Design of Peptide-Modified Aluminum Nanoparticles with Enhanced Antimicrobial, Antibiofilm, Antioxidant, and DNA-Cleaving Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis and Characterization
2.1.2. In Vitro Experiments
2.1.3. Bacterial Strains and Cells
2.2. Methods
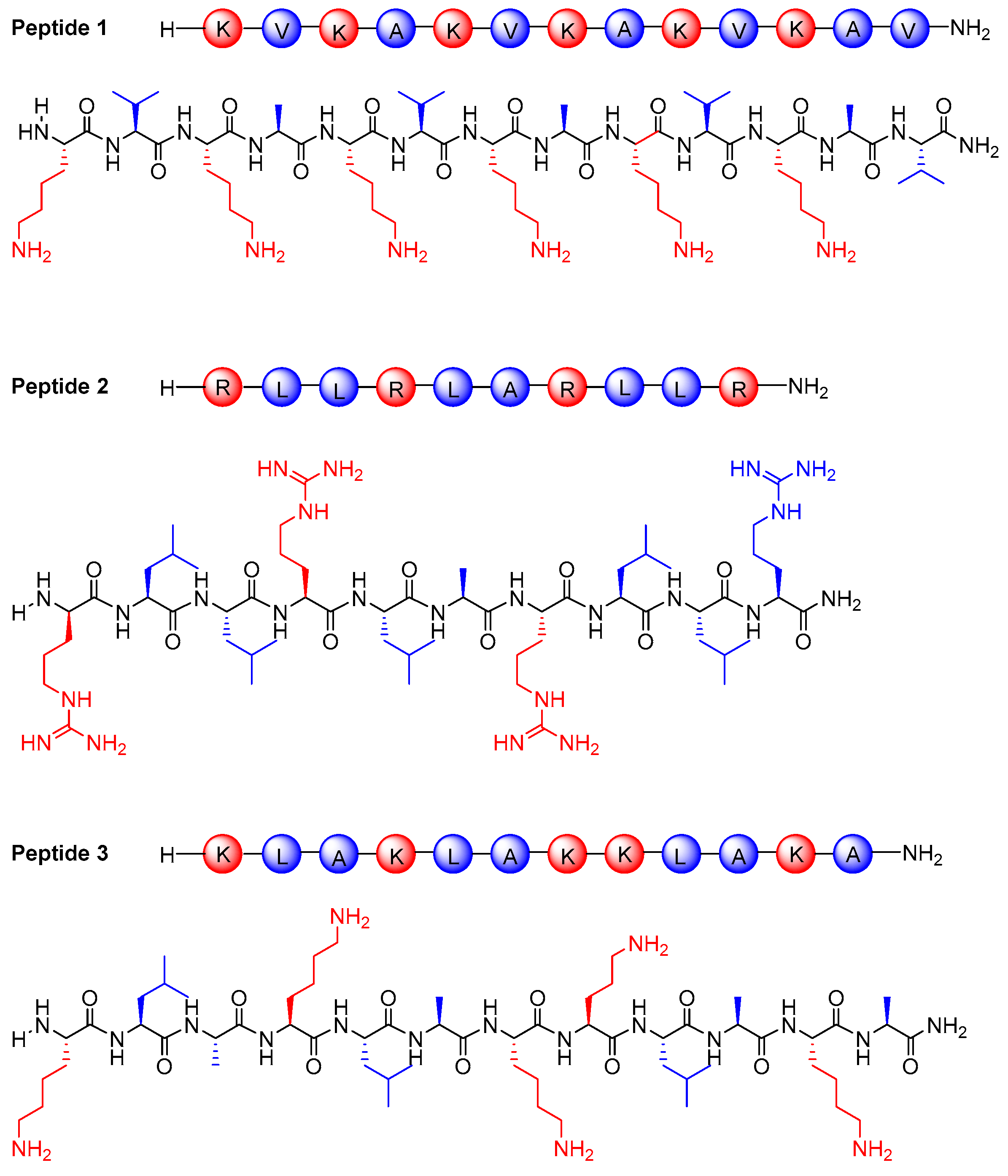
2.2.1. Peptide Synthesis and Characterization
2.2.2. Preparation of Aluminum Nanoparticles
2.2.3. Surface Modification of Aluminum Nanoparticles with Peptides
2.2.4. Physicochemical Characterization of Nanoparticles
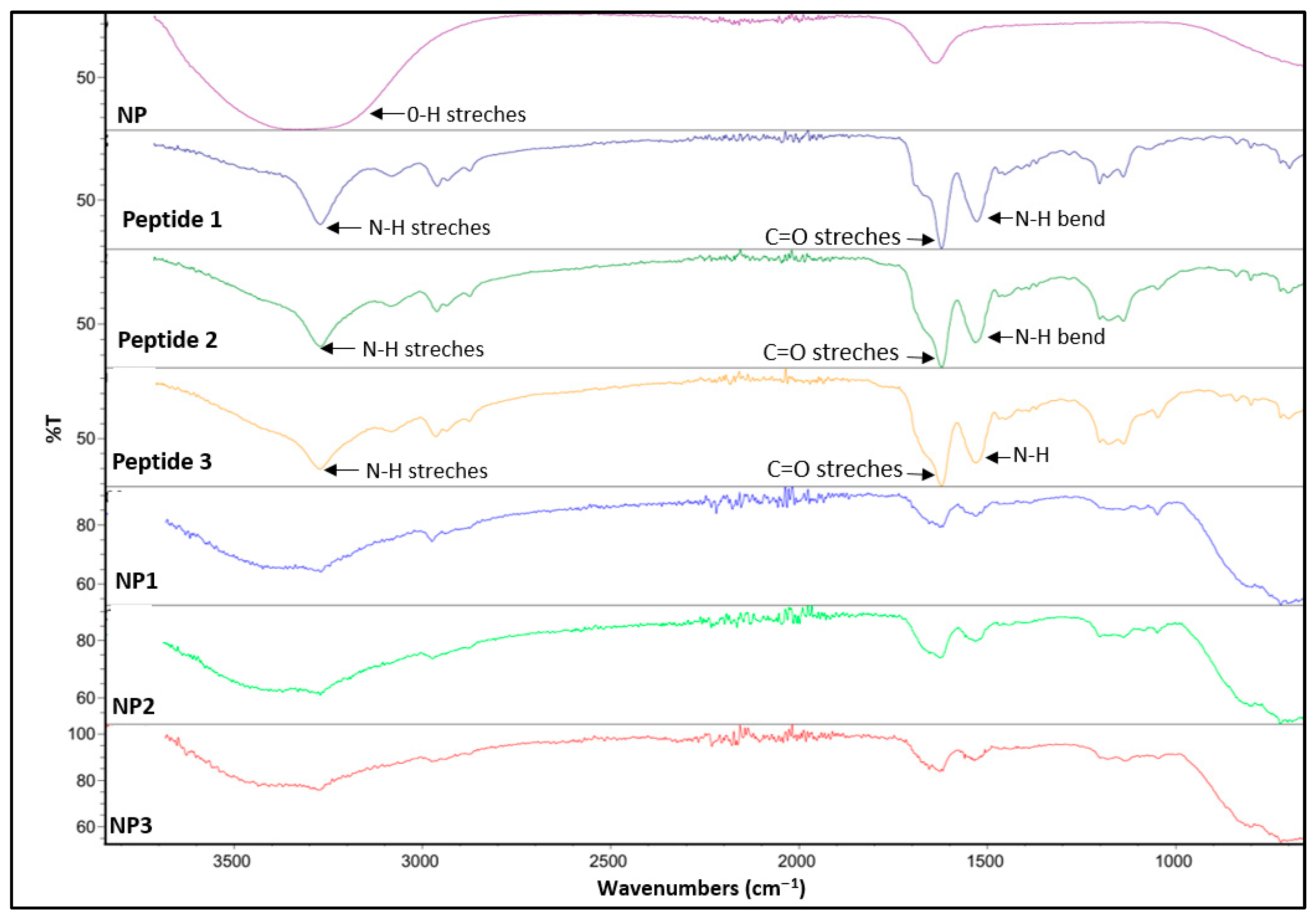
Fourier Transform Infrared Spectroscopy (FTIR)
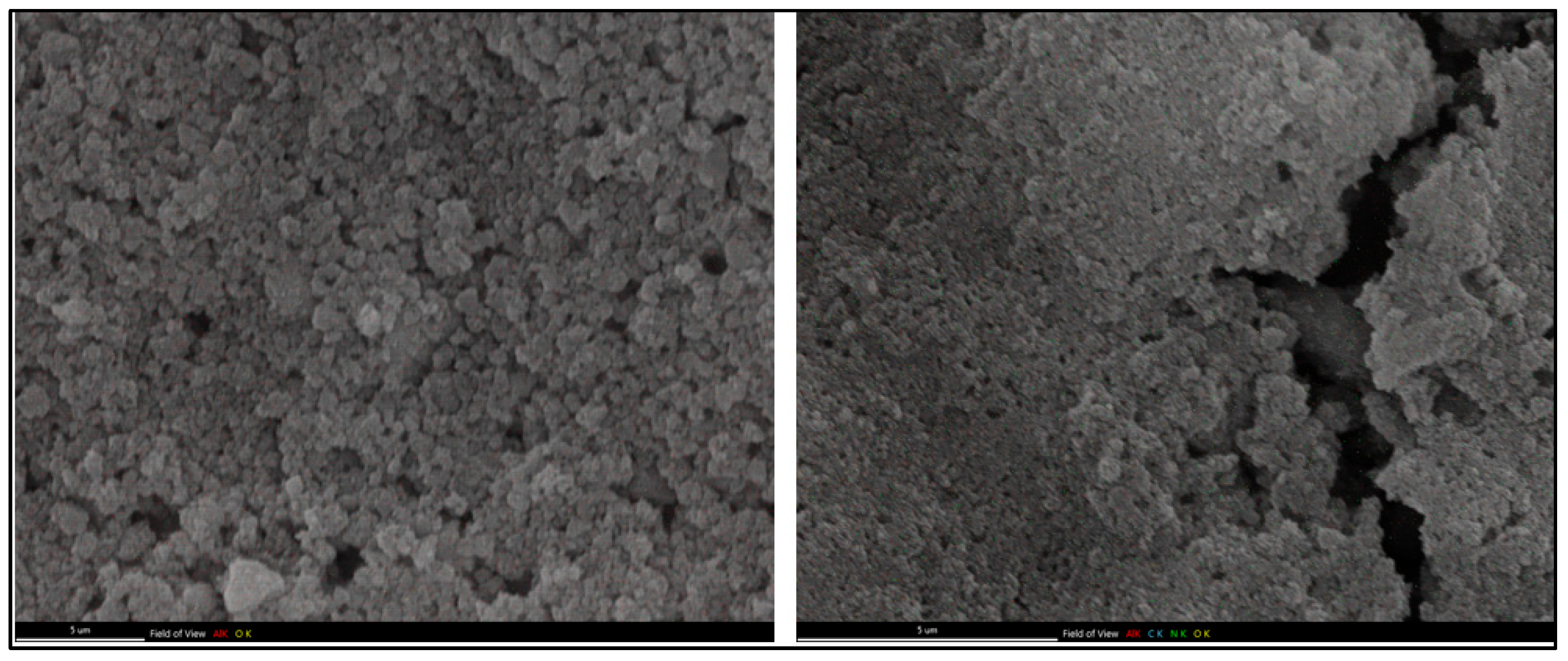
Scanning Electron Microscopy (SEM)
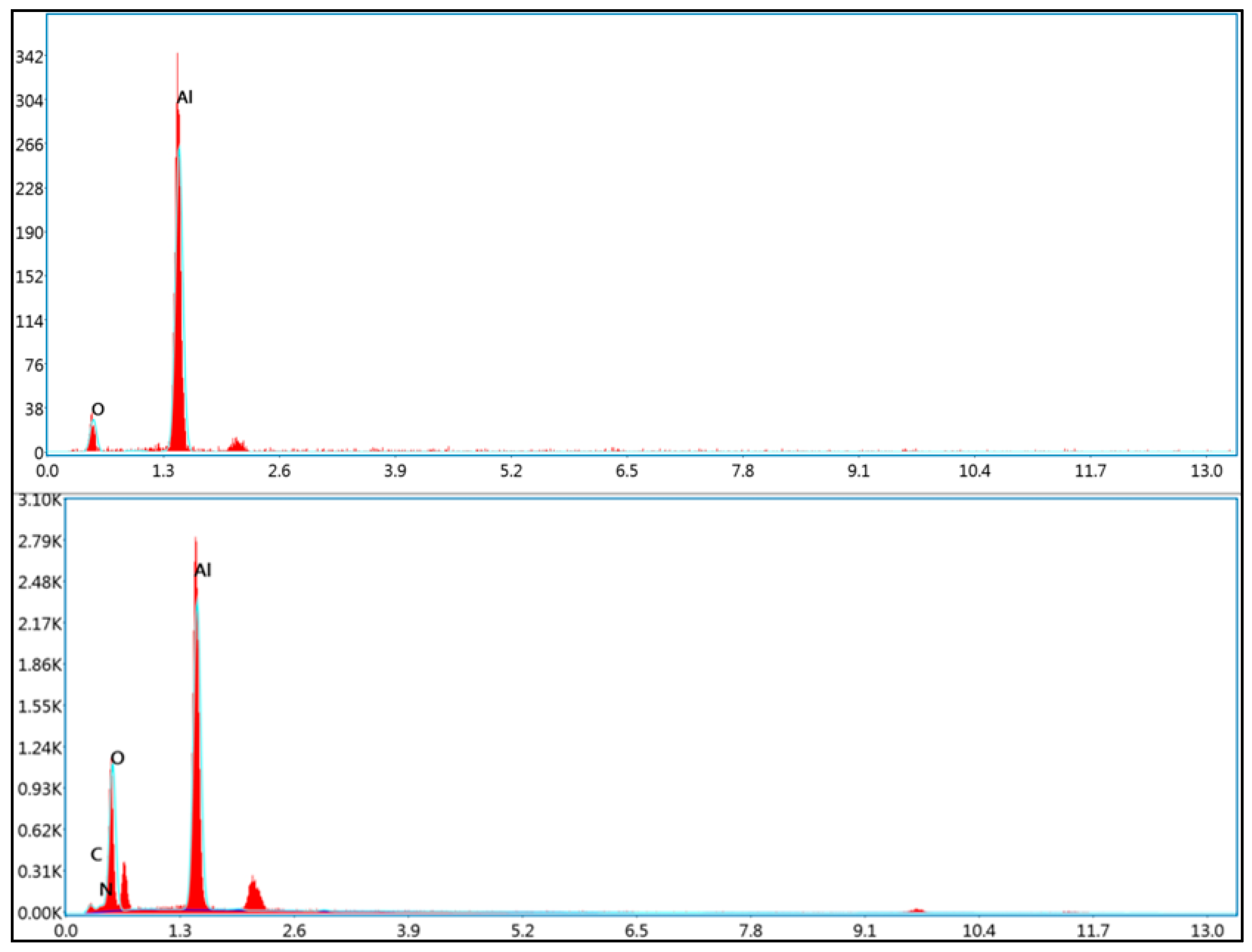
Energy Dispersive X-Ray Spectroscopy (EDS)
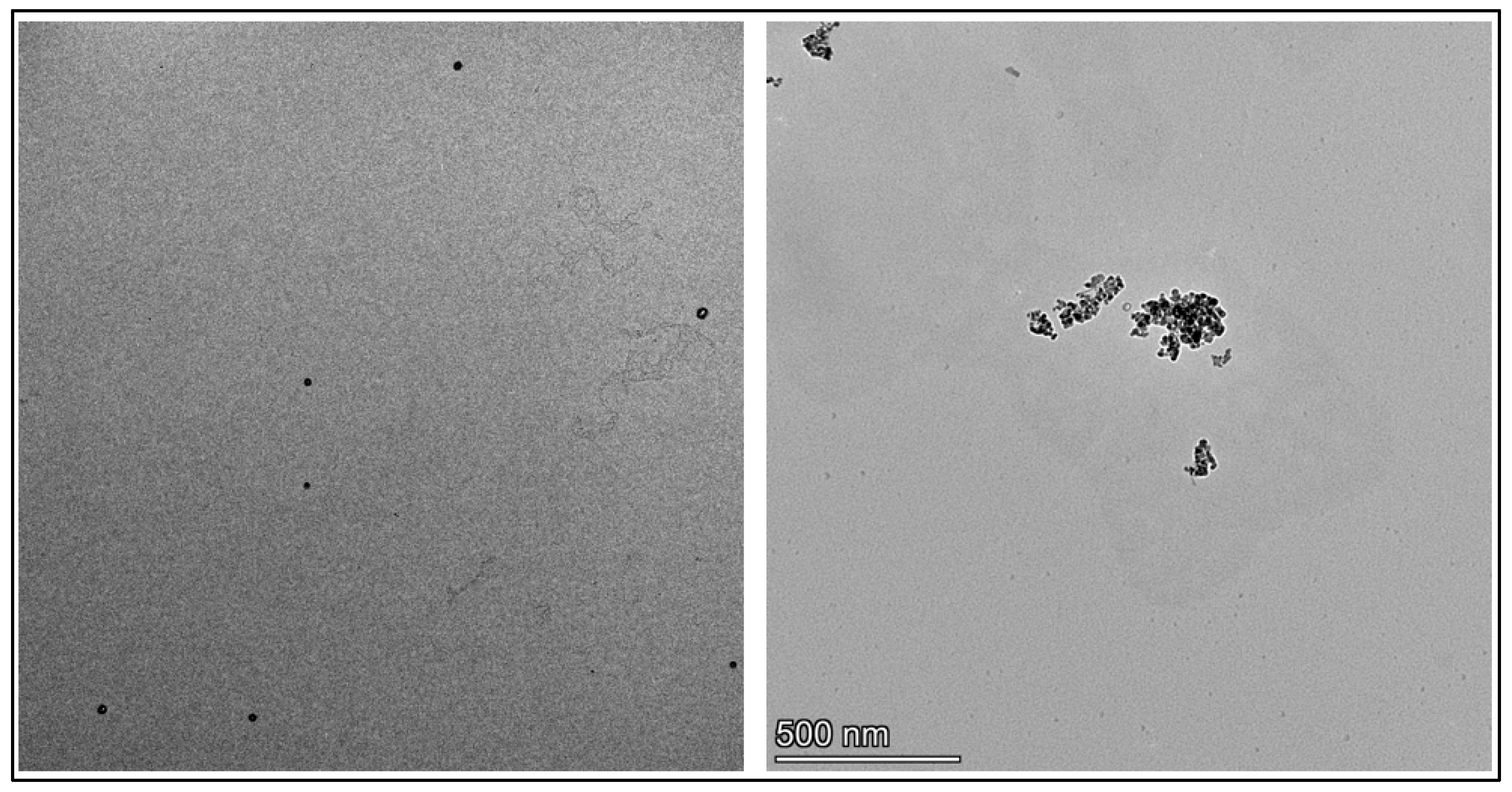
Transmission Electron Microscopy (TEM)
Zeta-Potential
2.2.5. Antimicrobial Activity
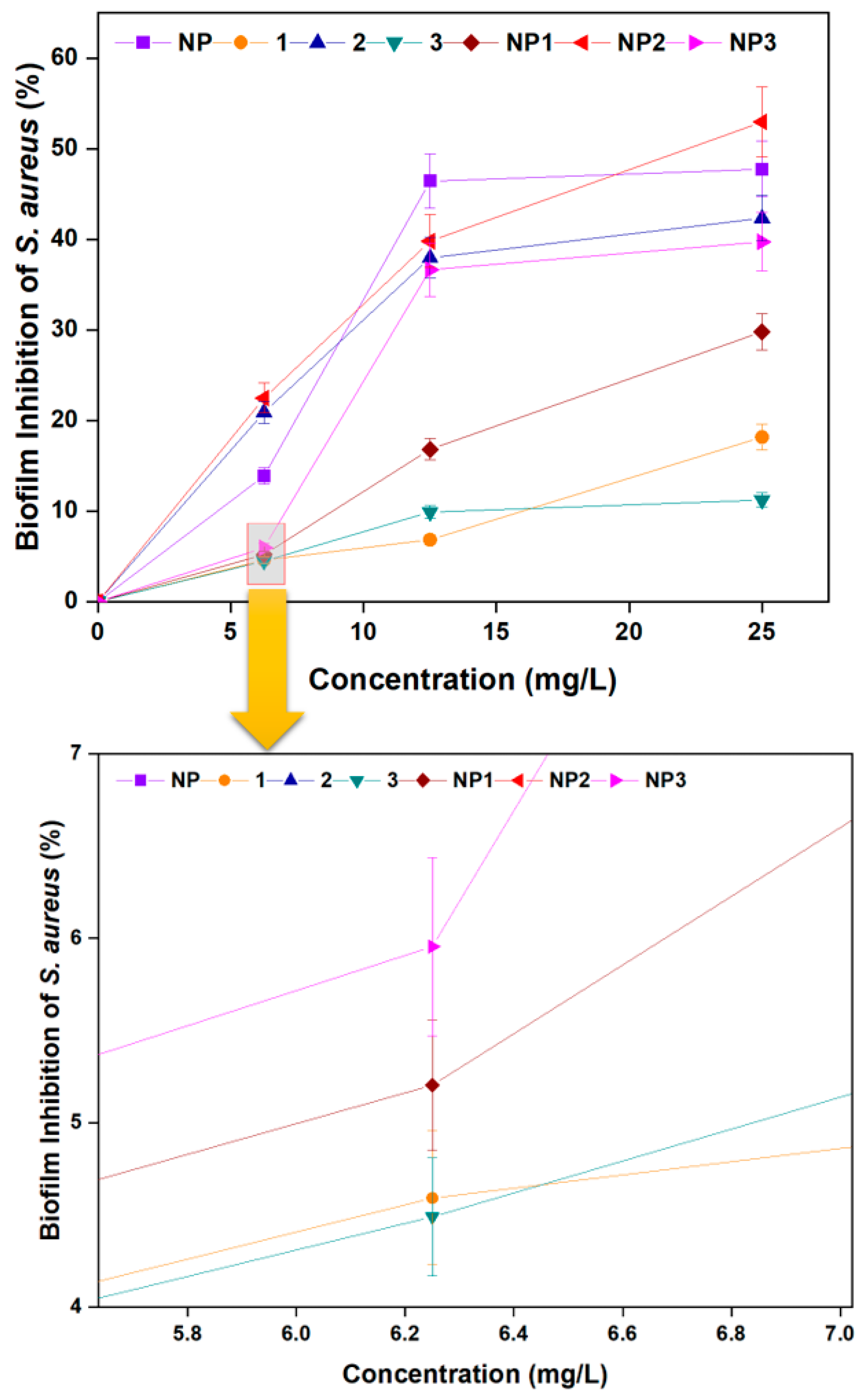
2.2.6. Biofilm Inhibition Activity
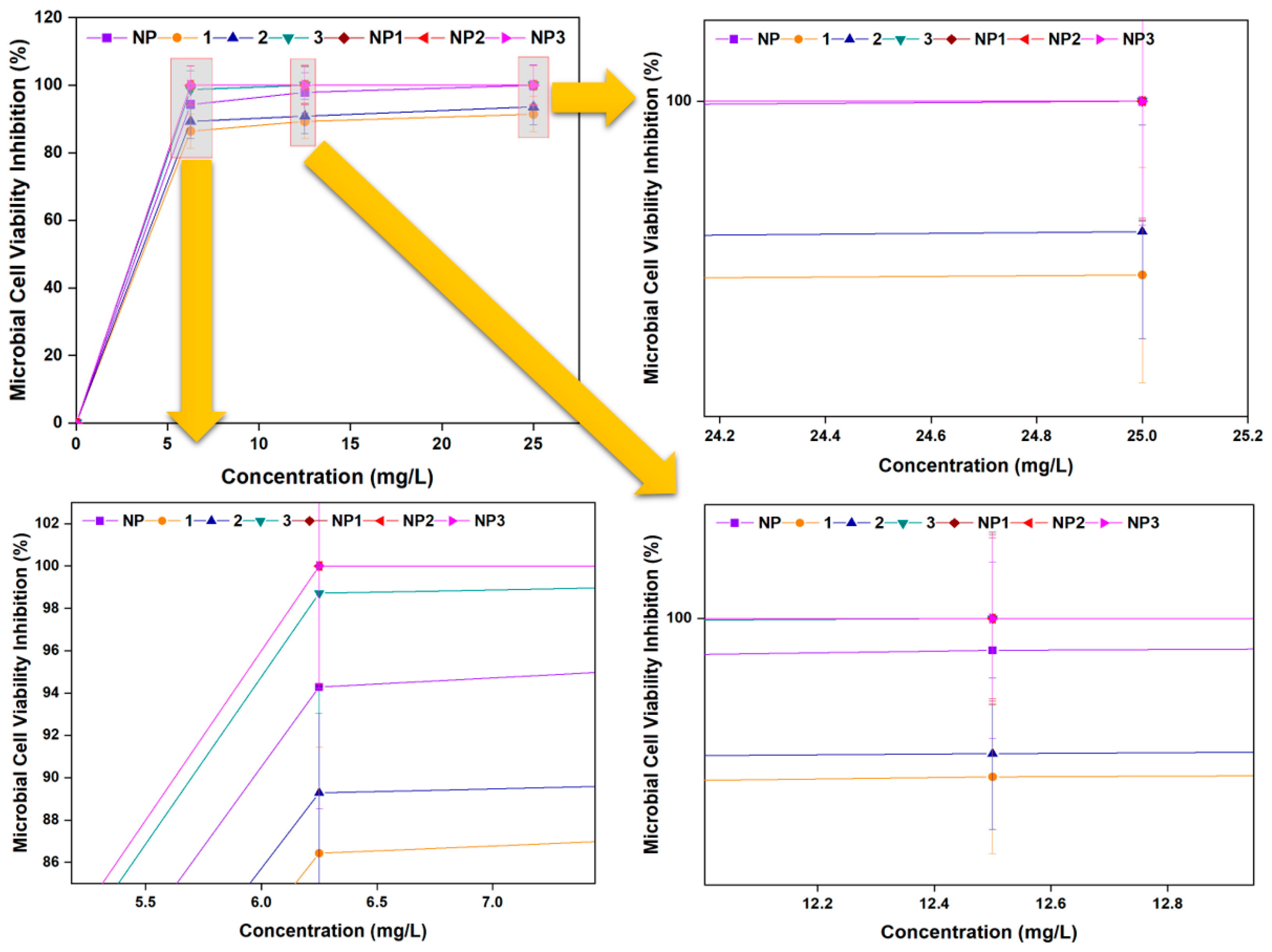
2.2.7. Microbial Cell Viability Inhibition
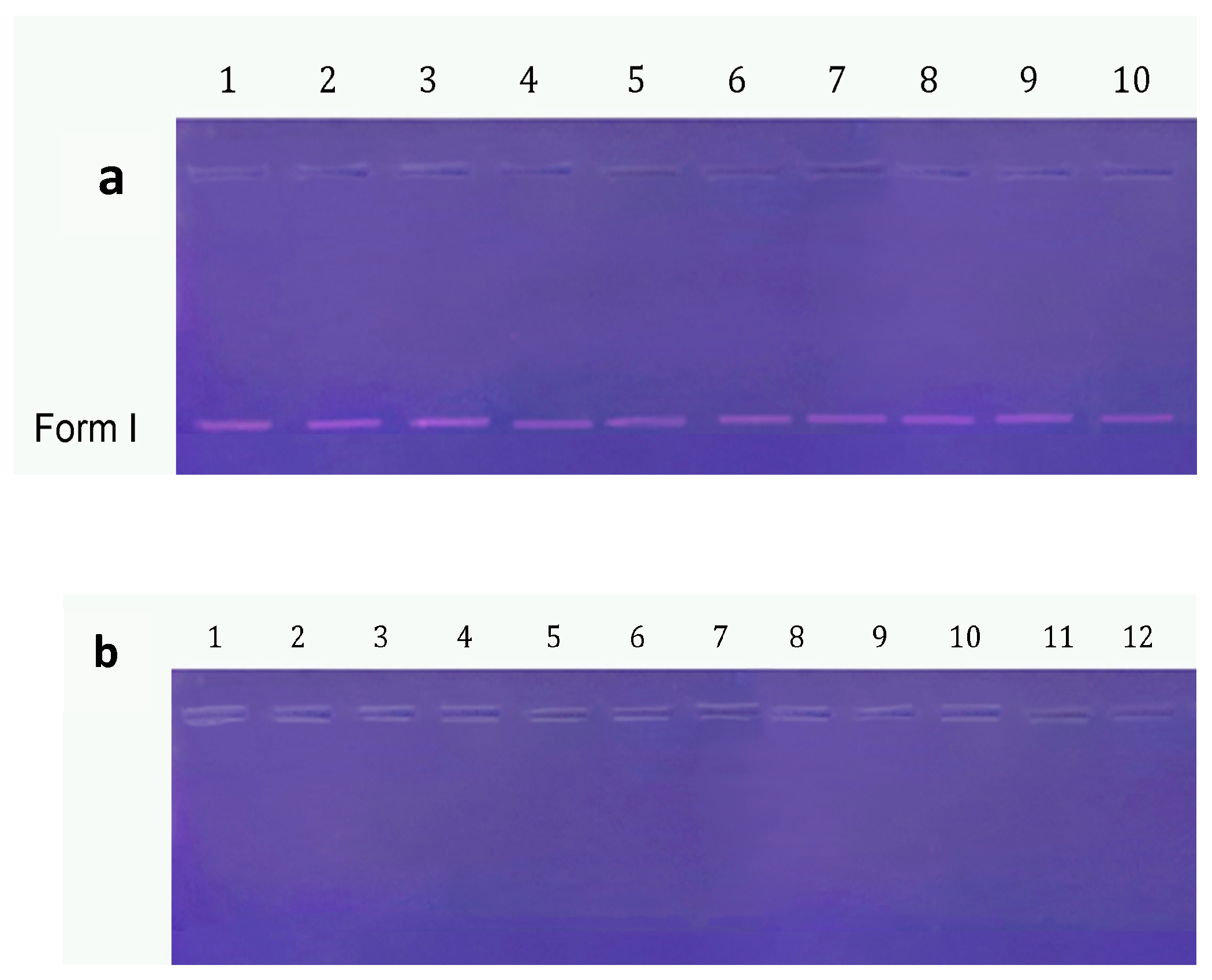
2.2.8. DNA Cleavage Activity
2.2.9. Antioxidant Activity
2.2.10. Amylolytic Activity
3. Results and Discussion
3.1. Synthesis and Characterization of Peptides
3.2. Preparation and Characterization of Peptide-Conjugated Aluminum Oxide Nanoparticles
3.3. Biological Studies
3.3.1. Antimicrobial Activity
3.3.2. Biofilm Inhibition Activity
3.3.3. Microbial Cell Viability Inhibition
3.3.4. DNA Cleavage Activity
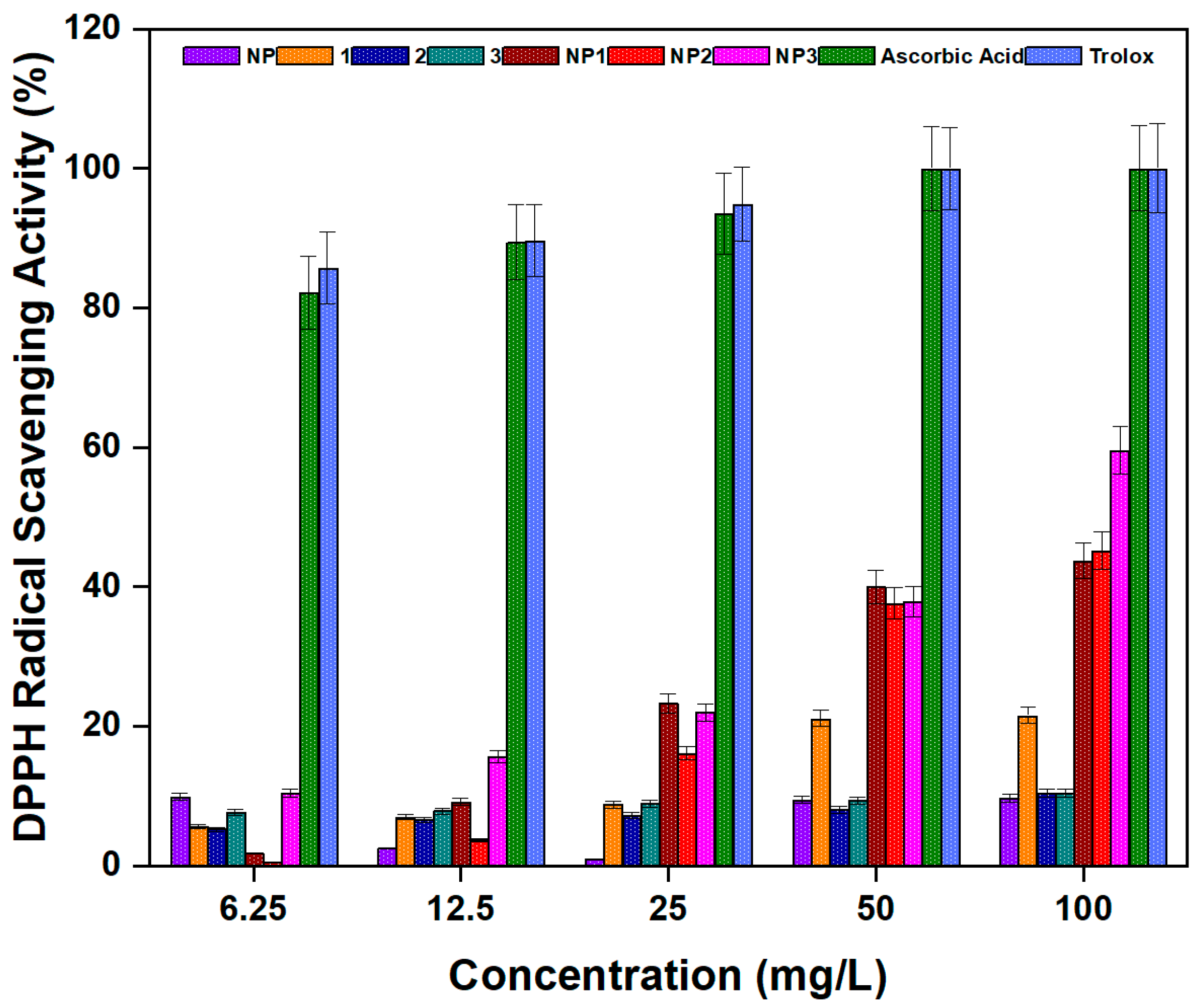
3.3.5. Antioxidant Activity
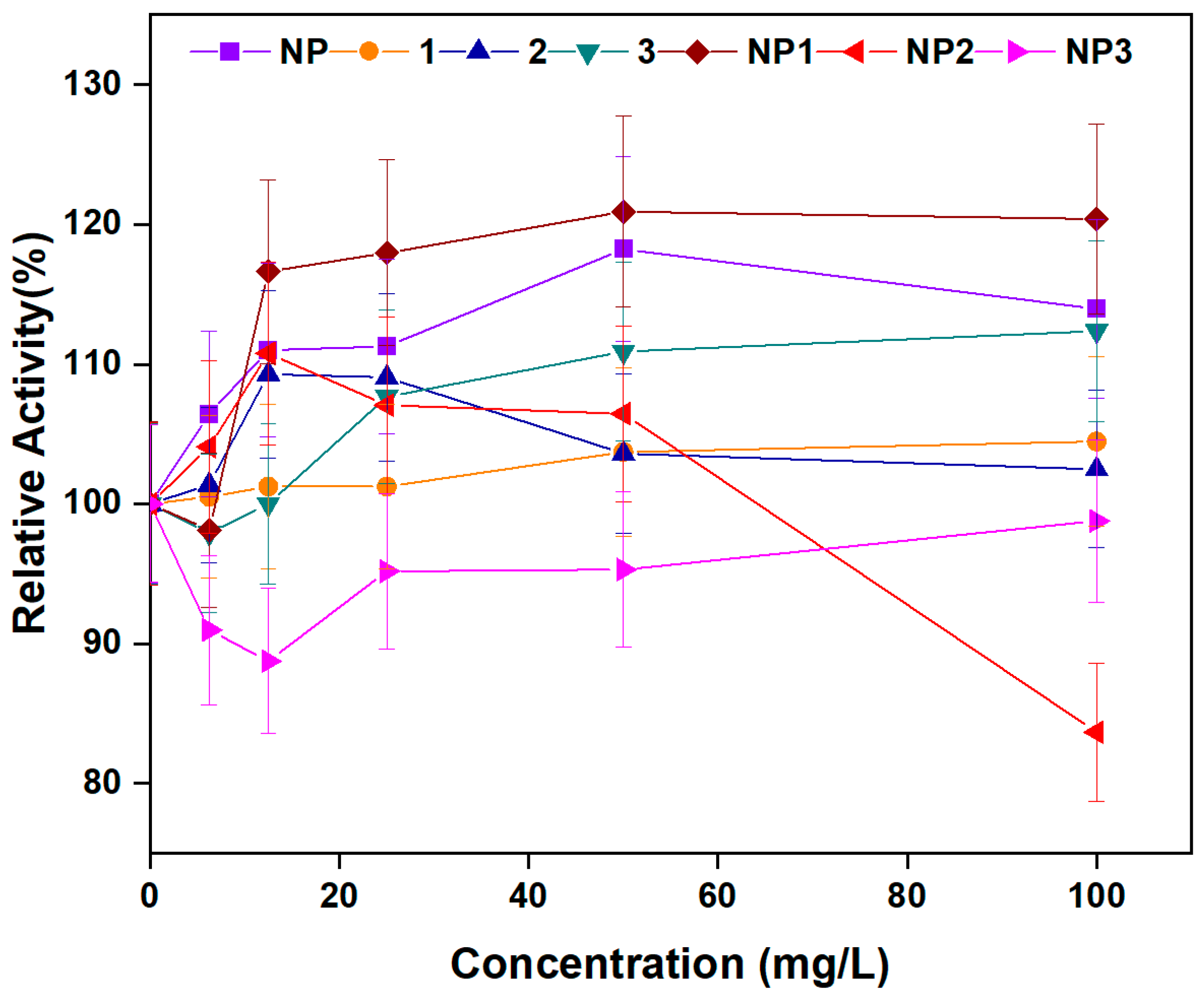
3.3.6. Amylolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alsfouk, A.A.; Othman, I.M.M.; Anwar, M.M.; Saleh, A.; Tashkandi, N.Y.; Nossier, E.S. New quinazolinone-based heterocyclic compounds as promising antimicrobial agents: Development, DNA gyrase B/topoisomerase IV inhibition activity, and in silico computational studies. J. Mol. Struct. 2025, 1344, 142953. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical applications of antimicrobial peptides (AMPs): Where do we stand now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Midura-Nowaczek, K.; Markowska, A. Antimicrobial Peptides and Their Analogs: Searching for New Potential Therapeutics. Perspect. Med. Chem. 2014, 6, 73–80. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wu, H.; Yin, T.; Wang, L.; Shan, A. Nanostructured Antimicrobial Peptides: Crucial Steps of Overcoming the Bottleneck for Clinics. Front. Microbiol. 2021, 12, 710199. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjug. Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Hu, D.; Deng, Y.; Chen, T.; Jin, Q.; Ji, J. Bacteria-targeted supramolecular photosensitizer delivery vehicles for photodynamic ablation against biofilms. Macromol. Rapid Commun. 2019, 40, e1800763. [Google Scholar] [CrossRef]
- Zhang, Q. Antimicrobial peptides: From discovery to developmental applications. Appl. Environ. Microbiol. 2025, 91, e02115-24. [Google Scholar] [CrossRef]
- Al Edhari, B.; Mashreghi, M.; Makhdoumi, A.; Darroudi, M. Antibacterial and antibiofilm efficacy of Ag NPs, Ni NPs and Al2O3 NPs singly and in combination against multidrug-resistant Klebsiella pneumoniae isolates. J. Trace Elem. Med. Biol. 2021, 68, 126840. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Jalal, M.; Ali, S.G.; Pal, R.; Musarrat, J. Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2015, 31, 153–164. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.E.; Beni, B.H.; Vashaee, D.; Tayebi, L. Controlling the degradation rate of bioactive magnesium implants by electrophoretic deposition of akermanite coating. Ceram. Int. 2014, 40, 3865–3872. [Google Scholar] [CrossRef]
- Torres, L.M.F.C.; Almeida, M.T.; Santos, T.L.; Marinho, L.E.S.; de Mesquita, J.P.; da Silva, L.M.; Verly, R.M. Antimicrobial aluminum nanobiostructures of disulfide- and triazole-linked peptides: Synthesis, characterization, membrane interactions and biological activity. Colloids Surf. B-Biointerfaces 2019, 177, 94–104. [Google Scholar] [PubMed]
- Ferreira, C.S.; Costa, L.M.F.; Nunes, L.O.; de Souza, K.R.; Araújo, G.P.; Salnikov, E.S.; Kato, K.C.; Martins, H.R.; de Castro Pimenta, A.M.; Resende, J.M.; et al. Influence of Peptide Conjugation Sites on Lunatin–Aluminum Nanoparticles: Implications for Membrane Interaction and Antimicrobial Activity. Pharmaceuticals 2025, 18, 952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Shrotriya, P.; Dassanayake, R.P. NK-lysin antimicrobial peptide-functionalized nanoporous aluminum membranes as biosensors for label-free bacterial endotoxin detection. Biochem. Biophys. Res. Commun. 2022, 636, 18–23. [Google Scholar]
- Hiemstra, T.; Yong, H.; Van Riemsdijk, W.H. Interfacial Charging Phenomena of Aluminum (Hydr)oxides. Langmuir 1999, 15, 5942–5955. [Google Scholar] [CrossRef]
- Bennett, G.; Beatty, M.W.; Simetich, B. Evaluation of Printability, Color Difference, Translucency, and Surface Roughness over Time in a 3D-Printed TiO2-Containing Denture Base Resin: A Pilot Study. Materials 2025, 18, 3683. [Google Scholar] [CrossRef]
- Ozay, Y.; Alterkaoui, A.; Kahya, K.; Ozdemir, S.; Serpil, G.; Ocakoglu, K.; Kulekcig, M.K. Antifouling and antibacterial performance evaluation of polyethersulfone membranes modified with AZ63 alloy. Water Sci. Technol. 2023, 87, 1616–1629. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, A.; Ouyang, X.; Li, B.; Yang, P.; Ba, Z.; Yang, Y.; Mao, W.; Zhong, C.; Gou, S.; et al. Rationally designed highly amphipathic antimicrobial peptides demonstrating superior bacterial selectivity relative to the corresponding α-helix peptide. Eur. J. Med. Chem. 2025, 286, 117310. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Ma, Z.; Ma, J.; Chen, J. Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules 2025, 30, 1529. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kang, W.; Li, W.; Chen, S.; Gao, Y. Oral delivery of protein and peptide drugs: From non-specific formulation approaches to intestinal cell targeting strategies. Theranostics 2022, 12, 1419–1439. [Google Scholar] [CrossRef]
- Isfahani, T.D.; Javadpour, J.; Khavandi, A.; Dinnebier, R.; Goodarzi, M.; Rezaie, H.R. Mechanochemical synthesis of aluminum nanoparticles: Formation mechanism and phase transformation. Powder Technol. 2012, 229, 17–23. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Synthesis and characterization of aluminum nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Purif. Technol. 2012, 89, 245–251. [Google Scholar] [CrossRef]
- Li, S.; Wan, H.; Lin, J.; Min, J. Physicochemical interactions between amorphous metal oxide and polymer in metal–polymer hybrid materials. Mater. Des. 2023, 230, 111993. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Chen, Y.; Li, C.; Jiang, H.; Wang, X. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Chen, H.; Wang, Z.; Guo, Y.; Wu, Y.; Hu, Y.; Liu, Q. Carrier-free cryptotanshinone-peptide conjugates self-assembled nanoparticles: An efficient and low-risk strategy for acne vulgaris. Asian J. Pharm. Sci. 2024, 19, 100946. [Google Scholar] [CrossRef]
- Olesk, J.; Donahue, D.; Ross, J.; Sheehan, C.; Bennett, Z.; Armknecht, K.; Nallathamby, P.D. Antimicrobial peptide-conjugated phage-mimicking nanoparticles exhibit potent bactericidal action against in murine wound infection models. Nanoscale Adv. 2024, 6, 1145–1162. [Google Scholar] [CrossRef]
- Primo, L.M.D.G.; Roque-Borda, C.A.; Canales, C.S.C.; Caruso, I.P.; de Lourenco, I.O.; Colturato, V.M.M.; Pavan, F.R. Antimicrobial peptides grafted onto the surface of N-acetylcysteine-chitosan nanoparticles can revitalize drugs against clinical isolates of Mycobacterium tuberculosis. Carbohydr. Polym. 2024, 323, 121449. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Adamcik, J.; Katanaev, V.L. Bactericidal and Antiviral Bionic Metalized Nanocoatings. Nanomaterials 2022, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef]
- Keikhosravani, P.; Khodaei, A.; Bollen, T.; Nazmi, K.; Bikker, F.J.; van Steenbergen, M.; Yavari, S.A. Developing antibacterial HB43 peptide-loaded chitosan nanoparticles for biofilm treatment. Int. J. Biol. Macromol. 2025, 310, 143397. [Google Scholar] [CrossRef]
- Saha, S.; Kar, R.; Sikder, K.; Manna, D.; Pal, R.R.; Chakraborti, S.; Basu, A. Deciphering the inhibitory mechanism of antimicrobial peptide pexiganan conjugated with sodium-alginate chitosan-cholesterol nanoparticle against the opportunistic pathogen. J. Drug Deliv. Sci. Technol. 2024, 101, 106305. [Google Scholar] [CrossRef]
- Sattari-Maraji, A.; Nikchi, M.; Shahmiri, M.; Ahadi, E.M.; Firoozpour, L.; Moazeni, E.; Kharrazi, S. Jellein-I-conjugated gold nanoparticles: Insights into the antibacterial, antibiofilm activities against MRSA, and anticancer properties. J. Drug Deliv. Sci. Technol. 2024, 101, 106218. [Google Scholar] [CrossRef]
- Alhosani, F.; Islayem, D.; Almansoori, S.; Zaka, A.; Nayfeh, L.; Rezk, A.; Yousef, A.F.; Pappa, A.M.; Nayfeh, A. Antibiofilm activity of ZnO–Ag nanoparticles against Pseudomonas aeruginosa. Sci. Rep. 2025, 15, 17321. [Google Scholar] [CrossRef]
- Zattarin, E.; Sotra, Z.; Wiman, E.; Bas, Y.; Rakar, J.; Berglund, L.; Starkenberg, A.; Björk, E.M.; Khalaf, H.; Oksman, K.; et al. Controlled release of antimicrobial peptides from nanocellulose wound dressings for treatment of wound infections. Mater. Today Bio 2025, 32, 101756. [Google Scholar] [CrossRef]
- Alcalde-Ordóñez, A.; Barreiro-Piñeiro, N.; McGorman, B.; Gómez-González, J.; Bouzada, D.; Rivadulla, F.; Vázquez, M.E.; Kellett, A.; Martínez-Costas, J.; López, M.V. A copper(II) peptide helicate selectively cleaves DNA replication foci in mammalian cells. Chem. Sci. 2023, 14, 14082–14091. [Google Scholar] [CrossRef]
- Chakraborty, I.; Saha, U.; Rakshit, R.; Talukdar, S.; Kumar, G.S.; Mandal, K. Design and development of bioactive α-hydroxy carboxylate group modified MnFe2O4 nanoparticle: Comparative fluorescence study, magnetism and DNA nuclease activity. Mater. Today Chem. 2017, 5, 92–100. [Google Scholar] [CrossRef]
- Al-Mamary, M.A.; Moussa, Z. Antioxidant activity: The presence and impact of hydroxyl groups in small molecules of natural and synthetic origin. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; pp. 253–280. [Google Scholar] [CrossRef]
- Dilek, Ö. Investigation of antioxidant activities of novel fluorine-based azo compounds combined with in silico molecular docking and in vitro CUPRAC method. J. Indian Chem. Soc. 2025, 102, 101513. [Google Scholar] [CrossRef]
- Haldar, D.; Shabbirahmed, A.M.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Ponnusamy, V.K.; Patel, A.K. Understanding the management of household food waste and its engineering for sustainable valorization- A state-of-the-art review. Bioresour. Technol. 2022, 358, 127390. [Google Scholar] [CrossRef] [PubMed]
- Ugwuoji, E.T.; Nwagu, T.N.T.; Ezeogu, L.I. Detergent-stable amylase production by Paenibacillus lactis strain OPSA3 isolated from soil; optimization by response surface methodology. Biotechnol. Rep. 2025, 39, e00808. [Google Scholar] [CrossRef]
- Mishra, A.; Ahmad, R.; Singh, V.; Gupta, M.N.; Sardar, M. Preparation, characterization and biocatalytic activity of a nanoconjugate of alpha amylase and silver nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 5028–5033. [Google Scholar] [CrossRef]
- Abdel-Nasser, M.; Abdel-Maksoud, G.; Abdel-Aziz, M.S.; Darwish, S.S.; Hamed, A.A.; Youssef, A.M. Evaluation of the efficiency of nanoparticles for increasing α-amylase enzyme activity for removing starch stain from paper artifacts. J. Cult. Herit. 2022, 53, 14–23. [Google Scholar] [CrossRef]

| Peptide 1 | Peptide 2 | Peptide 3 | ||||
|---|---|---|---|---|---|---|
| Calculated Mass (Da) | Observed Mass (Da) | Calculated Mass (Da) | Observed Mass (Da) | Calculated Mass (Da) | Observed Mass (Da) | |
| M | 1394.980 | - | 1278.670 | - | 1280.900 | - |
| [M + 1H]+ | 1395.987 | - | 1279.677 | - | 1281.907 | - |
| [M + 2H]2+ | 698.497 | 698.031 | 640.342 | 640.469 | 641.457 | 641.960 |
| [M + 3H]3+ | 466.001 | 466.034 | 427.231 | 427.281 | 427.974 | 427.974 |
| [M + 4H]4+ | 349.752 | - | 320.675 | - | 321.232 | - |
| MIC Values (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | 1 | 2 | 3 | NP | NP1 | NP2 | NP3 | AMP * | FLZ * |
| Staphylococcus aureus | 64 | 64 | 128 | 128 | 32 | 64 | 32 | 0.5 | - |
| Bacillus spizizenii | 64 | 32 | 64 | 128 | 32 | 16 | 16 | 0.5 | - |
| Enterococcus faecalis | 64 | 32 | 128 | 128 | 64 | 16 | 16 | 0.5 | - |
| Escherichia coli | 32 | 32 | 64 | 64 | 16 | 32 | 32 | 0.5 | - |
| Pseudomonas aeruginosa | 32 | 32 | 64 | 64 | 16 | 32 | 32 | 0.5 | - |
| Legionella pneumophila | 64 | 32 | 32 | 128 | 16 | 16 | 32 | 0.5 | - |
| Candida albicans | 128 | 128 | 128 | 256 | 128 | 64 | 64 | - | 1 |
| Candida glabrata | 128 | 64 | 256 | 256 | 64 | 64 | 64 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanlidere, Z.; Öztürk, N.F.; Yalçın, M.S.; Özdemir, S. Design of Peptide-Modified Aluminum Nanoparticles with Enhanced Antimicrobial, Antibiofilm, Antioxidant, and DNA-Cleaving Properties. Pharmaceutics 2025, 17, 1490. https://doi.org/10.3390/pharmaceutics17111490
Kanlidere Z, Öztürk NF, Yalçın MS, Özdemir S. Design of Peptide-Modified Aluminum Nanoparticles with Enhanced Antimicrobial, Antibiofilm, Antioxidant, and DNA-Cleaving Properties. Pharmaceutics. 2025; 17(11):1490. https://doi.org/10.3390/pharmaceutics17111490
Chicago/Turabian StyleKanlidere, Zeynep, Nazlı Farajzadeh Öztürk, M. Serkan Yalçın, and Sadin Özdemir. 2025. "Design of Peptide-Modified Aluminum Nanoparticles with Enhanced Antimicrobial, Antibiofilm, Antioxidant, and DNA-Cleaving Properties" Pharmaceutics 17, no. 11: 1490. https://doi.org/10.3390/pharmaceutics17111490
APA StyleKanlidere, Z., Öztürk, N. F., Yalçın, M. S., & Özdemir, S. (2025). Design of Peptide-Modified Aluminum Nanoparticles with Enhanced Antimicrobial, Antibiofilm, Antioxidant, and DNA-Cleaving Properties. Pharmaceutics, 17(11), 1490. https://doi.org/10.3390/pharmaceutics17111490







