- Article
Pulmonary Arterial Hypertension and Cancer: Unveiling Parallels in Epidemiology, Clinical Pathways, and Therapeutic Strategies
- Karim EI-Kersh,
- Nadine Zawadzki and
- Jason Shafrin
- + 5 authors
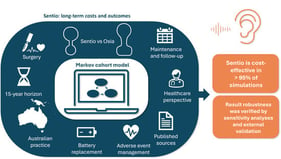
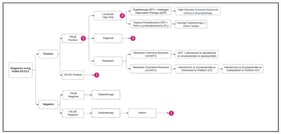
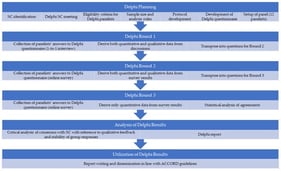
Pulmonary arterial hypertension (PAH) and cancer share high mortality and complex prognoses. Due to PAH’s rarity, these parallels may be underrecognized by healthcare stakeholders. This study explored similarities between PAH and cancer across epidemiological, clinical, therapeutic, and healthcare resource utilization (HCRU) considerations. A four-step approach was employed: (1) inclusion/exclusion criteria were applied to identify potential PAH cancer analogs; (2) characteristics for comparison were categorized as epidemiologic, clinical, therapeutic landscape, and HCRU; (3) a targeted literature review extracted data on disease characteristics; (4) a similarity ranking was calculated as the absolute difference between each cancer’s and PAH’s characteristics. Fourteen cancers met the inclusion criteria. Well-differentiated thyroid cancer (WDTC) had the highest number (5) of characteristics closest to PAH. WDTC and medullary thyroid cancer were most similar to PAH in epidemiology; gastrointestinal stromal tumor was most similar in clinical and HCRU characteristics, and anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer and renal cell carcinoma were most similar in therapeutic landscape. Although no single cancer fully mirrors PAH, the identification of multiple analogs underscores PAH’s multidimensional complexity and confirms its overlap with oncological conditions. Cancer analogs could serve as a valuable framework for enhancing recognition of PAH’s clinical, therapeutic, and HRCU implications among healthcare stakeholders.
6 February 2026