Nanoparticle-Based Modification of the DNA Methylome: A Therapeutic Tool for Atherosclerosis?
Abstract
:1. Introduction
2. Atherosclerosis: The Essentials
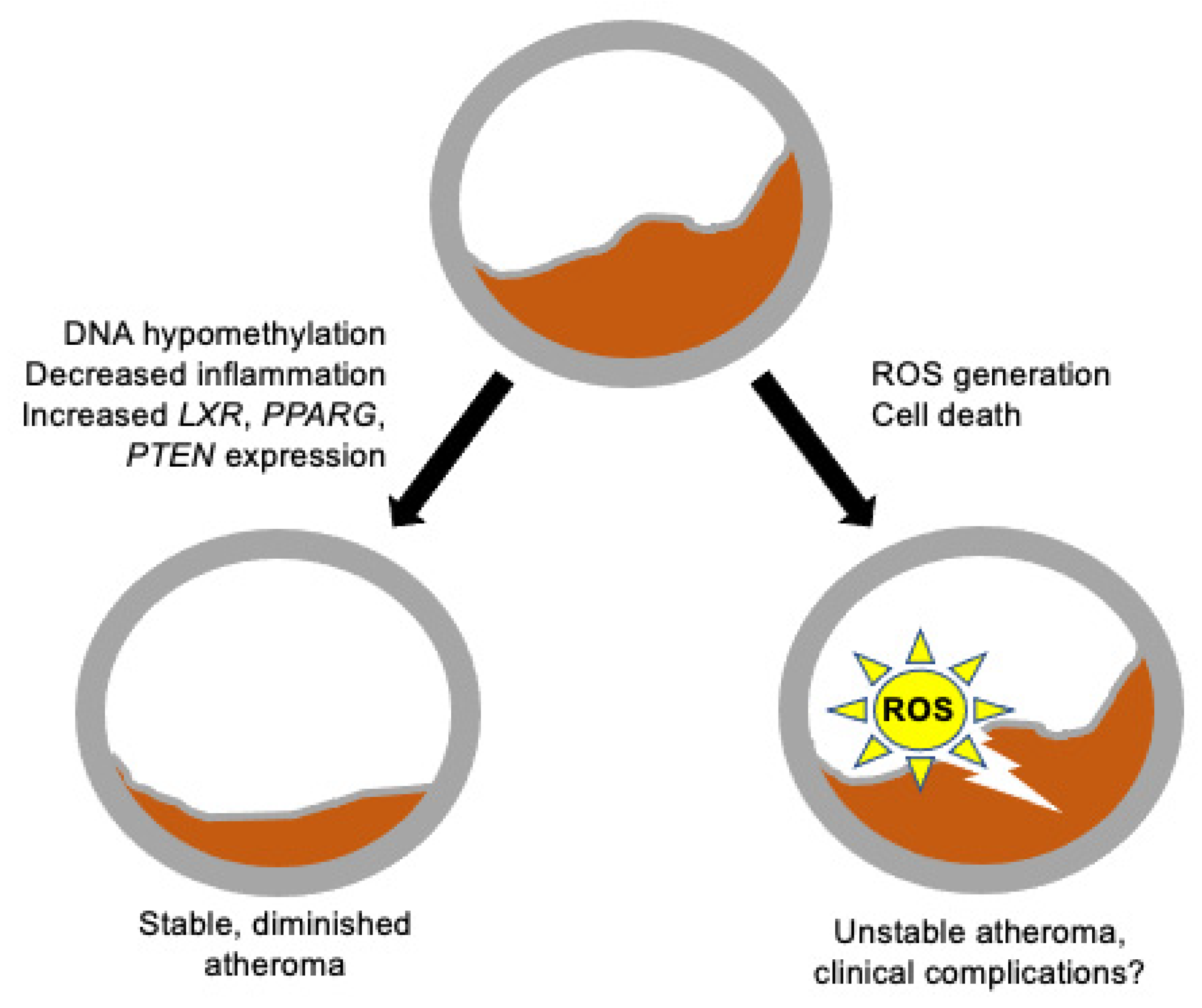
3. The DNA Methylome of AS
4. Possible NP-Based Strategies for the Modification of the AS DNA Methylome
4.1. Candidate NP Cargo Molecules
4.2. Candidate and Tested NP Delivery Systems in AS
5. NP-Based DNAm Targeting in Cancer-Associated Inflammation
6. The Other Edge of the Sword: Environmental NPs as Modifiers of the Epigenome
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Kanthi, Y.; de la Zerda, A.; Smith, B.R. Nanotherapeutic Shots through the Heart of Plaque. ACS Nano 2020, 14, 1236–1242. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Strahl, B.D. Interpreting the language of histone and DNA modifications. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 627–643. [Google Scholar] [CrossRef] [Green Version]
- Jeltsch, A.; Broche, J.; Bashtrykov, P. Molecular processes connecting dna methylation patterns with DNA methyltransferases and histone modifications in mammalian genomes. Genes 2018, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Irizarry, R. A Evolution in health and medicine Sackler colloquium: Stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 1757–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, J.; Georgomanolis, T.; Selich, A.; Kuo, C.-C.; Stöger, R.; Brant, L.; Mulabdić, M.S.; Fernandez-Rebollo, E.; Grezella, C.; Ostrowska, A.; et al. DNA methylation changes during long-term in vitro cell culture are caused by epigenetic drift. Commun. Biol. 2021, 4, 598. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K. Inflammation, infection and atherosclerosis. Trends Cardiovasc. Med. 2019, 29, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B particles and cardiovascular disease: A narrative review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Bornfeldt, K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Stavenow, L. Differences in bovine aortic smooth muscle cells cultured from spontaneous atherosclerotic lesions of different severity within the same vessel. Atherosclerosis 1984, 53, 337–342. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a reassessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef]
- Depuydt, M.A.; Prange, K.H.; Slenders, L.; Örd, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Gill, P.K.; Dron, J.S.; Hegele, R.A. Genetics of hypertriglyceridemia and atherosclerosis. Curr. Opin. Cardiol. 2021, 36, 264–271. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, J.; Heyn, H.; Moran, S.; Serra-Musach, J.; Pujana, M.A.; Bibikova, M.; Esteller, M. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics 2011, 6, 692–702. [Google Scholar] [CrossRef]
- Moran, S.; Arribas, C.; Esteller, M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics 2016, 8, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Flores, A.M.; Ye, J.; Jarr, K.U.; Hosseini-Nassab, N.; Smith, B.R.; Leeper, N.J. Nanoparticle therapy for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 635–646. [Google Scholar] [CrossRef]
- Libby, P.; Bornfeldt, K.E. How far we have come, how far we have yet to go in atherosclerosis research. Circ. Res. 2020, 126, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Zaina, S.; Heyn, H.; Carmona, F.J.; Varol, N.; Sayols, S.; Condom, E.; Ramirez-Ruz, J.; Gomez, A.; Goncalves, I.; Moran, S.; et al. DNA Methylation Map of Human Atherosclerosis. Circ. Cardiovasc. Genet. 2014, 7, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Lund, G.; Andersson, L.; Lauria, M.; Lindholm, M.; Fraga, M.F.; Villar-Garea, A.; Ballestar, E.; Esteller, M.; Zaina, S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J. Biol. Chem. 2004, 279, 29147–29154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Pilar Valencia-Morales, M.; Zaina, S.; Heyn, H.; Carmona, F.J.; Varol, N.; Sayols, S.; Condom, E.; Ramírez-Ruz, J.; Gomez, A.; Moran, S.; et al. The DNA methylation drift of the atherosclerotic aorta increases with lesion progression. BMC Med. Genomics 2015, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Yang, Q.; Li, A.-F.; Li, R.-Q.; Wang, Z.; Liu, L.-S.; Ren, Z.; Zheng, X.-L.; Tang, X.-Q.; Li, G.-H.; et al. Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE/mice. Oncotarget 2016, 7, 76423–76436. [Google Scholar] [CrossRef]
- Zaina, S.; Gonçalves, I.; Carmona, F.J.; Gomez, A.; Heyn, H.; Mollet, I.G.; Moran, S.; Varol, N.; Esteller, M. DNA methylation dynamics in human carotid plaques after cerebrovascular events. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1835–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Qiu, Y.; Yang, J.; Bian, S.; Chen, G.; Deng, M.; Kang, H.; Huang, L. DNMT1-PPARγ pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci. Rep. 2016, 6, 30053. [Google Scholar] [CrossRef]
- Dunn, J.; Qiu, H.; Kim, S.; Jjingo, D.; Hoffman, R.; Kim, C.W.; Jang, I.; Son, D.J.; Kim, D.; Pan, C.; et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J. Clin. Investig. 2014, 124, 3187–3199. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Wang, X.; Jia, L.; Mondal, A.K.; Diallo, A.; Hawkins, G.A.; Das, S.K.; Parks, J.S.; Yu, L.; Shi, H.; et al. Inhibiting DNA Methylation by 5-Aza-2′-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology 2014, 155, 4925–4938. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liao, S.; Hu, Q.; Wu, S.; Qiu, S.; Cheng, G.; Li, X.; Lu, W.; Menczel, J.; Bezrukova, A.G. Effects of liposomal simvastatin nanoparticles on vascular endothelial function and arterial smooth muscle cell apoptosis in rats with arteriosclerotic occlusive disease of lower limb via p38 mitogen-activated protein kinase nuclear factor kappa-b pathway. J. Nanosci. Nanotechnol. 2021, 21, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; Martins Cardoso, R.; Boyle, A.L.; Kros, A.; Jiskoot, W.; Kuiper, J.; Bouwstra, J.; Van Eck, M.; Slütter, B. complement receptor targeted liposomes encapsulating the liver x receptor agonist GW3965 accumulate in and stabilize atherosclerotic plaques. Adv. Healthc. Mater. 2020, 9, e2000043. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Even-Or, O.; Xu, X.; van Rosmalen, M.; Lim, L.; Gadde, S.; Farokhzad, O.C.; Fisher, E.A. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv. Healthc. Mater. 2015, 4, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Gao, M.; Han, Z.; Jiang, W.; Gu, Y.; Liu, Y. Platelet-mimicking therapeutic system for noninvasive mitigation of the progression of atherosclerotic plaques. Adv. Sci. 2021, 8, 2004128. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.S.; Wairkar, S. Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int. J. Pharm. 2021, 598, 120395. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.S.; Chan Kwon, I.; Tae, G. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E/mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.-k.; Lin, J.; Fuchs, J.R.; Marcucci, G.; et al. Curcumin is a potent DNA hypomethylation agent. Bioorganic Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar] [CrossRef]
- Meng, N.; Gong, Y.; Zhang, J.; Mu, X.; Song, Z.; Feng, R.; Zhang, H. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J. Biomater. Appl. 2018, 33, 088532821881532. [Google Scholar] [CrossRef]
- Derissen, E.J.B.; Beijnen, J.H.; Schellens, J.H.M. Concise drug review: Azacitidine and decitabine. Oncologist 2013, 18, 619–624. [Google Scholar] [CrossRef] [Green Version]
- de Castro Leão, M.; Raffin Pohlmann, A.; Soares Alves, A.; Poiselli Farsky, S.; Uchiyama, M.; Araki, K.; Sandri, S.; Stanisçuaski Guterres, S.; Alves Castro, I. Docosahexaenoic acid nanoencapsulated with anti-PECAM-1 as co-therapy for atherosclerosis regression. Eur. J. Pharm. Biopharm. 2020, 159, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, A.J.P.; van Leent, M.M.T.; Prevot, G.; Brechbuhl, E.E.S.; Pérez-Medina, C.; Duivenvoorden, R.; Fayad, Z.A.; Mulder, W.J.M. Targeting trained innate immunity with nanobiologics to treat cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Hossaini Nasr, S.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Rho, J.G.; Um, W.; EK, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic acid nanoparticles as nanomedicine for treatment of inflammatory diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Venosa, A.; Lee, I.H.; Myers, D.; Holloway, J.A.; Prud’homme, R.K.; Gao, D.; Szekely, Z.; Laskin, J.D.; et al. A novel bivalent mannosylated targeting ligand displayed on nanoparticles selectively targets anti-inflammatory M2 macrophages. Pharmaceutics 2020, 12, 243. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, J.H.; VanWye, J.; Banerjee, R.; Wickline, S.A.; Pan, H.; Totary-Jain, H. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol. Ther. 2021, 29, 1744–1757. [Google Scholar] [CrossRef]
- Segers, F.M.; Yu, H.; Molenaar, T.J.; Prince, P.; Tanaka, T.; van Berkel, T.J.; Biessen, E.A. Design and validation of a specific scavenger receptor class AI binding peptide for targeting the inflammatory atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Esfandyari-Manesh, M.; Abdi, M.; Talasaz, A.H.; Ebrahimi, S.M.; Atyabi, F.; Dinarvand, R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. DARU J. Pharm. Sci. 2020, 28, 131–138. [Google Scholar] [CrossRef]
- Datta, J.; Ghoshal, K.; Denny, W.A.; Gamage, S.A.; Brooke, D.G.; Phiasivongsa, P.; Redkar, S.; Jacob, S.T. A new class of quinoline-based dna hypomethylating agents reactivates tumor suppressor genes by blocking DNA methyltransferase 1 activity and inducing its degradation. Cancer Res. 2009, 69, 4277–4285. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Shen, N.; Yang, Y.; Yu, H.; Xu, S.; Yang, Y.-W.; Liu, S.; Meguellati, K.; Yan, F. Targeting epigenetic pathway with gold nanoparticles for acute myeloid leukemia therapy. Biomaterials 2018, 167, 80–90. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Bridgeman, S.; Northrop, W.; Ellison, G.; Sabapathy, T.; Melton, P.E.; Newsholme, P.; Mamotte, C.D.S. Statins do not directly inhibit the activity of epigenetic modifying enzymes. Cancers 2019, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; Van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef] [Green Version]
- Hesari, M.; Mohammadi, P.; Khademi, F.; Shackebaei, D.; Momtaz, S.; Moasefi, N.; Farzaei, M.H.; Abdollahi, M. Current advances in the use of nanophytomedicine therapies for human cardiovascular diseases. Int. J. Nanomed. 2021, 16, 3293–3315. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef]
- Kalani, A.; Kamat, P.K.; Kalani, K.; Tyagi, N. Epigenetic impact of curcumin on stroke prevention. Metab. Brain Dis. 2015, 30, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prilepskii, A.Y.; Serov, N.S.; Kladko, D.V.; Vinogradov, V.V. Nanoparticle-based approaches towards the treatment of atherosclerosis. Pharmaceutics 2020, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Hossaini Nasr, S.; Huang, X. Nanotechnology for targeted therapy of atherosclerosis. Front. Pharmacol. 2021, 12, 755569. [Google Scholar] [CrossRef]
- Katsuki, S.; Koga, J.; Matoba, T.; Umezu, R.; Nakashiro, S.; Nakano, K.; Tsutsui, H.; Egashira, K. Nanoparticle-mediated delivery of pitavastatin to monocytes/macrophages inhibits angiotensin ii-induced abdominal aortic aneurysm formation in ApoE/mice. J. Atheroscler. Thromb. 2022, 29, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and nanoparticles for statin delivery: Current use and future perspectives in cardiovascular disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef]
- Yin, T.; Li, Y.; Ren, Y.; Fuad, A.R.M.; Hu, F.; Du, R.; Wang, Y.; Wang, G.; Wang, Y. Phagocytosis of polymeric nanoparticles aided activation of macrophages to increase atherosclerotic plaques in ApoE/mice. J. Nanobiotechnology 2021, 19, 121. [Google Scholar] [CrossRef]
- He, H.; Wang, J.; Yannie, P.J.; Korzun, W.J.; Yang, H.; Ghosh, S. Nanoparticle-based “Two-pronged” approach to regress atherosclerosis by simultaneous modulation of cholesterol influx and efflux. Biomaterials 2020, 260, 120333. [Google Scholar] [CrossRef] [PubMed]
- Seijkens, T.T.P.; van Tiel, C.M.; Kusters, P.J.H.; Atzler, D.; Soehnlein, O.; Zarzycka, B.; Aarts, S.A.B.M.; Lameijer, M.; Gijbels, M.J.; Beckers, L.; et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kumar, S.; Kang, D.-W.; Jo, H.; Park, J.-H. Affinity-driven design of cargo-switching nanoparticles to leverage a cholesterol-rich microenvironment for atherosclerosis therapy. ACS Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef]
- Schmidt, A.; Weber, O.F. In memoriam of Rudolf virchow: A historical retrospective including aspects of inflammation, infection and neoplasia. Contrib. Microbiol. 2006, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andalibi, A.; Liao, F.; Imes, S.; Fogelman, A.M.; Lusis, A.J. Oxidized lipoproteins influence gene expression by causing oxidative stress and activating the transcription factor NF-κB. Proceedings of the Biochemical Society Transactions. Biochem. Soc. Trans. 1993, 21, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Goswami, S.; Frissora, F.W.; Xie, Z.; Yan, P.S.; Bundschuh, R.; Walker, L.A.; Huang, X.; Mani, R.; Mo, X.M.; et al. ROR1-targeted delivery of miR-29b induces cell cycle arrest and therapeutic benefit in vivo in a CLL mouse model. Blood 2019, 134, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A.; Karlsson, H.L.; Coppedè, F.; Migliore, L. Epigenetic effects of nano-sized materials. Toxicology 2013, 313, 3–14. [Google Scholar] [CrossRef]
- Kunovac, A.; Hathaway, Q.A.; Pinti, M.V.; Goldsmith, W.T.; Durr, A.J.; Fink, G.K.; Nurkiewicz, T.R.; Hollander, J.M. ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure. Part. Fibre Toxicol. 2019, 16, 24. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Ye, H.; Huang, K.; Lv, Z.; Ke, Y. Different effects of titanium dioxide nanoparticles instillation in young and adult mice on DNA methylation related with lung inflammation and fibrosis. Ecotoxicol. Environ. Saf. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Lu, X.; Miousse, I.R.; Pirela, S.V.; Melnyk, S.; Koturbash, I.; Demokritou, P. Short-term exposure to engineered nanomaterials affects cellular epigenome. Nanotoxicology 2016, 10, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Tian, D.; He, J.; Yan, X.; Zhao, J.; Yuan, X.; Peng, S. Prolonged exposure to carbon nanoparticles induced methylome remodeling and gene expression in zebrafish heart. J. Appl. Toxicol. 2019, 39, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, K.; Grądzka, I.; Kruszewski, M. Silver, Gold, and Iron Oxide Nanoparticles Alter miRNA Expression but do not Affect DNA Methylation in HepG2 Cells. Materials 2019, 12, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoeb, M.; Kodali, V.K.; Farris, B.Y.; Bishop, L.M.; Meighan, T.G.; Salmen, R.; Eye, T.; Friend, S.; Schwegler-Berry, D.; Roberts, J.R.; et al. Oxidative stress, DNA methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact 2017, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Häfner, S.J.; Lund, A.H. Great expectations—Epigenetics and the meandering path from bench to bedside. Biomed. J. 2016, 39, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Gedda, M.R.; Babele, P.K.; Zahra, K.; Madhukar, P. Epigenetic aspects of engineered nanomaterials: Is the collateral damage inevitable? Front. Bioeng. Biotechnol. 2019, 7, 228. [Google Scholar] [CrossRef]
- Beldman, T.J.; Malinova, T.S.; Desclos, E.; Grootemaat, A.E.; Misiak, A.L.S.; Van Der Velden, S.; Van Roomen, C.P.A.A.; Beckers, L.; Van Veen, H.A.; Krawczyk, P.M.; et al. Nanoparticle-aided characterization of arterial endothelial architecture during atherosclerosis progression and metabolic therapy. ACS Nano 2019, 13, 13759–13774. [Google Scholar] [CrossRef] [PubMed]
| Advantages/Limitations or Main Functional Features | References | ||
|---|---|---|---|
| Core NP structure | Liposome | Well-characterized, comparatively easy to assemble, good in vivo tolerance. | [31,32,33] |
| Macrophage membrane-coated | Mimic a physiological structure. Effective targeting through macrophage–atheroma interactions. | [34] | |
| Platelet membrane-coated | Mimic a physiological structure. Effective targeting through platelet–atheroma interactions. | [35,36] | |
| Pluronic | Foam suppressant. Long-term in vivo tolerance to be determined. | [37] | |
| DNMT inhibitor cargo | Curcumin | Extremely well-characterized molecule, with a range of documented biological effects, including DNMT inhibition. | [38,39] |
| DNMT siRNA | Potentially highly specific. | - | |
| DNMTi | Several DNMTi available, some well-characterized in cancer therapy. | [40] | |
| Statins | Well-characterized cardiovascular drugs. | [31] | |
| Unknown plant-derived factor(s)? | A potential abundant source of novel drugs. | - | |
| Targeting system, non-peptidic | Anti-PECAM-1 antibody | Relatively expensive reagent. Binds to the cellular adhesion receptor PECAM-1/CD31. | [41] |
| Apo-AI | Physiological component of lipoproteins, easy to assemble into NP. Binds to the macrophage scavenger BI receptor. | [42] | |
| Hyaluronic acid | Well-characterized, good in vivo tolerance. Targets the atheroma by binding to CD44. | [43,44] | |
| Mannose | Well-tolerated. Targets the atheroma by binding to M2 macrophage mannose receptor. | [45] | |
| Targeting peptides | p5RHH | Receptor-independent cell penetration. Possible issues with cell type specificity. | [46] |
| cRGD | Targets integrin. | [37] | |
| Lyp-1 | Targets the foam cell-stage macrophage p32 receptor. | [33] | |
| PP1 | Well-characterized, targets both human and mouse scavenger AI receptor. | [47] | |
| S2P | Targets stabilin-2, an endothelial scavenger receptor. | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Sánchez, A.C.; Sánchez-Segura, L.; Lund, G.; Zaina, S. Nanoparticle-Based Modification of the DNA Methylome: A Therapeutic Tool for Atherosclerosis? Cardiogenetics 2022, 12, 12-23. https://doi.org/10.3390/cardiogenetics12010002
Márquez-Sánchez AC, Sánchez-Segura L, Lund G, Zaina S. Nanoparticle-Based Modification of the DNA Methylome: A Therapeutic Tool for Atherosclerosis? Cardiogenetics. 2022; 12(1):12-23. https://doi.org/10.3390/cardiogenetics12010002
Chicago/Turabian StyleMárquez-Sánchez, Ana Cristina, Lino Sánchez-Segura, Gertrud Lund, and Silvio Zaina. 2022. "Nanoparticle-Based Modification of the DNA Methylome: A Therapeutic Tool for Atherosclerosis?" Cardiogenetics 12, no. 1: 12-23. https://doi.org/10.3390/cardiogenetics12010002
APA StyleMárquez-Sánchez, A. C., Sánchez-Segura, L., Lund, G., & Zaina, S. (2022). Nanoparticle-Based Modification of the DNA Methylome: A Therapeutic Tool for Atherosclerosis? Cardiogenetics, 12(1), 12-23. https://doi.org/10.3390/cardiogenetics12010002






