Abstract
Bone conduction hearing implant systems (BCHIs) are established treatments for patients with conductive or mixed hearing loss or single-sided deafness when conventional hearing aids are unsuitable. This study evaluated the cost-effectiveness of the active transcutaneous system Sentio versus a similar system, i.e., Osia in an Australian setting. Scenario analyses also compared Sentio to other systems, i.e., Ponto and Baha Attract. A Markov cohort model was adapted from a previously published source to reflect Australian practice, incorporating device acquisition, surgery, maintenance, battery replacement and adverse event management over a 15-year horizon from a healthcare perspective. Effectiveness inputs were derived from published evidence using a naïve indirect comparison. Extensive sensitivity analyses and external validation tested robustness. In the base case, Sentio was associated with lower costs and a small modelled incremental quality-adjusted life years (QALYs) gain versus Osia. Scenario analyses confirmed cost-effectiveness relative to Ponto and Baha Attract, with outcomes below the Australian willingness-to-pay threshold. Health state utility, device price and reimplantation assumptions were the most influential drivers, yet Sentio remained cost-effective in over 95% of simulations. These findings support Sentio as a clinically and economically efficient BCHI in Australia and highlight the need for direct utility and long-term durability data.
1. Introduction
Bone conduction hearing implant systems (BCHIs) are well-established solutions for individuals with conductive hearing loss (CHL), mixed hearing loss (MHL), or single-sided deafness (SSD), particularly when traditional air conduction hearing aids are not suitable or effective [1,2,3]. These systems bypass the outer and middle ear by transmitting sound vibrations directly to the cochlea through the skull bone, offering an alternative route to auditory perception [1].
Two primary configurations exist: percutaneous and transcutaneous systems. Percutaneous systems (e.g., Oticon Medical Ponto) have an abutment that penetrates the skin, allowing direct mechanical transmission. While effective, this design poses a risk of skin-related complications, including infections and irritation at the implant site [4,5]. To address these issues, transcutaneous systems were introduced, employing either passive or active mechanisms. Passive transcutaneous implants (e.g., Cochlear Baha Attract) use magnetic coupling through intact skin but deliver lower output due to skin attenuation. Active transcutaneous systems (i.e., Cochlear Osia, MED-EL Bonebridge and Oticon Medical Sentio) place the transducer on the bone beneath the skin, improving sound transmission and reducing skin complications while maintaining qualitative audiological outcomes [6,7,8,9].
Technological differences exist between active transcutaneous systems. The Osia system features a piezoelectric transducer and is said to deliver greater audiological gain at high frequencies [10]. The implant requires minimal bone work but has a higher profile above the bone than Sentio and Bonebridge. Sentio and Bonebridge utilise electromagnetic transducers that provide stronger low-frequency output and a smoother frequency response, potentially resulting in a more natural sound quality and improved speech understanding [9,11,12]. Despite these recent innovations, mild adverse events such as skin discomfort or inflammation can still occur, particularly with implants that have a large protrusion above the bone [13,14,15]. Nevertheless, the clinical and audiological benefits of active transcutaneous bone conduction systems are well established, supporting their role in improving hearing outcomes and quality of life [16,17].
In Australia, BCHIs are offered to patients with CHL, MHL, or SSD when conventional hearing aids are inadequate. Referral is typically via ENT (Ear, Nose and Throat) or audiology services, with device selection guided by audiometric and clinical considerations. Although the initial approach to hearing rehabilitation involves fitting air conduction hearing aids, there are patients for whom these are either unsuitable or provide inadequate sound amplification. It has been reported that one in six Australians, over 3.6 million, live with some degree of hearing loss [18]. Furthermore, approximately 3.8% of the population has moderate or severe hearing loss in their worst ear [19], making them potentially eligible for BCHIs such as Sentio, Osia, or Ponto.
From a reimbursement perspective, health systems such as Australia’s require that new implantable technologies demonstrate at least comparable clinical effectiveness to existing alternatives and that any added costs are justified by improvements in outcomes [20]. Health economic evaluations are therefore critical to inform decisions about public funding and clinical adoption of new BCHIs.
While earlier studies, such as Brunner et al. [21], have evaluated the clinical and economic performance of BCHIs (i.e., Osia and Baha Attract), no published analysis has examined the cost-effectiveness of the newly introduced active transcutaneous Sentio system. Given the need for evidence to guide reimbursement decisions and support clinical adoption in Australia, this study evaluates the cost-effectiveness of the Sentio system compared with the Osia system. To provide a broader context, a scenario analysis is also conducted, incorporating additional potential comparators such as Ponto and Baha Attract.
2. Materials and Methods
2.1. Model Structure
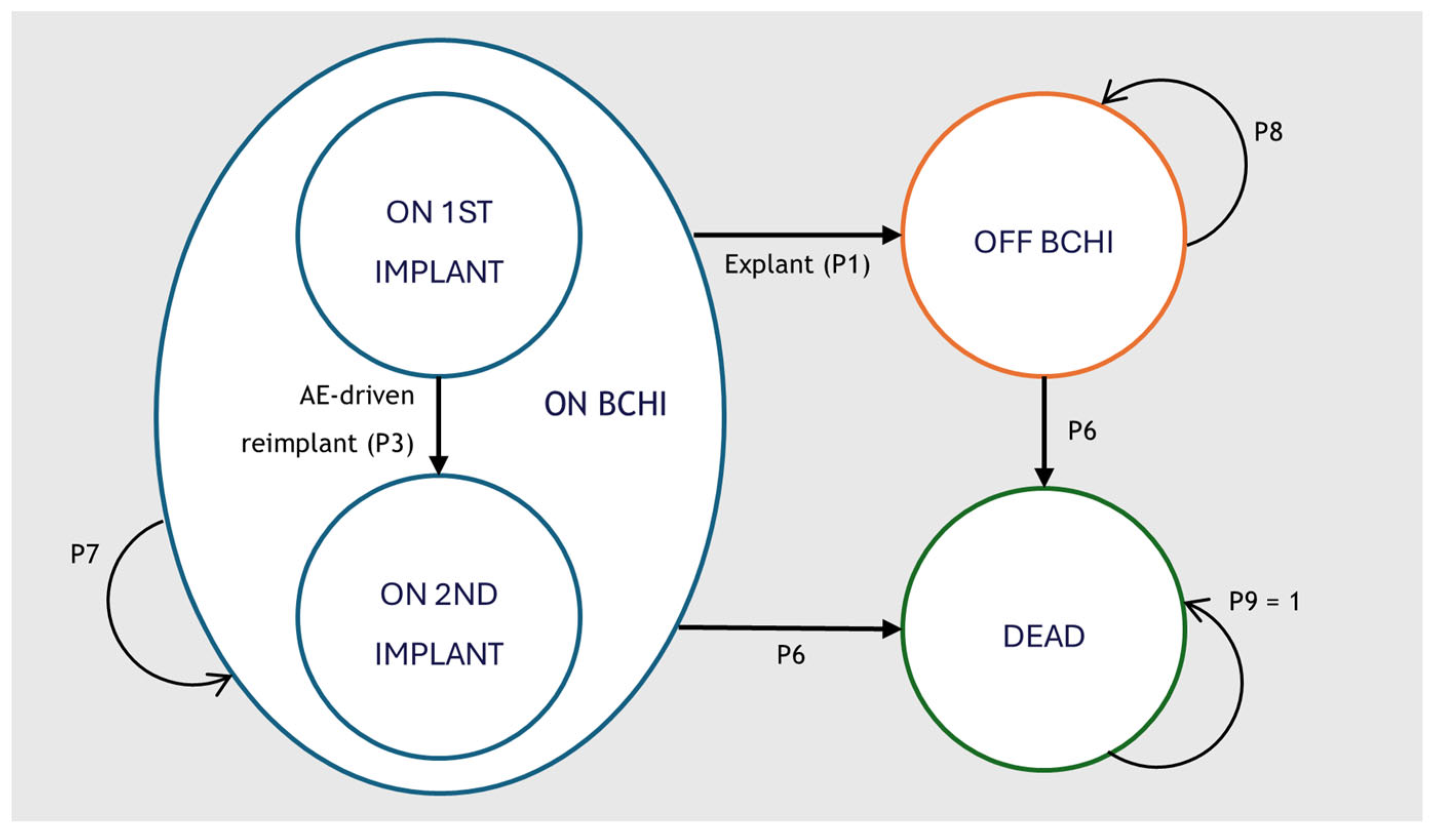
A Markov cohort model was developed in Microsoft Excel 2016 to capture the clinical and economic pathways of patients receiving a BCHI. The model structure was adapted from the cost-effectiveness model of the Osia system developed by Brunner et al. [21] and expanded to capture health care resource use costs, cost of battery replacement and anticipated reimplantation. An overview of the structure is available in Figure 1.
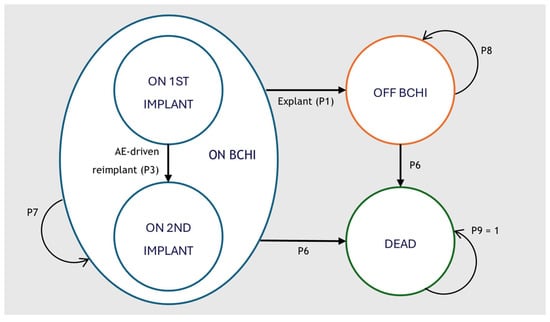
Figure 1.
Cohort market model structure for the analysis of BCHI. The model structure is equal in all comparator arms. BCHI = Bone Conducted Hearing Implant; AE = Adverse Event.
The model comprised three primary health states: on BCHI, off BCHI and dead. All patients entered the model in the on BCHI health state with a first implant. Patients could transition to a second implant due to an adverse event (AE)-driven reimplantation (P3). Reimplantation was assumed to occur only once, and no patients received a third implant. Patients undergoing explantation surgery (P1) transitioned permanently to the off BCHI state. Mortality was applied according to age- and sex-specific population rates (P6) and was unaffected by implant status.
While in the on BCHI state, patients could also experience additional events that did not alter the primary health state: revision surgery (P2), anticipated reimplantation (P4) and sound processor (SP) replacement. Anticipated reimplantation represented scheduled replacement at the end of device lifespan and/or due to progressive hearing loss and could occur multiple times for eligible patients. Revision surgery reflected procedures required to manage adverse events or device malfunctions. SP replacement reflected the exchange of the external audio processor, due to wear, failure, or technological upgrades. AEs included soft tissue complications and pain. A brief description of the model events is provided in Table 1.

Table 1.
Description of model events.
2.2. Economic Evaluation
This economic evaluation compared the Sentio system with the Osia system for patients with MHL, CHL and SSD in the Australian healthcare setting. Scenario analyses included comparisons with Ponto and Baha Attract. Model development followed the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Task Force recommendations on Good Modelling Practices [22] and adhered to the methodological requirements of Australia’s Health Technology Assessment (HTA) body, the Medical Services Advisory Committee (MSAC) [23].
2.3. Indirect Treatment Comparison of Clinical Effectiveness
As no direct head-to-head clinical trials were available across all relevant bone conduction hearing implant (BCHI) systems, an anchored indirect comparison was not feasible. Therefore, a naïve indirect treatment comparison (ITC) approach was implemented to estimate relative clinical effectiveness across devices. Meta-analysis was the primary source for comparison.
Effectiveness inputs were derived from published evidence for each comparator and applied directly in the model. A pooled evidence synthesis including 170 studies (>6000 implantations [24]) was used to inform follow-up–adjusted probabilities of adverse events, revisions and reimplantations for Osia, Ponto and Baha Attract. Sentio is a recently introduced device and was not included in this synthesis; instead, corresponding parameters were informed by data from its predecessor device (BCI [25]) and supplemented by available clinical trial data for Sentio [9].
Across included studies, patient populations were broadly comparable in terms of indication (CHL, MHL and SSD), baseline hearing severity and follow-up duration (Table A1 in Appendix A). The approximate distribution between CHL/MHL and SSD is five to one, and the majority of studies include adult patients only (Table A1 in Appendix A).
No formal statistical adjustment for between-study heterogeneity was performed, as the primary objective was to approximate relative effectiveness using the best available evidence. Variability in study design and definitions was explored through deterministic and probabilistic sensitivity analyses, which demonstrated the robustness of results to uncertainty in the input data.
2.4. Perspective, Discounting, Cycle Length and Time Horizon
The analysis was conducted from a healthcare system perspective, considering only direct medical costs; non-healthcare costs such as productivity losses or patient travel were excluded. Costs and health outcomes were discounted at an annual rate of 5% (three scenario analyses were conducted: 0% discounting for costs and QALYs; 3.5% discounting for both; and 5% for costs with 3% for QALYs), consistent with Australian health economic evaluation guidelines [23]. A half-cycle correction was applied to account for events occurring throughout the model cycle.
The model used a 6-month cycle length, with outcomes evaluated biannually. The base case time horizon was set to 15 years, which was considered sufficient to capture the lifetime costs and benefits of BCHIs while limiting long-term uncertainty. A baseline age of 40 years was selected to reflect the mean age of adults receiving BCHIs in published cohorts [14,17,25].
2.5. Model Inputs
2.5.1. Resource Usage and Costs
The model incorporated cost categories considered to reflect the key components of implantation and long-term management of a BCHI. These included:
- BCHI acquisition
- SP replacement
- Battery replacement
- Health care resource use (routine maintenance appointments)
- Surgery-related costs for implantation, revision, reimplantation (AE-driven or anticipated) and explantation
- AE management costs
For each category, costs were estimated by multiplying unit prices by the frequency of use within each model cycle. Cycle-specific costs were then aggregated to estimate total per-patient costs over the model’s time horizon.
Healthcare resource use costs were derived from the Medicare Benefits Schedule (MBS, effective 1 January 2025) [26] and the National Hospital Cost Data Collection (NHCDC) 2020–21 public sector report [27]. NHCDC costs were adjusted for inflation using the Consumer Price Index (CPI) for Medical products, appliances and equipment from June 2021 to September 2024 [28].
Surgery and hospitalisation costs were modelled as one-time costs per procedure (implantation, revision, reimplantation, or explantation). Hospitalisation included ward, operating room and overhead costs. Anaesthesia costs were based on the MBS tariff for 61–75 min, reflecting the average duration of transcutaneous BCHI implantation. Published surgery times were 53–64 min for Osia [29,30,31] and 58 min for Sentio [9]. Ponto implantation typically does not require general anaesthesia [32], while Baha Attract was assumed to require anaesthesia in 50% of cases [33].
Following all surgeries except explantation, two audiologist appointments and one surgical follow-up were assumed. SP replacement required one audiologist appointment. For AEs (soft tissue complications and pain), two specialist appointments were included [21]. After implantation, patients attended annual audiologist appointments for SP calibration and fine-tuning. The cost of SP upgrades was incorporated at five-year intervals, consistent with Australian clinical practice. Battery cost was included, where the time to replacement was determined by battery longevity and daily use. All systems use batteries with the same cost, estimated at AUD 0.43 per battery [34,35].
All cost inputs were sourced from the latest available Australian tariffs, as of 2024, and are summarised in Table 2.

Table 2.
Healthcare costs and sources for economic evaluation.
2.5.2. Model Inputs for Anticipated Reimplantation and Sound Processor Replacement
Despite BCHIs typically being lifelong treatments, there is a lack of long-term clinical evidence exploring patient trajectories over a longer period, exceeding 10 years. For this reason, anticipated reimplantation and SP replacement were modelled and included as recurring events in the model to reflect device ageing, expected hearing loss progression and clinical praxis in the Australian healthcare system. Expected hearing loss progression was considered due to the relatively high proportion of patients who experience a difference in pure-tone average exceeding 10 dB over a five-year period [43]. To capture variability across patients and to avoid discrete jumps in costs and outcomes, both events were modelled using a gamma distribution.
For Osia and Baha Attract, there are no options available for stronger outputs without going through surgery. For this reason, anticipated reimplantation was assumed to occur after approximately 10 years. For Sentio and Ponto, a longer interval of 15 years was assumed, reflecting the availability of a super-powerful Sentio implant and a Ponto Superpower sound processor that would reduce the need for additional surgery. Different time horizons (5, 10, 20 and lifelong) and variations in timing of anticipated reimplantation and SP replacement, excluding the parameter of anticipated reimplantation altogether, were explored as part of the sensitivity analyses.
Across all implants, SP replacement was assumed to occur at five-year intervals, consistent with standard Australian clinical practice [44]. These values were applied as base case inputs in the model.
2.5.3. Literature Search
To inform model parameters, targeted literature searches were performed in PubMed to identify evidence on utility weights, event rates and clinical effectiveness of BCHIs. While Brunner et al. [21] provided a key foundation for the Osia–Attract comparison, additional evidence was required to extend the model to include Sentio and Ponto and to identify any updated data for Osia and Baha Attract published after Brunner’s analysis.
The literature search specifically aimed to (1) include data for newer BCHI systems and comparators, (2) identify supplementary evidence on adverse event rates, device longevity and utility values, and (3) support sensitivity and scenario analyses assessing uncertainty in clinical and economic inputs.
Searches were restricted to English-language studies published from 2017 to 2024, covering Sentio, Osia (1 and 2), Baha Attract and Ponto. The strategy followed the relevant market introduction of BCHI and the approach described by Brunner et al. [21] to ensure the most recent data for comparators. Data were extracted from text and tables.
2.5.4. Results of Literature Search
The targeted literature search identified several relevant studies that were used to inform and validate model inputs for clinical effectiveness, event rates and utility parameters across BCHI systems. The search identified a number of studies that could provide more recent data than Brunner et al. [21] for the comparator. A summary of the included studies is provided in Table A1 in Appendix A. Overall, the evidence base demonstrated consistent patient characteristics, follow-up durations and outcome measures across studies, supporting the assumption that BCHI populations were broadly comparable in terms of indication (CHL, MHL, SSD), baseline severity and clinical outcomes. Functional gains, patient-reported improvements and safety profiles were similar across devices, justifying the use of pooled data and naïve indirect comparisons in the model.
Sentio
For Sentio, evidence was limited to recent clinical studies. Reinfeldt et al. [25] reported long-term outcomes for its predecessor (BCI), with stable functional gains (~30 dB) and no serious adverse events over five years, which were used as proxy data for durability. Hol et al. [9] conducted a multicentre study of Sentio in 51 adults, reporting a mean functional gain of 32.8 dB, significant improvements in speech recognition and high patient-reported quality-of-life benefits (96%), with only mild adverse events during the first 6 months of follow-up.
Osia
For Osia, base case transitions were derived from the systematic review and meta-regression by Caversaccio et al. [24], synthesising data from over 170 studies (>6000 implantations). Supporting evidence from a systematic review including several multicentre trials (Key et al. [17]), showed consistent functional gains and patient-reported benefits.
Ponto
For Ponto, base case transitions were derived from the systematic review and meta-regression by Caversaccio et al. [24]. Additional evidence from Lagerkvist et al. [4], a systematic review of 41 studies, reported mean functional gains of 33.9 dB, high implant survival and improvements in quality-of-life outcomes (GBI mean 32.6), supporting comparability with other active BCHI systems.
Baha Attract
For Baha Attract, base case transitions were derived from the systematic review and meta-regression by Caversaccio et al. [24]. Supporting evidence from Kruyt et al., which showed significant audiological and PROM improvements, 100% implant survival and a low rate of soft tissue complications (~5%), was applied in scenario analyses.
2.5.5. Transition Probabilities and Clinical Effectiveness Data
Transition probabilities for key events (explantation, revision surgery and AE-driven reimplantation) were sourced from systematic reviews and meta-analyses to obtain pooled, follow-up-adjusted estimates. For Osia, Ponto and Baha Attract, base case inputs were taken from Caversaccio et al. [24] to ensure consistency across comparators, while device-specific estimates from Key et al. [17] (Osia), Lagerkvist et al. [4] (Ponto) and Brunner et al. [21] (Baha Attract) were reserved for scenario analyses.
For Sentio, limited long-term data were available due to its recent introduction. Long-term outcomes from its predecessor (BCI, [25]) were therefore applied as base case inputs, assuming comparable surgical performance. Short-term Sentio data were incorporated only in scenario analyses, given the limited follow-up time of 6 months [9].
AE probabilities were derived in the same manner: pooled estimates from Caversaccio et al. [24] for Osia, Ponto and Baha Attract and Reinfeldt et al. [25] for Sentio. Minor complications in pooled sources were assumed to include both soft tissue reactions and pain, with probabilities split equally between the two categories.
For Sentio, several transition probabilities were set to zero in the base case. These represent empirical zeros based on the available evidence from Reinfeldt et al. (predecessor [25]), rather than structural assumptions of no risk. Given the limited sample size and follow-up, these zeros may reflect sparse data rather than the absence of events. To address this uncertainty, non-zero event probabilities were explored in scenario analyses by applying pooled rates for active bone conduction implants with electromagnetic transducers (aBCIem) from Caversaccio et al. [24] to Sentio.
Probabilities were assumed to be constant over the entire model time horizon. Detailed transition probabilities for key events are presented in Table 3 and AE probabilities are provided in Table 4; all estimates reflect base case inputs and are reported for a 6-month cycle length.

Table 3.
Transition probabilities for main events (explantation, revision surgery and AE-related reimplantation) applied in the base case analysis, reported for a 6-month cycle length.

Table 4.
Adverse event probabilities (soft tissue complications and pain) applied in the base case analysis, reported for a 6-month cycle length.
2.5.6. Utility—Health-Related Quality of Life
Utilities for on and off BCHI health states were derived from Brunner et al. [21], who reported Health Utility Index (HUI) values for Osia and Baha Attract patients. These values were applied across Osia, Baha Attract, Ponto and Sentio to ensure comparability, in line with the approach taken for event transitions using Caversaccio et al. [24]. Subgroup adjustments (CHL/MHL and SSD) were tested in scenario analyses.
For Sentio and Ponto, the use of Osia utilities as proxies was supported by a meta-analysis of specific quality of life outcomes for percutaneous and transcutaneous bone conduction devices, showing that clinically relevant differences are more linked to the type of hearing loss than the type of BCHIs [45]. For example, the Glasgow Benefit Inventory (GBI) total score for transcutaneous was 33, 95% confidence interval [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,45] which is similar to what is reported for Sentio: 29, 95% confidence interval [9,24,25,26,27,28,29,30,31,32,33,34] and Ponto (mean 32.6 across five studies) [4,45]. Furthermore, functional gain for Osia is reported to be between 29 and 41 dB [17] and for Sentio between 30 and 36 dB [9] indicating equivalent clinical outcomes. Also, patient-reported outcomes on perceived benefits measured by the Speech Spatial and hearing Qualities (SSQ) score indicate similar outcomes for both Sentio [9] and Osia [29]. Further support was provided by clinical expert validation, who unanimously confirmed the clinical relevance of the assumption of a generalizable utility score across BCHI brands (see Section 2.7).
For Baha Attract, published utility decrements from Brunner et al. [21] were incorporated, reflecting lower quality-of-life improvements relative to active systems.
Soft tissue complication disutility was sourced from Matza et al. [46], where superficial wound infection after orthopaedic surgery was considered clinically comparable to post-implantation tissue events. Pain disutility was sourced from Dixon et al. [47] as the mean decrement for moderate pain (levels 4–7 on a 0–10 scale). This decrement was scaled to three days (3/365 of an annual decrement) to reflect its short duration.
Scenario analyses tested subgroup-specific utilities (CHL/MHL vs. SSD) using Brunner’s stratified values, while base case utilities remained common across active BCHIs. In summary, the model assumed equal utility gains across systems (Sentio, Osia, Ponto), decrements for Baha Attract and short-term disutilities for AEs. These assumptions were validated by clinical experts (see Section 2.7) and are summarised in Table 5.

Table 5.
Utility weights for economic evaluation.
2.6. Sensitivity Analysis
2.6.1. Parameter and Scenario Analysis
A series of sensitivity and scenario analyses was conducted to explore the robustness of model outcomes.
For the one-way sensitivity analyses (SA), each parameter was varied independently within the bounds of its 95% confidence interval to assess its individual impact on incremental results. When standard errors were not available, an arbitrary standard error corresponding to ±10% of the parameter value was assumed
The scenario analyses were deterministic and focused on structural assumptions and treatment practice patterns and included the following:
- Discounting and cycle correction
- Alternative discount rates: 3.5% and 0% [23].
- Excluding half-cycle correction.
- Cycle length: 3-month and 12-month cycles.
- Time horizon: 5, 10, 20 and lifetime horizon.
- Patient demographics
- Younger and older cohorts.
- Gender-specific cohorts (100% male, 100% female).
- Device assumptions
- Utility gains specific to CHL/MHL and SSD subgroups.
- Alternative device acquisition costs for Sentio.
- Alternative transition probabilities from published sources.
- Variations in timing of anticipated reimplantation and SP replacement.
- Alternative distributions for SP replacement and anticipated reimplantation timing (Log-Normal).
- Exclusion of anticipated reimplantation.
- Course of care settings
- Different numbers of annual audiologist visits.
- Compliance variations for SP replacement and anticipated reimplantation.
- Alternative CPI adjustments for medical costs.
2.6.2. Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis (PSA) was conducted using Monte Carlo simulation with 10,000 iterations. All model parameters were varied simultaneously, with beta distributions assigned to utilities and event probabilities, and gamma distributions assigned to costs. When standard errors were not available, an arbitrary standard error corresponding to 10% of the parameter value was assumed. Parameters with zero observed events in the base case were treated as fixed in the PSA. Uncertainty related to these parameters was instead explored through dedicated scenario analyses, rather than through arbitrary continuity corrections, by assigning non-zero event probabilities derived from external pooled evidence.
2.7. Internal and External Validation
The model underwent internal validation through systematic quality checks to ensure technical accuracy and consistency. Programming logic was verified to confirm correct implementation of state transitions, event probabilities and discounting, while cross-checks of parameter inputs and outputs demonstrated consistency with source data. Plausibility testing of intermediate and overall results indicated that modelled outcomes followed expected patterns, supporting the reliability of the internal model structure.
For external validation, the model was benchmarked against the published framework by Brunner et al. [21], specifically replicating their Osia versus Baha Attract analysis. To ensure comparability, Brunner’s published parameters were implemented in our model. In addition, Sentio was benchmarked against Baha Attract within the Brunner framework. These steps allowed assessment of consistency with an established, peer-reviewed model. Finally, a semi-structured clinical validation process was conducted with four international experts in BCHIs, focusing on model structure, quality-of-life assumptions and anticipated reimplantation.
3. Results
3.1. Base Case Results of the Economic Evaluation
In the base case comparison, Sentio was associated with lower total costs and a small gain in QALYs, resulting in dominance over Osia. The main cost savings came from fewer anticipated reimplantations, revisions and explantations. These savings were only partly offset by higher sound processor replacement and routine healthcare use. Overall, Sentio remained both less costly and more effective than Osia. Table 6 presents the deterministic cost-effectiveness model results.

Table 6.
Deterministic cost-effectiveness model results, Sentio vs. Osia, discounted.
In the Australian context, this outcome falls well within the commonly accepted willingness-to-pay (WTP) threshold of AUD 50,000 per QALY [48,49], supporting Sentio’s potential inclusion on the Prostheses List and broader access for eligible patients.
3.2. Results of Sensitivity Analysis
3.2.1. Univariate Parameter and Scenario Analysis Results
The one-way sensitivity analysis examined the impact of varying key parameters within their 95% confidence intervals on the incremental net monetary benefit of Sentio compared to Osia. The most influential drivers were the utility assigned to the on BCHI state, the probability of explantation for Osia and the utility of the off BCHI state. Other influential parameters included the assumed time to anticipated reimplantation, the initial device acquisition cost of Sentio and the interval for sound processor replacement. Across all tested ranges, Sentio remained cost-effective compared to Osia, with the incremental cost-effectiveness ratio (ICER) consistently falling within the “less costly, more effective” quadrant. Even under the most conservative assumptions, the dominance of Sentio was preserved, confirming the robustness of the base case findings.
Scenario analyses were conducted to test the model’s robustness by varying key assumptions and input parameters, one at a time, in a deterministic manner. Table A4 in Appendix B summarises the incremental percentage changes in costs, QALYs and ICERs relative to the base case.
Time horizon had the greatest impact on incremental QALYs. Reducing the time horizon from 15 to 5 years decreased QALYs by 81%, whereas extending the horizon to a lifetime increased QALYs by 215%. Changing starting age and sex had only minor effects.
Device price also influenced results. Reducing the price to AUD 13,000 lowered incremental costs by −6.9%, while increasing to AUD 15,000 raised costs by +8.5%. In both cases, Sentio maintained dominance. At a device price of AUD 23,462, the ICER reached AUD 50,000 per QALY, corresponding to the commonly assumed WTP threshold in Australia. Discounting assumptions produced similar patterns; removing discounting increased incremental QALYs by +53.5%, while applying a 3.5% discount rate reduced them by −13.1%.
Excluding anticipated reimplantation reduced cost savings by 61.8%, yet Sentio still dominated Osia. Other care-related assumptions, excluding AE pain management and audiologist appointment frequency, had a negligible impact. Two scenario analyses addressed uncertainty related to zero observed event rates for Sentio. Transition probabilities were varied using short-term Sentio data from Hol et al. [9] and pooled aBCIem event rates from Caversaccio et al. [24]. In both cases, Sentio remained dominant compared with Osia.
Overall, the scenario analyses confirmed the robustness of the base case, with Sentio remaining economically favourable compared to Osia across all tested assumptions.
3.2.2. Probabilistic Sensitivity Results
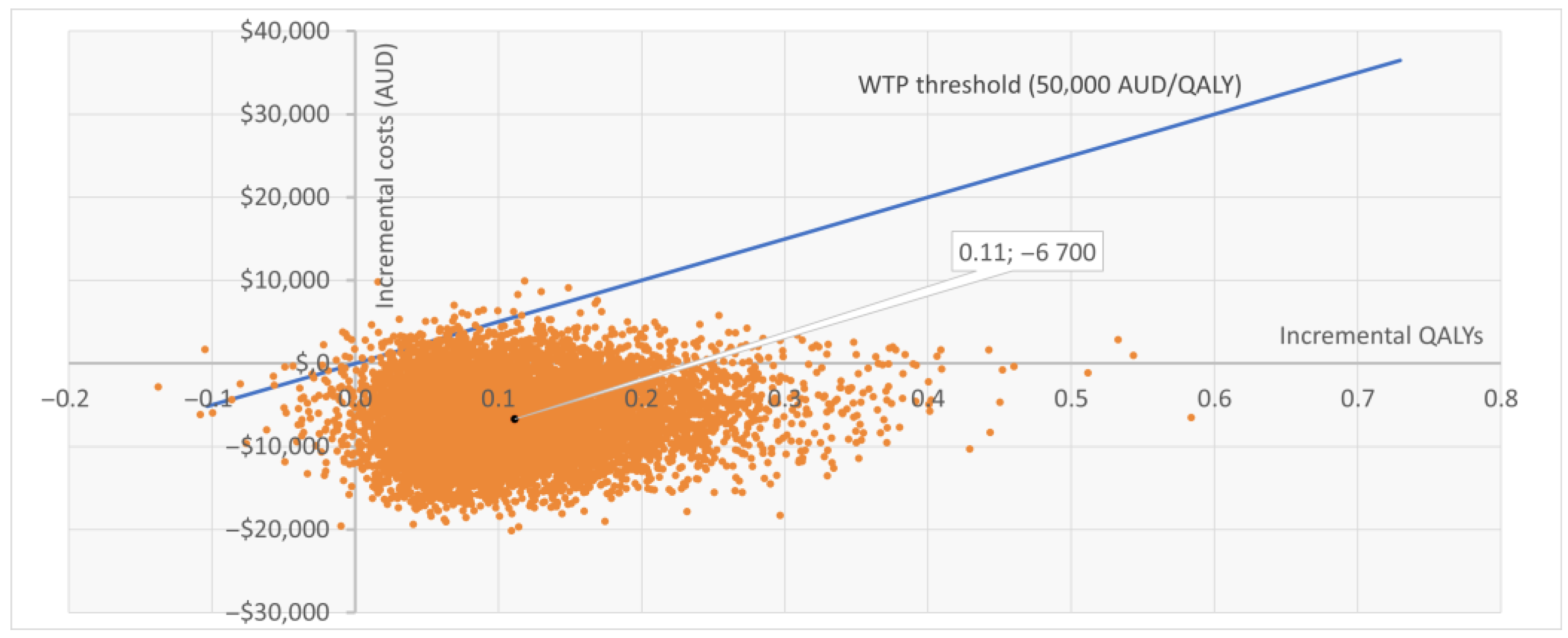
The PSA based on 10,000 iterations confirmed the robustness of the base case. Sentio incurred lower mean costs compared with Osia (AUD 39,109 vs. 45,808) and slightly higher QALYs (8.209 vs. 8.098), resulting in dominance. At a WTP threshold of AUD 50,000 per QALY, Sentio was cost-effective in 99.2% of simulations. Most iterations (92%) fell in the “less costly, more effective” quadrant, while 7% indicated “more costly, more effective,” and <1% suggested lower effectiveness. The cost-effectiveness scatter plot (Figure 2) showed nearly all iterations below the WTP threshold line. The cost-effectiveness acceptability curve (Figure A1 in Appendix B) indicated >95% probability of Sentio being cost-effective at thresholds well below AUD 50,000 per QALY, reaching near-certainty at the reference threshold.
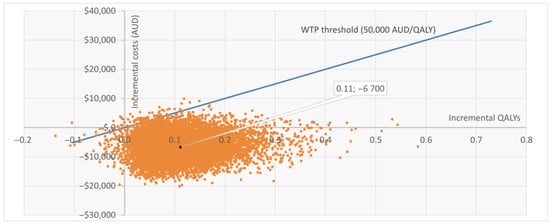
Figure 2.
Cost-effectiveness scatter plot (CE plane) for Sentio versus Osia. Each point represents one of 10,000 PSA iterations: the majority fall below the WTP threshold line. WTP = Willingness to Pay; QALY = Quality Adjusted Life Year; AUD = Australian Dollars; PSA = Probabilistic Sensitivity Analysis.
3.2.3. Results of External Validation
An external validation was conducted using the published Brunner et al. model (Osia vs. Baha Attract). Two tests were performed. First, Brunner’s published parameters were directly implemented in our model to replicate their base case. Second, Sentio was benchmarked against Baha Attract by introducing Sentio inputs into the Brunner model.
As shown in Table A5 in Appendix C, the first test produced almost identical results to Brunner’s published analysis, confirming that our model accurately replicated the original framework. In the second test, Sentio demonstrated a lower ICER than Osia when both were compared against the same benchmark (Baha Attract), reinforcing the main analysis and highlighting Sentio as the more efficient alternative.
The clinical expert validation of key model assumptions confirmed that the model structure is clinically plausible and appropriately reflects the treatment pathway, while acknowledging that it is also a necessary simplification of individual patient trajectories. Assumptions regarding utility values, anticipated reimplantation and the number of AE-driven reimplantations were judged to be reasonable and consistent with clinical practice.
3.3. Scenario Analyses: Sentio Compared to Ponto and Baha Attract
Scenario analyses were also conducted to assess the cost-effectiveness of the Sentio System relative to Ponto and Baha Attract (See Table A2 and Table A3 in Appendix B). The main analyses showed that, while Sentio dominated Osia in the primary comparison, the results differed when evaluated against Ponto and Baha Attract. Compared with Ponto, Sentio was associated with a positive ICER of AUD 24,200/QALY, reflecting higher incremental costs and health gains and suggesting a less favourable cost-effectiveness profile than observed in the Osia comparison. In comparison, Sentio, compared with Baha Attract, produced an ICER of AUD 11,052/QALY, indicating a slightly more favourable outcome. Both outcomes align with commonly accepted Australian WTP thresholds.
For the Sentio versus Ponto comparison, scenario analyses demonstrated that excluding anticipated reimplantation reduced the ICER (AUD 9702/QALY), highlighting the influence of long-term device replacement assumptions. In contrast, applying aBCIem-specific data from Caversaccio et al. [24] as a proxy for Sentio clinical inputs yielded minimal QALY gains (0.006 QALYs) and consequently a substantially less favourable cost-effectiveness outcome. When both adjustments were combined, the ICER increased markedly, reflecting a scenario in which small QALY differences drive disproportionate cost-effectiveness results. These relatively less favourable outcomes reflect the small incremental costs of Ponto together with the minimal incremental quality-of-life gains, which drive the ICERs to unfavourable levels under these assumptions.
For the Sentio versus Baha Attract comparison, Sentio consistently generated positive incremental QALYs with ICERs within ranges considered acceptable in Australia. Excluding anticipated reimplantation lowered the ICER further (AUD 13,632/QALY), while applying aBCIem-specific clinical inputs from Caversaccio [24] increased the ICER slightly (AUD 15,435/QALY). Combining these adjustments yielded an ICER of AUD 20,751/QALY. These results indicate that the relative cost-effectiveness of Sentio versus Baha Attract is robust, with outcomes primarily driven by assumptions on device reimplantation intervals and the size of incremental QALY gain.
4. Discussion
This study evaluated the cost-effectiveness of the Sentio system in the Australian setting, building on the model by Brunner et al. [21]. The findings indicate that Sentio is cost-effective compared with the Osia system, and in most scenarios even dominant, delivering a small modelled incremental QALY gain at lower costs. Scenario analyses against Ponto and Baha Attract confirmed robustness, with ICERs consistently below the Australian WTP threshold. External validation showed close concordance with Brunner’s Osia versus Attract analysis, supporting the credibility of the adapted model. Taken together, these findings strengthen the evidence base for Sentio as an efficient alternative to existing BCHIs.
The analysis has several strengths. It is based on a peer-reviewed model that was externally validated and has been expanded to incorporate additional long-term resource use and cost elements such as battery replacement and anticipated reimplantation. It is one of the first studies to systematically evaluate the cost-effectiveness of BCHIs using a structured approach to evidence collection and parameterization. External validation against Brunner’s results confirmed the accuracy of the adapted model, and the inclusion of clinical expert input further reinforced the appropriateness of the assumptions. The results are therefore highly relevant for both clinical and policy decision-making in the Australian context. A further strength of this analysis is that the model structure and key assumptions were validated by four clinical experts, who confirmed their plausibility in reflecting real-world clinical practice.
At the same time, important limitations must be acknowledged. Evidence generation in this field is constrained by the lack of head-to-head randomised controlled trials, leading to reliance on naïve comparisons. There is an inherent risk of biases of such approach stemming from disparate studies with heterogeneous designs, populations and outcome definitions. The model therefore assumes that patient populations across included studies are broadly comparable with respect to indication, baseline severity and treatment pathways. Discrepancies from population heterogeneity were mainly expected to influence clinical effectiveness and have a limited impact on safety-related parameters such as AE probabilities, reimplantation intervals and utility gains. However, Table A1 shows equivalent functional outcomes between studies, supporting the statement from clinical expertise that BCHIs is generally highly effective treatments for the targeted patient population. That is, CHL, MHL, or SSD, where traditional air conduction hearing aids are not suitable or effective.
To minimise biases from naïve comparisons and study heterogeneity, meta-analytic evidence was the first-hand choice for deriving input parameters. This reduces some of the inherited study variability and removes some of the problems with few and small individual studies. Furthermore, extensive sensitivity testing and scenario analyses were applied. Specifically, the relevant parameters were varied using wide probability distributions in a probabilistic sensitivity analysis, and alternative assumptions were explored in scenario analyses. In addition, the model assumes constant transition probabilities over time, as time-dependent event risks could not be reliably estimated due to limited long-term data. These methods ensured that uncertainty around clinical event rates was transparently reflected, allowing the robustness of the base case results to be evaluated.
A potential concern relates to the use of zero observed event rates for Sentio in the base case. These values reflect the currently available evidence rather than assumptions of no risk. To address this uncertainty, scenario analyses were conducted in which transition probabilities for Sentio were varied using both short-term Sentio data (Hol et al. [9]) and pooled aBCIem event rates from Caversaccio et al. [24]. While these scenarios substantially reduced incremental QALY gains compared with the base case, they did not alter the overall economic interpretation. This indicates that the conclusions are not driven by assumptions of zero event risk, but primarily by differences in long-term cost structure.
The analysis of patients with single-sided deafness (SSD) was limited to a single scenario and reflects a smaller patient subgroup with modest expected health gains, whereas the base case results are driven by the larger CHL/MHL population.
Health state utilities specific to Sentio were unavailable; however, reported clinical and patient-reported outcomes indicate results similar to those of Osia, as specified in Table A1 in Appendix A. Clinical expertise validation supports the assumption that the expected utilities are equal, regardless of the BCHI brand. This assumption implies that differences in device design, comfort, or cosmetic outcomes do not translate into clinically meaningful differences in generic health-related quality of life as captured by HUI. Consequently, incremental QALY differences in the model are driven by modelled event pathways and cost offsets rather than demonstrated differences in clinical effectiveness. To address the uncertainty of these assumptions, the model incorporated wide confidence intervals around the utility estimates in a probabilistic sensitivity analysis and conducted scenario analyses using subgroup-specific utilities for CHL/MHL versus SSD. Scenario analyses varying Sentio health state utilities by ±5–10% showed that Sentio remained economically favourable compared with Osia, either as a dominant option when utilities were higher or as a less costly, less effective option when utilities were lower. In addition, univariate sensitivity analysis showed that the utility weight for the on BCHI state was the most influential parameter, and the utility weight for the off BCHI state was the third most influential parameter. Overall, across all scenarios, Sentio remained economically favourable relative to Osia—either dominant or less costly and less effective, demonstrating robustness to uncertainty in utility assumptions.
Further uncertainty stems from the variability of inputs and definitions across studies and comparators, offset to some extent by the use of pooled safety evidence from Caversaccio et al. [24]. The model further assumes full compliance with sound processor replacement and anticipated reimplantation in the base case, which may not fully reflect real-world practice for all patients. Overall, these limitations contributed to uncertainty in cost-effectiveness outcomes, which was reflected in sensitivity analyses. Both the probabilistic sensitivity analysis and one-way sensitivity analysis demonstrated that incremental results varied significantly depending on assumptions, although the probability of Sentio being cost-effective remained above 95% at the Australian threshold.
Interpretation of sensitivity analyses requires caution, particularly in the context of very small incremental QALY differences. In the probabilistic sensitivity analysis, a high proportion of simulations fall within the dominance quadrant; however, this reflects the combination of modest cost differences and very small modelled QALY gains rather than evidence of consistent or clinically meaningful superiority. When incremental QALYs are close to zero, small changes in model inputs can lead to large proportional changes in ICERs, or shifts between dominance and non-dominance, despite minimal absolute differences in outcomes.
Scenario analyses further illustrate the dependence of dominance on key structural assumptions. Dominance over Osia was preserved across most scenarios; however, when assumptions related to health state utilities were altered, Sentio shifted from dominance to being less costly and less effective. This occurs because the modelled incremental QALY gain is small and largely driven by assumptions regarding utilities rather than differences in baseline clinical effectiveness. Similarly, comparisons with Ponto did not consistently result in dominance, reflecting smaller cost differentials and minimal modelled differences in QALYs. These findings highlight that dominance is not a fixed property of the intervention, but rather a function of specific modelling assumptions, particularly those related to utility inputs.
The findings raise important points for interpretation. Cost-effectiveness of Sentio was most evident in the base case, where anticipated reimplantation was included, resulting in dominance over Osia with a small modelled incremental QALY gain at lower overall costs. The magnitude of the incremental QALY gain was modest and likely within the measurement uncertainty of generic utility instruments, such as HUI, and therefore should not be interpreted as indicating clinically meaningful superiority. Differences in anticipated reimplantation rates and timing represented the largest contributor to incremental cost differences between Sentio and Osia in the model. When anticipated reimplantation was excluded, Sentio remained dominant, although the magnitude of cost savings was reduced compared with the base case. This underscores the importance of accounting for reimplantation in long-term models, since excluding it risks underestimating lifetime costs. Also, hearing loss is rarely a stable condition and a decline exceeding 10 dB in pure-tone average is relatively common over a five-year period [43] suggesting that a model should account for progressive hearing loss and associated interventions over time. Available long-term evidence furthermore suggests that reimplantation is a clinically relevant outcome [24]. This further supports its inclusion in economic analyses. However, the assumed timing of anticipated reimplantation is based on published evidence and expert opinion rather than device-specific long-term observational data and therefore represents an important modelling assumption.
Other key drivers were health-state utilities, system acquisition costs and the assumed interval to reimplantation or SP replacement, with battery replacement playing a secondary role. Pricing assumptions are particularly relevant for reimbursement considerations. At a device price of AUD 23,462, the ICER reached AUD 50,000 per QALY, corresponding to the commonly assumed WTP threshold in Australia [48,49]. This illustrates the sensitivity of cost-effectiveness to device pricing and provides a tangible benchmark for decision-makers when assessing the value of Sentio in the Australian context. These results point to clear priorities for further evidence generation, including the collection of direct utility data for Sentio using validated generic preference-based instruments such as EQ-5D or HUI, which have so far been lacking. However, these instruments may lack sensitivity to capture the full benefits of hearing implants, potentially underestimating utility gains. To strengthen future evaluations, mapping studies from device-specific patient-reported outcome measures to utility values may be more useful. The absence of Sentio-specific health state utility data increases uncertainty in the analysis, with potential impact on the ICER, and is therefore an important limitation. Such data are essential to reduce reliance on proxy measures, though alternative methods such as discrete choice experiments could provide complementary insights into WTP for specific system attributes. It should, however, be noted that the clinical expert validation revealed that there would be limited clinical relevant differences between utility scores and different brands of BCHIs. Typically, when a patient decides for treatment with BCHI, irrespective of type and brand, it is a well-grounded and motivated decision at a final step of a long journey, which usually leads to high level of satisfaction.
Future research should address the need for long-term follow-up data, particularly on reimplantation and device durability, which have a substantial impact on cost-effectiveness. Comparative evidence across CHL, MHL and SSD subgroups, as well as registry data on AEs and revisions, would improve the precision of future evaluations and reduce uncertainty.
In summary, compared with Osia, Sentio offers improved economic efficiency, and when benchmarked against Ponto and Baha Attract, it achieves favourable ICERs well below the national threshold. While the analysis necessarily relies on several structural and data-related assumptions, extensive sensitivity analyses suggest that the conclusions are robust to plausible parameter uncertainty. The results demonstrate economic favourability rather than clinical superiority and have direct implications for clinical practice and reimbursement policy, supporting Sentio as a preferred technology for patients with conductive and mixed hearing loss or single-sided deafness. The results also highlight the value of structured economic modelling in fields where randomised evidence is limited.
5. Conclusions
This study demonstrates that the Sentio system, assuming a baseline device price of AUD 14,000, is a cost-effective, and in many cases cost-saving, option for patients with CHL, MHL or SSD in Australia. In the base case, Sentio dominated Osia, providing higher health gains at lower overall costs. Scenario analyses against Ponto and Baha Attract confirmed robustness, with ICERs consistently below the commonly assumed national WTP threshold. Sensitivity analyses showed that results were resilient to uncertainty in key parameters, underscoring the reliability of the findings. These results support Sentio as a clinically and economically efficient alternative within the Australian healthcare setting and provide a benchmark for reimbursement and policy decisions. Future research should prioritise the collection of direct utility data and long-term evidence on reimplantation and device durability to reduce current uncertainties.
Author Contributions
Conceptualization, M.V., I.H. and J.H.; methodology, M.V. and J.H.; validation, M.V., I.H. and J.H.; formal analysis, M.V. and I.H.; data curation, M.V. and I.H.; visualisation, M.V. and I.H.; writing—original draft preparation, M.V.; writing—review and editing, M.V., I.H., J.H., M.Å.H. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Oticon Medical AB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data were extracted from published sources. Formulas extracted from public sources will be available on request, directed to M.V.
Acknowledgments
Karin Wahlberg provided medical writing support. Clinical expertise and validation were provided voluntarily and without compensation by four senior ENT/Audiology consultants from Europe/North America.
Conflicts of Interest
M.Å.H and H.L. are employees of Oticon Medical AB. M.V., I.H. and J.H. are employees of IHE—The Swedish Institute for Health Economics. IHE has received funding from Oticon Medical AB to conduct this study.
Abbreviations
The following abbreviations are used in this manuscript:
| aBCIem | Active bone conduction implant (electromagnetic) |
| AE | Adverse Event |
| AUD | Australian Dollar |
| BAHS | Bone Anchored Hearing System |
| BCHI/BCHIs | Bone Conduction Hearing Implant System |
| BCI | Bone Conduction Implant, predecessor to Sentio |
| CEAC | Cost-Effectiveness Acceptability Curve |
| CHL | Conductive Hearing Loss |
| CPI | Consumer Price Index |
| GBI | Glasgow Benefit Inventory |
| HCRU | Health Care Resource Use |
| HTA | Health Technology Assessment |
| HUI | Health Utilities Index |
| HUI3 | Health Utilities Index Mark 3 |
| ICER | Incremental Cost-Effectiveness Ratio |
| IHACPA | Independent Health and Aged Care Pricing Authority |
| ISPOR | International Society for Pharmacoeconomics and Outcomes Research |
| ITC | Indirect Treatment Comparison |
| MBS | Medicare Benefits Schedule |
| MHL | Mixed Hearing Loss |
| MSAC | Medical Services Advisory Committee |
| NHCDC | National Hospital Cost Data Collection |
| pBAHS | Percutaneous bone anchored hearing systems |
| PLB | Prosthesis List Benefit |
| PROMs | Patient-Reported Outcome Measures |
| PSA | Probabilistic Sensitivity Analysis |
| PTA4 | Pure-tone average (0.5, 1, 2 and 4 kHz) |
| QALY | Quality-Adjusted Life Year |
| SA | Sensitivity Analysis |
| SNR | Signal-to-Noise Ratio |
| SP | Sound Processor |
| SPL | Sound Pressure Level |
| SRS | Speech Recognition Score |
| SRT | Speech Reception Threshold |
| SSD | Single-Sided Deafness |
| SSQ | Speech Spatial and hearing Qualities |
| WTP | Willingness-to-Pay |
Appendix A. Studies Included in the Literature Search

Table A1.
Evidence table of BCHI Studies.
Table A1.
Evidence table of BCHI Studies.
| Refence | Design | Device | N/Studies Cohort | Follow-Up | Functional Gain | PROMs | Safety |
|---|---|---|---|---|---|---|---|
| Key et al. (2023) [17] | Systematic review and meta-analysis | Osia | 14 studies, 15 cohorts (n = 314) 80% CHL/MHL 20% SSD All ages | Varied | 35 dB SPL 95% CI [29; 41], n = 140 (37.7 dB SPL CHL/MHL, n = 97) | Comparable or improved | Moderate to severe 3% (n = 222) |
| Lagerkvist et al. (2020) [4] | Systematic review | Ponto | 41 studies (n = 1352) 85% CHL/MHL 15% SSD All ages | Up to 5 years | ~33.9 dB, n = 89 | 98% improved GBI score, n = 176 | Implant loss 2%, n = 1166 Skin reactions 15%, n = 863 |
| Kruyt et al. (2020) [50] | Prospective multicentre study | Baha Attract | n = 54 72% CHL/MHL 28% SSD Adults | 2 years | FG not mentioned, significant audiological improvement | Improved HUI3, APHAB, SSQ | Explantation 7%, skin reactions 5%, substantial pain 6% |
| Reinfeldt et al. (2022) [25] | Prospective multicentre study | BCI | n = 16 100% CHL/MHL Adults | Up to 5 years | 29.5 dB (std: 7.2 dB) | Comparable | No serious AEs |
| Hol et al. (2025) [9] | Prospective multicentre study | Sentio | n = 51 76% CHL/MHL 24% SSD Adults | 6 months | 33 dB 95% CI [30; 36] PTA4, n = 51, (CHL, MHL, SSD) | 96% improved GBI score | No serious adverse events |
| Caversaccio et al. (2025) [24] | Systematic review and meta-regression | All BCHI | 170 studies (n = 6215; 6451 implantations) CHL/MHL/SSD All ages | Adjusted for FU time | Not applicable (safety study) | Not reported | Lowest AE rates in aBCIem (9.1/100 pt-yrs), highest major/revisions in pBAHS |
| Brunner et al. (2024) [21] | ITC and cost–utility model | Osia vs. Baha Attract | Osia n = 80, Baha Attract n = 54 76% CHL/MHL 24% SSD Adults | 6–24 months | FG not reported, PTA4 + 7.05 dB; quiet + 15.35%; noise + 9.44 dB | HUI3: Osia 0.12, Attract: 0.06 | Osia 1 explantation, Baha Attract 4 explantations, soft tissue AE lower with Osia than Attract |
AE = adverse event; aBCIem = active bone conduction implant (electromagnetic); BCHI = bone conduction hearing implant system; CHL = conductive hearing loss; CI = confidence interval; FG = functional gain; FU = follow-up; HUI3 = Health Utilities Index Mark 3; MHL = mixed hearing loss; PROMs = patient-reported outcome measures; pBAHS = percutaneous bone anchored hearing system; PTA4 = pure-tone average (0.5, 1, 2 and 4 kHz); SPL = sound pressure level; SSD = single-sided deafness.
Appendix B. Results from the Scenario Analysis

Table A2.
Deterministic cost-effectiveness model results, Sentio vs. Ponto, discounted.
Table A2.
Deterministic cost-effectiveness model results, Sentio vs. Ponto, discounted.
| Deterministic Results | Sentio System | Ponto System | Incremental Change |
|---|---|---|---|
| Disaggregated Costs (AUD) | |||
| Implantation | 21,068 | 15,160 | 5909 |
| Revision | 0 | 5840 | −5840 |
| Anticipated reimplantation | 3152 | 1571 | 1581 |
| AE-driven reimplantation | 1206 | 609 | 598 |
| Explantation | 0 | 886 | −886 |
| SP replacement | 10,689 | 9127 | 1562 |
| HCRU | 1871 | 1716 | 155 |
| Battery replacement | 252 | 221 | 31 |
| AE | 28 | 498 | −470 |
| Total costs (AUD) | $38,267 | $35,627 | $2639 |
| Total QALYs | 8.205 | 8.096 | 0.109 |
| Total Life Years | 10.797 | 10.797 | 0.000 |
| ICER | 24,200 |
AUD = Australian Dollars; AE = Adverse Events; SP = Sound Processor; HCRU = Health Care Resource Use; QALY = Quality Adjusted Life-Years; ICER = Incremental Cost-Effectiveness Ratio.

Table A3.
Deterministic cost-effectiveness model results, Sentio vs. Baha Attract.
Table A3.
Deterministic cost-effectiveness model results, Sentio vs. Baha Attract.
| Deterministic Results | Sentio System | Baha Attract System | Incremental Change |
|---|---|---|---|
| Disaggregated Costs (AUD) | |||
| Implantation | 21,068 | 15,639 | 5429 |
| Revision | 0 | 976 | −976 |
| Anticipated reimplantation | 3152 | 4333 | −1180 |
| AE-driven reimplantation | 1206 | 609 | 597 |
| Explantation | 0 | 1447 | −1447 |
| SP replacement | 10,689 | 8480 | 2209 |
| HCRU | 1871 | 1622 | 249 |
| Battery replacement | 252 | 94 | 158 |
| AE | 28 | 458 | −430 |
| Total costs (AUD) | $38,267 | $33,660 | $4607 |
| Total QALYs | 8.205 | 7.769 | 0.436 |
| Total Life Years | 10.797 | 10.797 | 0.000 |
| ICER | 10,558 |
AUD = Australian Dollars; AE = Adverse Events; SP = Sound Processor; HCRU = Health Care Resource Use; QALY = Quality Adjusted Life-Years; ICER = Incremental Cost-Effectiveness Ratio.

Table A4.
Scenario analysis results for Sentio versus Osia.
Table A4.
Scenario analysis results for Sentio versus Osia.
| Setting | Scenario | Incremental Cost (% Change from Base Case) | Incremental QALY (% Change from Base Case) | ICER (AUD/QALY) |
|---|---|---|---|---|
| Core settings | ||||
| Population | MHL/CHL | +0.0% | +18.0% | Dominant |
| Population | SSD | +0.0% | −59.7% | Dominant |
| Health state transition probability source for Sentio | Hol et al. [9] | +12.9% | −3.9% | Dominant |
| Health state transition probability source for Sentio | Caversaccio et al. [24] | −4.7% | −92.0% | Dominant |
| Time horizon | 5 years | −73.3% | −81.0% | Dominant |
| Time horizon | 10 years | −16.5% | −44.3% | Dominant |
| Time horizon | 20 years | −29.0% | +45.3% | Dominant |
| Time horizon | Lifetime (70 years) | −20.8% | +215.3% | Dominant |
| Starting age | 70 years | −7.8% | −16.4% | Dominant |
| Starting age | 20 years | +0.4% | +1.0% | Dominant |
| Percent male | 0% | +0.2% | +0.3% | Dominant |
| Percent male | 100% | −0.2% | −0.3% | Dominant |
| Price of Sentio system | AUD 13,000 | +18.6% | +0.0% | Dominant |
| Price of Sentio system | AUD 14,369 | −6.9% | +0.0% | Dominant |
| Price of Sentio system | AUD 15,000 | −18.6% | +0.0% | Dominant |
| Utility values for Sentio compared to Osia | +5% | +0.0% | +368.1% | Dominant |
| Utility values for Sentio compared to Osia | +10% | +0.0% | +735.8% | Dominant |
| Utility values for Sentio compared to Osia | −5% | +0.0% | −368.1% | Less costly, Less effective (23,579 AUD saved for each QALY lost) |
| Utility values for Sentio compared to Osia | −10% | +0.0% | −735.8% | Less costly, Less effective (9944 AUD saved for each QALY lost) |
| Technical settings | ||||
| Distribution—SP replacement and anticipated reimplantation | Log-normal | −0.6% | +0.0% | Dominant |
| Half-cycle correction | No | +1.1% | +0.0% | Dominant |
| Cycle length | 3 months | −4.3% | −4.1% | Dominant |
| Cycle length | 12 months | +8.0% | +8.7% | Dominant |
| Discount rate (costs, QALYs) | 0% (undiscounted) | +32.4% | +53.5% | Dominant |
| Discount rate (costs, QALYs) | 3.5% | +8.8% | +13.1% | Dominant |
| Discount rate (costs, QALYs) | 5%; 3% | +0.0% | +17.9% | Dominant |
| CPI data | General CPI | +7.3% | +0.0% | Dominant |
| Course of care settings | ||||
| Include anticipated reimplantation | Exclude | −61.8% | +0.0% | Dominant |
| Anticipated reimplantation same for both comparators | 15 years | −69.5% | +0.0% | Dominant |
| Anticipated reimplantation same for both comparators | 10 years | −76.3% | +0.0% | Dominant |
| Include AE pain | Exclude | −2.2% | −2.0% | Dominant |
| Number of annual audiologist appointments | 0.8 | +0.5% | +0.0% | Dominant |
| Number of annual audiologist appointments | 1.2 | −0.5% | +0.0% | Dominant |
| SP replacement compliance rate | 0.8 | +3.6% | +0.0% | Dominant |
| Anticipated reimplantation rate | 0.8 | −12.4% | +0.0% | Dominant |
AUD = Australian Dollar; QALY = Quality Adjusted Life Year; ICER = Incremental Cost-Effectiveness Ratio; CHL = Conductive hearing loss; MHL = Mixed hearing loss; SSD = Single-sided deafness; AE = Adverse Event; SP = Sound Processor; CPI = Consumer Price Index.
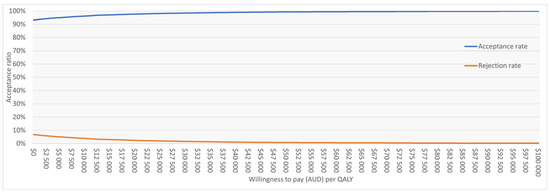
Figure A1.
Cost-effectiveness acceptability curve (CEAC) for Sentio versus Osia. Sentio shows >95% probability of being cost-effective at willingness-to-pay thresholds below AUD 50,000 per QALY. WTP = Willingness to Pay; QALY = Quality Adjusted Life Year.
Figure A1.
Cost-effectiveness acceptability curve (CEAC) for Sentio versus Osia. Sentio shows >95% probability of being cost-effective at willingness-to-pay thresholds below AUD 50,000 per QALY. WTP = Willingness to Pay; QALY = Quality Adjusted Life Year.
Appendix C. Results from External Validation

Table A5.
External validation results comparing outcomes from the Brunner et al. [21] model with those generated by the current analysis.
Table A5.
External validation results comparing outcomes from the Brunner et al. [21] model with those generated by the current analysis.
| External Validation Test | ICER (Model in This Article) | ICER (Model in Brunner et al. [21]) | ICER Deviation | Incremental Costs Change | Incremental QALYs Change |
|---|---|---|---|---|---|
| Test 1: Brunner parameters | 29,268 AUD/QALY | 29,301 AUD/QALY | −0.1% | +12% | +10% |
| Test 2: Sentio vs. Baha Attract (structural alignment) | 25,736 AUD/QALY | 29,301 AUD/QALY | −12% | +1% | +13% |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; AUD = Australian Dollars.
References
- Reinfeldt, S.; Hakansson, B.; Taghavi, H.; Eeg-Olofsson, M. New developments in bone-conduction hearing implants: A review. Med. Devices 2015, 8, 79–93. [Google Scholar] [CrossRef]
- Snik, A.F.; Mylanus, E.A.; Proops, D.W.; Wolfaardt, J.F.; Hodgetts, W.E.; Somers, T.; Niparko, J.K.; Wazen, J.J.; Sterkers, O.; Cremers, C.W.; et al. Consensus statements on the BAHA system: Where do we stand at present? Ann. Otol. Rhinol. Laryngol. Suppl. 2005, 195, 2–12. [Google Scholar] [CrossRef]
- Verhaert, N.; Desloovere, C.; Wouters, J. Acoustic hearing implants for mixed hearing loss: A systematic review. Otol. Neurotol. 2013, 34, 1201–1209. [Google Scholar] [CrossRef]
- Lagerkvist, H.; Carvalho, K.; Holmberg, M.; Petersson, U.; Cremers, C.; Hultcrantz, M. Ten years of experience with the Ponto bone-anchored hearing system—A systematic literature review. Clin. Otolaryngol. 2020, 45, 667–680. [Google Scholar] [CrossRef]
- Dun, C.A.; Faber, H.T.; de Wolf, M.J.; Mylanus, E.A.; Cremers, C.W.; Hol, M.K. Assessment of more than 1,000 implanted percutaneous bone conduction devices: Skin reactions and implant survival. Otol. Neurotol. 2012, 33, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Meyer, A.; Rivas, A.; Mowry, S.; Carvalho, D.; Chang, K.W.; Germiller, J.; Liu, Y.C.; Tejani, V. Outcomes Following Cochlear Osia 2 Implantation in Patients Ages 5-11 Years: A Multi-Center Trial. Laryngoscope 2025, 135, 2958–2966. [Google Scholar] [CrossRef]
- Sprinzl, G.; Lenarz, T.; Hagen, R.; Baumgartner, W.D.; Keintzel, T.; Keck, T.; Riechelmann, H.; Magele, A.; Salcher, R.; Maier, H.; et al. Long-Term, Multicenter Results with the First Transcutaneous Bone Conduction Implant. Otol. Neurotol. 2021, 42, 858–866. [Google Scholar] [CrossRef]
- Ordonez-Ordonez, L.; Caraballo, J.A.; Ortiz, J.G.C.; Eslait, F.G.; Saffon, R.J.; Lora, J.G.; Hernandez, S.; Guzman, J.; Rincon, L.A.; Buzo, B.C. Active Osseointegrated Steady-State Implant System: Surgical and Clinical Performance. Otol. Neurotol. 2025, 46, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Hol, M.K.S.; Aukema, T.W.; Ubbink, S.W.J.; Lindholm, D.; Holmberg, M.; Arndt, S.; Wolf, S.; Monksfield, P.; Marwa, M.; Lenarz, T.; et al. A Prospective, Multicenter Clinical Investigation on the Safety and Performance of a New Active Transcutaneous Bone-Anchored Implant System. Otol. Neurotol. 2025, 47, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choe, G.; Oh, H.; Choi, B.Y. A comparative study of audiological outcomes and compliance between the Osia system and other bone conduction hearing implants. Eur. Arch. Otorhinolaryngol. 2023, 280, 227–2224. [Google Scholar] [CrossRef]
- Lagerkvist, H.; Johansson, M.L. Whitepaper: The Sentio System—Almost Nothing to See, so Much to Experience; Oticon Medical AB: Askim, Sweden, 2024. [Google Scholar]
- Sikolova, S.; Urik, M.; Hosnova, D.; Kruntorad, V.; Bartos, M.; Motyka, O.; Jabandziev, P. Two Bonebridge bone conduction hearing implant generations: Audiological benefit and quality of hearing in children. Eur. Arch. Otorhinolaryngol. 2022, 279, 3387–3398. [Google Scholar] [CrossRef]
- Lein, A.; Baumgartner, W.D.; Landegger, L.D.; Riss, D.; Thurner, T.; Liu, D.T.; Kosec, A.; Vyskocil, E.; Brkic, F.F. A MAUDE database analysis on the new generation of active bone conduction hearing implants. Laryngoscope Investig. Otolaryngol. 2024, 9, e70010. [Google Scholar] [CrossRef]
- Lein, A.; Baumgartner, W.D.; Riss, D.; Gstottner, W.; Landegger, L.D.; Liu, D.T.; Thurner, T.; Vyskocil, E.; Brkic, F.F. Early Results with the New Active Bone-Conduction Hearing Implant: A Systematic Review and Meta-Analysis. Otolaryngol. Head. Neck Surg. 2024, 170, 1630–1647. [Google Scholar] [CrossRef]
- Jukic, A.; Munhall, C.C.; Stevens, S.M. A Systematic Review of Surgical Characteristics and Adverse Events of an Active, Transcutaneous Bone Conduction Device. Ann. Otol. Rhinol. Laryngol. 2024, 133, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Magele, A.; Schoerg, P.; Stanek, B.; Gradl, B.; Sprinzl, G.M. Active transcutaneous bone conduction hearing implants: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0221484. [Google Scholar] [CrossRef] [PubMed]
- Key, S.; Mohamed, N.; Da Cruz, M.; Kong, K.; Hasan, Z. Systematic Review and Meta-Analysis of a New Active Transcutaneous Bone Conduction Implant. Laryngoscope 2024, 134, 1531–1539. [Google Scholar] [CrossRef]
- Hearing Australia. Reflecting on a Year of Impact and Commitment to Hearing Health. 2024. Available online: https://www.hearing.com.au/news-and-articles/reflecting-on-a-year-of-impact-and-commitment-to-hearing-health/ (accessed on 7 October 2025).
- Deloitte Access Economics. The Social and Economic Cost of Hearing Loss in Australia. 2017. Available online: https://hcia.com.au/wp-content/uploads/2024/01/Social-and-Economic-Cost-of-Hearing-Health-in-Australia_June-2017.pdf (accessed on 4 February 2025).
- Department of Health Disability and Ageing. Prescribed List of Medical Devices and Human Tissue Products Guide—Draft. 2023. Available online: https://www.health.gov.au/resources/publications/prescribed-list-of-medical-devices-and-human-tissue-products-guide-draft (accessed on 4 February 2025).
- Brunner, M.; Schou, M.; Briggs, R.J.; Kingsford Smith, D. Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness. J. Mark. Access Health Policy 2024, 12, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Briggs, A.H.; Siebert, U.; Kuntz, K.M. ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—Overview: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med. Decis. Mak. 2012, 32, 667–677. [Google Scholar] [CrossRef]
- Medical Services Advisory Committee. Guidelines for Preparing Assessments for MSAC; Australian Government DoH, Disability and Ageing, Ed.; Medical Services Advisory Committee: Canberra, Australia, 2021. [Google Scholar]
- Caversaccio, M.; Wimmer, W.; Hoch, A.; Dejaco, T.; Schwab, B. Safety profiles of bone-conduction hearing implants revisited: A meta-analytic comparison adjusted for follow-up time. Eur. Arch. Otorhinolaryngol. 2025, 282, 5529–5538. [Google Scholar] [CrossRef]
- Reinfeldt, S.; Eeg-Olofsson, M.; Freden Jansson, K.J.; Persson, A.C.; Hakansson, B. Long-term follow-up and review of the Bone Conduction Implant. Hear. Res. 2022, 421, 108503. [Google Scholar] [CrossRef]
- Department of Health and Aged Care. Medicare Benefits Schedule (MBS). January 2025. Available online: https://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-250101 (accessed on 4 February 2025).
- Independent Health and Aged Care Pricing Authority (IHACPA). NHCDC Public Sector Cost Weights AR-DRG Version 11.0 2020-21. Available online: https://www.ihacpa.gov.au/resources/national-hospital-cost-data-collection-nhcdc-public-sector-report-2020-21 (accessed on 4 February 2025).
- Australian Bureau of Statistics (ABS). Medical Products, Appliances and Equipment; Australia, TABLE 7. CPI: Group, Sub-Group and Expenditure Class, Weighted Average of Eight Capital Cities. 2024. Available online: https://www.abs.gov.au/statistics/economy/price-indexes-and-inflation/consumer-price-index-australia/sep-quarter-2024#data-downloads (accessed on 10 October 2025).
- Briggs, R.; Birman, C.S.; Baulderstone, N.; Lewis, A.T.; Ng, I.H.Y.; Ostblom, A.; Rousset, A.; Tari, S.; Tong, M.C.F.; Cowan, R. Clinical Performance, Safety, and Patient-Reported Outcomes of an Active Osseointegrated Steady-State Implant System. Otol. Neurotol. 2022, 43, 827–834. [Google Scholar] [CrossRef]
- Rauch, A.K.; Wesarg, T.; Aschendorff, A.; Speck, I.; Arndt, S. Long-term data of the new transcutaneous partially implantable bone conduction hearing system Osia(R). Eur. Arch. Otorhinolaryngol. 2022, 279, 4279–4288. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Cismas, M.; Flores, N.; Stevens, S.M. Clinical Outcomes of an Active, Transcutaneous, Bone Conduction Hearing Device: A Retrospective Study. Otolaryngol. Head. Neck Surg. 2024, 171, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.D.S.; Danieli, F.; Hakansson, M.A.; Johansson, M.L.; Dos Santos, F.R.; Mirandola Barbosa Reis, A.C.; Hyppolito, M.A. Minimally invasive surgery as a new clinical standard for bone anchored hearing implants-real-world data from 10 years of follow-up and 228 surgeries. Front. Surg. 2023, 10, 1209927. [Google Scholar] [CrossRef]
- Dimitriadis, P.A.; Farr, M.R.; Allam, A.; Ray, J. Three year experience with the cochlear BAHA attract implant: A systematic review of the literature. BMC Ear Nose Throat Disord. 2016, 16, 12. [Google Scholar] [CrossRef]
- Hearing Aid Batteries Express. Zenipower 675. Available online: https://www.hearingaidbatteries.com.au/zenipower-675-60-cells.html (accessed on 5 February 2025).
- Hearing Aid Batteries Express. Zenipower 312. Available online: https://www.hearingaidbatteries.com.au/zenipower-312-60-cells.html (accessed on 5 February 2025).
- Department of Health and Aged Care. Prescribed List of Medical Devices and Human Tissue Products. November 2024. Available online: https://www.health.gov.au/resources/publications/prescribed-list-of-medical-devices-and-human-tissue-products?language=en (accessed on 4 February 2025).
- Oticon Medical Sentio 1 Mini—Product Information. 2024. Available online: https://sentio.oticonmedical.com/ (accessed on 10 October 2025).
- Cowan, R.; Lewis, A.T.; Hallberg, C.; Tong, M.C.F.; Birman, C.S.; Ng, I.H.; Briggs, R. Clinical performance, safety, and patient-reported outcomes of an active osseointegrated bone-conduction hearing implant system at 24-month follow-up. Eur. Arch. Otorhinolaryngol. 2024, 281, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Oticon Medical Ponto 5 Superpower—Product Information. 2024. Available online: https://www.oticonmedical.com/solutions/bone-conduction/ponto-5 (accessed on 10 October 2025).
- Teunissen, E.M.; Aukema, T.W.; Banga, R.; Eeg-Olofsson, M.; Hol, M.K.S.; Hougaard, D.D.; Tysome, J.R.; Johansson, M.L.; Svensson, S.; Powell, H.R.F. Evaluation of Clinical Performance of Ponto Implantation Using a Minimally Invasive Surgical Technique—A Prospective Multicenter Study. Otol. Neurotol. 2024, 45, 1037–1044. [Google Scholar] [CrossRef]
- den Besten, C.A.; Monksfield, P.; Bosman, A.; Skarzynski, P.H.; Green, K.; Runge, C.; Wigren, S.; Blechert, J.I.; Flynn, M.C.; Mylanus, E.A.M.; et al. Audiological and clinical outcomes of a transcutaneous bone conduction hearing implant: Six-month results from a multicentre study. Clin. Otolaryngol. 2019, 44, 144–157. [Google Scholar] [CrossRef]
- Cochlear Limited Datasheet Cochlear Baha 5 Power. 2020. Available online: https://cdn.hjm.aws.inera.se/83c26cb5-f5f2-4d28-8bdf-cc71b516e64a/8edf402c-6172-44ae-abee-98ba8816b098/Baha%205%20Power%20datasheet.pdf (accessed on 10 October 2025).
- Mitchell, P.; Gopinath, B.; Wang, J.J.; McMahon, C.M.; Schneider, J.; Rochtchina, E.; Leeder, S.R. Five-year incidence and progression of hearing impairment in an older population. Ear Hear. 2011, 32, 251–257. [Google Scholar] [CrossRef]
- Aukema, T.W.; Teunissen, E.M.; Janssen, A.M.; Hol, M.K.S.; Mylanus, E.A.M. Post-implantation clinical cost analysis between transcutaneous and percutaneous bone conduction devices. Eur. Arch. Otorhinolaryngol. 2024, 281, 117–127. [Google Scholar] [CrossRef]
- Gutierrez, J.A., 3rd; Shannon, C.M.; Nguyen, S.A.; Meyer, T.A.; Lambert, P.R. Comparison of Quality of Life Outcomes for Percutaneous Versus Transcutaneous Implantable Hearing Devices: A Systematic Review and Meta-analysis. Otol. Neurotol. 2024, 45, e129–e136. [Google Scholar] [CrossRef] [PubMed]
- Matza, L.S.; Kim, K.J.; Yu, H.; Belden, K.A.; Chen, A.F.; Kurd, M.; Lee, B.Y.; Webb, J. Health state utilities associated with post-surgical Staphylococcus aureus infections. Eur. J. Health Econ. 2019, 20, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.; Poole, C.D.; Odeyemi, I.; Retsa, P.; Chambers, C.; Currie, C.J. Deriving health state utilities for the numerical pain rating scale. Health Qual. Life Outcomes 2011, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Fasseeh, A.N.; Korra, N.; Elezbawy, B.; Sedrak, A.S.; Gamal, M.; Eldessouki, R.; Eldebeiky, M.; George, M.; Seyam, A.; Abourawash, A.; et al. Framework for developing cost-effectiveness analysis threshold: The case of Egypt. J. Egypt. Public Health Assoc. 2024, 99, 12. [Google Scholar] [CrossRef]
- CREST. Step by Step Guide to Economic Evaluation in Cancer Trials. Available online: https://www.uts.edu.au/globalassets/sites/default/files/2024-07/crest-factsheet_steps-in-an-economic-evaluation_june2024.pdf (accessed on 25 October 2025).
- Kruyt, I.J.; Monksfield, P.; Skarzynski, P.H.; Green, K.; Runge, C.; Bosman, A.; Blechert, J.I.; Wigren, S.; Mylanus, E.A.M.; Hol, M.K.S. Results of a 2-Year Prospective Multicenter Study Evaluating Long-term Audiological and Clinical Outcomes of a Transcutaneous Implant for Bone Conduction Hearing. Otol. Neurotol. 2020, 41, 901–911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.