Controlled Release Technologies for Diltiazem Hydrochloride: A Comprehensive Review of Solid Dosage Innovations
Abstract
1. Introduction
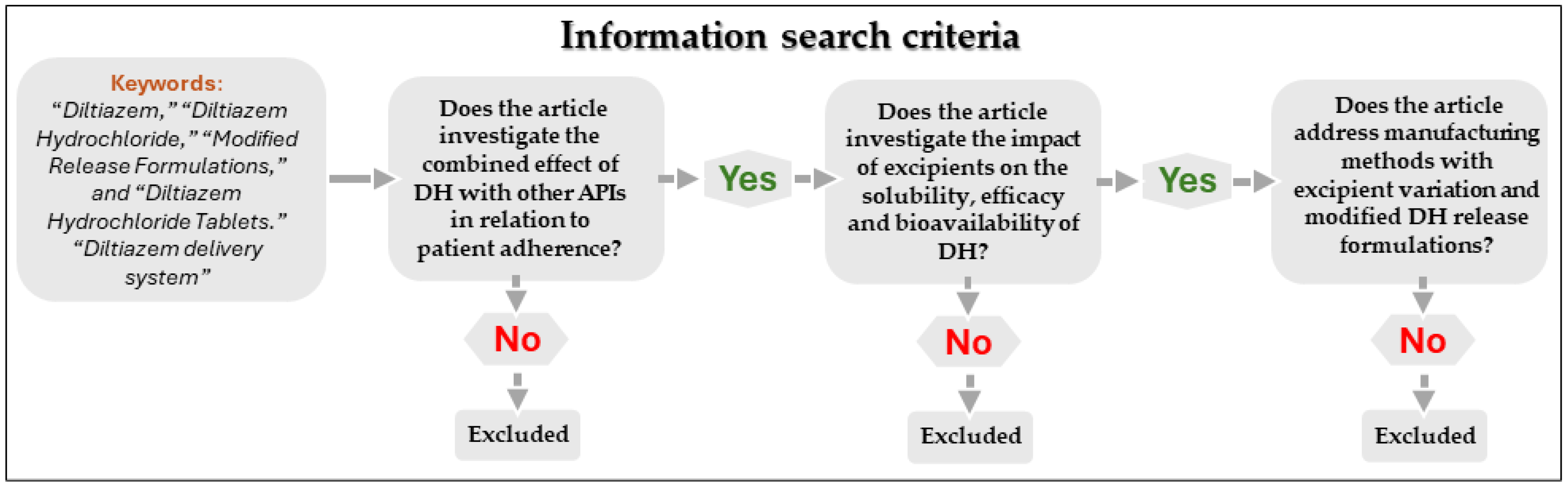
2. Methodology
3. Modified Release Dosage Forms (MRDFs)
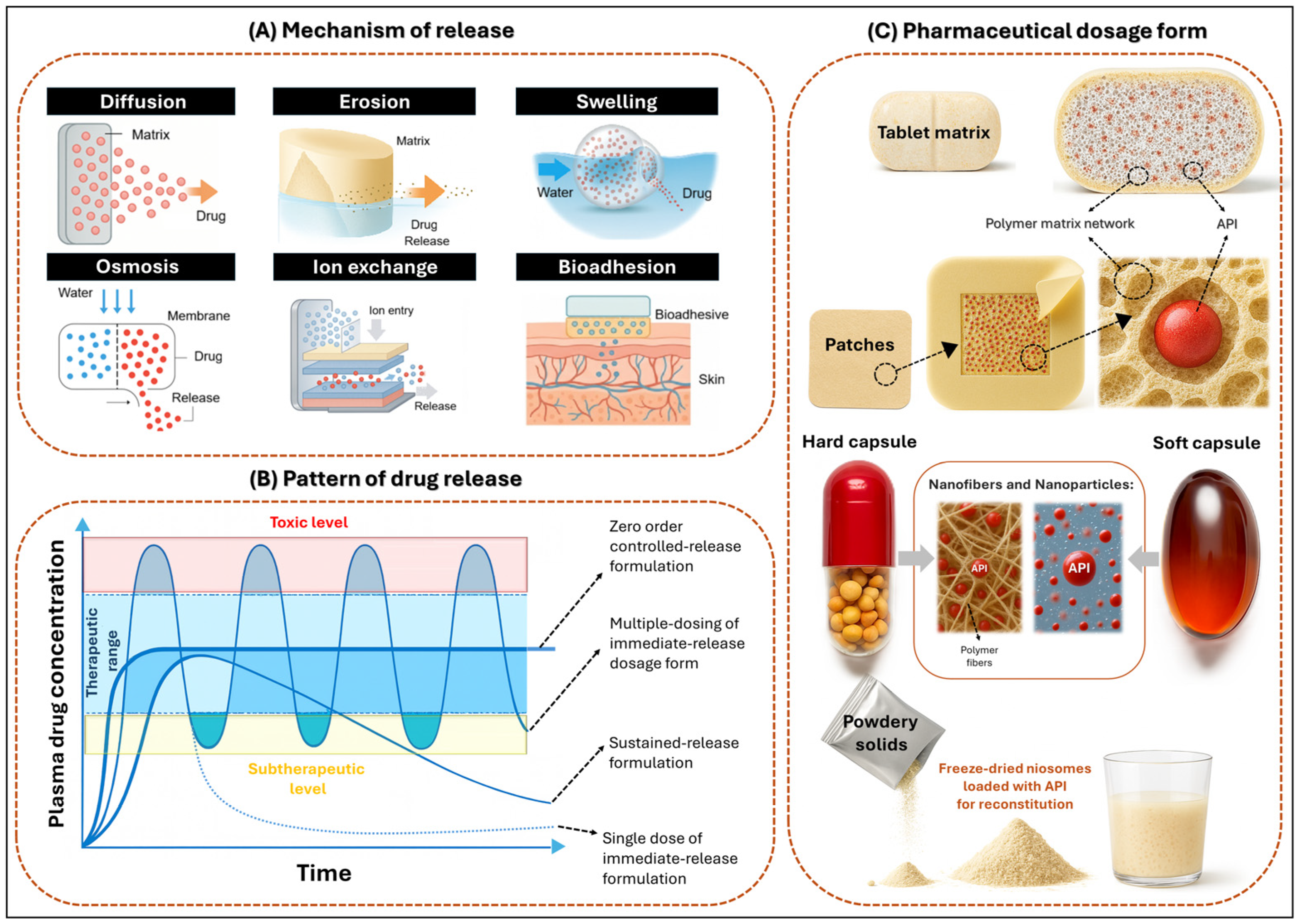
3.1. Mechanism of Release
3.2. Pattern of Drug Release
3.3. Pharmaceutical Dosage Form
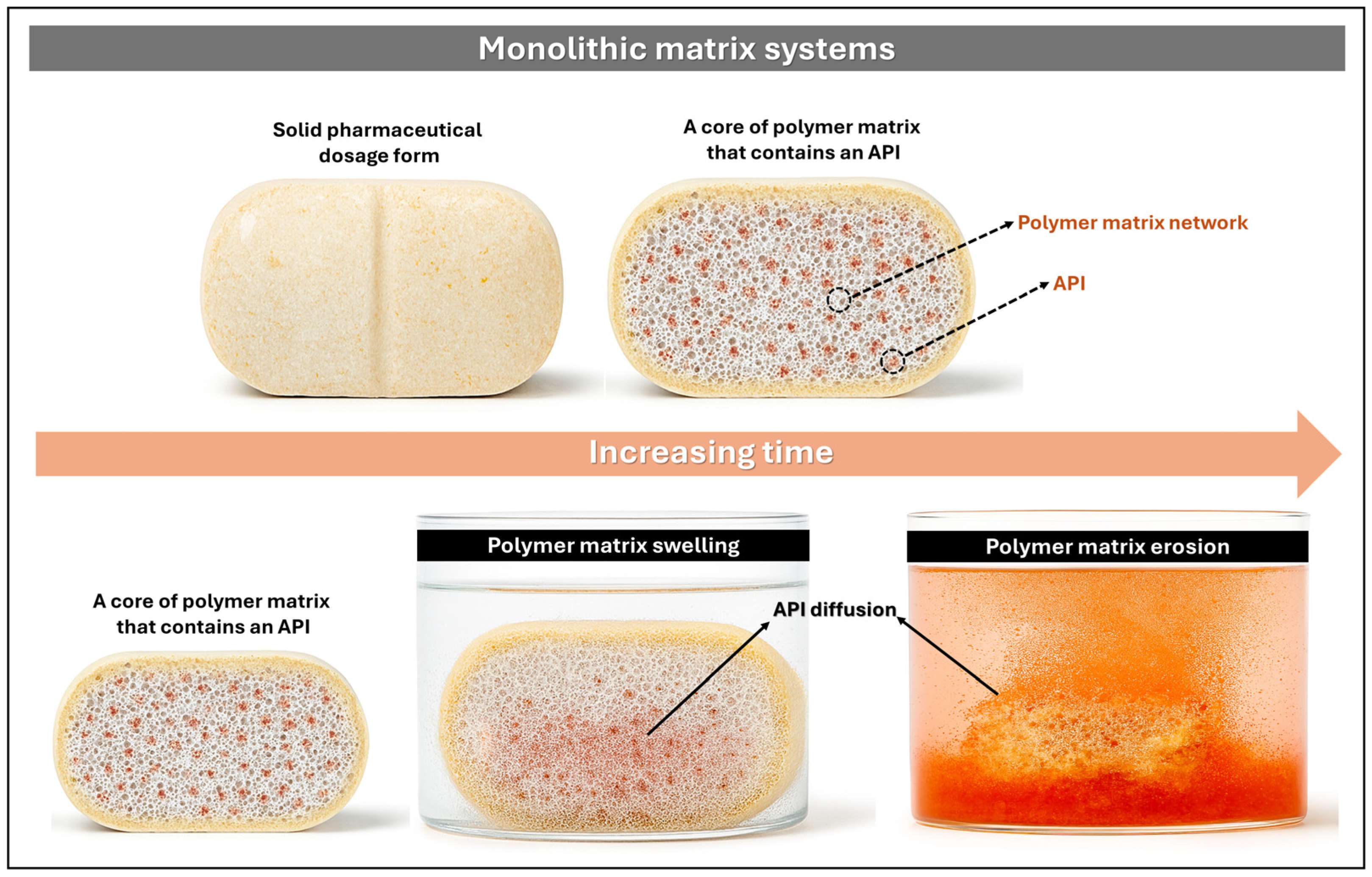
3.3.1. Monolithic Matrix Systems
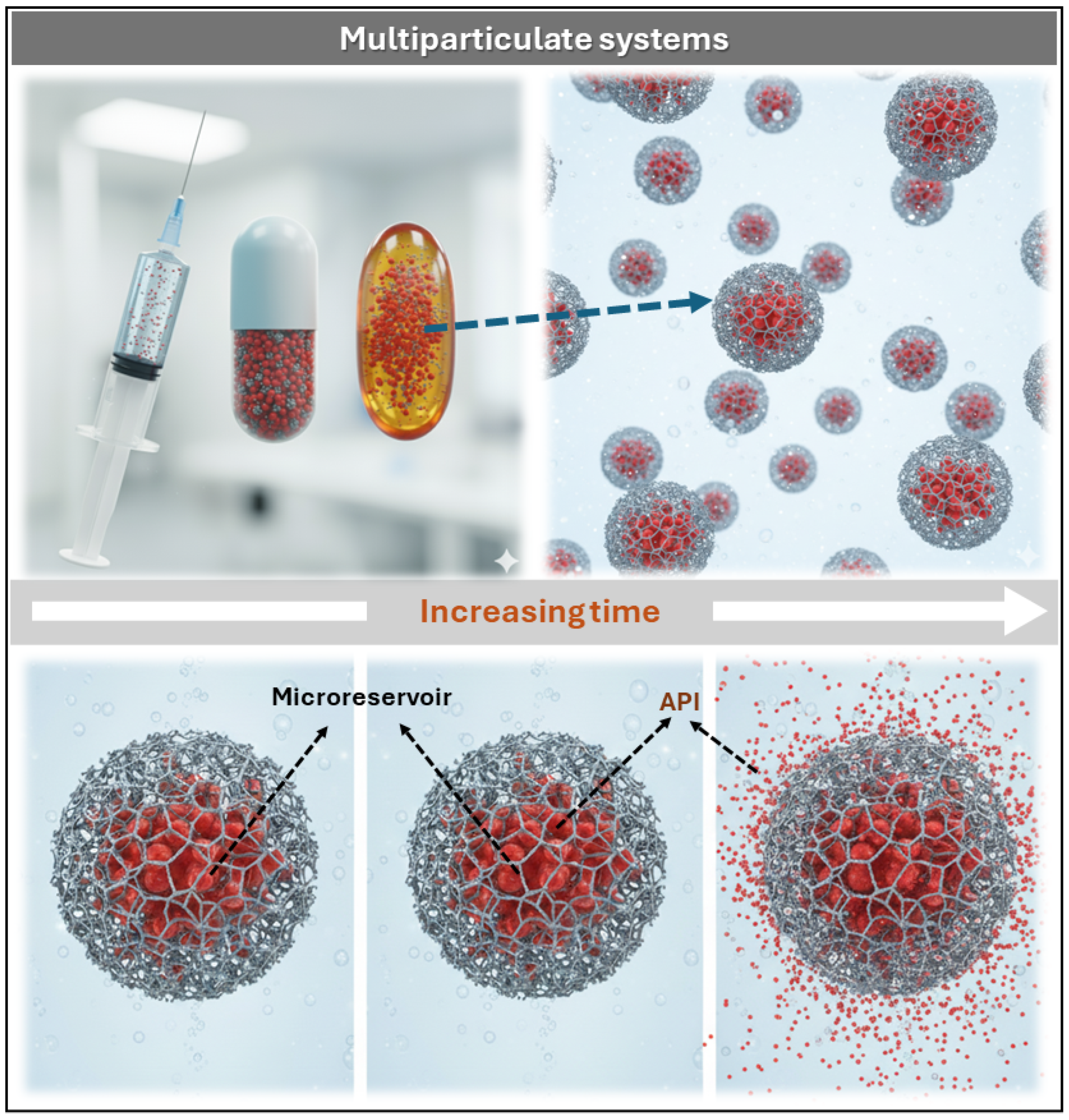
3.3.2. Multiparticulate Systems
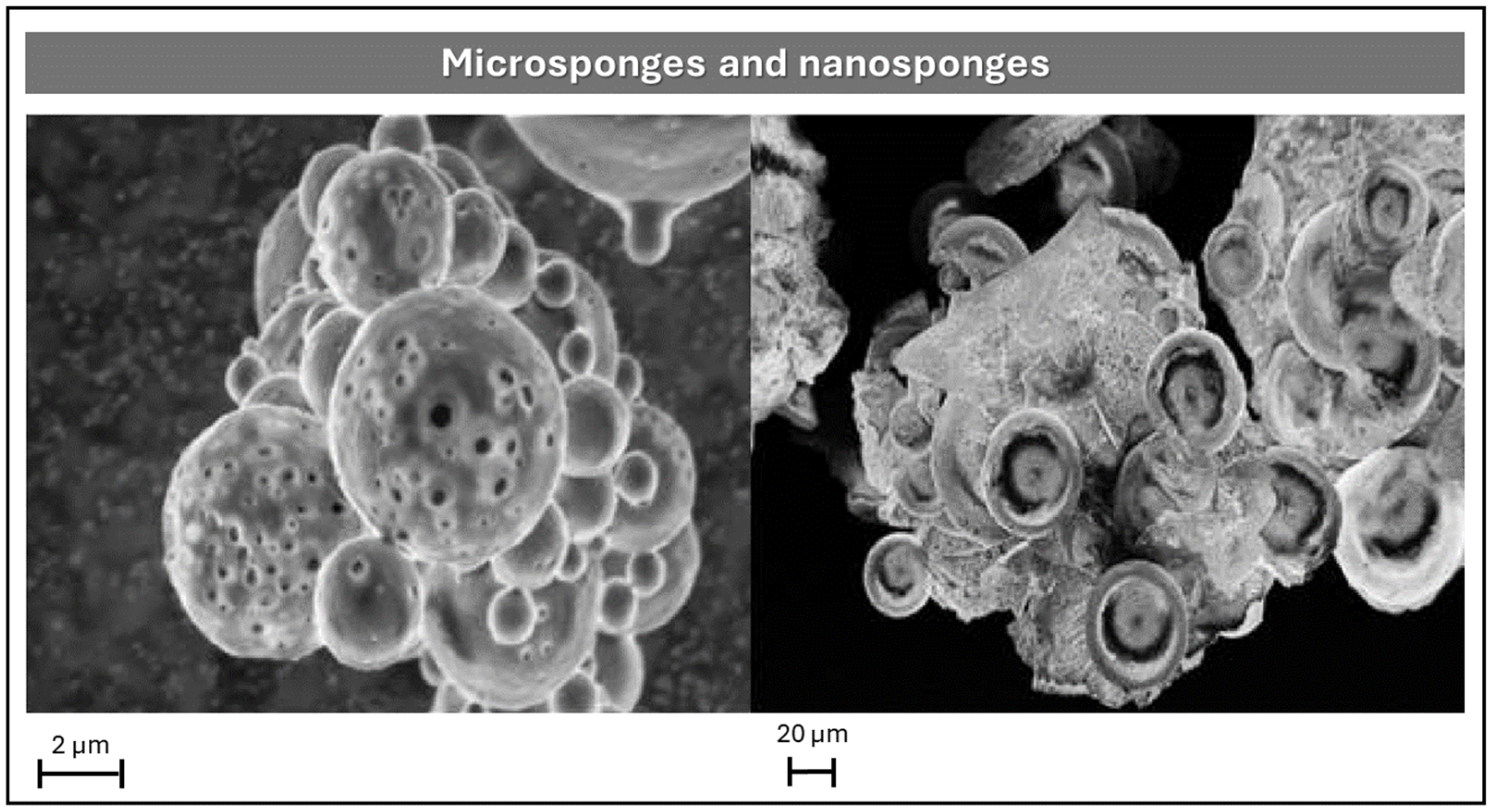
Microsponges and Nanosponges
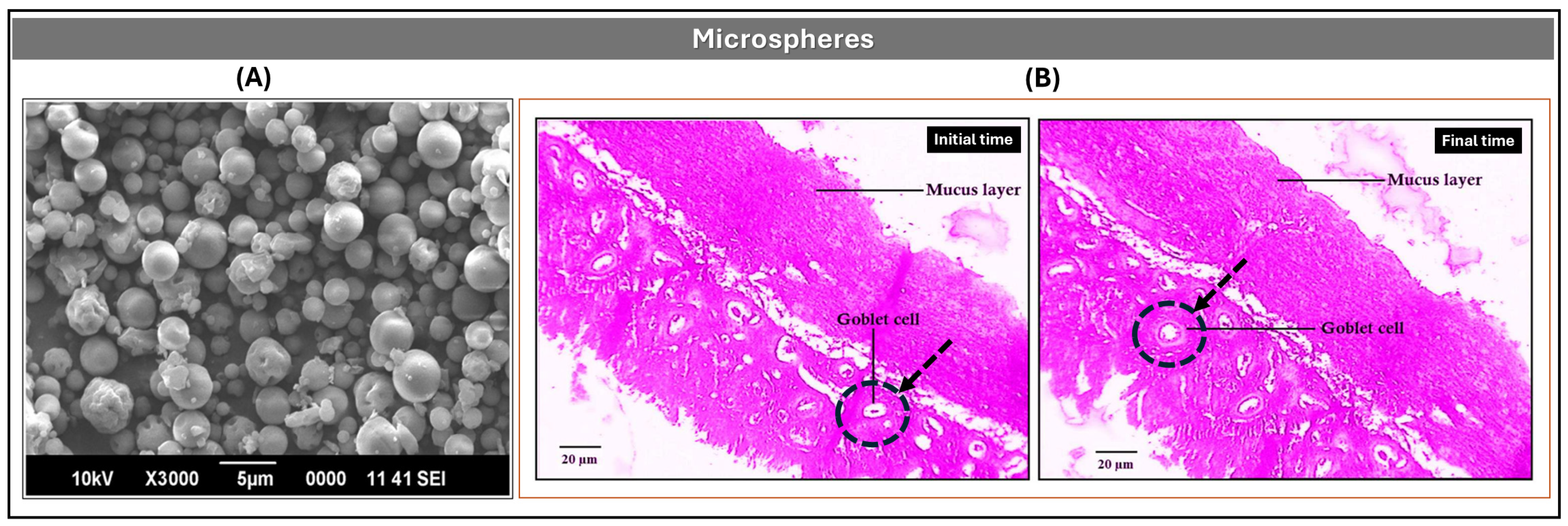
Microspheres
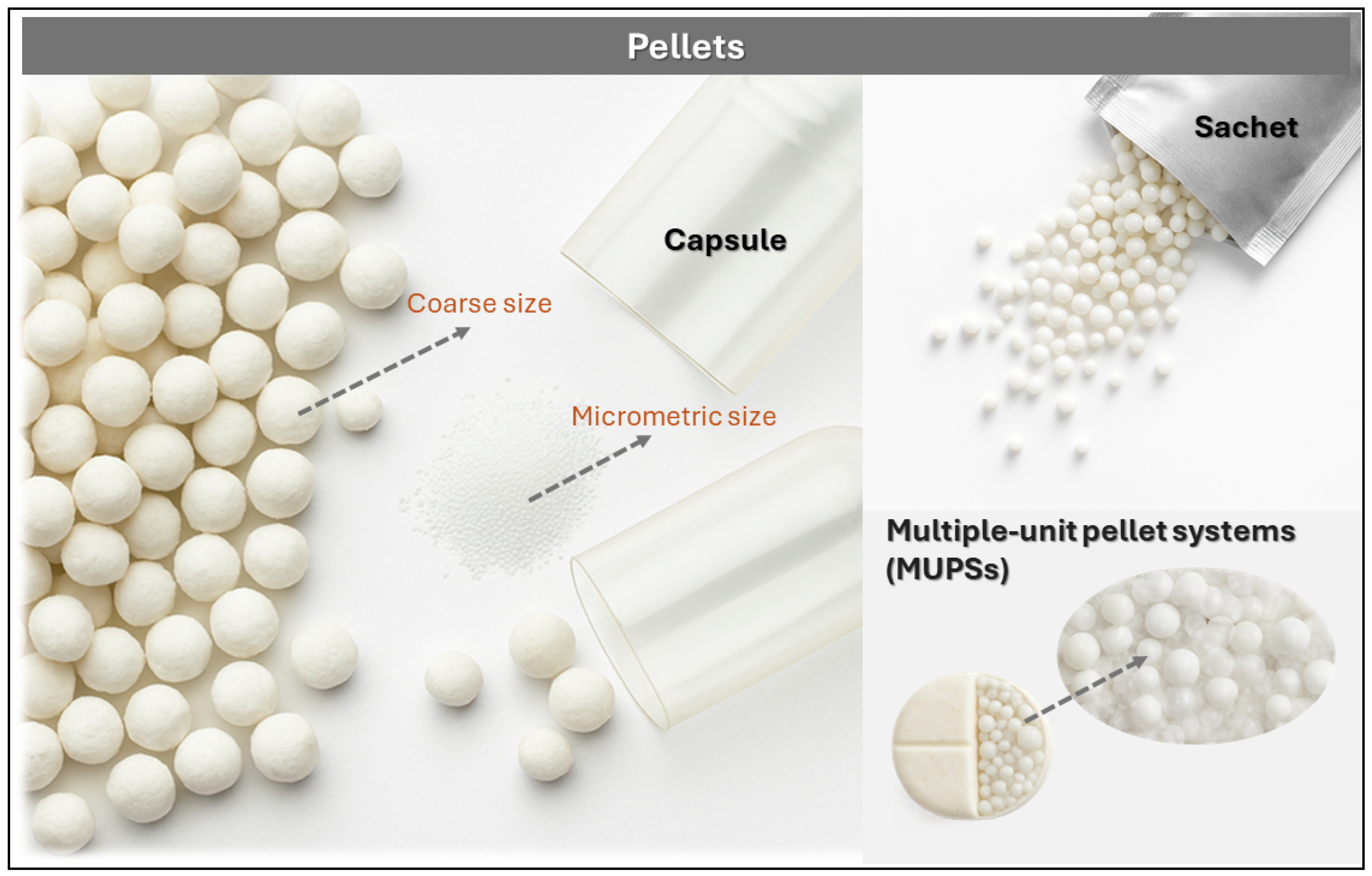
Pellets
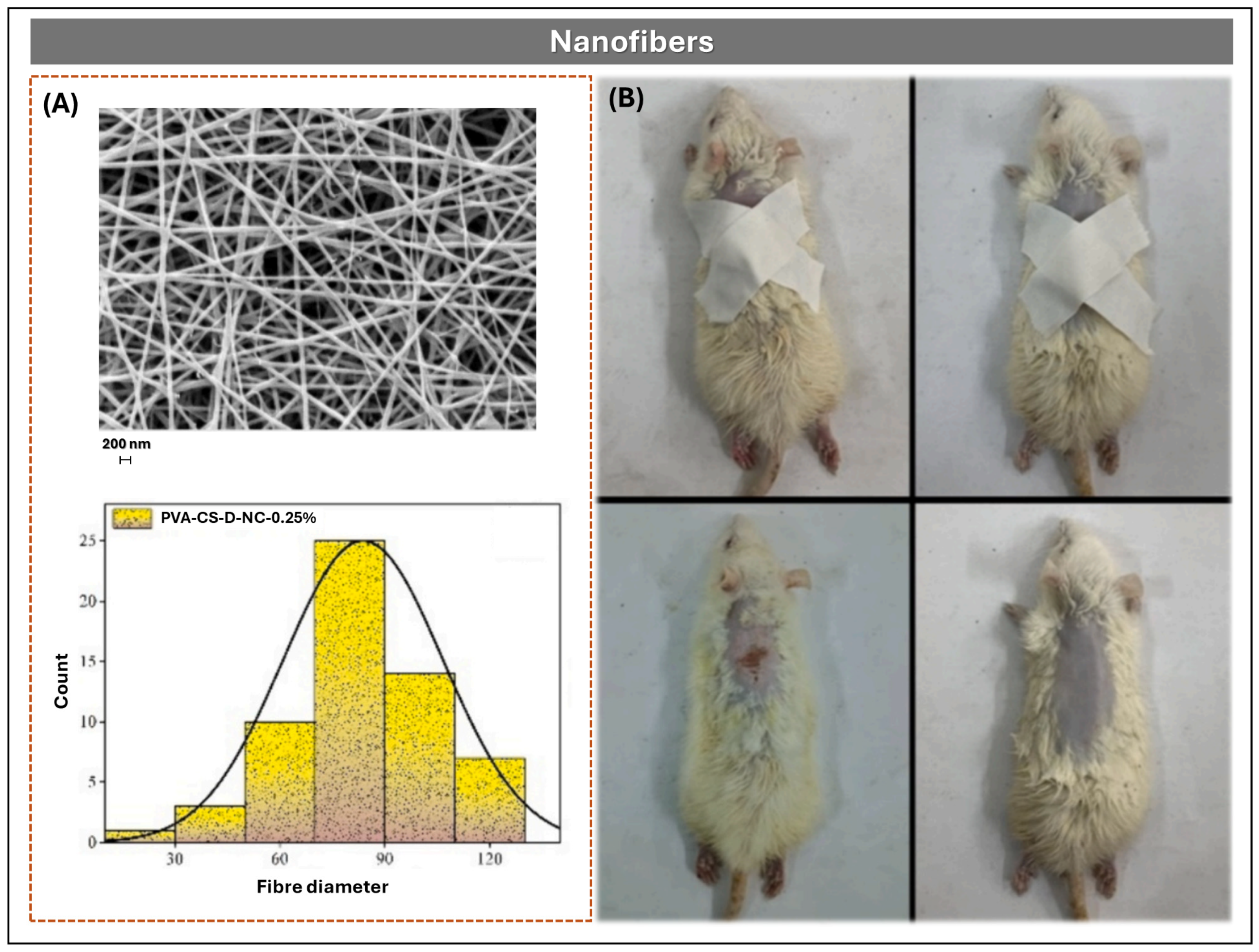
Nanofibers and Nanoparticles
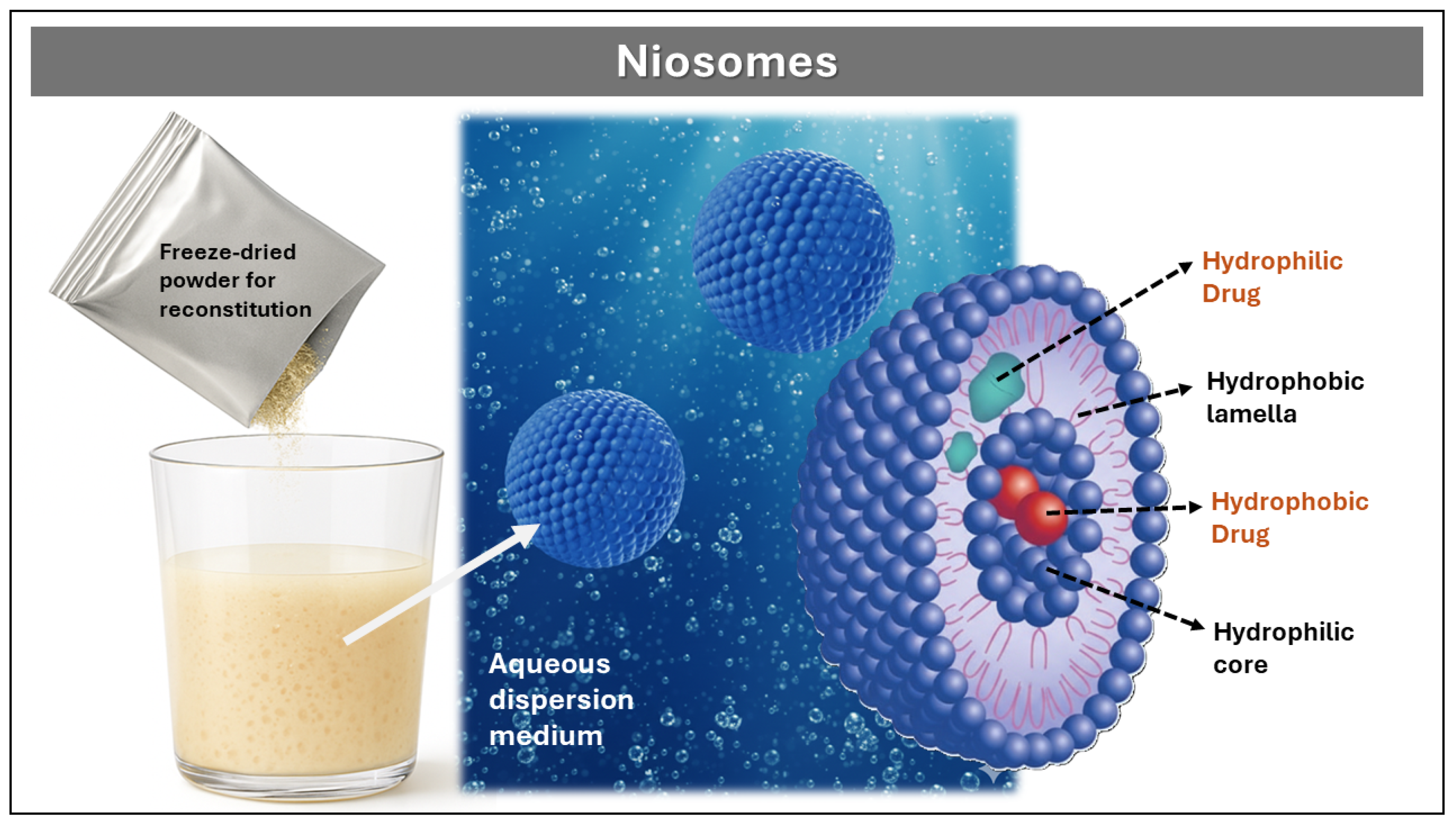
Niosomes
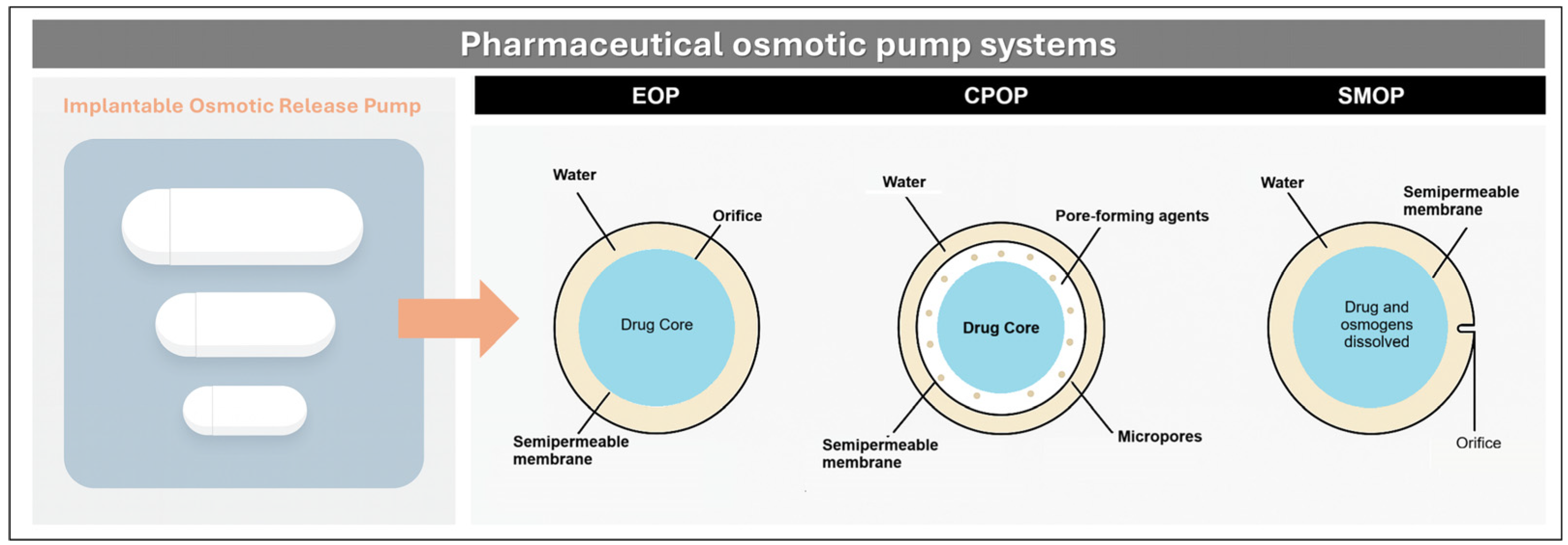
3.3.3. Reservoir-Type
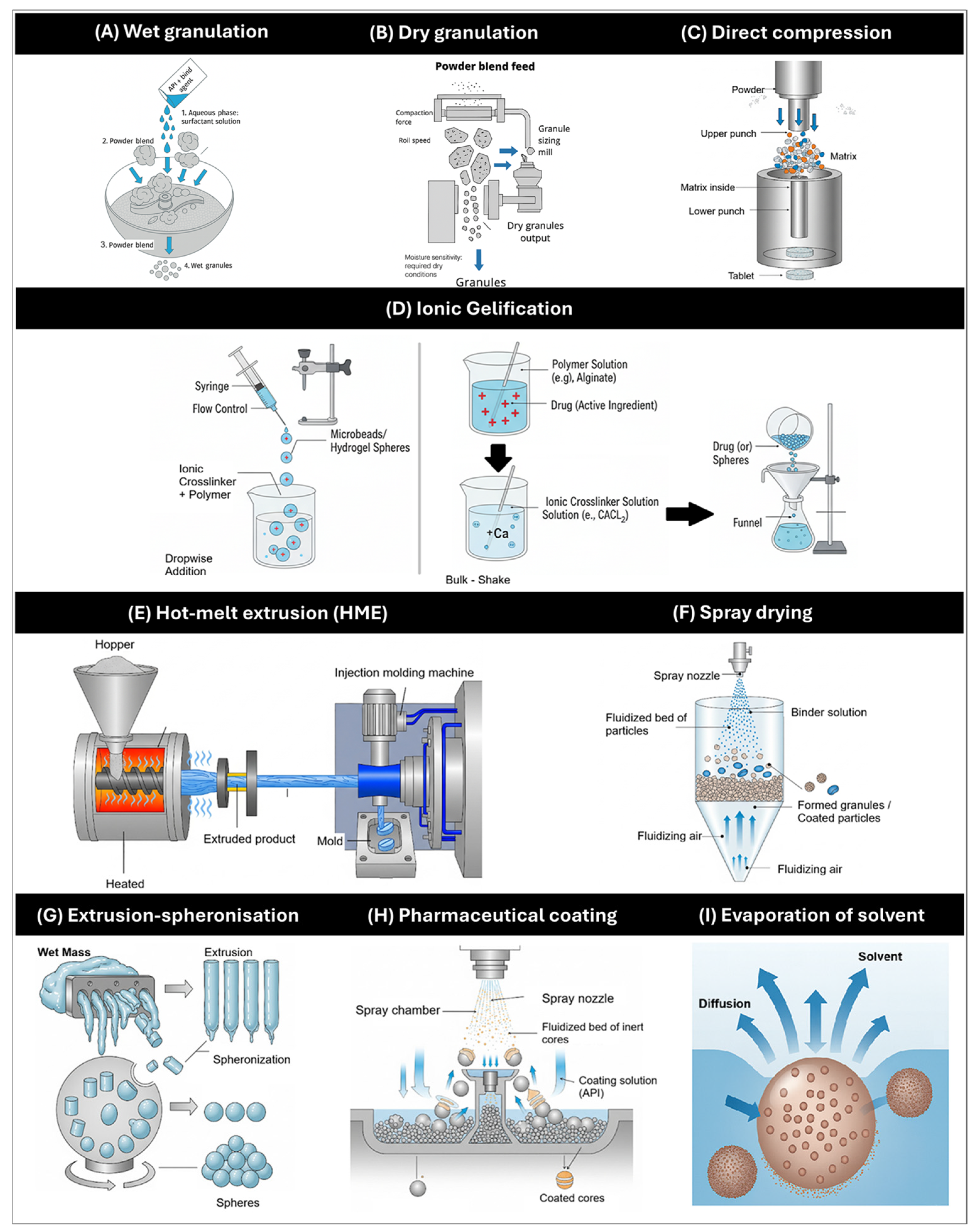
4. General Methods of Manufacture of MRDFs
4.1. Optimal Pharmacokinetics and Integration with Drug Delivery Technology
4.2. Wet and Dry Granulation
4.3. Direct Compression
4.4. Ionic Gelification
4.5. Hot-Melt Extrusion (HME)
4.6. Spray Drying
4.7. Extrusion-Spheronization
4.8. Pharmaceutical Coating
4.9. Evaporation of Solvent
4.10. Combination of Manufacturing Methods
4.11. Emerging Technologies: 3D Printing
4.12. Current Commercial Formulations of DH
4.13. Industrial Viability and Comparative Implementation Analysis
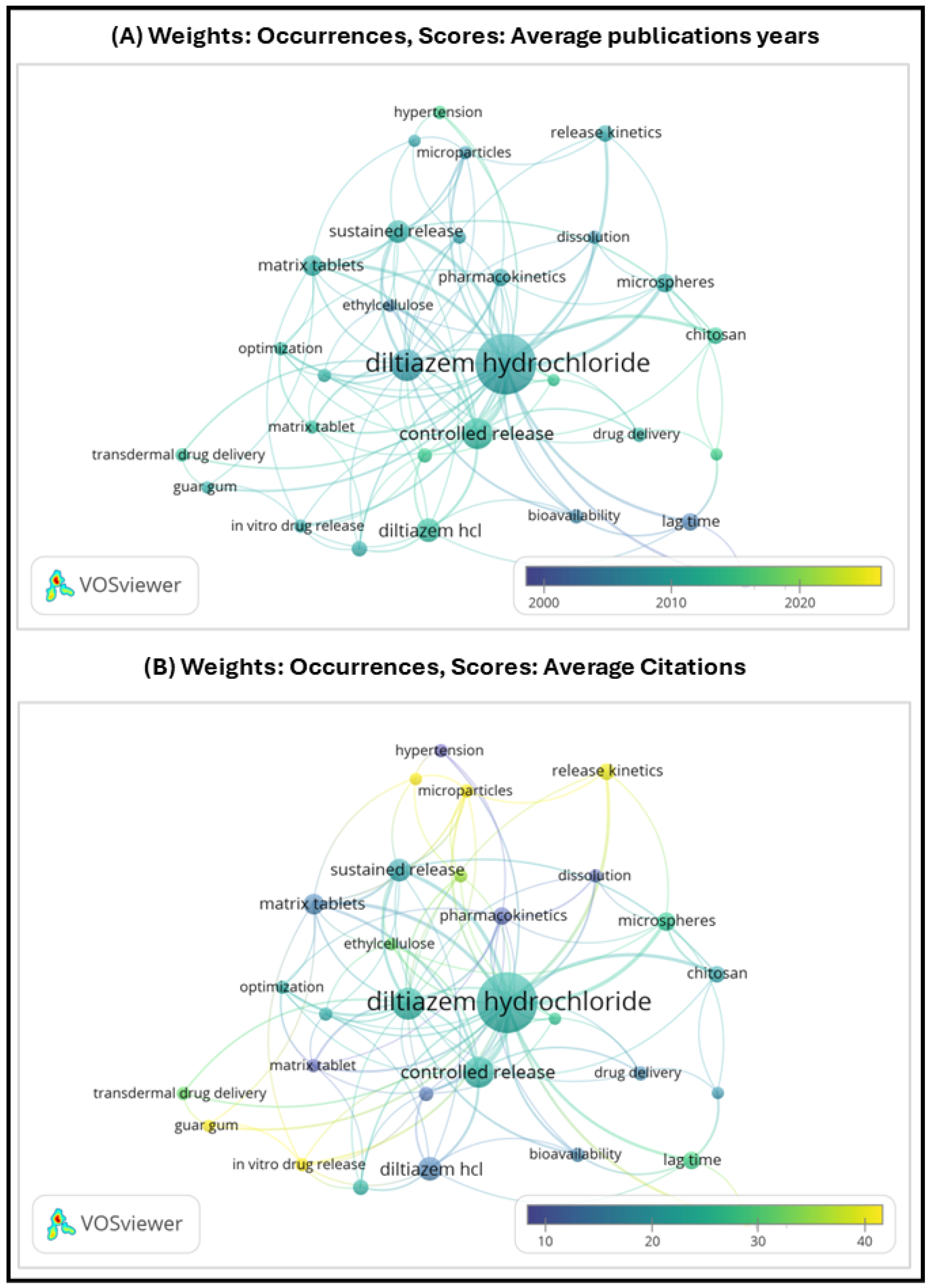
4.14. Market Traction and Bibliometric Visualization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| BCS | Biopharmaceutical Classification System |
| CAP | Cellulose Acetate Phthalate |
| CS | Chitosan |
| CPOP | Controlled Porosity Osmotic Pump |
| DH | Diltiazem Hydrochloride |
| DSC | Differential Scanning Calorimetry |
| ECM | Extracellular Matrix |
| EE | Encapsulation Efficiency |
| EOP | Elementary Osmotic Pump |
| FDM | Fused Deposition Modeling |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HME | Hot-Melt Extrusion |
| HPMC | Hydroxypropyl Methylcellulose |
| HPC | Hydroxypropylcellulose |
| ICH | International Council for Harmonisation |
| IVIVC | In Vitro–In Vivo Correlation |
| MCC | Microcrystalline Cellulose |
| MR | Modified Release |
| MRDFs | Modified Release Dosage Forms |
| MUPS | Multiple-Unit Pellet Systems |
| NaCMC | Sodium Carboxymethylcellulose |
| PCL | Polycaprolactone |
| PEC | Pectin |
| PEG | Polyethylene Glycol |
| PEO | Polyethylene Oxide |
| PVA | Polyvinyl Alcohol |
| QbD | Quality by Design |
| SEM | Scanning Electron Microscopy |
| SEDDS | Self-Emulsifying Drug Delivery System |
| SMOP | Solubility-Modulated Osmotic Pump |
| TGA | Thermogravimetric Analysis |
| TPP | Tripolyphosphate |
| USP | United States Pharmacopeia |
| XRD | X-ray Diffraction |
References
- Escobar, C.; Ariza, A.; Barrios, V.; Campuzano, R.; Freixa-Pamias, R.; Gámez, J.M.; Fernández Olmo, M.R.; Jorge-Pérez, P.; Tamargo, J. Actualización Del Uso de Los Fármacos Antianginosos En El Tratamiento Del Síndrome Coronario Crónico: Enfoque Práctico. Rev. Esp. Cardiol. 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Trapani, A.; Di Franco, C.; Mandracchia, D.; Trapani, G.; Franchini, C.; Corbo, F.; Tripodo, G.; Kolev, I.N.; Stoyanov, G.S.; et al. In Vitro and Ex Vivo Studies on Diltiazem Hydrochloride-Loaded Microsponges in Rectal Gels for Chronic Anal Fissures Treatment. Int. J. Pharm. 2019, 557, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, V.; Sharma, S.; Bhatt, P. Formulation and Evaluation of Sustained Release Tablet of Diltiazem Hydrochloride. Int. J. Pharm. Sci. Res. 2020, 11, 2193. [Google Scholar] [CrossRef]
- Jones, K.E.; Hayden, S.L.; Meyer, H.R.; Sandoz, J.L.; Arata, W.H.; Dufrene, K.; Ballaera, C.; Lopez Torres, Y.; Griffin, P.; Kaye, A.M.; et al. The Evolving Role of Calcium Channel Blockers in Hypertension Management: Pharmacological and Clinical Considerations. Curr. Issues Mol. Biol. 2024, 46, 6315–6327. [Google Scholar] [CrossRef] [PubMed]
- Kavishetti, R.; Halkeri, M.; Nargund, S.L.; Shahapur, A. Formulation and Evaluation of Mucoadhesive Tablets of Diltiazem Hydrochloride. World J. Pharm. Pharm. Sci. 2024, 13, 1022–1037. [Google Scholar]
- Rashmitha, N.; Somashekar, C.; Shravya, S.; Mani, T. Formulation and Evaluation of Pellets Loaded with Diltiazem Hydrochloride for Sustained Release. World J. Pharm. Res. 2019, 8, 1607. Available online: www.wjpr.Net (accessed on 24 May 2025).
- Aulton, M.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-7020-7005-1. [Google Scholar]
- Saxena, A.; Kitawat, S.; Gaur, K.; Singh, V. Formulation, Development and Characterization of Floating Beads of Diltiazem Hydrochloride. Int. J. Drug Deliv. Technol. 2016, 6, 1–6. [Google Scholar] [CrossRef][Green Version]
- Höskuldsdóttir, A.H.; Freydís, E.; Ína, T.; Gísladóttir, L.; Káradóttir, K. Diltiazem Hydrochloride with First Order Modified Release. Bachelor’s Thesis, University of Iceland, Reykjavík, Iceland, 2022. [Google Scholar]
- Kolev, I.; Ivanova, N.; Topouzova-Hristova, T.; Dimova, T.; Koseva, P.; Vasileva, I.; Ivanova, S.; Apostolov, A.; Alexieva, G.; Tzonev, A.; et al. Ammonio Methacrylate Copolymer (Type B)-Diltiazem Interactions in Solid Dispersions and Microsponge Drug-Delivery Systems. Polymers 2022, 14, 2125. [Google Scholar] [CrossRef]
- Gupta, M.K.; Khunteta, A. Optimization of the Release Kinetics of Diltiazem Hydrochloride from Tableted Microspheres. J. Drug Deliv. Ther. 2018, 8, 57–63. [Google Scholar] [CrossRef]
- Viramgama, P.H.; Modi, C.D.; Patel, D.J.; Chaudhary, A.B. Amalgamation of QbD and Alcohol Induced Dose Dumping Studies on Diltiazem Hydrochloride Modified Release Tablets. Int. J. Pharm. Investig. 2022, 12, 462–469. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Elgaied-Lamouchi, D.; Descamps, N.; Lefèvre, P.; Mackin-Mohamour, A.R.; Neut, C.; Siepmann, F.; Siepmann, J.; Muschert, S. Robustness of Controlled Release Tablets Based on a Cross-Linked Pregelatinized Potato Starch Matrix. AAPS PharmSciTech 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, Q.; Li, J. Comparative Study of Silica Nanoparticles and Surface Modified Silica Nanoparticles: Drug Adsorption Process and Controlled Release Behavior. Mater. Sci. Eng. B 2020, 259, 114609. [Google Scholar] [CrossRef]
- Emara, L.H.; El-Ashmawy, A.A.; Taha, N.F. Stability and Bioavailability of Diltiazem/Polyethylene Oxide Matrix Tablets. Pharm. Dev. Technol. 2018, 23, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, C.; Zhang, D.; Li, M.; Fu, Q.; He, Z. Formulation and Development of Ternary Hybrid Matrix Tablets of Diltiazem Hydrochloride. Powder Technol. 2016, 294, 66–70. [Google Scholar] [CrossRef]
- Samanta, A.P.; Ghosh, A.; Dutta, K.; Mandal, D.; Tudu, S.; Sarkar, K.; Das, B.; Ghosh, S.K.; Chattopadhyay, D. Biofabrication of Aminated Nanocellulose Reinforced Polyvinyl Alcohol/Chitosan Nanofibrous Scaffold for Sustained Release of Diltiazem Hydrochloride. Int. J. Biol. Macromol. 2024, 277, 134395. [Google Scholar] [CrossRef]
- Seyedian, R.; Shabankareh Fard, E.; Hashemi, S.S.; Hasanzadeh, H.; Assadi, M.; Zaeri, S. Diltiazem-Loaded Electrospun Nanofibers as a New Wound Dressing: Fabrication, Characterization, and Experimental Wound Healing. Pharm. Dev. Technol. 2021, 26, 167–180. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kang, J.H.; Se-Woon, C.; Yun, Y.H.; Yoon, S. Do Synthesis, Recognition Properties and Drug Release Behavior of Diltiazem-Imprinted Chitosan-Based Biomaterials. J. Appl. Polym. Sci. 2025, 142, e56307. [Google Scholar] [CrossRef]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems—A Comprehensive Review. Pharmaceuticals 2022, 15, 1430. [Google Scholar] [CrossRef]
- Monton, C.; Kulvanich, P. Push-Pull Osmotic Pumps Using Crosslinked Hard Gelatin Capsule as a Structural Assembly for Delivery of Drugs with Different Water Solubilities. J. Pharm. Innov. 2022, 17, 791–805. [Google Scholar] [CrossRef]
- Alkhamis, K.A.; Al-Nimry, S.S. Preparation of Diltiazem HCl-Modified Release Formulation Using Cation-Exchange Resin as a Single Excipient. Pharm. Dev. Technol. 2025, 30, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Allaboun, H.; Alkhamis, K.A.; Al-Nimry, S.S. Preparation of Sustained Release Formulation of Verapamil Hydrochloride Using Ion Exchange Resins. AAPS PharmSciTech 2023, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Bari, D.B.; Surana, S.J.; Pardeshi, C.V. In Vitro, Ex Vivo and in Vivo Performance of Chitosan-Based Spray-Dried Nasal Mucoadhesive Microspheres of Diltiazem Hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 31, 108–117. [Google Scholar] [CrossRef]
- Derle, D.V.; Barhate, R.S.; Umberkar, P.A.; Derle, N.D. Formulation Development and Evaluation of Diltiazem Hydrochloride Sustained Release Matrix Tablet. Indo Am. J. Pharm. Res. 2017, 7, 3. [Google Scholar]
- Ishrath, A.; Devadas, V.; Ahmed, M.M. Formulation Development and Characterization of Diltiazem Pulsin Cap for Pulsatile Drug Delivery. Am. J. Pharmtech Res. 2019, 9, 97–113. [Google Scholar]
- Varasteghan, H.; Shokri, J.; Asnaashari, S.; Javadzadeh, Y. Formulation and Evaluation of Novel Bilayer Floating and Sustained Release Drug Delivery System of Diltiazem HCl. Int. J. Drug Dev. Res. 2019, 11, 1–3. [Google Scholar] [CrossRef]
- Shaikh, N.; Zuber, P.; Khan, G.; Patel, S.; Zakir, S. Design and Evaluation of Bilayer Floating Tablets of Diltiazem HCl. Indo Am. J. Pharm. Sci. 2017, 4, 2612–2621. [Google Scholar] [CrossRef]
- Baviskar, D.; Sharma, R.; Jain, D. Formulation and in Vitro/in Vivo Evaluation of Sustained Release Diltiazem Matrix Tablets. Trop. J. Pharm. Res. 2013, 12, 311–316. [Google Scholar] [CrossRef][Green Version]
- Magnúsdóttir, I.L.; Halldórsdóttir, L.; Davíðsdóttir, N. Development of Zero-Order Release Diltiazem Tablets. Bachelor’s Thesis, University of Iceland, Reykjavík, Iceland, 2024. [Google Scholar]
- Kanteti, R.V.; Sarheed, O.; Yadav, H.; Islam, Q.; Boateng, J. Studies on Almond Gum and Gelucire-Based Pellets Prepared by Extrusion and Spheronization for Sustained Release. Turk. J. Pharm. Sci. 2022, 19, 521–529. [Google Scholar] [CrossRef]
- Bolourchian, N.; Bahjat, M. Design and in Vitro Evaluation of Eudragit-Based Extended Release Diltiazem Microspheres for Once- and Twice-Daily Administration: The Effect of Coating on Drug Release Behavior. Turk. J. Pharm. Sci. 2019, 16, 340–347. [Google Scholar] [CrossRef]
- Muhsin, M.D.A.; George, G.; Beagley, K.; Ferro, V.; Wang, H.; Islam, N. Effects of Chemical Conjugation of l -Leucine to Chitosan on Dispersibility and Controlled Release of Drug from a Nanoparticulate Dry Powder Inhaler Formulation. Mol. Pharm. 2016, 13, 1455–1466. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Ghasemi, M.; Eshaghi, M.; Eghbali, M.; Jafarkhani, B.; Rahimnia, S.M.; Negarandeh, R.; Babaei, A.; et al. An Eco-Friendly and Hopeful Promise Platform for Delivering Hydrophilic Wound Healing Agents in Topical Administration for Wound Disorder: Diltiazem-Loaded Niosomes. J. Pharm. Innov. 2023, 18, 1111–1127. [Google Scholar] [CrossRef]
- Subedi, G.; Shrestha, A.K.; Shakya, S. Study of Effect of Different Factors in Formulation of Micro and Nanospheres with Solvent Evaporation Technique. Open Pharm. Sci. J. 2016, 3, 182–195. [Google Scholar] [CrossRef]
- Mondal, N. The Role of Matrix Tablet in Drug Delivery System: Review Article. Int. J. Appl. Pharm. 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Tsuji, T.; Ono, T.; Taguchi, H.; Leong, K.; Hayashi, Y.; Kumada, S.; Okada, K.; Onuki, Y. Continuous Monitoring of the Hydration Behavior of Hydrophilic Matrix Using Time-Domain NMR. Chem. Pharm. Bull. 2023, 71, 576–583. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P. Drug-Polymers Composite Matrix Tablets: Effect of Hydroxypropyl Methylcellulose (HPMC) K-Series on Porosity, Compatibility, and Release Behavior of the Tablet Containing a BCS Class I Drug. Polymers 2022, 14, 3406. [Google Scholar] [CrossRef]
- Vasvári, G.; Kalmár, J.; Veres, P.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Haimhoffer, Á.; Rusznyák, Á.; Fenyvesi, F.; et al. Matrix Systems for Oral Drug Delivery: Formulations and Drug Release. Drug Discov. Today Technol. 2018, 27, 71–80. [Google Scholar] [CrossRef]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Rafiee, M.H.; Rasool, B.K.A. An Overview of Microparticulate Drug Delivery System and Its Extensive Therapeutic Applications in Diabetes. Adv. Pharm. Bull. 2022, 12, 730–746. [Google Scholar] [CrossRef]
- Kállai-Szabó, N.; Farkas, D.; Lengyel, M.; Basa, B.; Fleck, C.; Antal, I. Microparticles and Multi-Unit Systems for Advanced Drug Delivery. Eur. J. Pharm. Sci. 2024, 194, 106704. [Google Scholar] [CrossRef]
- Nidhi, K.; Verma, S.; Kumar, S. Microsponge. J. Clin. Sci. Res. 2021, 10, 108–111. [Google Scholar] [CrossRef]
- Biharee, A.; Bhartiya, S.; Yadav, A.; Thareja, S.; Jain, A.K. Microsponges as Drug Delivery System: Past, Present, and Future Perspectives. Curr. Pharm. Des. 2023, 29, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Reddy, R.; Fathima, R.; Preethi, P. Design, Development and Evaluation of Diltiazem Hydrochloride Loaded Nanosponges for Oral Delivery. Int. J. Curr. Pharm. Res. 2020, 12, 116–122. [Google Scholar] [CrossRef]
- Zárate-Hernández, E.; Hernández-Esquivel, R.A.; Pérez-Urizar, J.T. Microcápsulas y Microesferas: Una Visión a La Caracterización Integral y Aplicación Para La Liberación de Medicamentos Biotecnológicos. CienciaUAT 2021, 15, 21–36. [Google Scholar] [CrossRef]
- Tareq, A.Z.; Hussein, M.S. Sodium Alginate-Gelatin Cross-Linked Microspheres for Releasing Diltiazem Hcl. Sci. J. Univ. Zakho 2016, 4, 226–235. [Google Scholar] [CrossRef]
- Sahoo, J.; Mishra, B.; Kumar Biswal, P.; Dixit, P.K. Formulation, Evaluation and in-Vitro Release Study of Diltiazem Hcl Loaded Gelatinous Microsphere. Biopharm. J. 2016, 2016, 27–33. [Google Scholar]
- Tawde, V.V. Multiparticulate Drug Delivery Systems-a Brief Review on Pharmaceutical Pellets and Pelletization Techniques. J. Emerg. Technol. Innov. Res. 2024, 11, b386. [Google Scholar]
- Priese, F.; Wiegel, D.; Funaro, C.; Mondelli, G.; Wolf, B. Comparison of Mini-Tablets and Pellets as Multiparticulate Drug Delivery Systems for Controlled Drug Release. Coatings 2023, 13, 1891. [Google Scholar] [CrossRef]
- Etemadi, N.; Mehdikhani, M.; Huri, P.Y.; Poursamar, S.A.; Rafienia, M. Novel Electrospun Polyvinyl Alcohol/Chitosan/Polycaprolactone-Diltiazem Hydrochloride Nanocomposite Membranes for Wound Dressing Applications. Emergent Mater. 2024, 7, 1103–1113. [Google Scholar] [CrossRef]
- Khairnar, G.; Mokale, V.; Mujumdar, A.; Naik, J. Development of Nanoparticulate Sustained Release Oral Drug Delivery System for the Antihyperglycemic with Antihypertensive Drug. Mater. Technol. 2019, 34, 880–888. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Pandey, P.; Pal, R.; Khadam, V.K.R.; Chawra, H.S.; Singh, R.P. Advancement and Characteristics of Non-Ionic Surfactant Vesicles (Niosome) and Their Application for Analgesics. Int. J. Pharm. Investig. 2024, 14, 616–632. [Google Scholar] [CrossRef]
- Ammar, H.O.; Haider, M.; Ibrahim, M.; El Hoffy, N.M. In Vitro and in Vivo Investigation for Optimization of Niosomal Ability for Sustainment and Bioavailability Enhancement of Diltiazem after Nasal Administration. Drug Deliv. 2017, 24, 414–421. [Google Scholar] [CrossRef]
- Joshi, M.; Gokhale, C.; Kenjale, P.; Pokharkar, V. Optimization of Diltiazem Hydrochloride Osmotic Formulation Using QBD Approach. Braz. J. Pharm. Sci. 2022, 58, e19779. [Google Scholar] [CrossRef]
- Soliman, L.; Ibrahim, W. Effect of Coating Thickness and Polyethylene Glycol’s Molecular Weight on Diltiazem Hydrochloride Release from Controlled Porosity Osmotic Pump Tablets. Res. J. Pharm. Technol. 2022, 15, 4043–4047. [Google Scholar] [CrossRef]
- Kardile, R.D.; Bhagat, V.C.; Birajdar, R.S.; Shete, R.V.; Lathi, S.S.; Mathdevru, B.R.; Pawar, O.P.; Mhetre, R. A Comprehensive Review of Recent Studies on Matrix Tablets for Drug Delivery with Oral Controlled Release. Biol. Forum 2023, 15, 789–798. [Google Scholar]
- Yadav, R.P.; Sheeba, F.R.; Sharma, M.; Bhargav, A.; Kumar, Y.; Patel, A.K. The Role of Matrix Tablet of Drug De-Livery. J. Pharm. Res. Dev. 2021, 9, 80–86. [Google Scholar] [CrossRef]
- Tiwari, K.; Bhattacharya, S. The Ascension of Nanosponges as a Drug Delivery Carrier: Preparation, Characterization, and Applications. J. Mater. Sci. Mater. Med. 2022, 33, 28. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lai, W.C.; Chopra, H.; Agrawal, R.; Singh, T.; Chaudhary, R.; Dubey, B.N. Nanosponge: A Promising and Intriguing Strategy in Medical and Pharmaceutical Science. Heliyon 2024, 10, e23303. [Google Scholar] [CrossRef]
- Srinatha, N.; Battu, S.; Vishwanath, B.A. Microsponges: A Promising Frontier for Prolonged Release-Current Perspectives and Patents. Beni-Suef Univ. J. Basic. Appl. Sci. 2024, 13, 60. [Google Scholar] [CrossRef]
- Padhi, R.K.; Ahmed, F. Advances in Microsponge Technology: A Comprehensive Review. Res. J. Pharm. Life Sci. 2024, 5, 23–44. [Google Scholar]
- Yadav, N.; Verma, A. Pharmaceutical Pellets: A Versatile Carrier for Oral Controlled Delivery of Drugs. Indian. J. Pharm. Educ. Res. 2016, 50, 9–24. [Google Scholar] [CrossRef]
- Hiwrale, A.; Pingale, P.; Rajput, A. Nanofibers: A Current Era in Drug Delivery System. Heliyon 2023, 9, e18917. [Google Scholar] [CrossRef] [PubMed]
- Bathia, T.; Singh, P. Comparative versus Nanotechnology for Drug Delivery System—A Review. Int. J. Recent. Sci. Res. 2025, 16, 189–205. [Google Scholar]
- Petrovic, S.; Bita, B.; Barbinta-Patrascu, M.E. Nanoformulations in Pharmaceutical and Biomedical Applications: Green Perspectives. Int. J. Mol. Sci. 2024, 25, 5842. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Moacă, E.A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef]
- Deulkar, D.A.; Kubde, J.A.; Hatwar, P.R.; Bakal, R.L.; Motwani, A.N. Niosomes: A Promising Approach for Targeted Drug Delivery. GSC Biol. Pharm. Sci. 2024, 29, 179–195. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; Bostanudin, M.F.; Esmaeil, A.; Salam, A.; AbuRuz, S. In Vitro and in Vivo Evaluation of Oral Controlled Release Formulation of a BCS Class I Drug Using Polymer Matrix Sys-Tem. Pharmaceuticals 2021, 14, 929. [Google Scholar] [CrossRef]
- Taha, N.F.; Emara, L.H. Convolution- and Deconvolution-Based Approaches for Prediction of Pharmacokinetic Parameters of Diltiazem Extend-Ed-Release Products in Flow-through Cell Dissolution Tester. AAPS PharmSciTech 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Moin, A.; Gangadharappa, H.V.; Adnan, M.; Rizvi, S.M.; Ashraf, S.A.; Patel, M.; Abu Lila, A.S.; Allam, A.N. Modulation of Drug Release from Natural Polymer Matrices by Response Surface Methodology: In Vitro and in Vivo Evaluation. Drug Des. Devel Ther. 2020, 14, 5325–5336. [Google Scholar] [CrossRef]
- Deshmukh, M.; Shete, R.V.; Deshmukh, V.; Deshmukh, T. Preparation and Evaluation of Sustained Release Drug Delivery of Diltiazem Hydrochloride. J. Curr. Pharma Res. 2016, 6, 1882–1889. [Google Scholar] [CrossRef]
- George, M.; Joseph, L.; Shivade, S.; Anjana, M.N. Design and in Vitro Evaluation of Sustained Release Tablets of Diltiazem Hydrochloride by Dry Granulation Method. Int. J. Pharm. Ther. 2017, 8, 1–10. [Google Scholar]
- Onofre, F.; O’Donnell, K. Leveraging Direct Compression Technology to Improve Tableting Efficiency|American Pharmaceutical Review—The Review of American Pharmaceutical Business & Technology. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/581695-Leveraging-Direct-Compression-Technology-to-Improve-Tableting-Efficiency/ (accessed on 19 September 2025).
- Al-Zoubi, N.; Gharaibeh, S.; Aljaberi, A.; Nikolakakis, I. Spray Drying for Direct Compression of Pharmaceuticals. Processes 2021, 9, 267. [Google Scholar] [CrossRef]
- Qazi, F.; Shoaib, M.H.; Yousuf, R.I.; Qazi, T.M.; Mehmood, Z.A.; Muhammad, S.; Hasan, F. Formulation Development and Evaluation of Diltiazem HCl Sustained Release Matrix Tablets Using HPMC K4M and K100M. Pak. J. Pharm. Sci. 2013, 26, 653–663. [Google Scholar] [PubMed]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 108. [Google Scholar] [CrossRef]
- Maheswaran, A.; Padmavathy, J.; Nandhini, V.; Saravanan, D.; Angel, P. Formulation and Evaluation of Floating Oral in Situ Gel of Diltiazem Hydrochloride. Int. J. Appl. Pharm. 2017, 9, 50–53. [Google Scholar] [CrossRef][Green Version]
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-Purposable Filaments of HPMC for 3D Printing of Medications with Tailored Drug Release and Timed-Absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D Printing in Personalized Drug Delivery: An Overview of Hot-Melt Extrusion-Based Fused Deposition Modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef]
- Kamble, S.R.; Kothari, P.P.; Kajale, A.D.; Channawar, M.A.; Gawande, S.R. Formulation and Evaluation of Microspheres of Diltiazem Hydrochloride by Spray Drying Technique. Int. J. Pharm. Sci. Rev. Res. 2022, 77, 212–219. [Google Scholar] [CrossRef]
- Garekani, H.A.; Dolatabadi, R.; Akhgari, A.; Abbaspour, M.R.; Sadeghi, F. Evaluation of Ethylcellulose and Its Pseudolatex (Surelease) in Preparation of Matrix Pellets of Theophylline Using Extrusion-Spheronization. Iran. J. Basic. Med. Sci. 2017, 20, 9–16. [Google Scholar] [CrossRef]
- Salawi, A. Pharmaceutical Coating and Its Different Approaches, a Review. Polymers 2022, 14, 3318. [Google Scholar] [CrossRef]
- Kállai-szabó, N.; Lengyel, M.; Farkas, D.; Barna, Á.T.; Fleck, C.; Basa, B.; Antal, I. Review on Starter Pellets: Inert and Functional Cores. Pharmaceutics 2022, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Salpe, H.G.; Ghugre, A.; Devhare, L.D.; Singh, N. Formulation and Evaluation of HPMC Coated Diltiazem HCl Tablet and Its Comparison with Other Marketed Preparation. Res. Chron. Health Sci. 2017, 3, 8–14. [Google Scholar]
- Krishan, B.V.; Rao, C.B.; Kishore, V.S. Design and Development of Pulsatile Drug Delivery of Diltiazem Hydrochloride. Res. J. Pharm. Tech. 2020, 13, 2315. [Google Scholar] [CrossRef]
- Ahangaran, F. Microencapsulation: Solvent Evaporation. In Principles of Biomaterials Encapsulation: Volume 1; Woodhead Publishing: Cambridge, UK, 2023; Volume 1, pp. 377–392. ISBN 9780323859479. [Google Scholar]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of Microspheres for Biomedical Applications: A Review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Sharma, P. A Review on Microencapsulation as Method of Drug Delivery. BIO Web Conf. 2024, 86, 01033. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Aguiar, A.; dos Santos, R.G.; Bordado, J.C.; Pinho, I.; Marques, A.C. Design of Experiment for Optimizing Microencapsulation by the Solvent Evaporation Technique. Polymers 2023, 16, 111. [Google Scholar] [CrossRef]
- Chen, H.; Fang, D.; Wang, X.; Gong, Y.; Ji, Y.; Pan, H. Fabrication of Osmotic Pump Tablets Utilizing Semisolid Extrusion Three-Dimensional Printing Technology. Int. J. Pharm. 2024, 665, 124668. [Google Scholar] [CrossRef]
- Naikodi, S. An Osmotic Drug Delivery System as a Component of a Modified Release Dosage Form-A Comprehensive Review. Int. J. Pharm. Res. Appl. 2023, 8, 1190–1201. [Google Scholar]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D Printed Personalized Polypills for the Treatment of the Metabolic Syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an Osmotic 3D Printed Solid Dosage Form for Controlled Release of Active Pharmaceutical Ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef] [PubMed]
- Diltiazem (Oral Route)—Side Effects & Dosage—Mayo Clinic. Available online: https://www.mayoclinic.org/drugs-supplements/diltiazem-oral-route/description/drg-20071775 (accessed on 19 September 2025).
- Diltiazem: MedlinePlus Medicinas. Available online: https://medlineplus.gov/spanish/druginfo/meds/a684027-es.html (accessed on 19 September 2025).
- DailyMed—DH Capsule, Extended Release. Available online: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=1e2f2025-0350-40c4-ab42-3b57f3d105a6 (accessed on 19 September 2025).
- Patil, C.; Patil, R.; Patil, A. A Comprehensive Review of Matrix Tablets and Assessment Techniques. Int. J. Pharm. Sci. 2025, 3, 1047–1057. [Google Scholar] [CrossRef]
- FDA. Mechanistic Modeling of Complex Injectables: Recommendations to Navigate Regulatory Challenges; Office of Generic Drugs, CDER: Silver Spring, MD, USA, 2022.
- Alzhrani, R.F.; Fitaihi, R.A.; Majrashi, M.A.; Zhang, Y.; Maniruzzaman, M. Toward a Harmonized Regulatory Framework for 3D-Printed Pharmaceutical Products: The Role of Critical Feedstock Materials and Process Parameters. Drug Deliv. Transl. Res. 2025, 15, 4501–4518. [Google Scholar] [CrossRef]
- Forster, S.P.; Dippold, E.; Haser, A.; Emanuele, D.; Meier, R. Integrated Continuous Wet Granulation and Drying: Process Evaluation and Comparison with Batch Processing. Pharmaceutics 2023, 15, 2317. [Google Scholar] [CrossRef]
- Na, M.S.; Lee, Y.S.; Ha, E.S.; Kim, M.-S.; Park, H. Comprehensive Review in Continuous Direct Compression Process. J. Pharm. Investig. 2025, 55, 415–462. [Google Scholar] [CrossRef]
- Ortiz-Romero, N.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A. Advances in Researchinto Encapsulation through Ionic Gelation: A Systematic Review. TecnoLógicas 2021, 24, e1962. [Google Scholar] [CrossRef]
- Patil, H.; Vemula, S.K.; Narala, S.; Lakkala, P.; Munnangi, S.R.; Narala, N.; Jara, M.O.; Williams, R.O.; Terefe, H.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation—Where Are We Now? AAPS PharmSciTech 2024, 25, 1–25. [Google Scholar] [CrossRef]
- Gurrea, J. Formulation and Process Considerations for Optimising Spray-Dried Solid Dispersions. Int. Pharm. Ind. 2021, 13, 56–59. [Google Scholar]
- Thommes, M.; Kleinebudde, P. The Science and Practice of Extrusion-Spheronization. In Multiparticulate Drug Delivery. Advances in Delivery Science and Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 37–63. [Google Scholar]
- Dataintelo Consulting Pvt Ltd. Diltiazem Hydrochloride Market Report|Global Forecast From 2025 To 2033; Dataintelo: Ontario, CA, USA, 2024. [Google Scholar]
- Verified Market Reports. Diltiazem Hydrochloride Market 2026–2033; Verified Market Reports: Washington, DC, USA, 2024. [Google Scholar]
- Winarti, L.; Sari, L.O.R.K.; Nurahmanto, E.D.I.D.; Rosyidi, V.A.; Ameliana, L.; Barikah, K.Z.A.; Anjarani, R.A. Optimization of Diltiazem Hydrochloride Nanoparticles Formula and Its Release Kinetics Evaluation. Int. J. Appl. Pharm. 2021, 13, 194–199. [Google Scholar] [CrossRef]
- Suttee, A.; Pavani, S. Formulation and Evaluation of Buccoadhesive Tablets of Diltiazem Hydrochloride. Int. J. Drug Deliv. Technol. 2022, 12, 981–984. [Google Scholar] [CrossRef]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic Liquids as Potential Enhancers for Transdermal Drug Delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Suresh, P. Transdermal Delivery of Diltiazem HCl from Matrix Film: Effect of Penetration Enhancers and Study of Antihypertensive Activity in Rabbit Model. J. Adv. Res. 2016, 7, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Li, X.; Li, Y.; Wang, H.; Wang, Y.; Wang, T.; Pan, W.; Yang, X. In Vitro and in Vivo Evaluation of Controlled-Release Matrix Tablets of Highly Water-Soluble Drug Applying Different Mw Polyethylene Oxides (PEO) as Retardants. Drug Dev. Ind. Pharm. 2018, 44, 544–552. [Google Scholar] [CrossRef]
- Chandira, R.M.; Pethappachetty, P.; Murugan, T.; Antony Samy, D. Designing a Nanofiber Mats as a Targeted Approach of Diltiazem Hydrochloride for Management of Hypertensive Conditions. Asian J. Biol. Life Sci. 2022, 11, 570–577. [Google Scholar] [CrossRef]
- Shravani, S.; Battu, S.; Abbulu, K. Formulation and In-Vitro Evaluation of Diltiazem Hydrochloride Non Effervescent Floating Tablets. Int. J. Sci. Res. 2018, 9, 387–392. [Google Scholar]
- Surini, S.; Gotalia, F.; Putri, K.S.S. Formulation of Mucoadhesive Buccal Films Using Pregelatinized Cassava Starch Phthalate as a Film-Forming Polymer. Int. J. Appl. Pharm. 2018, 10, 225–229. [Google Scholar] [CrossRef]
- Garudaiahgari, S.; Shaik, N.; Kovvasu, S.P.; Gudhanti, S.; Kolapalli, M. Response Surface Optimization of Diltiazem HCl Gastric Floating Matrix Tablets. Asian J. Pharm. 2020, 14, 422. [Google Scholar]
- Sharma, G.; Verma, V.S.; Sharma, M.; Chandrakar, S.; Gupta, S.; Solanki, H.; Dewangan, K.; Sahu, V.D.; Majumdar, M.; Tripathi, D.K.; et al. Formulation and Evaluation of Extended Release Tablets of Diltiazem Hydrochloride. Res. J. Pharm. Technol. 2016, 9, 782–788. [Google Scholar] [CrossRef]
- Koradia, K.D.; Jotaniya, B.K.; Koradia, H.D. Diltiazem Hydrochloride Floating Matrix Tablet: Formulation and in Vitro-in Vivo Evaluation. Cardiovasc. Hematol. Disord. Drug Targets 2024, 24, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Bhukyanagaraju; Raj, M.A.; Nagaraju, B. Design & Evaluation of Pulsatile Drug Delivery of Diltiazem HCL. Int. J. Multidiscip. Res. 2023, 5, 1–29. [Google Scholar]
- Al-Zoubi, N.; Al-obaidi, G.; Tashtoush, B.; Malamataris, S. Sustained Release of Diltiazem HCl Tableted after Co-Spray Drying and Physical Mixing with PVAc and PVP. Drug Dev. Ind. Pharm. 2016, 42, 270–279. [Google Scholar] [CrossRef]
- Reddy, P.D.; Bharathi, D.V.; Swarnalatha, D.; Gopinath, C. Formulation and Characterization of Diltiazem Hydrochloride Matrix Tablets by Using Natural Polymers. Int. J. Pharm. Sci. Res. 2016, 7, 3764. [Google Scholar] [CrossRef]
- Alhawmdeh, E.H.; Barqawi, A.; Alkhatib, H.S. Effect of Casein Incorporation on the Release of Diltiazem HCl from Hypromellose-Based Matrix Tablets. Jordan J. Pharm. Sci. 2018, 11, 93–103. [Google Scholar]
- Badwaik, H.R.; Sakure, K.; Nakhate, K.T.; Kashayap, P.; Dhongade, H.; Alexander, A.; Ajazuddin; Tripathi, D.K. Effect of Ca+2 Ion on the Release of Diltiazem Hydrochloride from Matrix Tablets of Carboxymethyl Xanthan Gum Graft Polyacrylamide. Int. J. Biol. Macromol. 2017, 94, 691–697. [Google Scholar] [CrossRef]
- Yong-Bin, W.; Lian, Z.X.; Chen, M.N.; Zhang, L.; Zhou, C.Y.; Wei, W. Bioadhesive Drug Delivery System of Diltiazem Hydrochloride for Improved Bioavailability in Cardiac Therapy. Trop. J. Pharm. Res. 2016, 15, 1375–1380. [Google Scholar] [CrossRef]
| MDRFs | Advantages | Disadvantages | References |
|---|---|---|---|
| Matrix tablets | They can be produced in a wide variety of sizes and forms. Easy to manufacture. Increase the API’s stability to protect from hydrolysis into gastrointestinal tract. | Not favorable for APIs with poor solubility. Challenges in achieving uniform drug distribution and consistent release profile during the manufacture process. Gastric emptying, diet and other factors affect the release rate. Compatibility problems between API and polymeric material. | [60,61] |
| Nanosponges | Due to pore size (≤µm) bacteria cannot penetrate. They are stable at pH of 1–11 range and temperatures up to 300 °C. The scale-up process is easy; hence, they can be easily commercialized. The API is protected from the first-pass metabolism because of the use of crosslinkers. | The API loading capacity is altered by the crosslinking degree, which determinates the void space available. APIs must have a molecular weight between 100 and 400 Da and a melting point less than 250 °C. | [62,63] |
| Microsponges | They are free from harmful effects, non-irritating, non-mutagenic, and non-allergic. Adaptability to create innovative product shapes. They can prolong the API release up to 12 h. | API must not react with monomers and/or cause the preparation’s viscosity to rise while being formulated. | [64,65] |
| Pellets | They are less susceptible to dose dumping. Can be pellets with different release patterns in a single dosage form such as a capsule. | The use of granulating liquid such as water is necessary, requiring a drying phase and increasing the cost and time of manufacture. | [66] |
| Nanofibers | A high surface-to-volume ratio. Ease of fiber functionalization. Relatively low startup cost: A basic electrospinning system typically costs around $3000 to $4000. | The challenges in achieving in situ deposition of nanofibers on different substrates. It provides a low yield and needs a high working voltage. Little of material is deposited in terms of thickness, there is high electrical dispersion with high-conductive blends, and there are challenges with aqueous solutions and biomaterials. | [67] |
| Nanoparticles | Nanocarriers enhance solubility and bioavailability without altering the chemical structure of the API. Controlled and targeted release using stimuliresponsive nanocarriers. | Complex, expensive manufacturing with challenges in reproducibility and batch-to-batch consistency. Sensitive to environmental factors (temperature, pH, light), requiring specialized storage conditions. High research, development, and manufacturing costs due to advanced technologies. | [68,69] |
| Niosomes | Controlled shape, size, and composition. The API remains protected from gastrointestinal breakdown and first pass metabolism | Time-consuming process for niosome preparation. Physico-chemical instability. | [70,71] |
| Technology | Technological Level & Implementation | Industrial Viability | Regulatory Viability |
|---|---|---|---|
| Direct compression | Very basic/widely adopted | Very high (standard equipment, low cost) | Strong (QbD-ready, minimal validation) |
| Dry granulation | Basic/widely adopted | Very high (roller compaction, low cost) | Strong (simplified process, scalable) |
| Wet granulation | Basic/widely adopted | High (requires mixing and drying units) | Strong (CM-compatible, PAT integration) |
| Ionic gelification | Intermediate/selective use | Moderate (requires gelation setup) | Emerging (biopolymer-based, targeted systems) |
| Hot melt extrusion | Intermediate/growing adoption | High (specialized extruders, scalable) | Strong (solid dispersions, QbD, PAT) |
| Spray drying | Intermediate/widely used | High (atomization, scalable) | Strong (ASDs, inhalables, GMP-aligned) |
| Extrusion-spheronization | Intermediate/specialized | High (multiparticulates, dual equipment) | Strong (coating-ready, multiparticulate systems) |
| Pharmaceutical coating | Intermediate/standard practice | Very high (fluid-bed or pan coaters) | Strong (CR, enteric, masking, GMP compliant) |
| Solvent evaporation | Intermediate/common in R&D | Moderate (solvent recovery required) | Acceptable (depends on solvent and scale) |
| Combination methods | Advanced/case-dependent | Variable (requires integrated platforms) | Strong (flexible, QbD adaptable) |
| 3D printing | Advanced/emerging | Low–moderate (limited infrastructure) | Limited (FDA-approved cases, evolving framework) |
| Osmotic systems | Advanced/specialized | Moderate–high (membrane design, modular) | Strong (IVIVC, lifecycle management) |
| IVIVC/PBPK modeling | Advanced/indirect | High (strategic design optimization) | Strong (VBE, biowaivers, post-approval support) |
| Pharmaceutical Form | Preparation Method | Main Application | Composition Highlights | Reference |
|---|---|---|---|---|
| Buccal mucoadhesive tablets | Direct compression | Oral delivery; improve bioavailability | DH, mucoadhesive polymers (Carbopol-934, HPMC K4M, alginate, Na CMC, guar gum), talc, Mg stearate | [5] |
| PulsinCap® system | Capsule crosslinking + wet granulation | Chronotherapy; latency-controlled release | DH granules, formaldehyde-crosslinked capsule, hydrogel plug (HPMC, ethylcellulose), disintegrants, MCC | [27] |
| Capsule tablets | Wet granulation + compression | Staged release; analgesic potential | DH, two tablets (fast/slow release), HPMC (50 and 4000 mPa·s), ethylcellulose, MCC, wheat starch | [9] |
| Sustained-release matrix tablet | Direct compression | 24 h release; hypertension therapy | DH, Kollidon SR, HPMC K100, MCC, talc, Mg stearate | [26] |
| Nanoparticles | Ionic gelation | Enhance bioavailability; prolong half-life | DH, chitosan, Na TPP, Tween 80, glacial acetic acid | [115] |
| Pellets | Extrusion–spheronization | 12 h sustained release | DH, MCC, CMC, demineralized water | [6] |
| Matrix pellets | Extrusion–spheronization | MCC-free sustained release for soluble drugs | DH, almond gum, Gelucire 43/01, lactose, water | [32] |
| Oroadhesive tablets | Direct compression | Prolong action; bypass hepatic metabolism | DH, kondagogu gum, guar gum, lactose, talc, Mg stearate | [116] |
| Electrospun nanofibers | Electrospinning | Transdermal delivery; wound healing | DH, PVA, CS, glutaraldehyde | [18] |
| Ionic liquid formulations | Solution preparation | Transdermal/topical vehicles | DH free base (from DH HCl + NaOH), ionic liquids | [117] |
| Microsponges | Evaporation diffusion | Rectal gels for anal fissures | DH, Eudragit RS100, DCM, PVA, methylcellulose or Poloxamer 407 hydrogels | [2] |
| Mucoadhesive microspheres | Spray drying | Nasal delivery; enhance residence and permeation | DH, low-MW chitosan, acetic acid, nitrous acid | [25] |
| Transdermal film/matrix | Solvent casting | Antihypertensive transdermal therapy | DH, HPMC K4M, Eudragit RS100, plasticizers (glycerol, DBP, PG), enhancers (cineole, capsaicin, DMSO, NMP) | [118] |
| Hydrophilic matrix | Direct compression | Stability and biopharmaceutical evaluation | DH, PEO (0.9–8 MDa), PSTPP, KCl, Na2CO3, talc, Mg stearate | [24] |
| Niosomes | Thin film hydration | Intranasal delivery; ↑ bioavailability and prolonged action | DH, Span 60, Brij-52, cholesterol | [57] |
| Drug-resin complex | Rotary bottle adsorption | Modified release using resin only | DH, Dowex 50WX8 resin, phosphate buffer | [23] |
| Matrix tablets | Wet granulation | SR profile comparable to commercial SR | DH, karaya gum, locust bean gum | [74] |
| Nanofibrous mats | Electrospinning | Wound healing; ↑ fibroblast proliferation, antioxidant effect | DH, PVA, glutaraldehyde, PBS | [19] |
| Hydrophilic matrix tablets | Direct compression | Avoid lag time; ~zero-order release | DH, PEO WSR 303, PEO N750 | [119] |
| Nanoparticles | Water-in-oil emulsion | Pulmonary delivery; improved aerosolization | DH, CS, CS–L-leucine, glutaraldehyde, PBS | [34] |
| Nanofibrous mats | Electrospinning | Rapid/modulated release; hypertension | DH, RRP K30, HPMC K4M, aspartame, menthol, DMF | [120] |
| Matrix tablets | Wet granulation + compression | Oral SR; stable plasma levels | DH, PEC, guar gum, lactose, starch, Emcompress, Mg stearate | [75] |
| Microspheres | Emulsion–solvent evaporation | 12–24 h release; reduced burst effect | DH, Eudragit RL/RS | [33] |
| Floating tablets | Direct compression | Gastroretention; prolonged gastric residence | DH, Methocel K4M/K15M, CS, Accurel, MCC, talc, Mg stearate | [121] |
| Tablets | Dry granulation + compression | SR tablets; in vitro evaluation | DH, HPC, HPMC, Eudragit/MAC | [76] |
| Nanoparticles | Ionotropic gelation | Oral SR; co-loaded with repaglinide | DH, CS, TPP, Tween 80, acetic acid | [115] |
| Microsponges | Solvent diffusion | Compatibility, amorphization, controlled release | DH (base/HCl), Eudragit RS100, organic solvents | [54] |
| Tablets | Compression + solvent coating | ~8 h extended release; Ph. Eur. compliant | DH, NaCl, PEO/HPMC | [31] |
| Pull osmotic pump | Compression + coating | Solubility-independent delivery; IVIVC | DH, crosslinked capsule, PEO, NaCl, HPMC E5, PEG 4000, cellulose acetate | [22] |
| Mucoadhesive oral films | Pour molding | Oral retention; ↑ bioavailability | DH, PCSPh, aspartame, glycerol, propylene glycol | [122] |
| Matrix tablets | Direct compression | Robust SR under GI variability | DH, crosslinked potato starch (PI10), HPMC | [14] |
| Nanoparticles | Coating + solvent evaporation | Controlled release; reduced burst | DH, Silica-01/03, KH-570, acetone, capsules | [15] |
| Pellets | Extrusion–spheronization | MCC-free SR pellets | DH, Gelucire 43/01, almond gum | [32] |
| Microsponges | Quasi-emulsion solvent diffusion | Rectal gel systems | DH base, Eudragit RS100, PVA, DCM, ethanol | [10] |
| Tablets | Coating | Extended release; comparison with commercial tablets | DH, HPMC, CAP, acrylates, EC, PVP, NaCMC | [89] |
| Matrix tablets | Wet granulation | 100% release in 12 h; ↑ bioavailability | DH, HEC, Na bicarbonate, lactose, PVP K30, talc, Mg stearate | [123] |
| Matrix tablets | Direct compression | 24 h SR for hypertension, arrhythmia, angina | DH, HPMC, povidone, Tragacanth, Talc, Magnesium stearate, Lactose: fructose (1:1) | [124] |
| Floating matrix tablets | Direct compression | Extended release; ↑ gastric residence | DH, HPMC K4M/K15M, Na carbonate, lubricants | [125] |
| Pulsatile tablets | Capsular systems + pulsatile coatings | Chronotherapy (asthma, angina, hypertension) | DH, Eudragit, HPMC, polymeric alcohols, effervescent agents | [126] |
| Matrix tablets | Co-spray drying + compression | Enhanced SR vs. physical mixtures | DH, Kollicoat SR 30D, Kollidon SR, PVP | [127] |
| Matrix tablets | Wet granulation + compression | SR for hypertension/angina | DH, karaya gum, kondagogu gum, MCC, talc, Mg stearate | [128] |
| Matrix tablets | Wet granulation + compression | pH-independent SR | DH, casein, HPMC, lactose, purified water | [129] |
| Matrix tablets | Graft polymerization + granulation | Ca2+-responsive controlled release | DH, xanthan gum, acrylamide, ammonium persulfate, CaCl2 | [130] |
| Bioadhesive buccal films | Solvent coating | Oral delivery; avoid first-pass metabolism | DH, PVA, PVP K30, Na CMC, glycerol | [131] |
| Electrospun nanocomposite membranes | Electrospinning | Wound healing; controlled release | DH, PVA, CS, PCL, ethanol, acetic acid, chloroform | [53] |
| Microspheres | Emulsion + crosslinking | pH-dependent controlled release | DH, sodium alginate, gelatin, glutaraldehyde, HCl | [49] |
| Nanosponges | Emulsion + solvent diffusion | Oral SR; ↑ bioavailability | DH, β-cyclodextrin, ethylcellulose, PVA, DCM, water | [8,47] |
| Floating pearls | External ionic gelation | Gastroretentive SR system | DH, Na alginate, CaCl2, sunflower oil, LMP | [8] |
| Osmotic tablets | Wet granulation + osmotic coating | Zero-order SR up to 24 h | DH, HPMC E3, lactose, MCC, cellulose acetate, PEG 400, triacetin, Mg stearate, silicon dioxide, Opadry® | [58] |
| Bilayer tablets | Direct layer compression | Bilayer gastroretentive SR system | DH, Avicel, lactose, Mg stearate, HPMC K4M, ethylcellulose, tragacanth, Na bicarbonate | [28] |
| Gelatinous microspheres | Ionic gelation | Sustained release; controlled in vitro profile | DH, gelatin, glutaraldehyde, water | [50] |
| Microspheres | Emulsion + compression | SR up to 12 h; reduced dissolution variability | DH, Eudragit RL100, RS100, RLPO, RSPO, Mg stearate | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troches-Mafla, E.; Salamanca, C.H.; Ciro, Y. Controlled Release Technologies for Diltiazem Hydrochloride: A Comprehensive Review of Solid Dosage Innovations. Pharmaceutics 2025, 17, 1491. https://doi.org/10.3390/pharmaceutics17111491
Troches-Mafla E, Salamanca CH, Ciro Y. Controlled Release Technologies for Diltiazem Hydrochloride: A Comprehensive Review of Solid Dosage Innovations. Pharmaceutics. 2025; 17(11):1491. https://doi.org/10.3390/pharmaceutics17111491
Chicago/Turabian StyleTroches-Mafla, Estefanía, Constain H. Salamanca, and Yhors Ciro. 2025. "Controlled Release Technologies for Diltiazem Hydrochloride: A Comprehensive Review of Solid Dosage Innovations" Pharmaceutics 17, no. 11: 1491. https://doi.org/10.3390/pharmaceutics17111491
APA StyleTroches-Mafla, E., Salamanca, C. H., & Ciro, Y. (2025). Controlled Release Technologies for Diltiazem Hydrochloride: A Comprehensive Review of Solid Dosage Innovations. Pharmaceutics, 17(11), 1491. https://doi.org/10.3390/pharmaceutics17111491






