunoreactivity of resident Iba1+ and phagocytic CD68+ microglial cells and a transient increase in the infiltration of Ly6G+ immune cells increased 4 and 72 h after FUS-BBB opening [].
Recently, research on various changes after FUS-mediated BBB opening has been explored using sequencing techniques such as transcriptomics and proteomics [,,]. McMahon et al. showed that many pro-inflammatory genes were upregulated, and BBB transporter genes were down-regulated 6 h after BBB opening, which returned to baseline within 24 h. However, angiogenesis-related genes were upregulated at 6 and 24 h []. McMahon et al. also emphasized the importance of the optimization of FUS parameters because FUS induces BBB opening regardless of the upregulation of the NFκB signaling pathway, although a damaging inflammatory response was detected at high MB doses []. Recently, Ji et al. investigated changes in the relative gene expression of mouse inflammatory cytokines and receptors over time (6 h, 24 h, and 72 h) []. Significant changes were observed in all cavitation groups at 6 and 24 h and returned to baseline at 72 h. According to the results, inflammatory responses caused by FUS depend on the cavitation dose of MB, so careful monitoring for MB cavitation will be critically required []. It is still unclear how FUS-mediated BBB opening affects the induction of neuroinflammation. Choi et al. recently reported changes in the inflammatory response according to FUS parameters (0.25 MPa and 0.42 MPa) []. Although micro-bleeding and tissue damage were observed, the BBB disruption effect was three times higher in the 0.42 MPa-treated group. As a result of transcriptome analysis, the expression level of NF-kB pathway-related genes was regulated in a time-dependent manner only in the 0.42 MPa treatment group. In addition, the induction of neuroinflammation through glial cell activation was confirmed in the 0.42 MPa group, but neuroprotective effects were specified by the expression of A2-type astrocytes. Therefore, the non-excessive 0.25 MPa parameter can control the BBB without a sterile inflammatory response. In addition, when excessive FUS parameters are used, a sterile inflammatory response can be induced through the activation of glial cells, suggesting that A2-type astrocytes affect the homeostasis of the brain microenvironment.
Since immune responses by FUS-mediated BBB opening depend on various factors, including differences in parameters and cavitation dose, inflammatory responses occur at different time points that can be prolonged or quickly vanish. As clinical studies on FUS-mediated BBB opening are actively expanding, we need to better understand varying inflammatory responses affected by FUS.
4. Conclusions and Future Directions
This review briefly summarizes how FUS-mediated BBB opening is currently being studied in AD, brain tumors, and PD. Moreover, the neurogenesis or immune response induced when the BBB is opened and the glymphatic system associated with recent clearance were briefly introduced. Although not mentioned in this review, FUS studies are underway in various diseases, such as amyotrophic lateral sclerosis, traumatic brain injury, and stroke. They will be expanded to more diseases in the future.
Currently, FUS is considered an innovative treatment method that effectively treats various neurological disorders that have been challenging to overcome for a long time. The effects and safety of FUS-mediated BBB opening on the brain have already been studied extensively. However, despite many technological advances in FUS over the past 20 years, research on the clinical applications of FUS-mediated BBB opening is only just beginning. Recently, clinical trials of FUS-mediated BBB opening have confirmed its safety in patients with AD [,], PD [,], and brain tumors [,]. However, the exact mechanism of drug delivery by FUS-mediated BBB opening has not yet been elucidated. In addition, there is a need to identify the mechanisms underlying the various biological effects of BBB opening. Furthermore, since various effects of FUS stimulation without MB have been reported, comparative studies of BBB opening with MB are needed. Clinical optimization studies are needed to standardize FUS-mediated BBB opening as a new treatment modality, and preclinical research studies are needed to confirm the clinical effect of this modality.
Among the latest medical technologies being developed for the treatment of many neurological disorders, one of the major directions is a noninvasive or minimally invasive treatment. The main advantage of FUS is that it is a non-invasive technology; therefore, it is relatively safe and can be used repeatedly. FUS may be established as a representative treatment technique for neurological disorders in the near future.
Author Contributions
C.K. and W.S.C. conceptualized the topic of the review. C.K. wrote the manuscript and designed the figures. W.S.C. critically proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF), (NRF-2022R1A6A3A01087249). In addition, this work was supported by the Young Medical Scientist Research Grant through the Daewoong Foundation (DFY2212P).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data listed in this review article are publicly accessible on PubMed.
Acknowledgments
We would like to thank Minkyung Park for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Allen, B.D.; Limoli, C.L. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic. Biol. Med. 2022, 178, 189–201. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing bbb heterogeneity: A new paradigm for drug delivery to brain tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’brien, J.; Boccia, J.; Casals, D.; Bertino, J.; Melamed, M. Expression of the multidrug resistance gene product (p-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 1990, 38, 1277–1287. [Google Scholar] [CrossRef]
- Schinkel, A.H. P-glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Shawkat, H.; Westwood, M.-M.; Mortimer, A. Mannitol: A review of its clinical uses. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 82–85. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive mr imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Kong, C. Combined Therapy of Focused Ultrasound and Aducanumab Induces Neurogenesis and Decreases of Beta-Amyloid Plaques in a Mouse Model of Alzheimer’s Disease; Graduate School, Yonsei University: Seoul, Republic of Korea, 2022. [Google Scholar]
- Vyas, N.; Manmi, K.; Wang, Q.; Jadhav, A.J.; Barigou, M.; Sammons, R.L.; Kuehne, S.A.; Walmsley, A.D. Which parameters affect biofilm removal with acoustic cavitation? A review. Ultrasound Med. Biol. 2019, 45, 1044–1055. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Jolesz, F.A.; McDannold, N.J. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release 2012, 162, 134–142. [Google Scholar] [CrossRef]
- Geerts, H.; Grossberg, G.T. Pharmacology of acetylcholinesterase inhibitors and n-methyl-d-aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J. Clin. Pharmacol. 2006, 46, 8S–16S. [Google Scholar] [CrossRef]
- Asher, S.; Priefer, R. Alzheimer’s disease failed clinical trials. Life Sci. 2022, 306, 120861. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y. The antibody aducanumab reduces aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Walsh, S.; Merrick, R.; Milne, R.; Brayne, C. Aducanumab for Alzheimer’s disease? BMJ 2021, 374, n1682. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J.F.; Ayala-Grosso, C.A.; Markham, K.; Huang, Y.; Chopra, R.; McLaurin, J.; Hynynen, K.; Aubert, I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the tgcrnd8 mouse model of Alzheimer’s disease. PLoS ONE 2010, 5, e10549. [Google Scholar] [CrossRef] [PubMed]
- Alecou, T.; Giannakou, M.; Damianou, C. Amyloid β plaque reduction with antibodies crossing the blood-brain barrier, which was opened in 3 sessions of focused ultrasound in a rabbit model. J. Ultrasound Med. 2017, 36, 2257–2270. [Google Scholar] [CrossRef] [PubMed]
- Xhima, K.; Markham-Coultes, K.; Nedev, H.; Heinen, S.; Saragovi, H.; Hynynen, K.; Aubert, I. Focused ultrasound delivery of a selective trka agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci. Adv. 2020, 6, eaax6646. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Lin, Y.-T.; Chung, Y.-H.; Lin, K.-J.; Yang, L.-Y.; Yen, T.-C.; Liu, H.-L. Focused ultrasound-induced blood-brain barrier opening enhances gsk-3 inhibitor delivery for amyloid-beta plaque reduction. Sci. Rep. 2018, 8, 12882. [Google Scholar] [CrossRef]
- Xhima, K.; Markham-Coultes, K.; Hahn Kofoed, R.; Saragovi, H.U.; Hynynen, K.; Aubert, I. Ultrasound delivery of a trka agonist confers neuroprotection to Alzheimer-associated pathologies. Brain 2022, 145, 2806–2822. [Google Scholar] [CrossRef]
- Dubey, S.; Heinen, S.; Krantic, S.; McLaurin, J.; Branch, D.R.; Hynynen, K.; Aubert, I. Clinically approved ivig delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32691–32700. [Google Scholar] [CrossRef]
- Mi, X.; Du, H.; Guo, X.; Wu, Y.; Shen, L.; Luo, Y.; Wang, D.; Su, Q.; Xiang, R.; Yue, S.; et al. Asparagine endopeptidase-targeted ultrasound-responsive nanobubbles alleviate tau cleavage and amyloid-β deposition in an Alzheimer’s disease model. Acta Biomater. 2022, 141, 388–397. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, X.; Chen, B.; Liao, Y.; Guan, X.; He, Y.; Cui, H.; Rong, Y.; Liu, Z.; Xu, Y. Ultrasound-targeted microbubbles destruction assists dual delivery of beta-amyloid antibody and neural stem cells to restore neural function in transgenic mice of Alzheimer’s disease. Med. Phys. 2022, 49, 1357–1367. [Google Scholar] [CrossRef]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra233. [Google Scholar] [CrossRef]
- Leinenga, G.; Koh, W.K.; Götz, J. Scanning ultrasound in the absence of blood-brain barrier opening is not sufficient to clear β-amyloid plaques in the app23 mouse model of Alzheimer’s disease. Brain Res. Bull. 2019, 153, 8–14. [Google Scholar] [CrossRef]
- Poon, C.T.; Shah, K.; Lin, C.; Tse, R.; Kim, K.K.; Mooney, S.; Aubert, I.; Stefanovic, B.; Hynynen, K. Time course of focused ultrasound effects on β-amyloid plaque pathology in the tgcrnd8 mouse model of Alzheimer’s disease. Sci. Rep. 2018, 8, 14061. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Kugelman, T.; Ji, R.; Murillo, M.; Wang, S.; Niimi, Y.; Small, S.A.; Duff, K.E.; Konofagou, E.E. Unilateral focused ultrasound-induced blood-brain barrier opening reduces phosphorylated tau from the rtg4510 mouse model. Theranostics 2019, 9, 5396. [Google Scholar] [CrossRef]
- Pandit, R.; Leinenga, G.; Götz, J. Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions. Theranostics 2019, 9, 3754–3767. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer disease in a mouse model: Mr imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014, 273, 736. [Google Scholar] [CrossRef]
- Leinenga, G.; Götz, J. Safety and efficacy of scanning ultrasound treatment of aged app23 mice. Front. Neurosci. 2018, 12, 55. [Google Scholar] [CrossRef]
- Shin, J.; Kong, C.; Cho, J.S.; Lee, J.; Koh, C.S.; Yoon, M.-S.; Na, Y.C.; Chang, W.S.; Chang, J.W. Focused ultrasound–mediated noninvasive blood-brain barrier modulation: Preclinical examination of efficacy and safety in various sonication parameters. Neurosurg. Focus 2018, 44, E15. [Google Scholar] [CrossRef]
- Shen, Y.; Hua, L.; Yeh, C.K.; Shen, L.; Ying, M.; Zhang, Z.; Liu, G.; Li, S.; Chen, S.; Chen, X.; et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer’s disease. Theranostics 2020, 10, 11794–11819. [Google Scholar] [CrossRef]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.-L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Kong, C.; Yang, E.-J.; Shin, J.; Park, J.; Kim, S.-H.; Park, S.-W.; Chang, W.S.; Lee, C.-H.; Kim, H.; Kim, H.-S. Enhanced delivery of a low dose of aducanumab via fus in 5× fad mice, an ad model. Transl. Neurodegener. 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, T.; Jordão, J.F.; O’reilly, M.A.; Ellens, N.; Hynynen, K.; Aubert, I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kong, C.; Lee, J.; Choi, B.Y.; Sim, J.; Koh, C.S.; Park, M.; Na, Y.C.; Suh, S.W.; Chang, W.S. Focused ultrasound-induced blood-brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimer Res. Ther. 2019, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, D.G.; Turpin, F.; Palliyaguru, T.; Evans, H.T.; Chicoteau, A.; Lee, W.; Pelekanos, M.; Nguyen, N.; Song, J.; Sullivan, R.K. Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including nmdar signaling. Mol. Psychiatry 2021, 26, 6975–6991. [Google Scholar] [CrossRef]
- Mooney, S.J.; Shah, K.; Yeung, S.; Burgess, A.; Aubert, I.; Hynynen, K. Focused ultrasound-induced neurogenesis requires an increase in blood-brain barrier permeability. PLoS ONE 2016, 11, e0159892. [Google Scholar] [CrossRef]
- Niu, X.; Yu, K.; He, B. Transcranial focused ultrasound induces sustained synaptic plasticity in rat hippocampus. Brain Stimul. 2022, 15, 352–359. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, J.; Xiao, Y.; Li, F.; Niu, L.; Liu, X.; Meng, L.; Zheng, H. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics 2021, 11, 4351. [Google Scholar] [CrossRef]
- Wang, F.; Wei, X.-X.; Chang, L.-S.; Dong, L.; Wang, Y.-L.; Li, N.-N. Ultrasound combined with microbubbles loading bdnf retrovirus to open bloodbrain barrier for treatment of Alzheimer’s disease. Front. Pharmacol. 2021, 12, 615104. [Google Scholar] [CrossRef]
- Leinenga, G.; Koh, W.K.; Götz, J. A comparative study of the effects of aducanumab and scanning ultrasound on amyloid plaques and behavior in the app23 mouse model of Alzheimer disease. Alzheimer’s Res. Ther. 2021, 13, 76. [Google Scholar] [CrossRef]
- Poon, C.; Pellow, C.; Hynynen, K. Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments. Theranostics 2021, 11, 1655. [Google Scholar] [CrossRef]
- Sun, T.; Shi, Q.; Zhang, Y.; Power, C.; Hoesch, C.; Antonelli, S.; Schroeder, M.K.; Caldarone, B.J.; Taudte, N.; Schenk, M. Focused ultrasound with anti-pglu3 aβ enhances efficacy in Alzheimer’s disease-like mice via recruitment of peripheral immune cells. J. Control. Release 2021, 336, 443–456. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Y.; Chen, W.-S.; Feng, X.; Liao, Y.; Chen, S.; Liu, Y.; Liao, C.; Chen, M.; Ao, L. Treatment combining focused ultrasound with gastrodin alleviates memory deficit and neuropathology in an Alzheimer’s disease-like experimental mouse model. Neural Plast. 2022, 2022, 5241449. [Google Scholar] [CrossRef]
- Bathini, P.; Sun, T.; Schenk, M.; Schilling, S.; McDannold, N.J.; Lemere, C.A. Acute effects of focused ultrasound-induced blood-brain barrier opening on anti-pyroglu3 abeta antibody delivery and immune responses. Biomolecules 2022, 12, 951. [Google Scholar] [CrossRef]
- Bajracharya, R.; Cruz, E.; Götz, J.; Nisbet, R.M. Ultrasound-mediated delivery of novel tau-specific monoclonal antibody enhances brain uptake but not therapeutic efficacy. J. Control. Release 2022, 349, 634–648. [Google Scholar] [CrossRef]
- Rodrigues e Silva, A.M.; Geldsetzer, F.; Holdorff, B.; Kielhorn, F.W.; Balzer-Geldsetzer, M.; Oertel, W.H.; Hurtig, H.; Dodel, R. Who was the man who discovered the “lewy bodies”? Mov. Disord. 2010, 25, 1765–1773. [Google Scholar] [CrossRef]
- Appel-Cresswell, S.; Vilarino-Guell, C.; Encarnacion, M.; Sherman, H.; Yu, I.; Shah, B.; Weir, D.; Thompson, C.; Szu-Tu, C.; Trinh, J. Alpha-synuclein p. H50q, a novel pathogenic mutation for Parkinson’s disease. Mov. Disord. 2013, 28, 811–813. [Google Scholar] [CrossRef]
- Choi-Lundberg, D.L.; Lin, Q.; Chang, Y.-N.; Chiang, Y.L.; Hay, C.M.; Mohajeri, H.; Davidson, B.L.; Bohn, M.C. Dopaminergic neurons protected from degeneration by gdnf gene therapy. Science 1997, 275, 838–841. [Google Scholar] [CrossRef]
- Kearns, C.M.; Gash, D.M. Gdnf protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995, 672, 104–111. [Google Scholar] [CrossRef]
- Burke, R.E. GDNF as a candidate striatal target-derived neurotrophic factor for the development of substantia nigra dopamine neurons. J. Neural. Transm. Suppl. 2006, 70, 41–45. [Google Scholar]
- Kordower, J.H.; Emborg, M.E.; Bloch, J.; Ma, S.Y.; Chu, Y.; Leventhal, L.; McBride, J.; Chen, E.-Y.; Palfi, S.; Roitberg, B.Z. Neurodegeneration prevented by lentiviral vector delivery of gdnf in primate models of Parkinson’s disease. Science 2000, 290, 767–773. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F. Functional recovery in Parkinsonian monkeys treated with gdnf. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Grondin, R.; Zhang, Z.; Yi, A.; Cass, W.A.; Maswood, N.; Andersen, A.H.; Elsberry, D.D.; Klein, M.C.; Gerhardt, G.A.; Gash, D.M. Chronic, controlled gdnf infusion promotes structural and functional recovery in advanced Parkinsonian monkeys. Brain 2002, 125, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsieh, H.Y.; Chen, C.M.; Wu, S.R.; Tsai, C.H.; Huang, C.Y.; Hua, M.Y.; Wei, K.C.; Yeh, C.K.; Liu, H.L. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J. Control. Release 2016, 235, 72–81. [Google Scholar] [CrossRef]
- Fan, C.-H.; Ting, C.-Y.; Lin, C.Y.; Chan, H.-L.; Chang, Y.-C.; Chen, Y.-Y.; Liu, H.-L.; Yeh, C.-K. Noninvasive, targeted and non-viral ultrasound-mediated gdnf-plasmid delivery for treatment of Parkinson’s disease. Sci. Rep. 2016, 6, 19579. [Google Scholar] [CrossRef]
- Yue, P.; Miao, W.; Gao, L.; Zhao, X.; Teng, J. Ultrasound-triggered effects of the microbubbles coupled to gdnf plasmid-loaded pegylated liposomes in a rat model of Parkinson’s disease. Front. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef]
- Grondin, R.; Zhang, Z.; Ai, Y.; Ding, F.; Walton, A.; Surgener, S.; Gerhardt, G.; Gash, D. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of mptp-lesioned rhesus monkeys. Cell Transplant. 2008, 17, 373–381. [Google Scholar] [CrossRef]
- Samiotaki, G.; Acosta, C.; Wang, S.; Konofagou, E.E. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound—Mediated blood—Brain barrier opening in vivo. J. Cereb. Blood Flow Metab. 2015, 35, 611–622. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Wang, S.; Samiotaki, G.; Kugelman, T.; Olumolade, O.O.; Acosta, C.; Sun, T.; Han, Y.; Kamimura, H.A.; Jackson-Lewis, V. Amelioration of the nigrostriatal pathway facilitated by ultrasound-mediated neurotrophic delivery in early Parkinson’s disease. J. Control. Release 2019, 303, 289–301. [Google Scholar] [CrossRef]
- Noroozian, Z.; Xhima, K.; Huang, Y.; Kaspar, B.K.; Kügler, S.; Hynynen, K.; Aubert, I. Mri-guided focused ultrasound for targeted delivery of raav to the brain. In Adeno-Associated Virus Vectors: Design and Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 177–197. [Google Scholar]
- Ji, R.; Smith, M.; Niimi, Y.; Karakatsani, M.E.; Murillo, M.F.; Jackson-Lewis, V.; Przedborski, S.; Konofagou, E.E. Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model. Sci. Rep. 2019, 9, 19402. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, Y.-C.; Huang, C.-Y.; Wu, S.-R.; Chen, C.-M.; Liu, H.-L. Ultrasound-responsive neurotrophic factor-loaded microbubble-liposome complex: Preclinical investigation for Parkinson’s disease treatment. J. Control. Release 2020, 321, 519–528. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain delivery of curcumin through low-intensity ultrasound-induced blood–brain barrier opening via lipid-plga nanobubbles. Int. J. Nanomed. 2021, 16, 7433. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, K.; Li, J.; Liao, Y.; Liao, C.; Chen, W.-S.; Chen, M.; Ao, L. Focused ultrasound promotes the delivery of gastrodin and enhances the protective effect on dopaminergic neurons in a mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2022, 16, 884788. [Google Scholar] [CrossRef]
- Trinh, D.; Nash, J.; Goertz, D.; Hynynen, K.; Bulner, S.; Iqbal, U.; Keenan, J. Microbubble drug conjugate and focused ultrasound blood brain barrier delivery of aav-2 sirt-3. Drug Deliv. 2022, 29, 1176–1183. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused ultrasound strategies for brain tumor therapy. Oper. Neurosurg. 2020, 19, 9–18. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using mri-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef]
- Wei, K.-C.; Chu, P.-C.; Wang, H.-Y.J.; Huang, C.-Y.; Chen, P.-Y.; Tsai, H.-C.; Lu, Y.-J.; Lee, P.-Y.; Tseng, I.-C.; Feng, L.-Y. Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: A preclinical study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef]
- Liu, H.-L.; Huang, C.-Y.; Chen, J.-Y.; Wang, H.-Y.J.; Chen, P.-Y.; Wei, K.-C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Chen, P.-Y.; Chu, P.-C.; Pan, C.-H.; Yang, H.-W.; Huang, C.-Y.; Wang, J.-J.; Yen, T.-C.; Wei, K.-C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Hsieh, H.-Y.; Huang, C.-Y.; Lin, C.-Y.; Wei, K.-C.; Liu, H.-L. Focused ultrasound-induced blood–brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: A preclinical feasibility study. J. Transl. Med. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Wei, K.-C.; Liu, H.-L. Neural immune modulation and immunotherapy assisted by focused ultrasound induced blood-brain barrier opening. Hum. Vaccines Immunother. 2015, 11, 2682–2687. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not her2/neu-positive breast cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Zhang, Y.Z.; Vykhodtseva, N.; McDannold, N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release 2012, 163, 277–284. [Google Scholar] [CrossRef]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef]
- Schoen, S.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on p-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Kerbel, R.; Wels, W.S.; Hynynen, K. Early treatment of her2-amplified brain tumors with targeted nk-92 cells and focused ultrasound improves survival. Neuro-Oncology 2016, 18, 974–981. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, Q.; Wang, F.; Zhang, X.; Hu, J.; Tan, Y.; Huang, N.; Wang, Z.; Wang, Z.; Cheng, Y. Targeted shrna-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth. Cancer Lett. 2018, 418, 147–158. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.; Power, C.; Alexander, P.M.; Sutton, J.T.; Aryal, M.; Vykhodtseva, N.; Miller, E.L.; McDannold, N.J. Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model. Proc. Natl. Acad. Sci. USA 2017, 114, E10281–E10290. [Google Scholar] [CrossRef]
- Curley, C.T.; Mead, B.P.; Negron, K.; Kim, N.; Garrison, W.J.; Miller, G.W.; Kingsmore, K.M.; Thim, E.A.; Song, J.; Munson, J.M. Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection. Sci. Adv. 2020, 6, eaay1344. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C. Ultrasound-mediated delivery of paclitaxel for glioma: A comparative study of distribution, toxicity, and efficacy of albumin-bound versus cremophor formulationsus-delivered abx extends survival in gbm pdx mouse model. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene therapy for drug-resistant glioblastoma via lipid-polymer hybrid nanoparticles combined with focused ultrasound. Int. J. Nanomed. 2021, 16, 185. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Blood-brain barrier disruption and delivery of irinotecan in a rat model using a clinical transcranial mri-guided focused ultrasound system. Sci. Rep. 2020, 10, 8766. [Google Scholar] [CrossRef]
- Englander, Z.K.; Wei, H.-J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.-I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M. Focused ultrasound mediated blood–brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 6521. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Breza, V.R.; Paul, S.; McCauley, K.S.; Berr, S.S.; Miller, G.W.; Neumann, K.D.; Price, R.J. Immunopet-informed sequence for focused ultrasound-targeted mcd47 blockade controls glioma. J. Control. Release 2021, 331, 19–29. [Google Scholar] [CrossRef]
- Ye, D.; Yuan, J.; Yue, Y.; Rubin, J.B.; Chen, H. Focused ultrasound-enhanced delivery of intranasally administered anti-programmed cell death-ligand 1 antibody to an intracranial murine glioma model. Pharmaceutics 2021, 13, 190. [Google Scholar] [CrossRef]
- Chen, K.-T.; Chai, W.-Y.; Lin, Y.-J.; Lin, C.-J.; Chen, P.-Y.; Tsai, H.-C.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef]
- Moon, H.; Hwang, K.; Nam, K.M.; Kim, Y.-S.; Ko, M.J.; Kim, H.R.; Lee, H.J.; Kim, M.J.; Kim, T.H.; Kang, K.-S. Enhanced delivery to brain using sonosensitive liposome and microbubble with focused ultrasound. Biomater. Adv. 2022, 141, 213102. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Witter, A.R.; Garrison, W.J.; Miller, G.W.; Price, R.J.; Bullock, T.N. Profiling of the immune landscape in murine glioblastoma following blood brain/tumor barrier disruption with mr image-guided focused ultrasound. J. Neuro-Oncol. 2022, 156, 109–122. [Google Scholar] [CrossRef]
- Mooney, S.J.; Nobrega, J.N.; Levitt, A.J.; Hynynen, K. Antidepressant effects of focused ultrasound induced blood-brain-barrier opening. Behav Brain Res. 2018, 342, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 2019, 24, 974–982.e973. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599.e585. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Chen, H.-L.; Chen, P.-C.; Lu, C.-H.; Tsai, N.-W.; Yu, C.-C.; Chou, K.-H.; Lai, Y.-R.; Taoka, T.; Lin, W.-C. Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (dti-alps) in patients with Parkinson’s disease. Oxidative Med. Cell. Longev. 2021, 2021, 4034509. [Google Scholar] [CrossRef]
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R. Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke 2017, 48, 2301–2305. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Plog, B.A.; Mestre, H.; Olveda, G.E.; Sweeney, A.M.; Kenney, H.M.; Cove, A.; Dholakia, K.Y.; Tithof, J.; Nevins, T.D.; Lundgaard, I. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 2018, 3, e120922. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef]
- Liu, D.-x.; He, X.; Wu, D.; Zhang, Q.; Yang, C.; Liang, F.-y.; He, X.-f.; Dai, G.-y.; Pei, Z.; Lan, Y. Continuous theta burst stimulation facilitates the clearance efficiency of the glymphatic pathway in a mouse model of sleep deprivation. Neurosci. Lett. 2017, 653, 189–194. [Google Scholar] [CrossRef]
- von Holstein-Rathlou, S.; Petersen, N.C.; Nedergaard, M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett. 2018, 662, 253–258. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Park, E.-J.; Kwon, S.; Kim, H.; Lee, J.Y.; Lee, D.S. Improvement of glymphatic–lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci. Rep. 2020, 10, 16144. [Google Scholar] [CrossRef]
- Meng, Y.; Abrahao, A.; Heyn, C.C.; Bethune, A.J.; Huang, Y.; Pople, C.B.; Aubert, I.; Hamani, C.; Zinman, L.; Hynynen, K. Glymphatics visualization after focused ultrasound-induced blood–brain barrier opening in humans. Ann. Neurol. 2019, 86, 975–980. [Google Scholar] [CrossRef]
- Ye, D.; Chen, S.; Liu, Y.; Weixel, C.; Hu, Z.; Chen, H. Mechanically manipulate glymphatic transportation by ultrasound combined with microbubbles. bioRxiv 2022. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, ra111–ra147. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Burks, S.R.; Frank, J.A. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 2018, 8, 2245. [Google Scholar] [CrossRef]
- Sinharay, S.; Tu, T.-W.; Kovacs, Z.I.; Schreiber-Stainthorp, W.; Sundby, M.; Zhang, X.; Papadakis, G.Z.; Reid, W.C.; Frank, J.A.; Hammoud, D.A. In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound:[18f] dpa-714 pet study. J. Neuroinflammation 2019, 16, 155. [Google Scholar] [CrossRef]
- McMahon, D.; Hynynen, K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 2017, 7, 3989. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.S.; Gorick, C.M.; Price, R.J. Multiple regression analysis of a comprehensive transcriptomic data assembly elucidates mechanically-and biochemically-driven responses to focused ultrasound blood-brain barrier disruption. Theranostics 2021, 11, 9847. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Bendayan, R.; Hynynen, K. Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci. Rep. 2017, 7, 45657. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Karakatsani, M.E.; Burgess, M.; Smith, M.; Murillo, M.F.; Konofagou, E.E. Cavitation-modulated inflammatory response following focused ultrasound blood-brain barrier opening. J. Control. Release 2021, 337, 458–471. [Google Scholar] [CrossRef]
- Choi, H.J.; Han, M.; Seo, H.; Park, C.Y.; Lee, E.-H.; Park, J. The new insight into the inflammatory response following focused ultrasound-mediated blood–brain barrier disruption. Fluids Barriers CNS 2022, 19, 103. [Google Scholar] [CrossRef]
- D’Haese, P.F.; Ranjan, M.; Song, A.; Haut, M.W.; Carpenter, J.; Dieb, G.; Najib, U.; Wang, P.; Mehta, R.I.; Chazen, J.L.; et al. Β-amyloid plaque reduction in the hippocampus after focused ultrasound-induced blood-brain barrier opening in Alzheimer’s disease. Front. Hum. Neurosci. 2020, 14, 593672. [Google Scholar] [CrossRef]
- Park, S.H.; Baik, K.; Jeon, S.; Chang, W.S.; Ye, B.S.; Chang, J.W. Extensive frontal focused ultrasound mediated blood–brain barrier opening for the treatment of Alzheimer’s disease: A proof-of-concept study. Transl. Neurodegener. 2021, 10, 44. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Huang, Y.; Jones, R.M.; Ottoy, J.; Goubran, M.; Oliveira, L.M.; Davidson, B.; Lawrence, L.S.; Lau, A.Z. Putaminal recombinant glucocerebrosidase delivery with magnetic resonance–guided focused ultrasound in Parkinson’s disease: A phase i study. Mov. Disord. 2022, 37, 2134–2139. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, Y.; Pople, C.B.; Bethune, A.; Jones, R.M.; Abrahao, A.; Hamani, C.; Kalia, S.K.; Kalia, L.V.; Lipsman, N. Cavitation feedback control of focused ultrasound blood-brain barrier opening for drug delivery in patients with Parkinson’s disease. Pharmaceutics 2022, 14, 2607. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J. Neurosurg. 2020, 134, 475–483. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. One-year outcome of multiple blood–brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front. Oncol. 2020, 10, 1663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).