Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition
Abstract
1. Introduction
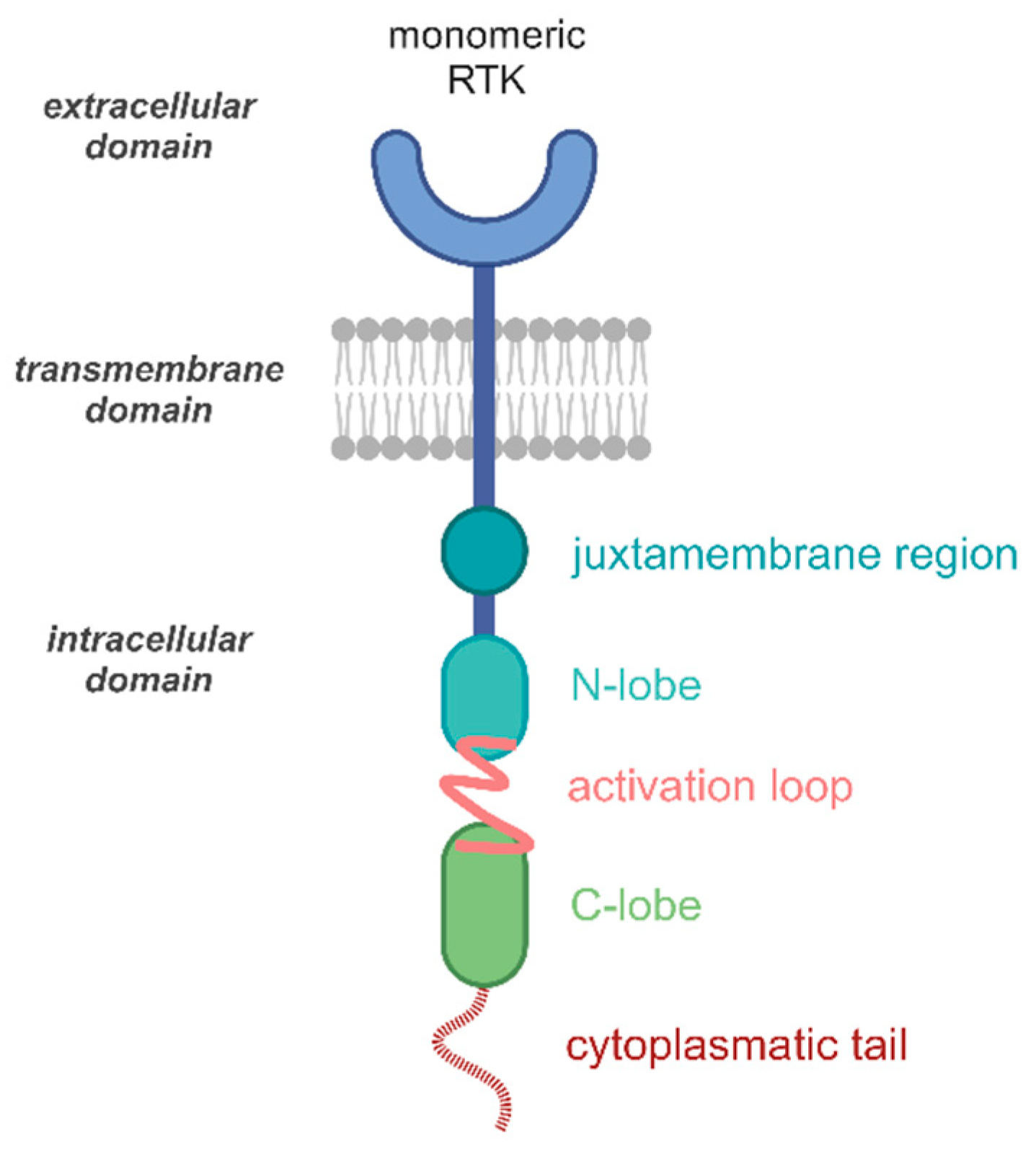
2. RTK Structure
2.1. RTK Activation Mechanism
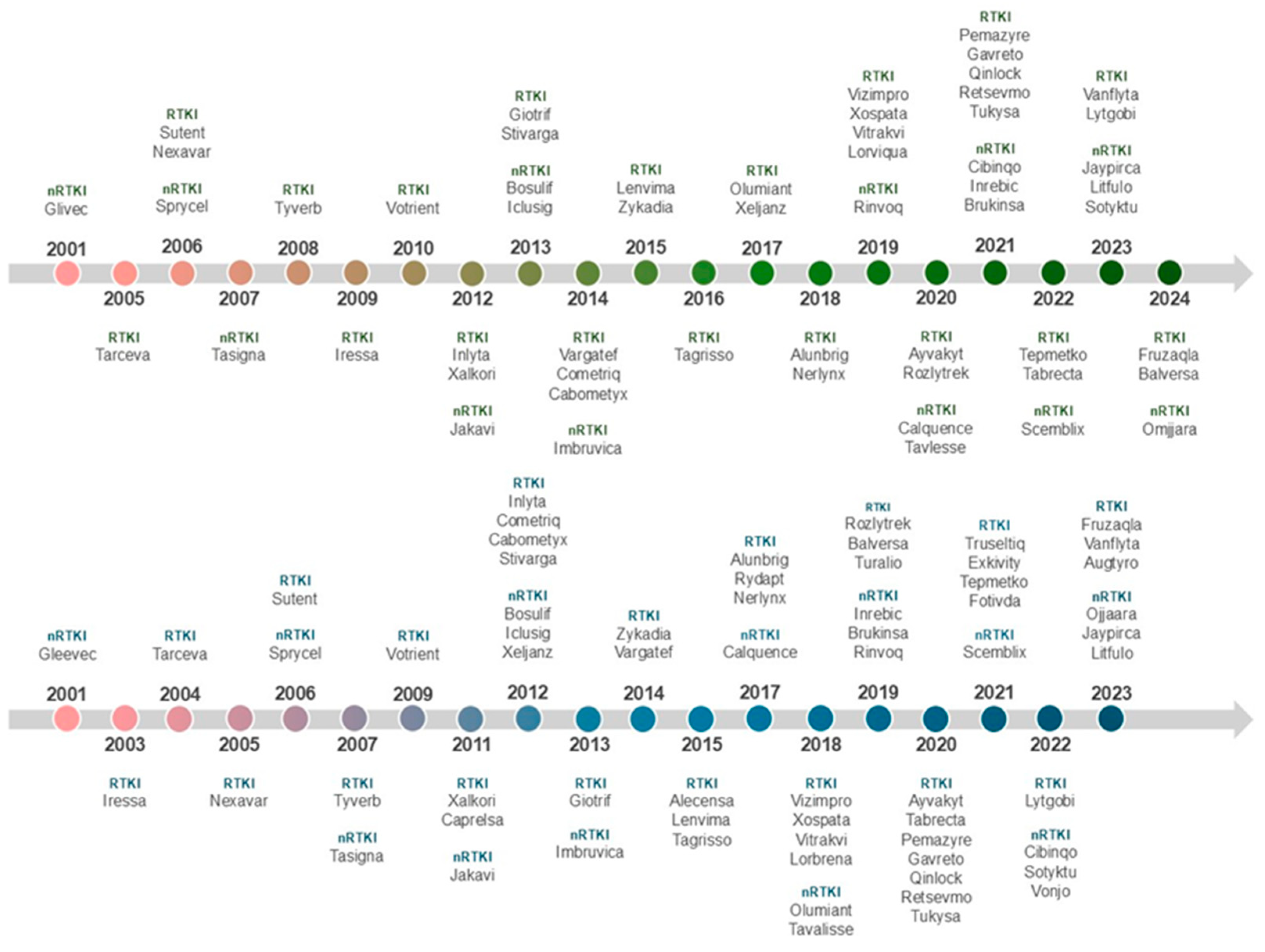
2.2. Tyrosine Kinase Inhibitors
2.3. Properties of the TKIs
2.4. Adverse Effects
2.5. Resistance
3. Nanomedicine for Cancer Therapy
3.1. Nanoparticles Characteristics
3.2. Clearance Properties
4. Nanoparticles in Cancer Treatment
4.1. Lipid-Based Nanoparticles
4.2. Polymer-Based Nanoparticles
4.3. Metal Nanoparticles
4.4. Platinum Nanoparticles
4.5. Silver Nanoparticles
4.6. Gold Nanoparticles
5. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | Adenocarcinomic human alveolar basal epithelial cells | K562 | Myelogenous leukemia cell line |
| AgNPs | Silver nanoparticles | LNPs | Lipid based nanoparticles |
| AI | Artificial intelligence | MCF-7 | Human breast cancer cells |
| ALK | Anaplastic lymphoma kinase | MDS | Midostaurin |
| ALL | Acute lymphoblastic / lymphocytic leukemia | MET | Proto-oncogene tyrosine kinase receptor |
| ATP | Adenosine triphosphate | MPS | Mononuclear phagocyte system |
| ATP | Adenosine triphosphate | MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| AuNPs | Gold nanoparticles | MuSK | Muscle-specific kinase |
| AuNRs | Gold nanorods | NIR | Near-infrared region |
| BBB | Blood–brain barrier | NPs | Nanoparticles |
| BSA | Bovine serum albumin | nRTKs | Non-receptor tyrosine kinases |
| BTK | Bruton tyrosine kinase | NSCLC | Non-small cell lung cancer |
| CML | Chronic myelogenous leukemia | PDGFR | Platelet-derived growth factor receptor |
| CT | Computed tomography | PdNPs | Palladium nanoparticles |
| DNA | Deoxyribonucleic Acid | PDT | Photodynamic therapy |
| DOX | Doxorubicin | PEG | Polyethylene glycol |
| EBC | Early breast cancer | PPG | Polypropylene glycol |
| EGF | Epidermal growth factor | PTK7 | Protein tyrosine kinase 7 |
| EGFR | Epidermal growth factor receptor | PTT | Photothermal therapy |
| EGFR | Epidermal growth factor receptor | PYR | Pyrotinib |
| EMA | European Medicines Agency | RCC | Renal cell carcinoma |
| Eph | Erythropoietin-producing human hepatocellular receptor | RES | Reticuloendothelial system |
| Ephrin | Eph receptor-interacting protein | RET | Proto-oncogene receptor |
| FCS | Fluorescence correlation spectroscopy | ROR | Receptor Tyrosine Kinase Like Orphan Receptor |
| FDA | Food and Drug Administration | ROS | Reactive oxygen species |
| FGF | Fibroblast growth factor | RTKs | Receptor tyrosine kinases |
| FGFR | Fibroblast growth factor receptor | RYK | Receptor like tyrosine kinase |
| FLT3 | Fms-like tyrosine kinase 3 | SEF | Surface-enhanced fluorescence |
| GBM | Glioblastoma | SERS | Surface-enhanced Raman scattering |
| HD | Hydrodynamic diameter | SSc-ILD | Scleroderma-Associated Interstitial Lung Disease |
| HER | Human epidermal growth factor receptor | TEM | Transmission electron microscopy |
| HGF | Hepatocyte growth factor | TKD | Tyrosine kinase domain |
| HNSCC | Head and neck squamous cell carcinoma | TKIs | Tyrosine kinase inhibitors |
| HSA | Human serum albumin | TKs | Tyrosine kinases |
| IR | Insulin receptor | TRK | Tropomyosin receptor kinase |
| JAK | Janus kinase | VEGF | Vascular endothelial growth factor |
| JMR | Juxtamembrane regulatory region | VEGFR | Vascular endothelial growth factor receptor |
References
- Yaron-Barir, T.M.; Joughin, B.A.; Huntsman, E.M.; Kerelsky, A.; Cizin, D.M.; Cohen, B.M.; Regev, A.; Song, J.; Vasan, N.; Lin, T.-Y.; et al. The Intrinsic Substrate Specificity of the Human Tyrosine Kinome. Nature 2024, 629, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Hedger, G.; Sansom, M.S.P.; Koldsø, H. The Juxtamembrane Regions of Human Receptor Tyrosine Kinases Exhibit Conserved Interaction Sites with Anionic Lipids. Sci. Rep. 2015, 5, 9198. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Baek, M.; Joon Kim, D. Protein Tyrosine Signaling and Its Potential Therapeutic Implications in Carcinogenesis. Curr. Pharm. Des. 2017, 23, 4226–4246. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Wang, W.; Qin, J.; Shi, Z.; Hao, L.; Ma, Y.; Xu, H.; Wu, Z.; Pan, D.; Chen, Z.; et al. Role of Protein Phosphorylation in Cell Signaling, Disease, and the Intervention Therapy. MedComm (2020) 2022, 3, e175. [Google Scholar] [CrossRef]
- Roskoski, R. Hydrophobic and Polar Interactions of FDA-Approved Small Molecule Protein Kinase Inhibitors with Their Target Enzymes. Pharmacol. Res. 2021, 169, 105660. [Google Scholar] [CrossRef]
- Mingione, V.R.; Paung, Y.; Outhwaite, I.R.; Seeliger, M.A. Allosteric Regulation and Inhibition of Protein Kinases. Biochem. Soc. Trans. 2023, 51, 373–385. [Google Scholar] [CrossRef]
- Karpov, O.A.; Fearnley, G.W.; Smith, G.A.; Kankanala, J.; McPherson, M.J.; Tomlinson, D.C.; Harrison, M.A.; Ponnambalam, S.; Karpov, O.A.; Fearnley, G.W.; et al. Receptor Tyrosine Kinase Structure and Function in Health and Disease. AIMS Biophys. 2015, 2, 476–502. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring Receptor Tyrosine Kinases-Inhibitors in Cancer Treatments. Egypt. J. Med. Hum. Genet. 2019, 20, 35. [Google Scholar] [CrossRef]
- Smidova, V.; Michalek, P.; Goliasova, Z.; Eckschlager, T.; Hodek, P.; Adam, V.; Heger, Z. Nanomedicine of Tyrosine Kinase Inhibitors. Available online: https://www.thno.org/v11p1546.htm (accessed on 14 January 2025).
- Wang, Z.; Cole, P.A. Catalytic Mechanisms and Regulation of Protein Kinases. Methods Enzymol. 2014, 548, 1–21. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein Tyrosine Kinase Inhibitor Resistance in Malignant Tumors: Molecular Mechanisms and Future Perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Till, J.H. Protein Tyrosine Kinase Structure and Function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Solouki, S.; August, A.; Huang, W. Non-Receptor Tyrosine Kinase Signaling in Autoimmunity and Therapeutic Implications. Pharmacol. Ther. 2019, 201, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Arienti, C.; Pignatta, S.; Tesei, A. Epidermal Growth Factor Receptor Family and Its Role in Gastric Cancer. Front. Oncol. 2019, 9, 1308. [Google Scholar] [CrossRef]
- Choi, E.; Bai, X.-C. The Activation Mechanism of the Insulin Receptor: A Structural Perspective. Annu. Rev. Biochem. 2023, 92, 247–272. [Google Scholar] [CrossRef]
- Berenstein, R. Class III Receptor Tyrosine Kinases in Acute Leukemia—Biological Functions and Modern Laboratory Analysis. Biomark. Insights 2015, 10, BMI.S22433. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent Progress on Vascular Endothelial Growth Factor Receptor Inhibitors with Dual Targeting Capabilities for Tumor Therapy. J. Hematol. Oncol. 2022, 15, 89. [Google Scholar] [CrossRef]
- Krook, M.A.; Reeser, J.W.; Ernst, G.; Barker, H.; Wilberding, M.; Li, G.; Chen, H.-Z.; Roychowdhury, S. Fibroblast Growth Factor Receptors in Cancer: Genetic Alterations, Diagnostics, Therapeutic Targets and Mechanisms of Resistance. Br. J. Cancer 2021, 124, 880–892. [Google Scholar] [CrossRef]
- Dessaux, C.; Ganier, L.; Guiraud, L.; Borg, J.-P. Recent Insights into the Therapeutic Strategies Targeting the Pseudokinase PTK7 in Cancer. Oncogene 2024, 43, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin Receptor Kinase (TRK) Biology and the Role of NTRK Gene Fusions in Cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Endo, M.; Kamizaki, K.; Minami, Y. The Ror-Family Receptors in Development, Tissue Regeneration and Age-Related Disease. Front. Cell Dev. Biol. 2022, 10, 891763. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Gnanasambandan, K. Structure and Activation of MuSK, a Receptor Tyrosine Kinase Central to Neuromuscular Junction Formation. Biochim. et Biophys. Acta (BBA)—Proteins Proteom. 2013, 1834, 2166–2169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Liao, H.-Y.; Zhang, H.-H. Roles of MET in Human Cancer. Clin. Chim. Acta 2022, 525, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.M.A.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM Receptor Tyrosine Kinases: Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2008; Volume 100, pp. 35–83. [Google Scholar]
- Meltzer, M.; Eliash, N.; Azoulay, Z.; Hadad, U.; Papo, N. In Vitro Inhibition of Cancer Angiogenesis and Migration by a Nanobody That Targets the Orphan Receptor Tie1. Cell. Mol. Life Sci. 2022, 79, 312. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Structure and Physiology of the RET Receptor Tyrosine Kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a009134. [Google Scholar] [CrossRef]
- Zapata-García, J.A.; Jave-Suárez, L.F.; Aguilar-Lemarroy, A. Delving into the Role of Receptor-like Tyrosine Kinase (RYK) in Cancer: In Silico Insights into Its Diagnostic and Prognostic Utility. J. Mol. Pathol. 2024, 5, 66–80. [Google Scholar] [CrossRef]
- Fu, H.-L.; Valiathan, R.R.; Arkwright, R.; Sohail, A.; Mihai, C.; Kumarasiri, M.; Mahasenan, K.V.; Mobashery, S.; Huang, P.; Agarwal, G.; et al. Discoidin Domain Receptors: Unique Receptor Tyrosine Kinases in Collagen-Mediated Signaling. J. Biol. Chem. 2013, 288, 7430–7437. [Google Scholar] [CrossRef]
- Kato, S.; Alsafar, A.; Walavalkar, V.; Hainsworth, J.; Kurzrock, R. Cancer of Unknown Primary in the Molecular Era. Trends Cancer 2021, 7, 465–477. [Google Scholar] [CrossRef]
- Mórotz, G.M.; Bradbury, N.A.; Caluseriu, O.; Hisanaga, S.; Miller, C.C.J.; Swiatecka-Urban, A.; Lenz, H.-J.; Moss, S.J.; Giamas, G. A Revised Nomenclature for the Lemur Family of Protein Kinases. Commun. Biol. 2024, 7, 57. [Google Scholar] [CrossRef]
- Ueno, H.; Hirano, N.; Kozutsumi, H.; Sasaki, K.; Tanaka, T.; Yazaki, Y.; Hirai, H. An Epidermal Growth Factor Receptor-Leukocyte Tyrosine Kinase Chimeric Receptor Generates Ligand-Dependent Growth Signals through the Ras Signaling Pathway. J. Biol. Chem. 1995, 270, 20135–20142. [Google Scholar] [CrossRef]
- Du, X.; Shao, Y.; Qin, H.-F.; Tai, Y.-H.; Gao, H.-J. ALK-Rearrangement in Non-Small-Cell Lung Cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fu, Y.; Wang, Y. Downregulation of GLUT3 Impairs STYK1/NOK-mediated Metabolic Reprogramming and Proliferation in NIH-3T3 Cells. Oncol. Lett. 2021, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural Features of the Protein Kinase Domain and Targeted Binding by Small-Molecule Inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef]
- Combarel, D.; Dousset, L.; Bouchet, S.; Ferrer, F.; Tetu, P.; Lebbe, C.; Ciccolini, J.; Meyer, N.; Paci, A. Tyrosine Kinase Inhibitors in Cancers: Treatment Optimization—Part I. Crit. Rev. Oncol./Hematol. 2024, 199, 104384. [Google Scholar] [CrossRef]
- Bashton, M.; Nobeli, I.; Thornton, J.M. Cognate Ligand Domain Mapping for Enzymes. J. Mol. Biol. 2006, 364, 836–852. [Google Scholar] [CrossRef]
- Ullrich, A.; Schlessinger, J. Signal Transduction by Receptors with Tyrosine Kinase Activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Knösel, T.; Kampmann, E.; Kirchner, T.; Altendorf-Hofmann, A. Tyrosinkinasen in Weichgewebstumoren. Pathologe 2014, 35, 198–201. [Google Scholar] [CrossRef]
- Gnagni, L.; Ruscito, I.; Zizzari, I.G.; Nuti, M.; Napoletano, C.; Rughetti, A. Precision Oncology Targeting FGFRs: A Systematic Review on Pre-Clinical Activity and Clinical Outcomes of Pemigatinib. Crit. Rev. Oncol./Hematol. 2024, 202, 104464. [Google Scholar] [CrossRef]
- Receptor Tyrosine Kinases (RTKs). Available online: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=304 (accessed on 5 October 2024).
- Reinecke, M.; Brear, P.; Vornholz, L.; Berger, B.-T.; Seefried, F.; Wilhelm, S.; Samaras, P.; Gyenis, L.; Litchfield, D.W.; Médard, G.; et al. Chemical Proteomics Reveals the Target Landscape of 1,000 Kinase Inhibitors. Nat. Chem. Biol. 2024, 20, 577–585. [Google Scholar] [CrossRef]
- Ryszkiewicz, P.; Malinowska, B.; Schlicker, E. Polypharmacology: Promises and New Drugs in 2022. Pharmacol. Rep. 2023, 75, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M.; Lee, J.K.; Witte, O.N. Clinical Targeting of Mutated and Wild-Type Protein Tyrosine Kinases in Cancer. Mol. Cell Biol. 2014, 34, 1722–1732. [Google Scholar] [CrossRef]
- Shawver, L.K.; Slamon, D.; Ullrich, A. Smart Drugs: Tyrosine Kinase Inhibitors in Cancer Therapy. Cancer Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y. Advances in Studies of Tyrosine Kinase Inhibitors and Their Acquired Resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Saraon, P.; Pathmanathan, S.; Snider, J.; Lyakisheva, A.; Wong, V.; Stagljar, I. Receptor Tyrosine Kinases and Cancer: Oncogenic Mechanisms and Therapeutic Approaches. Oncogene 2021, 40, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Classification of Small Molecule Protein Kinase Inhibitors Based upon the Structures of Their Drug-Enzyme Complexes. Pharmacol. Res. 2016, 103, 26–48. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2024 Update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 5 October 2024).
- Medicines|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines (accessed on 5 October 2024).
- Resources for Information|Approved Drugs. WITHDRAWN: FDA Grants Accelerated Approval to Infigratinib for Metastatic Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on 5 October 2024).
- Sarah, A.; Dondi, E.; De Francia, S. Tyrosine Kinase Inhibitors: The Role of Pharmacokinetics and Pharmacogenetics. Expert Opin. Drug Metab. Toxicol. 2023, 19, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Di Gion, P.; Kanefendt, F.; Lindauer, A.; Scheffler, M.; Doroshyenko, O.; Fuhr, U.; Wolf, J.; Jaehde, U. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors. Clin. Pharmacokinet. 2011, 50, 551–603. [Google Scholar] [CrossRef]
- Dostálek, M.; Juřica, J.; Janoštíková, E.; Zahradníková, L. Farmakokinetika (Pharmacokinetics), 1st ed.; Grada Publishing: Praha, Czech Republic, 2006; ISBN 80-247-1464-7. [Google Scholar]
- Eckstein, N.; Röper, L.; Haas, B.; Potthast, H.; Hermes, U.; Unkrig, C.; Naumann-Winter, F.; Enzmann, H. Clinical Pharmacology of Tyrosine Kinase Inhibitors Becoming Generic Drugs: The Regulatory Perspective. J. Exp. Clin. Cancer Res. 2014, 33, 15. [Google Scholar] [CrossRef]
- Hsyu, P.-H.; Pignataro, D.S.; Matschke, K. Absolute Bioavailability of Bosutinib in Healthy Subjects from an Open-Label, Randomized, 2-Period Crossover Study. Clin. Pharmacol. Drug Dev. 2018, 7, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, H.; Li, W.; Zhang, Y. Clinical Pharmacokinetics and Drug–Drug Interactions of Tyrosine-Kinase Inhibitors in Chronic Myeloid Leukemia: A Clinical Perspective. Crit. Rev. Oncol./Hematol. 2024, 195, 104258. [Google Scholar] [CrossRef] [PubMed]
- Chaar, M.; Kamta, J.; Ait-Oudhia, S. Mechanisms, Monitoring, and Management of Tyrosine Kinase Inhibitors-Associated Cardiovascular Toxicities. OTT 2018, 11, 6227–6237. [Google Scholar] [CrossRef] [PubMed]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse Effects of Tyrosine Kinase Inhibitors in Cancer Therapy: Pathophysiology, Mechanisms and Clinical Management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Hamnvik, O.-P.R.; Choueiri, T.K.; Turchin, A.; McKay, R.R.; Goyal, L.; Davis, M.; Kaymakcalan, M.D.; Williams, J.S. Clinical Risk Factors for the Development of Hypertension in Patients Treated with Inhibitors of the VEGF Signaling Pathway. Cancer 2015, 121, 311–319. [Google Scholar] [CrossRef]
- Jansook, P.; Loftsson, T.; Stefánsson, E. Drug-like Properties of Tyrosine Kinase Inhibitors in Ophthalmology: Formulation and Topical Availability. Int. J. Pharm. 2024, 655, 124018. [Google Scholar] [CrossRef]
- Kucharczuk, C.R.; Ganetsky, A.; Vozniak, J.M. Drug-Drug Interactions, Safety, and Pharmacokinetics of EGFR Tyrosine Kinase Inhibitors for the Treatment of Non–Small Cell Lung Cancer. J. Adv. Pract. Oncol. 2018, 9, 189–200. [Google Scholar]
- Kozuki, T. Skin Problems and EGFR-Tyrosine Kinase Inhibitor. Jpn. J. Clin. Oncol. 2016, 46, 291–298. [Google Scholar] [CrossRef]
- Zuo, R.C.; Apolo, A.B.; DiGiovanna, J.J.; Parnes, H.L.; Keen, C.M.; Nanda, S.; Dahut, W.L.; Cowen, E.W. Cutaneous Adverse Effects Associated with the Tyrosine-Kinase Inhibitor Cabozantinib. JAMA Dermatol. 2015, 151, 170–177. [Google Scholar] [CrossRef]
- Alemán, J.O.; Farooki, A.; Girotra, M. Effects of Tyrosine Kinase Inhibition on Bone Metabolism: Untargeted Consequences of Targeted Therapies. Endocr. Relat. Cancer 2014, 21, R247–R259. [Google Scholar] [CrossRef]
- Lin, W.; Wang, X.; Diao, M.; Wang, Y.; Zhao, R.; Chen, J.; Liao, Y.; Long, Q.; Meng, Y. Promoting Reactive Oxygen Species Accumulation to Overcome Tyrosine Kinase Inhibitor Resistance in Cancer. Cancer Cell Int. 2024, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gu, Z.; Yu, X.; Cheng, T.; Liu, B. Research Progress on the Role of Bypass Activation Mechanisms in Resistance to Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Front. Oncol. 2024, 14, 1447678. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine Kinase Inhibitors for Solid Tumors in the Past 20 Years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef]
- Rahban, M.; Joushi, S.; Bashiri, H.; Saso, L.; Sheibani, V. Characterization of Prevalent Tyrosine Kinase Inhibitors and Their Challenges in Glioblastoma Treatment. Front. Chem. 2024, 11, 1325214. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bernards, R. Rational Combinations of Targeted Cancer Therapies: Background, Advances and Challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Wu, P.; Han, J.; Gong, Y.; Liu, C.; Yu, H.; Xie, N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics 2022, 14, 1990. [Google Scholar] [CrossRef]
- Rana, I.; Oh, J.; Baig, J.; Moon, J.H.; Son, S.; Nam, J. Nanocarriers for Cancer Nano-Immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 1936–1954. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology of Tyrosine Kinase Inhibitors in Cancer Therapy: A Perspective. Int. J. Mol. Sci. 2021, 22, 6538. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, P.S.; Chabukswar, A.R.; Raut, K.G.; Gaikwad-Pawar, B.; Jagdale, S.C. Nanoparticle-Based Targeted Therapy through EGFR Tyrosine Kinase Inhibitors and Their Recent Advances in Lung Cancer Therapy. Explor. Med. 2024, 5, 513–529. [Google Scholar] [CrossRef]
- Bofinger, R.; Weitsman, G.; Evans, R.; Glaser, M.; Sander, K.; Allan, H.; Hochhauser, D.; Kalber, T.L.; Årstad, E.; Hailes, H.C.; et al. Drug Delivery, Biodistribution and Anti-EGFR Activity: Theragnostic Nanoparticles for Simultaneous in Vivo Delivery of Tyrosine Kinase Inhibitors and Kinase Activity Biosensors. Nanoscale 2021, 13, 18520–18535. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Bryant, A.A.; Vanden Berg-Foels, W.S.; Wen, X. Chapter One—Bioengineering Strategies for Designing Targeted Cancer Therapies. In Advances in Cancer Research; Tew, K.D., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 118, pp. 1–59. [Google Scholar]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced Permeability and Retention Effect: A Key Facilitator for Solid Tumor Targeting by Nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Xu, W.; Ye, C.; Qing, X.; Liu, S.; Lv, X.; Wang, W.; Dong, X.; Zhang, Y. Multi-Target Tyrosine Kinase Inhibitor Nanoparticle Delivery Systems for Cancer Therapy. Mater. Today Bio 2022, 16, 100358. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef]
- Narum, S.M.; Le, T.; Le, D.P.; Lee, J.C.; Donahue, N.D.; Yang, W.; Wilhelm, S. Chapter 4—Passive Targeting in Nanomedicine: Fundamental Concepts, Body Interactions, and Clinical Potential. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–53. ISBN 978-0-12-816662-8. [Google Scholar]
- Kaushik, N.; Borkar, S.B.; Nandanwar, S.K.; Panda, P.K.; Choi, E.H.; Kaushik, N.K. Nanocarrier Cancer Therapeutics with Functional Stimuli-Responsive Mechanisms. J. Nanobiotechnol. 2022, 20, 152. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, T.; Liu, X.; Fang, X.; Mo, Y.; Xie, N.; Nie, G.; Zhang, B.; Fan, X. Smart Nanoplatforms Responding to the Tumor Microenvironment for Precise Drug Delivery in Cancer Therapy. Int. J. Nanomed. 2024, 19, 6253–6277. [Google Scholar] [CrossRef]
- Fatima, M.; Almalki, W.H.; Khan, T.; Sahebkar, A.; Kesharwani, P. Harnessing the Power of Stimuli-Responsive Nanoparticles as an Effective Therapeutic Drug Delivery System. Adv. Mater. 2024, 36, 2312939. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as Promising Theranostic Tools in Nanomedicine and Their Applications in Clinical Disease Diagnosis and Treatment. Nanomaterials 2021, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Kuang, J.; Ren, Y.; Li, X.; Li, C. Fabrication of Transparent Super-Hydrophilic Coatings with Self-Cleaning and Anti-Fogging Properties by Using Dendritic Nano-Silica. Ceram. Int. 2021, 47, 18743–18750. [Google Scholar] [CrossRef]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and Targeted Delivery of Hydrophilic Drug Compounds: A Review of Existing and Novel Technologies from Bench to Bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.-C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of Biomolecule-Modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef]
- Pearson, R.M.; Juettner, V.V.; Hong, S. Biomolecular Corona on Nanoparticles: A Survey of Recent Literature and Its Implications in Targeted Drug Delivery. Front. Chem. 2014, 2, 108. [Google Scholar] [CrossRef]
- Zielińska, A.; Cano, A.; Andreani, T.; Martins-Gomes, C.; Silva, A.M.; Szalata, M.; Słomski, R.; Souto, E.B. Lipid-Drug Conjugates and Nanoparticles for the Cutaneous Delivery of Cannabidiol. Int. J. Mol. Sci. 2022, 23, 6165. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rangaraj, N.; Singh, S.B.; Srivastava, S. Exploring the Unexplored Avenues of Surface Charge in Nano-Medicine. Colloid Interface Sci. Commun. 2021, 42, 100406. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Lu, B.; Wang, J.; Hendriks, A.J.; Nolte, T.M. Clearance of Nanoparticles from Blood: Effects of Hydrodynamic Size and Surface Coatings. Environ. Sci. Nano 2024, 11, 406–417. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted Elimination of Gold Nanoparticles from Mouse Liver. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine Therapeutic Approaches to Overcome Cancer Drug Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Front. Drug Deliv. 2022, 2, 901281. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.-F.; Zhou, X. Lipid-Based Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Ali, S.R.; Deori, C.; Gogoi, G.K.; Borah, N.J.; Kalita, P.P.; Chowdhury, R.; Kaur, S.; Kakoti, B.B. Liposomic Nano Particles in the Treatment of Colorectal and Ovarian Cancer. Eur. J. Med. Chem. Rep. 2024, 11, 100149. [Google Scholar] [CrossRef]
- Shah, N.N.; Schafer, E.S.; Heym, K.M.; Place, A.E.; Burns, M.A.; Gossai, N.; Shaw, P.; Burke, M.J.; Chang, B.H.; Hermiston, M.L.; et al. Vincristine Sulfate Liposome Injection (VSLI, Marqibo®) in Combination with UK ALL-R3 Induction Chemotherapy for Children, Adolescents and Young Adults with Relapsed Acute Lymphoblastic Leukemia (ALL): A Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) Consortium Trial. Blood 2021, 138, 3402. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, X.; Li, B.; Yan, C.; Wu, C.; He, L.; Cao, J.; Lu, F.; Chen, H.; Li, W. Potent Cancer Therapy by Liposome Microstructure Tailoring with Active-to-Passive Targeting and Shell-to-Core Thermosensitive Features. Mater. Today Bio 2024, 26, 101035. [Google Scholar] [CrossRef]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid Polymer Hybrid Nanoparticles as Potent Vehicles for Drug Delivery in Cancer Therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-Chitosan Hybrid Nanoparticles for Controlled Delivery of Cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef]
- Shao, H.; Liu, M.; Jiang, H.; Zhang, Y. Polysaccharide-Based Drug Delivery Targeted Approach for Colon Cancer Treatment: A Comprehensive Review. Int. J. Biol. Macromol. 2025, 302, 139177. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin Nanoparticle of Paclitaxel (Abraxane) Decreases While Taxol Increases Breast Cancer Stem Cells in Treatment of Triple Negative Breast Cancer. Mol. Pharm. 2020, 17, 2275–2286. [Google Scholar] [CrossRef]
- Blackwell, C.W.; Castillo, H.L. Injectable Cabotegravir: A New Approach to HIV Pre-Exposure Prophylaxis. J. Nurse Pract. 2022, 18, 947–950. [Google Scholar] [CrossRef]
- APRETUDE (Cabotegravir Extended-Release Injectable Suspension) 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/215499s008lbl.pdf (accessed on 5 October 2024).
- Mayer, K.H.; Molina, J.-M.; Thompson, M.A.; Anderson, P.L.; Mounzer, K.C.; De Wet, J.J.; DeJesus, E.; Jessen, H.; Grant, R.M.; Ruane, P.J.; et al. Emtricitabine and Tenofovir Alafenamide vs Emtricitabine and Tenofovir Disoproxil Fumarate for HIV Pre-Exposure Prophylaxis (DISCOVER): Primary Results from a Randomised, Double-Blind, Multicentre, Active-Controlled, Phase 3, Non-Inferiority Trial. Lancet 2020, 396, 239–254. [Google Scholar] [CrossRef]
- ESTRASORB® (Estradiol Topical Emulsion) 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/021371s009lbl.pdf (accessed on 5 October 2024).
- Lee, E.; Anselmo, M.; Tahsin, C.T.; Vanden Noven, M.; Stokes, W.; Carter, J.R.; Keller-Ross, M.L. Vasomotor Symptoms of Menopause, Autonomic Dysfunction, and Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1270–H1280. [Google Scholar] [CrossRef] [PubMed]
- Hheidari, A.; Mohammadi, J.; Ghodousi, M.; Mahmoodi, M.; Ebrahimi, S.; Pishbin, E.; Rahdar, A. Metal-Based Nanoparticle in Cancer Treatment: Lessons Learned and Challenges. Front. Bioeng. Biotechnol. 2024, 12, 1436297. [Google Scholar] [CrossRef]
- Roshani, M.; Rezaian-Isfahni, A.; Lotfalizadeh, M.H.; Khassafi, N.; Abadi, M.H.J.N.; Nejati, M. Metal Nanoparticles as a Potential Technique for the Diagnosis and Treatment of Gastrointestinal Cancer: A Comprehensive Review. Cancer Cell Int. 2023, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Introduction to Nanoparticles. In Microwaves in Nanoparticle Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–24. ISBN 978-3-527-64812-2. [Google Scholar]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Beirne, D.F.; Dalla Via, M.; Velasco-Torrijos, T.; Montagner, D. Metal-Tyrosine Kinase Inhibitors: Targeted Metal-Drug Conjugates. Coord. Chem. Rev. 2022, 469, 214655. [Google Scholar] [CrossRef]
- Kao, H.-F.; Liao, B.-C.; Huang, Y.-L.; Huang, H.-C.; Chen, C.-N.; Chen, T.-C.; Hong, Y.-J.; Chan, C.-Y.; Chia, J.-S.; Hong, R.-L. Afatinib and Pembrolizumab for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (ALPHA Study): A Phase II Study with Biomarker Analysis. Clin. Cancer Res. 2022, 28, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, Y.; Mei, F.; Li, X.; Zhang, M.; Yao, B.; Wu, R.; You, J.; Pei, F. SPP1 Overexpression Is Associated with Poor Outcomes in ALK Fusion Lung Cancer Patients without Receiving Targeted Therapy. Sci. Rep. 2021, 11, 14031. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.-M.; Kim, H.; Pyo, K.-H.; Sun, J.-M.; Ahn, M.-J.; Park, K.; Keam, B.; Kwon, N.-J.; Yun, H.J.; et al. Phase II Clinical and Exploratory Biomarker Study of Dacomitinib in Recurrent and/or Metastatic Esophageal Squamous Cell Carcinoma. Oncotarget 2015, 6, 44971–44984. [Google Scholar] [CrossRef]
- Dolman, M.E.M.; Harmsen, S.; Pieters, E.H.; Sparidans, R.W.; Lacombe, M.; Szokol, B.; Őrfi, L.; Kéri, G.; Storm, G.; Hennink, W.E.; et al. Targeting of a Platinum-Bound Sunitinib Analog to Renal Proximal Tubular Cells. IJN 2012, 7, 417–433. [Google Scholar] [CrossRef]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver Nanoparticle for Biomedical Applications: A Review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef] [PubMed]
- Janith, G.I.; Herath, H.S.; Hendeniya, N.; Attygalle, D.; Amarasinghe, D.A.S.; Logeeshan, V.; Wickramasinghe, P.M.T.B.; Wijayasinghe, Y.S. Advances in Surface Plasmon Resonance Biosensors for Medical Diagnostics: An Overview of Recent Developments and Techniques. J. Pharm. Biomed. Anal. Open 2023, 2, 100019. [Google Scholar] [CrossRef]
- Piergies, N.; Oćwieja, M.; Paluszkiewicz, C.; Kwiatek, W. Identification of Erlotinib Adsorption Pattern onto Silver Nanoparticles: SERS Studies. J. Raman Spectrosc. 2018, 49, 1265–1273. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fahim, J.R.; Masri, R.R.E.; Salem, M.A.; Desoukey, S.Y.; Ahmed, S.; Kamel, M.S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Abdelmohsen, U.R. Chemical and Biological Studies on the Soft Coral Nephthea Sp. RSC Adv. 2021, 11, 23654–23663. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Protein-Guided Formation of Silver Nanoclusters and Their Assembly with Graphene Oxide as an Improved Bioimaging Agent with Reduced Toxicity. J. Phys. Chem. Lett. 2017, 8, 2291–2297. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent Advances Using Gold Nanoparticles as a Promising Multimodal Tool for Tissue Engineering and Regenerative Medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window. Nano-Micro Lett. 2020, 12, 38. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of Photodynamic Therapy (PDT) and Anti-Tumor Immunity in Cancer Therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-Gold Nanoparticle Conjugates for Photodynamic Therapy of Cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, R.-A.; Tigu, A.B.; Feder, R.; Tatar, A.-S.; Gulei, D.; Tomuleasa, C.; Boca, S. In Vivo Imaging System (IVIS) Therapeutic Assessment of Tyrosine Kinase Inhibitor-Loaded Gold Nanocarriers for Acute Myeloid Leukemia: A Pilot Study. Front. Pharmacol. 2024, 15, 1382399. [Google Scholar] [CrossRef] [PubMed]
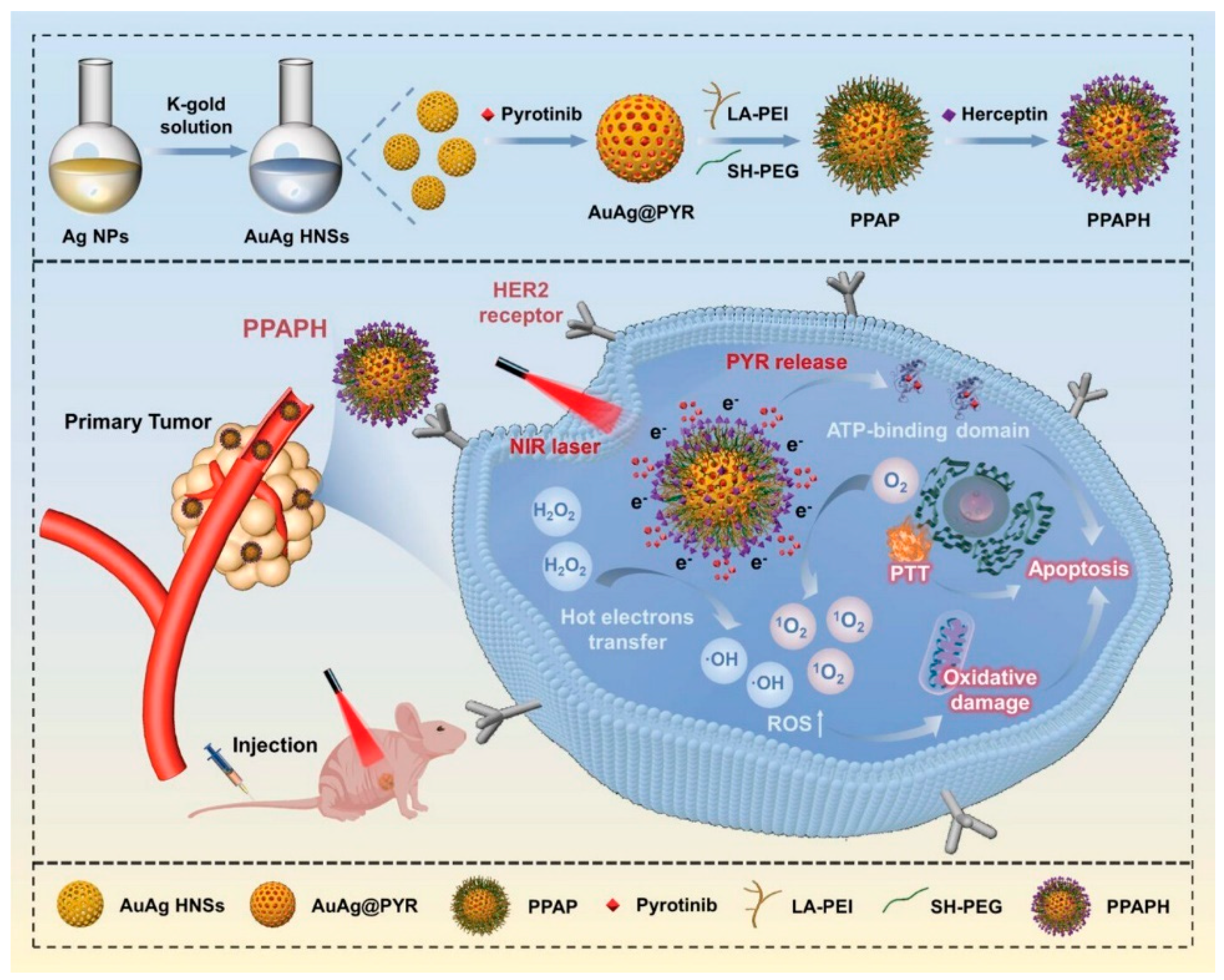
- Zhao, L.; Chang, F.; Tong, Y.; Yin, J.; Xu, J.; Li, H.; Du, L.; Jiang, Y. A Multifunctional Bimetallic Nanoplatform for Synergic Local Hyperthermia and Chemotherapy Targeting HER2-Positive Breast Cancer. Adv. Sci. 2024, 11, 2308316. [Google Scholar] [CrossRef]
- Xuhong, J.-C.; Qi, X.-W.; Zhang, Y.; Jiang, J. Mechanism, Safety and Efficacy of Three Tyrosine Kinase Inhibitors Lapatinib, Neratinib and Pyrotinib in HER2-Positive Breast Cancer. Am. J. Cancer Res. 2019, 9, 2103–2119. [Google Scholar]
- Wilson, F.R.; Coombes, M.E.; Brezden-Masley, C.; Yurchenko, M.; Wylie, Q.; Douma, R.; Varu, A.; Hutton, B.; Skidmore, B.; Cameron, C. Herceptin® (Trastuzumab) in HER2-Positive Early Breast Cancer: A Systematic Review and Cumulative Network Meta-Analysis. Syst. Rev. 2018, 7, 191. [Google Scholar] [CrossRef] [PubMed]
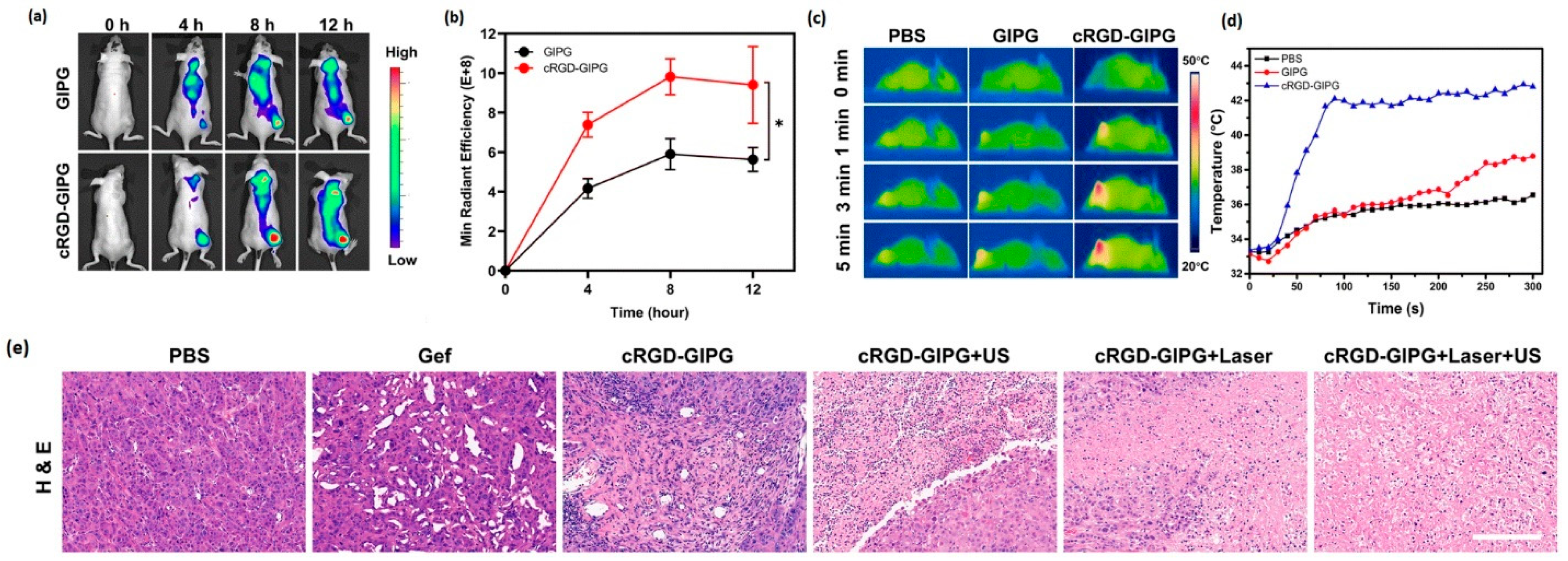
- Lv, W.; Wu, H.; Zhang, Y.; Li, H.; Shu, H.; Su, C.; Zhu, Y.; Wang, T.; Nie, F. cRGD-Targeted Gold-Based Nanoparticles Overcome EGFR-TKI Resistance of NSCLC via Low-Temperature Photothermal Therapy Combined with Sonodynamic Therapy. Biomater. Sci. 2023, 11, 1677–1691. [Google Scholar] [CrossRef]
- Kang, S.; Yim, G.; Min, D.-H.; Jang, H. Wavelength Independent Photo-Chemo Tri-Modal Combinatorial Renal Cell Carcinoma Therapy with Biocompatible Gold-Titania Nanostars. Adv. Ther. 2022, 5, 2100204. [Google Scholar] [CrossRef]
- Molinari, A.; Iovenitti, G.; Mancini, A.; Gravina, G.L.; Chebbi, M.; Caruana, M.; Vignaroli, G.; Orofino, F.; Rango, E.; Angelucci, A.; et al. AuNP Pyrazolo[3,4-d]Pyrimidine Nanosystem in Combination with Radiotherapy against Glioblastoma. ACS Med. Chem. Lett. 2020, 11, 664–670. [Google Scholar] [CrossRef]
- Gauld, S.B.; Cambier, J.C. Src-Family Kinases in B-Cell Development and Signaling. Oncogene 2004, 23, 8001–8006. [Google Scholar] [CrossRef]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [Google Scholar] [CrossRef]
- Liu, A. Fierce Pharma Asia—Hengrui’s FDA Reprimands; Sanofi’s India Site Expansion; Aslan’s Downfall|Fierce Pharma. Available online: https://www.fiercepharma.com/pharma/hengrui-fda-reprimands-sanofi-india-site-expansion-aslan-downfall (accessed on 5 October 2024).
- Liu, A. 20 Years in, Singapore Still Searches for Its Biotech Success Story. Available online: https://www.fiercebiotech.com/biotech/20-years-singapore-still-searches-its-biotech-success-story (accessed on 5 October 2024).
- Codullo, V.; Cova, E.; Pandolfi, L.; Breda, S.; Morosini, M.; Frangipane, V.; Malatesta, M.; Calderan, L.; Cagnone, M.; Pacini, C.; et al. Imatinib-Loaded Gold Nanoparticles Inhibit Proliferation of Fibroblasts and Macrophages from Systemic Sclerosis Patients and Ameliorate Experimental Bleomycin-Induced Lung Fibrosis. J. Control. Release 2019, 310, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Cryer, A.M.; Chan, C.; Eftychidou, A.; Maksoudian, C.; Mahesh, M.; Tetley, T.D.; Spivey, A.C.; Thorley, A.J. Tyrosine Kinase Inhibitor Gold Nanoconjugates for the Treatment of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2019, 11, 16336–16346. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.N.; Tian, H.; Schoen, C.; Winograd, N. Label-Free Visualization of Nilotinib-Functionalized Gold Nanoparticles within Single Mammalian Cells by C60- SIMS Imaging. Anal. Bioanal. Chem. 2017, 409, 3067–3076. [Google Scholar] [CrossRef]
- Sarkar, S.; Konar, S.; Prasad, P.N.; Rajput, S.; Kumar, B.N.P.; Rao, R.R.; Pathak, A.; Fisher, P.B.; Mandal, M. Micellear Gold Nanoparticles as Delivery Vehicles for Dual Tyrosine Kinase Inhibitor ZD6474 for Metastatic Breast Cancer Treatment. Langmuir 2017, 33, 7649–7659. [Google Scholar] [CrossRef]
- Vinhas, R.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticles for BCR-ABL1Gene Silencing: Improving Tyrosine Kinase Inhibitor Efficacy in Chronic Myeloid Leukemia. Mol. Ther. Nucleic Acids 2017, 7, 408–416. [Google Scholar] [CrossRef]
- Schiller, G.J.; Tuttle, P.; Desai, P. Allogeneic Hematopoietic Stem Cell Transplantation in FLT3-ITD–Positive Acute Myelogenous Leukemia: The Role for FLT3 Tyrosine Kinase Inhibitors Post-Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 982–990. [Google Scholar] [CrossRef]
- Suarasan, S.; Simon, T.; Boca, S.; Tomuleasa, C.; Astilean, S. Gelatin-Coated Gold Nanoparticles as Carriers of FLT3 Inhibitors for Acute Myeloid Leukemia Treatment. Chem. Biol. Drug Des. 2016, 87, 927–935. [Google Scholar] [CrossRef]
- Gossai, N.P.; Naumann, J.A.; Li, N.-S.; Zamora, E.A.; Gordon, D.J.; Piccirilli, J.A.; Gordon, P.M. Drug Conjugated Nanoparticles Activated by Cancer Cell Specific mRNA. Oncotarget 2016, 7, 38243–38256. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Abshire, C.; Carry, C.; Sholl, A.B.; Mandava, S.H.; Datta, A.; Ranjan, M.; Callaghan, C.; Peralta, D.V.; Williams, K.S.; et al. Nanotechnology Combined Therapy: Tyrosine Kinase-Bound Gold Nanorod and Laser Thermal Ablation Produce a Synergistic Higher Treatment Response of Renal Cell Carcinoma in a Murine Model. BJU Int. 2017, 119, 342–348. [Google Scholar] [CrossRef]
- Ding, H.X.; Liu, K.K.-C.; Sakya, S.M.; Flick, A.C.; O’Donnell, C.J. Synthetic Approaches to the 2011 New Drugs. Bioorganic Med. Chem. 2013, 21, 2795–2825. [Google Scholar] [CrossRef]
- Lian, S.; Gao, X.; Song, C.; Li, H.; Lin, J. Chemical Enhancement Effect of Icotinib–Au Complex Studied by Combined Density Functional Theory and Surface-Enhanced Raman Scattering. Langmuir 2021, 37, 12907–12918. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Soccio, L.; Kim, H.; Gadina, M.; Schwartzberg, P.L.; Laurence, A.; O’Shea, J.J. Protein Kinases: Drug Targets for Immunological Disorders. Nat. Rev. Immunol. 2023, 23, 787–806. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Zhou, H.; Liu, J.; Xia, X.; Ha, W.; Jiang, Y.; Liu, Q.; Xiong, H. Kinase Inhibitors and Kinase-Targeted Cancer Therapies: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 5489. [Google Scholar] [CrossRef]
- Taherdoost, H.; Ghofrani, A. AI’s Role in Revolutionizing Personalized Medicine by Reshaping Pharmacogenomics and Drug Therapy. Intell. Pharm. 2024, 2, 643–650. [Google Scholar] [CrossRef]
- Ghebrehiwet, I.; Zaki, N.; Damseh, R.; Mohamad, M.S. Revolutionizing Personalized Medicine with Generative AI: A Systematic Review. Artif. Intell. Rev. 2024, 57, 128. [Google Scholar] [CrossRef]
- Fattahi, M.R.; Dehghani, M.; Paknahad, S.; Rahiminia, S.; Zareie, D.; Hoseini, B.; Oroomi, T.R.; Motedayyen, H.; Arefnezhad, R. Clinical Insights into Nanomedicine and Biosafety: Advanced Therapeutic Approaches for Common Urological Cancers. Front. Oncol. 2024, 14, 1438297. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cardoso Delfino, C.; de Paula Pereira, M.C.; dos Santos Oliveira, M.; de Carvalho Favareto, I.; Valladão, V.S.; de Oliveira Mota, M.; Costa, M.V.B.; Sousa-Batista, A.J.; Balbino, T.A. Scaling Nanopharmaceutical Production for Personalized Medicine: Challenges and Strategies. J. Nanopart. Res. 2025, 27, 108. [Google Scholar] [CrossRef]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef]



| Type of RTKs | Name of the Receptor Family | Receptors | Function and Main Characterization | Reference |
|---|---|---|---|---|
| I | EPIDERMAL GROWTH FACTOR ErbB | ErbB-1 (HER1) or EGFR (epidermal growth factor receptor) ErbB-2 (HER2) ErbB-3 (HER3) ErbB-4 (HER4) | Regulation of cell growth, proliferation and migration of tumors. EGFR and HER2 are overexpressed in gastric cancer, HER3 preferentially activates the phosphatidylinositol 3-kinase (PI3K) pathway. | [14] |
| II | INSULIN RECEPTOR IR | IGF1R (insulin-like growth factor I receptor) InsR (insulin receptor) IRR (IR-related receptor) | Regulation of metabolism (main targets of action are liver, muscle and adipose tissue), growth, and proliferation. IR in the brain regulates cognitive behavior, food intake, dysfunction leads to diabetes, cancer or Alzheimer’s disease. | [15] |
| III | PDGFR, CSFR, Kit, FLT3 | PDGFR α/β (platelet-derived growth factor receptor α/β) CSF1R (colony-stimulating factor 1 receptor) c-Kit FLT3 (fms-related tyrosine kinase 3) | Mutations have a major impact on leukemic transformation of acute myeloid leukemia (AML) cells. PDGFR α/β also regulates bone formation, tissue repair, and fibroblast proliferation. | [16] |
| IV | VASCULAR ENDOTHELIAL GROWTH FACTOR VEGF | VEGFR-1 or Flt-1 VEGFR-2 or KDR VEGFR-3 or Flt-4 (vascular endothelial growth factor receptors 1/2/3) or (fms related receptor tyrosine kinase 1/4 and kinase insert domain receptor) | Regulation of tumor-induced angiogenesis. VEGFRs are essential for the development of hematopoietic cells, vascular endothelial cells and lymphatic endothelial cells, VEGFR3 plays a critical role in lymphangiogenesis and the spread of tumor cells to regional lymph nodes. | [17] |
| V | FIBROBLAST GROWTH FACTOR FGF | FGFR1/2/3/4 (fibroblast growth factor receptor 1/2/3/4) | Promotion of cell survival, proliferation, development, angiogenesis and differentiation. Highest alteration frequency of FGFR was found in urothelial cancer, cholangiocarcinoma, endometrial cancer, squamous lung cancers, breast cancer and cervical cancer. | [18] |
| VI | PTK7/CCK4 | PTK7 (tyrosine-protein kinase-like 7) or CCK4 (colon carcinoma kinase 4) | PTK7 influences the establishment of cell polarity, regulation of cell movement and migration and cell invasion. Pseudotyrosine kinase PTK7 is overexpressed in several solid tumors and hematological malignancies and linked to metastasis, poor prognosis, and resistance to treatment. | [19] |
| VII | TROPOMYOSIN RECEPTOR KINASE TRK | TRKA/B/C | TRKs are encoded by the NTRK genes (neurotrophins) and play a role in the development and normal functioning of the nervous system. NTRK gene fusions occur in thyroid cancer, colorectal and appendiceal cancer, lung cancer, sarcoma, central nervous system or gastrointestinal stromal tumors. | [20] |
| VIII | ROR | ROR1/2 (receptor tyrosine kinase like orphan receptor 1/2) | Regulation of cell polarity, migration, proliferation and differentiation during developmental morphogenesis, tissue-/organo-genesis and regeneration of adult tissues following injury. RORs are implicated in age-related diseases, including tissue fibrosis, atherosclerosis (or arteriosclerosis), neurodegenerative diseases, and cancers. | [21] |
| IX | MuSK | MuSK (muscle-specific tyrosine kinase receptor) | Regulation of formation and stabilization of neuromuscular junctions (NMJs). MuSK is expressed in mammalian tissues other than skeletal muscle, including excitatory neurons in the central nervous system. | [22] |
| X | HEPATOCYTE GROWTH FACTOR HGF or SCATTER FACTOR SF | MET (proto-oncogene tyrosine kinase receptor) | MET is expressed in all human cell types, overexpressed in multisystem tumors, including respiratory, digestive, reproductive, nervous and epithelial tissue tumors. | [23] |
| XI | TAM | AXL TYRO3 MER | Alteration of TAM receptor function can lead to autoimmune disease, retinitis pigmentosa, and cancers (myeloid and lymphoblastic leukemias, melanoma, breast, lung, colon, liver, gastric, kidney, ovarian, uterine, and brain) | [24] |
| XII | TIE | TIE1/2 | TIEs are expressed in endothelial cells and are key regulators of normal blood and lymphatic vessel development and of pathological processes, including tumor angiogenesis (Lewis lung carcinoma, melanoma, EL4 leukemia/lymphoma), progression and metastasis, atherosclerosis, and vascular leakage. | [25] |
| XIII | EPH RECEPTOR- -INTERACTING PROTEIN Ephrin | EphA1 to EphA8 and inactive EphA10 EphB1 to EphB4 and inactive EphB6 (erythroprotein- -producing human hepatocellular receptors) | The largest of the RTK families. Ephs are expressed in most adult tissues and on immune system cells and have complex roles in embryonic and neural developmental processes such as cell segregation and migration, spatial organization of cell populations, tissue boundary formation, axonal guidance, and angiogenesis. Eph receptors are involved in the pathogenesis of various diseases, e.g., atherosclerosis, fibrosis, CNS diseases and cancer (EphA10 expressed on breast cancer cells) | [26] |
| XIV | RET | RET (proto-oncogene receptor) | Mutations in the RET gene have been found in several different cancers of neuroendocrine origin (papillary thyroid carcinoma, medullary thyroid carcinoma, multiple endocrine neoplasias) and a gut syndrome characterized by intestinal obstruction known as Hirschsprung’s disease. | [27] |
| XV | RYK | RYK (receptor like tyrosine kinase) | RYK is highly expressed in various malignancies, including mesothelioma, small cell lung cancer, gastric cancer, glioblastoma, liver cancer, acute leukemias and breast cancer. | [28] |
| XVI | DDR | DDR1/2 (discoidin domain receptor tyrosine kinase 1/2) | DDRs recognize collagens as their ligands and regulate cell-collagen interactions in normal and pathological conditions. | [29] |
| XVII | ROS | ROS1 (proto-oncogene 1) | ROS1 overexpression is observed in 80−100% of metastatic oral squamous cell carcinomas, upregulated in E-cadherin-deficient breast cancers, glioblastoma, NSCLC, Spitzoid neoplasms and inflammatory myofibroblastic tumors. | [30] |
| XVIII | LMK | LMTK1-a LMTK1-b LMTK2 LMTK3 (lemur tyrosine kinase 1-a/1-b/2/3) | Regulation of axonal transport and endosomal trafficking, modulation of synaptic functions, memory and learning. LMTKs are involved in various diseases including cancer (breast, prostate, lung, colorectal, renal, testis and ovarian, thyroid, pancreatic, bladder, gastric, glio- and neuroblastoma, and leukemia), cystic fibrosis, Alzheimer’s disease, amyotrophic lateral sclerosis/frontotemporal dementia and global developmental delay/intellectual disability. | [31] |
| XIX | LTK | LTK (leukocyte receptor tyrosine kinase) ALK (anaplastic lymphoma kinase receptor) | Very little is known about the physiological role of LTK tyrosine kinase. The LTK gene is preferentially expressed in leukemias with no cell lineage specificity, but not in other neoplasms. Most mutations of the ALK gene are in the form of a translocation with another partner gene leading to a fusion oncogene. ALK-rearrangement was identified in many different cancers, including inflammatory myofibroblastic tumors, diffuse large B-cell lymphoma, non-small-cell lung cancer, and esophageal squamous cell, colorectal, and breast carcinomas. | [32,33] |
| XX | STYK | STYK1 (serine/threonine/ tyrosine kinase 1) | STYK1 is a potent oncogene that enhances cell proliferation in vitro and drives both tumorigenesis and metastasis in animal model systems and aberrant expression has been identified in a wide range of cancer types, including lung, ovarian, breast, colorectal, prostate and renal cell cancer. | [34] |
| International Name | Brand Name | Company | Year Approved | Primary Targets | Therapeutic Indications | ||
|---|---|---|---|---|---|---|---|
| FDA | EMA | ||||||
| 1 | Fruquintinib | Fruzaqla | Takeda Pharma | 2023 | 2024 | VEGFR | Metastatic colorectal cancer |
| 2 | Quizartinib | Vanflyta | Daiichi Sankyo | 2023 | 2023 | FLT3 | Acute myeloid leukemia |
| 3 | Repotrectinib | Augtyro | Bristol Myers | 2023 | disapproved | ROS1 | Non-small-cell lung carcinoma |
| 4 | Futibatinib | Lytgobi | Taiho Pharma Netherlands B.V. | 2022 | 2023 | FGFR2 | Cholangiocarcinoma |
| 5 | Infigratinib | Truseltiq | QED Therapeutics | 2021 a | - | FGFR2 | Cholangiocarcinoma |
| 6 | Mobocertinib | Exkivity | Takeda Pharma | 2021 | application withdrawn | EGFR | Non-small-cell lung carcinoma |
| 7 | Tepotinib | Tepmetko | Merck | 2021 | 2022 | MET | Non-small-cell lung carcinoma |
| 8 | Tivozanib | Fotivda | AVEO Pharma | 2021 | 2017 | VEGFR | Kidney cancer |
| 9 | Avapritinib | Ayvakyt | Blueprint Medicines | 2020 | 2020 | PDGFRα KIT | Gastrointestinal stromal tumors Mastocytosis |
| 10 | Capmatinib | Tabrecta | Novartis | 2020 | 2022 | MET | Non-small-cell lung carcinoma |
| 11 | Pemigatinib | Pemazyre | Incyte | 2020 | 2021 | FGFR | Cholangiocarcinoma |
| 12 | Pralsetinib | Gavreto | Rigel Pharma | 2020 | 2021 | RET | Non-small-cell lung carcinoma |
| 13 | Ripretinib | Qinlock | Decipera Pharma | 2020 | 2021 | KIT PDGFRα | Gastrointestinal stromal tumor Stomach and bowel cancer |
| 14 | Selpercatinib | Retsevmo | Eli Lilly | 2020 | 2021 | RET | Non-small-cell lung carcinoma Thyroid cancer Solid tumors |
| 15 | Tucatinib | Tukysa | Pfizer | 2020 | 2021 | ErbB2/HER2 | HER2-positive breast cancer |
| 16 | Entrectinib | Rozlytrek | Genentech (Roche) | 2019 | 2020 | TRK ROS1 | Solid tumors Non-small-cell lung carcinoma |
| 17 | Erdafitinib | Balversa | Janssen Pharma | 2019 | 2024 | FGFR | Urothelial bladder and urinary cancer |
| 18 | Pexidartinib | Turalio | Daiichi Sankyo | 2019 | refused | CSF1R KIT FLT3 | Tenosynovial giant cell tumors |
| 19 | Ftinib | Vizimpro | Pfizer | 2018 | 2019 | EGFR | Non-small-cell lung carcinoma |
| 20 | Gilteritinib | Xospata | Astellas Pharma | 2018 | 2019 | FLT3 | Acute myeloid leukemia |
| 21 | Larotrectinib | Vitrakvi | Bayer | 2018 | 2019 | TRK | Lungs, thyroid glands and intestines carcinomas |
| 22 | Lorlatinib | Lorbrena (US, Canada, Japan) Lorviqua (EU) | Pfizer | 2018 | 2019 | ALK | Non-small-cell lung carcinoma |
| 23 | Brigatinib | Alunbrig | Takeda Pharma | 2017 | 2018 | ALK | Non-small-cell lung carcinoma |
| 24 | Midostaurin | Rydapt | Novartis | 2017 | 2017 | FLT3 KIT | Acute myeloid leukemia |
| 25 | Neratinib | Nerlynx | Puma Biotech | 2017 | 2018 | ErbB2/HER2 | HER2-positive breast cancer |
| 26 | Alectinib | Alecensa | Roche | 2015 | 2017 | ALK | Non-small-cell lung carcinoma |
| 27 | Lenvatinib | Lenvima | Easai | 2015 | 2015 | VEGFR FGFR RET | Thyroid Neoplasms Hepatocellular carcinoma Endometrial carcinoma |
| 28 | Osimertinib | Tagrisso | AstraZeneca | 2015 | 2016 | EGFR | Non-small-cell lung carcinoma |
| 29 | Ceritinib | Zykadia | Novartis | 2014 | 2015 | ALK | Non-small-cell lung carcinoma |
| 30 | Nintedanib | Vargatef | Boehringer Ingelheim | 2014 | 2014 | VEGFR FGFR PDGFR | Non-small-cell lung carcinoma |
| 31 | Afatinib | Giotrif | Boehringer Ingelheim | 2013 | 2013 | ErbB | Non-small-cell lung carcinoma |
| 32 | Axitinib | Inlyta | Pfizer | 2012 | 2012 | VEGFR | Renal cell carcinoma |
| 33 | Cabozantinib | Cometriq capsule Cabometyx tablet form | Exelixis | 2012 | 2014 | VEGFR MET RET | Thyroid neoplasms Renal cell carcinoma Hepatocellular carcinoma |
| 34 | Regorafenib | Stivarga | Bayer | 2012 | 2013 | VEGFR | Colorectal cancer Gastrointestinal stromal tumor Hepatocellular carcinoma |
| 35 | Crizotinib | Xalkori | Pfizer | 2011 | 2012 | ALK ROS1 | Non-small-cell lung carcinoma |
| 36 | Vandetanib | Caprelsa (US) Zactima (EU) | Sanofi | 2011 | application withdrawn | EGFR VEGFR | Medullary thyroid cancer |
| 37 | Pazopanib | Votrient | GSK | 2009 | 2010 | VEGFR PDGFR FGFR | Renal cell carcinoma Soft-tissue sarcomas |
| 38 | Lapatinib | Tyverb | GSK | 2007 | 2008 | ErbB2/HER2 | Breast neoplasms |
| 39 | Sunitinib | Sutent | Pfizer | 2006 | 2006 | PDGFR VEGFR KIT | Gastrointestinal stromal tumors Renal cell carcinoma Neuroendocrine tumors |
| 40 | Sorafenib | Nexavar | Bayer | 2005 | 2006 | VEGFR KIT FLT3 | Hepatocellular carcinoma Renal cell carcinoma Differentiated thyroid carcinoma |
| 41 | Erlotinib | Tarceva | Genentech (Roche Group) | 2004 | 2005 | EGFR | Non-small-cell lung carcinoma Pancreatic neoplasms |
| 42 | Gefitinib | Iressa | AstraZeneca | 2003 | 2009 | EGFR | Non-small-cell lung carcinoma |
| International Name | Name of Medicine | Company | Year Approved | Primary Targets | Therapeutic Indications | ||
|---|---|---|---|---|---|---|---|
| FDA | EMA | ||||||
| 1 | Momelotinib | Ojjaara (US) Omjjara (EU) | GlaxoSmith Kline | 2023 | 2024 | JAK | Splenomegaly Myeloproliferative disorders |
| 2 | Pirtobrutinib | Jaypirca | Eli Lilly | 2023 | 2023 | BTK | Mantle cell lymphoma |
| 3 | Ritlecitinib | Litfulo | Pfizer | 2023 | 2023 | JAK3 | Alopecia areata |
| 4 | Abrocitinib | Cibinqo | Pfizer | 2022 | 2021 | JAK | Atopic dermatitis |
| 5 | Deucravacitinib | Sotyktu | Bristol-Myers Squibb | 2022 | 2023 | TYK2 JAK | Psoriasis |
| 6 | Pacritinib | Vonjo | CTI Biopharma | 2022 | disapproved | JAK | Post-polycythemia vera Post-essential thrombocythemia |
| 7 | Asciminib | Scemblix | Novartis | 2021 | 2022 | BCR-ABL | Chronic myeloid leukemia |
| 8 | Fedratinib | Inrebic | Bristol-Myers Squibb | 2019 | 2021 | JAK | Myelofibrosis |
| 9 | Zanubrutinib | Brukinsa | BeiGene USA | 2019 | 2021 | BTK | Waldenström’s macroglobulinemia Marginal zone lymphoma Chronic lymphocytic leukemia Follicular lymphoma |
| 10 | Upadacitinib | Rinvoq | AbbVie | 2019 | 2019 | JAK | Rheumatoid arthritis Psoriatic arthritis Atopic dermatitis Axial spondyloarthritis Ulcerative colitis |
| 11 | Fostamatinib | Tavalisse (US) Tavlesse (EU) | Rigel Pharma | 2018 | 2020 | SYK (SRC family) | Chronic immune thrombocytopenia |
| 12 | Baricitinib | Olumiant | Eli Lilly | 2018 | 2017 | JAK | Rheumatoid arthritis Atopic dermatitis Alopecia areata Juvenile idiopathic arthritis |
| 13 | Acalabrutinib | Calquence | Astra Zeneca | 2017 | 2020 | BTK | Chronic lymphocytic leukemia Blood cancer affecting B cells |
| 14 | Ibrutinib | Imbruvica | Pharmacyclics LLC | 2013 | 2014 | BTK | Mantle cell lymphoma Chronic lymphocytic leukemia Waldenström’s macroglobulinemia |
| 15 | Bosutinib | Bosulif | Pfizer | 2012 | 2013 | BCR-ABL | Chronic lymphocytic leukemia |
| 16 | Ponatinib | Iclusig | Takeda Pharms | 2012 | 2013 | BCR-ABL | Chronic lymphocytic leukemia Acute lymphoblastic leukemia |
| 17 | Tofacitinib | Xeljanz | Pfizer | 2012 | 2017 | JAK | Rheumatoid arthritis Psoriatic arthritis Juvenile idiopathic arthritis Ulcerative colitis Ankylosing spondylitis |
| 18 | Ruxolitinib | Jakafi (US) Jakavi (EU) | Incyte | 2011 | 2012 | JAK | Splenomegaly Polycythemia vera Acute or chronic graft-versus-host disease |
| 19 | Nilotinib | Tasigna | Novartis | 2007 | 2007 | BCR-ABL | Chronic lymphocytic leukemia |
| 20 | Dasatinib | Sprycel | Bristol Myers Squibb | 2006 | 2006 | BCR-ABL | Chronic myeloid leukemia Ph+ acute lymphoblastic leukemia |
| 21 | Imatinib | Gleevec (US) Glivec (EU) | Novartis | 2001 | 2001 | BCR-ABL | Chronic myeloid leukemia Ph+ acute lymphoblastic leukemia Myeloproliferative diseases Advanced hypereosinophilic syndrome Gastrointestinal stromal tumors Dermatofibrosarcoma protuberans |
| Type of Metal NPs | TKI | Treatment | Nanosystem Enhancement Effect | Reference, Year |
|---|---|---|---|---|
| platinum | sunitinib | Renal fibrosis | Accelerated drug accumulation, longer time in circulation | [131], 2012 |
| silver | erlotinib | - (SERS analysis) | - | [135], 2018 |
| imatinib | - (X-ray CT imaging, FCS measurement) | - | [137], 2017 | |
| gold | midostaurin | Acute myeloid leukemia (AML) | Inhibition of tumor formation, increased cytotoxicity against tumor cells | [144], 2024 |
| pyrotinib | Early breast cancer (EBC) | Inhibition of cell proliferation, photothermal effect, tumor shrinkage Inhibition of cell proliferation, photothermal effect, tumor shrinkage Inhibition of cell proliferation, photothermal effect, tumor shrinkage Inhibition of cell proliferation, photothermal effect, tumor shrinkage | [145], 2024 | |
| gefitinib | Non-small cell lung cancer (NSCLC) | Almost complete removal of tumor tissue by the photothermal effect, reducing the viability of the tumor cell line | [148], 2023 | |
| sorafenib | Renal cancer | High percentage of drug released, tumor growth suppression, reduction in hepatotoxicity High percentage of drug released, tumor growth suppression, reduction of hepatotoxicity | [149], 2021 | |
| icotinib | - (UV/vis spectroscopy, TEM, DFT, SERS analysis) (SERS analysis) | - | [165], 2021 | |
| SI306 | Glioblastoma (GBM) | Reduction in tumor cell viability in combination with radiotherapy | [150], 2020 | |
| varlitinib | Pancreatic cancer | Reduction in tumor cell viability | [152], 2019 | |
| imatinib | Scleroderma-associated interstitial lung disease (SSc-ILD) | Controlled drug release, selective drug activity against c-Abl and PDGFR | [155], 2019 | |
| afatinib | Non-small cell lung cancer (NSCLC) | Improved biocompatibility and efficacy of the drug, reduced tumor cell viability | [156], 2019 | |
| nilotinib | - (Secondary ion mass spectrometry imaging) | - | [157], 2017 | |
| vandetanib | Metastatic breast cancer (MBC) | Reduction in migration and viability of tumor cells, induction of apoptosis | [158], 2017 | |
| imatinib | Chronic myelogenous leukemia (CML) | Decrease in IC50 of the drug, decrease in viability of drug-resistant cells | [159], 2017 | |
| midostaurin sorafenib lestaurtinib quizartinib | Acute myeloid leukemia (AML) | Decrease in the viability of tumor cells due to the effect of the gelatinous coating | [161], 2016 | |
| dasatinib | Acute myeloid leukemia (AML) | Increase in drug efficacy, decrease in toxicity | [162], 2016 | |
| sorafenib | Renal cell carcinoma (RCC) | Necrosis induced by the photothermal effect, significant reduction in the tumor | [163], 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurillová, A.; Kvítek, L.; Panáček, A. Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition. Pharmaceutics 2025, 17, 783. https://doi.org/10.3390/pharmaceutics17060783
Kurillová A, Kvítek L, Panáček A. Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition. Pharmaceutics. 2025; 17(6):783. https://doi.org/10.3390/pharmaceutics17060783
Chicago/Turabian StyleKurillová, Antónia, Libor Kvítek, and Aleš Panáček. 2025. "Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition" Pharmaceutics 17, no. 6: 783. https://doi.org/10.3390/pharmaceutics17060783
APA StyleKurillová, A., Kvítek, L., & Panáček, A. (2025). Innovative Approaches in Cancer Treatment: Emphasizing the Role of Nanomaterials in Tyrosine Kinase Inhibition. Pharmaceutics, 17(6), 783. https://doi.org/10.3390/pharmaceutics17060783








