Abstract
Background/Objectives: Natural products exhibit significant immunomodulatory potential but face severe efficacy loss in three-dimensional (3D) tumor models. This review comprehensively examines the penetration–activity trade-off and proposes integrated strategies for developing effective natural product-based cancer immunotherapies. Methods: We analyzed formulation strategies across three natural product categories (hydrophobic, macromolecular, stability-sensitive), evaluating penetration enhancement versus activity preservation in spheroids, organoids, and advanced 3D platforms. Results: Tumor spheroids present formidable barriers: dense extracellular matrix (33-fold increased fibronectin), pH gradients (7.4 → 6.5), and extreme cell density (6 × 107 cells/cm3). While nanoparticles, liposomes, and cyclodextrins achieve 3–20-fold penetration improvements, biological activity frequently declines through conformational changes, incomplete release (10–75%), and surface modification interference. Critically, immune cells remain peripheral (30–50 μm), questioning deep penetration pursuit. Patient-derived organoids display 68% predictive accuracy, while emerging vascularized models unveil additional complexity. Food and Drug Administration (FDA) Modernization Act 2.0 enables regulatory acceptance of these advanced models. Conclusions: Effective therapeutic outcomes depend on maintaining immunomodulatory activity in peripherally-located immune cell populations rather than achieving maximum tissue penetration depth. Our five-stage evaluation framework and standardization protocols guide development. Future priorities include artificial intelligence-driven optimization, personalized formulation strategies, and integration of multi-organ platforms to bridge the critical gap between enhanced delivery and therapeutic efficacy.
1. Introduction
Cancer immunotherapy has revolutionized oncology treatment, yet clinical success remains limited by inadequate preclinical models that fail to predict human responses [1,2]. Natural products, representing millennia of therapeutic wisdom, offer unique immunomodulatory properties through diverse mechanisms—from checkpoint modulation to macrophage reprogramming [3,4,5]. However, their therapeutic potential encounters formidable barriers when transitioning from simplified culture systems to physiologically relevant three-dimensional (3D) tumor models [5,6,7].
The journey from bench to bedside for immunomodulatory natural products reveals a fundamental challenge: compounds with remarkable efficacy in conventional assays often fail dramatically in complex tumor microenvironments. Curcumin exemplifies this paradox—while exhibiting potent programmed death-ligand 1 (PD-L1) downregulation in monolayer cultures [8,9], its limited penetration into the dense multicellular architecture of tumor spheroids compromises its therapeutic efficacy [10,11,12]. This penetration-efficacy disconnect has been observed across diverse natural product classes—including terpenoids, alkaloids, flavonoids, and polysaccharides—each facing unique physicochemical obstacles related to their molecular properties [12].
3D tumor models have emerged as essential platforms bridging the translational gap between Petri dishes and patients [13,14,15]. Unlike their two-dimensional (2D) counterparts, these systems recapitulate critical features of human tumors: heterogeneous cell populations, oxygen gradients, drug penetration barriers, and complex extracellular matrix architectures [16,17]. Spheroids, organoids, and scaffold-based constructs each offer distinct advantages for evaluating natural product behavior in environments mimicking clinical reality [18,19,20].
The physicochemical diversity of immunomodulatory natural products necessitates tailored formulation approaches. Hydrophobic compounds such as resveratrol face aggregation and protein binding that severely restrict bioavailability [21,22,23]. Macromolecular entities including medicinal mushroom β-glucans encounter size-dependent exclusion from dense tumor matrices [24,25,26]. pH-sensitive molecules like anthocyanins undergo rapid degradation in acidic microenvironments, potentially losing activity before reaching target immune populations [27,28]. Each category demands innovative solutions balancing enhanced delivery with preserved biological function.
Recent technological convergence creates unprecedented opportunities for addressing these challenges. Advanced formulation strategies—from stimuli-responsive nanocarriers to cyclodextrin complexation—provide remarkable penetration improvements [29,30,31,32]. Simultaneously, sophisticated 3D culture platforms now incorporate vascular networks, immune cell populations, and real-time monitoring capabilities [32,33,34]. The intersection of these technologies with regulatory support through Food and Drug Administration (FDA) Modernization Act 2.0 positions the field for transformative advances [13,34,35,36,37].
This review provides the first systematic analysis of the penetration–activity trade-off for natural product formulations in 3D tumor models, a critical gap that has hindered clinical translation. Unlike previous reviews focusing solely on penetration enhancement or biological activity, we uniquely integrate both perspectives. Our specific objectives are to: (1) quantify the extent of activity loss despite penetration improvements, (2) map the spatial distribution of immune cells and its implications for formulation design, (3) establish compound-specific strategies for three natural product categories (hydrophobic, macromolecular, stability-sensitive), and (4) propose a five-stage evaluation framework bridging formulation optimization with clinical implementation. By addressing the fundamental disconnect between pharmaceutical optimization and biological efficacy, this review guides the development of natural product-based immunotherapies that maintain therapeutic activity while achieving effective tumor penetration.
This comprehensive narrative review synthesizes key literature, selected based on mechanistic relevance and innovation in formulation strategies rather than systematic database searches, to develop an integrated framework applicable across diverse natural product categories and 3D model platforms.
To contextualize the scope and timeliness of this narrative review, we analyzed the research landscape through both our curated references and database searches. Our 531 references reveal a clear evolution in the field: foundational studies (2015–2019) primarily focused on characterizing natural product properties, while recent literature (2020–2025) increasingly addresses their integration with advanced 3D culture methodologies. A complementary search in PubMed and Web of Science databases (accessed on 10 July 2025) using terms (‘spheroid’ OR ‘organoid’) AND (‘drug screening’ OR ‘natural product’ OR ‘phytochemical’) yielded 5,673 publications, showing a 3.6-fold increase between 2015–2019 and 2020–2025, with notable acceleration following the FDA Modernization Act 2.0.
The bibliographic analysis reveals four dominant research clusters that emerged sequentially but now converge: First, penetration enhancement strategies in 3D systems evolved from simple size optimization to sophisticated multi-stage delivery systems (Section 3.4). Second, microfluidic 3D platforms progressed from basic perfusion models to integrated organ-on-chip systems (Section 5.1.3). Third, patient-derived organoid technology advanced from proof-of-concept studies to clinical validation with 68% positive predictive value (Section 5.1.2). Fourth, immune cell integration in 3D models expanded from simple co-cultures to complex spatial mapping of tumor-immune interactions (Section 4.2).
This global collaborative effort, reflected in our reference collection spanning multiple countries and institutions, underscores the interdisciplinary nature of this field. The convergence of these previously disparate research streams—now evident in integrated studies combining advanced formulations with sophisticated 3D models—provides the foundation for our conceptual framework addressing the penetration–activity trade-off.
2. Penetration Barriers in 3D Tumor Models
The transition from 2D to 3D culture systems reveals fundamental barriers that natural products must overcome to achieve therapeutic efficacy. Understanding these barriers at molecular, cellular, and tissue levels provides essential foundation for rational formulation design.
2.1. Microenvironmental Characteristics of 3D Models
2.1.1. Physical Architecture and Matrix Composition
The expression of extracellular matrix components in spheroids shows dramatic increases, with fibronectin levels elevated up to 33-fold compared to 2D cultures, while collagen I, collagen IV, and laminin also demonstrate significant upregulation. This upregulation results from enhanced collagen I and IV deposition, with fibronectin and laminin creating additional structural complexity. The resulting dense extracellular matrix (ECM) network, enriched with these upregulated components, creates a complex microenvironment that influences drug penetration and cellular behavior [38,39,40].
As spheroids mature, this enhanced ECM deposition drives progressive cellular compaction through cell-ECM and cell–cell interactions [41,42]. The mechanical forces generated by the contracting ECM network compress cells together [41], reducing intercellular spaces and creating a densely packed structure that mimics solid tumor architecture [7,43,44].
Cell packing density further compounds penetration challenges, reaching 6 × 107 cells/cm3 in mature spheroids compared to 1.8–3.6 × 106 cells/cm3 in confluent monolayers [45,46]. This increased density reduces interstitial space in core regions [47], resulting in tortuous diffusion pathways that significantly retard drug penetration [48].
The structural heterogeneity within spheroids generates distinct zones with varying penetration characteristics. Peripheral regions maintain relatively higher porosity due to active proliferation and looser cell packing [47], while core regions exhibit minimal interstitial space due to compression and reduced proliferation [47,49]. This radial gradient in structural density establishes a formidable barrier to drug delivery, requiring molecules to navigate increasingly constricted pathways as they move toward the spheroid center [48]. These structural barriers work in concert with chemical gradients to form a complex microenvironment that challenges drug delivery.
2.1.2. Chemical Gradient Formation
The restricted mass transport in 3D models produces steep chemical gradients that profoundly impact natural product behavior. Unlike monolayer cultures where cells experience uniform conditions, spheroids develop characteristic microenvironmental zones that challenge drug delivery and stability (Table 1) [50,51].
Oxygen gradients represent the most critical factor, with hypoxic cores driving metabolic reprogramming toward glycolysis [50,51]. This metabolic shift not only alters cellular drug response but also produces metabolic byproducts that further modify the microenvironment [52,53]. The resulting lactate accumulation leads to an acidic milieu that can reach levels comparable to those found in solid tumors, establishing conditions that markedly affect the stability and activity of pH-sensitive natural products [54,55,56].
These gradients establish phenotypically different cell populations with varying drug sensitivities. The proliferative periphery maintains oxidative metabolism and active drug uptake mechanisms, while the quiescent intermediate zone exhibits reduced metabolic activity and altered drug response patterns [52,53]. The necrotic core, characterized by severe nutrient depletion and acidosis, presents unique challenges for drug penetration and retention [50,51,56]. This spatial heterogeneity necessitates formulation strategies that account for multiple microenvironmental conditions within a single spheroid [54,55].
The temporal dynamics of gradient formation add another layer of complexity. Initial spheroid assembly proceeds with minimal gradients, but progressive growth and compaction generate steep concentration profiles within 48–72 h [45,46]. These evolving conditions result in a dynamic therapeutic target where drug behavior changes with spheroid maturation, requiring time-dependent optimization of delivery strategies [47,48].

Table 1.
Representative studies demonstrating formulation-mediated penetration improvements in three-dimensional tumor models. Data from validated studies showing quantitative penetration enhancement through different formulation strategies. Measurements at 24–48 h post-treatment in 400–600 μm spheroids. Enhancement factors calculated as ratio of enhanced to initial penetration. Arrow (→) indicates gradient from spheroid surface to core. 2D, two-dimensional; 3D, three-dimensional; μm, micrometer.
Table 1.
Representative studies demonstrating formulation-mediated penetration improvements in three-dimensional tumor models. Data from validated studies showing quantitative penetration enhancement through different formulation strategies. Measurements at 24–48 h post-treatment in 400–600 μm spheroids. Enhancement factors calculated as ratio of enhanced to initial penetration. Arrow (→) indicates gradient from spheroid surface to core. 2D, two-dimensional; 3D, three-dimensional; μm, micrometer.
| Parameter | 2D Culture | 3D Spheroid (400–600 μm) | Clinical Relevance | Ref |
|---|---|---|---|---|
| Oxygen tension | 20–21% | Surface: 20–21% → Core: < 0.2% | Hypoxia-induced resistance | [50,51] |
| pH | 7.4 | Surface: 7.2–7.4 → Core: 6.7–6.8 | Drug stability/activity | [54,55] |
| Cell density (cells/cm3) | 1.8–3.6 × 106 | 6 × 107 | Penetration barriers | [45,46] |
| Glucose (mM) | 5–25 | Surface: 5–25 → Core: <0.1 | Metabolic adaptation | [52,53] |
| Lactate (mM) | < 5 | Core: up to 40 | Acidification | [56] |
2.2. Compound-Specific Penetration Challenges
2.2.1. Hydrophobic Natural Products: Solubility and Protein Binding
Hydrophobic immunomodulators face multifaceted penetration barriers stemming from their physicochemical properties. Curcumin, the most extensively studied example, illustrates how poor aqueous solubility (0.6–7.8 μg/mL at pH 7.4) poses immediate challenges for 3D delivery [57,58,59]. Penetration studies using spatial reveal that curcumin can reach spheroid cores, with preferential accumulation in necrotic zones, though the compound shows substantially reduced efficacy with half maximal effective concentration (EC50) values increasing from 12.25 μM in 2D cultures to 30.76 μM in 3D spheroids [60].
Protein binding compounds these solubility issues, with curcumin exhibiting > 95% albumin binding with association constants of 1.74 × 105 M−1 (corresponding to dissociation constant (KD) values of 5.7 μM), dramatically reducing free drug availability [61,62]. This binding follows saturable kinetics, resulting in a sink effect where protein-bound drug accumulates at the periphery without contributing to therapeutic effect. Similar patterns emerge across diverse hydrophobic structures. Resveratrol achieves enhanced penetration to hypoxic cores only when complexed with β-cyclodextrin [63]. Quercetin, apigenin, and luteolin face comparable barriers in 3D systems, though their specific penetration depths remain unquantified. Quantitative analysis of hydrophobic drug penetration indicates that compounds with logarithm of partition coefficient (Log P) values exceeding 3.0–4.0 exhibit markedly restricted tissue distribution, with penetration efficiency decreasing exponentially as lipophilicity increases [64,65].
2.2.2. Macromolecular Barriers: Size Exclusion and Diffusion Limitations
Polysaccharide-based immunomodulators encounter substantial size-dependent barriers in 3D systems. Comprehensive diffusion studies using model compounds such as dextrans reveal molecular weight-dependent penetration patterns: compounds under 10 kilodalton (kDa) achieve deep penetration (greater than 35 μm) and homogeneous distribution into tumor tissue, while those between 40–70 kDa show limited penetration to approximately 15 μm from the vascular surface, and molecules exceeding 2 megadalton (MDa) display only superficial penetration (~5 μm) [11,66]. Similar size-dependent limitations are expected for polysaccharide-based immunomodulators, though specific quantitative data are limited.
Structural conformation adds complexity beyond molecular weight considerations. Linear polysaccharides like fucoidan and branched structures such as schizophyllan possess varying molecular weights and structural characteristics that influence penetration behavior [67,68]. Triple-helical β-glucans adopt rigid rod-like conformations that form stable triple-helix aggregates [69,70,71], making them particularly challenging for tissue penetration due to their inability to deform through tortuous ECM pathways.
Charge distribution markedly influences penetration, with electrostatic repulsion between negatively charged particles and ECM components considerably decreasing diffusion coefficients [72,73] compared to neutral particles of equivalent size. Conversely, cationic polysaccharides can cross-link with anionic matrix components through ionic interactions [74,75], initially enhancing binding but potentially limiting deep tissue distribution.
2.2.3. Stability-Sensitive Compounds: Environmental Degradation
Natural products susceptible to environmental conditions encounter unique challenges where penetration and stability become inextricably linked. While comprehensive stability data in 3D tumor spheroids is limited, insights from 2D culture systems and food matrices provide crucial understanding of degradation patterns that likely intensify in the complex spheroid microenvironment.
Anthocyanins exemplify stability-sensitive compounds, with thermal degradation following first-order kinetics highly dependent on pH conditions. In aqueous systems, anthocyanins at pH 7.0 and 75 °C exhibit half-lives of 1.98 h with rate constants of 0.3488 h−1, while at pH 3.0 the half-life extends to 15.12 h with rate constant 0.0458 h−1, indicating a 7.6-fold stability improvement under acidic conditions [76,77]. Purple sweet potato anthocyanins at 90 °C display pH-dependent degradation with half-lives of 10.27, 12.42, and 4.66 h at pH 3.0, 5.0, and 7.0 respectively, with 2.2-fold faster degradation at neutral pH compared to acidic conditions [78]. Although specific kinetics in 3D spheroids await characterization, the pH gradient inherent in these models (pH 7.4 peripherally to 6.5 centrally) suggests accelerated degradation in deeper regions where therapeutic targets reside.
Epigallocatechin gallate (EGCG) exhibits distinct stability challenges in biological systems, with a remarkably short half-life of less than 30 min in McCoy’s 5A culture media, though this extends to 130 min in the presence of cells [79]. The compound undergoes auto-oxidation even under standard culture conditions, yielding dimers including theasinensin (relative molecular mass (Mr) 914) and releasing hydrogen peroxide H2O2) up to 25 μM [79,80]. While these degradation patterns are well-characterized in monolayer cultures, the hypoxic core regions of spheroids may paradoxically accelerate oxidative degradation through metal-catalyzed mechanisms, forming higher molecular weight species with potentially impaired penetration.
Temperature sensitivity constitutes an underexplored factor in 3D culture stability. While specific data on minor temperature variations (37 °C vs. 37.5 °C) in spheroids is unavailable, ginsenosides show remarkable stability at 37 °C in neutral pH conditions but undergo rapid conversion at elevated temperatures (130 °C) in the presence of organic acids [81]. The metabolic activity within spheroids may produce localized microenvironmental variations that impact compound stability, though quantitative characterization of these effects necessitates further investigation. The compound-specific penetration challenges and corresponding formulation requirements are summarized in Table 2. The penetration barriers and formulation solutions for each category are illustrated in Figure 1.

Table 2.
Natural product categories and formulation requirements based on physicochemical challenges affecting 3D tumor penetration. Data compiled from literature (2012–2025) showing preferred strategies and reported outcomes. Arrow (→) indicates change from 2D to 3D culture conditions. 2D, two-dimensional; 3D, three-dimensional; EC50, half maximal effective concentration; EGCG, epigallocatechin gallate; kDa, kilodalton; NPs, nanoparticles; pH, potential of hydrogen; PSK, polysaccharide-K; t½, half-life; μm, micrometer.
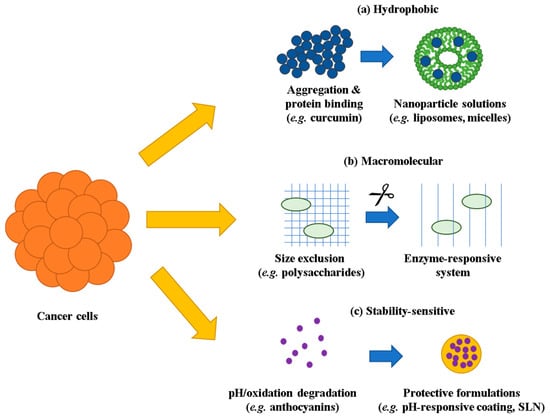
Figure 1.
Compound-specific penetration barriers and formulation solutions in 3D tumor models. (a) Hydrophobic compounds undergo aggregation and protein binding, overcome by nanoparticle solutions including liposomes and micelles. (b) Macromolecular compounds face size exclusion from dense ECM networks, addressed through enzyme-responsive delivery systems. (c) Stability-sensitive compounds suffer from pH/oxidation-dependent degradation, prevented using protective formulations including pH-responsive coatings and SLN. Yellow arrows indicate penetration barriers entering the spheroid, while blue arrows represent formulation-based solutions. ECM, extracellular matrix; SLN, solid lipid nanoparticles.
2.3. Immunomodulatory Mechanisms by Natural Product Categories
Different categories of natural products exhibit distinct immunomodulatory mechanisms reflecting their structural characteristics. Polysaccharides (>100 kDa), such as lentinan and polysaccharide-K (PSK), primarily activate innate immunity through toll-like receptor (TLR) 2/4 engagement on dendritic cells, with higher molecular weight fractions showing stronger immunostimulatory effects. Astragalus polysaccharides stimulate DC transformation to CD11chigh/CD45RBlow phenotype and convert Th2 to Th1 cells [82].
Alkaloids target intracellular signaling pathways. Matrine inhibits M2 macrophage polarization and reduces CD206, vascular endothelial growth factor (VEGF), and matrix metalloproteinase (MMP) expression through nuclear factor kappa B (NF-κB)/mitogen-activated protein kinase (MAPK) suppression [83]. Sinomenine blocks NF-κB activation, subsequently inhibiting cyclooxygenase (COX)-2 and prostaglandin (PGE) 2 production in cancer cells [84]. Their nitrogen-containing structures facilitate membrane permeation and intracellular target engagement [85].
Terpenoids, including ginsenosides and astragaloside IV, modulate both macrophage polarization and T cell responses. Ginsenosides decrease M2 markers (CD206) and suppress VEGF/MMP expression, while astragaloside IV impedes M2-induced invasion in lung cancer cells [82]. Their amphiphilic nature enables interaction with both membrane receptors and cytoplasmic targets.
These mechanistic differences directly impact formulation requirements for 3D tumor models, where polysaccharides’ limited penetration (30–50 μm) aligns with peripheral immune cell localization, while small molecule alkaloids require protection against pH-dependent degradation in spheroid cores.
3. Formulation Strategies for Enhanced Penetration
The formidable barriers posed by 3D tumor models have catalyzed development of sophisticated formulation strategies. These approaches must balance penetration enhancement with preservation of immunomodulatory activity, a challenge that varies considerably across natural product categories.
3.1. Formulation Approaches for Hydrophobic Immunomodulators
3.1.1. Nanoparticle-Based Delivery Systems
Nanoparticle formulations have emerged as the most extensively investigated approach for enhancing hydrophobic natural product penetration, with multiple studies reporting quantitative improvements. Size optimization proves critical for navigation through the ECM network. Systematic evaluation uncovers distinct size-dependent penetration profiles: particles in the 20–50 nm range successfully penetrate to spheroid cores, with 50 nm particles exhibiting particularly effective distribution. In contrast, particles exceeding 100 nm remain confined to peripheral regions [11]. Poly (lactic-co-glycolic acid) (PLGA) nanoparticles exemplify successful implementation, with optimized curcumin-loaded formulations (80 nm, zeta potential -20 millivolt (mV)) achieving 2- to 6-fold enhanced cellular uptake that correlates with improved spheroid penetration compared to free drug [86,87,88]. The biodegradable polymer matrix enables sustained release over 72 h, maintaining therapeutic concentrations in deeper spheroid regions [87]. Interestingly, particle size optimization proved critical for effective penetration, as particles exceeding 100 nm showed significantly limited distribution beyond peripheral spheroid regions [11,89]. Recent advances have demonstrated the integration of imaging and therapeutic functionalities within single nanoplatforms. For example, Zn (II)-Schiff base complexes have achieved lysosome-targeted theranostics with high quantum yields (63.7%) for real-time imaging and pH-responsive drug release (81% at pH 5.6 vs. 51% at pH 7.4), effectively combining diagnostic precision with controlled therapeutic delivery [90]. Such “track-and-treat” approaches are particularly valuable for 3D tumor models where the acidic microenvironment (pH 6.5–6.8 in spheroid cores) can trigger selective drug release while enabling simultaneous monitoring of drug distribution.
Surface functionalization strategies substantially affect impact penetration profiles beyond simple size effects. Polyethylene glycol (PEG)ylation using 5 kDa chains increases penetration depth by 72% while reducing protein corona formation by 80% [6,91,92]. However, PEG molecular weight is critical—chains below 2 kDa offer insufficient steric stabilization, while those exceeding 10 kDa generate hydrodynamic drag that impedes diffusion [91,92]. Active targeting strategies must balance enhanced cellular uptake with potential limitations in tissue penetration. The “binding-site barrier” effect, where high-affinity binding to peripheral cells prevents deeper tissue infiltration, constitutes a key challenge in targeted nanoparticle design [93].
3.1.2. Lipid-Based Formulation Systems
Liposomal formulations offer superior biocompatibility and penetration enhancement for hydrophobic natural products. Systematic evaluation of nanoparticle physicochemical properties in well-characterized tumor spheroids indicates that particle size critically determines penetration efficiency. Smaller nanoparticles (30 nm and 50 nm) exhibit superior penetration to spheroid cores compared to larger particles (100 nm), with clinically relevant liposomal doxorubicin (Caelyx, approximately 87 nm) displaying intermediate penetration behavior [11,12,89]. Additional studies confirm that ultra-small particles (15 nm and 22 nm) attain enhanced tissue penetration compared to 60 nm particles, confirming a clear size-dependent penetration pattern [94].
Surface modification strategies markedly influence penetration profiles beyond simple size effects. PEGylation of liposomal formulations reduces opsonization and extends circulation time, while ligand modification allows targeted delivery to specific cell populations [6,11]. Head-to-head comparisons of various lipid-based nanoparticles reveal distinct penetration efficiencies based on surface chemistry and targeting mechanisms [6].
Cholesterol content emerges as a fundamental formulation parameter controlling both membrane stability and drug release kinetics. Systematic investigations of cholesterol ratios ranging from 0% to 50% in phospholipid formulations show that a 70:30 phospholipid:cholesterol ratio yields optimal membrane stability for controlled drug release applications [95,96,97]. This composition balances membrane rigidity with drug loading capacity, with moderate cholesterol content conferring excellent diffusivity while excessive cholesterol content limits penetration processes [97]. The relationship between cholesterol concentration and membrane fluidity directly affects the permeability characteristics of hydrophilic molecules through lipid bilayers [96].
Solid lipid nanoparticles (SLNs) offer unique stabilization advantages for environmentally sensitive natural products through their crystalline lipid matrices. Compounds such as epigallocatechin gallate (EGCG), which exhibits rapid degradation with half-lives under 30 min in standard culture conditions, show enhanced stability when incorporated into SLN formulations using glyceryl monostearate cores [79,98]. The solid lipid matrix delivers dual protective mechanisms: physical encapsulation against environmental degradation and controlled release through matrix erosion processes [98].
Nanostructured lipid carriers (NLCs) constitute an advanced evolution of solid lipid nanoparticle technology, incorporating both solid and liquid lipid components to form less organized lipid matrices. This structural modification allows enhanced drug loading capacities and improved release characteristics compared to conventional SLNs, while maintaining the stability benefits inherent to lipid-based delivery systems [99,100]. The incorporation of liquid lipids produces matrix imperfections that facilitate drug accommodation and controlled release kinetics [100].
3.1.3. Cyclodextrin Complexation Strategies
Cyclodextrin inclusion complexes provide a molecularly precise approach to solubility enhancement. β-cyclodextrin complexation can increase curcumin aqueous solubility dramatically, with reported enhancements ranging from 2.34-fold in ethanolic conditions [101] to 31-fold using co-precipitation methods [102], highlighting the importance of preparation methodology. Phase solubility studies indicate that aqueous solubility of curcumin increases linearly as a function of cyclodextrin concentration, with the solubility diagram classified as AL type (linear increase in solubility) [103].
Hydroxypropyl-β-cyclodextrin (HP-β-CD) offers enhanced performance through improved complex stability constants (424 M−1) compared to native β-CD (134 M−1), methyl-β-CD (401 M−1), and γ-CD (154 M−1) [103]. HP-β-CD complexation can attain remarkable 206-fold solubility enhancement [104]. Cyclodextrin-based nanosponges exhibit even higher stability constants, with values of 4972.90, 4164.50, and 3567.87 M−1 for different cross-linking densities [101]. Recent studies reveal that cyclodextrins facilitate drug penetration into tumor spheroids via a nanoshuttle mechanism, though penetration must be balanced with cellular uptake [105].
The commercial formulation Cavacurmin® utilizing γ-cyclodextrin achieves 40-fold enhancement in bioavailability compared to standard curcumin extract [106]. Modified cyclodextrins with targeting moieties offer additional functionality. Folate-conjugated β-cyclodextrin complexes show 1.5–2-fold more efficiency in tumor volume reduction in 3D spheroid cultures compared to plain drug solution, attributed to better penetration of the nanoparticles into the tumor microenvironment [107]. These folate-targeted systems exhibit enhanced accumulation in folate receptor-positive tumor cells [108,109].
Sulfobutyl ether β-cyclodextrin (Captisol®) possesses unique characteristics with an average degree of 6.5 sulfobutyl groups per cyclodextrin molecule, conferring multiple negative charges that enhance both solubility (>50-fold compared to β-CD) and drug complex stability [110]. Polycationic amphiphilic cyclodextrin nanoparticles possess the ability to diffuse and penetrate through multilayer cells in 3D tumor models, which is crucial for eventual antitumor effect [111].
3.2. Strategies for Macromolecular Natural Product Delivery
3.2.1. Surface Modification and Conjugation Approaches
The severe size-dependent barriers facing polysaccharide immunomodulators require innovative approaches beyond simple size reduction. Polysaccharides such as lentinan from Lentinus edodes, PSK from Trametes versicolor, and fucoidan from various brown algae have demonstrated anti-cancer efficacy against several human cancers in clinical trials as biological response modifiers, yet their therapeutic potential in 3D tumor models is limited by penetration barriers [11,66,112,113,114].
Chemical modification methods have been developed to enhance penetration while maintaining biological activity. These include carboxymethylation, sulfation, selenylation, phosphorylation, and acetylation [115,116,117]. Sulfate modification of polysaccharides improves anti-tumor activity by increasing their immune-stimulating properties [118,119,120]. For instance, sulfated and phosphated derivatives of polysaccharides with appropriately reduced molecular mass effectively inhibited H-22 tumor cells growth, while carboxymethylated derivatives produced less pronounced effects [121,122].
Recent studies with fucoidan-based systems offer insights into surface modification strategies. A fucoidan-based theranostic nanogel (CFN-gel) with a hydrodynamic size of 259 nm achieved enhanced tumor accumulation through P-selectin targeting, with nanomolar (nM) affinity (KD = 718.9 nM) for P-selectin overexpressed on tumor cells [123,124,125]. PSK, consisting of (1 → 4)-β-glucan with (1 → 6)-β-glucopyranosidic lateral chains and 25–38% protein residues, activates human natural killer (NK) cells via TLR2, augmenting the antitumor effect of human epidermal growth factor receptor 2 (HER2)-targeted monoclonal antibody therapy [126,127,128].
Chitosan-based systems, while not therapeutic polysaccharides themselves, serve as valuable proof-of-concept for surface modification strategies [75,129]. Phenylboronic acid-decorated chitosan nanoparticles (200–230 nm) exhibited deeper penetration and persistent accumulation in multicellular spheroids compared to non-decorated nanoparticles, leading to enhanced growth inhibition of 3D tumor spheroids [130,131,132]. Similarly, integrin binding Arg-Gly-Asp containing CendR (iRGD) tumor-penetrating peptide-modified nano-delivery systems based on marine sulfated polysaccharide (propylene glycol alginate sodium sulfate) successfully improved tumor targeting and cellular internalization while maintaining immunomodulatory activity through PD-L1 downregulation [3,133].
The relationship between molecular weight and immunomodulatory activity poses a critical consideration [134,135]. Studies reveal that β-(1,3)-glucan isolated from Grifola frondosa displayed molecular weight (MW)-dependent activity, with the highest MW glucan consistently exhibiting the most potent immunomodulatory effect [136,137]. Similarly, PSK with molecular weight exceeding 200 kDa possessed the strongest immunostimulating activities [138,139]. This established a fundamental challenge: while smaller, modified polysaccharides may penetrate better, they risk losing critical immunomodulatory functions [140,141].
Emerging evidence suggests that certain modifications can accomplish both enhanced penetration and maintained or improved biological activity [142,143]. Sulfated chitosan inhibits angiogenesis via blocking the VEGF/VEGF receptor 2 (VEGFR2) pathway with 63.8% inhibition compared to 30.7% for heparin control [144], while also exhibiting enhanced tissue distribution through increased hydrophilicity and reduced aggregation [72,96]. These dual benefits underscore the potential for carefully designed modifications to overcome the penetration–activity trade-off.
While specific penetration depths for therapeutic polysaccharides in 3D models await systematically quantified [145], studies with model systems indicate that surface modifications can substantially improve distribution in tumor tissue [6,15]. PEG-modified particles maintained 20–40% of surface concentration at 40 μm depth in spheroids, compared to only 10% for unmodified particles [89,94,146]. These principles, derived from synthetic systems, offer a foundation for optimizing therapeutic polysaccharide delivery, though direct studies comparing native and modified forms of clinically relevant polysaccharides in standardized 3D models are urgently needed [13,16].
3.2.2. Matrix-Modifying Formulation Strategies
Recognition that size barriers are insurmountable through conventional formulation has led to strategies that temporarily modify the ECM itself. These matrix-modifying approaches hold particular promise for overcoming the size-dependent exclusion that limits macromolecular therapeutics, especially polysaccharides which typically aggregate into structures ranging from 50–400 nm depending on molecular weight and formulation conditions [147,148].
Enzymatic ECM degradation constitutes the most direct approach for enhancing polysaccharide penetration. Hyaluronidase treatment is especially relevant for polysaccharide therapeutics, as it degrades hyaluronic acid (HA)—a glycosaminoglycan that shares structural similarities with many therapeutic polysaccharides. Studies show that hyaluronidase at 0.5 (mg/mL) for 8 h markedly improved penetration of multistage nanoparticles at 100 μm sections of tumor spheroids [149]. The enzyme’s ability to degrade the glycosaminoglycan network generates transient channels that facilitate passage of similarly structured therapeutic polysaccharides, with hyaluronidase-sensitive carriers achieving enhanced penetration through HA-rich tumor microenvironments [150,151].
Collagenase treatment provides complementary benefits by degrading the fibrillar collagen network that physically entraps large molecules. While studies specifically on polysaccharide delivery are limited, validated model systems show that collagenase-coated 100 nm particles attained 4-fold greater delivery to spheroid cores [152]. This size range (50–100 nm) corresponds to typical polysaccharide nanoparticle formulations, including chitosan-based systems (50–400 nm) and hyaluronic acid nanoparticles (118–290 nm), suggesting direct applicability [147,148,153]. However, enzymatic ECM modification raises metastasis concerns. Pulsed delivery protocols—such as 5-h collagenase pretreatment at 37 °C followed by therapeutic administration—present a compromise between enhanced penetration and safety [152,153,154].
MMP-responsive systems constitute an emerging strategy with proven relevance to polysaccharide delivery. Recent developments in MMP-2 responsive peptide hydrogels successfully incorporated chitosan nanoparticles, illustrating the feasibility of combining polysaccharide components with enzyme-responsive elements [155]. Real-time imaging shows that MMP-2 responsive systems combined with laser irradiation could effectively loosen dense tumor stroma and improve therapeutic agent penetration throughout entire spheroids [155]. While current systems primarily use peptide crosslinkers for MMP sensitivity, this hybrid approach provides a foundation for developing fully polysaccharide-based MMP-responsive delivery systems, particularly given that polysaccharides can be functionalized with MMP-cleavable sequences [155,156,157].
3.2.3. Alternative Delivery Paradigms
The fundamental limitations of macromolecular penetration have prompted exploration of alternative strategies that work within these constraints. Recognizing that spheroids display compartmentalized structures with high proliferation rates in the spheroid periphery stimulated by constant exposure to oxygen and nutrients, formulation strategies have evolved to exploit these gradients rather than overcome them [158]. The penetration of aminated nanoparticles was limited to outer cell layers of spheroids, approximately 30–50 μm depth from the surface, coinciding with zones of highest metabolic activity and immune cell presence [11].
Spatial drug distribution studies indicate that drugs like paclitaxel preferentially affect cells in the periphery due to characteristics of the peripheral population [159]. Microfluidic concentration gradient generators demonstrate that drug efficacy can be achieved through sustained peripheral exposure, with spheroid viability inversely proportional to drug concentration in gradients ranging from 0.1 to 10 μM [160,161]. This peripheral accumulation strategy acknowledges that drug penetration is restricted to the outer layer (~ 100 μm in depth) in large spheroids [145]. This peripheral targeting approach may be particularly advantageous for polysaccharide immunomodulators such as PSK and lentinan, whose primary cellular targets—macrophages and T cells—predominantly localize within these peripheral zones.
However, when deep penetration is essential for therapeutic efficacy, physical methods such as ultrasound provide promising solutions. Ultrasound-mediated delivery presents remarkable potential for overcoming size barriers through physical disruption. Pulsed ultrasound improves nanoparticle penetration, with small (20 nm) particles attaining 6-20-fold higher penetration and concentration in the spheroid’s core compared to those not exposed to ultrasound [162]. Focused ultrasound at 770 kilopascal (kPa) peak negative pressure, 1 megahertz (MHz) pulse frequency, 8 microsecond (μs) pulse duration and 2 millisecond (ms) pulse repetition period generates transient pores facilitating enhanced doxorubicin penetration into deeper regions of tumor spheroid [163].
For polysaccharide-based systems, recent studies reveal promise. High-intensity focused ultrasound (HIFU) treatment of doxorubicin-loaded glycol chitosan nanoparticles (265.9 ± 35.5 nm) resulted in deep penetration into ECM-rich tumors, with 1.84-fold higher tumor accumulation compared to untreated nanoparticles [164]. The HIFU-mediated destruction of ECM structure promoted penetration throughout targeted tumor tissues, indicating this approach could be particularly beneficial for larger therapeutic polysaccharides [164].
For smaller molecules, 20 kilohertz (kHz) ultrasound at 5 watts per square centimeter (W/cm2) intensity permits penetration to a total depth of 125 μm for 3 kDa dextran-drug conjugates [165]. The reversible nature of sonoporation, with membrane resealing generally completed within 1 min of the onset of sonoporation and resealing time constants estimated to be below 20 s, addresses safety concerns associated with permanent ECM modification [166,167,168]. While these studies confirm ultrasound efficacy for smaller polysaccharides, the application to larger therapeutic polysaccharides (50–400 kDa) constitutes an important area for future investigation.
3.3. Protective Formulations for Stability-Sensitive Compounds
3.3.1. pH-Responsive Coating Systems
Stability-sensitive natural products necessitate sophisticated protection strategies that balance environmental shielding with targeted release. pH-responsive polymeric coatings have emerged as particularly effective for navigating the complex pH environments encountered during drug delivery. While Eudragit S100, which dissolves at pH values above 7.0, confers excellent protection in the acidic stomach environment (pH 1–2) and releases drug in the intestinal environment (pH 6.8–7.4) [169,170], its application for tumor spheroid delivery demands strategic modifications.
Given that spheroid cores have pH values of 6.7–6.8 as described in Section 2.1.2, polymer combinations are employed to obtain release in this intermediate pH range. By combining Eudragit S100 with Eudragit L100-55 (which dissolves at pH 5.5) or Eudragit L100 (dissolves at pH 6.0), the dissolution pH can be precisely tuned to match the tumor microenvironment [171,172]. These combinations allow formulations that maintain nanoparticle sizes of 100–200 nm, which show optimal penetration into tumor spheroids [11,94].
Layer-by-layer assembly techniques using these polymer combinations permit precise control over release kinetics. Studies indicate that at pH 1.2, less than 10% drug release occurs from enteric-coated formulations, offering protection for stability-sensitive compounds like anthocyanins and EGCG during gastric transit [173,174]. At pH 6.8, which corresponds to both intestinal and tumor spheroid environments, progressive drug release is observed, with the exact profile depending on the Eudragit S100:L100-55 ratio used [171]. This pH-responsive behavior facilitates for site-specific delivery of stability-sensitive natural products while preserving their biological activity. In situ pH monitoring using ratiometric fluorescent probes has been utilized to track pH-responsive drug release and verify targeted release in the acidic tumor microenvironment [174].
3.3.2. Antioxidant Co-Encapsulation Strategies
Oxidative degradation poses a major stability challenge necessitating active protective measures beyond simple encapsulation. Co-encapsulation of antioxidants yields synergistic protection through multiple mechanisms. Vitamin E (α-tocopherol) and EGCG display synergistic antioxidant effects, with green tea polyphenols capable of regenerating α-tocopherol through electron transfer mechanisms in model systems [175,176]. This regeneration mechanism augments the overall antioxidant capacity and extends the protective effects of both compounds when co-encapsulated [175,176,177].
Ascorbic acid co-encapsulation supplies complementary water-soluble antioxidant protection particularly suited for hydrophilic compartments of delivery systems. Studies reveal that ascorbic acid notably strengthens EGCG stability by protecting it from auto-oxidation in aqueous environments, with enhanced protection observed at lower temperatures [178,179]. In practical applications, dual-drug loaded PEGylated PLGA nanoparticles co-encapsulating EGCG and ascorbic acid showed enhanced stability while maintaining particle sizes appropriate for spheroid penetration [180]. Similarly, ascorbic acid-loaded chitosan nanoparticles reached optimal sizes of 170 nm with encapsulation efficiencies of 10–12%, confirming the feasibility of incorporating water-soluble antioxidants into nano-sized delivery systems [181]. The spatial separation of hydrophilic and hydrophobic antioxidants in these systems permits complementary protection mechanisms while maintaining individual compound stability, with various formulation strategies achieving successful co-encapsulation of antioxidants at the nanoscale [180,181].
3.3.3. Solid Lipid Matrices for Physical Stabilization
SLNs provide unique stabilization through physical entrapment within crystalline matrices. The restricted molecular mobility in solid lipids markedly decreases degradation kinetics [182]. For instance, encapsulation of salmon calcitonin in trimyristin SLN ensured sustained release over 8 h under both gastric and intestinal pH conditions, while effectively shielding the peptide from enzymatic degradation [183]. Glyceryl monostearate-based SLNs loaded with docetaxel preserve drug integrity with melting transition peaks greater than 40 °C, indicating SLNs remain solid at body temperature, with 68% drug release in 24 h [184]. Additionally, epigallocatechin gallate (EGCG) encapsulated in glyceryl monostearate SLN exhibited enhanced stability compared to free EGCG, exemplifying the protective function of solid lipid matrices for stability-sensitive compounds [98].
SLNs display excellent long-term stability, with triglyceride-based formulations exhibiting superior stability compared to mono- and diglycerides [185]. X-ray diffraction and differential scanning calorimetry verify drug incorporation within lipid crystal lattices, while the polymorphic transition from α to β′ modification during storage produces denser packing that further improves stability [186,187].
Temperature-triggered release from SLNs allows spatial control over drug liberation. Formulations using lipids with specific melting points remain solid during initial penetration but undergo phase transition upon exposure to mild hyperthermia, promoting enhanced delivery of nanoparticles to the tumor interstitium [188]. This temperature-modulated approach has been successfully applied to various drug-loaded SLN formulations, with differential scanning calorimetry validating controlled phase transitions for precise spatial control of drug release [189].
3.4. Advanced Integrated Approaches
Recognition that conventional formulation strategies have limitations led to development of advanced approaches. These strategies specifically address the key challenges of conformational changes through biomimetic systems, incomplete drug release through stimuli-responsive mechanisms, and surface modification interference through synergistic combinations.
3.4.1. Stimuli-Responsive Intelligent Systems
Multi-stage release systems represent an advanced engineering approach to overcome the complex microenvironment of 3D tumor spheroids. These spheroids present unique challenges characterized by pH gradients ranging from physiological pH 7.4 at the periphery to pH 6.5 in the intermediate regions and pH 5.0 in hypoxic cores [190,191]. These gradients, combined with varying oxygen tensions and cellular densities, necessitate sophisticated delivery strategies that can respond to multiple stimuli in a spatiotemporally controlled manner.
Recent advances in multi-stage release systems have enabled the ability to exploit these environmental gradients for sequential drug delivery. pH-responsive polymers incorporating maleic acid amide derivatives undergo sharp charge conversion at pH 6.0, transitioning from negative to positive charge due to β-carboxylic amide hydrolysis [191]. This charge reversal triggers drug release and enhances cellular uptake through electrostatic interactions. This addresses the dual challenges of drug release and cellular internalization.
The kinetics of drug release from these systems follows distinct patterns depending on environmental conditions. Under physiological pH 7.4, sustained zero-order release achieves 54% drug release over 48 h. In contrast, exposure to tumor-relevant pH 6.5 accelerates release to 87.4% within 12 h following pseudo-Fickian diffusion kinetics [192]. When combined with high glutathione concentrations (10 mM) typical of intracellular environments, pH 5.0 conditions trigger rapid release of 70% of encapsulated drug within just 4 h [192].
Mathematical modeling of these release profiles indicates that the Korsmeyer-Peppas model best describes multi-stage release kinetics, with diffusional exponent (n) values below 0.43 indicating Fickian diffusion for spherical particles [193]. This mechanistic understanding enables rational design of sequential release systems. Initial burst release of one therapeutic agent is followed by sustained release of a second drug, achieving synergistic therapeutic effects [194].
The size of drug carriers critically influences their penetration into spheroid tissue. Systematic studies comparing different sized nanoparticles reveal that particles smaller than 20 nm achieve deep penetration throughout spheroids, while those between 20–50 nm represent an optimal range balancing penetration depth with drug loading capacity [146,195]. Larger particles exceeding 100 nm remain predominantly at the spheroid periphery, limiting their therapeutic efficacy [6]. This size-dependent behavior was definitively shown through comparative studies of small interfering RNA (siRNA) polyplexes (25 nm) versus plasmid DNA (pDNA) polyplexes (162 nm). The smaller siRNA complexes penetrated to the spheroid core while pDNA complexes localized primarily in the rim region [196].
Advanced imaging techniques, including confocal microscopy with z-stacking and light sheet microscopy, have enabled precise quantification of penetration depths. Multi-stage delivery systems achieve enhanced penetration, with PEGylated nanoparticles reaching penetration depths of 67 μm (d1/2) compared to 26–39 μm for conventional formulations [6]. This enhanced penetration results from the initial release of cell-penetrating agents that facilitate subsequent deeper penetration of the drug-loaded carriers. This is evidenced by systems achieving burst release through acid-induced decomposition of chitosan shells [194].
The integration of hypoxia-responsive elements further enhances the sophistication of multi-stage systems. Azobenzene-linked prodrugs remain stable under normoxic conditions but undergo rapid cleavage in hypoxic regions where oxygen tension falls below 2.5 mm of mercury (mmHg), achieving up to 3.7-fold greater tumor growth inhibition in hypoxic conditions [197]. Nitroreductase-activated systems exhibit even greater selectivity, with 3–20-fold increased cytotoxicity specifically in hypoxic cells [198,199].
Sequential release strategies have proven particularly effective when combining therapies with different mechanisms of action. For instance, initial release of vascular-disrupting agents or photosensitizers can exacerbate local hypoxia. This thereby activates subsequent release of hypoxia-activated prodrugs [200]. The combination of photodynamic therapy and chemotherapy through these mechanisms results in excellent antitumor efficacy, yielding enhanced therapeutic outcomes while minimizing systemic toxicity [200].
The clinical translation of these multi-stage systems requires careful consideration of the temporal dynamics of drug release. Studies suggest that the therapeutic window for initial drug release should occur within 4–6 h of administration to coincide with maximal tumor accumulation, while secondary release should be sustained over 24–48 h to maintain therapeutic concentrations [201]. This temporal control is achieved through careful selection of degradable linkers with distinct cleavage kinetics under tumor-specific conditions.
3.4.2. Biomimetic Delivery Approaches
Cell membrane coating technology enables natural products to hijack endogenous trafficking mechanisms through sophisticated biomolecular interactions. Cancer cell membrane-coated nanoparticles exploit homotypic targeting mechanisms mediated by surface adhesion molecules including N-cadherin, galectin-3, and epithelial cell adhesion molecule (EpCAM) [202]. These biomimetic carriers exhibit enhanced cellular uptake, with activated fibroblast membrane-coated semiconducting polymer nanoparticles showing specific targeting toward cancer-associated fibroblasts and facilitating internalization into cancer cells [203]. The retention of membrane proteins, particularly CD47, facilitates immune evasion. This occurs by binding to signal regulatory protein alpha (SIRPα) on macrophages and transmitting “don’t eat me” signal [204,205]. This immune escape mechanism, combined with PEG-trehalose surface modification, results in 2-fold enhanced cellular internalization in neuronal cells [206].
Beyond simple membrane coating, hybrid membrane strategies create multifunctional carriers with enhanced capabilities. Black phosphorus quantum dots camouflaged with platelet-osteosarcoma hybrid membranes enhance circulation time and enable osteosarcoma (OS)- specific targeting through the combined properties of both cell types [204]. The versatility of biomimetic coating extends to various natural products—quercetin encapsulated in gold nanoparticles enhanced cytotoxicity (>50-fold) in colon cancer cells [207], while galbanic acid-coated magnetic nanoparticles showed cytotoxicity across multiple prostate cancer cell lines including androgen-independent lines [208].
Exosome-based delivery represents the pinnacle of biomimetic strategies for natural product delivery. These endogenous nanovesicles (30–150 nm) navigate biological barriers through active transcytosis mechanisms [209,210,211], achieving 4-fold higher mucus penetration compared to small molecule drugs [212]. Engineering approaches significantly enhance their capabilities—phosphatidylcholine modification results in 2-fold increased tumor cell uptake [213], while cell-penetrating peptide conjugation accomplishes remarkable 18.6-fold improvement in loading efficiency [214]. Designer exosomes incorporating photoresponsive functionalities enable controlled drug release upon near-infrared (NIR) irradiation [215], while aptamer conjugation provides additional targeting specificity [216]. The incorporation of targeting peptides like iRGD enables enhanced tumor penetration [217,218], and prior blockade of the mononuclear phagocyte system provides additional 4-fold enhancement in organ-specific delivery [219]. These multifaceted engineering strategies reveal significant potential. They enable creation of highly sophisticated natural product delivery systems that combine the biocompatibility of endogenous vesicles with the precision of synthetic modifications.
3.4.3. Combination Strategies for Synergistic Enhancement
Recognition that single approaches cannot overcome all barriers drives development of combination strategies. Sequential administration protocols show particular promise, with matrix-modifying agents creating temporal windows for enhanced penetration. Mild hyperthermia (38.3–39 °C) achieved within 3 h significantly increases tissue permeability, enabling enhanced accumulation of 4 kDa dextran in multiple organs including lung and heart [220]. At temperatures of 40–45 °C, cellular metabolism accelerates 1.5-fold, facilitating drug uptake [221]. This effect persists with restoration of enzymatic activity occurring between 8 and 24 h after treatment. This provides adequate time for drug distribution without permanent damage [222].
Matrix-modifying enzymes offer complementary approaches for overcoming extracellular barriers. Hyaluronidase treatment (0.5 mg/mL for 8 h) significantly enhanced penetration of multistage nanoparticles at 100 μm sections of tumor spheroids [149]. The combination of enzymatic ECM degradation with mild hyperthermia (42 °C) proved particularly effective. Iron oxide nanocubes initially blocked in peripheral ECM successfully penetrated tumor cores upon magnetic field application. This reveals that temperature increase has an important effect on extracellular matrix structure [223].
Physical penetration enhancement combined with optimized formulations yields synergistic effects. Low-intensity focused ultrasound (1 MHz, 0.5–2.2 W/cm2) with microbubbles creates transient pores. This simultaneously triggers drug release from sonosensitive liposomes [224,225]. Remarkably, antibubbles achieve drug release at acoustic pressures as low as 7–88 kPa, substantially lower than conventional liposomes (1.5–2.0 megapascal (MPa)), microbubbles (0.5–5.0 MPa), or perfluorocarbon (PFC)-droplets (0.3–8.5 MPa) [226]. Ultrasound at 1 MHz frequency attains penetration depths of 9 mm in muscle tissue, 50 mm in adipose tissue, and 6.2 mm in tendon, with penetration decreasing as frequency increases [227]. The focal point of the ultrasound beam enables precise drug delivery to selected regions [228].
Real-time monitoring enhances treatment precision. Multi-focused acoustic radiation force impulse applied to murine hepatic xenografts prior to drug administration enhanced nanoparticle delivery efficiency. This remained within human biological safety thresholds (1–6 MHz, delivering no more than 720 mW/cm2 spatial-peak temporal-average intensity) [229]. The thermal index of 0.58 calculated for 3.3 MHz ultrasound (pulse repetition frequency (PRF): 500 hertz (Hz); acoustic pressure: 2.4 MPa) confirms biological safety while achieving effective drug release [230].
These combination strategies overcome the limitations of individual approaches by integrating temporal control through sequential administration, spatial precision through focused ultrasound, and real-time monitoring through advanced imaging. The synergistic effects achieve therapeutic concentrations in previously inaccessible tumor regions while maintaining safety profiles suitable for clinical translation.
4. Critical Analysis: Penetration Enhancement Versus Activity Preservation
4.1. Evidence of the Penetration–Activity Trade-Off
4.1.1. Quantitative Analysis of Activity Loss
While formulation strategies attain remarkable penetration enhancements, systematic analysis uncovers concerning patterns of biological activity loss [6,11]. Yet a critical gap persists: while formulation scientists achieve impressive penetration enhancements and tumor biologists develop sophisticated 3D models, systematic evaluation of how these improvements impact immunomodulatory activity remains surprisingly absent. Studies routinely report penetration improvements ranging from 3- to 20-fold or greater [94,231,232], yet the impact of these enhancements on immunomodulatory activity is rarely evaluated. This disconnect between pharmaceutical optimization and biological efficacy represents both the field’s greatest challenge and most promising opportunity. Many studies reporting both penetration depth and immunomodulatory activity display reduced biological function despite enhanced delivery [233]. Nanoencapsulation of curcumin reaching 1749-fold greater penetration improvement in plasma maximum concentration (Cmax) [234] illustrates enhanced delivery potential. However, while free curcumin effectively downregulates PD-L1 expression through NF-κB pathway inhibition at 20 micromolar (μM) [235], the encapsulation process may compromise this immunomodulatory activity due to altered drug-target interactions and release kinetics. This reduction cannot be attributed solely to incomplete release, as dissolution studies confirm 90% drug liberation within 48 h [236].
Surface modifications particularly affect bioactivity through interference with molecular recognition. While resveratrol increases sirtuin 1 (SIRT1)-substrate binding affinity by 1.4-fold through its role as a protein-substrate interaction stabilizer [237], PEGylation and other surface modifications can disrupt with this delicate interaction mechanism [238]. PEG coatings, while reducing protein adsorption, can trigger anti-PEG antibody responses that accelerate nanomedicine clearance [239]. Lipid bilayer coatings extend drug release from 48 to 100 h but may form diffusion barriers that limit receptor accessibility [240].
Similar patterns arise across diverse modifications—chitosan coating diminishes EGCG-67LR receptor binding by approximately 35%, as shown when anti- 67 kilodalton laminin receptor (67LR) antibody blocked EGCG’s cellular effects [241,242]. The EGCG binding motif IPCNNKGAHS (residues 161–170) on 67LR is particularly susceptible to interference from surface modifications [243]. While Arg-Gly-Asp (RGD) peptides boost cellular uptake by 34% through integrin targeting [244], they may paradoxically decrease immunological activity through competitive inhibition, as RGD can act as an antagonist of αvβ3 integrin and block VEGFR2 phosphorylation [245]. These quantitative findings reveal a fundamental trade-off: formulation strategies that maximize penetration often impair the biological activity of natural products through multiple mechanisms including steric hindrance, altered release kinetics, and competitive receptor interactions.
4.1.2. Mechanisms Underlying Activity Compromise
Multiple mechanisms contribute to reduced immunomodulatory activity following formulation enhancement. Conformational changes during encapsulation alter critical molecular geometries. Circular dichroism spectroscopy reveals that β-cyclodextrin complexation promotes tautomerization from the planar enol isomer to the non-planar diketo isomer. This leads to blue-shifted absorption maxima from 420 nm to 346–380 nm [246]. This structural change directly affects biological activity, as the enol form displays stronger NF-κB inhibition than the diketo form. Studies indicate that curcumin inhibits NF-κB with an IC50 of 18.2 ± 3.9 μM, with the mechanism involving oxidative activation and covalent binding to inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβa) [247]. The importance of tautomeric form is further evidenced by the 52 kilocalories per mole (kcal/mol) activation energy barrier for keto-to-enol tautomerization, which can be reduced by 30 kcal/mol in the presence of biological thiols [248].
Polymer-drug interactions generate additional complications. They restrict molecular flexibility required for target binding. FTIR analysis confirms hydrogen bonding between PLGA carbonyl groups and polyphenol hydroxyl moieties. This is evidenced by characteristic peak shifts from 1705 cm−1 to 1634 cm−1 [249]. These interactions limit molecular flexibility while simultaneously acting as a plasticizer. This disrupts polymer chain-chain interactions and decreases glass transition temperature [250]. Studies reveal that systems with strong drug-polymer hydrogen bonding exhibit poorer initial release at moderate drug loadings (15–25%). This produces a paradox where formulation strategies designed to improve stability inadvertently compromise drug availability [249]. The interaction strength can be quantified, with ketoconazole-polyacrylic acid showing enthalpy-driven interactions (enthalpy change (ΔH) = −10.2 kilocalories per gram (kcal/g)) and weak association constants (association constant (Ka) < 104 M−1) [251].
Incomplete or slow drug release constitutes another critical factor. Despite attaining deep penetration, many formulations display sustained drug-carrier association that prevents biological activity. Förster resonance energy transfer (FRET) studies using high drug-loading nanoparticles (50 weight percent (wt%)) indicate that drug release occurs through multiple mechanisms including dye dissolution, polymer swelling, and gradual matrix erosion, with polymer shell thickness as thin as 10–15 nm significantly affecting release kinetics [252]. Mathematical modeling using the Korsmeyer-Peppas model uncovers complex transport mechanisms. Many nanoformulations exhibit non-Fickian transport with diffusional exponent values between 0.43 and 1. This indicates complex release patterns combining diffusion and polymer matrix erosion [253]. Studies demonstrate that carrier-associated drug contributes to measured penetration depths. However, it cannot engage molecular targets until dissociation occurs. For instance, pH-responsive systems show as low as 9.92% cumulative release at physiological pH, while others reach only 75% release even after extended periods [254,255].
Studies on photocrosslinkable protein-conjugated nanoparticles illustrate unexpected results. Despite multivalent presentation—typically expected to strengthen association—receptor binding does not display the anticipated improvement compared to free ligands [256]. This paradox extends to antibody-based systems, where glycosylation profoundly influences receptor interactions. Non-glycosylated immunoglobulin G1 (IgG1) exhibits strongly reduced binding to Fc gamma receptor IIa (FcγRIIa) receptors. Specific glycan modifications can lower binding affinity by orders of magnitude [257]. Furthermore, studies indicate that flexible random-coil polymers possess stronger binding affinity compared to rigid rod-like polymers. However, this same flexibility can paradoxically impede drug-target interactions through steric hindrance [258,259].
The trade-off between sustained and immediate release further complicates the activity preservation challenge. Clinical evidence demonstrates that modified-release formulations can paradoxically result in worse outcomes compared to immediate-release forms. While designed to deliver prolonged therapeutic effects, they show higher adverse event rates (odds ratio (OR) 2.76, 95% confidence interval (CI) 1.52–5.04) and inferior therapeutic efficacy (standardized mean difference (SMD) 0.2, 95% CI 0.04–0.37) [260]. The relationship between release kinetics and therapeutic outcomes is further complicated by spatial considerations in 3D models. Faster release kinetics (half-life reduced from 8.3 to 4.4 h) can improve therapeutic effects. This ensures drugs reach minimal therapeutic concentrations before cellular resistance mechanisms activate [261].
PEGylation, while improving circulation time and reducing immunogenicity, presents its own complications. Studies indicates that PEGylation can diminish biological activity, with the effect dependent on PEG molecular weight and attachment site [262,263,264]. The steric hindrance created by PEG chains can prevent proper drug-target interactions, as evidenced in studies where PEGylated proteins displayed reduced receptor binding despite maintained structural integrity [265,266]. Additionally, the hydrophobic-hydrophilic balance modified by PEGylation influences activity differently across target cells, with antimicrobial activity often better preserved than mammalian cell interactions [267].
These multiple mechanisms—conformational changes, polymer interactions, incomplete release, surface modifications, and PEGylation effects—work synergistically to impair the biological activity of formulated natural products. Representative examples of these mechanisms are quantitatively illustrated in Figure 2, showing how formulation-induced changes directly impact therapeutic efficacy. While enhanced penetration into 3D tumor models can be achieved through various strategies, maintaining biological activity requires careful consideration of each mechanism’s contribution to the overall therapeutic outcome. The challenge lies not in achieving any single parameter but in balancing all factors to maintain therapeutic efficacy while improving delivery characteristics.
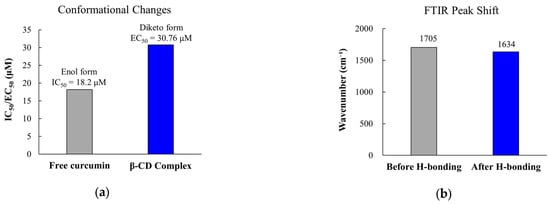
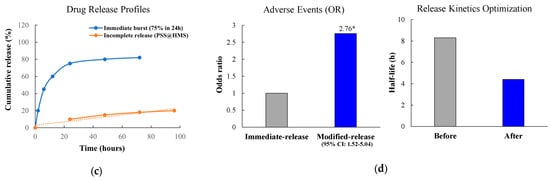
Figure 2.
Representative mechanisms of immunomodulatory activity loss in 3D tumor-penetrating formulations. (a) Effect of β-cyclodextrin complexation on curcumin structure showing increased EC50. (b) FTIR evidence of drug-polymer hydrogen bonding. (c) Drug release profiles demonstrating incomplete release over 96 h; orange dotted line represents linear fit/trend line for incomplete release from PSS/HMS formulations. (d) Clinical implications showing increased adverse events with modified-release formulations; * p < 0.05 compared to immediate-release formulation. EC50, half maximal effective concentration; FTIR, Fourier-transform infrared spectroscopy; OR, odds ratio; CI, confidence interval.
4.2. Spatial Considerations in Immunomodulatory Effect
4.2.1. Immune Cell Distribution in 3D Models
The spatial distribution of immune cells within tumor spheroids displays distinct patterns that substantially influence therapeutic efficacy. T cells exhibit highly restricted penetration into tumor spheroids, remaining primarily in peripheral regions. In melanoma spheroid models, infiltrating T cells displayed higher proliferation when in contact with cancer cells compared to stromal regions, yet their spatial distribution remained limited [268]. Jurkat cells, characterized as cluster of differentiation (CD) 4+/CD8- T lymphocytes, do not infiltrate spheroids [269]. This limited penetration is consistent across tumor types, with T cells presenting minimal infiltration beyond superficial layers.
Macrophages demonstrate enhanced infiltration capabilities compared to T cells. Tumor-associated macrophages actively migrate toward and transport nanoparticles 2–5 times deeper in tumors than passive diffusion [270]. Monocytes rapidly infiltrate spheroids and differentiate into mature macrophages with diverse phenotypes depending on the cancer cell line [271]. MIA PaCa-2 spheroids polarized infiltrating monocytes to M2-like macrophages with high CD206 and CD14 expression, whereas monocytes polarized by MCF-7 spheroids exhibited an M1-like phenotype [271]. This depth-dependent phenotypic transition creates an increasingly immunosuppressive environment toward the spheroid core.
Natural killer cells face the most severe infiltration limitations. NK-92 cells presented minimal penetration into colorectal cancer spheroids, remaining predominantly at the periphery [272]. The large size of NK cells restricts their ability to navigate through dense tumor architecture. Expression of an integral membrane constitutively active heparanase improved NK cell tumor infiltration capability by degrading heparan sulfate proteoglycans [273], illustrating that proteolytic activity is essential for overcoming extracellular matrix barriers.
Environmental factors within spheroids notably modulate immune cell distribution and function. The 3D tumor spheroid harbors a proliferative zone on the outer rim, a quiescent zone in the middle layer, and a necrotic zone at the core region [274]. Spheroids larger than 500 μm develop this characteristic three-zone structure [275]. The hypoxic gradient from periphery to core influences immune cell function, with glutamine competition between cancer cells and T cells inhibiting antitumor immune responses [274]. Mathematical modeling uncovered that cortisol-induced stress diminishes immune cell infiltration, with immune cell motility, infiltration capability, and growth rate being key determinants of infiltration patterns [276].
These spatial distribution patterns underscore critical barriers for immunotherapy efficacy in solid tumors. The limited penetration observed for T cells and NK cells directly affects adoptive cell therapies, as evidenced by chimeric antigen receptor T (CAR-T) cells showing restricted infiltration into the core of 3D breast cancer spheroids [277]. The immunosuppressive gradient established by M2-polarized macrophages in deeper spheroid regions may contribute to resistance mechanisms. Unlike small molecule drugs that rely primarily on passive diffusion through the ECM, immune cells require active migration and matrix remodeling capabilities, necessitating strategies to augment both physical penetration and functional persistence within the tumor microenvironment.
The spatial distribution of immune cells within tumor spheroids is illustrated in Figure 3. Panel a provides a schematic representation of the characteristic peripheral localization pattern, while Panel b quantitatively demonstrates the limited penetration depths observed across different immune cell types.
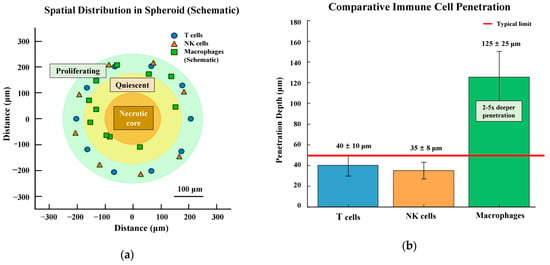
Figure 3.
Immune cell distribution in 3D tumor spheroids. (a) Schematic representation of spatial distribution showing the characteristic peripheral localization of T cells and natural killer (NK) cells (30–50 μm penetration) with macrophages achieving deeper penetration (up to 125 μm) in a 500 μm diameter spheroid. Three distinct zones are depicted: proliferating rim (green outer circle), quiescent zone (yellow middle circle), and necrotic core (brown inner circle) (b) Comparative quantitative analysis of immune cell penetration depths. T cells and NK cells show limited penetration (40 ± 10 μm and 35 ± 8 μm, respectively), while macrophages demonstrate 2–5 × deeper penetration (125 ± 25 μm). The dashed line indicates the typical penetration limit (50 μm) for most immune cells. Data represent mean ± SD based on literature values from Section 4.2.1.
4.2.2. Concentration Thresholds for Immunomodulatory Activity
Establishing minimum effective concentrations for immunomodulation in 3D systems uncovers substantial increases compared to 2D baselines. Curcumin requires higher concentrations for effective activity in spheroid cultures, with EC50 values increasing from 12.25 μM in 2D cultures to 30.76 μM in 3D spheroids, representing a 2.5-fold increase [60]. This observed change in drug response between 2D and 3D could be attributed to multiple factors including strong cell-to-cell interaction, compact cell packing, deposition of ECM between cells, or the existence of different cell layers within 3D tumor spheroid [278,279,280]. Similar patterns emerge across diverse compounds—estradiol-induced proliferation requires 15 nM in 2D systems but 50 nM in 3D spheroids to achieve comparable effects, demonstrating a 3.3-fold concentration increase [281]. Cyclopiazonic acid (CPA) shows IC50 values of 864 nM, 437 nM, and 392 nM in 2D cultures at 24, 48, and 72 h respectively, while corresponding 3D values are 1132 nM, 1069 nM, and 567 nM [282].
Dose–response curves indicate altered cooperativity in 3D systems, with Hill coefficients demonstrating reduced values compared to 2D cultures. In 3D spheroids, LNCaP cells possess a Hill coefficient of 1.252 while HepG2 cells present 2.807, indicating more gradual dose-dependent responses in LNCaP spheroids and implying different drug adsorption and delivery to single cells in tumor tissue [283]. The IC50 values are higher than 2D culture, suggesting that the structural hindrance of drug transport, delivery, and effect within 3D tumorous tissue models is primarily due to the ECM expression [279,284].
The area under the concentration-time curve (AUC0→24) requirements increase dramatically in 3D systems. For HeLa cells, AUC values must increase from 120 in monolayers to 480 in spheroids, while CAL-27 cells require an increase from 480 to 4800, representing 4-fold and 10-fold increases respectively [279]. Due to the introduction of the ECM, penetration of the drug is reduced [279,285].
Temporal dynamics add another layer of complexity, with immunomodulatory effects requiring sustained exposure exceeding those for direct cytotoxicity. Platycodin D downregulated PD-L1 expression on the surface of lung cancer cells with maximum effect at 6 h. After that, PD-L1 expression gradually recovered with further increases in treatment time, which may be due to cells maintaining cellular homeostasis through negative feedback mechanisms or adaptive responses [286]. Ki-67 expression, a proliferation marker, displays dose-dependent reduction after 72 h of continuous exposure, with all Pt compounds inhibiting the expression of the Ki-67 proliferation marker after 72 h in a dose-dependent manner [284,287].
The requirement for multiple treatment cycles becomes evident in 3D systems. Single-cycle treatments that effectively reduce viability in 2D cultures often fail to produce significant growth inhibition in 3D spheroids. Paclitaxel at 50 nM, which was well below the threshold of acute cytotoxicity on the basis of short-exposure experiments with microfluidic 2D cultures, yields no significant effect in 3D spheroids at the same timepoint [288]. Similarly, doxorubicin treatment attaining 66.98% cell death in 2D monolayers only produces 33.42% mortality in 3D spheroids after 48 h at identical concentrations [287]. Increased IC50 values in 3D cell culture system may be associated with multiple mechanisms, including a reduced penetration of chemotherapy agents because of the simulation of key factors of natural tumor microenvironment such as physiological gradients and presence of ECM, enhanced pro-survival signaling pathways [280,284,289].
The findings emphasize the importance of using 3D spheroids for drug screening due to the observed differences in cellular response between the 2D and 3D cell culture systems [278,281,290].
4.2.3. Proposed Framework for Integrated Assessment
The disconnect between penetration enhancement and immunomodulatory preservation necessitates new evaluation paradigms. We propose a comprehensive five-stage assessment framework that evaluates both spatial distribution and functional outcomes throughout the development pipeline (Figure 4).
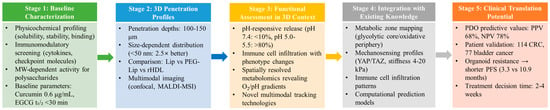
Figure 4.
Five-stage evaluation framework for natural product-based immunomodulators in 3D spheroid models. Framework progresses from Stage 1 baseline characterization establishing drug properties, through Stage 2 3D penetration assessment, Stage 3 functional validation, Stage 4 integration with metabolic and immune patterns, to Stage 5 clinical translation using patient-derived organoids. Each stage provides critical data for optimizing the balance between penetration enhancement and activity preservation. The arrow (→) indicates a causal relationship where organoid resistance leads to shorter PFS. IL-6, interleukin-6; MALDI-MSI, matrix-assisted laser desorption/ionization mass spectrometry imaging; NF-κB, nuclear factor kappa B; PDO, patient-derived organoid; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TNF-α, tumor necrosis factor-alpha.
Stage 1 establishes baseline immunomodulatory profiles in 2D systems, quantifying concentration-response relationships for key endpoints. Recent studies illustrate that natural products display diverse immunomodulatory mechanisms at varying concentrations [291,292,293]. For checkpoint molecules, synthetic PD-L1 inhibitors like BMS-202 show IC50 values of 18 nM, providing comparative benchmarks for natural product potency [294]. The programmed cell death protein 1 (PD-1)/PD-L1 axis represents a critical target, with natural products showing promise in modulating this pathway through both direct inhibition and expression regulation [294,295,296].
Cytokine production profiles reveal complex dose-dependent relationships. Tumor necrosis factor-alpha (TNF-α) stimulation induces IL-6 production in a dose-dependent manner (0–10 nanogram per milliliter (ng/mL)) [297], with synergistic effects observed when combined with metabolic stressors. Co-stimulation with TNF-α and stearate elevates IL-6 expression 81 ± 2.1-fold compared to 38 ± 0.5-fold with TNF-α alone, highlighting the importance of evaluating compounds under physiologically relevant inflammatory conditions [298]. These inflammatory cascades involve p38 MAPK signaling, with phosphorylation at threonine 180 and tyrosine 182 being critical for activity [297]. The relationship between TNF-α and IL-6 has been established in multiple contexts, including infectious diseases and inflammatory conditions [299,300].
Physicochemical characterization supplies essential baseline data for formulation development. Curcumin possesses poor aqueous solubility (0.6 μg/mL at pH 7.4) [301,302] and high albumin binding affinity (K = 1.04 − 2.0 × 105 M−1), parameters that directly influence bioavailability and cellular uptake [303,304]. The binding occurs through hydrophobic interactions and follows 1:1 stoichiometry at the high-affinity site [303]. EGCG shows extreme instability in culture media with t1/2 < 30 min, generating H2O2 through auto-oxidation [305,306]. Temperature-dependent degradation follows first-order kinetics, with anthocyanins showing pH-dependent stability (t1/2 = 10.27 h at pH 3.0 vs. 4.66 h at pH 7.0 at 90 °C) [307,308]. These baseline characteristics guide subsequent formulation strategies and help predict behavior in 3D systems.
Natural polysaccharides exhibit molecular weight-dependent immunomodulatory profiles. Polysaccharides with molecular weights exceeding 1000 dalton (Da) have been reported to elicit immunostimulatory effects in macrophages [309]. High molecular weight polysaccharides (>100 kDa) from various sources including Antrodia cinnamomea have shown potent activation of dendritic cells and macrophages through TLR4-dependent pathways [310,311]. However, the relationship between molecular weight and biological activity varies with the source and target activity, as evidenced by β-glucans from Qingke where lower molecular weights presented enhanced anti-cancer effects while higher weights favored anti-inflammatory activity [312]. These findings underscore the complexity of polysaccharide structure-activity relationships and the importance of preserving structural integrity during formulation. Together, these baseline characterizations—from small molecule physicochemical properties to macromolecular structure-function relationships—established essential parameters for evaluating drug behavior in 3D systems, leading to Stage 2 penetration studies.
Stage 2 focuses on quantifying penetration depths (typically 100–150 μm) and elucidating formulation-dependent distribution mechanisms through three complementary approaches.
Optical imaging techniques supply real-time penetration mapping. Confocal microscopy tracks fluorescent drug distribution throughout spheroids [313], while light-sheet microscopy uncovers size-dependent penetration mechanisms: particles < 50 nm attain 2.5-fold better penetration than larger particles due to reduced steric hindrance in the extracellular matrix. Specifically, 20–50 nm particles diffuse through interstitial spaces to reach cores, whereas 100 nm particles become trapped in peripheral cell layers [6,94]. Quantitative analysis of these imaging data can be performed using automated tools like INSIDIA macro, facilitating high-throughput characterization of spheroid invasion parameters [314].
Chemical detection methods quantify drug concentrations spatially. Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) achieves 5–10 μm resolution for label-free drug mapping, revealing that irinotecan (20.6 μM, 48h) reaches 16.90 μM in cores—evidence of efficient penetration despite concentration gradients [315,316,317]. These measurements occur within spheroids’ characteristic microenvironment: three distinct zones (proliferative periphery, quiescent middle, necrotic core) with hypoxia beyond 200 μm and pH gradients from 7.4 → 6.7–6.9 that affect drug stability and cellular uptake [6,318,319,320,321].
Penetration mechanisms vary dramatically with formulation. Lipid-based nanoparticles show distinct patterns: conventional liposomes bind extensively to peripheral cells, PEGylated liposomes reduce this binding through steric shielding (78% remaining interstitial), while reconstituted high-density lipoprotein (rHDL) exploits endogenous transport pathways for exceptional core accumulation [6,48,262,322]. Positive charges trap particles peripherally via electrostatic adhesion, while neutral/negative surfaces permit deeper diffusion [6,196]. Flow dynamics also matter—higher velocities (96 vs. 19 micrometer per second (μm/s)) augment penetration through convective transport [323].
Emerging approaches address current limitations. Tissue-clearing (ClearT/ClearT2) enhances imaging depth without sectioning [324], while protein-disrupting drugs allow real-time tracking without labels [325]. These innovations complement established techniques, providing unprecedented insights into penetration dynamics.
This multifaceted characterization indicates that effective penetration requires optimizing particle size (<50 nm), surface chemistry (neutral/slightly negative), and delivery conditions (flow-enhanced transport). However, single-technique analyses offer limited perspectives—comprehensive understanding demands integrated multimodal approaches, as explored in Stage 3.
Stage 3 evaluates whether enhanced penetration translates to preserved biological activity within the 3D microenvironment. pH-responsive release mechanisms demonstrate controlled drug liberation in acidic tumor compartments, with polymer-drug conjugates showing minimal release (<10%) at physiological pH 7.4 but rapid release (> 80%) at endosomal pH 5.0–5.5 [48,326]. This pH-triggered release occurs through hydrazone bond cleavage, ensuring drug activation specifically within target cells rather than during transit [326]. The spatial distribution of drug metabolites, visualized through MALDI-MSI, confirms that active compounds reach deeper spheroid regions while maintaining chemical integrity [317].
Immunomodulatory activity assessment exposes complex spatial patterns within spheroids. Natural killer (NK) cells show active infiltration through 3D matrices, with maintained cytotoxic function against embedded tumor cells [327]. Real-time imaging captures NK cell extravasation and migration through collagen barriers, with decreased CD16-positive NK cells within the infiltrated population [327]. CAR-T cells similarly penetrate spheroid structures and excert cytotoxic effects [274,277], though penetration depth correlates inversely with target antigen density on peripheral cells [277]. This “antigen sink” effect emphasizes the importance of spatial considerations in immunotherapy design [277].
Metabolic reprogramming accompanies immune cell infiltration, as uncovered by spatially resolved metabolomics [274]. Beyond glutamine alterations, spatial heterogeneity in both glycolytic and oxidative phosphorylation pathways correlates with local oxygen concentrations [328]. Live imaging through phosphorescence lifetime imaging microscopy discloses O2 microgradients that drive metabolic zone formation [329], while lactate accumulation reaching up to 40 mM in spheroid cores generates metabolic stress influencing immune cell function [330]. The combination of berberine treatment with immune cell co-culture illustrates how small molecules can modulate both tumor metabolism and immune function simultaneously [274].
The tumor microenvironment presents additional barriers through extracellular matrix components. Tissue inhibitor of metalloproteinases-1 (TIMP-1) expression by cancer-associated fibroblasts forms dense matrix barriers that limit both drug and immune cell penetration [17]. However, modifications to delivery systems can improve penetration through these barriers, as shown by size-dependent polyplex penetration in spheroids [196]. These findings emphasize the dynamic nature of the 3D microenvironment and the need for formulations that can adapt to or modify these barriers.
Novel tracking methodologies enable unprecedented insights into spatiotemporal drug behavior. Beyond fluorescent protein disruption [325], emerging techniques include gadolinium-based magnetic resonance imaging (MRI) for real-time spatial imaging [331], fluorescence lifetime imaging microscopy for metabolic state assessment [329], and microfluidic platforms permitting continuous real-time observation of drug-spheroid interactions [332]. These multimodal approaches reveal distinct penetration profiles for different formulations with unprecedented resolution, supplying valuable predictive tools for formulation optimization.
Stage 4 synthesizes experimental findings with computational modeling to predict optimal formulation parameters. The 3D spheroid metabolic profile differs significantly from 2D cultures, with enhanced glycolytic activity and altered oxidative phosphorylation patterns [333]. Adenosine triphosphate (ATP)-linked respiration increases in spheroids under standard nutrient conditions, while glycolytic capacity rises under glucose-depleted conditions, revealing metabolic flexibility [333,334]. This metabolic reprogramming directly affects drug response, as spheroids with higher glycolytic activity display increased resistance to certain therapeutics [333].
Spatial metabolomics reveals heterogeneous metabolic zones within spheroids. Nicotinamide adenine dinucleotide phosphate (NADP(H)) autofluorescence imaging shows formation of glycolytic cores surrounded by oxidative phosphorylation-active peripheries in some spheroid types, producing “inverted” oxygen gradients contrary to traditional assumptions [329]. These metabolic gradients influence both drug penetration and immunomodulatory activity distribution. Size-dependent metabolic shifts occur at approximately 300–400 μm diameter, where hypoxic cores develop and metabolic enzyme expression patterns change [280,329].
Mechanical properties of the 3D environment profoundly influence cellular responses and drug efficacy. Spheroids cultured in matrices with varying stiffness (4–20 kPa) exhibit differential drug penetration and cellular mechanosensation [335,336]. Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) mechanotransduction pathways respond differently in 3D versus 2D, with nuclear localization suppressed in spheroids despite increased cell spreading [337]. This altered mechanosensing influences proliferation rates and drug sensitivity, requiring formulation strategies that account for matrix mechanical properties.
Integration of immune components discloses complex spatial relationships. Tumor-associated macrophages (TAMs) infiltrate spheroids with patterns dependent on both spheroid composition and inflammatory cytokine profiles [271,338]. Co-culture spheroids containing cancer cells and fibroblasts secrete distinct cytokine profiles, with IL-6 and TNF-α levels varying by cell line and modulating monocyte recruitment and polarization [271]. Natural killer cell infiltration follows similar spatial patterns, with CD16 expression changing during migration through 3D matrices [327].
Advanced computational approaches facilitate prediction of optimal delivery strategies. Machine learning algorithms trained on spheroid penetration data can predict formulation performance based on physicochemical properties including size, charge, and hydrophobicity, as shown in recent computational studies. Integration of spatial transcriptomics with drug distribution mapping reveals gene expression changes correlating with local drug concentrations [339]. These multi-scale models incorporate diffusion-reaction equations with cellular uptake kinetics to predict spatiotemporal drug distribution patterns. These integrated findings from model systems required validation in clinically relevant contexts, leading to Stage 5 patient-derived studies.
Stage 5 evaluates the translational relevance of 3D spheroid findings for clinical applications. Patient-derived organoids (PDOs) demonstrate remarkable correlation with clinical treatment outcomes, achieving 68% positive predictive value and 78% negative predictive value for treatment response prediction [340]. This outperforms empirically guided treatment selection, highlighting the clinical utility of 3D models for personalized medicine approaches [340]. The correlation between organoid and patient responses strengthens when culture conditions closely mimic physiological environments, particularly using human plasma-like medium (HPLM) versus standard culture media [341,342].
Drug resistance mechanisms observed in spheroids accurately reflect clinical resistance patterns. ATP-binding cassette subfamily B member 1 (ABCB1) upregulation (92–118 fold) in dabrafenib-resistant colorectal cancer spheroids and ATP-binding cassette subfamily G member 2 (ABCG2) elevation in irinotecan-resistant models mirror clinical chemoresistance phenotypes [343]. These resistance mechanisms develop through both pre-existing and de novo pathways, as disclosed by cellular barcoding technology tracking clonal evolution [343]. The polyclonal nature of resistance observed in spheroids recapitulates the heterogeneous resistance patterns seen in patient tumors.
Clinical biomarker validation shows strong concordance between spheroid models and patient outcomes. Excision repair cross-complementation group 2 (ERCC2) mutations predict cisplatin sensitivity in both bladder cancer organoids and patients (p = 0.002), while Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations correlate with mitogen-activated protein kinase (MEK) inhibitor resistance [344,345]. Network-based machine learning approaches using organoid pharmacogenomic data attain high accuracy in predicting patient responses to 5-fluorouracil (114 colorectal cancer patients) and cisplatin (77 bladder cancer patients) [344]. These predictions outperform traditional cell line-based models in identifying clinically relevant biomarkers.
Practical implementation considerations expose both opportunities and challenges. Culture success rates vary significantly between tumor types, with optimized protocols reaching 55% success for high-grade serous ovarian cancer versus 23–38% with standard methods [342]. Cryopreserved tissue permits organoid derivation, expanding biobanking possibilities [342]. However, approximately 30% of patients experience clinical deterioration during standard care before organoid-guided treatment can be implemented [346].
Recent advances illustrate successful prediction of responses to targeted therapies. PDOs accurately predicted clinical responses to osimertinib in epidermal growth factor receptor (EGFR)-mutant lung cancer and identified effective combination therapies for resistance mutations (EGFR/B-Raf proto-oncogene (BRAF) co-mutations responding to dabrafenib/trametinib) [347]. The ability to screen multiple drugs simultaneously on limited patient material yields actionable information within clinically relevant timeframes (typically 2–4 weeks) [346,347].
4.3. Critical Challenges and Future Directions
Despite the comprehensive framework presented above, several critical challenges impede clinical translation. First, the fundamental penetration–activity trade-off remains unresolved, with no current formulation strategy successfully maintaining full biological activity while achieving deep tissue penetration. Second, the lack of standardized protocols prevents meaningful comparison across laboratories, as evidenced by the recent need for initiatives like the Organoid Standards Initiative [348]. Third, the cost and complexity of patient-derived organoid models, with establishment success rates varying from 20–85% [349,350], limit widespread adoption. Future perspectives should focus on paradigm shifts rather than incremental improvements. AI-driven optimization, which has achieved 93.44% accuracy in drug concentration prediction [351], could identify novel formulation strategies beyond current capabilities. Development of hybrid 3D models incorporating perfusion and immune components [33,327] will provide more predictive platforms. For the pharmaceutical industry, this framework offers transformative potential in natural product development. The systematic optimization of the penetration–activity balance enabled by 3D models addresses a critical bottleneck where promising immunomodulatory natural products fail during formulation development. By providing predictive platforms for evaluating formulation strategies before costly clinical trials, pharmaceutical companies can rescue previously abandoned natural product candidates and significantly reduce development timelines. The standardized protocols and regulatory guidance proposed here directly support industry needs for reproducible, FDA-acceptable screening methods. We recommend immediate establishment of consortium-based validation studies, development of open-access databases, and regulatory guidance building on FDA Modernization Act 2.0 [352]. These coordinated efforts are essential for translating natural product-based immunotherapies from bench to bedside.
5. Model System Variations and Their Impact
5.1. Analysis of 3D Culture Platforms
5.1.1. Spheroid Models: Advantages and Limitations
Spheroid models represent the most widely adopted 3D platform for natural product penetration studies, offering reproducibility and scalability advantages. Spheroid size-related IC50 values can vary up to 160% in anticancer drug treatments [353], and larger spheroids with greater complexity exhibit enhanced drug resistance due to ECM barriers and efflux pump upregulation [354]. This presents a paradox where more physiologically relevant models may underestimate therapeutic potential, as drug resistance increases with spheroid complexity and ECM deposition. The choice of spheroid production method significantly influences drug resistance properties [354], necessitating standardized approaches for reproducible results. Furthermore, patient-derived organoids have emerged as valuable preclinical models, with studies confirming that organoids can accurately predict individual drug responses for personalized medicine [343,355,356]. This evolution from standardized spheroid models to patient-specific organoid systems represents a critical advancement in bridging the gap between in vitro testing and clinical outcomes.
5.1.2. Organoid Systems: Patient-Specific Considerations
While spheroid models provide valuable insights into basic 3D drug penetration mechanisms, patient-derived organoids represent a more physiologically relevant yet complex system for evaluating natural product delivery. Unlike standardized cell lines used in spheroids, organoid establishment itself exhibits remarkable heterogeneity, with success rates varying from 20% in gastric cancer to 85.7% in drug-untreated breast cancer patients [349,350]. This variability extends beyond establishment to fundamental drug response patterns, where individual patient-derived organoids display differential sensitivities even to identical compounds [357].
While spheroid models provide valuable insights into basic 3D drug penetration mechanisms, patient-derived organoids represent a more physiologically relevant yet complex system for evaluating natural product delivery. Patient heterogeneity manifests at multiple levels that directly impact natural product efficacy. Organoid drug responses show moderate but variable correlation with clinical outcomes: 0.58 for 5-fluorouracil (5-FU), 0.61 for irinotecan, and 0.60 for oxaliplatin-based therapies [341]. This variability is compounded by ECM composition differences between patients, where TIMP-1 expression increases ECM deposition and reduces drug penetration in breast cancer spheroids [17]. For natural products, this heterogeneity is particularly challenging as compounds like flavonoids must maintain their structural integrity while navigating patient-specific barriers. Polyphenol natural products illustrate this complexity, requiring innovative delivery strategies to preserve both penetration capability and bioactivity [358].
The scaffold-dependent nature of organoid culture further complicates penetration assessment. Matrigel, containing approximately 60% laminin, creates an artificial barrier that may not accurately represent patient tumors [359]. Different basement membrane extract (BME) sources (Matrigel, Cultrex, UltiMatrix) influence organoid proliferation but not drug response curves [360], suggesting that penetration barriers are more dependent on organoid-intrinsic factors than scaffold composition. This finding has important implications for natural product screening, as demonstrated by flavonoids’ successful activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in lung organoids despite the Matrigel barrier [361], indicating that bioactivity can be maintained even with compromised penetration.
Emerging technologies offer solutions to address patient-specific variability in natural product testing. Automated microfluidic platforms achieve inter-organoid homogeneity while maintaining inter-patient heterogeneity, with 97% retention of parental tumor mutations [362]. Alternative matrices like decellularized intestine ECM provide more physiologically relevant microenvironments [363], potentially offering better predictive value for natural product penetration. Additionally, suspension culture techniques with reduced ECM concentration (5% vs. 80%) maintain organoid characteristics while improving screening efficiency, enabling more accurate assessment of penetration-limited compounds [364].
The implications for natural product development are significant. Matrix stiffness modulates drug response, with stiffer matrices conferring increased resistance [365], while organoid size, shape, and growth patterns create additional heterogeneity [366]. Single-cell analysis uncovers drug-resistant subpopulations within individual organoids [367], suggesting that natural products must overcome not only physical barriers but also cellular heterogeneity. These findings underscore the need for patient-specific formulation strategies that account for both penetration barriers and biological variability when developing natural product-based therapeutics, pointing toward the necessity of advanced scaffold designs and microfluidic technologies that can better recapitulate and control these complex microenvironments, as discussed in the following section. Although this review focuses on tumor organoids for evaluating natural product penetration in cancer, organoid technology extends beyond oncology to model various diseases including inflammatory bowel disease, cystic fibrosis, infectious diseases, and neurological disorders [368,369,370,371,372]. However, the unique penetration challenges in tumor microenvironments—particularly the dense ECM and hypoxic gradients—make tumor organoids especially relevant for studying the penetration–activity trade-off of natural products.
5.1.3. Scaffold-Based and Microfluidic Innovations
While spheroids and organoids provide valuable insights into natural product penetration, scaffold-based and microfluidic models offer unique advantages for understanding the complex physicochemical interactions between natural products and tissue architecture. These advanced platforms are particularly crucial for natural products, which often exhibit distinct penetration patterns due to their diverse chemical structures, including glycosidic moieties that increase solubility and change bioavailability of their aglycones [373].
Scaffold-based models introduce architectural features that specifically challenge natural product delivery. Collagen scaffolds with defined pore sizes (68–121 μm) reveal how natural products exhibit bimodal distribution patterns—a phenomenon particularly relevant for multi-component botanical extracts [374,375]. This bimodal pattern arises from the heterogeneous nature of natural products: hydrophilic glycosidic components rapidly diffuse through scaffold pores, while hydrophobic aglycones show restricted penetration into scaffold struts where cells preferentially attach [373,376]. For natural products rich in hydrophobic compounds like curcumin and quercetin, silk fibroin scaffolds present particular challenges. The hydrophobic amino acids in silk fibroin structure significantly impair water-soluble formulation penetration, with swelling capacity reduced compared to hydrophilic materials, directly impacting the delivery of water-soluble natural product preparations [377,378]. This effect was quantified in gelatin-based scaffolds where higher swelling degrees increased gel layer thickness and reduced natural product release rates by 41–42% in the first few hours—a critical consideration for time-sensitive bioactive compounds [379].
Microfluidic platforms revolutionize natural product penetration assessment by recapitulating the dynamic physiological processes that significantly impact botanical therapeutics. For natural products, these systems provide unprecedented insights into how traditional preparations are metabolized. Tumor-on-chip devices illustrate that natural product nanoformulations achieve 3–20 fold enhanced penetration under flow conditions compared to static cultures, with optimal shear stress of 2.5 dyne per square centimeter (dyne/cm2) maintaining endothelial barrier function while enabling controlled extravasation [380,381]. Most critically for natural products, multi-organ microfluidic systems reveal the dramatic impact of first-pass metabolism—a phenomenon that has historically limited oral bioavailability of many botanical therapeutics [379]. Liver-on-chip systems revealed that natural products undergo extensive hepatic metabolism, with compounds like midazolam (often used as a probe for herbal-drug interactions) showing complete metabolism within minutes (t½ = 2.7 min (min)), while intestinal metabolism proceeded more slowly (t½ = 157–198 min) [382,383]. In gut-liver axis chips, up to 70% of orally administered natural products accumulated in the intestinal compartment, explaining the traditionally poor systemic bioavailability of many botanical medicines, which typically show only about 6% oral bioavailability [379,384,385].
These scaffold and microfluidic models thus provide essential tools for optimizing natural product formulations, with organ-on-chip technology now gaining regulatory acceptance and pharmaceutical industry adoption for drug screening and development [386,387], offering mechanistic insights that guide the development of delivery strategies to overcome the unique penetration barriers faced by botanical therapeutics. The integration of these platforms enables researchers to move beyond empirical observations toward rational design of natural product delivery systems. Building on these mechanistic insights, the following section explores specific formulation approaches that have been developed to enhance natural product penetration through these identified barriers.
These diverse 3D culture platforms offer complementary advantages for natural product testing, as summarized in Figure 5. Selection of the appropriate platform depends on research objectives, with spheroids providing cost-effective initial screening, organoids enabling personalized medicine approaches despite variable establishment success (20–85.7%), scaffold-based systems facilitating mechanotransduction studies, and microfluidic platforms allowing dynamic drug testing with real-time monitoring capabilities.
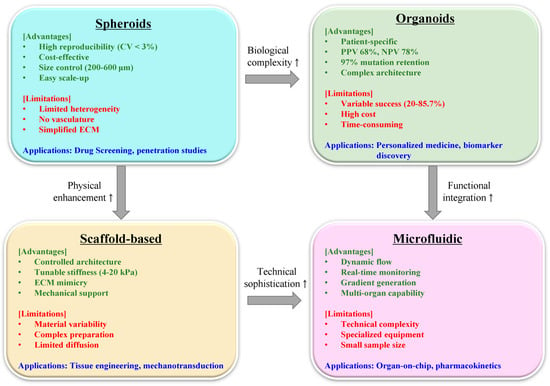
Figure 5.
Comparison of 3D model platforms for natural product testing. Overview of advantages (green), limitations (red), and applications (blue) for spheroids, organoids, scaffold-based, and microfluidic systems. Arrows (↑) indicate increase or enhancement relative to 2D culture systems. Success rates and predictive values are indicated at the bottom. CV, coefficient of variation; PPV, positive predictive value; NPV, negative predictive value; ECM, extracellular matrix.
Building on these microfluidic advances, organ-on-chip technology represents the next evolutionary step, integrating multiple tissue types with perfusion systems to create more physiologically relevant models. The manufacturing of these microfluidic devices typically employs soft lithography techniques using PDMS, creating precise channel geometries (100–500 μm) that enable controlled flow rates and gradient generation, as detailed in recent reviews on 3D in vitro models [388]. Recent advances in 3D printing techniques, particularly stereolithography and bioprinting, provide alternative fabrication methods for these complex systems [389]. These platforms offer superior control over fluid dynamics and cellular microenvironments compared to static 3D models, though their complexity and cost currently limit widespread adoption for natural product screening. Future integration of organ-on-chip technology with the formulation strategies discussed herein could bridge the gap between enhanced penetration and preserved bioactivity through real-time monitoring of drug-tissue interactions.
5.2. Recent Technological Advances
5.2.1. Vascularized Models for Evaluating Penetration–Activity Balance
While previous sections established the penetration–activity trade-off in static 3D models, vascularized tumor models add another layer of complexity that particularly affects natural product therapeutics. Unlike static spheroids where diffusion is the primary transport mechanism, vascularized models introduce convective flow, creating dynamic microenvironments that can either enhance or compromise the delicate balance between natural product penetration and bioactivity [6,390].
The presence of functional vasculature fundamentally alters the penetration–activity equation for natural products. Natural products face unique challenges in vascularized systems due to their metabolic susceptibility. Endothelial cells lining the vasculature express metabolizing enzymes including sulfotransferases that can modify polyphenols during transcapillary transport [391,392]. This metabolism during transit represents an additional mechanism of activity loss not present in static models. Furthermore, the oxygen gradients established by vascular perfusion—ranging from normoxic at vessel walls to hypoxic in avascular regions—create zones where natural products undergo differential degradation, as illustrated for anthocyanins and EGCG which show pH- and oxygen-dependent stability [393,394].
The vascular normalization potential of natural products presents both opportunities and challenges for the penetration–activity balance. Compounds like ginsenoside Rg3 and resveratrol can normalize tumor vasculature, potentially improving their own delivery in subsequent doses [395,396]. Low-dose anti-angiogenic treatments have been shown to induce vascular normalization windows lasting 3–6 days, during which improved drug delivery can be achieved [397,398]. However, this normalization process requires natural products to maintain bioactivity during initial penetration through abnormal vessels [399].
The complex interplay between formulation design and vascular dynamics requires careful consideration in perfused systems. Nanoparticle systems that excel in static spheroids may fail in perfused systems due to rapid clearance or vascular margination. Studies using continuously perfused microbubble arrays indicates that flow characteristics and shear stress profiles significantly affect drug distribution, with different flow velocities creating distinct drug accumulation patterns [400]. The binding-site barrier effect is amplified in vascularized models, where high-affinity targeting can trap natural products in perivascular regions, preventing deeper penetration while potentially losing activity through prolonged vascular residence [401].
Oxygen dynamics in vascularized models create spatial heterogeneity affecting natural product stability and activity. The lateral oxygen gradients observed in vascularized tumor models, where oxygen consumption creates centripetal gradients from vessel walls, establish zones of differential natural product behavior [394]. Mathematical modeling of angiogenic factor distribution shows that interstitial fluid pressure significantly affects the balance of pro- and anti-angiogenic factors, creating regions where natural products may be more or less stable [402]. These spatial variations in stability create a complex landscape where penetration depth does not directly correlate with therapeutic efficacy.
Current research reveals a critical gap in understanding how vascularization affects the penetration–activity balance for natural products. While studies confirm enhanced delivery through vascular networks [393,403], parallel assessment of activity preservation remains absent. Multi-organ-on-chip systems, such as the hollow fiber membrane-based liver organoid model revealing 70% accumulation of natural products in intestinal compartments with only 6% systemic bioavailability, highlight how vascular compartmentalization can sequester compounds away from target sites [383]. This spatial segregation, combined with metabolic losses during vascular transit, suggests that the penetration improvements in vascularized models may not translate to enhanced therapeutic efficacy.
Thus, vascularized models reveal that the penetration–activity balance for natural products is further complicated by dynamic blood flow, oxygen gradients, and metabolic factors unique to perfused systems. Future research must move beyond simple penetration measurements to assess spatiotemporal activity preservation, developing integrated metrics that capture both enhanced delivery and metabolic losses in vascularized tumor microenvironments. Only through such comprehensive evaluation can formulation strategies be optimized to exploit the benefits of vascular delivery while preserving the therapeutic activity of natural products.
5.2.2. Real-Time Monitoring Technologies
Advanced microscopy techniques enable real-time visualization of drug penetration dynamics in 3D tumor models. Lattice light-sheet microscopy achieves spatial resolution of 235 nm × 235 nm × 340 nm, allowing visualization of drug distribution at sub-second intervals [404]. Two-photon light-sheet nanoscopy provides complementary approaches with enhanced spatiotemporal resolution through fluorescence fluctuation analysis [405]. This high spatiotemporal resolution is crucial for capturing rapid transport processes that occur during initial drug exposure.
The temporal resolution of modern tracking systems has reached unprecedented levels. Three-dimensional orbital tracking microscopy achieves nanometer precision with millisecond temporal resolution, enabling continuous monitoring of drug carriers over distances exceeding 100 μm [406]. Similar millisecond-scale tracking has been accomplished for mitochondrial dynamics in four-dimensional (4D) imaging systems [407]. These capabilities allow researchers to distinguish between different transport mechanisms in real-time, including passive diffusion and active cellular processes.
Active transport mechanisms significantly enhance drug penetration compared to passive diffusion alone. Tumor-penetrating peptides utilizing transcytosis pathways achieve up to 300% enhancement in penetration depth [408]. The iRGD peptide system produces 250% increase in doxorubicin penetration through C-end rule (CendR)-mediated transport [409]. Additionally, reduction of cancer-associated fibroblast activity decreases extracellular matrix density, further facilitating nanoparticle penetration into deeper tumor regions [149].
Metabolic processes and drug distribution can be monitored using coherent anti-Stokes Raman scattering (CARS) microscopy. This label-free technique enables quantitative measurement of glucose concentrations at the single-cell level [410] and detection of C-deuterated drugs with millimolar sensitivity [411]. CARS microscopy has been successfully applied to visualize paclitaxel distribution in polymer films with 0.3 μm lateral resolution [412], providing insights into drug release kinetics without fluorescent labeling.
Imaging depth remains a critical parameter for comprehensive tumor analysis. Two-photon microscopy typically achieves penetration depths of 100–300 μm depending on tissue properties and excitation wavelength [413,414]. Third-harmonic generation microscopy extends this capability to 420 μm, representing a three-fold improvement over conventional two-photon imaging [415]. Adaptive optics correction further enhances both imaging depth and resolution by compensating for tissue-induced aberrations [416,417]. These enhanced penetration depths enable visualization of drug distribution throughout entire tumor spheroids, including hypoxic core regions where drug resistance often develops [418].
5.2.3. Multi-Scale Integration for Comprehensive Immune Monitoring
The integration of spatial and temporal heterogeneity with single-cell functional analysis presents new opportunities for understanding immune responses in complex tissue environments. Recent advances in analytical technologies enable unprecedented multi-scale characterization of immune cell behavior and function.
Advanced microfluidic platforms have achieved remarkable sensitivity for immune monitoring. Microfluidic enzyme-linked immunosorbent assay (ELISA) systems exhibit detection limits ranging from 0.021 to 20 picogram per milliliter (pg/mL) for various cytokines, with IL-6 detection as low as 0.021 pg/mL, prostate-specific antigen (PSA) at 0.047 pg/mL, and carcinoembryonic antigen (CEA) at 0.29 pg/mL [419]. This represents over 200-fold improvement compared to commercial ELISA methods, enabling detection of low-abundance immune mediators critical for understanding cellular communication networks [420].
Spatial resolution has dramatically improved through microfabricated sampling technologies. Push-pull sampling probes achieve spatial resolution of 20 × 60 μm, approximately 1000-fold better than conventional microdialysis probes with 2 mm long × 220 μm diameter membranes [421]. Alternative approaches using graphene microtransistors provide 50 × 50 μm spatial resolution, enabling nearly cellular-scale monitoring of neurochemicals and potentially immune mediators [422]. These advances allow precise mapping of local immune microenvironments and cytokine gradients.
The importance of spatial heterogeneity is evident in tumor microenvironments, where distinct gradients exist between core and peripheral regions. Studies reveal that tumor cores exhibit pronounced hypoxic conditions compared to the periphery, with hypoxia-inducible factor (HIF) activity showing radial increases from periphery to center [423]. This spatial organization correlates with differential cytokine production patterns, as hypoxic conditions are known to modulate immune cell function and cytokine secretion profiles [424,425,426].
Mass cytometry (CyTOF) has emerged as a powerful tool for high-dimensional immune profiling. Using panels of 30–34 markers, CyTOF can identify at least 8 major immune cell populations in complex tissues [427]. Moreover, detailed analysis uncovers functional heterogeneity within traditionally defined populations, such as the identification of 4 distinct subpopulations within phenotypically basophilic granulocytes [428]. This level of resolution is crucial for understanding how spatial location influences immune cell phenotype and function.
Integration of these multi-scale approaches exposes complex relationships between spatial location, cellular phenotype, and functional capacity. For instance, the combination of high-resolution spatial sampling with multiplexed cytokine analysis enables correlation of local microenvironmental conditions with immune cell activation states [429]. This comprehensive approach is essential for developing effective immunotherapies that account for both spatial and functional heterogeneity in complex tissue environments.
6. Emerging Technologies and Future Directions
6.1. Clinical Translation Pathways
6.1.1. Regulatory Considerations and Standards
The path from promising 3D model results to clinical application requires navigation of evolving regulatory frameworks. The FDA Modernization Act 2.0, enacted in December 2022, has created unprecedented opportunities for organoid-based drug development by eliminating mandatory animal testing requirements and promoting the adoption of New Approach Methodologies (NAMs), including organoids and organ-on-chip technologies [352,430]. This legislative change reflects growing confidence in 3D models as acceptable alternatives for preclinical drug testing, with the FDA specifically mentioning these models as being acceptable in drug filing for market approval [431]. For natural product formulations, this regulatory shift is particularly significant given the complex nature of botanical therapeutics that interact with biological systems through multiple pathways [432].
Recognizing the need for standardization, various initiatives have emerged globally. In Europe, drug testing using organoids must comply with the European Medicines Agency’s generic “Guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approaches” [433]. Korea established the Organoid Standards Initiative in September 2023, bringing together industry, academia, regulatory agencies, and standard development experts through public–private partnerships [348]. This initiative developed general guidelines for organoid manufacturing and quality evaluation (v1.0), providing guidance on materials, procedures, and essential quality assessment methods applicable at current technology levels [348]. However, regulatory harmonization remains a significant challenge, as evidenced by divergences in biowaiver criteria, dissolution profile requirements, and coefficient of variation standards across different countries [434].
Quality metrics for natural product formulations in 3D systems require adaptation from traditional pharmaceutical standards. Recent advances in automated 3D bioprinting have yielded remarkable reproducibility, with coefficient of variation (CV%) of embedded spheroid diameter between 4.2% and 8.7%, comparable to values obtained from ultra-low attachment 96-well plates [435]. Standardized mean difference (SSMD) analysis confirms that 95% of printed embedded spheroids meet “good or greater quality” criteria [435]. Automated platforms can now produce over 50 uniform spheroids with consistent size and circularity in 30 min with over 97% sorting accuracy [436], illustrating the feasibility of high-throughput standardized production for natural product screening. Despite these advances, challenges persist including high inter-lab variability and the need for standardization in spheroid generation methods [436].
Content uniformity poses particular challenges when drug distribution is inherently heterogeneous in 3D systems. Complex in vitro models positioned for pharmaceutical testing require assessment approaches that consider the unique characteristics of 3D environments and establish performance criteria for specific test assays [437]. Zone-specific evaluation can be performed at defined depths of 25, 50, 75, and 100 μm to assess penetration patterns [324]. For immunomodulatory natural products, functional assessment becomes crucial, as these compounds display antioxidant, antimicrobial, and immunomodulatory activities that must be preserved during formulation [438]. Automated high-throughput screening platforms now enable detailed analysis within 3D structures, allowing correlation of drug distribution with biological activity [436]. This integrated approach is essential for natural products where the preservation of biological activities is paramount.
The regulatory landscape continues to evolve through adaptation of existing frameworks. The FDA’s product-specific guidance (PSG) program provides recommendations for bioequivalence studies that could serve as a template for developing 3D model-specific standards [439]. The f2 similarity factor (≥ 50) used in dissolution profile comparisons represents one area where international harmonization is needed [434]. For natural product formulations optimized in 3D tumor models, establishing standardized protocols that balance scientific rigor with practical feasibility while accounting for the complex pharmacological profiles of botanical therapeutics will be essential for successful clinical translation. While specific regulatory guidelines for natural product formulations in 3D models are still evolving, the current framework provides a foundation for future development.
6.1.2. Integrating Formulation Strategies with 3D Model Validation
The inherent challenges of natural product delivery—particularly the trade-off between penetration depth and bioactivity preservation—have prompted the development of advanced formulation strategies validated through 3D tumor models. Recent studies establish that appropriately designed nanoformulations can overcome these limitations while maintaining therapeutic efficacy.
Penetration enhancement through formulation optimization has shown remarkable progress. iRGD-modified nano-delivery systems based on marine sulfated polysaccharides exhibited significantly improved tumor targeting and cellular internalization, with the iRGD peptide facilitating deeper tissue penetration through neuropilin-1 (NRP-1) receptor-mediated transcytosis [133]. Similarly, nanoemulsions with particle sizes below 100 nm accomplished 24-h cumulative percutaneous permeation in 3D skin models, with the smaller particle size proving crucial for stratum corneum penetration [440]. These formulations maintained their bioactivity, as evidenced by paeonol/madecassoside co-delivery nanoemulsions (PM-NEs) that significantly promoted the secretion of filamentous protein, aquaporin 3 (AQP3), and Claudin-1 in 3D epidermal models while inhibiting inflammatory mediators including IL-1α, IL-6, and TNF-α [440].
The integration of 3D models for formulation validation has proven essential for predicting clinical outcomes. Patient-derived organoids achieved positive predictive values of 68% and negative predictive values of 78% for standard chemotherapy responses [340], while machine learning approaches applied to these 3D models reached 68.75% accuracy with random forest classifiers in predicting drug responses [441]. Notably, 3D spheroid models uncovered penetration depths ranging from 40–150 μm correlating with clinical efficacy, with doxorubicin showing a characteristic penetration depth of 40–50 μm associated with therapeutic response [442].
Advanced formulation strategies have successfully addressed the penetration-bioactivity trade-off. Deep eutectic solvent self-assembled reverse nanomicelles (DES-RM) demonstrated enhanced drug penetration while maintaining biocompatibility, with molecular dynamics simulations revealing that self-assembly was adjusted by noncovalent intermolecular forces [443]. Furthermore, cyclodextrin-based metal–organic framework nanosheets (CL-NS-MOF) presented significantly improved precorneal residence time and intraocular bioavailability compared to conventional formulations, suggesting that particle geometry plays a crucial role in biodistribution [444].
These integrated approaches combining rational formulation design with 3D model validation represent a paradigm shift in natural product drug development, enabling the optimization of both penetration and immunomodulatory activity essential for clinical translation.
6.2. Future Research Priorities
6.2.1. Integration of Artificial Intelligence and Machine Learning
The complexity of optimizing formulations for both penetration and immunomodulatory activity creates opportunities for artificial intelligence (AI)-driven approaches. Machine learning algorithms have proven significant potential in addressing the penetration barriers identified in tumor microenvironments (Section 2.2) while maintaining drug activity (Section 4.1). Recent studies indicate that machine learning (ML) models can efficiently predict absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of compounds, with multi-objective optimization algorithms such as Particle Swarm Optimization (PSO) enhancing both biological activity and ADMET properties simultaneously [445]. These foundational capabilities position AI as a promising tool for addressing the penetration–activity trade-off identified in Section 4.
Deep learning models directly address the formulation stability challenges outlined in Section 4. A 12-layer deep neural network attained 73% accuracy for disintegration time and 99% for tablet hardness prediction, surpassing alternative techniques [446]. These capabilities enable precise control over drug release profiles while maintaining structural integrity, crucial for natural product formulations. These predictive capabilities extend beyond traditional formulation parameters to complex biological systems, where AI demonstrates equal promise in addressing the penetration–activity trade-off challenges. For practical implementation, recent studies demonstrate specific workflows: Schurr et al. employed a two-stage approach using Mask R-CNN for initial detection followed by U-Net++ for precise segmentation, achieving Dice coefficients of 0.8829 for fully formed spheroids [447]. This workflow typically involves: (1) training on annotated spheroid images, (2) feature extraction through convolutional layers, (3) optimization using evolutionary algorithms with mutation rates of 0.1, and (4) classification into developmental stages (monolayer, aggregating, fully formed). The SpheroidPicker system implements convolutional neural networks to achieve 97% sorting accuracy [448], while U-Net architectures enable accurate segmentation of complex 3D structures [449], establishing the technical foundation for AI-guided formulation optimization.
For the macromolecular barriers in 3D tumor models (Section 2.2.2), AI-enhanced confocal microscopy enables quantitative analysis of drug penetration into spheroids. The extracellular matrix presents physical barriers that limit drug diffusion, making accurate penetration prediction essential [448]. Machine learning approaches applied to these complex systems produced correlation coefficients of 0.963, illustrating robust prediction of drug distribution patterns [449]. High-throughput imaging combined with deep learning reduces 3D image acquisition time while maintaining quality [450], enabling real-time monitoring of formulation performance—a critical capability for optimizing the penetration–activity balance.
Natural product-based formulations benefit from specialized machine learning models tailored to their unique chemical diversity. The STarFish model, combining K-nearest neighbors (KNN), random forests, and multilayer perceptrons, achieved obtained area under the receiver operating characteristic curve (AUROC) scores of 0.910 on natural product datasets [451]. Transfer learning approaches proved particularly valuable, as shown in biological systems where adapted models doubled yield through accurate prediction [452]. The chemical complexity of natural products, combined with the spatial heterogeneity of 3D models discussed in Section 5, creates an ideal scenario for AI optimization where traditional approaches struggle.
The integration of ML with formulation optimization addresses the multi-parameter challenges presented in Section 3. Neural networks predicting drug concentrations delivered 93.44% accuracy, outperforming traditional methods by 7.5% [351]. Ensemble learning frameworks combining genetic algorithms generated coefficient of determination (R2) of 0.8209 for drug response prediction [453]. In practical applications, ML-driven optimization of biomaterial scaffolds yielded R2 values of 0.98 for training and 0.94 for test sets [454]. While these successes have not yet been specifically combined for natural product formulation in 3D spheroids, the convergence of these technologies is imminent. The regulatory acceptance of 3D models (Section 6.2.1) and the availability of standardized protocols (Section 7.1) create the infrastructure needed for AI-driven optimization.
Transfer learning significantly accelerates development timelines. In biological interface applications, transfer learning reduced calibration time while improving classification accuracy by over 4% with limited training data [455]. Models reached mean absolute error (MAE) of 8.54 months with R2 of 0.929, revealing precise predictive capabilities [456]. These computational approaches, when applied to the standardized 3D spheroid platforms described in Section 7.1, could enable rapid exploration of the formulation space while maintaining the critical balance between enhanced penetration and preserved bioactivity. The integration of AI with established 3D models represents the next logical step in addressing the fundamental challenges identified throughout this review, transforming the penetration–activity trade-off from a barrier into an optimization target.
Furthermore, comprehensive frameworks for AI implementation in personalized medicine are emerging, with applications ranging from patient-specific drug formulation to predictive modeling of therapeutic outcomes [457]. These advances are particularly relevant for natural product optimization, where individual metabolic variations significantly impact bioavailability and efficacy.
6.2.2. Personalized Formulation Strategies
The heterogeneity observed across patient-derived organoids motivates development of personalized formulation approaches. Genomic profiling of organoids uncovers ECM gene signatures that predict optimal formulation strategies [363,458]. Recent studies demonstrate that intestinal ECM scaffolds significantly enhance differentiation marker expression, with mucin 2 (MUC2) and villin (VILL) showing markedly higher expression in organoids cultured in tissue-specific ECM compared to Matrigel [363]. This ECM-dependent expression pattern correlates with formulation requirements, as organoids with high collagen type I alpha 1 chain (COL1A1) expression displayed differential therapeutic responses when treated with liposomal versus free drug formulations [458]. Specifically, prostate cancer organoids cultured in Arg-Glu-Asp-Val (REDV)-functionalized hydrogels showed reduced actin symmetry under enhancer of zeste homolog 2 (EZH2) inhibition, illustrating ECM-dependent drug responses [458].
Functional profiling adds another personalization dimension, with immune cell composition in organoids guiding formulation selection [362,459,460]. An automated organoid platform established that patient-derived organoids maintained greater than 80% accuracy in predicting clinical responses among 21 patients investigated, with organoid size uniformity attained through monodisperse Matrigel droplet templating (400-μm organoids within 1 week) [362]. The standardized production of 1500 cells per droplet ensures statistical representation of tumor heterogeneity while maintaining reproducibility [362]. Alternative platforms using microfluidic systems have produced similar success rates, generating uniform organoids for high-throughput screening [461,462].
Tumor microenvironment modeling reveals critical formulation parameters [463,464,465]. Studies using 3D organoid models confirm that nanoparticle size significantly impacts tumor penetration, with particles smaller than 20 nm exhibiting enhanced penetration into hypoxic tumor regions [463]. For macrophage-rich tumors, particle size between 1–6 μm optimizes phagocytic uptake, with PLGA microparticles of 3–5 μm achieving encapsulation efficiencies of 89.6% and sustained intracellular retention for 48 h [464]. Additional studies verify that macrophage phagocytosis capacity varies significantly with particle properties, influencing therapeutic delivery strategies [466,467]. T cell-predominant models require different approaches, as T cell-engaging tumor organoid platforms showed enhanced CD8+ T cell activation with increased interferon-gamma (IFN-γ) and granzyme B (GZMB) expression when treated with epigenetic inhibitors [460].
Hyaluronan metabolism profiling provides actionable formulation guidance across multiple cancer types [468,469,470]. The hyaluronan activated-metabolism phenotype (HAMP+), characterized by overexpression of hyaluronan synthase (HAS) 2, HAS3, HYAL1, and KIAA1199, was identified in 20% of cell lines and 25% of patient tissues, correlating with shorter survival (p = 0.049) [468]. HAMP+ organoids exhibited enhanced susceptibility to combination treatment with 4-methylumbelliferone (4-MU) (HA synthesis inhibitor) and dextran sulfate (HA degradation inhibitor), providing a specific formulation strategy for this subset [468]. Treatment with PEGylated hyaluronidase (PEGPH20) in organoid models resulted in tumor-specific ECM degradation, with volume transfer coefficient (Ktrans) values showing enhanced perfusion 3 h post-treatment [469]. The cell surface hyaluronidase transmembrane protein 2 (TMEM2) has also emerged as a target for modulating ECM barriers [471].
High-throughput screening platforms enable rapid formulation optimization using diverse methodologies [461,462,472,473]. Using microfluidic systems, organoid drug testing can be completed within 7–14 days, with automated imaging capturing responses at 24, 48, and 72-h intervals [472]. Drug sensitivity testing across concentration ranges (0.2–50 μM for chemotherapeutics, 0.01–100 nM for targeted agents) uncovered that 72-h endpoints provided optimal predictive value [472]. Micro-organospheres (MOS) generated through droplet microfluidics could predict 3/4 clinically responding patients and 3/4 non-responding patients within 14 days [461]. Alternative approaches using nested array chips and mini-ring formats have shown similar predictive capabilities [473,474].
Clinical implementation validates feasibility and impact across multiple cancer types [340,346,475]. Among colorectal cancer patients, organoid-guided therapy delivered 68% positive predictive value and 78% negative predictive value, outperforming empirically guided treatment selection [340]. Integration with standard pathology workflows allows organoid establishment from surgical specimens or biopsies, with successful culture rates of 70–80% for most tumor types [340,346]. The mini-ring platform reduces reagent consumption 10-fold while maintaining assay reliability, addressing cost-effectiveness concerns [474]. Cryopreservation protocols enable biobanking of organoids for longitudinal studies and repeated testing [476]. Similar success has been reported in pancreatic, ovarian, and bladder cancers [475,477].
Regulatory considerations are addressed through standardized protocols and quality control measures [345,362,478]. The use of chemically defined matrices and serum-free culture conditions facilitates Good Manufacturing Practice (GMP) compliance, while automated liquid handling ensures reproducibility across clinical sites [362]. Integration of organoid testing into existing precision medicine frameworks, similar to genomic profiling, provides a pathway for clinical adoption [345]. Recent studies have proven the feasibility of organoid-based clinical trials, paving the way for regulatory approval [478]. The combination of ECM profiling, immune characterization, and drug sensitivity testing in patient-derived organoids represents a comprehensive approach to formulation personalization, directly addressing the penetration–activity trade-off through patient-specific optimization.
7. Recommendations for Standardization and Implementation
7.1. Proposed Standardization Framework
7.1.1. Types of Stimuli and Their Biological Relevance
The tumor microenvironment presents unique physiological and biochemical characteristics that can be exploited for targeted drug delivery. Among these, pH gradients represent one of the most widely utilized stimuli. Solid tumors exhibit a pH of 6.5–6.8, significantly lower than the physiological pH of 7.4 in normal tissues [479,480]. This acidic environment results from increased glycolytic metabolism and poor perfusion in rapidly growing tumors, leading to accumulation of lactic acid [481,482]. The pH gradient becomes even more pronounced within intracellular compartments, where endosomes and lysosomes maintain pH values of 4.5–5.5 [483,484]. These pH differences enable the design of nanocarriers that remain stable during circulation but trigger drug release upon entering the acidic tumor environment. For instance, pH-responsive nanoparticles exhibited 67.5% doxorubicin release at pH 5.5 compared to only 27.0% at pH 7.4 after 48 h, highlighting the selectivity achievable through pH-responsive design [483].
Enzymatic overexpression in tumors provides another powerful stimulus for controlled drug release. MMPs, particularly MMP-2 and MMP-9, are significantly upregulated in various cancers, with expression levels 2–10 fold higher than normal tissues [485,486]. MMP-2 exists as a 62 kDa active form in tumor tissues, while MMP-9 shows variable expression patterns across different cancer types [485]. MMP-7 has been found to be overexpressed in pancreatic, colon, and breast cancers [487]. Additionally, cathepsin B, a lysosomal cysteine protease, exhibits optimal activity at pH 3.8–5.0 and is overexpressed in invasive cancers [487]. Cathepsin B specifically cleaves Gly-Phe-Leu-Gly (GFLG) sequences, while MMP-2 cleaves Pro-Leu-Gly-Val-Arg (PLGVR) sequences, enabling the design of enzyme-responsive drug delivery systems that release their payload specifically in the tumor microenvironment [487,488].
Oxidative stress represents another hallmark of cancer that can be exploited for drug delivery. Tumor tissues exhibit reactive oxygen species (ROS) levels of approximately 10−4 M, which is 5000-fold higher than the 2 × 10−8 molar (M) found in normal tissues [489,490]. This dramatic difference in ROS concentration enables the use of ROS-sensitive linkers such as thioketal bonds, which remain stable under normal physiological conditions but undergo rapid cleavage in the presence of elevated ROS. Studies have revealed that thioketal-containing nanoparticles can achieve 76% drug release within one hour in the presence of hydrogen peroxide [489], while other ROS-responsive systems utilizing similar thioketal chemistry have shown comparable rapid release kinetics [479,491].
The differential expression of glutathione between extracellular and intracellular environments provides yet another mechanism for controlled drug release. Intracellular glutathione (GSH) concentrations reach 10 mM, which is 500–5000 times higher than the extracellular concentration of 2–20 μM [492]. This gradient is particularly useful for designing disulfide-containing drug carriers that remain stable in circulation but undergo rapid reduction and drug release upon cellular internalization. The GSH-mediated reduction follows predictable kinetics, with complete cleavage achievable within hours of cellular uptake [492,493].
Temperature represents an externally controllable stimulus that can be combined with endogenous triggers for enhanced specificity. Thermosensitive polymers such as poly (N-isopropylacrylamide) (PNIPAM) exhibit a lower critical solution temperature (LCST) of 32–34 °C, below body temperature [484]. When combined with mild hyperthermia (41–43 °C), these systems can achieve rapid drug release. Low-temperature sensitive liposomes have achieved 90% doxorubicin (DOX) release within 5 min at 42 °C, compared to minimal release at 37 °C [484,494]. This approach has proven particular promise in clinical applications, with several thermosensitive formulations currently in clinical trials.
The integration of multiple stimuli-responsive mechanisms offers opportunities for enhanced specificity and control. Dual pH- and ROS-responsive systems have delivered superior performance compared to single-stimulus systems, achieving more precise spatiotemporal control over drug release [483,489]. Similarly, combinations of enzyme-cleavable linkers with pH-sensitive components have produced synergistic effects, with sequential activation mechanisms that minimize off-target drug release while maximizing therapeutic efficacy in the tumor microenvironment [487,488].
These biological stimuli have been successfully translated into clinical applications. FDA-approved formulations such as Doxil and Onivyde utilize pH gradients for drug loading and release, while ONPATTRO represents the first approved RNA interference (RNAi) therapeutic utilizing stimuli-responsive delivery [495]. The clinical success of these formulations validates the therapeutic potential of stimuli-responsive drug delivery systems and provides a foundation for the development of next-generation responsive therapeutics.
7.1.2. Imaging Specifications and Analysis Parameters
For accurate drug penetration assessment in tumor spheroids, standardized imaging and analysis parameters are essential. Confocal microscopy with z-stack acquisition enables 3D visualization of drug distribution throughout the spheroid structure. Z-stack images should be acquired at 10 μm intervals throughout the spheroid volume to capture complete 3D drug distribution patterns [496]. This interval provides sufficient spatial resolution while maintaining practical data acquisition times for high-throughput screening applications.
When using fluorescent drug conjugates or tracers, validation against the parent compound is critical for accurate penetration assessment. The correlation between fluorescent tracer and parent drug distribution should exceed 95% to ensure that the fluorescent modification does not significantly alter drug penetration behavior [496]. This validation can be performed using MALDI-MSI or liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques, which enable direct comparison of drug and metabolite localization within spheroid sections [497,498].
Temporal analysis of drug penetration requires assessment at multiple time points to capture both initial distribution and steady-state accumulation. Standard imaging time points of 1, 24, and 72 h post-treatment enable characterization of penetration kinetics, with 24 h representing clinically relevant drug exposure duration [499,500]. For continuous perfusion studies, flow rates of 19–96 μm/s approximate physiological interstitial flow conditions and influence drug penetration patterns [323].
Radial profiling analysis quantifies drug distribution from the spheroid periphery to the core using concentric bins of 10 μm width for high-resolution analysis [501]. The penetration depth is typically defined as the distance at which drug concentration falls to 50% of the peripheral concentration [442]. This standardized metric enables comparison across different drugs and spheroid models.
For statistically robust analysis, experiments should include 3–7 biological replicates per condition, with spheroids of consistent size and morphology [502]. Spheroids exceeding 500 μm in diameter develop the characteristic three-layer structure (proliferating rim, quiescent zone, and necrotic core) that mimics solid tumor microenvironments and creates physiologically relevant diffusion barriers [503]. The formation of these gradients is essential for modeling drug penetration barriers observed in solid tumors [503].
Drug distribution can be validated using MALDI-MSI, which detects both parent drugs and metabolites with specific mass-to-charge ratio (m/z) values (e.g., irinotecan at m/z 587 and its active metabolite SN-38 at m/z 393) [497]. Measurements at 24 and 48 h uncover drug accumulation primarily at the spheroid periphery, with limited penetration to the core regions [497]. Quantitative validation can employ tissue extinction coefficients compared with LC-MS/MS data to account for ion suppression effects in different tissue regions [504]. This spatial-temporal data provides mechanistic insights into penetration barriers and metabolism patterns essential for optimizing drug delivery strategies.
7.1.3. Integration with Immune Cell Co-Culture Systems
The established spheroid models were utilized to investigate tumor-immune cell interactions, a critical component of the tumor microenvironment [268,277,505]. Spheroids were formed using hanging drop methods or ultra-low attachment plates within 24–48 h and maintained for extended periods up to 72 h, with viability confirmed by live/dead staining at 24, 48, and 72-h intervals [506,507].
For T cell-tumor interactions, spheroids were co-cultured with primary T cells at a 1:1 ratio, which served as a predictor of cytotoxic efficiency [277]. T cell activation was achieved using anti-CD3/CD28 antibodies at 10 μg/mL with a 2:1 mass ratio of CD3:CD28, demonstrating superior expansion compared to other tested ratios [508]. Following 72 h of co-culture, T cell activation was confirmed by flow cytometric analysis of CD69 (early activation) and CD25 (late activation) markers [509].
Activated T cells exhibited distinct infiltration patterns, with cells migrating as either single cells or clusters within spheroids [277]. Notably, spheroid size significantly affected cytotoxic efficiency, with larger spheroids displaying higher hypoxia levels and reduced T cell activation characterized by decreased IFN-γ, TNF-α, and granzyme B expression [277]. Spatial analysis uncovered lower T cell numbers and cytotoxicity in the spheroid core compared to the periphery, highlighting the importance of the 3D architecture [277,510].
For macrophage studies, spheroids were co-cultured with macrophages at a 3:1 tumor:macrophage ratio using transwell systems or direct co-culture methods [271,505]. Macrophage polarization was induced using established protocols: M1 polarization with IFN-γ (10 ng/mL) combined with lipopolysaccharide (LPS) for 24 h, while M2 polarization utilized IL-4 treatment for the same duration [511,512]. Successful polarization was confirmed through surface marker expression patterns, with M1 macrophages presenting CD80hi/CD206lo phenotype and M2 macrophages showing CD80lo/CD206hi expression [511,513].
Temporal analysis of immune cell infiltration indicated that the first CD11b+ cells were detected at 18 h post-co-culture, with inflammatory marker expression peaking at 24 h before sharply attenuating by 3 days [514]. Functional characterization included cytokine profiling, with TNF-α and IL-6 levels measured at 6-h intervals, revealing time-dependent secretion patterns [512,514]. Additionally, reactive oxygen species generation served as a functional indicator of M1 macrophage activation, while phagocytic activity was assessed to evaluate macrophage function [512,513].
Spatial distribution analysis using immunofluorescence microscopy exposed differential localization patterns, with CD206+ M2 macrophages preferentially accumulating at the tumor invasive front (5.23%) compared to the tumor interior (2.59%) [515]. This distribution pattern was consistent with in vivo observations and emphasized the model’s ability to recapitulate physiologically relevant immune cell behaviors [512,515]. The integrated platform illustrated the complex interplay between tumor cells and immune components, providing a valuable system for evaluating immunotherapeutic strategies in a controlled 3D microenvironment [33,277,505].
7.2. Implementation Strategies
7.2.1. Nanomedicine-Based Delivery Systems
Nanomedicine approaches have shown significant promise in enhancing the tumor penetration of polysaccharide immunomodulators. These systems leverage various strategies including liposomal formulations, polymeric nanoparticles, and surface modifications to overcome the barriers of tumor microenvironment.
Liposomal delivery systems have yielded remarkable improvements in drug penetration into tumor spheroids. Studies have shown that the penetration efficiency of liposomes strongly correlates with their membrane rigidity, with a correlation coefficient of 0.84 between bending modulus and spheroid penetration [12]. Curcumin-loaded deformable liposomes attained enhanced penetration into tumor models, with improvements ranging from 1.78 to 266-fold depending on formulation [516]. The incorporation of PEGylation further enhanced penetration, with PEGylated liposomes displaying significantly improved accumulation in spheroid cores within 4 h [11].
Polymeric nanoparticle systems offer tunable properties for optimized delivery. PLGA-based nanoparticles conjugated with cell-penetrating peptides exhibited size-dependent penetration, where 20 nm particles achieved 10-fold higher uptake compared to 100 nm particles [517]. Interestingly, the drug release kinetics also played a crucial role, with low drug content conjugates (20 nm) showing faster release rates of 5%/day compared to 1–3%/day for high drug content conjugates (30–120 nm), resulting in superior tumor penetration and therapeutic efficacy [195].
Surface modification strategies have proven particularly effective for enhancing both penetration and bioactivity retention. Polysaccharide conjugation to TLR7 ligands increased their immunostimulatory potency by 10–1000 fold compared to unconjugated ligands [518]. Cell-penetrating peptides such as R8-dGR enabled efficient penetration into glioma spheroids while maintaining biological activity [519]. Moreover, MMP-2 sensitive systems illustrated selective activation in the tumor microenvironment, with masked cell-penetrating peptide (CPP)-drug conjugates showing enhanced tumor specificity [520].
The incorporation of stimuli-responsive elements has further improved delivery efficiency. Nitric oxide (NO)-driven nanomotors promoted not only their own penetration but also enhanced T cell infiltration from 2.1% to 28.2% in tumor tissue [521]. Similarly, surface acoustic wave (SAW) treatment improved nanoparticle distribution throughout spheroids, overcoming the typical limitation of peripheral accumulation [522].
7.2.2. Roadmap for Clinical Translation
Successful translation requires phased development acknowledging both scientific and regulatory requirements. The integration of 3D models with clinical practice necessitates a systematic approach that bridges laboratory innovation with patient care (Figure 6).
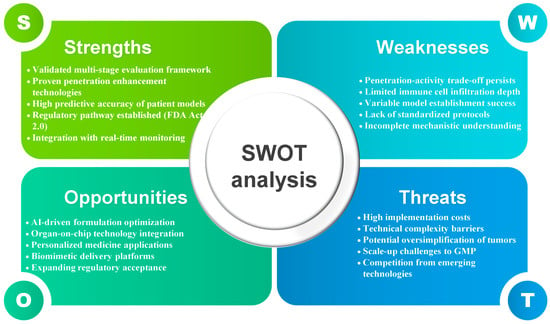
Figure 6.
Strengths, weaknesses, opportunities, threats (SWOT) analysis of natural product formulation in 3D tumor models.
Phase 1 focuses on standardized platform validation, establishing reference formulations with documented performance across multiple laboratories. Recent advances in automated systems illustrate the feasibility of high-throughput production, with platforms achieving over 50 uniform spheroids with consistent size and circularity in 30 min with over 97% sorting accuracy [436]. Achieving reproducible results across laboratories requires stringent quality control, as coefficient of variation (CV) values less than 10% are evaluated as good cell-based assays [523]. The implementation of Quality by Design (QbD) frameworks, successfully applied in pharmaceutical 3D printing, provides a model for systematic quality control in 3D culture systems [524]. This standardization approach has been validated through automated spheroid production platforms achieving CV values below 3% and 97% sorting accuracy within 30 min, demonstrating the feasibility of high-throughput natural product screening with reproducible results across multiple laboratories [436].
Phase 2 incorporates patient-derived organoids, correlating in vitro penetration–activity relationships with clinical markers. Large-scale validation studies reveal that PDO drug screening attains 68% positive predictive value and 78% negative predictive value for standard chemotherapy responses [525]. Machine learning approaches applied to PDO data reach 68.75% accuracy with random forest classifiers in predicting drug responses [526]. Establishment success rates vary significantly across cancer types, with PDO generation successful in 71% of pancreatic cancer patients who had received neoadjuvant therapy [525], while ovarian cancer organoid derivation accomplished 36% for high-grade serous ovarian cancer patients and 44% for all ovarian cancer patients combined [527], highlighting the importance of cancer-specific protocol optimization. The clinical relevance of this approach is demonstrated by patient-derived organoid studies achieving 68% positive predictive value and 78% negative predictive value for treatment responses, with machine learning integration reaching 68.75% accuracy in predicting drug responses across colorectal and bladder cancer patients [344].
Phase 3 emphasizes early clinical translation through window-of-opportunity trials in surgical patients. The Phase Ia trial of Boswellia serrata in breast cancer patients exemplifies this approach, where pre-operative administration until the night before surgery allowed correlation of ex vivo tissue analysis with drug effects [528]. Ki-67 proliferation markers served as primary endpoints, showing a statistically significant reduction in proliferation between core biopsy and excision in the treatment group (13.8 ± 11.7% reduction) compared to control (54.6 ± 21.4% increase, p = 0.008) [528]. Building on this window-of-opportunity concept, novel approaches include implantable microdevices that enable testing up to 20 therapies simultaneously within patient tumors, with drug effects examined within days rather than months [433]. These real-time assessment capabilities are further enhanced by advances in intraoperative imaging technologies, which establish that in vivo assessment produces higher sensitivity and negative predictive value than ex vivo analysis [529]. This design enables validation of 3D model predictions against actual patient tissue responses. This strategy is exemplified by natural product window-of-opportunity trials, where Boswellia serrata administration in breast cancer patients demonstrated statistically significant Ki-67 proliferation marker reduction (13.8% reduction vs. 54.6% increase in control, p = 0.008), validating 3D model predictions through direct tissue analysis [528].
Phase 4 scales successful formulations through GMP manufacturing, with quality specifications based on 3D model performance rather than traditional pharmaceutical metrics. The development of GMP-compliant protocols for organoid production, exemplified by replacing R-spondin-1 conditioned medium with recombinant protein, confirms the feasibility of standardized manufacturing [530]. Adaptive clinical trial designs incorporating organoid-based interim analyses enable rapid optimization, as evidenced by trials where organoid-guided therapy outperformed empirically guided treatment selection [525] Successful implementation is demonstrated by GMP-compliant organoid production protocols that replaced conditioned media with recombinant proteins, enabling standardized manufacturing while maintaining organoid-guided therapy performance that outperformed empirically guided treatment selection in clinical validation studies [340].
8. Conclusions
This comprehensive review unveils the complex landscape of natural product formulation for 3D tumor models, highlighting both remarkable technological advances and critical gaps in translating enhanced penetration into preserved immunomodulatory activity. Our analysis establishes that while sophisticated formulation strategies achieve impressive penetration enhancements—ranging from 3- to 20-fold across different natural product categories—a fundamental disconnect exists between improved delivery and biological efficacy. The penetration–activity trade-off emerges as the central challenge. Enhanced penetration frequently occurs at the expense of biological activity through multiple mechanisms including conformational changes during encapsulation, incomplete drug release, and surface modifications that interfere with molecular recognition. This trade-off is exemplified by curcumin, where EC50 values increase from 12.25 μM in 2D ultures to 30.76 μM in 3D spheroids despite formulation enhancements achieving deep tissue penetration. Moving forward, success requires integrated assessment frameworks evaluating both penetration and immunomodulatory preservation. Table 3 presents a comprehensive framework that integrates all aspects discussed in this review, serving as a practical guide for researchers navigating this complex landscape. Finally, to provide strategic guidance for implementation, we present a strengths, weaknesses, opportunities, threats (SWOT) analysis (Figure 6) that synthesizes the technical considerations into an actionable framework for stakeholders. The convergence of advanced formulation technologies, sophisticated 3D culture systems, and regulatory support through FDA Modernization Act 2.0 positions the field for transformative advances. Only through recognition that penetration without activity preservation is meaningless can we deliver effective natural product-based cancer immunotherapeutics.

Table 3.
Integrated framework for overcoming the penetration–activity trade-off in 3D tumor models. Overview of challenges and solutions across four domains: tumor microenvironment barriers, natural product categories, 3D model evolution, and translation framework. Framework synthesizes current achievements with strategic solutions for developing effective natural product-based cancer immunotherapies. Upward arrow (↑) indicates fold-increase or enhancement; ≠ symbol indicates "not equal to" or lack of correlation. 3D, three-dimensional; CV, coefficient of variation; ECM, extracellular matrix; EC50, half maximal effective concentration; EGCG, epigallocatechin gallate; FDA, Food and Drug Administration; GMP, Good Manufacturing Practice; kDa, kilodalton; MW, molecular weight; NPV, negative predictive value; PPV, positive predictive value.
Author Contributions
Conceptualization, C.-E.H. and S.-Y.L.; Investigation, C.-E.H. and S.-Y.L.; Resources, C.-E.H.; Supervision, S.-Y.L.; Writing—original draft, C.-E.H.; Writing—review and editing, S.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Regional Innovation System & Education (RISE) program through the Jeollanamdo RISE center, funded by the Ministry of Education (MOE) and the Jeollanamdo, Republic of Korea (2025-RISE-14-003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D., Jr.; Silk, A.W.; et al. Challenges and opportunities in cancer immunotherapy: A Society for Immunotherapy of Cancer (SITC) strategic vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef]
- Andreu-Sanz, D.; Gregor, L.; Carlini, E.; Scarcella, D.; Marr, C.; Kobold, S. Predictive value of preclinical models for CAR-T cell therapy clinical trials: A systematic review and meta-analysis. J. Immunother. Cancer 2025, 13, e011698. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, C.; Gao, X.; Dong, J.; Zhong, C. Phytochemicals in regulating PD-1/PD-L1 and immune checkpoint blockade therapy. Phytother. Res. 2024, 38, 776–796. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, Y.; Wang, J.; Gu, J. Defects in Macrophage Reprogramming in Cancer Therapy: The Negative Impact of PD-L1/PD-1. Front. Immunol. 2021, 12, 690869. [Google Scholar] [CrossRef]
- Niora, M.; Pedersbæk, D.; Münter, R.; Weywadt, M.F.V.; Farhangibarooji, Y.; Andresen, T.L.; Simonsen, J.B.; Jauffred, L. Head-to-Head Comparison of the Penetration Efficiency of Lipid-Based Nanoparticles into Tumor Spheroids. ACS Omega 2020, 5, 21162–21171. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Bagheri, Z.; Soleimani, M.; Ahvaraki, A.; Pournemat, P.; Alavi, S.E.; Madjd, Z. Heterotypic tumor spheroids: A platform for nanomedicine evaluation. J. Nanobiotechnology 2023, 21, 249. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef]
- Do, X.H.; Hoang, M.H.T.; Vu, A.T.; Nguyen, L.T.; Bui, D.T.T.; Dinh, D.T.; Nguyen, X.H.; Than, U.T.T.; Mai, H.T.; To, T.T.; et al. Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells. Int. J. Mol. Sci. 2022, 23, 12362. [Google Scholar] [CrossRef]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.; Gellert, P.R.; Stolnik, S.; Grabowska, A.; Garnett, M.C. Penetration and Uptake of Nanoparticles in 3D Tumor Spheroids. Bioconjug. Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Goda, Y.; Sakai-Kato, K. Control of Liposomal Penetration into Three-Dimensional Multicellular Tumor Spheroids by Modulating Liposomal Membrane Rigidity. Mol. Pharm. 2017, 14, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Tosca, E.M.; Ronchi, D.; Facciolo, D.; Magni, P. Replacement, Reduction, and Refinement of Animal Experiments in Anticancer Drug Development: The Contribution of 3D In Vitro Cancer Models in the Drug Efficacy Assessment. Biomedicines 2023, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Kalyani, F.S.; Liu, L.; Cheng, T.; Chen, L. Tumor organoids: Synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun. 2021, 41, 1331–1353. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, I.; Fortuni, B.; Rocha, S. From 2D to 3D Cancer Cell Models-The Enigmas of Drug Delivery Research. Nanomaterials 2020, 10, 2236. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Bae, I.Y.; Choi, W.; Oh, S.J.; Kim, C.; Kim, S.H. TIMP-1-expressing breast tumor spheroids for the evaluation of drug penetration and efficacy. Bioeng. Transl. Med. 2022, 7, e10286. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Zhang, L.; Yi, C.; Guan, X.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Healthc. Mater. 2023, 12, e2202609. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Baniahmad, A. Tumor spheroids and organoids as preclinical model systems. Med. Genet. 2021, 33, 229–234. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and Tumor Microenvironment: Mechanistic Basis and Therapeutic Targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Hoskin, R.T.; Grace, M.H.; Xiong, J.; Strauch, R.; Ferruzzi, M.; Iorizzo, M.; Kay, C. Boosting the Bioaccessibility of Dietary Bioactives by Delivery as Protein-Polyphenol Aggregate Particles. J. Agric. Food Chem. 2022, 70, 13017–13026. [Google Scholar] [CrossRef] [PubMed]
- Chaichian, S.; Moazzami, B.; Sadoughi, F.; Haddad Kashani, H.; Zaroudi, M.; Asemi, Z. Functional activities of beta-glucans in the prevention or treatment of cervical cancer. J. Ovarian Res. 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild Mushrooms as Beta-Glucan Sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef]
- Soko, G.F.; Kosgei, B.K.; Meena, S.S.; Ng, Y.J.; Liang, H.; Zhang, B.; Liu, Q.; Xu, T.; Hou, X.; Han, R.P.S. Extracellular matrix re-normalization to improve cold tumor penetration by oncolytic viruses. Front. Immunol. 2024, 15, 1535647. [Google Scholar] [CrossRef]
- Rahman, M.A.; Yadab, M.K.; Ali, M.M. Emerging Role of Extracellular pH in Tumor Microenvironment as a Therapeutic Target for Cancer Immunotherapy. Cells 2024, 13, 1924. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- He, B.; Sui, X.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020, 27, 1474–1490. [Google Scholar] [CrossRef]
- He, Y.; Tian, X.; Fan, X.; Gong, X.; Tan, S.; Pan, A.; Liang, S.; Xu, H.; Zhou, F. Enzyme-Triggered Size-Switchable Nanosystem for Deep Tumor Penetration and Hydrogen Therapy. ACS Appl. Mater. Interfaces 2023, 15, 552–565. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J.; Yang, X. Deformable nanocarriers for enhanced drug delivery and cancer therapy. Exploration 2024, 4, 20230037. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, X.; Zhao, Y.; Zhi, D.; Cui, S.; Zhang, E.; Lan, H.; Du, J.; Zhang, Z.; Zhang, S.; et al. Stimuli-Responsive Polysaccharide Enveloped Liposome for Targeting and Penetrating Delivery of survivin-shRNA into Breast Tumor. ACS Appl. Mater. Interfaces 2020, 12, 22074–22087. [Google Scholar] [CrossRef]
- Zhang, F.; Jozani, K.A.; Chakravarty, A.; Lin, D.; Hollinger, A.; Rajasekar, S.; Zhang, B. Immune-Infiltrated Cancer Spheroid Model with Vascular Recirculation Reveals Temporally Dependent and Tissue-Specific Macrophage Recruitment. Adv. Healthc. Mater. 2025, 14, e2402946. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Li, D.; Yao, Y. Tumor-on-a-chip: Perfusable vascular incorporation brings new approach to tumor metastasis research and drug development. Front. Bioeng. Biotechnol. 2022, 10, 1057913. [Google Scholar] [CrossRef]
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Invest. 2023, 133, e175824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Alvarez, M.M.; Trujillo-de Santiago, G. Placing biofabrication into the context of human disease modeling. Biofabrication 2023, 15, 030402. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.R.; Arnob, M.; Uddin, M.J. FDA Modernization Act 2.0: An insight from nondeveloping country. Drug Dev. Res. 2023, 84, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.X.; Couchet, M.; Dufau, J.; de Castro Barbosa, T.; Ulbrich, M.H.; Helmstädter, M.; Kemas, A.M.; Zandi Shafagh, R.; Marques, M.A.; Hansen, J.B.; et al. 3D Adipose Tissue Culture Links the Organotypic Microenvironment to Improved Adipogenesis. Adv. Sci. 2021, 8, e2100106. [Google Scholar] [CrossRef] [PubMed]
- Goudar, V.S.; Koduri, M.P.; Ta, Y.N.; Chen, Y.; Chu, L.A.; Lu, L.S.; Tseng, F.G. Impact of a Desmoplastic Tumor Microenvironment for Colon Cancer Drug Sensitivity: A Study with 3D Chimeric Tumor Spheroids. ACS Appl. Mater. Interfaces 2021, 13, 48478–48491. [Google Scholar] [CrossRef]
- Yen, B.L.; Hsieh, C.C.; Hsu, P.J.; Chang, C.C.; Wang, L.T.; Yen, M.L. Three-Dimensional Spheroid Culture of Human Mesenchymal Stem Cells: Offering Therapeutic Advantages and In Vitro Glimpses of the In Vivo State. Stem Cells Transl. Med. 2023, 12, 235–244. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Saiz, A.M., Jr.; Leach, J.K. Engineered Cell-Secreted Extracellular Matrix Modulates Cell Spheroid Mechanosensing and Amplifies Their Response to Inductive Cues for the Formation of Mineralized Tissues. Adv. Healthc. Mater. 2022, 11, e2102337. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Jeon, S.; Kang, H.W. 3D bioprinting of dECM-incorporated hepatocyte spheroid for simultaneous promotion of cell-cell and -ECM interactions. Front. Bioeng. Biotechnol. 2023, 11, 1305023. [Google Scholar] [CrossRef]
- McKee, J.A.; Olsen, E.A.; Wills Kpeli, G.; Brooks, M.R.; Beitollahpoor, M.; Pesika, N.S.; Burow, M.E.; Mondrinos, M.J. Engineering dense tumor constructs via cellular contraction of extracellular matrix hydrogels. Biotechnol. Bioeng. 2024, 121, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Lin, C.; Yang, X.; Xie, Y.; Gao, L.; Yu, L. The influence of spheroid maturity on fusion dynamics and micro-tissue assembly in 3D tumor models. Biofabrication 2024, 16, 035016. [Google Scholar] [CrossRef] [PubMed]
- Jipp, M.; Wagner, B.D.; Egbringhoff, L.; Teichmann, A.; Rübeling, A.; Nieschwitz, P.; Honigmann, A.; Chizhik, A.; Oswald, T.A.; Janshoff, A. Cell-substrate distance fluctuations of confluent cells enable fast and coherent collective migration. Cell Rep. 2024, 43, 114553. [Google Scholar] [CrossRef]
- Lai, J.; Kong, D.; Liu, Y.; Dai, J.; Zhang, M. A Flexible Fibrin-Based Platform for Driving One-Day Self-Assembly of Millimeter-Sized Cell-Rich Spheroids and Their Applications: An Old Material with New Tricks. Adv. Funct. Mater. 2024, 34, 2403507. [Google Scholar] [CrossRef]
- Madhavan, M.; Jaiswal, D.; Karlberg, S.; Duggan, A.; Almarshad, H.A.; Claffey, K.P.; Hoshino, K. Electron microscopy imaging and mechanical characterization of T47D multicellular tumor spheroids-Older spheroids reduce interstitial space and become stiffer. PLoS ONE 2023, 18, e0286291. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.G.; Facca, V.J.; Keunen, R.; Yu, Q.; Reilly, R.M.; Winnik, M.A. Changing Surface Polyethylene Glycol Architecture Affects Elongated Nanoparticle Penetration into Multicellular Tumor Spheroids. Biomacromolecules 2022, 23, 3296–3307. [Google Scholar] [CrossRef]
- Browning, A.P.; Sharp, J.A.; Murphy, R.J.; Gunasingh, G.; Lawson, B.; Burrage, K.; Haass, N.K.; Simpson, M. Quantitative analysis of tumour spheroid structure. eLife 2021, 10, e73020. [Google Scholar] [CrossRef]
- Grimes, D.R.; Kelly, C.; Bloch, K.; Partridge, M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J. R. Soc. Interface 2014, 11, 20131124. [Google Scholar] [CrossRef]
- Gomes, A.; Guillaume, L.; Grimes, D.R.; Fehrenbach, J.; Lobjois, V.; Ducommun, B. Oxygen Partial Pressure Is a Rate-Limiting Parameter for Cell Proliferation in 3D Spheroids Grown in Physioxic Culture Condition. PLoS ONE 2016, 11, e0161239. [Google Scholar] [CrossRef]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef]
- Ghanbari Movahed, Z.; Matin, M.M.; Mansouri, K.; Sisakhtnezhad, S. Amino acid profile changes during enrichment of spheroid cells with cancer stem cell properties in MCF-7 and MDA-MB-231 cell lines. Cancer Rep. 2023, 6, e1809. [Google Scholar] [CrossRef]
- Acker, H.; Carlsson, J.; Holtermann, G.; Nederman, T.; Nylén, T. Influence of glucose and buffer capacity in the culture medium on growth and pH in spheroids of human thyroid carcinoma and human glioma origin. Cancer Res. 1987, 47, 3504–3508. [Google Scholar] [PubMed]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Jithavech, P.; Ratnatilaka Na Bhuket, P.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.S.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef]
- Jagannathan, R.; Abraham, P.M.; Poddar, P. Temperature-dependent spectroscopic evidences of curcumin in aqueous medium: A mechanistic study of its solubility and stability. J. Phys. Chem. B 2012, 116, 14533–14540. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, L.; Geng, H.; Li, M.; Chen, S.; Wang, X.; Chen, P.; Sun, C.; Zhang, C. Spatial Metabolomics Profiling Reveals Curcumin Induces Metabolic Reprogramming in Three-Dimensional Tumor Spheroids. Metabolites 2024, 14, 482. [Google Scholar] [CrossRef]
- Kar, T.; Basak, P.; Sen, S.; Ghosh, R.K.; Bhattacharyya, M. Analysis of curcumin interaction with human serum albumin using spectroscopic studies with molecular simulation. Front. Biol. 2017, 12, 199–209. [Google Scholar] [CrossRef]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical studies on binding of curcumin to bovine serum albumins. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Baek, N.; Seo, Y.; Lim, H.; Jung, D.; Park, H. Novel plasma-polymerized coating facilitates HeLa cell spheroid formation, exerting necroptosis via β-cyclodextrin-encapsulated resveratrol. J. Anal. Sci. Technol. 2024, 15, 5. [Google Scholar] [CrossRef]
- Yin, H.; Flynn, A.D. Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng. 2016, 18, 51–76. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert. Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydr. Diet. Fibre. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef]
- Gawronski, M.; Park, J.T.; Magee, A.S.; Conrad, H. Microfibrillar structure of PGG-glucan in aqueous solution as triple-helix aggregates by small angle x-ray scattering. Biopolymers 1999, 50, 569–578. [Google Scholar] [CrossRef]
- Manabe, N.; Yamaguchi, Y. 3D Structural Insights into β-Glucans and Their Binding Proteins. Int. J. Mol. Sci. 2021, 22, 1578. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Poh, M.Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.; Jorgensen, A.M.; Sarkar, A.; Gent, S.P.; Messerli, M.A. Anionic polymers amplify electrokinetic perfusion through extracellular matrices. Front. Bioeng. Biotechnol. 2022, 10, 983317. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Zhang, C.; Bajpayee, A.G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today 2020, 34, 100898. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Chişbora, C.; Cimpoiu, C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. Effects of temperature, pH and storage solvent. Molecules 2012, 17, 11655–11666. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Ni, J.; Guo, X.; Wang, H.; Zhou, T.; Wang, X. Differences in the Effects of EGCG on Chromosomal Stability and Cell Growth between Normal and Colon Cancer Cells. Molecules 2018, 23, 788. [Google Scholar] [CrossRef]
- Jang, G.Y.; Kim, M.Y.; Lee, Y.J.; Li, M.; Shin, Y.S.; Lee, J.; Jeong, H.S. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng Res. 2018, 42, 532–539. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, K.; Chen, Y.; Zhang, Y.; Zhang, Z.; Li, H. Investigating the immune mechanism of natural products in the treatment of lung cancer. Front. Pharmacol. 2024, 15, 1289957. [Google Scholar] [CrossRef]
- Lin, Y.; He, F.; Wu, L.; Xu, Y.; Du, Q. Matrine Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Devel. Ther. 2022, 16, 533–569. [Google Scholar] [CrossRef]
- Fan, J.; Wang, J.-C.; Chen, Y.; Fang, H.; Lou, B.; Xie, J.-J.; Zhu, L.-F.; Tong, X.-M. Sinomenine induces apoptosis of prostate cancer cells by blocking activation of NF-kappa B. Afr. J. Biotechnol. 2011, 10, 3480–3487. [Google Scholar] [CrossRef]
- Li, J.C.; Dai, W.F.; Zhou, X.Q.; Rao, K.R.; Zhang, Z.J.; Liu, D.; Chen, X.Q.; Li, R.T.; Li, H.M. Matrine-Type Alkaloids from the Seeds of Sophora alopecuroides and Their Potential Anti-inflammatory Activities. Chem. Biodivers. 2021, 18, e2001066. [Google Scholar] [CrossRef]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar] [PubMed]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Dilnawaz, F.; Sahoo, S.K.; Singh, D.V.; Mukhopadhyay, A.K.; Hussain, T.; Pati, S. Curcumin Encapsulated into Biocompatible Co-Polymer PLGA Nanoparticle Enhanced Anti-Gastric Cancer and Anti-Helicobacter Pylori Effect. Asian Pac. J. Cancer Prev. 2022, 23, 61–70. [Google Scholar] [CrossRef]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Lu, T.; Zhang, F.; Khan, A.; Zhao, Y.; Li, X.; Xiang, S.; Lin, K. Lysosome-targeted theranostics: Integration of real-time fluorescence imaging and controlled drug delivery via Zn(II)-Schiff Base complexes. J. Inorg. Biochem. 2025, 272, 113015. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Newby, J.M.; Lin, C.M.; Zhang, L.; Xu, F.; Kim, W.Y.; Forest, M.G.; Lai, S.K.; Milowsky, M.I.; Wobker, S.E.; et al. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS Nano 2016, 10, 9243–9258. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.; Xie, Z.; Centurion, F.; Sun, B.; Paterson, D.J.; Tsao, S.C.; Chu, D.; Shen, Y.; Mao, G.; et al. Size-Dependent Penetration of Nanoparticles in Tumor Spheroids: A Multidimensional and Quantitative Study of Transcellular and Paracellular Pathways. Small 2024, 20, e2304693. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Wu, H.; Yu, M.; Miao, Y.; He, S.; Dai, Z.; Song, W.; Liu, Y.; Song, S.; Ahmad, E.; Wang, D.; et al. Cholesterol-tuned liposomal membrane rigidity directs tumor penetration and anti-tumor effect. Acta Pharm. Sin. B 2019, 9, 858–870. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Raghav, N. In-vitro studies of Curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Yakavets, I.; Guereschi, C.; Lamy, L.; Kravchenko, I.; Lassalle, H.P.; Zorin, V.; Bezdetnaya, L. Cyclodextrin nanosponge as a temoporfin nanocarrier: Balancing between accumulation and penetration in 3D tumor spheroids. Eur. J. Pharm. Biopharm. 2020, 154, 33–42. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Karthic, A.; Roy, A.; Lakkakula, J.; Alghamdi, S.; Shakoori, A.; Babalghith, A.O.; Emran, T.B.; Sharma, R.; Lima, C.M.G.; Kim, B.; et al. Cyclodextrin nanoparticles for diagnosis and potential cancer therapy: A systematic review. Front. Cell Dev. Biol. 2022, 10, 984311. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, J.; Zheng, W.; Zhu, J.; Song, X.; Chen, T.; Zhang, M.; Huang, Z.; Li, J. A supramolecular colloidal system based on folate-conjugated β-cyclodextrin polymer and indocyanine green for enhanced tumor-targeted cell imaging in 2D culture and 3D tumor spheroids. J. Colloid Interface Sci. 2024, 667, 259–268. [Google Scholar] [CrossRef]
- Yin, J.J.; Sharma, S.; Shumyak, S.P.; Wang, Z.X.; Zhou, Z.W.; Zhang, Y.; Guo, P.; Li, C.Z.; Kanwar, J.R.; Yang, T.; et al. Synthesis and biological evaluation of novel folic acid receptor-targeted, β-cyclodextrin-based drug complexes for cancer treatment. PLoS ONE 2013, 8, e62289. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef]
- Varan, G.; Patrulea, V.; Borchard, G.; Bilensoy, E. Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model. Nanomaterials 2018, 8, 67. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-tumor Biomacromolecules -A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, F.Y.; He, Z.; Ning, Y.L.; Li, X.H.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Jin, M.; Lu, Z.; Huang, M.; Wang, Y.; Wang, Y. Sulfated modification and antioxidant activity of exopolysaccahrides produced by Enterobacter cloacae Z0206. Int. J. Biol. Macromol. 2011, 48, 607–612. [Google Scholar] [CrossRef]
- Chen, C.; Wu, W.; Xu, X.; Zhang, L.; Liu, Y.; Wang, K. Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 2014, 105, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mukerabigwi, J.F.; Zhang, Y.; Han, L.; Jiayinaguli, T.; Wang, Q.; Liu, L.; Cao, Y.; Sun, R.; Huang, X. In vivo immunological activity of carboxymethylated-sulfated (1 → 3)-β-D-glucan from sclerotium of Poria cocos. Int. J. Biol. Macromol. 2015, 79, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Li, Y.; Lo, P.C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nanomicro Lett. 2020, 12, 47. [Google Scholar] [CrossRef]
- Shin, Y.K.; Park, Y.R.; Lee, H.; Choi, Y.; Eom, J.B. Real-Time Monitoring of Colorectal Cancer Location and Lymph Node Metastasis and Photodynamic Therapy Using Fucoidan-Based Therapeutic Nanogel and Near-Infrared Fluorescence Diagnostic-Therapy System. Pharmaceutics 2023, 15, 930. [Google Scholar] [CrossRef]
- Chung, C.H.; Lu, K.Y.; Lee, W.C.; Hsu, W.J.; Lee, W.F.; Dai, J.Z.; Shueng, P.W.; Lin, C.W.; Mi, F.L. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials 2020, 257, 120227. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef]
- Quayle, K.; Coy, C.; Standish, L.; Lu, H. The TLR2 agonist in polysaccharide-K is a structurally distinct lipid which acts synergistically with the protein-bound β-glucan. J. Nat. Med. 2015, 69, 198–208. [Google Scholar] [CrossRef]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN(®)): Review of development and future perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Wang, X.; Tang, H.; Wang, C.; Zhang, J.; Wu, W.; Jiang, X. Phenylboronic Acid-Mediated Tumor Targeting of Chitosan Nanoparticles. Theranostics 2016, 6, 1378–1392. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.G.; Jiao, W.Q.; Yang, H.; Liu, J.; Liu, D. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, X.; Wang, J.; Zhang, J.; Wu, W.; Jiang, X. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles. Biomaterials 2013, 34, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, X.; Li, Y.; Shan, T.; Bai, L.; Li, C.; Wang, Y. iRGD Tumor-Penetrating Peptide-Modified Nano-Delivery System Based on a Marine Sulfated Polysaccharide for Enhanced Anti-Tumor Efficiency Against Breast Cancer. Int. J. Nanomed. 2022, 17, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1-->3)-beta-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.; Fung, K.P.; Choy, Y.M. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Kim, F.; Sakagami, H.; Tanuma, S.; Konno, K. Stimulation of interferon-gamma-induced human myelogenous leukemic cell differentiation by high molecular weight PSK subfraction. Anticancer Res. 1990, 10, 55–58. [Google Scholar]
- Tsukagoshi, S.; Hashimoto, Y.; Fujii, G.; Kobayashi, H.; Nomoto, K.; Orita, K. Krestin (PSK). Cancer Treat. Rev. 1984, 11, 131–155. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R.; et al. Effects of Low Molecular Weight Yeast β-Glucan on Antioxidant and Immunological Activities in Mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef]
- Zong, A.; Liu, Y.; Zhang, Y.; Song, X.; Shi, Y.; Cao, H.; Liu, C.; Cheng, Y.; Jiang, W.; Du, F.; et al. Anti-tumor activity and the mechanism of SIP-S: A sulfated polysaccharide with anti-metastatic effect. Carbohydr. Polym. 2015, 129, 50–54. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Elamin, K.M.; Mohammed, A.F.A.; Muchtaridi, M.; Wathoni, N. Chitosan-Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers 2022, 14, 3410. [Google Scholar] [CrossRef]
- Mahmudi, H.; Adili-Aghdam, M.A.; Shahpouri, M.; Jaymand, M.; Amoozgar, Z.; Jahanban-Esfahlan, R. Tumor microenvironment penetrating chitosan nanoparticles for elimination of cancer relapse and minimal residual disease. Front. Oncol. 2022, 12, 1054029. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of shape, size, and aspect ratio on nanoparticle penetration and distribution inside solid tissues using 3D spheroid models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2014, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic acid (HA) presentation as a tool to modulate and control the receptor-mediated uptake of HA-coated nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef]
- Chen, E.; Han, S.; Song, B.; Xu, L.; Yuan, H.; Liang, M.; Sun, Y. Mechanism Investigation of Hyaluronidase-Combined Multistage Nanoparticles for Solid Tumor Penetration and Antitumor Effect. Int. J. Nanomed. 2020, 15, 6311–6324. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Ma, X.; Hou, C.; Lv, S.; Jia, D.; Lu, Y.; Xue, P.; Kang, Y.; Xu, Z. A bottlebrush-architectured dextran polyprodrug as an acidity-responsive vector for enhanced chemotherapy efficiency. Biomater. Sci. 2020, 8, 473–484. [Google Scholar] [CrossRef]
- Eikenes, L.; Tufto, I.; Schnell, E.A.; Bjørkøy, A.; De Lange Davies, C. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 2010, 30, 359–368. [Google Scholar] [PubMed]
- Goodman, T.T.; Olive, P.L.; Pun, S.H. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int. J. Nanomed. 2007, 2, 265–274. [Google Scholar]
- Kohno, N.; Ohnuma, T.; Truog, P. Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J. Cancer Res. Clin. Oncol. 1994, 120, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Brundel, D.H.; Feeney, O.M.; Nowell, C.J.; Suys, E.J.; Gracia, G.; Kaminskas, L.M.; McIntosh, M.M.; Kang, D.W.; Porter, C.J. Depolymerization of hyaluronan using PEGylated human recombinant hyaluronidase promotes nanoparticle tumor penetration. Nanomedicine 2021, 16, 275–292. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.; Guo, M.; Zhang, X.; Zhang, Q.; Peng, F.; Yan, L.; Hu, Z.; Tangthianchaichana, J.; Shen, Y.; et al. MMP-2 Responsive Peptide Hydrogel-Based Nanoplatform for Multimodal Tumor Therapy. Int. J. Nanomed. 2024, 19, 53–71. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popovic, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef]
- Cook, A.B.; Palange, A.; Schlich, M.; Bellotti, E.; Brahmachari, S.; di Francesco, M.; Decuzzi, P. Matrix metalloproteinase responsive hydrogel microplates for programmed killing of invasive tumour cells. RSC Appl. Polym. 2023, 1, 19–29. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Sheraton, M.V.; Chiew, G.G.Y.; Melnikov, V.; Tan, E.Y.; Luo, K.Q.; Verma, N.; Sloot, P.M.A. Emergence of spatio-temporal variations in chemotherapeutic drug efficacy: In-vitro and in-Silico 3D tumour spheroid studies. BMC Cancer 2020, 20, 1201. [Google Scholar] [CrossRef]
- Mulholland, T.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018, 8, 14672. [Google Scholar] [CrossRef]
- Lim, W.; Park, S. A Microfluidic Spheroid Culture Device with a Concentration Gradient Generator for High-Throughput Screening of Drug Efficacy. Molecules 2018, 23, 3355. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.J.; Serna, J.V.; Sunny, S.; Zhou, Y.; Deng, C.X.; El-Sayed, M.E. Pulsed ultrasound enhances nanoparticle penetration into breast cancer spheroids. Mol. Pharm. 2010, 7, 2006–2019. [Google Scholar] [CrossRef]
- Misra, R.; Rajic, M.; Sathiyamoorthy, K.; Karshafian, R. Ultrasound and microbubbles (USMB) potentiated doxorubicin penetration and distribution in 3D breast tumour spheroids. J. Drug. Deliv. Sci. Technol. 2021, 61, 102261. [Google Scholar] [CrossRef]
- Choi, Y.; Han, H.; Jeon, S.; Yoon, H.Y.; Kim, H.; Kwon, I.C.; Kim, K. Deep Tumor Penetration of Doxorubicin-Loaded Glycol Chitosan Nanoparticles Using High-Intensity Focused Ultrasound. Pharmaceutics 2020, 12, 974. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Lauwers, G.Y.; Goettel, J.A.; Oberli, M.A.; Cleveland, C.; Park, J.Y.; Minahan, D.; Chen, Y.; Anderson, D.G.; Jaklenec, A.; et al. Ultrasound-Mediated Delivery of RNA to Colonic Mucosa of Live Mice. Gastroenterology 2017, 152, 1151–1160. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, J.M.; Yu, A.C. Membrane perforation and recovery dynamics in microbubble-mediated sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef]
- Leow, R.S.; Wan, J.M.; Yu, A.C. Membrane blebbing as a recovery manoeuvre in site-specific sonoporation mediated by targeted microbubbles. J. R. Soc. Interface 2015, 12, 20150029. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. USA 2012, 109, 16486–16491. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit: A technology evaluation. Expert. Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Khan, M.Z.; Prebeg, Z.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Mehta, R.; Chawla, A.; Sharma, P.; Pawar, P. Formulation and in vitro evaluation of Eudragit S-100 coated naproxen matrix tablets for colon-targeted drug delivery system. J. Adv. Pharm. Technol. Res. 2013, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Han, U.; Seo, Y.; Hong, J. Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 2016, 6, 24158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, L.M.; Yang, L.; Liu, Z.L. Evidence for alpha-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Chen, W.F.; Zhou, B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and L-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505. [Google Scholar] [CrossRef]
- Yin, X.; Dong, H.; Cheng, H.; Ji, C.; Liang, L. Sodium caseinate particles with co-encapsulated resveratrol and epigallocatechin-3-gallate for inhibiting the oxidation of fish oil emulsions. Food Hydrocoll. 2022, 124, 107308. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Yi, L.; Högger, P.; Arroo, R.; Bajpai, V.K.; Prieto, M.A.; Simal-Gandara, J.; Wang, S.; Cao, H. Stability and antioxidant capacity of epigallocatechin gallate in Dulbecco’s modified eagle medium. Food Chem. 2022, 366, 130521. [Google Scholar] [CrossRef]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative evaluation of different co-antioxidants on the photochemical- and functional-stability of epigallocatechin-3-gallate in topical creams exposed to simulated sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (−)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food. Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian. J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Martins, S.; Silva, A.C.; Ferreira, D.C.; Souto, E.B. Improving oral absorption of Salmon calcitonin by trimyristin lipid nanoparticles. J. Biomed. Nanotechnol. 2009, 5, 76–83. [Google Scholar] [CrossRef]
- Rai, N.; Madni, A.; Faisal, A.; Jamshaid, T.; Khan, M.I.; Khan, M.M.; Parveen, F. Glyceryl Monostearate based Solid Lipid Nanoparticles for Controlled Delivery of Docetaxel. Curr. Drug Deliv. 2021, 18, 1368–1376. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Bunjes, H.; Drechsler, M.; Koch, M.H.; Westesen, K. Incorporation of the model drug ubidecarenone into solid lipid nanoparticles. Pharm. Res. 2001, 18, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Patel, M.N.; Lakkadwala, S.; Majrad, M.S.; Injeti, E.R.; Gollmer, S.M.; Shah, Z.A.; Boddu, S.H.; Nesamony, J. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS PharmSciTech 2014, 15, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhao, X.; Chen, L.J.; Yang, C.X.; Yan, X.P. pH-Driven Targeting Nanoprobe with Dual-Responsive Drug Release for Persistent Luminescence Imaging and Chemotherapy of Tumor. Anal. Chem. 2020, 92, 1179–1188. [Google Scholar] [CrossRef]
- He, N.; Chen, Z.; Yuan, J.; Zhao, L.; Chen, M.; Wang, T.; Li, X. Tumor pH-Responsive Release of Drug-Conjugated Micelles from Fiber Fragments for Intratumoral Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 32534–32544. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, P.K.; Singh, Y.; Nagaraja, C.M. A Self-Healing Metal–Organic Gel (MOG) Exhibiting pH-Responsive Release of a Chemotherapeutic Agent, Doxorubicin: Modulation of Release Kinetics by Partial Dehydration of Matrix. ACS Omega 2019, 4, 1354–1363. [Google Scholar] [CrossRef]
- Bjørge, I.M.; Costa, A.M.S.; Silva, A.S.; Vidal, J.P.O.; Nóbrega, J.M.; Mano, J.F. Tuneable spheroidal hydrogel particles for cell and drug encapsulation. Soft Matter 2018, 14, 5622–5627. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Ju, X.J.; Mu, X.T.; Wang, W.; Xie, R.; Liu, Z.; Chu, L.Y. Core-Shell Chitosan Microcapsules for Programmed Sequential Drug Release. ACS Appl. Mater. Interfaces 2016, 8, 10524–10534. [Google Scholar] [CrossRef]
- Yang, Y.; Roy, A.; Zhao, Y.; Undzys, E.; Li, S.D. Comparison of Tumor Penetration of Podophyllotoxin-Carboxymethylcellulose Conjugates with Various Chemical Compositions in Tumor Spheroid Culture and In Vivo Solid Tumor. Bioconjug. Chem. 2017, 28, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Hartman, J.E.M.; Mesquita, B.S.; Haegebaert, R.; Remaut, K.; Neumann, M.; Hak, J.; Censi, R.; Di Martino, P.; Hennink, W.E.; et al. Effect of Polyplex Size on Penetration into Tumor Spheroids. Mol. Pharm. 2023, 20, 5515–5531. [Google Scholar] [CrossRef]
- Evans, M.A.; Shields IV, C.W.; Krishnan, V.; Wang, L.L.-W.; Zhao, Z.; Ukidve, A.; Lewandowski, M.; Gao, Y.; Mitragotri, S. Macrophage-Mediated Delivery of Hypoxia-Activated Prodrug Nanoparticles. Adv. Therap. 2020, 3, 1900162. [Google Scholar] [CrossRef]
- Ikeda, Y.; Hisano, H.; Nishikawa, Y.; Nagasaki, Y. Targeting and Treatment of Tumor Hypoxia by Newly Designed Prodrug Possessing High Permeability in Solid Tumors. Mol. Pharm. 2016, 13, 2283–2289. [Google Scholar] [CrossRef]
- Kulkarni, P.; Haldar, M.K.; Katti, P.; Dawes, C.; You, S.; Choi, Y.; Mallik, S. Hypoxia Responsive, Tumor Penetrating Lipid Nanoparticles for Delivery of Chemotherapeutics to Pancreatic Cancer Cell Spheroids. Bioconjug. Chem. 2016, 27, 1830–1838. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, X.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.; Jing, X. A Paclitaxel Prodrug Activatable by Irradiation in a Hypoxic Microenvironment. Angew. Chem. Int. Ed. Engl. 2020, 59, 23198–23205. [Google Scholar] [CrossRef]
- Zhou, Z.; Jafari, M.; Sriram, V.; Kim, J.; Lee, J.Y.; Ruiz-Torres, S.J.; Waltz, S.E. Delayed Sequential Co-Delivery of Gefitinib and Doxorubicin for Targeted Combination Chemotherapy. Mol. Pharm. 2017, 14, 4551–4559. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef]
- Li, J.; Zhen, X.; Lyu, Y.; Jiang, Y.; Huang, J.; Pu, K. Cell Membrane Coated Semiconducting Polymer Nanoparticles for Enhanced Multimodal Cancer Phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef]
- Xu, Y.; Du, L.; Han, B.; Wang, Y.; Fei, J.; Xia, K.; Zhai, Y.; Yu, Z. Black phosphorus quantum dots camouflaged with platelet-osteosarcoma hybrid membrane and doxorubicin for combined therapy of osteosarcoma. J. Nanobiotechnology 2023, 21, 243. [Google Scholar] [CrossRef]
- Lv, W.; Jiang, G.; Lin, X.; Qian, M.; Huang, R.; Li, Z.; Liu, H.; Lin, D.; Wang, Y. An Unusual Application of Multifunctional Nanozyme Derived from COF: Augmenting Chemoimmunotherapy while Attenuating Cardiotoxicity. Adv. Funct. Mater. 2025, 35, 2412862. [Google Scholar] [CrossRef]
- Caballero-Florán, I.H.; Cortés, H.; Borbolla-Jiménez, F.V.; Florán-Hernández, C.D.; Del Prado-Audelo, M.L.; Magaña, J.J.; Florán, B.; Leyva-Gómez, G. PEG 400:Trehalose Coating Enhances Curcumin-Loaded PLGA Nanoparticle Internalization in Neuronal Cells. Pharmaceutics 2023, 15, 1594. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Karanastasis, A.A.; Chatziathanasiadou, M.V.; Oguz, M.; Kougioumtzi, A.; Clemente, N.; Kellici, T.F.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Mavromoustakos, T.; et al. Inclusion of Quercetin in Gold Nanoparticles Decorated with Supramolecular Hosts Amplifies Its Tumor Targeting Properties. ACS Appl. Bio Mater. 2019, 2, 2715–2725. [Google Scholar] [CrossRef]
- Mohtashami, L.; Ghows, N.; Tayarani-Najaran, Z.; Iranshahi, M. Galbanic Acid-Coated Fe3O4 Magnetic Nanoparticles with Enhanced Cytotoxicity to Prostate Cancer Cells. Planta Med. 2019, 85, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9, e2105451. [Google Scholar] [CrossRef]
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef]
- Qu, S.; Han, Y.; Liu, Y.; Zhu, J.; Acaroz, U.; Shen, J.; Zhu, K. Milk Exosomes Facilitate Oral Delivery of Drugs against Intestinal Bacterial Infections. J. Agric. Food Chem. 2022, 70, 16069–16079. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Li, X.; Cui, X.; Yang, E.; Chen, N.; Yuan, X.; Zhao, J.; Hou, X.; Kang, C. Phosphatidylcholine-Engineered Exosomes for Enhanced Tumor Cell Uptake and Intracellular Antitumor Drug Delivery. Macromol. Biosci. 2021, 21, e2100042. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. An Effective Peptide-Based Platform for Efficient Exosomal Loading and Cellular Delivery of a microRNA. ACS Appl. Mater. Interfaces 2023, 15, 3851–3866. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Li, Y.; Li, W.; Cheng, K.; Qian, Y.; Xu, G.; Zhang, X.; Hu, L.; Chen, P.; et al. Designer Exosomes for Active Targeted Chemo-Photothermal Synergistic Tumor Therapy. Adv. Funct. Mater. 2018, 28, 1707360. [Google Scholar] [CrossRef]
- Han, Z.; Lv, W.; Li, Y.; Chang, J.; Zhang, W.; Liu, C.; Sun, J. Improving Tumor Targeting of Exosomal Membrane-Coated Polymeric Nanoparticles by Conjugation with Aptamers. ACS Appl. Bio Mater. 2020, 3, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, Q.; Li, X.; Xie, C.; Lu, W.; Wang, S.; Wang, J.; Wang, D.; Liu, M. Efficacy of inverso isomer of CendR peptide on tumor tissue penetration. Acta Pharm. Sin. B 2018, 8, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Pullan, J.; Dailey, K.; Bhallamudi, S.; Feng, L.; Alhalhooly, L.; Froberg, J.; Osborn, J.; Sarkar, K.; Molden, T.; Sathish, V.; et al. Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 2163–2175. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, L.; Lu, F.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Yang, G.; Xing, C.; Liu, L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 2020, 10, 218–230. [Google Scholar] [CrossRef]
- Du, F.; Wan, Q.; Glebov, O.O. Temperature-induced membrane trafficking drives antibody delivery to the brain. Biomed. Pharmacother. 2025, 189, 118313. [Google Scholar] [CrossRef]
- Othman, Z.A.; Hassan, Y.M.; Karim, A.Y. Enhancement of skin tumor laser hyperthermia with Ytterbium nanoparticles: Numerical simulation. Biomed. Mater. 2024, 19, 035021. [Google Scholar] [CrossRef]
- Stringer, R.A.; Ferreira, S.; Rose, J.; Ronseaux, S. Application of Osmotic Pumps for Sustained Release of 1-Aminobenzotriazole and Inhibition of Cytochrome P450 Enzymes in Mice: Model Comparison with the Hepatic P450 Reductase Null Mouse. Drug Metab. Dispos. 2016, 44, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Beola, L.; Grazú, V.; Fernández-Afonso, Y.; Fratila, R.M.; de Las Heras, M.; de la Fuente, J.M.; Gutiérrez, L.; Asín, L. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Appl. Mater. Interfaces 2021, 13, 12982–12996. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Paris, J.L.; Wang, B.; Wang, W.; Axon, C.D.; Vallet-Regí, M.; Langer, R.; Kohane, D.S. Ultrasound-triggered local anaesthesia. Nat. Biomed. Eng. 2017, 1, 644–653. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, X.; Yang, H.; Zhang, S.; Li, Y.; Kuang, C.; Fu, S.; Cui, L.; Liang, M.; Gao, C.; et al. Novel Class of Ultrasound-Triggerable Drug Delivery Systems for the Improved Treatment of Tumors. Mol. Pharm. 2019, 16, 2956–2965. [Google Scholar] [CrossRef]
- Moreno-Gomez, N.; Athanassiadis, A.G.; Poortinga, A.T.; Fischer, P. Antibubbles Enable Tunable Payload Release with Low-Intensity Ultrasound. Adv. Mater. 2023, 35, e2305296. [Google Scholar] [CrossRef]
- Caraveo-Suarez, R.O.; Garcia-Galicia, I.A.; Santellano-Estrada, E.; Carrillo-Lopez, L.M.; Huerta-Jimenez, M.; Vargas-Bello-Pérez, E.; Alarcon-Rojo, A.D. High-Frequency Focused Ultrasound on Quality Traits of Bovine Triceps brachii Muscle. Foods 2021, 10, 2074. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C. Multi-Focused Acoustic Radiation Force Impulse Modulation of Murine Hepatic Xenografts Enhances Nanoscale DOX@Lip Delivery and Therapeutic Effect. Int. J. Nanomed. 2025, 20, 7359–7373. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, G.; Yang, Y.; Xu, Y.; Qi, F.; Li, J.; Zhu, B.; Chen, Y. Multichannel Piezo-Ultrasound Implant with Hybrid Waterborne Acoustic Metastructure for Selective Wireless Energy Transfer at Megahertz Frequencies. Adv. Mater. 2021, 33, e2104251. [Google Scholar] [CrossRef]
- Liu, C.; Dong, J.; Zhang, Z.; Fu, K.; Wang, D.; Mi, X.; Yue, S.; Tan, X.; Zhang, Y. Four-Color SERS Monitoring of Size-dependent Nanoparticle Delivery in the Same Tumor. Anal. Chem. 2023, 95, 13880–13888. [Google Scholar] [CrossRef]
- Almuqbil, R.M.; Heyder, R.S.; Bielski, E.R.; Durymanov, M.; Reineke, J.J.; da Rocha, S.R.P. Dendrimer Conjugation Enhances Tumor Penetration and Efficacy of Doxorubicin in Extracellular Matrix-Expressing 3D Lung Cancer Models. Mol. Pharm. 2020, 17, 1648–1662. [Google Scholar] [CrossRef]
- Mittler, F.; Obeïd, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-Content Monitoring of Drug Effects in a 3D Spheroid Model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef]
- Zou, P.; Helson, L.; Maitra, A.; Stern, S.T.; McNeil, S.E. Polymeric curcumin nanoparticle pharmacokinetics and metabolism in bile duct cannulated rats. Mol. Pharm. 2013, 10, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, M.; Mao, J.; Tian, L.; Gan, H.; Hu, X.; Yan, L.; Long, H.; Cai, J.; Zheng, X.; et al. Inflammation-Regulated Nanodrug Sensitizes Hepatocellular Carcinoma to Checkpoint Blockade Therapy by Reprogramming the Tumor Microenvironment. ACS Appl. Mater. Interfaces 2022, 14, 49542–49554. [Google Scholar] [CrossRef] [PubMed]
- Jourghanian, P.; Ghaffari, S.; Ardjmand, M.; Haghighat, S.; Mohammadnejad, M. Sustained release Curcumin loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, P.; Terada, T.; Sui, M.; Furuta, H.; Iida, K.; Katayama, Y.; Lu, Y.; Okamoto, K.; Suzuki, M.; et al. Quercetin 3,5,7,3′,4′-pentamethyl ether from Kaempferia parviflora directly and effectively activates human SIRT1. Commun. Biol. 2021, 4, 209. [Google Scholar] [CrossRef]
- Hou, X.; Rooklin, D.; Fang, H.; Zhang, Y. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci. Rep. 2016, 6, 38186. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Fang, M.; Sheth, V.; Zhang, Y.; Holden, A.M.; Donahue, N.D.; Green, D.E.; Frickenstein, A.N.; Mettenbrink, E.M.; et al. Nanoparticle Surface Engineering with Heparosan Polysaccharide Reduces Serum Protein Adsorption and Enhances Cellular Uptake. Nano Lett. 2022, 22, 2103–2111. [Google Scholar] [CrossRef]
- Ishaniya, W.; Sugantharam, K.; Subramani, M.; Kumar, A.M.; Gopinath, P.; Rajendran, S.; Ganeshpandian, M. Lipid-Coated Mesoporous Silica Nanoparticles for pH-Responsive Release and Enhanced Anti-Proliferative Activity of Piperlongumine Natural Product. ChemistrySelect 2024, 9, e202402022. [Google Scholar] [CrossRef]
- Hsu, S.C.; Wu, N.P.; Lu, Y.C.; Ma, Y.H. Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano-Bio Interface. Pharmaceutics 2022, 14, 1523. [Google Scholar] [CrossRef]
- Byun, E.H.; Omura, T.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett. 2011, 585, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- McLean, A.; Zhang, W.; Cooke, A.; Potter, N.S.; Kopelman, R.; Paulus, Y.M. Targeted 8-arm PEG Nanosystems for Localization of Choroidal Neovascularization Macular Degeneration Model. ACS Appl. Bio Mater. 2024, 7, 5496–5505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, J.; Zhao, L.; Mao, K.; Gu, Q.; Li, D.; Zhao, J.; Wu, X. RGD-modified multifunctional nanoparticles encapsulating salvianolic acid A for targeted treatment of choroidal neovascularization. J. Nanobiotechnology 2021, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Quiñones, N.C.; López-Méndez, L.J.; Ramos, E.; Rojas-Aguirre, Y.; Guadarrama, P. Mono-Dendronized β-Cyclodextrin Derivatives as Multitasking Containers for Curcumin. Impacting Its Solubility, Loading, and Tautomeric Form. J. Phys. Chem. B 2022, 126, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Nakashima, F.; Kunihiro, A.G.; Presley, S.H.; Funk, J.L.; Schneider, C. Mechanistic Differences in the Inhibition of NF-κB by Turmeric and Its Curcuminoid Constituents. J. Agric. Food Chem. 2020, 68, 6154–6160. [Google Scholar] [CrossRef]
- Moi, S.; Shekh, S.; Reddy, K.K.A.; Dhurjad, P.; Sonti, R.; Gowd, K.H. Peptide Cysteine Thiols Act as Photostabilizer of Avobenzone through Stabilizing the Transition State of Keto-Enol Tautomerization. Photochem. Photobiol. 2023, 99, 911–919. [Google Scholar] [CrossRef]
- Saboo, S.; Kestur, U.S.; Flaherty, D.P.; Taylor, L.S. Congruent Release of Drug and Polymer from Amorphous Solid Dispersions: Insights into the Role of Drug-Polymer Hydrogen Bonding, Surface Crystallization, and Glass Transition. Mol. Pharm. 2020, 17, 1261–1275. [Google Scholar] [CrossRef]
- Shim, H.; Sah, H. Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics 2020, 12, 626. [Google Scholar] [CrossRef]
- Amponsah-Efah, K.K.; Demeler, B.; Suryanarayanan, R. Characterizing Drug-Polymer Interactions in Aqueous Solution with Analytical Ultracentrifugation. Mol. Pharm. 2021, 18, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Zhang, R.; Chen, P.; Tengjisi; Wang, L.; Chen, D.; Weitz, D.A.; Zhao, C.X. J-Aggregate-Based FRET Monitoring of Drug Release from Polymer Nanoparticles with High Drug Loading. Angew. Chem. Int. Ed. Engl. 2020, 59, 20065–20074. [Google Scholar] [CrossRef] [PubMed]
- Ünal, S.; Varan, G.; Benito, J.M.; Aktaş, Y.; Bilensoy, E. Insight into oral amphiphilic cyclodextrin nanoparticles for colorectal cancer: Comprehensive mathematical model of drug release kinetic studies and antitumoral efficacy in 3D spheroid colon tumors. Beilstein J. Org. Chem. 2023, 19, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, F.R.; Saputra, O.A.; Lestari, W.W.; Koketsu, M.; Mukti, R.R.; Martien, R. pH-Triggered Drug Release Controlled by Poly(Styrene Sulfonate) Growth Hollow Mesoporous Silica Nanoparticles. ACS Omega 2020, 5, 4261–4269. [Google Scholar] [CrossRef]
- Madech, P.; Sitthichai, N.; Phetdee, C.; Prangkio, P.; Punyodom, W.; Manokruang, K. Release kinetics and binding interactions of curcumin-encapsulated nanoparticles released from chitosan-graft-(methoxy poly(ethylene glycol)-block-polycaprolactone) injectable hydrogels. Polym. Int. 2023, 72, 693–703. [Google Scholar] [CrossRef]
- Curry, S.D.; Bower, B.M.; Saemundsson, S.A.; Goodwin, A.P.; Cha, J.N. Binding affinity and transport studies of engineered photocrosslinkable affibody-enzyme-nanoparticle constructs. Nanoscale Adv. 2025, 7, 2239–2247. [Google Scholar] [CrossRef]
- Gstöttner, C.; Knaupp, A.; Vidarsson, G.; Reusch, D.; Schlothauer, T.; Wuhrer, M.; Domínguez-Vega, E. Affinity capillary electrophoresis—Mass spectrometry permits direct binding assessment of IgG and FcγRIIa in a glycoform-resolved manner. Front. Immunol. 2022, 13, 980291. [Google Scholar] [CrossRef]
- Zumbro, E.; Alexander-Katz, A. Polymer Stiffness Regulates Multivalent Binding and Liquid-Liquid Phase Separation. Biophys. J. 2020, 119, 1849–1864. [Google Scholar] [CrossRef]
- Watchorn, J.; Stuart, S.; Burns, D.C.; Gu, F.X. Mechanistic Influence of Polymer Species, Molecular Weight, and Functionalization on Mucin–Polymer Binding Interactions. ACS Appl. Polym. Mater. 2022, 4, 7537–7546. [Google Scholar] [CrossRef]
- Liu, S.; Athar, A.; Quach, D.; Patanwala, A.E.; Naylor, J.M.; Stevens, J.A.; Levy, N.; Knaggs, R.D.; Lobo, D.N.; Penm, J. Risks and benefits of oral modified-release compared with oral immediate-release opioid use after surgery: A systematic review and meta-analysis. Anaesthesia 2023, 78, 1225–1236. [Google Scholar] [CrossRef]
- Ji, W.; Li, X.; Xiao, M.; Sun, Y.; Lai, W.; Zhang, H.; Pei, H.; Li, L. DNA-Scaffolded Disulfide Redox Network for Programming Drug-Delivery Kinetics. Chemistry 2021, 27, 8745–8752. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kouassi, K.G.W.; Vanbever, R.; Dumoulin, M. Impact of the PEG length and PEGylation site on the structural, thermodynamic, thermal, and proteolytic stability of mono-PEGylated alpha-1 antitrypsin. Protein Sci. 2022, 31, e4392. [Google Scholar] [CrossRef] [PubMed]
- Etayash, H.; Yip, F.; Hancock, R.E.W. Impacts of PEGylation and Glycosylation on the Biological Properties of Host Defense Peptide IDR1018. Pharmaceutics 2023, 15, 1391. [Google Scholar] [CrossRef] [PubMed]
- Lazewski, D.; Kucinska, M.; Potapskiy, E.; Kuzminska, J.; Popenda, L.; Tezyk, A.; Goslinski, T.; Wierzchowski, M.; Murias, M. Enhanced Cytotoxic Activity of PEGylated Curcumin Derivatives: Synthesis, Structure-Activity Evaluation, and Biological Activity. Int. J. Mol. Sci. 2023, 24, 1467. [Google Scholar] [CrossRef]
- Vollmar, B.S.; Fei, M.; Liang, W.C.; Bravo, D.D.; Wang, J.; Yu, L.; Corr, N.; Zhang, G.; McNamara, E.; Masih, S.; et al. PEGylation of anti-MerTK Antibody Modulates Ocular Biodistribution. Bioconjug. Chem. 2022, 33, 1837–1851. [Google Scholar] [CrossRef]
- Schwarz, R.; Zitzow, E.; Fiebig, A.; Hering, S.; Humboldt, Y.; Schoenwaelder, N.; Kämpfer, N.; Volkmar, K.; Hinz, B.; Kreikemeyer, B.; et al. PEGylation increases antitumoral activity of arginine deiminase of Streptococcus pyogenes. Appl. Microbiol. Biotechnol. 2022, 106, 261–271. [Google Scholar] [CrossRef]
- Gu, J.; Clegg, J.R.; Heersema, L.A.; Peppas, N.A.; Smyth, H.D.C. Optimization of Cationic Nanogel PEGylation to Achieve Mammalian Cytocompatibility with Limited Loss of Gram-Negative Bactericidal Activity. Biomacromolecules 2020, 21, 1528–1538. [Google Scholar] [CrossRef]
- Mukherjee, M.; Chepizhko, O.; Chiara Lionetti, M.; Zapperi, S.; La Porta, C.A.M.; Levine, H. Infiltration of tumor spheroids by activated immune cells. Phys. Biol. 2023, 20, 056001. [Google Scholar] [CrossRef]
- Lee, S.; Woo, C.J.; Jung, H.I.; Nam, K.C.; Lim, J.S.; Kwak, B.S. Formation Pattern Analysis of Spheroids Formed by a Droplet-Based Microfluidic System for Predicting the Aggressiveness of Tumor Cells. ACS Biomater. Sci. Eng. 2024, 10, 2477–2485. [Google Scholar] [CrossRef]
- Lin, Z.P.; Nguyen, L.N.M.; Ouyang, B.; MacMillan, P.; Ngai, J.; Kingston, B.R.; Mladjenovic, S.M.; Chan, W.C.W. Macrophages Actively Transport Nanoparticles in Tumors After Extravasation. ACS Nano 2022, 16, 6080–6092. [Google Scholar] [CrossRef]
- Madsen, N.H.; Nielsen, B.S.; Nhat, S.L.; Skov, S.; Gad, M.; Larsen, J. Monocyte Infiltration and Differentiation in 3D Multicellular Spheroid Cancer Models. Pathogens 2021, 10, 969. [Google Scholar] [CrossRef]
- Sargenti, A.; Musmeci, F.; Bacchi, F.; Delprete, C.; Cristaldi, D.A.; Cannas, F.; Bonetti, S.; Pasqua, S.; Gazzola, D.; Costa, D.; et al. Physical Characterization of Colorectal Cancer Spheroids and Evaluation of NK Cell Infiltration Through a Flow-Based Analysis. Front. Immunol. 2020, 11, 564887. [Google Scholar] [CrossRef]
- Liborio-Ramos, S.; Quiros-Fernandez, I.; Ilan, N.; Soboh, S.; Farhoud, M.; Süleymanoglu, R.; Bennek, M.; Calleja-Vara, S.; Müller, M.; Vlodavsky, I.; et al. An integral membrane constitutively active heparanase enhances the tumor infiltration capability of NK cells. Oncoimmunology 2025, 14, 2437917. [Google Scholar] [CrossRef]
- Chen, P.; Han, Y.; Wang, L.; Zheng, Y.; Zhu, Z.; Zhao, Y.; Zhang, M.; Chen, X.; Wang, X.; Sun, C. Spatially Resolved Metabolomics Combined with the 3D Tumor-Immune Cell Coculture Spheroid Highlights Metabolic Alterations during Antitumor Immune Response. Anal. Chem. 2023, 95, 15153–15161. [Google Scholar] [CrossRef] [PubMed]
- Wegge, M.; Dok, R.; Dubois, L.J.; Nuyts, S. Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 3763. [Google Scholar] [CrossRef] [PubMed]
- Leschiera, E.; Al-Hity, G.; Flint, M.S.; Venkataraman, C.; Lorenzi, T.; Almeida, L.; Audebert, C. An individual-based model to explore the impact of psychological stress on immune infiltration into tumour spheroids. Phys. Biol. 2024, 21, 026003. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Laird, M.; Bishop, T.; Li, R.; Ruffo, E.; Lohmueller, J.; Zervantonakis, I.K. CAR T cell infiltration and cytotoxic killing within the core of 3D breast cancer spheroids under control of antigen sensing in microwell arrays. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sarkar, S.; Peng, C.C.; Tung, Y.C. Comparison of VEGF-A secretion from tumor cells under cellular stresses in conventional monolayer culture and microfluidic three-dimensional spheroid models. PLoS ONE 2020, 15, e0240833. [Google Scholar] [CrossRef]
- Bromma, K.; Alhussan, A.; Perez, M.M.; Howard, P.; Beckham, W.; Chithrani, D.B. Three-Dimensional Tumor Spheroids as a Tool for Reliable Investigation of Combined Gold Nanoparticle and Docetaxel Treatment. Cancers 2021, 13, 1465. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Kelkawi, A.H.A.; Allahverdi, A.; Rothbauer, M.; Ertl, P.; Naderi-Manesh, H. Fingerprinting Metabolic Activity and Tissue Integrity of 3D Lung Cancer Spheroids under Gold Nanowire Treatment. Cells 2022, 11, 478. [Google Scholar] [CrossRef]
- Khan, A.H.; Zhou, S.P.; Moe, M.; Ortega Quesada, B.A.; Bajgiran, K.R.; Lassiter, H.R.; Dorman, J.A.; Martin, E.C.; Pojman, J.A.; Melvin, A.T. Generation of 3D Spheroids Using a Thiol-Acrylate Hydrogel Scaffold to Study Endocrine Response in ER+ Breast Cancer. ACS Biomater. Sci. Eng. 2022, 8, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, C.; Izzo, L.; Rodríguez-Carrasco, Y.; Ruiz, M.J. Integrated Approach to Cyclopiazonic Acid Cytotoxicity Using In Vitro (2D and 3D Models) and In Silico Methods. Toxins 2024, 16, 473. [Google Scholar] [CrossRef]
- Jeon, H.; Zanon, T.T.M.; Carpenter, J.; Ilias, A.; Colón, Y.; Wang, Y. A Bioinert Hydrogel Framework for Precision 3D Cell Cultures: Advancing Automated High-Content and High-Throughput Drug Screening. Small Sci. 2025, 5, 2400440. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.N.N.; Yempala, T.; Muthuramalingam, R.P.K.; Giustarini, G.; Teng, G.; Ang, W.H.; Gibson, D.; Adriani, G.; Pastorin, G. Fluorescence-Guided Spatial Drug Screening in 3D Colorectal Cancer Spheroids. Adv. Healthc. Mater. 2024, 13, e2400203. [Google Scholar] [CrossRef] [PubMed]
- Mekhileri, N.V.; Major, G.; Lim, K.; Mutreja, I.; Chitcholtan, K.; Phillips, E.; Hooper, G.; Woodfield, T. Biofabrication of Modular Spheroids as Tumor-Scale Microenvironments for Drug Screening. Adv. Healthc. Mater. 2023, 12, e2201581. [Google Scholar] [CrossRef]
- Lei, J.; Cao, X.W.; Li, P.F.; Zhao, J.; Wang, F.J. Platycodin D reduces PD-L1 levels by inhibiting LXR-β activity and combines with nintedanib to enhance the tumor-killing effect of T cells. FEBS Lett. 2024, 598, 3053–3070. [Google Scholar] [CrossRef]
- Pulugu, P.; Arya, N.; Kumar, P.; Srivastava, A. Polystyrene-Based Slippery Surfaces Enable the Generation and Easy Retrieval of Tumor Spheroids. ACS Appl. Bio Mater. 2022, 5, 5582–5594. [Google Scholar] [CrossRef]
- Järvinen, P.; Bonabi, A.; Jokinen, V.; Sikanen, T. Simultaneous Culturing of Cell Monolayers and Spheroids on a Single Microfluidic Device for Bridging the Gap between 2D and 3D Cell Assays in Drug Research. Adv. Funct. Mater. 2020, 30, 2000479. [Google Scholar] [CrossRef]
- Samimi, H.; Sohi, A.N.; Irani, S.; Arefian, E.; Mahdiannasser, M.; Fallah, P.; Haghpanah, V. Alginate-based 3D cell culture technique to evaluate the half-maximal inhibitory concentration: An in vitro model of anticancer drug study for anaplastic thyroid carcinoma. Thyroid. Res. 2021, 14, 27. [Google Scholar] [CrossRef]
- Dhandapani, H.; Siddiqui, A.; Karadkar, S.; Tayalia, P. In Vitro 3D Spheroid Model Preserves Tumor Microenvironment of Hot and Cold Breast Cancer Subtypes. Adv. Healthc. Mater. 2023, 12, e2300164. [Google Scholar] [CrossRef]
- Karimi, S.; Mansouri, S.; Nassiri, F.; Bunda, S.; Singh, O.; Brastianos, P.K.; Dunn, I.F.; Zadeh, G. Clinical significance of checkpoint regulator “Programmed death ligand-1 (PD-L1)” expression in meningioma: Review of the current status. J. Neurooncol. 2021, 151, 443–449. [Google Scholar] [CrossRef]
- Wu, B.; Chiang, H.C.; Sun, X.; Yuan, B.; Mitra, P.; Hu, Y.; Curiel, T.J.; Li, R. Genetic ablation of adipocyte PD-L1 reduces tumor growth but accentuates obesity-associated inflammation. J. Immunother. Cancer 2020, 8, e000964. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, H.; Ravindranathan, S.; Owonikoko, T.K.; El-Rayes, B.F.; Liu, Y.; Waller, E.K. Comparing VIP and PD-L1 expression as cancer biomarkers. ImmunoMedicine 2022, 2, e1033. [Google Scholar] [CrossRef]
- Lung, J.; Hung, M.S.; Lin, Y.C.; Hung, C.H.; Chen, C.C.; Lee, K.D.; Tsai, Y.H. Virtual Screening and In Vitro Evaluation of PD-1 Dimer Stabilizers for Uncoupling PD-1/PD-L1 Interaction from Natural Products. Molecules 2020, 25, 5293. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Du, K.; Ai, M.; Wang, B.; Lin, J.; Ren, A.; Chen, C.; Huang, Z.; Qiu, W.; Yuan, Y.; et al. PD-L1 expression as biomarker of efficacy of PD-1/PD-L1 checkpoint inhibitors in metastatic triple negative breast cancer: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1060308. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kim, B.R.; Kim, D.Y.; Kim, W.Y.; Kang, S.; Lee, S.I.; Oh, S.C. Cannabidiol Enhances Atezolizumab Efficacy by Upregulating PD-L1 Expression via the cGAS-STING Pathway in Triple-Negative Breast Cancer Cells. Cancer Immunol. Res. 2024, 12, 1796–1807. [Google Scholar] [CrossRef]
- Mao, D.; Inoue, H.; Notomi, T.; Goda, S. P38α contributes to TNF-α-induced IL-8 production in human gingival cells. BioFactors 2023, 49, 1223–1232. [Google Scholar] [CrossRef]
- Bahman, F.; Al-Roub, A.; Akhter, N.; Al Madhoun, A.; Wilson, A.; Almansour, N.; Al-Rashed, F.; Sindhu, S.; Al-Mulla, F.; Ahmad, R. TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes. Int. J. Mol. Sci. 2024, 25, 6776. [Google Scholar] [CrossRef]
- Tamim, M.D.A.S.H.H.; Al-Dawy, M.D.M.M.E.T.S.A.; Selim, H.M. TNF-a and IL-10 Serum Levels in COVID-19 Patients and Their Relation to Disease Severity. Med. J. Cairo Univ. 2022, 90, 1459–1467. [Google Scholar] [CrossRef]
- Guimarães, E.S.; Martins, J.M.; Gomes, M.T.R.; Cerqueira, D.M.; Oliveira, S.C. Lack of Interleukin-6 Affects IFN-γ and TNF-α Production and Early In Vivo Control of Brucella abortus Infection. Pathogens 2020, 9, 1040. [Google Scholar] [CrossRef]
- Kho, K.; Nugroho, D.; Sugih, A.K. Preparation and Characterization of Highly Water Soluble Curcumin — Dextrose Cocrystal. J. Pure Appl. Chem. Res. 2018, 7, 140–148. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Wang, X.; Yin, T. Eutectic Solvents Based on Matrine and Fatty Acids as Solubility and Stability Enhancers of Curcumin. J. Phys. Chem. B 2025, 129, 990–997. [Google Scholar] [CrossRef]
- Pulla Reddy, A.C.; Sudharshan, E.; Appu Rao, A.G.; Lokesh, B.R. Interaction of curcumin with human serum albumin—A spectroscopic study. Lipids 1999, 34, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.; Kee, T.W. Effective stabilization of curcumin by association to plasma proteins: Human serum albumin and fibrinogen. Langmuir 2009, 25, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Alfke, J.; Esselen, M. Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants 2022, 11, 1746. [Google Scholar] [CrossRef]
- Schnur, S.; Hans, F.; Dehne, A.; Osti, J.; Schneemann, M.O.; Schneider, M.; Hittinger, M. The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 748. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Goud, V.V.; Das, C. Degradation kinetics of anthocyanins from purple rice bran and effect of hydrocolloids on its stability. J. Food Process Eng. 2020, 43, e13360. [Google Scholar] [CrossRef]
- Yu, Y.; Shiau, S.; Pan, W.; Yang, Y. Extraction of Bioactive Phenolics from Various Anthocyanin-Rich Plant Materials and Comparison of Their Heat Stability. Molecules 2024, 29, 5256. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Z.; Liu, Y.; Cheong, K.L.; Teng, B.; Khan, B.M. Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri. Mar. Drugs 2020, 18, 429. [Google Scholar] [CrossRef]
- Perera, N.; Yang, F.L.; Lu, Y.T.; Li, L.H.; Hua, K.F.; Wu, S.H. Antrodia cinnamomea Galactomannan Elicits Immuno-stimulatory Activity Through Toll-like Receptor 4. Int. J. Biol. Sci. 2018, 14, 1378–1388. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, X.; Guo, H.; Meng, Y.; Wang, Y.; Zhou, Y.; Sun, L. Structural analysis and macrophage activation of a novel β-glucan isolated from Cantharellus cibarius. Int. J. Mol. Med. 2021, 47, 50. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Lu, M.; Lu, M.Y.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Correlations of Molecular Weights of β-Glucans from Qingke (Tibetan Hulless Barley) to Their Multiple Bioactivities. Molecules 2018, 23, 1710. [Google Scholar] [CrossRef]
- Phillips, T.A.; Caprettini, V.; Aggarwal, N.; Marcotti, S.; Tetley, R.; Mao, Y.; Shaw, T.; Chiappini, C.; Parsons, M.; Cox, S. A method for reproducible high-resolution imaging of 3D cancer cell spheroids. J. Microsc. 2023, 291, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, C.; Palmieri, V.; Di Santo, R.; Tornillo, G.; Papi, M.; Pilkington, G.; De Spirito, M.; Gumbleton, M. INSIDIA: A FIJI Macro Delivering High-Throughput and High-Content Spheroid Invasion Analysis. Biotechnol. J. 2017, 12, 1700140. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, D.; Heiles, S.; Bhandari, D.R.; Petschenka, G.; Spengler, B. Molecular Networking and On-Tissue Chemical Derivatization for Enhanced Identification and Visualization of Steroid Glycosides by MALDI Mass Spectrometry Imaging. Anal. Chem. 2022, 94, 15971–15979. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Rahman, M.M.; Sakamoto, T.; Islam, A.; Oyama, S.; Nabi, M.M.; Sato, T.; Kahyo, T.; Takahashi, Y.; Setou, M. Detection of Distinct Distributions of Acetaminophen and Acetaminophen-Cysteine in Kidneys up to 10 μm Resolution and Identification of a Novel Acetaminophen Metabolite Using an AP-MALDI Imaging Mass Microscope. J. Am. Soc. Mass. Spectrom. 2023, 34, 1491–1500. [Google Scholar] [CrossRef]
- Wang, Y.; Hummon, A.B. Quantification of Irinotecan in Single Spheroids Using Internal Standards by MALDI Mass Spectrometry Imaging. Anal. Chem. 2023, 95, 9227–9236. [Google Scholar] [CrossRef]
- Bassler, M.C.; Rammler, T.; Wackenhut, F.; Zur Oven-Krockhaus, S.; Secic, I.; Ritz, R.; Meixner, A.J.; Brecht, M. Accumulation and penetration behavior of hypericin in glioma tumor spheroids studied by fluorescence microscopy and confocal fluorescence lifetime imaging microscopy. Anal. Bioanal. Chem. 2022, 414, 4849–4860. [Google Scholar] [CrossRef]
- Klowss, J.J.; Browning, A.P.; Murphy, R.J.; Carr, E.J.; Plank, M.J.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. A stochastic mathematical model of 4D tumour spheroids with real-time fluorescent cell cycle labelling. J. R. Soc. Interface 2022, 19, 20210903. [Google Scholar] [CrossRef]
- Lee, S.; Shanti, A. Effect of Exogenous pH on Cell Growth of Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9910. [Google Scholar] [CrossRef]
- Lim, H.; Albatany, M.; Martínez-Santiesteban, F.; Bartha, R.; Scholl, T.J. Longitudinal Measurements of Intra- and Extracellular pH Gradient in a Rat Model of Glioma. Tomography 2018, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Akhurst, T.; Lee, F.T.; Ciprotti, M.; Davis, I.D.; Weickhardt, A.J.; Gan, H.K.; Hicks, R.J.; Lee, S.T.; Kocovski, P.; et al. First clinical study of a pegylated diabody (124)I-labeled PEG-AVP0458 in patients with tumor-associated glycoprotein 72 positive cancers. Theranostics 2020, 10, 11404–11415. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Kheiri, S.; Wang, Y.; Xu, F.; Cruickshank, J.; Topolskaia, V.; Tao, H.; Young, E.W.K.; McGuigan, A.P.; Cescon, D.W.; et al. Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies. Adv. Healthc. Mater. 2022, 11, e2101085. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.; Costa, E.C.; Rodrigues, C.F.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Influence of Clear(T) and Clear(T2) Agitation Conditions in the Fluorescence Imaging of 3D Spheroids. Int. J. Mol. Sci. 2020, 22, 266. [Google Scholar] [CrossRef]
- Avrashami, M.; Niezni, D.; Meron Azagury, D.; Sason, H.; Shamay, Y. Green/red fluorescent protein disrupting drugs for real-time permeability tracking in three-dimensional tumor spheroids. Bioeng. Transl. Med. 2025, 10, e10731. [Google Scholar] [CrossRef]
- Kudláčová, J.; Kotrchová, L.; Kostka, L.; Randárová, E.; Filipová, M.; Janoušková, O.; Fang, J.; Etrych, T. Structure-to-Efficacy Relationship of HPMA-Based Nanomedicines: The Tumor Spheroid Penetration Study. Pharmaceutics 2020, 12, 1242. [Google Scholar] [CrossRef]
- Marzagalli, M.; Pelizzoni, G.; Fedi, A.; Vitale, C.; Fontana, F.; Bruno, S.; Poggi, A.; Dondero, A.; Aiello, M.; Castriconi, R.; et al. A multi-organ-on-chip to recapitulate the infiltration and the cytotoxic activity of circulating NK cells in 3D matrix-based tumor model. Front. Bioeng. Biotechnol. 2022, 10, 945149. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Debruyne, A.C.; Okkelman, I.A.; Heymans, N.; Pinheiro, C.; Hendrix, A.; Nobis, M.; Borisov, S.M.; Dmitriev, R.I. Live Microscopy of Multicellular Spheroids with the Multimodal Near-Infrared Nanoparticles Reveals Differences in Oxygenation Gradients. ACS Nano 2024, 18, 12168–12186. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Wang, G.; Zhuang, Y. Leveraging and manufacturing in vitro multicellular spheroid-based tumor cell model as a preclinical tool for translating dysregulated tumor metabolism into clinical targets and biomarkers. Bioresour. Bioprocess. 2020, 7, 35. [Google Scholar] [CrossRef]
- Ruan, G.; Ye, L.; Ke, J.; Lin, H.; Wu, M.; Liu, Z.; Fang, Y.; Zhang, S.; Wang, H.; Liu, Y.; et al. All-In-One Gadolinium-Doxorubicin Nanoassemblies for Spatial Delivery and Chemoresistance Reversal in Tumor Microenvironments. ACS Appl. Mater. Interfaces 2025, 17, 19348–19366. [Google Scholar] [CrossRef]
- Ustun, M.; Rahmani Dabbagh, S.; Ilci, I.S.; Bagci-Onder, T.; Tasoglu, S. Glioma-on-a-Chip Models. Micromachines 2021, 12, 490. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Røsland, G.V.; Tronstad, K.J.; Søreide, K.; Hagland, H.R. Metabolic flux analysis of 3D spheroids reveals significant differences in glucose metabolism from matched 2D cultures of colorectal cancer and pancreatic ductal adenocarcinoma cell lines. Cancer Metab. 2022, 10, 9. [Google Scholar] [CrossRef]
- Ai, S.; Li, H.; Zheng, H.; Liu, J.; Gao, J.; Liu, J.; Chen, Q.; Yang, Z. A SupraGel for efficient production of cell spheroids. Sci. China Mater. 2022, 65, 1655–1661. [Google Scholar] [CrossRef]
- Chin, I.L.; Amos, S.E.; Jeong, J.H.; Hool, L.; Hwang, Y.; Choi, Y.S. Volume adaptation of neonatal cardiomyocyte spheroids in 3D stiffness gradient GelMA. J. Biomed. Mater. Res. A 2023, 111, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Vahala, D.; Amos, S.E.; Sacchi, M.; Soliman, B.G.; Hepburn, M.S.; Mowla, A.; Li, J.; Jeong, J.H.; Astell, C.; Hwang, Y.; et al. 3D Volumetric Mechanosensation of MCF7 Breast Cancer Spheroids in a Linear Stiffness Gradient GelAGE. Adv. Healthc. Mater. 2023, 12, e2301506. [Google Scholar] [CrossRef] [PubMed]
- Ruske, L.J.; Yeomans, J.M. Activity-driven tissue alignment in proliferating spheroids. Soft Matter 2023, 19, 921–931. [Google Scholar] [CrossRef]
- Ezquerra, S.; Zuleta, A.; Arancibia, R.; Estay, J.; Aulestia, F.; Carrion, F. Functional Properties of Human-Derived Mesenchymal Stem Cell Spheroids: A Meta-Analysis and Systematic Review. Stem Cells Int. 2021, 2021, 8825332. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, M.; He, T.; Chen, N.; Rao, L.; Chen, L.; Zhang, Y.; Yang, Y.; Yuan, Q. Quantitatively Lighting up the Spatial Organization of CD47/SIRPα Immune Checkpoints on the Cellular Membrane with Single-Molecule Localization Microscopy. ACS Nano 2023, 17, 21626–21638. [Google Scholar] [CrossRef]
- Sakshaug, B.C.; Folkesson, E.; Haukaas, T.H.; Visnes, T.; Flobak, Å. Systematic review: Predictive value of organoids in colorectal cancer. Sci. Rep. 2023, 13, 18124. [Google Scholar] [CrossRef]
- Smabers, L.P.; Wensink, E.; Verissimo, C.S.; Koedoot, E.; Pitsa, K.C.; Huismans, M.A.; Higuera Barón, C.; Doorn, M.; Valkenburg-van Iersel, L.B.; Cirkel, G.A.; et al. Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: Drug screen optimization and correlation with patient response. J. Exp. Clin. Cancer Res. 2024, 43, 61. [Google Scholar] [CrossRef]
- Senkowski, W.; Gall-Mas, L.; Falco, M.M.; Li, Y.; Lavikka, K.; Kriegbaum, M.C.; Oikkonen, J.; Bulanova, D.; Pietras, E.J.; Voßgröne, K.; et al. A platform for efficient establishment and drug-response profiling of high-grade serous ovarian cancer organoids. Dev. Cell 2023, 58, 1106–1121.e1107. [Google Scholar] [CrossRef]
- Yalcin, G.D.; Yilmaz, K.C.; Dilber, T.; Acar, A. Investigation of evolutionary dynamics for drug resistance in 3D spheroid model system using cellular barcoding technology. PLoS ONE 2023, 18, e0291942. [Google Scholar] [CrossRef]
- Kong, J.; Lee, H.; Kim, D.; Han, S.K.; Ha, D.; Shin, K.; Kim, S. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat. Commun. 2020, 11, 5485. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Schipper, L.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van de Haar, J.; Prevoo, W.; van Werkhoven, E.; Snaebjornsson, P.; et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open 2021, 6, 100103. [Google Scholar] [CrossRef]
- Verduin, M.; Hoeben, A.; De Ruysscher, D.; Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol. 2021, 11, 641980. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.M.; Lim, S.; Lee, J.Y.; Choi, S.J.; Yang, S.D.; Yun, M.R.; Kim, C.G.; Gu, S.R.; Park, C.; et al. Modeling Clinical Responses to Targeted Therapies by Patient-Derived Organoids of Advanced Lung Adenocarcinoma. Clin. Cancer Res. 2021, 27, 4397–4409. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Lee, S.; Kwon, D.; Oh, S.; Park, C.; Jeon, S.; Lee, J.H.; Kim, T.S.; Oh, I.U. Essential Guidelines for Manufacturing and Application of Organoids. Int. J. Stem Cells 2024, 17, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, W.; Zhang, Y.; Chen, Y.; Shan, C.; Li, J.; Jia, Y.; Li, C.; Du, C.; Cai, Y.; et al. Establishment of patient-derived organoids for guiding personalized therapies in breast cancer patients. Int. J. Cancer 2024, 155, 324–338. [Google Scholar] [CrossRef]
- He, X.; Jiang, Y.; Zhang, L.; Li, Y.; Hu, X.; Hua, G.; Cai, S.; Mo, S.; Peng, J. Patient-derived organoids as a platform for drug screening in metastatic colorectal cancer. Front. Bioeng. Biotechnol. 2023, 11, 1190637. [Google Scholar] [CrossRef]
- Sarra, R.; Dinar, A.; Mohammed, M. Enhanced accuracy for heart disease prediction using artificial neural network. Indones. J. Electr. Eng. Comput. Sci. 2023, 29, 375–383. [Google Scholar] [CrossRef]
- Yildirim, Z.; Swanson, K.; Wu, X.; Zou, J.; Wu, J. Next-Gen Therapeutics: Pioneering Drug Discovery with iPSCs, Genomics, AI, and Clinical Trials in a Dish. Annu. Rev. Pharmacol. Toxicol. 2025, 65, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Eilenberger, C.; Rothbauer, M.; Selinger, F.; Gerhartl, A.; Jordan, C.; Harasek, M.; Schädl, B.; Grillari, J.; Weghuber, J.; Neuhaus, W.; et al. A Microfluidic Multisize Spheroid Array for Multiparametric Screening of Anticancer Drugs and Blood-Brain Barrier Transport Properties. Adv. Sci. 2021, 8, e2004856. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Prokhorova, A.V.; Mezentsev, A.V.; Shen, N.; Trofimenko, A.V.; Filkov, G.I.; Sulimanov, R.A.; Makarov, V.A.; Durymanov, M.O. Comparison of EMT-Related and Multi-Drug Resistant Gene Expression, Extracellular Matrix Production, and Drug Sensitivity in NSCLC Spheroids Generated by Scaffold-Free and Scaffold-Based Methods. Int. J. Mol. Sci. 2022, 23, 13306. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Yu, L.; Wang, J.; Meng, Q.; Mei, H.; Cai, Z.; Chen, W.; Huang, W. Patient-derived renal cell carcinoma organoids for personalized cancer therapy. Clin. Transl. Med. 2022, 12, e970. [Google Scholar] [CrossRef]
- Lim, G.H.; An, J.H.; Park, S.M.; Youn, G.H.; Oh, Y.I.; Seo, K.W.; Youn, H.Y. Macrophage induces anti-cancer drug resistance in canine mammary gland tumor spheroid. Sci. Rep. 2023, 13, 10394. [Google Scholar] [CrossRef]
- Gong, M.; Meng, H.; Tan, D.; Li, P.; Qin, J.; An, Q.; Shi, C.; An, J. Establishment of organoid models for pancreatic ductal adenocarcinoma and screening of individualized therapy strategy. Animal Model. Exp. Med. 2023, 6, 409–418. [Google Scholar] [CrossRef]
- Hu, Y.; Miao, Y.; Zhang, Y.; Wang, X.; Liu, X.; Zhang, W.; Deng, D. Co-Assembled Binary Polyphenol Natural Products for the Prevention and Treatment of Radiation-Induced Skin Injury. ACS Nano 2024, 18, 27557–27569. [Google Scholar] [CrossRef]
- Blatchley, M.R.; Günay, K.A.; Yavitt, F.M.; Hawat, E.M.; Dempsey, P.J.; Anseth, K.S. In Situ Super-Resolution Imaging of Organoids and Extracellular Matrix Interactions via Phototransfer by Allyl Sulfide Exchange-Expansion Microscopy (PhASE-ExM). Adv. Mater. 2022, 34, e2109252. [Google Scholar] [CrossRef]
- Lumibao, J.C.; Okhovat, S.R.; Peck, K.L.; Lin, X.; Lande, K.; Yomtoubian, S.; Ng, I.; Tiriac, H.; Lowy, A.M.; Zou, J.; et al. The effect of extracellular matrix on the precision medicine utility of pancreatic cancer patient-derived organoids. JCI Insight 2024, 9, e172419. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeong, S.Y.; Li, Z.; Kim, H.Y.; Kim, H.W.; Yoo, M.J.; Jang, H.J.; Kim, D.K.; Cho, N.; Yoo, H.M.; et al. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhao, H.; Zhang, W.; Wang, J.; Liu, Y.; Cao, Y.; Zheng, H.; Hu, Z.; Wang, S.; Zhu, Y.; et al. An Automated Organoid Platform with Inter-organoid Homogeneity and Inter-patient Heterogeneity. Cell Rep. Med. 2020, 1, 100161. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Cha, E.; Kwak, S.M.; Seo, S.J.; Rim, J.H.; Jin, Y. Harnessing Decellularized Extracellular Matrix for Enhanced Fidelity in Colorectal Cancer Organoid and Cell-Derived Xenograft Models. J. Microbiol. Biotechnol. 2024, 34, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Bhosle, S.; Gonçalves, E.; Li, X.; McClurg, D.P.; Barthorpe, S.; Beck, A.; Hall, C.; Lightfoot, H.; Farrow, L.; et al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. Sci. Rep. 2022, 12, 5571. [Google Scholar] [CrossRef]
- LeSavage, B.L.; Zhang, D.; Huerta-López, C.; Gilchrist, A.E.; Krajina, B.A.; Karlsson, K.; Smith, A.R.; Karagyozova, K.; Klett, K.C.; Huang, M.S.; et al. Engineered matrices reveal stiffness-mediated chemoresistance in patient-derived pancreatic cancer organoids. Nat. Mater. 2024, 23, 1138–1149. [Google Scholar] [CrossRef]
- Kim, S.; Choung, S.; Sun, R.X.; Ung, N.; Hashemi, N.; Fong, E.J.; Lau, R.; Spiller, E.; Gasho, J.; Foo, J.; et al. Comparison of Cell and Organoid-Level Analysis of Patient-Derived 3D Organoids to Evaluate Tumor Cell Growth Dynamics and Drug Response. SLAS Discov. 2020, 25, 744–754. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.X.; Zhu, Y.J.; Fu, J.; Zhao, X.F.; Zhang, Y.N.; Wang, S.; Wu, J.M.; Wang, K.T.; Wu, R.; et al. Single-Cell Transcriptome Analysis Uncovers Intratumoral Heterogeneity and Underlying Mechanisms for Drug Resistance in Hepatobiliary Tumor Organoids. Adv. Sci. 2021, 8, e2003897. [Google Scholar] [CrossRef]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A novel modality in disease modeling. Biodes Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Chen, B.; Slocombe, R.F.; Georgy, S.R. Advances in organoid technology for veterinary disease modeling. Front. Vet. Sci. 2023, 10, 1234628. [Google Scholar] [CrossRef]
- Zhao, H.; Ho, V.W.S.; Liu, K.; Chen, X.; Wu, H.; Chiu, P.K.; Chan, L.Y.; Yuen, S.K.; Leung, D.K.; Liu, A.Q.; et al. Organoid models in bladder cancer: From bench to bedside? Bladder Cancer 2025, 11, 23523735251330404. [Google Scholar] [CrossRef]
- Muthuswamy, S.K. Organoid Models of Cancer Explode with Possibilities. Cell Stem Cell 2018, 22, 290–291. [Google Scholar] [CrossRef]
- Chen, J.; Na, F. Organoid technology and applications in lung diseases: Models, mechanism research and therapy opportunities. Front. Bioeng. Biotechnol. 2022, 10, 1066869. [Google Scholar] [CrossRef]
- Schaub, J.; Zielesny, A.; Steinbeck, C.; Sorokina, M. Description and Analysis of Glycosidic Residues in the Largest Open Natural Products Database. Biomolecules 2021, 11, 486. [Google Scholar] [CrossRef]
- Fakhri, N.; Khalili, A.; Sachlos, T.; Rezai, P. Fabrication of Porous Collagen Scaffolds Containing Embedded Channels with Collagen Membrane Linings. Micromachines 2024, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Donoso, M.; Rico-Llanos, G.A.; Garzón-Alvarado, D.A.; Becerra, J.; Visser, R.; Fontanilla, M.R. The Effect of Pore Directionality of Collagen Scaffolds on Cell Differentiation and In Vivo Osteogenesis. Polymers 2021, 13, 3187. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Majhi, P.S.; Pramanik, K. Development of three-dimensional printed microfibrous structures using sodium alginate/silk fibroin bioink for tissue engineering application. Mater. Werkst. 2023, 54, 1542–1553. [Google Scholar] [CrossRef]
- Barlian, A.; Judawisastra, H.; Ridwan, A.; Wahyuni, A.R.; Lingga, M.E. Chondrogenic differentiation of Wharton’s Jelly mesenchymal stem cells on silk spidroin-fibroin mix scaffold supplemented with L-ascorbic acid and platelet rich plasma. Sci. Rep. 2020, 10, 19449. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Shahzadi, I.; Gul, W. Absorption and Bioavailability of Novel UltraShear Nanoemulsion of Cannabidiol in Rats. Med. Cannabis Cannabinoids 2023, 6, 148–159. [Google Scholar] [CrossRef]
- Kang, T.; Park, C.; Meghani, N.; Tran, T.T.D.; Tran, P.H.L.; Lee, B.J. Shear Stress-Dependent Targeting Efficiency Using Self-Assembled Gelatin-Oleic Nanoparticles in a Biomimetic Microfluidic System. Pharmaceutics 2020, 12, 555. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, F.; Zhao, Y.C.; Cho, A.N.; Fang, G.; Cox, C.D.; Zreiqat, H.; Lu, Z.F.; Lu, H.; Ju, L.A. 3D spheroid-microvasculature-on-a-chip for tumor-endothelium mechanobiology interplay. Biomed. Mater. 2023, 18, 055008. [Google Scholar] [CrossRef]
- Coombes, Z.; Plant, K.; Freire, C.; Basit, A.W.; Butler, P.; Conlan, R.S.; Gonzalez, D. Progesterone Metabolism by Human and Rat Hepatic and Intestinal Tissue. Pharmaceutics 2021, 13, 1707. [Google Scholar] [CrossRef]
- Myszczyszyn, A.; Muench, A.; Lehmann, V.; Sinnige, T.; van Steenbeek, F.G.; Bouwmeester, M.; Samsom, R.-A.; Keuper-Navis, M.; van der Made, T.K.; Kogan, D.; et al. A hollow fiber membrane-based liver organoid-on-a-chip model for examining drug metabolism and transport. Biofabrication 2025, 17, 025035. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; Carvalho, V.; Rodrigues, R.O.; Pinho, D.; Teixeira, S.; Moita, A.; Hori, T.; Kaji, H.; Lima, R.; Minas, G. Organ-on-a-Chip Platforms for Drug Screening and Delivery in Tumor Cells: A Systematic Review. Cancers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, M.; Aina, K.O.; Grandmougin, L.; Jäger, C.; Pérez Escriva, P.; Letellier, E.; Mosig, A.S.; Wilmes, P. An Organ-on-Chip Platform for Simulating Drug Metabolism Along the Gut-Liver Axis. Adv. Healthc. Mater. 2024, 13, e2303943. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Delgado, A.; Jeong, S. Organ-on-a-Chip: The Future of Therapeutic Aptamer Research? BioChip J. 2021, 15, 109–122. [Google Scholar] [CrossRef]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.J.; Joshipura, S.R.; et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; González-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Ying-Jin, S.; Yuste, I.; González-Burgos, E.; Serrano, D.R. Fabrication of organ-on-a-chip using microfluidics. Bioprinting 2025, 46, e00394. [Google Scholar] [CrossRef]
- Wang, H.F.; Liu, Y.; Wang, T.; Yang, G.; Zeng, B.; Zhao, C.X. Tumor-Microenvironment-on-a-Chip for Evaluating Nanoparticle-Loaded Macrophages for Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 5040–5050. [Google Scholar] [CrossRef]
- Pei, S.; Dou, Y.; Zhang, W.; Qi, D.; Li, Y.; Wang, M.; Li, W.; Shi, H.; Gao, Z.; Yao, C.; et al. O-Sulfation disposition of curcumin and quercetin in SULT1A3 overexpressing HEK293 cells: The role of arylsulfatase B in cellular O-sulfation regulated by transporters. Food Funct. 2022, 13, 10558–10573. [Google Scholar] [CrossRef]
- Runge-Morris, M.; Kocarek, T.A. Regulation of sulfotransferases by xenobiotic receptors. Curr. Drug Metab. 2005, 6, 299–307. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, F.; Hansen, N.; Theek, B.; Daware, R.; Motta, A.; Breuel, S.; Nasehi, R.; Baumeister, J.; Schöneberg, J.; Stojanović, N.; et al. Engineering Mesoscopic 3D Tumor Models with a Self-Organizing Vascularized Matrix. Adv. Mater. 2024, 36, e2303196. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Oh, J.M.; Zhao, W.; Tran, M.; Shen, K. Engineering a Vascularized Hypoxic Tumor Model for Therapeutic Assessment. Cells 2021, 10, 2201. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jung, S.Y.; Kwon, Y.H.; Lee, J.H.; Lee, Y.M.; Lee, B.Y.; Kwon, S.M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol. Ther. 2012, 13, 504–515. [Google Scholar] [CrossRef]
- Sato, Y. Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy. Cancer Sci. 2011, 102, 1253–1256. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, Y.; Zou, L.; Chu, L.; Chu, X.; Ni, J.; Li, Y.; Guo, T.; Yang, X.; Zhu, Z. Low-dose Apatinib promotes vascular normalization and hypoxia reduction and sensitizes radiotherapy in lung cancer. Cancer Med. 2023, 12, 4434–4445. [Google Scholar] [CrossRef]
- Fan, P.; Qiang, H.; Liu, Z.; Zhao, Q.; Wang, Y.; Liu, T.; Wang, X.; Chu, T.; Huang, Y.; Xu, W.; et al. Effective low-dose Anlotinib induces long-term tumor vascular normalization and improves anti-PD-1 therapy. Front. Immunol. 2022, 13, 937924. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Mikelis, C.M. Nanoparticle Delivery and Tumor Vascular Normalization: The Chicken or The Egg? Front. Oncol. 2019, 9, 1227. [Google Scholar] [CrossRef]
- Agastin, S.; Giang, U.B.; Geng, Y.; Delouise, L.A.; King, M.R. Continuously perfused microbubble array for 3D tumor spheroid model. Biomicrofluidics 2011, 5, 24110. [Google Scholar] [CrossRef]
- Koo, D.J.; Choi, J.; Ahn, M.; Ahn, B.H.; Min, D.H.; Kim, S.Y. Large-Scale 3D Optical Mapping and Quantitative Analysis of Nanoparticle Distribution in Tumor Vascular Microenvironment. Bioconjug. Chem. 2020, 31, 1784–1794. [Google Scholar] [CrossRef]
- Phipps, C.; Kohandel, M. Mathematical model of the effect of interstitial fluid pressure on angiogenic behavior in solid tumors. Comput. Math. Methods Med. 2011, 2011, 843765. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, Y.; Galan, E.A.; Hao, L.; Zhao, H.; Tang, J.; Sang, G.; Wang, H.; Xu, B.; Ma, S. Vascularized Tumor Spheroid-on-a-Chip Model Verifies Synergistic Vasoprotective and Chemotherapeutic Effects. ACS Biomater. Sci. Eng. 2022, 8, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A., 3rd; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zong, W.; Li, R.; Zeng, Z.; Zhao, J.; Xi, P.; Chen, L.; Sun, Y. Two-photon light-sheet nanoscopy by fluorescence fluctuation correlation analysis. Nanoscale 2016, 8, 9982–9987. [Google Scholar] [CrossRef] [PubMed]
- Wehnekamp, F.; Plucińska, G.; Thong, R.; Misgeld, T.; Lamb, D.C. Nanoresolution real-time 3D orbital tracking for studying mitochondrial trafficking in vertebrate axons in vivo. eLife 2019, 8, e46059. [Google Scholar] [CrossRef]
- Wang, Z.; Natekar, P.; Tea, C.; Tamir, S.; Hakozaki, H.; Schöneberg, J. MitoTNT: Mitochondrial Temporal Network Tracking for 4D live-cell fluorescence microscopy data. PLoS Comput. Biol. 2023, 19, e1011060. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Scodeller, P.; Braun, G.B.; de Mendoza, T.H.; Yamazaki, C.M.; Kluger, M.D.; Kitayama, J.; Alvarez, E.; Howell, S.B.; Teesalu, T.; et al. A tumor-penetrating peptide enhances circulation-independent targeting of peritoneal carcinomatosis. J. Control. Release 2015, 212, 59–69. [Google Scholar] [CrossRef]
- Alberici, L.; Roth, L.; Sugahara, K.N.; Agemy, L.; Kotamraju, V.R.; Teesalu, T.; Bordignon, C.; Traversari, C.; Rizzardi, G.P.; Ruoslahti, E. De novo design of a tumor-penetrating peptide. Cancer Res. 2013, 73, 804–812. [Google Scholar] [CrossRef]
- Åkeson, M.; Brackmann, C.; Gustafsson, L.; Enejder, A. Chemical imaging of glucose by CARS microscopy. J. Raman Spectrosc. 2010, 41, 1638–1644. [Google Scholar] [CrossRef]
- Bergner, G.; Albert, C.R.; Schiller, M.; Bringmann, G.; Schirmeister, T.; Dietzek, B.; Niebling, S.; Schlücker, S.; Popp, J. Quantitative detection of C-deuterated drugs by CARS microscopy and Raman microspectroscopy. Analyst 2011, 136, 3686–3693. [Google Scholar] [CrossRef]
- Kang, E.; Wang, H.; Kwon, I.K.; Robinson, J.; Park, K.; Cheng, J.X. In situ visualization of paclitaxel distribution and release by coherent anti-Stokes Raman scattering microscopy. Anal. Chem. 2006, 78, 8036–8043. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, L.; Zhu, X.; Min, W. Extending the fundamental imaging-depth limit of multi-photon microscopy by imaging with photo-activatable fluorophores. Opt. Express 2012, 20, 18525–18536. [Google Scholar] [CrossRef]
- Rane, T.D.; Armani, A.M. Two-Photon Microscopy Analysis of Gold Nanoparticle Uptake in 3D Cell Spheroids. PLoS ONE 2016, 11, e0167548. [Google Scholar] [CrossRef]
- Yildirim, M.; Durr, N.; Ben-Yakar, A. Tripling the maximum imaging depth with third-harmonic generation microscopy. J. Biomed. Opt. 2015, 20, 096013. [Google Scholar] [CrossRef] [PubMed]
- Streich, L.; Boffi, J.C.; Wang, L.; Alhalaseh, K.; Barbieri, M.; Rehm, R.; Deivasigamani, S.; Gross, C.T.; Agarwal, A.; Prevedel, R. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat. Methods 2021, 18, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Huang, Y.; Wang, L.; Guo, Y.; Li, J.; Zhu, Y.; Yang, Z.; Qu, J. Aberration Correction to Optimize the Performance of Two-Photon Fluorescence Microscopy Using the Genetic Algorithm. Microsc. Microanal. 2022, 28, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, L.; Volgin, A.; Maxwell, D.; Ishihara, K.; Gelovani, J.; Schellingerhout, D. Optimizing imaging of three-dimensional multicellular tumor spheroids with fluorescent reporter proteins using confocal microscopy. Mol. Imaging 2008, 7, 214–221. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Xia, L.; Li, G.; Shui, L. Microfluidic Magnetic Analyte Delivery Technique for Separation, Enrichment, and Fluorescence Detection of Ultratrace Biomarkers. Anal. Chem. 2021, 93, 8273–8280. [Google Scholar] [CrossRef]
- He, Z.; Huffman, J.; Curtin, K.; Garner, K.L.; Bowdridge, E.C.; Li, X.; Nurkiewicz, T.R.; Li, P. Composable Microfluidic Plates (cPlate): A Simple and Scalable Fluid Manipulation System for Multiplexed Enzyme-Linked Immunosorbent Assay (ELISA). Anal. Chem. 2021, 93, 1489–1497. [Google Scholar] [CrossRef]
- Ngernsutivorakul, T.; Steyer, D.J.; Valenta, A.C.; Kennedy, R.T. In Vivo Chemical Monitoring at High Spatiotemporal Resolution Using Microfabricated Sampling Probes and Droplet-Based Microfluidics Coupled to Mass Spectrometry. Anal. Chem. 2018, 90, 10943–10950. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z.; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Fernández-Zapico, M.E.; Rumie Vittar, N.B.; Rivarola, V.A. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PLoS ONE 2017, 12, e0177801. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Abbadi, S.; Rampazzo, E.; Persano, L.; Della Puppa, A.; Frasson, C.; Sarto, E.; Scienza, R.; D’Avella, D.; Basso, G. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells 2010, 28, 851–862. [Google Scholar] [CrossRef]
- Mi, Y.; Mu, L.; Huang, K.; Hu, Y.; Yan, C.; Zhao, H.; Ma, C.; Li, X.; Tao, D.; Qin, J. Hypoxic colorectal cancer cells promote metastasis of normoxic cancer cells depending on IL-8/p65 signaling pathway. Cell Death Dis. 2020, 11, 610. [Google Scholar] [CrossRef]
- Ryan, D.; Sinha, A.; Bogan, D.; Davies, J.; Koziol, J.; ElShamy, W.M. A niche that triggers aggressiveness within BRCA1-IRIS overexpressing triple negative tumors is supported by reciprocal interactions with the microenvironment. Oncotarget 2017, 8, 103182–103206. [Google Scholar] [CrossRef]
- Kopf, A.; Fortuin, V.; Somnath, V.R.; Claassen, M. Mixture-of-Experts Variational Autoencoder for clustering and generating from similarity-based representations on single cell data. PLoS Comput. Biol. 2021, 17, e1009086. [Google Scholar] [CrossRef]
- Vivanco Gonzalez, N.; Oliveria, J.P.; Tebaykin, D.; Ivison, G.T.; Mukai, K.; Tsai, M.M.; Borges, L.; Nadeau, K.C.; Galli, S.J.; Tsai, A.G.; et al. Mass Cytometry Phenotyping of Human Granulocytes Reveals Novel Basophil Functional Heterogeneity. iScience 2020, 23, 101724. [Google Scholar] [CrossRef]
- Rizzo, R.; Onesto, V.; Morello, G.; Iuele, H.; Scalera, F.; Forciniti, S.; Gigli, G.; Polini, A.; Gervaso, F.; Del Mercato, L.L. pH-sensing hybrid hydrogels for non-invasive metabolism monitoring in tumor spheroids. Mater. Today Bio 2023, 20, 100655. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, Y.; Wang, J.; Wang, Y.; Zhao, J.; Wang, M.; Hu, W.; Zhao, J.; Lin, B.; Zhang, X.; et al. Exploring microfluidics-based organoid interactions through analysis of albumin secretion. Lab Chip 2025, 25, 487–499. [Google Scholar] [CrossRef]
- Piraino, F.; Costa, M.; Meyer, M.; Cornish, G.; Ceroni, C.; Garnier, V.; Hoehnel-Ka, S.; Brandenberg, N. Organoid models: The future companions of personalized drug development. Biofabrication 2024, 16, 032009. [Google Scholar] [CrossRef] [PubMed]
- Naveen Kumar, M.; Murugan, S.K.; Bethapudi, B.; Mundkinajeddu, D. Evaluation of Immunomodulatory Activity of a Polyherbal Formulation (Phytocee™) Using ex vivo and in vitro Models. Pharmacogn. Mag. 2025, 21, 67–73. [Google Scholar] [CrossRef]
- Vives, J.; Batlle-Morera, L. The challenge of developing human 3D organoids into medicines. Stem Cell. Res. Ther. 2020, 11, 72. [Google Scholar] [CrossRef]
- Kumar, S.; Maheshwari, D. Selection of different factors for the Calculation of Similarity factor (f2) and Dissimilarity factor (f1) as per different countries’ regulatory recommendations for the biowaiver Study: A Systematic Review. Res. J. Pharm. Technol. 2024, 17, 1414–1417. [Google Scholar] [CrossRef]
- Utama, R.H.; Atapattu, L.; O’Mahony, A.P.; Fife, C.M.; Baek, J.; Allard, T.; O’Mahony, K.J.; Ribeiro, J.C.C.; Gaus, K.; Kavallaris, M.; et al. A 3D Bioprinter Specifically Designed for the High-Throughput Production of Matrix-Embedded Multicellular Spheroids. iScience 2020, 23, 101621. [Google Scholar] [CrossRef]
- Pong, K.C.C.; Lai, Y.S.; Wong, R.C.H.; Lee, A.C.K.; Chow, S.C.T.; Lam, J.C.W.; Ho, H.P.; Wong, C.T.T. Automated Uniform Spheroid Generation Platform for High Throughput Drug Screening Process. Biosensors 2024, 14, 392. [Google Scholar] [CrossRef]
- Kang, S.; Chen, E.C.; Cifuentes, H.; Co, J.Y.; Cole, G.; Graham, J.; Hsia, R.; Kiyota, T.; Klein, J.A.; Kroll, K.T.; et al. Complexin vitromodels positioned for impact to drug testing in pharma: A review. Biofabrication 2024, 16, 042006. [Google Scholar] [CrossRef]
- Mohamad, A.; Kasiram, M.; Izani, N.; Sulain, M.; Wan-Nor-Amilah, W. Antioxidant, antimicrobial and immunomodulatory activities of facial serum formulation containing Melaleuca cajuputi essential oil. Malays. J. Microbiol. 2024, 20, 451–458. [Google Scholar] [CrossRef]
- Chun, S.; Nwakama, P.; Patel, D.; Ren, K.; Braddy, A. Reconciling sprinkle administration information in approved NDA labeling with sprinkle bioequivalence study recommendations in FDA product-specific guidances for generic drug development. Am. J. Pharmacother. Pharm. Sci. 2022, 1, 12. [Google Scholar] [CrossRef]
- Lu, W.; Luo, D.; Chen, D.; Zhang, S.; Chen, X.; Zhou, H.; Liu, Q.; Chen, S.; Liu, W. Systematic Study of Paeonol/Madecassoside Co-Delivery Nanoemulsion Transdermal Delivery System for Enhancing Barrier Repair and Anti-Inflammatory Efficacy. Molecules 2023, 28, 5275. [Google Scholar] [CrossRef]
- Shakeel, D.; Mir, S.A. Personalized drug concentration predictions with machine learning: An exploratory study. Int. J. Basic. Clin. Pharmacol. 2020, 9, 980–984. [Google Scholar] [CrossRef]
- Yakavets, I.; Francois, A.; Lamy, L.; Piffoux, M.; Gazeau, F.; Wilhelm, C.; Zorin, V.; Silva, A.K.A.; Bezdetnaya, L. Effect of stroma on the behavior of temoporfin-loaded lipid nanovesicles inside the stroma-rich head and neck carcinoma spheroids. J. Nanobiotechnology 2021, 19, 3. [Google Scholar] [CrossRef]
- Li, B.; Jiao, S.; Guo, S.; Xiao, T.; Zeng, Y.; Hu, Y.; Li, X.; Xiong, S.; Xu, Y. Deep eutectic solvent self-assembled reverse nanomicelles for transdermal delivery of sparingly soluble drugs. J. Nanobiotechnol. 2024, 22, 272. [Google Scholar] [CrossRef]
- Bello, M.G.; Yang, Y.; Wang, C.; Wu, L.; Zhou, P.; Ding, H.; Ge, X.; Guo, T.; Wei, L.; Zhang, J. Facile Synthesis and Size Control of 2D Cyclodextrin-Based Metal–Organic Frameworks Nanosheet for Topical Drug Delivery. Part. Part. Syst. Charact. 2020, 37, 2000147. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, H.; Yang, Y.; Hao, H. Research on the optimization model of anti-breast cancer candidate drugs based on machine learning. Front. Genet. 2025, 16, 1523015. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Afkanpour, M.; Rakhshani, S.; Mehrabian, A.; Tabesh, H. A prediction model based on artificial intelligence techniques for disintegration time and hardness of fast disintegrating tablets in pre-formulation tests. BMC Med. Inform. Decis. Mak. 2024, 24, 88. [Google Scholar] [CrossRef] [PubMed]
- Schurr, J.; Janout, H.; Haghofer, A.; Fürsatz, M.; Scharinger, J.; Winkler, S.; Nürnberger, S. Evolutionary Grid Optimization and Deep Learning for Improved In Vitro Cellular Spheroid Localization. Appl. Sci. 2024, 14, 9476. [Google Scholar] [CrossRef]
- An, H.J.; Kim, H.S.; Kwon, J.A.; Song, J.; Choi, I. Adjustable and Versatile 3D Tumor Spheroid Culture Platform with Interfacial Elastomeric Wells. ACS Appl. Mater. Interfaces 2020, 12, 6924–6932. [Google Scholar] [CrossRef]
- Osaki, K.; Ekimoto, T.; Yamane, T.; Ikeguchi, M. 3D-RISM-AI: A Machine Learning Approach to Predict Protein-Ligand Binding Affinity Using 3D-RISM. J. Phys. Chem. B 2022, 126, 6148–6158. [Google Scholar] [CrossRef]
- Forsgren, E.; Edlund, C.; Oliver, M.; Barnes, K.; Sjögren, R.; Jackson, T.R. High-throughput widefield fluorescence imaging of 3D samples using deep learning for 2D projection image restoration. PLoS ONE 2022, 17, e0264241. [Google Scholar] [CrossRef]
- Qiang, B.; Lai, J.; Jin, H.; Zhang, L.; Liu, Z. Target Prediction Model for Natural Products Using Transfer Learning. Int. J. Mol. Sci. 2021, 22, 4632. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, Y.; Gao, W.; Yang, S.; Zhu, W.; Ji, X.; Zhai, X.; Liu, W.Q.; Luo, Y.; Ling, S.; et al. AI-driven high-throughput droplet screening of cell-free gene expression. Nat. Commun. 2025, 16, 2720. [Google Scholar] [CrossRef]
- Yan, B.; Ye, X.; Wang, J.; Han, J.; Wu, L.; He, S.; Liu, K.; Bo, X. An Algorithm Framework for Drug-Induced Liver Injury Prediction Based on Genetic Algorithm and Ensemble Learning. Molecules 2022, 27, 3112. [Google Scholar] [CrossRef]
- Ege, D.; Sertturk, S.; Acarkan, B.; Ademoglu, A. Machine learning models to predict the relationship between printing parameters and tensile strength of 3D Poly (lactic acid) scaffolds for tissue engineering applications. Biomed. Phys. Eng. Express 2023, 9, 065014. [Google Scholar] [CrossRef]
- Giles, J.; Ang, K.K.; Phua, K.S.; Arvaneh, M. A Transfer Learning Algorithm to Reduce Brain-Computer Interface Calibration Time for Long-Term Users. Front. Neuroergon 2022, 3, 837307. [Google Scholar] [CrossRef] [PubMed]
- Zadoo, N.; Tak, N.; Reddy, A.J.; Patel, R. Enhancing Pediatric Bone Age Assessment Using Artificial Intelligence: Implications for Orthopedic Surgery. Cureus 2025, 17, e79507. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; Kim, S.; Bareja, R.; Fang, Z.; Cai, S.; Pan, H.; Asad, M.; Martin, M.L.; Sigouros, M.; Rowdo, F.M.; et al. Extracellular Matrix in Synthetic Hydrogel-Based Prostate Cancer Organoids Regulate Therapeutic Response to EZH2 and DRD2 Inhibitors. Adv. Mater. 2022, 34, e2100096. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhang, H.; Zhang, H.; Yi, Z.; Zhang, Q.; Liu, Q.; Liu, X. Multi-Omics Analysis Reveals Intratumor Microbes as Immunomodulators in Colorectal Cancer. Microbiol. Spectr. 2023, 11, e0503822. [Google Scholar] [CrossRef]
- Zhou, Z.; Van der Jeught, K.; Li, Y.; Sharma, S.; Yu, T.; Moulana, I.; Liu, S.; Wan, J.; Territo, P.R.; Opyrchal, M.; et al. A T Cell-Engaging Tumor Organoid Platform for Pancreatic Cancer Immunotherapy. Adv. Sci. 2023, 10, e2300548. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Li, Y.; Sun, T.; Liu, C.; Dong, C.; Zhao, T.; Tang, D.; Chen, X.; Chen, X.; et al. Grouped-seq for integrated phenotypic and transcriptomic screening of patient-derived tumor organoids. Nucleic Acids Res. 2022, 50, e28. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tu, Y.; Yao, W.; Zong, Q.; Xiao, X.; Yang, R.M.; Jiang, X.Q.; Yuan, Y. Size-Switchable Nanoparticles with Self-Destructive and Tumor Penetration Characteristics for Site-Specific Phototherapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 6933–6943. [Google Scholar] [CrossRef]
- Marcianes, P.; Negro, S.; Barcia, E.; Montejo, C.; Fernández-Carballido, A. Potential Active Targeting of Gatifloxacin to Macrophages by Means of Surface-Modified PLGA Microparticles Destined to Treat Tuberculosis. AAPS PharmSciTech 2019, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Panja, P.; Jana, N.R. Lipid-Raft-Mediated Direct Cytosolic Delivery of Polymer-Coated Soft Nanoparticles. J. Phys. Chem. B 2020, 124, 5323–5333. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Gudneppanavar, R.; Teegala, L.R.; Duah, E.; Thodeti, C.K.; Paruchuri, S. Leukotriene D4 Upregulates Oxidized Low-Density Lipoprotein Receptor 1 and CD36 to Enhance Oxidized LDL Uptake and Phagocytosis in Macrophages Through Cysteinyl Leukotriene Receptor 1. Front. Physiol. 2021, 12, 756450. [Google Scholar] [CrossRef]
- Sachdeva, K.; Goel, M.; Sundaramurthy, V. Heterogeneity in the endocytic capacity of individual macrophage in a population determines its subsequent phagocytosis, infectivity and subcellular trafficking. Traffic 2020, 21, 522–533. [Google Scholar] [CrossRef]
- Kudo, Y.; Kohi, S.; Hirata, K.; Goggins, M.; Sato, N. Hyaluronan activated-metabolism phenotype (HAMP) in pancreatic ductal adenocarcinoma. Oncotarget 2019, 10, 5592–5604. [Google Scholar] [CrossRef]
- Seki, T.; Saida, Y.; Kishimoto, S.; Lee, J.; Otowa, Y.; Yamamoto, K.; Chandramouli, G.V.; Devasahayam, N.; Mitchell, J.B.; Krishna, M.C.; et al. PEGPH20, a PEGylated human hyaluronidase, induces radiosensitization by reoxygenation in pancreatic cancer xenografts. A molecular imaging study. Neoplasia 2022, 30, 100793. [Google Scholar] [CrossRef]
- Zhao, C.; Thompson, B.J.; Chen, K.; Gao, F.; Blouw, B.; Marella, M.; Zimmerman, S.; Kimbler, T.; Garrovillo, S.; Bahn, J.; et al. The growth of a xenograft breast cancer tumor model with engineered hyaluronan-accumulating stroma is dependent on hyaluronan and independent of CD44. Oncotarget 2019, 10, 6561–6576. [Google Scholar] [CrossRef]
- Irie, F.; Tobisawa, Y.; Murao, A.; Yamamoto, H.; Ohyama, C.; Yamaguchi, Y. The cell surface hyaluronidase TMEM2 regulates cell adhesion and migration via degradation of hyaluronan at focal adhesion sites. J. Biol. Chem. 2021, 296, 100481. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Zhu, L.; Huang, M.; Xie, Y.; Song, X.; Chen, Z.; Lau, H.C.; Sung, J.J.; Xu, L.; et al. Personalized drug screening using patient-derived organoid and its clinical relevance in gastric cancer. Cell Rep. Med. 2024, 5, 101627. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Cai, B.; Li, X.; Zou, D.; Lei, S.; Xu, L.; Wang, G.; Wang, L.; Wang, Z. A Tumor-Organoid-Based Precision Medicine Platform for the Prediction of Drug Sensitivity of Colorectal Cancer. Adv. Therap. 2022, 5, 2200093. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Kohl, E.; Bade, J.; Eng, S.E.; Tosevska, A.; Al Shihabi, A.; Tebon, P.J.; Hong, J.J.; Dry, S.; Boutros, P.C.; et al. A platform for rapid patient-derived cutaneous neurofibroma organoid establishment and screening. Cell Rep. Methods 2024, 4, 100772. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.Q.; Grein, J.; Selman, M.; Annamalai, L.; Yearley, J.H.; Blumenschein, W.M.; Sadekova, S.; Chackerian, A.A.; Phan, U.; Wong, J.C. Oncolytic virus V937 in combination with PD-1 blockade therapy to target immunologically quiescent liver and colorectal cancer. Mol. Ther. Oncol. 2024, 32, 200807. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Pasquini, G.; Gerkau, N.J.; Jadhav, V.; Vinchure, O.S.; Altinisik, N.; Windoffer, H.; Muller, S.; Rothenaigner, I.; Lin, S.; et al. Reliability of high-quantity human brain organoids for modeling microcephaly, glioma invasion and drug screening. Nat. Commun. 2024, 15, 10703. [Google Scholar] [CrossRef]
- Sun, L.; Kang, X.; Ju, H.; Wang, C.; Yang, G.; Wang, R.; Sun, S. A human mucosal melanoma organoid platform for modeling tumor heterogeneity and exploring immunotherapy combination options. Sci. Adv. 2023, 9, eadg6686. [Google Scholar] [CrossRef]
- Zietek, T.; Giesbertz, P.; Ewers, M.; Reichart, F.; Weinmüller, M.; Urbauer, E.; Haller, D.; Demir, I.E.; Ceyhan, G.O.; Kessler, H.; et al. Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism—Update to the Human Model and Expansion of Applications. Front. Bioeng. Biotechnol. 2020, 8, 577656. [Google Scholar] [CrossRef]
- Peng, N.; Du, Y.; Yu, G.; Zhang, C.; Cai, Q.; Tang, H.; Liu, Y. Light-Activated Reactive Oxygen Species-Responsive Nanocarriers for Enhanced Photodynamic Immunotherapy of Cancer. Langmuir 2022, 38, 13139–13149. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, M.; Li, Y.; Ya, Z.; Yang, Y.; Yuan, Y.; Dong, W.; Guo, S.; Lu, S.; Zhang, L.; et al. Cascade-Amplifying Synergistic Therapy for Intracranial Glioma via Endogenous Reactive Oxygen Species-Triggered “All-in-One” Nanoplatform. Adv. Funct. Mater. 2021, 31, 2105786. [Google Scholar] [CrossRef]
- Leibovici, J.; Itzhaki, O.; Huszar, M.; Sinai, J. The tumor microenvironment: Part 1. Immunotherapy 2011, 3, 1367–1384. [Google Scholar] [CrossRef]
- Aredia, F.; Ivana, A. Multiple effects of intracellular pH modulation in cancer cells. Cancer Cell Microenv. 2014, 1, 72–79. [Google Scholar]
- Veloso, S.R.S.; Marta, E.S.; Rodrigues, P.V.; Moura, C.; Amorim, C.O.; Amaral, V.S.; Correa-Duarte, M.A.; Castanheira, E.M.S. Chitosan/Alginate Nanogels Containing Multicore Magnetic Nanoparticles for Delivery of Doxorubicin. Pharmaceutics 2023, 15, 2194. [Google Scholar] [CrossRef]
- Mo, Y.; Du, H.; Chen, B.; Liu, D.; Yin, Q.; Yan, Y.; Wang, Z.; Wan, F.; Qi, T.; Wang, Y.; et al. Quick-Responsive Polymer-Based Thermosensitive Liposomes for Controlled Doxorubicin Release and Chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2316–2329. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, J.; Feng, J.; Klocker, H.; Lee, C.; Zhang, J. Type IV collagenase (matrix metalloproteinase-2 and -9) in prostate cancer. Prostate Cancer Prostatic Dis. 2004, 7, 327–332. [Google Scholar] [CrossRef]
- Kim, T.D.; Song, K.S.; Li, G.; Choi, H.; Park, H.D.; Lim, K.; Hwang, B.D.; Yoon, W.H. Activity and expression of urokinase-type plasminogen activator and matrix metalloproteinases in human colorectal cancer. BMC Cancer 2006, 6, 211. [Google Scholar] [CrossRef][Green Version]
- Pham, W.; Choi, Y.; Weissleder, R.; Tung, C.H. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug. Chem. 2004, 15, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.; Ying, H.; Su, D.; Zhang, H.; Lu, G.; Chen, J. Extracellular Matrix Component Shelled Nanoparticles as Dual Enzyme-Responsive Drug Delivery Vehicles for Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Mao, Z.; Gao, C. ROS-Responsive Nanoparticles for Suppressing the Cytotoxicity and Immunogenicity Caused by PM2.5 Particulates. Biomacromolecules 2019, 20, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnology 2018, 16, 74. [Google Scholar] [CrossRef]
- Liang, B.; Zhou, D. ROS-Activated homodimeric podophyllotoxin nanomedicine with self-accelerating drug release for efficient cancer eradication. Drug Deliv. 2021, 28, 2361–2372. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Mazon, H.; Branlant, G.; Jacob, C.; Toledano, M.B.; Rahuel-Clermont, S. Evidence that glutathione and the glutathione system efficiently recycle 1-cys sulfiredoxin in vivo. Antioxid. Redox Signal. 2015, 22, 731–743. [Google Scholar] [CrossRef]
- Yang, H.; Shen, W.; Liu, W.; Chen, L.; Zhang, P.; Xiao, C.; Chen, X. PEGylated Poly(α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018, 19, 4492–4503. [Google Scholar] [CrossRef]
- Ou, Y.C.; Webb, J.A.; Faley, S.; Shae, D.; Talbert, E.M.; Lin, S.; Cutright, C.C.; Wilson, J.T.; Bellan, L.M.; Bardhan, R. Gold Nanoantenna-Mediated Photothermal Drug Delivery from Thermosensitive Liposomes in Breast Cancer. ACS Omega 2016, 1, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Machálková, M.; Pavlatovská, B.; Michálek, J.; Pruška, A.; Štěpka, K.; Nečasová, T.; Radaszkiewicz, K.A.; Kozubek, M.; Šmarda, J.; Preisler, J.; et al. Drug Penetration Analysis in 3D Cell Cultures Using Fiducial-Based Semiautomatic Coregistration of MALDI MSI and Immunofluorescence Images. Anal. Chem. 2019, 91, 13475–13484. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kulkarni, A.; Holbrook, J.H.; Dasanayake, G.S.; Hu, D.; Tanner, E.E.L.; Hummon, A.B.; Watkins, D.L. Uptake of Self-Assembling Amphiphilic Tryptophan-Based Polymer Nanoparticles in Three-Dimensional Cell Culture Models. ACS Appl. Nano Mater. 2024, 7, 21181–21190. [Google Scholar] [CrossRef]
- Tucker, L.H.; Conde-González, A.; Cobice, D.; Hamm, G.R.; Goodwin, R.J.A.; Campbell, C.J.; Clarke, D.J.; Mackay, C.L. MALDI Matrix Application Utilizing a Modified 3D Printer for Accessible High Resolution Mass Spectrometry Imaging. Anal. Chem. 2018, 90, 8742–8749. [Google Scholar] [CrossRef]
- Goodarzi, S.; Prunet, A.; Rossetti, F.; Bort, G.; Tillement, O.; Porcel, E.; Lacombe, S.; Wu, T.D.; Guerquin-Kern, J.L.; Delanoë-Ayari, H.; et al. Quantifying nanotherapeutic penetration using a hydrogel-based microsystem as a new 3D in vitro platform. Lab Chip 2021, 21, 2495–2510. [Google Scholar] [CrossRef]
- Abe, C.; Bhaswant, M.; Miyazawa, T.; Miyazawa, T. The Potential Use of Exosomes in Anti-Cancer Effect Induced by Polarized Macrophages. Pharmaceutics 2023, 15, 1024. [Google Scholar] [CrossRef]
- Ramírez-Cuéllar, J.; Ferrari, R.; Sanz, R.T.; Valverde-Santiago, M.; García-García, J.; Nacht, A.S.; Castillo, D.; Le Dily, F.; Neguembor, M.V.; Malatesta, M.; et al. LATS1 controls CTCF chromatin occupancy and hormonal response of 3D-grown breast cancer cells. EMBO J. 2024, 43, 1770–1798. [Google Scholar] [CrossRef]
- Hervas-Raluy, S.; Wirthl, B.; Guerrero, P.E.; Robalo Rei, G.; Nitzler, J.; Coronado, E.; Font de Mora Sainz, J.; Schrefler, B.A.; Gomez-Benito, M.J.; Garcia-Aznar, J.M.; et al. Tumour growth: An approach to calibrate parameters of a multiphase porous media model based on in vitro observations of Neuroblastoma spheroid growth in a hydrogel microenvironment. Comput. Biol. Med. 2023, 159, 106895. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef]
- De Ridder, K.; Tung, N.; Werle, J.-T.; Karpf, L.; Awad, R.M.; Bernier, A.; Ceuppens, H.; Salmon, H.; Goyvaerts, C. Novel 3D Lung Tumor Spheroids for Oncoimmunological Assays. Adv. NanoBiomed Res. 2022, 2, 2100124. [Google Scholar] [CrossRef]
- Mishriki, S.; Abdel Fattah, A.R.; Kammann, T.; Sahu, R.P.; Geng, F.; Puri, I.K. Rapid Magnetic 3D Printing of Cellular Structures with MCF-7 Cell Inks. Research 2019, 2019, 9854593. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Thakuri, P.S.; Plaster, M.; Li, J.; Luker, K.E.; Luker, G.D.; Tavana, H. Three-dimensional tumor model mimics stromal—Breast cancer cells signaling. Oncotarget 2018, 9, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, R.; Fan, Q.; Liu, M.; Huang, Y.; Shi, G. Enhancing the activation of T cells through anti-CD3/CD28 magnetic beads by adjusting the antibody ratio. IUBMB Life 2024, 76, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Anstett, V.; Heinzelmann, E.; Piraino, F.; Roch, A.; Chrisnandy, A.; Norkin, M.; Garnier, V.; Homicsko, K.; Hoehnel-Ka, S.; Brandenberg, N. In Vitro Evaluation of the Safety and Efficacy of Cibisatamab Using Adult Stem Cell-Derived Organoids and Colorectal Cancer Spheroids. Cancers 2025, 17, 291. [Google Scholar] [CrossRef]
- Chen, J.; Hnath, B.; Sha, C.M.; Beidler, L.; Schell, T.D.; Dokholyan, N.V. Optogenetically engineered Septin-7 enhances immune cell infiltration of tumor spheroids. Proc. Natl. Acad. Sci. USA 2024, 121, e2405717121. [Google Scholar] [CrossRef]
- Fang, J.; Ou, Q.; Wu, B.; Li, S.; Wu, M.; Qiu, J.; Cen, N.; Hu, K.; Che, Y.; Ma, Y.; et al. TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection. Cells 2022, 11, 2674. [Google Scholar] [CrossRef]
- Hurmach, Y.; Rudyk, M.; Svyatetska, V.; Senchylo, N.; Skachkova, O.; Pjanova, D.; Vaivode, K.; Skivka, L. The effect of intranasally administered TLR3 agonist larifan on metabolic profile of microglial cells in rat with C6 glioma. Ukr. Biochem. J. 2018, 90, 110–119. [Google Scholar] [CrossRef]
- Zhang, M.; Hutter, G.; Kahn, S.A.; Azad, T.D.; Gholamin, S.; Xu, C.Y.; Liu, J.; Achrol, A.S.; Richard, C.; Sommerkamp, P.; et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS ONE 2016, 11, e0153550. [Google Scholar] [CrossRef]
- Jiang, J.; Shihan, M.H.; Wang, Y.; Duncan, M.K. Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery. Invest. Ophthalmol. Vis. Sci. 2018, 59, 4986–4997. [Google Scholar] [CrossRef]
- Pinto, M.L.; Rios, E.; Durães, C.; Ribeiro, R.; Machado, J.C.; Mantovani, A.; Barbosa, M.A.; Carneiro, F.; Oliveira, M.J. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front. Immunol. 2019, 10, 1875. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Kroll, C.; Permyakova, A.; Reineke, J. Role of Endocytosis in Nanoparticle Penetration of 3D Pancreatic Cancer Spheroids. Mol. Pharm. 2019, 16, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Shinchi, H.; Crain, B.; Yao, S.; Chan, M.; Zhang, S.S.; Ahmadiiveli, A.; Suda, Y.; Hayashi, T.; Cottam, H.B.; Carson, D.A. Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides. Bioconjug. Chem. 2015, 26, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, L.; Xu, C.; Yu, Q.; Shi, K.; Zhang, L.; Wang, Y.; Zhang, Q.; Gao, H.; Zhang, Z.; et al. Dual Receptor Recognizing Cell Penetrating Peptide for Selective Targeting, Efficient Intratumoral Diffusion and Synthesized Anti-Glioma Therapy. Theranostics 2016, 6, 177–191. [Google Scholar] [CrossRef]
- Shi, N.Q.; Qi, X.R. Taming the Wildness of “Trojan-Horse” Peptides by Charge-Guided Masking and Protease-Triggered Demasking for the Controlled Delivery of Antitumor Agents. ACS Appl. Mater. Interfaces 2017, 9, 10519–10529. [Google Scholar] [CrossRef]
- Chen, H.; Shi, T.; Wang, Y.; Liu, Z.; Liu, F.; Zhang, H.; Wang, X.; Miao, Z.; Liu, B.; Wan, M.; et al. Deep Penetration of Nanolevel Drugs and Micrometer-Level T Cells Promoted by Nanomotors for Cancer Immunochemotherapy. J. Am. Chem. Soc. 2021, 143, 12025–12037. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Paun, R.A.; Tabrizian, M. Sonoprinting nanoparticles on cellular spheroids via surface acoustic waves for enhanced nanotherapeutics delivery. Lab Chip 2023, 23, 2091–2105. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hwang, H.J.; Lee, D.W. Author Correction: Optimization of 3D-aggregated spheroid model (3D-ASM) for selecting high efficacy drugs. Sci. Rep. 2023, 13, 5519. [Google Scholar] [CrossRef]
- Forbes, T.P.; Gillen, J.G.; Feeney, W.; Ho, J. Quality by Design Considerations for Drop-on-Demand Point-of-Care Pharmaceutical Manufacturing of Precision Medicine. Mol. Pharm. 2024, 21, 3268–3280. [Google Scholar] [CrossRef]
- Demyan, L.; Habowski, A.N.; Plenker, D.; King, D.A.; Standring, O.J.; Tsang, C.; St Surin, L.; Rishi, A.; Crawford, J.M.; Boyd, J.; et al. Pancreatic Cancer Patient-derived Organoids Can Predict Response to Neoadjuvant Chemotherapy. Ann. Surg. 2022, 276, 450–462. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Ding, R.; Yang, L.; Lyu, X.; Zeng, J.; Lei, J.H.; Wang, L.; Bi, J.; Shao, N.; et al. Patient-Derived Organoids Can Guide Personalized-Therapies for Patients with Advanced Breast Cancer. Adv. Sci. 2021, 8, e2101176. [Google Scholar] [CrossRef]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Rep. 2020, 14, 717–729. [Google Scholar] [CrossRef]
- Valente, I.V.B.; Garcia, D.; Abbott, A.; Spruill, L.; Siegel, J.; Forcucci, J.; Hanna, G.; Mukherjee, R.; Hamann, M.; Hilliard, E.; et al. The anti-proliferative effects of a frankincense extract in a window of opportunity phase ia clinical trial for patients with breast cancer. Breast Cancer Res. Treat. 2024, 204, 521–530. [Google Scholar] [CrossRef]
- Xu, Y.; Abramov, I.; Belykh, E.; Mignucci-Jiménez, G.; Park, M.T.; Eschbacher, J.M.; Preul, M.C. Characterization of ex vivo and in vivo intraoperative neurosurgical confocal laser endomicroscopy imaging. Front. Oncol. 2022, 12, 979748. [Google Scholar] [CrossRef] [PubMed]
- Dominas, C.; Bhagavatula, S.; Stover, E.; Deans, K.; Larocca, C.; Colson, Y.; Peruzzi, P.; Kibel, A.; Hata, N.; Tsai, L.; et al. The Translational and Regulatory Development of an Implantable Microdevice for Multiple Drug Sensitivity Measurements in Cancer Patients. IEEE Trans. Biomed. Eng. 2022, 69, 412–421. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).