Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems
Abstract
1. Introduction
2. Chemical Structure and Physicochemical Properties
3. Alterations of HPMC Structure by Other Chemical Compounds
3.1. Alterations Produced by Ionic Salt
3.2. Chemical Interaction with Surfactants
- Ethane-1,2-diyl bis (N, N-dimethyl-N-hexadecylammoniumacetoxy) dichloride.
- Pentanediyl-1,5-bis dimethylcetylammonium bromide.
- Hexanediyl-1,6-bis dimethylcetylammonium bromide.
- Cetyltrimethylammonium bromide, a conventional surfactant, has been studied employing surface tension and rheology evaluation [45].
3.3. Incompatibility with Strong Acids and Bases
3.4. Interaction with Oxidizing Agents
4. HPMC Applications in Pharmaceutical Technology
4.1. Binding Properties
4.2. Film-Coating Material
4.3. Biological Adhesive
4.4. Gelling Agent
4.5. Encapsulation Material
- They are semi-synthetic in nature, derived from plant cellulose.
- No cross-linking issues.
- They are easier to swallow.
- They form a flexible film.
- Water does not act as a plasticiser for HPMC, exhibiting enhanced stability in various conditions (temperature, humidity).
- Their non-ionic nature makes them compatible with most of the commonly used excipients as well as APIs.
- They are preservative-free, allergen-free, starch-free, and gluten-free.
- The adhesion properties and optimal shell texture of HPMC films enable the application of a modified-release uniform coating.
4.6. Suspending Agent
4.7. Thickening Agent
4.8. Controlled-Release Polymer
5. HPMC as a Key Excipient in the Development of Coated Tablets
- It produces an aqueous polymeric film.
- It is an easily processable excipient.
- It produces a transparent, resistant, and flexible film that protects the core.
- It upgrades the tablet’s appearance by improving the shininess and providing a homogenous aspect that can be easily evaluated macroscopically.
- It improves the organoleptic properties (taste and smell).
- It provides protection of the API from the action of environmental factors (air, O2, temperature).
- It avoids incompatibilities by incorporating one ingredient in the core and one in the coating polymeric mixture.
- It facilitates the easy identification of the pharmaceutical product, improving administration efficacy.
- It has improved flowability, which is desired in the pharmaceutical industry to enhance the production yield.
- It has resistance to external factors such as abrasion [92].
- Disintegration ability.
- Diffusion of water through the pores.
- A diffusion and erosion combination for the different particle sizes of HPMC.
- Drug/HPMC ratio.
- Drug solubility.
- Compression force.
- HPMC viscosity grade.
- Spraying rate.
- Inlet air temperature.
- Residual moisture.
- Pan speed.
- Atomization process.
- Drug properties [93].
6. Hypromellose as a Film-Forming Agent
- The use of low molecular polymers (HPMC E3 instead of HPMC E50).
- The use of surfactants (usually, the disintegration time tends to improve by using hydrophilic surfactants such as Tween 80, but palatability needs to be considered, and the taste needs to be improved while using this type of excipient; Tween might have a bitter aftertaste).
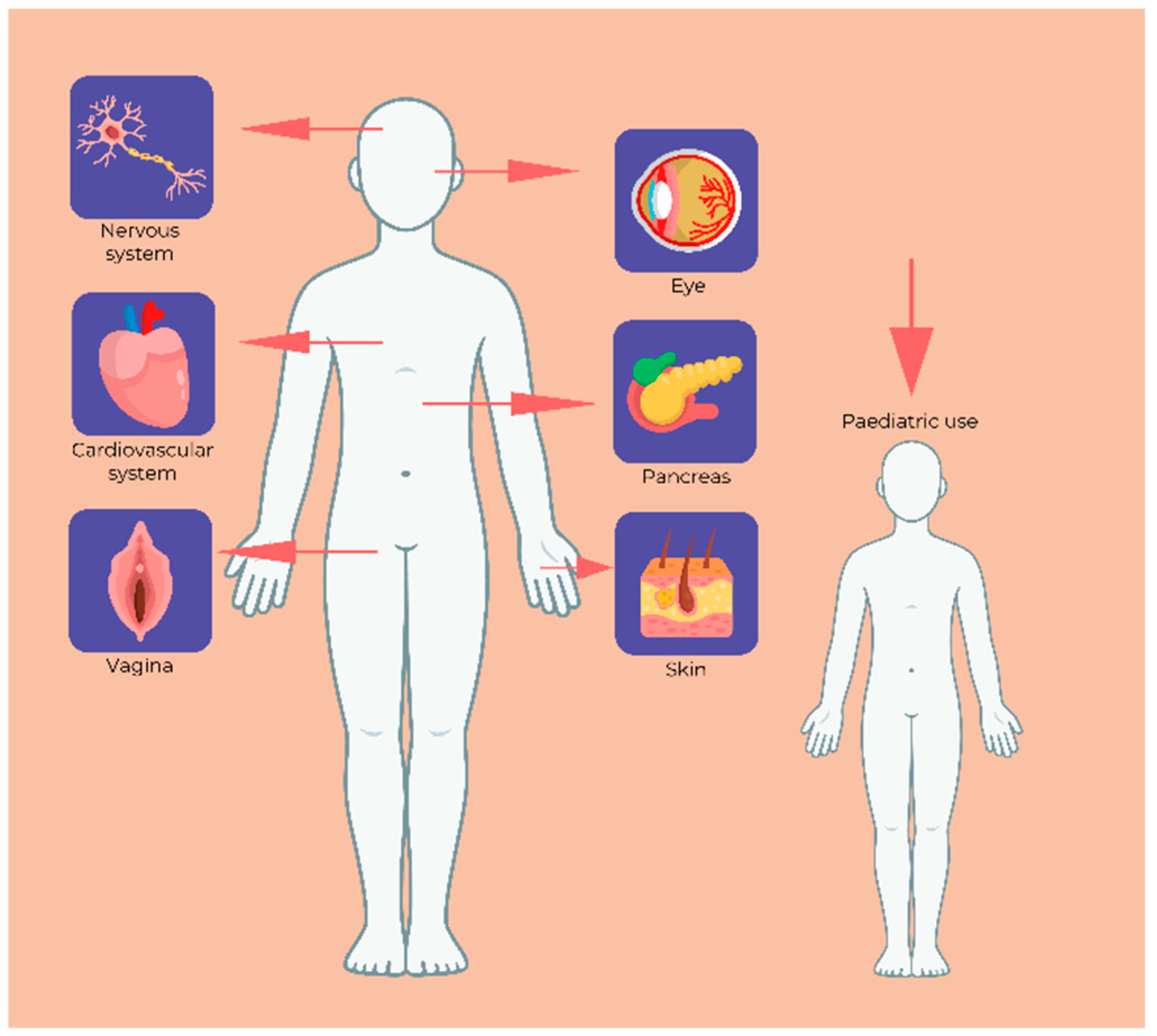
7. HPMC Formulations Used in the Medical Field
7.1. Biocompatibility
7.2. Ophthalmology
7.3. Diabetes
7.4. Neurology
7.5. Gynaecology
7.6. Paediatric Therapy
7.7. Dermatology and Dermato-Cosmetic Products
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Song, J.; Feng, H.; Wu, M.; Chen, L.; Xia, W.; Zhang, W. Preparation and Characterization of Arginine-Modified Chitosan/Hydroxypropyl Methylcellose Antibacterial Film. Int. J. Biol. Macromol. 2020, 145, 750–758. [Google Scholar] [CrossRef]
- Timmins, P.; Pygall, S.R.; Melia, C.D. Hydrophilic Matrix Dosage Forms: Definitions, General Attributes, and the Evolution of Clinical Utilization. In Hydrophilic Matrix Tablets for Oral Controlled Release; Timmins, P., Pygall, S.R., Melia, C.D., Eds.; Springer: New York, NY, USA, 2014; pp. 1–15. ISBN 978-1-4939-1519-4. [Google Scholar]
- Yu, X.; Liu, Q.; Jin, Z.; Jiao, A. Preparation and Characterization of Hydroxypropyl Methylcellulose/Hydroxypropyl Starch Composite Films Reinforced by Chitosan Nanoparticles of Different Sizes. Mater. Today Commun. 2023, 35, 105714. [Google Scholar] [CrossRef]
- Owusu-Ware, S.K.; Boateng, J.S.; Chowdhry, B.Z.; Antonijevic, M.D. Glassy State Molecular Mobility and Its Relationship to the Physico-Mechanical Properties of Plasticized Hydroxypropyl Methylcellulose (HPMC) Films. Int. J. Pharm. X 2019, 1, 100033. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-Micro Structure Characterization and Molecular Properties of Emulsion-Templated Polysaccharide Oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A Novel Electrospun Hydroxypropyl Methylcellulose/Polyethylene Oxide Blend Nanofibers: Morphology and Physicochemical Properties. Carbohydr. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef]
- NITIN; Chaudhary, V. A State of the Art an Exploration of Hpmc and Its Opportunities in the Construction Industry 2025. Available online: https://ssrn.com/abstract=5127042 (accessed on 1 June 2025). [CrossRef]
- Yu, J.Y.; Roh, S.H.; Park, H.J. Characterization of Ferulic Acid Encapsulation Complexes with Maltodextrin and Hydroxypropyl Methylcellulose. Food Hydrocoll. 2021, 111, 106390. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Zúñiga, R.N.; Osorio, F.; Pedreschi, F. Physical Properties of Emulsion-Based Hydroxypropyl Methylcellulose/Whey Protein Isolate (HPMC/WPI) Edible Films. Carbohydr. Polym. 2015, 123, 27–38. [Google Scholar] [CrossRef]
- Burdock, G.A. Safety Assessment of Hydroxypropyl Methylcellulose as a Food Ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 978-0-12-809261-3. [Google Scholar]
- De Simone, V.; Dalmoro, A.; Lamberti, G.; Caccavo, D.; d’Amore, M.; Barba, A.A. Effect of Binder and Load Solubility Properties on HPMC Granules Produced by Wet Granulation Process. J. Drug Deliv. Sci. Technol. 2019, 49, 513–520. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent Advances in Colon Drug Delivery Systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, X.; Jasti, B.R. Role of Physicochemical Properties of Some Grades of Hydroxypropyl Methylcellulose on in Vitro Mucoadhesion. Int. J. Pharm. 2021, 609, 121218. [Google Scholar] [CrossRef]
- Borandeh, S.; Van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric Drug Delivery Systems by Additive Manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Lim, C.; Song, Y.H.; Song, Y.; Seo, J.H.; Hwang, D.S.; Lee, D.W. Adaptive Amphiphilic Interaction Mechanism of Hydroxypropyl Methylcellulose in Water. Appl. Surf. Sci. 2021, 565, 150535. [Google Scholar] [CrossRef]
- Wang, L.; Dong, W.; Xu, Y. Synthesis and Characterization of Hydroxypropyl Methylcellulose and Ethyl Acrylate Graft Copolymers. Carbohydr. Polym. 2007, 68, 626–636. [Google Scholar] [CrossRef]
- Erothu, H.; Kumar, A.C. Hydrophilic Polymers. In Biomedical Applications of Polymeric Materials and Composites; Francis, R., Sakthi Kumar, D., Eds.; Wiley: Chichester, UK, 2016; pp. 163–185. ISBN 978-3-527-33836-8. [Google Scholar]
- Tundisi, L.L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Adden, R.; Müller, R.; Mischnick, P. Analysis of the Substituent Distribution in the Glucosyl Units and along the Polymer Chain of Hydroxypropylmethyl Celluloses and Statistical Evaluation. Cellulose 2006, 13, 459–476. [Google Scholar] [CrossRef]
- Lismeri, L.; Karina, A.P.; Taharuddin, T.; Darni, Y.; Azhar, A.; Arfiana, A.; Sudibyo, S. Production of Hydroxypropyl Methylcellulose (HPMC) from α-Cellulose Derived from Cassava Stems. In Proceedings of the 1st International Conference on Industry Science Technology and Sustainability (IConISTS 2023), Bandar Lampung, Indonesia, 11–12 October 2023; Zakaria, A., Herison, A., Anwar, H., Sari, D.K., Chairani, Z., Khadafi, F., Eds.; Advances in Engineering Research. Atlantis Press International BV: Dordrecht, The Netherlands, 2024; Volume 235, pp. 68–80. [Google Scholar]
- Yuan, K.; Zhao, Y.; Hu, Q.; Liu, M.; Li, D.; Zheng, H. Controllable Synthesis and Rheological Characterization of Hydroxypropyl Methyl Cellulose. J. Polym. Environ. 2024, 32, 5142–5156. [Google Scholar] [CrossRef]
- Khwaldia, K. Physical and Mechanical Properties of Hydroxypropyl Methylcellulose–Coated Paper as Affected by Coating Weight and Coating Composition. BioResources 2013, 8, 3438–3452. [Google Scholar] [CrossRef]
- Chiaregato, C.G.; Bernardinelli, O.D.; Shavandi, A.; Sabadini, E.; Petri, D.F.S. The Effect of the Molecular Structure of Hydroxypropyl Methylcellulose on the States of Water, Wettability, and Swelling Properties of Cryogels Prepared with and without CaO2. Carbohydr. Polym. 2023, 316, 121029. [Google Scholar] [CrossRef]
- Olechno, K.; Basa, A.; Winnicka, K. “Success Depends on Your Backbone”—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef]
- Perez-Robles, S.; Carotenuto, C.; Minale, M. Effect on the Thermo-Gelation Process of the Degree and Molar Substitution of HPMC Polymer Hydrogels. Macromol. Symp. 2022, 405, 2100277. [Google Scholar] [CrossRef]
- Hypromellose, Orodispersible Tablets, 2.9.1 Disintegration of Tablets and Capsules. In European Pharmacopoeia; Ph. Eur.; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2022; pp. 5657–5658.
- Ford, J.L. Design and Evaluation of Hydroxypropyl Methylcellulose Matrix Tablets for Oral Controlled Release: A Historical Perspective. In Hydrophilic Matrix Tablets for Oral Controlled Release; Timmins, P., Pygall, S.R., Melia, C.D., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014; Volume 16, pp. 17–51. ISBN 978-1-4939-1518-7. [Google Scholar]
- Marani, P.L.; Bloisi, G.D.; Petri, D.F.S. Hydroxypropylmethyl Cellulose Films Crosslinked with Citric Acid for Control Release of Nicotine. Cellulose 2015, 22, 3907–3918. [Google Scholar] [CrossRef]
- METHOCELTM-Industrial Cellulosics by IFF. Available online: https://www.industrialcellulosics.com/products/methocel (accessed on 7 June 2025).
- Pinheiro, V.A.; Kaneko, T.M.; Velasco, M.V.R.; Consiglieri, V.O. Development and in Vitro Evaluation of Extended-Release Theophylline Matrix Capsules. Rev. Bras. Cienc. Farm. 2007, 43, 253–261. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The Role of Oral Controlled Release Matrix Tablets in Drug Delivery Systems. BioImpacts 2012, 2, 175–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levina, M.; Rajabi-Siahboomi, A.R. An Industrial Perspective on Hydrophilic Matrix Tablets Based on Hyproxypropyl Methylcellulose (Hypromellose). In Hydrophilic Matrix Tablets for Oral Controlled Release; Timmins, P., Pygall, S.R., Melia, C.D., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014; Volume 16, pp. 53–85. ISBN 978-1-4939-1518-7. [Google Scholar]
- Krese, A.; Kovačič, N.N.; Kapele, T.; Mrhar, A.; Bogataj, M. Influence of Ionic Strength and HPMC Viscosity Grade on Drug Release and Swelling Behavior of HPMC Matrix Tablets. J. Appl. Polym. Sci. 2016, 133, app.43604. [Google Scholar] [CrossRef]
- Celotech | Viscosity Properties of HPMC. Available online: https://www.celotech.com/news/viscosity-properties-of-hydroxypropyl-methylcellulose-hpmc/ (accessed on 6 June 2025).
- Jin, C.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Zhao, L.; Feng, Y. Updates on Applications of Low-Viscosity Grade Hydroxypropyl Methylcellulose in Coprocessing for Improvement of Physical Properties of Pharmaceutical Powders. Carbohydr. Polym. 2023, 311, 120731. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose–A Traditional Pharmaceutical Excipient with Modern Applications in Oral and Oromucosal Drug Delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- PHARMACOAT® | For Pharmaceutical | Shin-Etsu. Available online: https://www.metolose.jp/en/pharmaceutical/tc-5.html (accessed on 6 June 2025).
- BenecelTM Methylcellulose and Hypromellose. Available online: https://www.ashland.com/industries/pharmaceutical/oral-solid-dose/benecel-methylcellulose-and-hypromellose (accessed on 6 June 2025).
- Lotte-Cellulose. Available online: https://www.lotte-cellulose.com/product/anycoat/overview/ (accessed on 6 June 2025).
- Mori, D.; Dudhat, K.; Soniwala, M.; Parmar, R.; Suthar, D.; Jayani, R.; Shah, S.; Borkhataria, C.; Patel, K.; Dudhrejiya, A. Polymers Used in Pharmaceutical Industry for Oral Delivery: Insight to Synthesis, Structure–Activity Relationship, and Recent Applications. Polym. Bull. 2024, 81, 16373–16413. [Google Scholar] [CrossRef]
- Almeida, N.; Rakesh, L.; Zhao, J. Monovalent and Divalent Salt Effects on Thermogelation of Aqueous Hypromellose Solutions. Food Hydrocoll. 2014, 36, 323–331. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kamil, M.; Panda, M. Surfactant-Polymer Interaction: Effect of Hydroxypropylmethyl Cellulose on the Surface and Solution Properties of Gemini Surfactants. Colloid. Polym. Sci. 2018, 296, 1879–1889. [Google Scholar] [CrossRef]
- Kraisit, P.; Hirun, N.; Limpamanoch, P.; Sawaengsuk, Y.; Janchoochai, N.; Manasaksirikul, O.; Limmatvapirat, S. Effect of Cremophor RH40, Hydroxypropyl Methylcellulose, and Mixing Speed on Physicochemical Properties of Films Containing Nanostructured Lipid Carriers Loaded with Furosemide Using the Box–Behnken Design. Polymers 2024, 16, 1605. [Google Scholar] [CrossRef]
- Rede, K.; Felicijan, T.; Bogataj, M. Exploring the Unexpected Behavior of HPMC Matrix Tablets in Dissolution Media with SDS. J. Drug Deliv. Sci. Technol. 2021, 66, 102801. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Khosrowshahi Asl, A. Effect of Various Additives on the Properties of the Films and Coatings Derived from Hydroxypropyl Methylcellulose—A Review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef]
- Punitha, S.; Uvarani, R.; Panneerselvam, A. Effect of pH in Aqueous (Hydroxy Propyl Methyl Cellulose) Polymer Solution. Results Mater. 2020, 7, 100120. [Google Scholar] [CrossRef]
- Hydroxypropyl Methyl Cellulose. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3225318.htm (accessed on 6 June 2025).
- Zupanc, A.; Petkovšek, M.; Zdovc, B.; Žagar, E.; Zupanc, M. Degradation of Hydroxypropyl Methylcellulose (HPMC) by Acoustic and Hydrodynamic Cavitation. Ultrason. Sonochemistry 2024, 109, 107020. [Google Scholar] [CrossRef]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Biopharmaceutical Understanding of Excipient Variability on Drug Apparent Solubility Based on Drug Physicochemical Properties: Case Study—Hypromellose (HPMC). AAPS J. 2020, 22, 49. [Google Scholar] [CrossRef]
- Tank, D.; Karan, K.; Gajera, B.Y.; Dave, R.H. Investigate the Effect of Solvents on Wet Granulation of Microcrystalline Cellulose Using Hydroxypropyl Methylcellulose as a Binder and Evaluation of Rheological and Thermal Characteristics of Granules. Saudi Pharm. J. 2018, 26, 593–602. [Google Scholar] [CrossRef]
- Sahoo, C.K.; Rao, S.R.M.; Sudhakar, M. HPMC a Biomedical Polymer in Pharmaceutical Dosage Forms. J. Chem. Pharm. Sci. 2015, 8, 875–881. [Google Scholar]
- Fristiohady, A.; Suryani, S.; Fitrawan, L.O.M.; Andriani, R.; Aspadiah, V.; Saripuddin, S.; Purnama, L.O.M.J.; Hamsidi, R.; Indalifiany, A. Characterization, Formulation, and Evaluation of Tablet Containing Johar Leaves (Cassia siamea Lamk.) Extract with Hydroxypropyl Methylcellulose (HPMC) as Tablet Binder. Lett. Appl. NanoBioSci. 2020, 10, 2070–2077. [Google Scholar] [CrossRef]
- Fernández-Cancelo, P.; Giné-Bordonaba, J.; Pérez-Gago, M.B.; Palou, L.; Torres, R.; Echeverria, G.; Teixidó, N. A Hydroxypropyl Methylcellulose (HPMC)-Based Coating Inhibits Ethylene-Dependent Quality Changes and Reduces Superficial Scald Incidence and Blue Mould Severity during Postharvest Handling of Two Apple Varieties. Postharvest Biol. Technol. 2024, 207, 112610. [Google Scholar] [CrossRef]
- Bharadia, D.P.D.; Pandya, D.V.M. A Review on Aqueous Film Coating Technology. Indian J. Pharm. Pharmacol. 2014, 1, 65–106. [Google Scholar]
- Kwok, T.S.H.; Sunderland, B.V.; Heng, P.W.S. An Investigation on the Influence of a Vinyl Pyrrolidone/Vinyl Acetate Copolymer on the Moisture Permeation, Mechanical and Adhesive Properties of Aqueous-Based Hydroxypropyl Methylcellulose Film Coatings. Chem. Pharm. Bull. 2004, 52, 790–796. [Google Scholar] [CrossRef]
- Hiremath, P.; Nuguru, K.; Agrahari, V. Material Attributes and Their Impact on Wet Granulation Process Performance. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–315. ISBN 978-0-12-810460-6. [Google Scholar]
- Iswandana, R.; Lestari, D.A.T.; Cholimi, S. Combination of HPMC and Peg 400 as a Taste Masking Agent of Film-Coated Tablets Containing Momordica charantia Linn Extract. Int. J. App. Pharm. 2018, 10, 8–12. [Google Scholar] [CrossRef]
- Abbaspour, M.A.; Sharif Makhmalzadeh, B.S.M.; Jalali, S.J. Study of Free-Films and Coated Tablets Based on Hpmc and Microcrystalline Cellulose, Aimed for Improve Stability of Moisture-Sensitive Drugs. Jundishapur J. Nat. Pharm. Prod. 2010, 5, 6–17. [Google Scholar]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric Acid Crosslinked Cyclodextrin/Hydroxypropylmethylcellulose Hydrogel Films for Hydrophobic Drug Delivery. Int. J. Biol. Macromol. 2016, 93, 75–86. [Google Scholar] [CrossRef]
- Pan, P.; Svirskis, D.; Waterhouse, G.I.N.; Wu, Z. Hydroxypropyl Methylcellulose Bioadhesive Hydrogels for Topical Application and Sustained Drug Release: The Effect of Polyvinylpyrrolidone on the Physicomechanical Properties of Hydrogel. Pharmaceutics 2023, 15, 2360. [Google Scholar] [CrossRef]
- Mady, O.Y.; Dewedar, O.; Abdine, N.; Zaytoon, H.; Haggag, Y. Bioadhesive Behaviors of HPMC E5: Comparative Analysis of Various Techniques, Histological and Human Radiological Evidence. Sci. Rep. 2024, 14, 1840. [Google Scholar] [CrossRef]
- Peh, K.K.; Wong, C.F. Polymeric Films as Vehicle for Buccal Delivery: Swelling, Mechanical, and Bioadhesive Properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar]
- De Araujo, G.R.S.; Porfírio, L.D.O.; Silva, L.A.S.; Santana, D.G.; Barbosa, P.F.; Dos Santos, C.P.; Narain, N.; Sarmento, V.H.V.; Nunes, R.D.S.; Ting, E.; et al. In Situ Microemulsion-Gel Obtained from Bioadhesive Hydroxypropyl Methylcellulose Films for Transdermal Administration of Zidovudine. Colloids Surf. B Biointerfaces 2020, 188, 110739. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Singh, R. Formulation and Evaluation of Aloe Vera Topical Gels. Int. J. Pharm. Sci. 2010, 2, 551–555. [Google Scholar]
- Rohmani, S.; Yugatama, A.; Wijayanti, I.; Ermawati, D.E.; Artanti, A.N.; Kundarto, W.; Zulpadly, M.F. Characteristics of Eugenol Products and in Vitro Release in Gel Base With Hydroxypropyl Methylcellulose (HPMC) Variant as Gelling Agent. Int. J. App. Pharm. 2021, 13, 106–111. [Google Scholar] [CrossRef]
- Noval, N.; Rosyifa, R.; Annisa, A. Effect of HPMC Concentration Variation as Gelling Agent on Physical Stability of Formulation Gel Ethanol Extract Bundung Plants (Actinuscirpus Grossus). In Proceedings of the Proceedings of the Proceedings of the First National Seminar Universitas Sari Mulia (NS-UNISM 2019), Banjarmasin, South Kalimantan, Indonesia, 23 November 2019; EAI: Banjarmasin, Indonesia, 2020. [Google Scholar]
- Tanwar, Y.S.; Jain, A.K. Formulation and Evaluation of Topical Diclofenac Sodium Gel Using Different Gelling Agent. Asian J. Pharm. Res. Health Care 2012, 4, 1–6. [Google Scholar]
- Daood, N.M.; E Jassim, Z.; M Gareeb, M.; Zeki, H. Studying the Effect of Different Gelling Agent on the Preparation and Characterization of Metronidazole as Topical Emulgel. Asian. J. Pharm. Clin. Res. 2019, 12, 571–577. [Google Scholar] [CrossRef]
- Nursal, F.K.; Nining; Rahmah, A.S. Formulation and Development of Grape Seed Oil (Vitis vinifera L) Emulgel Peel-Off Mask Using Gelling Agent Hydroxy Propyl Methyl Cellulose (HPMC). IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012046. [Google Scholar] [CrossRef]
- Tuleu, C.; Khela, M.K.; Evans, D.F.; Jones, B.E.; Nagata, S.; Basit, A.W. A Scintigraphic Investigation of the Disintegration Behaviour of Capsules in Fasting Subjects: A Comparison of Hypromellose Capsules Containing Carrageenan as a Gelling Agent and Standard Gelatin Capsules. Eur. J. Pharm. Sci. 2007, 30, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Barham, A.S.; Tewes, F.; Healy, A.M. Moisture Diffusion and Permeability Characteristics of Hydroxypropylmethylcellulose and Hard Gelatin Capsules. Int. J. Pharm. 2015, 478, 796–803. [Google Scholar] [CrossRef]
- Hadi, M.; Rao, N.G.; Avanapu, S.R.; Shiva, G.; Akram, J.W. Impact of Capsules as a Carrier for Multiple Unit Drug Delivery and the Importance of HPMC Capsules. Res. J. Pharm. Technol. 2013, 6, 34–43. [Google Scholar]
- Yang, N.; Chen, H.; Jin, Z.; Hou, J.; Zhang, Y.; Han, H.; Shen, Y.; Guo, S. Moisture Sorption and Desorption Properties of Gelatin, HPMC and Pullulan Hard Capsules. Int. J. Biol. Macromol. 2020, 159, 659–666. [Google Scholar] [CrossRef]
- Faulhammer, E.; Kovalcik, A.; Wahl, V.; Markl, D.; Stelzer, F.; Lawrence, S.; Khinast, J.G.; Paudel, A. Multi-Methodological Investigation of the Variability of the Microstructure of HPMC Hard Capsules. Int. J. Pharm. 2016, 511, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Al-Tabakha, M.M. HPMC Capsules: Current Status and Future Prospects. J. Pharm. Pharm. Sci. 2010, 13, 428. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Biswas, G.R. HPMC as Capsule Shell Material: Physicochemical, Pharmaceutical and Biopharmaceutical Properties. Int. J. Pharm. Pharm. Sci. 2017, 9, 1. [Google Scholar] [CrossRef]
- Wu, H.; Du, S.; Lu, Y.; Li, Y.; Wang, D. The Application of Biomedical Polymer Material Hydroxy Propyl Methyl Cellulose(HPMC) in Pharmaceutical Preparations. J. Chem. Pharm. Res. 2014, 6, 155–160. [Google Scholar]
- Wang, H.; Sun, Y.; Yang, B.; Li, S. Association between the Physical Stability of Flurbiprofen Suspension and the Interaction of HPMC/SDS. Asian J. Pharm. Sci. 2018, 13, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tagliari, M.; Stulzer, H.; Assreuy, J.; Bresolin, T.; Silva, M. Evaluation of Physicochemical Characteristics of Suspensions Containing Hydrochlorothiazide Developed for Pediatric Use. Lat. Am. J. Pharm. 2009, 28, 734–740. [Google Scholar]
- Murthy, P.; Devi, M.; Sahoo, S.K.; Mahapatra, A.K.; Khandai, M. Rapid Assessment of Sedimentation Stability in Paracetamol Suspension Formulations with Different Suspending Agents Using near Infrared Transmission Measurements. J. Chem. Pharm. Res. 2015, 7, 186–195. [Google Scholar]
- Stolić-Jovanović, A.; Martinović, M.; Nešić, I. The Influence of Selected Thickeners on the Textural Properties of Oil-in-Water Emulsions. Acta Fac. Medic. Naissensis 2022, 39, 57–65. [Google Scholar] [CrossRef]
- El-Kamel, A.H. In Vitro and in Vivo Evaluation of Pluronic F127-Based Ocular Delivery System for Timolol Maleate. Int. J. Pharm. 2002, 241, 47–55. [Google Scholar] [CrossRef]
- Zhou, D.; Law, D.; Reynolds, J.; Davis, L.; Smith, C.; Torres, J.L.; Dave, V.; Gopinathan, N.; Hernandez, D.T.; Springman, M.K.; et al. Understanding and Managing the Impact of HPMC Variability on Drug Release from Controlled Release Formulations. J. Pharm. Sci. 2014, 103, 1664–1672. [Google Scholar] [CrossRef]
- Martínez González, I.; Villafuerte Robles, L. Influence of Enteric Citric Acid on the Release Profile of 4-Aminopyridine from HPMC Matrix Tablets. Int. J. Pharm. 2003, 251, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous Twin Screw Granulation of Controlled Release Formulations with Various HPMC Grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Pani, N.R.; Nath, L.K. Development of Controlled Release Tablet by Optimizing HPMC: Consideration of Theoretical Release and RSM. Carbohydr. Polym. 2014, 104, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Kitamura, S.; Kitagawa, T.; Terada, K. Dissolution Mechanism of Poorly Water-Soluble Drug from Extended Release Solid Dispersion System with Ethylcellulose and Hydroxypropylmethylcellulose. Int. J. Pharm. 2005, 302, 95–102. [Google Scholar] [CrossRef]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Biopharmaceutical Aspects and Implications of Excipient Variability in Drug Product Performance. Eur. J. Pharm. Biopharm. 2017, 111, 1–15. [Google Scholar] [CrossRef]
- Mohamed, F.A.A.; Roberts, M.; Seton, L.; Ford, J.L.; Levina, M.; Rajabi-Siahboomi, A.R. The Effect of HPMC Particle Size on the Drug Release Rate and the Percolation Threshold in Extended-Release Mini-Tablets. Drug Dev. Ind. Pharm. 2015, 41, 70–78. [Google Scholar] [CrossRef]
- Seo, K.-S.; Bajracharya, R.; Lee, S.H.; Han, H.-K. Pharmaceutical Application of Tablet Film Coating. Pharmaceutics 2020, 12, 853. [Google Scholar] [CrossRef]
- Wang, J.; Hemenway, J.; Chen, W.; Desai, D.; Early, W.; Paruchuri, S.; Chang, S.-Y.; Stamato, H.; Varia, S. An Evaluation of Process Parameters to Improve Coating Efficiency of an Active Tablet Film-Coating Process. Int. J. Pharm. 2012, 427, 163–169. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, D.-W.; Kuk, Y.-M.; Park, C.-W.; Rhee, Y.-S.; Oh, T.-O.; Weon, K.-Y.; Park, E.-S. Investigation of an Active Film Coating to Prepare New Fixed-Dose Combination Tablets for Treatment of Diabetes. Int. J. Pharm. 2012, 427, 201–208. [Google Scholar] [CrossRef]
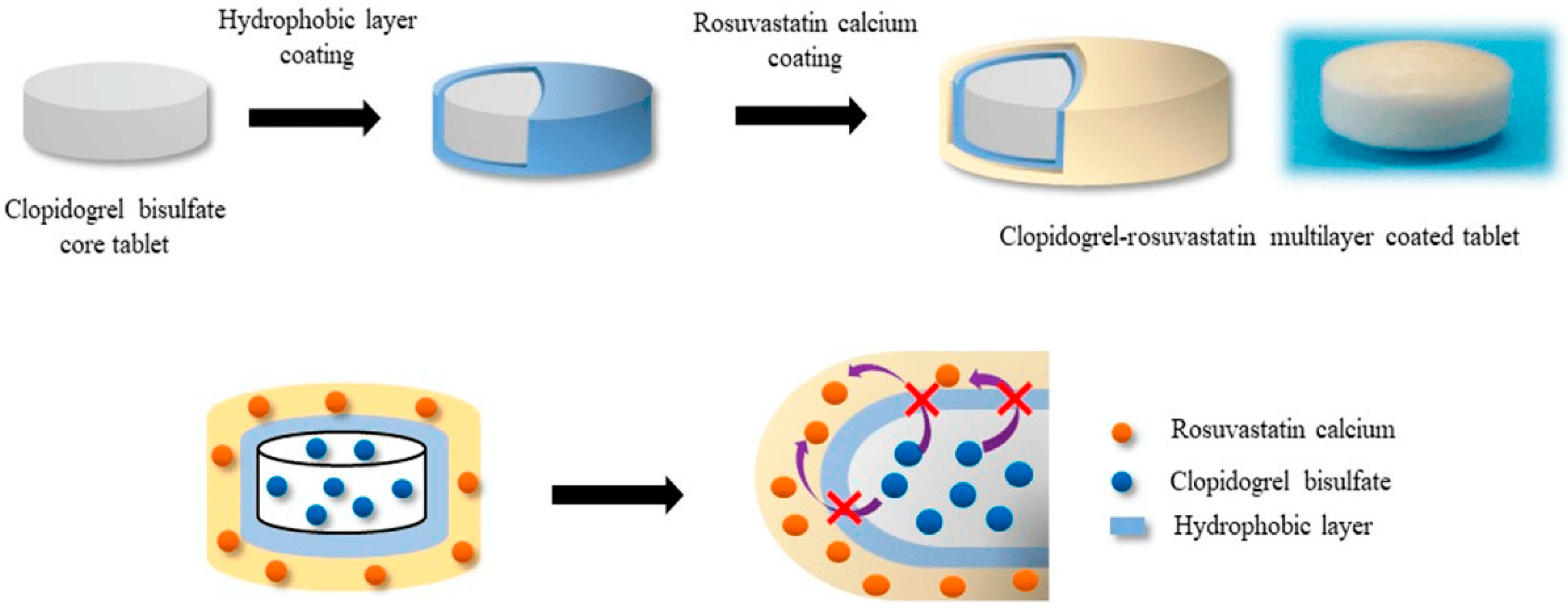
- Seo, K.-S.; Han, H.-K. Multilayer-Coated Tablet of Clopidogrel and Rosuvastatin: Preparation and In Vitro/In Vivo Characterization. Pharmaceutics 2019, 11, 313. [Google Scholar] [CrossRef]
- Silva, P.C.D.; Colucci, L.A.; Silva, L.L.D.; Molina, C.; Duque, M.D.; Rodrigues, L.N.C. Mechanical, Optical, and Physicochemical Properties of HPMC-Based Doxazosin Mesylate Orodispersible Films. Braz. J. Pharm. Sci. 2023, 59, e21114. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Aodsab, N.; Promyos, P.; Panraksa, P.; Udomsom, S.; Jantrawut, P. Fabrication of Hydroxypropyl Methylcellulose Orodispersible Film Loaded Mirtazapine Using a Syringe Extrusion 3D Printer. Sci. Pharm. 2022, 90, 68. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. Disintegration. In USP-NF; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2022. [Google Scholar]
- Ferlak, J.; Guzenda, W.; Osmałek, T. Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics 2023, 15, 361. [Google Scholar] [CrossRef]
- 3D Printing of Orodispersible Film for Poorly Soluble Drug. Available online: https://www.roquette.com/innovation-hub/pharma/technical-illustration/3d-printing-of-orodispersible-film-for-poorly-soluble-drug (accessed on 6 June 2025).
- Jadach, B.; Misek, M.; Ferlak, J. Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels 2023, 9, 687. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Pintea, A.; Coaicea, M.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Ciurba, A. Preparation and Evaluation of Caffeine Orodispersible Films: The Influence of Hydrotropic Substances and Film-Forming Agent Concentration on Film Properties. Polymers 2023, 15, 2034. [Google Scholar] [CrossRef]
- Vlad, R.-A. Structural and Thermal Analysis of Cannabidiol Orodispersible Formulations. Farmacia 2021, 69, 426–433. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Antonoaea, P.; Todoran, N.; Muntean, D.-L.; Rédai, E.M.; Silași, O.A.; Tătaru, A.; Bîrsan, M.; Imre, S.; Ciurba, A. Pharmacotechnical and Analytical Preformulation Studies for Cannabidiol Orodispersible Tablets. Saudi Pharm. J. 2021, 29, 1029–1042. [Google Scholar] [CrossRef]
- Vlad, R.-A. Obţinerea de Preparate Orodispersabile Cu Canabidiol; University Press: Târgu Mureş, Romania, 2022. [Google Scholar]
- Hussain, A.; Ahmad, Z.; Mahmood, A.; Shchinar, S.; Khan, M.I.; Akhtar, A.; Qavi, Y.; Siddique, W. Nystatin, Aspirin and Lidocaine Containing Orodispersible Films for Pronounced Drug Delivery: Development and Characterization. BioNanoScience 2024, 14, 2391–2402. [Google Scholar] [CrossRef]
- Khan, Q.; Siddique, M.I.; Rasool, F.; Naeem, M.; Usman, M.; Zaman, M. Development and Characterization of Orodispersible Film Containing Cefixime Trihydrate. Drug Dev. Ind. Pharm. 2020, 46, 2070–2080. [Google Scholar] [CrossRef]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef]
- Aswathy, K.V.; Beulah, K.C.; Nalina, M.; Ambedkar, D.S.; Sairam, A.L.; Priyadarshini, P.; Panneerselvam, A.; Rao, P.J. Hydroxypropyl Methylcellulose Stabilized Clove Oil Nanoemulsified Orodispersible Films: Study of Physicochemical Properties, Release Profile, Mucosal Permeation, and Anti-Bacterial Activity. Int. J. Biol. Macromol. 2024, 283, 137577. [Google Scholar] [CrossRef] [PubMed]
- ElMeshad, A.N.; El Hagrasy, A.S. Characterization and Optimization of Orodispersible Mosapride Film Formulations. AAPS PharmSciTech 2011, 12, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hayakawa, F.; Takeuchi, H. Formulation Design of Orally Disintegrating Film Using Two Cellulose Derivatives as a Blend Polymer. Pharmaceutics 2025, 17, 84. [Google Scholar] [CrossRef]
- Woertz, C.; Kleinebudde, P. Development of Orodispersible Polymer Films Containing Poorly Water Soluble Active Pharmaceutical Ingredients with Focus on Different Drug Loadings and Storage Stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Rédai, E.-M.; Antonoaea, P.; Todoran, N.; Vlad, R.A.; Bîrsan, M.; Tătaru, A.; Ciurba, A. Development and Evaluation of Fluoxetine Fast Dissolving Films: An Alternative for Noncompliance in Pediatric Patients. Processes 2021, 9, 778. [Google Scholar] [CrossRef]
- Shen, B.; Shen, C.; Yuan, X.; Bai, J.; Lv, Q.; Xu, H.; Dai, L.; Yu, C.; Han, J.; Yuan, H. Development and Characterization of an Orodispersible Film Containing Drug Nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 1348–1356. [Google Scholar] [CrossRef]
- Arpa, M.D.; Seçen, İ.M.; Erim, Ü.C.; Hoş, A.; Üstündağ Okur, N. Azelaic Acid Loaded Chitosan and HPMC Based Hydrogels for Treatment of Acne: Formulation, Characterization, in Vitro-Ex Vivo Evaluation. Pharm. Dev. Technol. 2022, 27, 268–281. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S.; Alany, R.G.; Nokhodchi, A. Polymeric Inserts Containing Eudragit® L100 Nanoparticle for Improved Ocular Delivery of Azithromycin. Biomedicines 2020, 8, 466. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Popescu, R.; Irimia, T.; Dinu-Pîrvu, C.-E. Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes 2021, 9, 1694. [Google Scholar] [CrossRef]
- Jaipal, A.; Pandey, M.M.; Charde, S.Y.; Raut, P.P.; Prasanth, K.V.; Prasad, R.G. Effect of HPMC and Mannitol on Drug Release and Bioadhesion Behavior of Buccal Discs of Buspirone Hydrochloride: In-Vitro and in-Vivo Pharmacokinetic Studies. Saudi Pharm. J. 2015, 23, 315–326. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In Situ Ophthalmic Gel Forming Systems of Poloxamer 407 and Hydroxypropyl Methyl Cellulose Mixtures for Sustained Ocular Delivery of Chloramphenicole: Optimization Study by Factorial Design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Shahien, M.M.; El-Horany, H.E.-S.; Ahmed, E.H.; El-Nahas, H.M.; Abdulla, N.A.; Ibrahim, T.M. Evaluation of Mucoadhesive Nano-Bilosomal In Situ Gels Containing Anti-Psychotic Clozapine for Treatment of Schizophrenia: In Vitro and In Vivo Studies. Pharmaceuticals 2024, 17, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, V.R.; Dahiya, L.; Sarwal, A. Transdermal Delivery of Duloxetine-Sulfobutylether-β-Cyclodextrin Complex for Effective Management of Depression. Int. J. Pharm. 2021, 594, 120129. [Google Scholar] [CrossRef] [PubMed]
- Jaimini, R.; Jaimini, M. Formulation and Optimization of Gastro-Retentive Floating Tablets of Enalapril Maleate and Losartan for Enhanced Bioavailability and Therapeutic Efficacy. Trop. J. Pharm. Life Sci. 2025, 12, 1–10. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Kedar, A.; Yadav, K.S.; Krishna, A.; Akhir, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. Chitosan/HPMC-Based Mucoadhesive Film Co-Loaded with Fluconazole and Ofloxacin for Management of Polymicrobial Keratitis. Int. J. Biol. Macromol. 2022, 222, 2785–2795. [Google Scholar] [CrossRef]
- Sampathi, S.; Prajapati, S.; Junnuthula, V.; Dyawanapelly, S. Pharmacokinetics and Anti-Diabetic Studies of Gliclazide Nanosuspension. Pharmaceutics 2022, 14, 1947. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, X.; Liu, T.; Lu, X.; Qiu, G.; Shea, K.J. Synthesis of Surfactant-Free Hydroxypropyl Methylcellulose Nanogels for Controlled Release of Insulin. Carbohydr. Polym. 2016, 151, 1006–1011. [Google Scholar] [CrossRef]
- Bhairam, M.; Prasad, J.; Verma, K.; Jain, P.; Gidwani, B. Formulation of Transdermal Patch of Losartan Potassium & Glipizide for the Treatment of Hypertension & Diabetes. Mater. Today Proc. 2023, 83, 59–68. [Google Scholar] [CrossRef]
- Fitriani, L.; Abdillah, R.; Ben, E.S. Formulation of Metformin HCl Floating Tablet Using HPC, HPMC K100M, and the Combinations. J. Sains. Far. Klin 2017, 4, 79. [Google Scholar] [CrossRef][Green Version]
- Hassan, A.S.; Soliman, G.M.; Ali, M.F.; El-Mahdy, M.M.; El-Gindy, G.E.-D.A. Mucoadhesive Tablets for the Vaginal Delivery of Progesterone: In Vitro Evaluation and Pharmacokinetics/Pharmacodynamics in Female Rabbits. Drug Dev. Ind. Pharm. 2018, 44, 224–232. [Google Scholar] [CrossRef]
- Ellakwa, T.E.; Abu-Khadra, A.S.; Ellakwa, D.E.-S. Influence of Physico-Chemical Properties of Hydroxypropyl Methylcellulose on Quetiapine Fumarate Release from Sustained Release Matrix Tablets. BMC Chem. 2024, 18, 219. [Google Scholar] [CrossRef]
- Srinivasa Rao, B.; Ratnam, B.V. Formulation and Optimization of Sustained Release Tablets of Rosuvastatin Using HPMC K4M, HPMC K100M and Carrageenan. Int. J. ChemTech Res. 2018, 11, 376–386. [Google Scholar] [CrossRef]
- Manna, S.; Lakshmi, U.; Racharla, M.; Sinha, P.; Kanthal, L.; Kumar, S. Bioadhesive HPMC Gel Containing Gelatin Nanoparticles for Intravaginal Delivery of Tenofovir. J. App. Pharm. Sci. 2016, 6, 022–029. [Google Scholar] [CrossRef]
- Bigdeli, A.; Makhmalzadeh, B.S.; Feghhi, M.; SoleimaniBiatiani, E. Cationic Liposomes as Promising Vehicles for Timolol/Brimonidine Combination Ocular Delivery in Glaucoma: Formulation Development and in Vitro/in Vivo Evaluation. Drug Deliv. Transl. Res. 2023, 13, 1035–1047. [Google Scholar] [CrossRef]
- Shiehzadeh, F.; Mohebi, D.; Chavoshian, O.; Daneshmand, S. Formulation, Characterization, and Optimization of a Topical Gel Containing Tranexamic Acid to Prevent Superficial Bleeding: In Vivo and In Vitro Evaluations. Turk. J. Pharm. Sci. 2023, 20, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Cho, I.; Young, S.A.; Anderson, W.H.K.; Waters, B.J.; Hung, S.-C.; Gao, Z.; Mahana, D.; Bihan, M.; Alekseyenko, A.V.; et al. The Nonfermentable Dietary Fiber Hydroxypropyl Methylcellulose Modulates Intestinal Microbiota. FASEB J. 2013, 27, 692–702. [Google Scholar] [CrossRef]
- Morsi, N.; Ghorab, D.; Refai, H.; Teba, H. Nanodispersion-Loaded Mucoadhesive Polymeric Inserts for Prolonged Treatment of Post-Operative Ocular Inflammation. J. Microencapsul. 2017, 34, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A Novel FK506 Loaded Nanomicelles Consisting of Amino-Terminated Poly(Ethylene Glycol)-Block-Poly(D,L)-Lactic Acid and Hydroxypropyl Methylcellulose for Ocular Drug Delivery. Int. J. Pharm. 2019, 562, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef]
- Kouchak, M.; Mahmoodzadeh, M.; Farrahi, F. Designing of a pH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation. AAPS PharmSciTech. 2019, 20, 210. [Google Scholar] [CrossRef]
- Sharma, M.; Kohli, S.; Dinda, A. In-Vitro and in-Vivo Evaluation of Repaglinide Loaded Floating Microspheres Prepared from Different Viscosity Grades of HPMC Polymer. Saudi Pharm. J. 2015, 23, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Xu, J.; Xu, S.; Li, Y.; Sun, R.; Huang, J.; Peng, J.; Gong, Z.; Wang, J.; et al. Development of a Hydroxypropyl Methyl Cellulose/Polyacrylic Acid Interpolymer Complex Formulated Buccal Mucosa Adhesive Film to Facilitate the Delivery of Insulin for Diabetes Treatment. Int. J. Biol. Macromol. 2024, 269, 131876. [Google Scholar] [CrossRef]
- Fathi, A.M.; Eissa, R.G.; Balata, G.F.; Ghazy, F.-E.S.; Eissa, N.G. Intranasal Thermosensitive Hydrogel of Agomelatine Solid Dispersion for Better Management of Depression. J. Drug Deliv. Sci. Technol. 2023, 88, 104974. [Google Scholar] [CrossRef]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Antipsychotic Potential and Safety Profile of TPGS-Based Mucoadhesive Aripiprazole Nanoemulsion: Development and Optimization for Nose-To-Brain Delivery. J. Pharm. Sci. 2021, 110, 1761–1778. [Google Scholar] [CrossRef]
- Abdallah, M.H.; El-Horany, H.E.-S.; El-Nahas, H.M.; Ibrahim, T.M. Tailoring Risperidone-Loaded Glycethosomal In Situ Gels Using Box–Behnken Design for Treatment of Schizophrenia-Induced Rats via Intranasal Route. Pharmaceutics 2023, 15, 2521. [Google Scholar] [CrossRef] [PubMed]
- Deshkar, S.S.; Palve, V.K. Formulation and Development of Thermosensitive Cyclodextrin-Based in Situ Gel of Voriconazole for Vaginal Delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-Hydroxypropyl Methylcellulose Tioconazole Films: A Promising Alternative Dosage Form for the Treatment of Vaginal Candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef]
- Saedi, H. Preparation and Assessment of Prostaglandin E2 Formulated with Hydroxypropyl Methyl Cellulose and Span 60 Sustained Release Pessaries (Vaginal Suppositories). Bull. Chem. Soc. Eth. 2018, 32, 361. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of Tenofovir Release from Mucoadhesive Vaginal Tablets by Polymer Combination to Prevent Sexual Transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef]
- Cavelier, M.; Gondé, H.; Costa, D.; Lamoureux, F.; Pereira, T.; Buchbinder, N.; Varin, R.; Hervouët, C. Development of an Oral Liquid Formulation of Nicardipine Hydrochloride Compounded with Simple Excipients for the Treatment of Pediatric Hypertension. Pharmaceutics 2023, 15, 446. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D Printing of Gummy Drug Formulations Composed of Gelatin and an HPMC-Based Hydrogel for Pediatric Use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.L.; Pandey, M.; Choudhury, H.; Lim, W.M.; Bhattamisra, S.K.; Gorain, B. Development of In-Situ Spray for Local Delivery of Antibacterial Drug for Hidradenitis Suppurativa: Investigation of Alternative Formulation. Polymers 2021, 13, 2770. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, H.A.; Mohamed, M.S.; Zayed, G.M.; Abdelaty, L.N.; Makki, M.A.; Abdel-Aleem, H.L.; El-Mokhtar, M.A.; Hetta, H.F.; Abdullah, N.; Saddik, M.S. HPMC-Zein Film-Forming Gel Loaded with 5-Fluorouracil Coupled with CO2 Laser Dermabrasion for Managing Stable Vitiligo. AAPS PharmSciTech 2024, 25, 225. [Google Scholar] [CrossRef]
- Hydroxypropyl Methylcellulose MakingCosmetics. Available online: https://www.makingcosmetics.com/THK-HYPMCEL-01.html?lang=en_US (accessed on 6 June 2025).
- Rhein, L.; Chaudhuri, B.; Jivani, N.; Fares, H.; Davis, A. Targeted Delivery of Salicylic Acid from Acne Treatment Products into and through Skin: Role of Solution and Ingredient Properties and Relationships to Irritation. Intern J. Cosmet. Sci. 2004, 26, 218–219. [Google Scholar] [CrossRef]
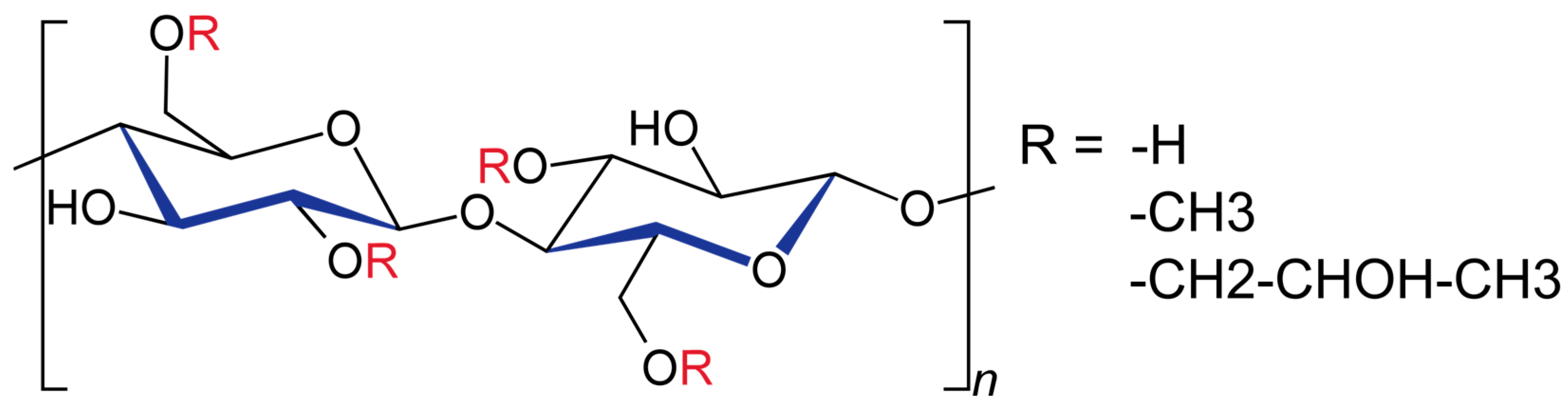
| Substitution Type | Methoxy (%) | Hydroxypropoxy (%) | Methocel® Type * | Viscosity (mPa·s) * | Widespread Applications | |
|---|---|---|---|---|---|---|
| 1828 | 16.5 to 20.0 | 23.0 to 32.0 | J | 12,000, 75,000 | Used as a thickener and rheology modifier. | |
| 2208 | 19.0 to 24.0 | 4.0 to 12.0 | K | 3, 100, 4000, 15,000, 100,000 | Highly used for manufacturing controlled-release dosage forms, especially hydrophilic matrices, and also used for the development of capsule shells. HPMC 4M possesses mucoadhesive properties. HPMC K15M and K100M provide thickening and gelling properties. | |
| 2906 | 27.0 to 30.0 | 4.0 to 7.5 | F | 50, 4000 | Binder, thickener, and rheology modifier. Commonly used in food, ceramics, coatings, and inks industries. | |
| 2910 | 28.0 to 30.0 | 7.0 to 12.0 | E | 3, 5, 6, 15, 50, 4000, 10,000, 15,000 | HPMC E3, E5, E15, E50: popular representatives used as film-forming agents [32,33]. | |
| Methoxy (%) | Hydroxypropoxy (%) | Methocel® type * | Viscosity (mPa·s) * | Widespread applications. | ||
| 1828 | 16.5 to 20.0 | 23.0 to 32.0 | J | 12,000, 75,000 | Used as a thickener and rheology modifier. | |
| 2208 | 19.0 to 24.0 | 4.0 to 12.0 | K | 3, 100, 4000, 15,000, 100,000 | Highly used for manufacturing controlled-release dosage forms, especially hydrophilic matrices, and also used for the development of capsule shells. HPMC 4M possesses mucoadhesive properties. HPMC K15M and K100M provide thickening and gelling properties | |
| 2906 | 27.0 to 30.0 | 4.0 to 7.5 | F | 50, 4000 | Binder, thickener, and rheology modifier. Commonly used in food, ceramics, coatings, and inks industries. | |
| 2910 | 28.0 to 30.0 | 7.0 to 12.0 | E | 3, 5, 6, 15, 50, 4000, 10,000, 15,000 | HPMC E3, E5, E15, E50: popular representatives used as film-forming agents [32]. | |
| Trademark Name | Substitution Type | Frequently Used Grades | Viscosity (mPa·s) * | Applications | Observations |
|---|---|---|---|---|---|
| Metolose® | 2910 | 60SH | 50–10,000 | Thickening agent, manufacturing oral films. | The viscosity of the solution generated due to the water-soluble nature of the polymer depends on the amount and grade used. |
| 2906 | 65SH | 50–4000 | Used as a binder, thickener and film-forming agent in solid dosage forms. | ||
| 2208 | 90SH | 4000–100,000 | |||
| 2208 | 90SH-SR | 100SR 4000SR 15000SR 100000SR | Sustained-release dosage forms for oral use—suitable for direct compression and wet granulation. | It is designed exclusively for a hydrophilic matrix agent. | |
| Pharmacoat® | 2910 | 603 | 3 | Pellet coating, for binder solutions used in wet granulation. | Low viscosity HPMC substitution grades. Developed in 1963, first used for film coating and later for manufacturing capsules in the place of gelatin or as a binder for granulation [40]. |
| 645 | 4.5 | Manufacturing capsule shells and amorphous solid dispersions. | |||
| 606 | 6 | Tablet coating, manufacturing capsule shells, oral films. | |||
| 615 | 15 | Taste masking by coating with enteric polymer and pore former, coating fragile tablets. |
| HPMC Type | Concentration | Active Ingredient | Method (s) | Observations | References |
|---|---|---|---|---|---|
| HPMC E5, HPMC E15 | 10–20% | Phenytoin (30 mg) | Syringe extrusion—3D printing | 20% (w/v) HPMC E5 and 10% (w/v) HPMC E15 suitable for the drug incorporation and 3D-printing. By loading the phenytoin into the HPMC E15 matrix, good physical appearance, good mechanical strength, rapid disintegration time, and a fast release of the API were obtained. | [109] |
| HPMC E15 | 10% | Mirtazapine | Solvent casting method 3D-printing method | Through both methods, an amorphous form was obtained in the case of the final mixture. The 3D-printing method outlined a porous matrix, good mechanical properties, a fast disintegration time, and an immediate release of mirtazapine (>80% in five minutes). | [98] |
| HPMC (Colorcon) | 3% | Meloxicam (5.16–5.51 mg) | Solvent casting method | ODFs with 3% HPMC and 2% microcrystalline cellulose and 3% HPMC and 2% Kolidon® were prepared. Disintegration times higher than 30 s for all the formulations developed; amounts of API < 60% were released at 120 min in comparison to the formulations where sodium alginate was used, and almost 80% of the active ingredient was released at the same time frame. | [102] |
| HPMC E5 | 8–9% | Caffeine | Solvent casting method | Four formulations coded CAF1 (8% HPMC E5, only caffeine), CAF2 (8% HPMC E5, caffeine + citric acid [1:1]), CAF3 (9% HPMC E5, caffeine + citric acid [1:1]), and CAF 4 (9% HPMC E5, caffeine + sodium benzoate [1:1]. Very fast release of the active ingredient, especially in the CAF4 formulation, where sodium benzoate was used as a hydrotropic ingredient (almost 100% of API release at 3 min). Disintegration times lower than 180 s were noticed in the case of both methods used (slide-frame method and basket-rack method). CAF2 and CAF3 exhibited good mechanical properties, with CAF2 outlining the highest number of folds needed to produce the rupture of the film, and similar tensile strengths for CAF2 and CAF3. | [103] |
| HPMC E5 | 20% | Loratadine | 3D printing | The amorphous state was outlined through a DSC study, which highlighted the lack of the endothermic peak that was obtained in the case of loratadine in its crystal form. Loratadine-ODFs were obtained with an average mass of 60.22 ± 5.02 mg, 164 ± 15 µm, and a disintegration time lower than the maximum admitted by the Ph. Eur. 11 for ODTs (180 s) of 146 ± 23 s. | [101] |
| HPMC—viscosity 28 cP (2% in H2 O) | 2% | Clove oil (nano-sized) | Solvent casting method | The influence of additives maltodextrin, pectin, and glycerol on the ODF was studied. A decrease in tensile strength and an increase in elongation at break and opacity were observed in clove oil ODFs compared to blank HPMC-ODFs. | [110] |
| HPMC E15 and maltodextrin | 3–5% | Mosapride citrate | Solvent casting method | Mosapride citrate solid dispersion with poloxamer 188 was incorporated in ODFs. To establish an optimal formulation, the type of plasticiser, its concentration, and the ratio between the selected film-forming agents were varied. Thicknesses between 0.17 ± 0.09 (F20: 15% propylene glycol and 1:9 maltodextrin–HPMC ratio) and 0.35 ± 0.08 mm (25% propylene glycol and 5:5 maltodextrin–HPMC ratio). Good mechanical properties in terms of tensile strength ranging between 2.6 MPa for F12 (25% glycerol and 5:5 maltodextrin–HPMC ratio) and 29.9 MPa F8 (15% glycerol and 5:5 maltodextrin–HPMC ratio). Elongation varied between 17.3% F13 (15% glycerol and 3:7 maltodextrin–HPMC ratio) and 82.8% in the case of F12, whose composition is mentioned above. Fast dissolution rates were registered in most of the formulations, with the formulation that contained 25% glycerol and a 3:7 maltodextrin–HPMC ratio exhibiting the fastest amount of API release. | [111] |
| HPMC viscosity of 6 mPa and HPC mixtures | 20–90% | Donepezil hydrochloride | Solvent casting method | Different blends of HPMC and HPC were used to develop ODFs. The 40/60 and 20/80 HPMC/HPC ratios outlined lower tensile strength and elongation, whilst blends > 40% HPC exhibited shorter disintegration times. The addition of donepezil hydrochloride reduced tensile strength and elongation. | [112] |
| HPMC (Pharmacoat® 606) and three types of HPC | 12.5–17.5/ 10/15 | Loperamide hydrochloride (LPH) and Ibuprofen | Solvent casting method | Dispersions were characterised in terms of viscosity and particle sedimentation, whilst the ODFs were verified for uniformity of content, thickness, mass, and stability. ODF obtained from low viscous suspensions, loaded with 10 mg/6 cm2 LPH/film, while the ones obtained from high viscous suspensions were loaded with up to 5 mg LPH/6 cm2 film. ODFs with 50 mg/6 cm2 IBU resulted in acceptable parameters, while higher concentrations of IBU in ODFs were less feasible. | [113] |
| HPMC E5, PVP K30 and maltodextrin | 8–12% | Fluoxetine | Solvent casting method | Twelve formulations of ODFs (six blank and six with fluoxetine) were developed by varying the amount of HPMC (between 8 and 12% w/w) with good mechanical properties and good disintegration ability. FX1 and FX2 (10% HPMC E5 with different amounts of PG 10% for FX1 and 12% FX2) released >97% of fluoxetine within 15 min. | [114] |
| HPMC E50, HPC | 5% | Herpetrione (nanosuspension) | Solvent casting method | Disintegration time < 30 s with reconstituted nanosuspension particle size of 280 ± 11 nm. Good redispersibility of the herpetrione ODFs. ODF containing herpetrione nanoparticles, an opportunity to transform drug nanosuspensions into a solid dosage form. ODFs with nano-sized API enhancing the dissolution rate of poorly water-soluble drugs. | [115] |
| Nr. | Active Pharmaceutical Ingredient | Formulation Type | Application | Reference |
|---|---|---|---|---|
| 1 | Azelaic acid | hydrogel | acne | [116] |
| 2 | Azithromycin | nanoparticle | ocular infections | [117] |
| 3 | Bupivacaine | in situ gel | ocular anaesthetic | [118] |
| 4 | Buspirone | buccal discs | anxiety | [119] |
| 5 | Chloramphenicol | gel | ocular | [120] |
| 6 | Clozapine | bilosomal gels | schizophrenia | [121] |
| 7 | Duloxetine | cyclodextrin | depression | [122] |
| 8 | Enalapril | floating tablet | hypertension | [123] |
| 9 | Fluconazole/ofloxacin | mucoadhesive film | polymicrobial keratitis | [124] |
| 10 | Gliclazide | nanosuspension | diabetes | [125] |
| 11 | Insulin | nanogel | diabetes | [126] |
| 12 | Losartan | transdermal patch | hypertension | [127] |
| 13 | Metformin | floating tablet | diabetes | [128] |
| 14 | Progesterone | mucoadhesive tablets | reproductive therapy | [129] |
| 15 | Quetiapine | tablets | Bipolar disorder | [130] |
| 16 | Rosuvastatin | tablet | hypercholesterolemia | [131] |
| 17 | Tenofovir | bioadhesive gel | HIV | [132] |
| 18 | Timolol/Brimonidine | liposome | glaucoma | [133] |
| 19 | Tranexamic acid | gel | local haemorrhages | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad, R.-A.; Pintea, A.; Pintea, C.; Rédai, E.-M.; Antonoaea, P.; Bîrsan, M.; Ciurba, A. Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems. Pharmaceutics 2025, 17, 784. https://doi.org/10.3390/pharmaceutics17060784
Vlad R-A, Pintea A, Pintea C, Rédai E-M, Antonoaea P, Bîrsan M, Ciurba A. Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems. Pharmaceutics. 2025; 17(6):784. https://doi.org/10.3390/pharmaceutics17060784
Chicago/Turabian StyleVlad, Robert-Alexandru, Andrada Pintea, Cezara Pintea, Emőke-Margit Rédai, Paula Antonoaea, Magdalena Bîrsan, and Adriana Ciurba. 2025. "Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems" Pharmaceutics 17, no. 6: 784. https://doi.org/10.3390/pharmaceutics17060784
APA StyleVlad, R.-A., Pintea, A., Pintea, C., Rédai, E.-M., Antonoaea, P., Bîrsan, M., & Ciurba, A. (2025). Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems. Pharmaceutics, 17(6), 784. https://doi.org/10.3390/pharmaceutics17060784









