Next-Generation Natural Hydrogels in Oral Tissue Engineering
Abstract
1. Introduction
1.1. Importance of Oral Tissue Engineering
- -
- Restore the structure and function of native tissue, rather than replacing it with inert materials;
- -
- Promote natural healing and regeneration through the administration of cells, growth factors, and supportive scaffolds;
- -
- Reduce the need for autografts or allografts, minimizing donor site morbidity and the risks of immune rejection;
- -
- Enable personalized treatment strategies, particularly due to advances in 3D bioprinting and patient-specific scaffold design.
1.2. Challenges in Oral Tissue Regeneration
2. Natural Hydrogels
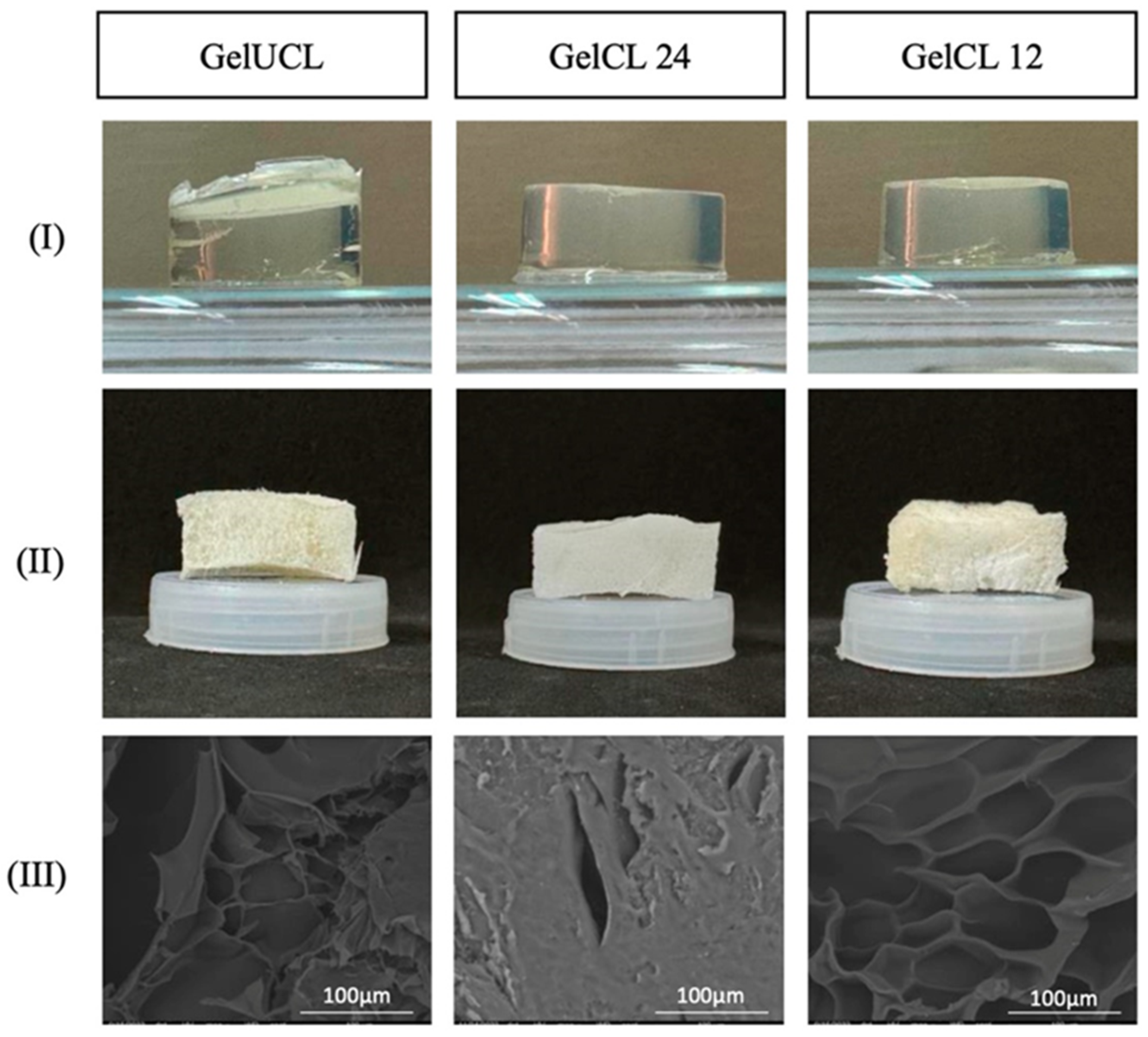
2.1. Composition, Properties and Manufacturing Methods
2.2. Advantages and Constraints of NHGs in Dentistry
- (i)
- Biocompatibility. NHGs are derived from biological sources such as collagen, gelatin, chitosan, alginate, hyaluronic acid, and fibrin, which are inherently compatible with living tissues. They present a minimized immune response and in general, these materials are well tolerated by the body, reducing the risk of inflammation or rejection. In addition, it is a cell-friendly environment, supporting the growth and function of oral cells such as fibroblasts, keratinocytes, osteoblasts, and dental pulp stem cells (DPSCs) [67].
- (ii)
- Biomimetics of the ECM. NHGs closely mimic the structure and composition of the ECM found in soft and hard oral tissues. Their 3D porous architecture allows for the diffusion of nutrients and oxygen, promoting cell survival. Many NHGs retain bioactive motifs within the structure of biopolymers and constituents, which can interact directly with cell receptors, enhancing cell adhesion and signaling [68].
- (iii)
- Biodegradability and controlled degradation. Another advantage of NHGs is their ability to degrade in a controlled and predictable manner, matching the physiological rhythm of tissue regeneration. Degradation rates can be adjusted to allow the scaffold to gradually disappear as new tissue forms, reducing the need for surgical removal. HGs degradation products are usually non-toxic and can be metabolized or excreted naturally [69].
- (iv)
- Improved cell adhesion and proliferation. NGHs often contain cell adhesion molecules, such as arginine–glycine–aspartic acid (RGD) sequences, which promote better cell-skeleton interaction. At the same time, improved cell adhesion facilitates proliferation and differentiation, accelerating healing and regeneration. From epithelial to mesenchymal stem cells, NHGs can support various types of oral cells [70].
- (v)
- Injectable and thermoresponsive properties. Many NHGs, such as gelatin or chitosan-based formulations, can be administered in a minimally invasive manner as injectable gels. They can transition from liquid to gel at body temperature, perfectly filling irregular defects. Not to be overlooked, this property is particularly useful for applications in delicate oral tissues or in areas that are difficult to access [71,72].
- (vi)
- Versatility in applications. NHGs are adaptable to a wide range of applications in dental tissue engineering, including periodontal regeneration (supporting the regeneration of cementum, periodontal ligament and alveolar bone) and pulp and dentin regeneration (as carriers for DPSC-derived stem cells and growth factors to restore pulp vitality). Also, HGs are tunable for bone grafts (supporting mineralization and osteogenesis of maxillofacial and jaw bone defects) and oral mucosa repair (helping with re-epithelialization in cases of trauma or ulceration) [73].
- (vii)
- Potential for delivery of growth factors and drugs. HGs can act as carriers for bioactive molecules, including growth factors (e.g., BMPs, VEGF, TGF-β), antimicrobial agents, or anti-inflammatory drugs. They can be engineered to release these agents in a controlled and sustained manner, improving local therapeutic outcomes. By delivering bioactive factors, enhanced tissue regeneration is supported [74].
- (viii)
- Reduced risk of chronic inflammation and fibrosis. Compared to synthetic materials, NHGs are less likely to induce chronic inflammatory responses or fibrosis, both of which can hinder the long-term success of oral implants or grafts [75].
- (ix)
- Eco-friendly and sustainable materials. Derived from natural sources, these NHGs are often renewable, eco-friendly, and cost-effective, which is beneficial for sustainable clinical practices and commercial production [76].
- (i)
- Weak Structural Integrity and Wear: NHGs often lack the mechanical strength and durability required for load-bearing applications in dentistry. For example, they might not withstand the forces exerted during chewing or other oral functions. Over time, hydrogels can degrade under mechanical stress, leading to wear and tear, which reduces their long-term effectiveness as dental materials.
- (ii)
- Inconsistent Composition and Source Variability: NHGs, derived from biological sources like collagen, alginate, or chitosan, can have variable properties depending on the source and method of extraction. This can result in inconsistent performance across different batches. The availability and quality of natural materials can vary depending on environmental conditions and sourcing, which can also lead to supply chain issues or fluctuations in prices.
- (iii)
- Variable Swelling Behavior: NHGs may absorb water differently based on environmental factors, which can influence their stability and performance in the oral environment.
- (iv)
- Lack of Control Over Premature Degradation: While biodegradability is generally considered a positive feature, the rate of degradation can sometimes be too rapid for certain applications in dentistry. If the hydrogel degrades before the intended therapeutic or restorative effect is achieved, the treatment may fail. It can be challenging to precisely control the rate of biodegradation, leading to inconsistent outcomes in dental procedures.
- (v)
- Microbial Colonization and Infection Risk: NHGs, particularly those derived from polysaccharides and proteins, can be more susceptible to microbial colonization and infection compared to synthetic materials. This is a significant concern in the oral cavity, where the presence of bacteria is prevalent. If the hydrogel degrades prematurely or has poor antimicrobial properties, it may increase the risk of secondary infections, particularly in periodontal treatments or wound healing after surgery.
- (vi)
- Sensitivity to Heat and Chemicals and Limited Shelf Life: NHGs can be sensitive to heat and chemical sterilization methods. Traditional sterilization processes might degrade or alter their properties, compromising their effectiveness as dental materials. Due to their natural origins, some hydrogels may have a shorter shelf life compared to synthetic materials, which can be a logistical issue for dental practices.
- (vii)
- Production and Processing Costs: NHGs may be more expensive to produce, especially if they are derived from complex or rare biological sources. This could make them less cost-effective compared to synthetic alternatives. The fabrication of NHGs for dental applications can involve complex processes to ensure uniformity and desired properties. This adds to the difficulty and cost of incorporating them into dental treatments.
- (viii)
- Unknown Long-Term Effects: While NHGs show promise in short-term studies, there is often a lack of comprehensive long-term data regarding their stability, biocompatibility, and potential adverse effects when used in dentistry. This makes clinicians hesitant to adopt them for more permanent dental treatments.
- (ix)
- Compatibility Issues: When used in conjunction with other materials, such as dental composites, adhesives, or metals, NHGs may not always exhibit optimal compatibility. This could affect the longevity and performance of restorative procedures like fillings, crowns, or implants. Table 1 outlines the main benefits and limitations of natural and synthetic hydrogels when applied in dental treatments.
2.3. The Role of Essential Oils in NHGs for Oral Tissue Regeneration
- -
- In periodontal regeneration, it can support infection control, modulate inflammation and promote the formation of new alveoli [81].
- -
- In post-extraction alveoli healing, it leads to a reduction in the incidence of dry socket, accelerating soft tissue closure [94].
- -
- It contributes to implant site preparation by reducing peri-implant microbial load and promotes osseointegration [95].
- -
- Repair of mucosal defects in cases of oral ulcers or trauma, promoting epithelial coverage and comfort [96].
3. Overview of Oral Tissues and Regeneration
3.1. Gingival Tissue
3.2. Periodontal Ligament (PDL)
3.3. Alveolar Bone
3.4. Oral Mucosa
3.5. Dental Pulp
3.6. Dentin
4. Applications of NHGs in Oral Tissue Engineering
4.1. Periodontal Regeneration
4.2. Bone and Alveolar Ridge Regeneration
4.3. Soft Tissue Repair (Gingiva and Oral Mucosa)
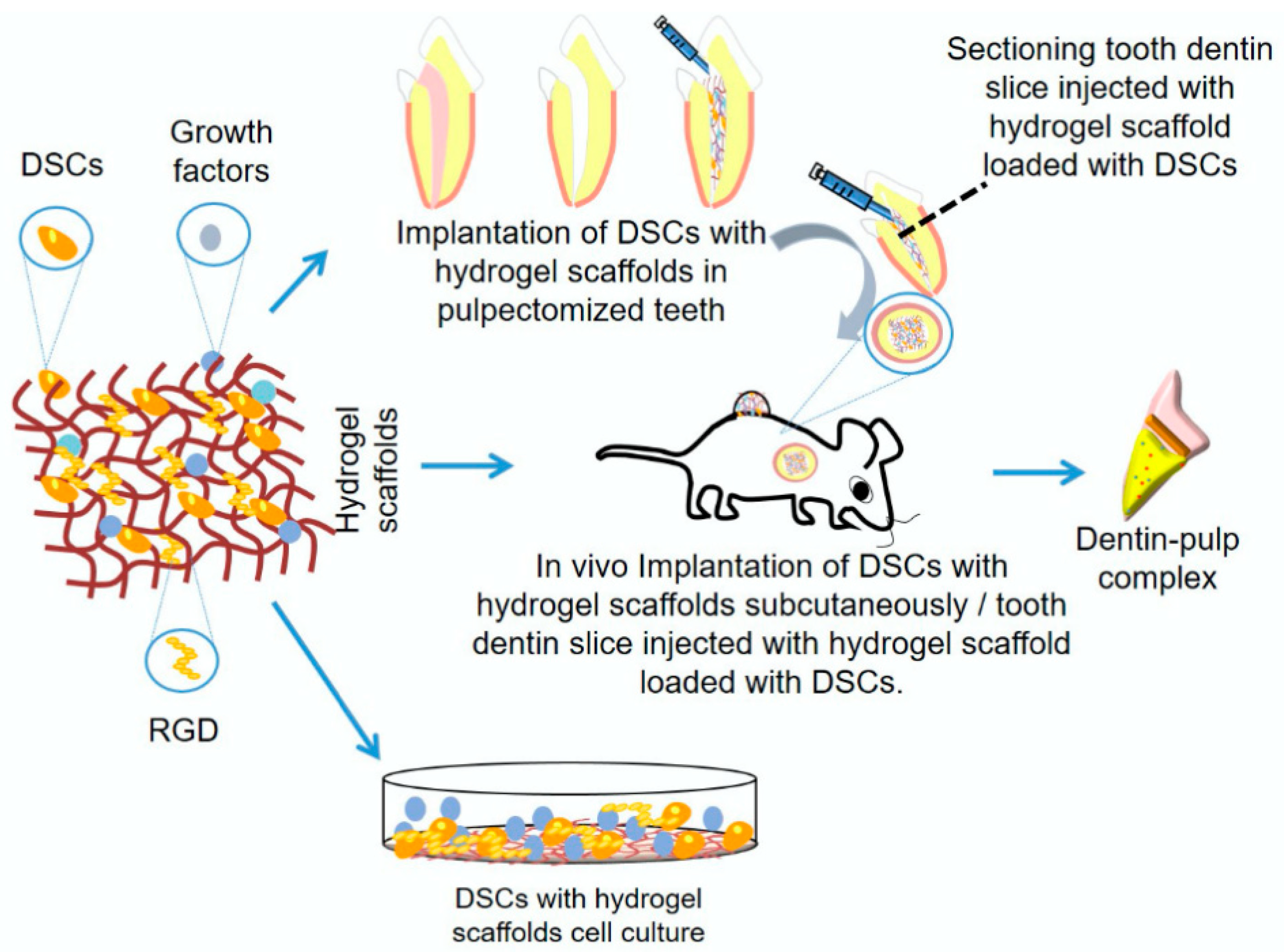
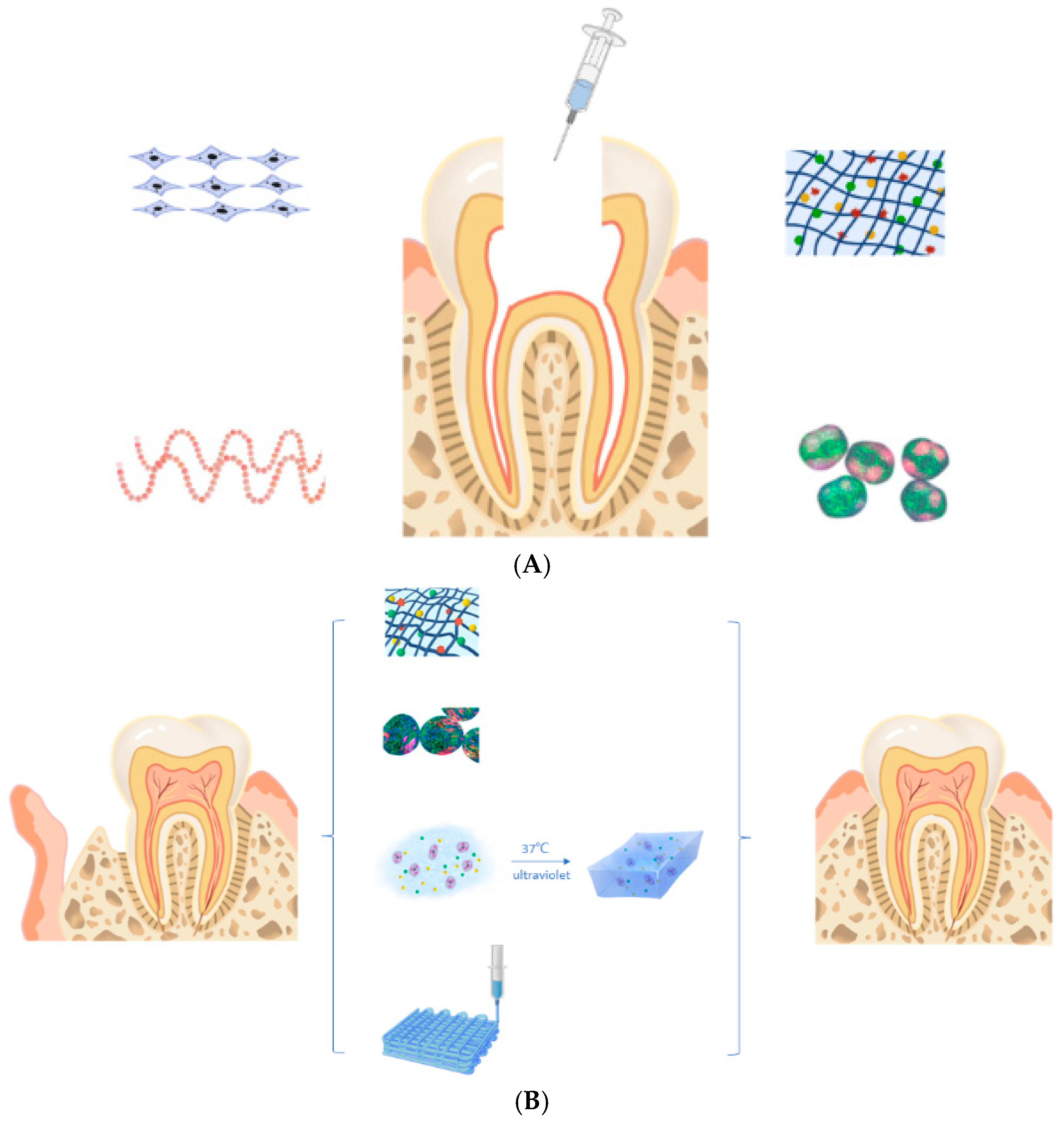
4.4. Dental Pulp Regeneration
4.5. Drug and Growth Factor Delivery Systems
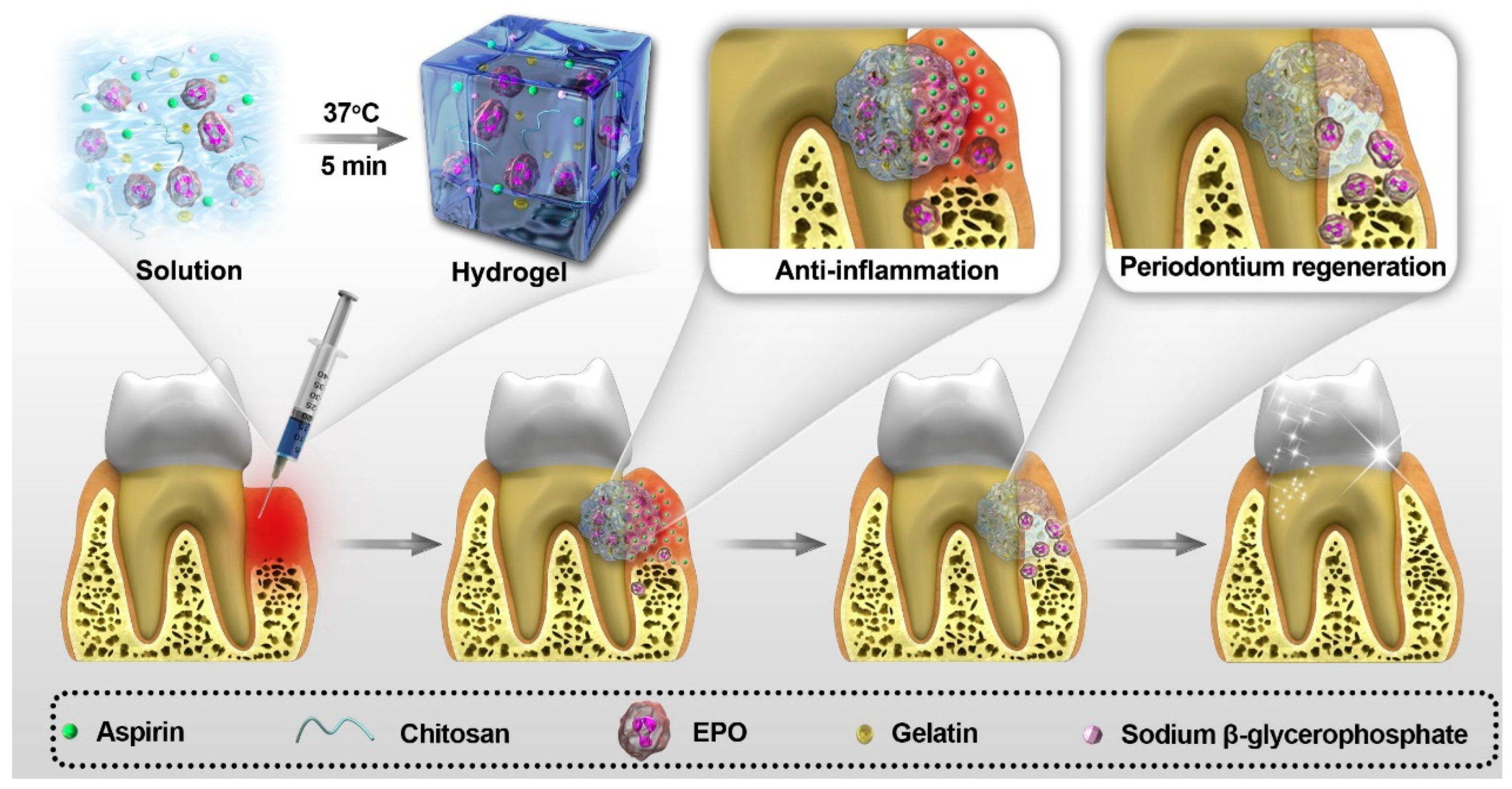
4.6. Wound Healing and Anti-Inflammatory Applications
5. Challenges and Limitations
5.1. Mechanical Deficiencies
5.2. Immunogenicity and Control of Biodegradation Rate
5.3. Scaling up and Clinical Translation
5.4. Regulatory and Ethical Considerations
6. Future Directions—Overcoming Challenges for NHGs in Oral Tissue Engineering
- (i)
- Improving mechanical strength to withstand masticatory forces through composite or cross-linked hydrogel systems.
- (ii)
- Optimizing degradation rates to align with oral wound healing time and tissue regeneration.
- (iii)
- Improving antimicrobial properties to resist the oral microbiome and prevent infection.
- (iv)
- Standardizing bio fabrication for consistent and scalable production suitable for intraoral applications.
- (v)
- Incorporating bioactive molecules (e.g., enamel matrix proteins, salivary peptides) to promote site-specific cellular differentiation.
- (vi)
- 3D bioprinting of patient-specific scaffolds for precise anatomical fit to complex oral defects.
- (vii)
- Using low-temperature or non-destructive sterilization methods to preserve the bioactivity of NHGs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. Available online: https://iris.who.int/handle/10665/269475 (accessed on 1 August 2025).
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef]
- Li, W.; Hu, J.; Chen, C.; Li, X.; Zhang, H.; Xin, Y.; Tian, Q.; Wang, S. Emerging advances in hydrogel-based therapeutic strategies for tissue regeneration. Regen. Ther. 2023, 24, 459–471. [Google Scholar] [CrossRef]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Pitterou, I.; Kalogeropoulou, F.; Tzani, A.; Tsiantas, K.; Gatou, M.A.; Pavlatou, E.; Batrinou, A.; Fountzoula, C.; Kriebardis, A.; Zoumpoulakis, P.; et al. Development of Alginate Hydrogels Incorporating Essential Oils Loaded in Chitosan Nanoparticles for Biomedical Applications. Molecules 2024, 29, 5318. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, F.; Guo, W. Research Advances on Hydrogel-Based Materials for Tissue Regeneration and Remineralization in Tooth. Gels 2023, 9, 245. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Makkar, H.; Dal-Fabbro, R.; Malda, J.; Sriram, G.; Bottino, M.C. Biofabrication Strategies for Oral Soft Tissue Regeneration. Adv. Healthc. Mater. 2024, 13, e2304537. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef]
- Ye, S.; Wei, B.; Zeng, L. Advances on Hydrogels for Oral Science Research. Gels 2022, 8, 302. [Google Scholar] [CrossRef]
- Chen, F.M.; Wu, L.A.; Zhang, M.; Zhang, R.; Sun, H.H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011, 32, 3189–3209. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Su, H.; Si, M.; Xu, J.; Chang, X.; Lv, J.; Zhai, Y. Tissue Engineering in Stomatology: A Review of Potential Approaches for Oral Disease Treatments. Front. Bioeng. Biotechnol. 2021, 8, 662418. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wang, Z.; Li, Z.; Shi, X.; Xiao, M.; Xu, Y. Formation, architecture, and persistence of oral biofilms: Recent scientific discoveries and new strategies for their regulation. Front. Microbiol. 2025, 16, 1602962. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef]
- Chen, A.; Deng, S.; Lai, J.; Li, J.; Chen, W.; Varma, S.N.; Zhang, J.; Lei, C.; Liu, C.; Huang, L. Hydrogels for Oral Tissue Engineering: Challenges and Opportunities. Molecules 2023, 28, 3946. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Yazdanpanah, S.; Petillo, O.; Conte, R.; Sepe, F.; Peluso, G.; Calarco, A. Sustainable Hydrogels for Medical Applications: Biotechnological Innovations Supporting One Health. Gels 2025, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Chapter I.2.5—Hydrogels, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Biomaterials Science; Academic Press: Cambridge, MA, USA, 2013; pp. 166–179. ISBN 9780123746269. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, S.; Alam Rakib, R.; Khan, A.R.; Yesmin, F.; Shakil, S.; Khan, M.M.R. Progress in hydrogel toughening: Addressing structural and crosslinking challenges for biomedical applications. Discov. Mate 2025, 5, 5. [Google Scholar] [CrossRef]
- Maleki, B.; Ghamari Kargar, P.; Sedigh Ashrafi, S.; Ghani, M. Perspective Chapter: Introduction to Hydrogels—Definition, Classifications, Applications and Methods of Preparation; Ionic Liquids Recent Advances; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.S.M.; Pina, A.S.; Roque, A.C.A. Affinity-triggered hydrogels: Developments and prospects in biomaterials science. Biomaterials 2021, 268, 120563. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Pinelli, F.; Nespoli, T.; Rossi, F. Sensing Materials: Hydrogels, 1st ed.; Narayan, R., Ed.; Encyclopedia of Sensors and Biosensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 148–166. ISBN 9780128225493. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, C.; Wang, H.; Wang, D. Swollen hydrogel nanotechnology: Advanced applications of the rudimentary swelling properties of hydrogels. ChemPhysMater 2024, 3, 357–375. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; Oyen, M.L. Hydrogel Composite Materials for Tissue Engineering Scaffolds. JOM 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Ivanov, D. Natural and Synthetic Polymers for Designing Composite Materials. In Handbook of Bioceramics and Biocomposites; Antoniac, I., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.; Chen, J.-P.; Yu, C. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef]
- Calderón Moreno, J.M.; Chelu, M.; Popa, M. Eco-Friendly Conductive Hydrogels: Towards Green Wearable Electronics. Gels 2025, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Zanrè, E.; Dalla Valle, E.; D’Angelo, E.; Sensi, F.; Agostini, M.; Cimetta, E. Recent Advancements in Hydrogel Biomedical Research in Italy. Gels 2024, 10, 248. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Cimpean, A. A Recent Insight into Research Pertaining to Collagen-Based Hydrogels as Dressings for Chronic Skin Wounds. Gels 2025, 11, 527. [Google Scholar] [CrossRef]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z. Hydrogel Nanocomposites: Biomedical Applications, Biocompatibility, and Toxicity Analysis. In Safety of Nanoparticles. Nanostructure Science and Technology; Webster, T., Ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Csóka, I. Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications. Gels 2025, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Azmir, M.S.N.A.; Moni Md, N.; Gobetti, A.; Ramorino, G.; Dey, K. Advances in modulating mechanical properties of gelatin-based hydrogel in tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 215–250. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Liao, Z.; Makrypidis, A.; Papathanasiou, M.M.; Charalambides, M.N. On the pseudoelastic-viscoelastic behavior of starch hydrogels at various degrees of gelatinization and retrogradation. Phys. Fluids 2025, 37, 027185. [Google Scholar] [CrossRef]
- Li, Z.; Ren, K.; Chen, J.; Zhuang, Y.; Dong, S.; Wang, J.; Liu, H.; Ding, J. Bioactive hydrogel formulations for regeneration of pathological bone defects. J. Control. Release 2025, 380, 686–714. [Google Scholar] [CrossRef]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef]
- Miao, X.; Davoudi, M.; Sadegh-Nejadi, S.; Ghahari, S.A.; Bagherieh, M.; Afrisham, R. Skin regenerative potential of hydrogel matrices incorporated with stem cell-derived extracellular vesicles enriched with MicroRNAs: A systematic review. Mol. Cell. Biochem. 2025, 480, 4035–4067. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Popa, M.; Calderón Moreno, J.M. Applications of Hydrogels in Emergency Therapy. Gels 2025, 11, 234. [Google Scholar] [CrossRef]
- Oyervides-Guajardo, V.G.; Claudio-Rizo, J.A.; Cabrera-Munguia, D.A.; Cano-Salazar, L.F.; León-Campos, M.I.; Tamayo-Ordoñez, M.C. Protein–Polysaccharide Hydrogels: A Review on Smart Materials for the Future of Biotechnology. Polym. Adv. Technol. 2025, 36, e70295. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in natural polymer-based hydrogels: Synthesis, applications, and future directions in biomedical and environmental fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon nanotubes hybrid hydrogels in drug delivery: A perspective review. BioMed Res. Int. 2014, 2014, 825017. [Google Scholar] [CrossRef]
- Munyiri, C.N.; Madivoli, E.S.; Kisato, J.; Gichuki, J.; Kareru, P.G. Biopolymer based hydrogels: Crosslinking strategies and their applications. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 625–640. [Google Scholar] [CrossRef]
- Pruksawan, S.; Chong, Y.T.; Lee, Y.S.Y.; Lam, D.K.F.; Wang, F. Design Strategies and Perspectives on the Toughening of Hydrogels via Fully Physical Cross-Linking. Chem. Mater. 2025, 37, 5436–5453. [Google Scholar] [CrossRef]
- Lee, C.; Fiocco, G.; Vigani, B.; Recca, T.; Milanese, C.; Delledonne, C.; Licchelli, M.; Rossi, S.; Chung, Y.; Volpi, F.; et al. Chemically Crosslinked Alginate Hydrogel with Polyaziridine: Effects on Physicochemical Properties and Promising Applications. ChemPlusChem 2025, 90, e202400649. [Google Scholar] [CrossRef]
- Cai, B.; Fang, J.; Zhou, S.; Xie, M.; Zhang, K.; Li, J.; Yin, G. Enzyme-crosslinked hyaluronic acid hydrogel scaffolds for BMSCs microenvironment and wound healing. Int. J. Biol. Macromol. 2025, 295, 139566. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.K.; Jayakumar, R. Hydrogels in dentistry. In Hydrogel Tissue Analogues; Woodhead Publishing: Sawston, UK, 2025; pp. 421–429. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Peng, S.; Lin, Y.; Ye, Z. Hydrogels in Dental Medicine. Adv. Ther. 2024, 7, 2300128. [Google Scholar] [CrossRef]
- Xie, Z.; Zhan, P.; Zhang, X.; Huang, S.; Shi, X.; Lin, Z.; Gao, X. Providing biomimetic microenvironment for pulp regeneration via hydrogel-mediated sustained delivery of tissue-specific developmental signals. Mater. Today Bio 2024, 26, 101102. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, X.; Wang, A.; Gu, Q.; Sun, C.; Wang, F.; Lei, L.; Zhang, W. Biodegradable natural hydrogels: Design, crosslinking, and medical applications. Mater. Today Commun. 2025, 46, 112929. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Dunne, K.; Breyer, W.; Wu, Y.; Pashuck, E.T. Hydrogels with multiple RGD presentations increase cell adhesion and spreading. Acta Biomater. 2025, 199, 142–153. [Google Scholar] [CrossRef]
- Flores-Espinoza, A.I.; Garcia-Contreras, R.; Guzman-Rocha, D.A.; Aranda-Herrera, B.; Chavez-Granados, P.A.; Jurado, C.A.; Alfawaz, Y.F.; Alshabib, A. Gelatin–Chitosan Hydrogel Biological, Antimicrobial and Mechanical Properties for Dental Applications. Biomimetics 2023, 8, 575. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Mohd, N.; Abu Kasim, N.H.; Razali, M. 3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration. Int. J. Mol. Sci. 2025, 26, 3510. [Google Scholar] [CrossRef]
- Hao, M.; Wang, D.; Duan, M.; Kan, S.; Li, S.; Wu, H.; Xiang, J.; Liu, W. Functional drug-delivery hydrogels for oral and maxillofacial wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1241660. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Biomaterials-Based Hydrogels for Therapeutic Applications; Biomaterials in Microencapsulation; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Ailiesei, G.-L.; Yilmaz, C.; Brebu, M. Synthesis of Bioactive Materials by In Situ One-Step Direct Loading of Syzygium aromaticum Essential Oil into Chitosan-Based Hydrogels. Gels 2022, 8, 225. [Google Scholar] [CrossRef]
- Karimi, Y.; Rashidipour, M.; Iranzadasl, M.; Ahmadi, M.H.; Sarabi, M.M.; Farzaneh, F. Biofilm targeting with chitosan-based nanohydrogel containing Quercus infectoria G. Olivier extract against Streptococcus mutans: New formulations of a traditional natural product. BMC Complement. Med. Ther. 2024, 24, 398. [Google Scholar] [CrossRef]
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- Serra, E.; Saubade, F.; Ligorio, C.; Whitehead, K.; Sloan, A.; Williams, D.W.; Hidalgo-Bastida, A.; Verran, J.; Malic, S. Methylcellulose Hydrogel with Melissa officinalis Essential Oil as a Potential Treatment for Oral Candidiasis. Microorganisms 2020, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, C.; Skoufos, I.; Fotou, K.; Tzora, A.; Bayon, Y.; Mari, D.; Sarafi, E.; Nikolaou, K.; Zeugolis, D.I. Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels. Bioengineering 2025, 12, 89. [Google Scholar] [CrossRef]
- Arpa, M.D.; Kesmen, E.E.; Arslan, T.; Karadağ, A.E.; Biltekin, S.N.; Demirci, F. Ginger essential oil-loaded hydrogels: Preparation, characterization, cytotoxicity, antimicrobial and anti-inflammatory activity. J. Essent. Oil Res. 2024, 36, 16–29. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Rusu, A.G.; Niță, L.E.; Roșca, I.; Croitoriu, A.; Ghilan, A.; Mititelu-Tarțău, L.; Grigoraș, A.V.; Crețu, B.-E.-B.; Chiriac, A.P. Alginate-Based Hydrogels Enriched with Lavender Essential Oil: Evaluation of Physicochemical Properties, Antimicrobial Activity, and In Vivo Biocompatibility. Pharmaceutics 2023, 15, 2608. [Google Scholar] [CrossRef]
- Comini, S.; Scutera, S.; Sparti, R.; Banche, G.; Coppola, B.; Bertea, C.M.; Bianco, G.; Gatti, N.; Cuffini, A.M.; Palmero, P.; et al. Combination of Poly(ε-Caprolactone) Biomaterials and Essential Oils to Achieve Anti-Bacterial and Osteo-Proliferative Properties for 3D-Scaffolds in Regenerative Medicine. Pharmaceutics 2022, 14, 1873. [Google Scholar] [CrossRef]
- Fawal, G.E.; Hong, H.; Mo, X.; Wang, H. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha Piperita Essential Oils Loaded in a Chitosan Nanogel with Inhibitory Effect on Biofilm Formation against S. Mutans on the Dental Surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Lee, Y.; Gou, Y.; Pan, X.; Gu, Z.; Xie, H. Advances of multifunctional hydrogels for periodontal disease. Smart Mater. Med. 2023, 4, 460–467. [Google Scholar] [CrossRef]
- Li, A.; Khan, I.N.; Khan, I.U.; Yousaf, A.M.; Shahzad, Y. Gellan gum-based bilayer mucoadhesive films loaded with moxifloxacin hydrochloride and clove oil for possible treatment of periodontitis. Drug Des. Dev. Ther. 2021, 15, 3937–3952. [Google Scholar] [CrossRef]
- Mockdeci, H.R.; Junqueira, L.A.; Duarte, L.M.; de Souza Moreira, C.P.; de Oliveira, M.A.L.; Brandão, M.A.F.; Tavares, G.D.; Raposo, N.R.B. Improved anti-Candida activity of hydrogel containing tea tree oil-loaded solid lipid nanoparticles for the treatment of oropharyngeal candidiasis. RPS Pharm. Pharmacol. Rep. 2023, 2, rqac010. [Google Scholar] [CrossRef]
- Alkandari, M.; Barai, P.; Atia, G.A.N.; Mohamed, S.Z.; Ghobashy, M.M.; Shalaby, H.K.; Foda, T.; Rabbee, M.F.; Mallick, S.; Barai, H.R.; et al. Bioactive Functionalized Chitosan Thermo-Responsive Hydrogels as Promising Platforms for Therapeutic, Regenerative Oral, and Maxillofacial Applications. Biotechnol. J. 2025, 20, e202400653. [Google Scholar] [CrossRef]
- Serra, E. A Hydrogel in Combination with Essential Oils for Oral Therapy. Doctoral Thesis, Manchester Metropolitan University, Manchester, UK, 2018. Available online: https://e-space.mmu.ac.uk/622355/ (accessed on 15 July 2025).
- Allizond, V.; Comini, S.; Cuffini, A.M.; Banche, G. Current Knowledge on Biomaterials for Orthopedic Applications Modified to Reduce Bacterial Adhesive Ability. Antibiotics 2022, 11, 529. [Google Scholar] [CrossRef]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef]
- Cabaña-Muñoz, M.E.; Pelaz Fernández, M.J.; Parmigiani-Cabaña, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics 2023, 15, 2109. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Sanches, N.; Imani, A.; Wang, L.; Pacheco Vitória, O.A.; Reinert, H.; Panahipour, L.; Souza, F.Á.; Garcia Júnior, I.R.; Gruber, R. Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells. Int. J. Mol. Sci. 2025, 26, 5578. [Google Scholar] [CrossRef] [PubMed]
- Attik, N.; Basri, I.; Sohier, J.; Gauthier, R.; Villat, C.; Goutaudier, C. Aluminum-Free Borosilicate Glass Functionalized Hydrogels for Enhanced Dental Tissue Regeneration. Materials 2024, 17, 5862. [Google Scholar] [CrossRef]
- Min, B.M. Oral Mucosa and Gingiva. In Oral Biochemistry; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Bosco, A.F.; Bonfante, S.; Luize, D.S.; Bosco, J.M.D.; Garcia, V.G. Periodontal plastic surgery associated with treatment for the removal of gingival overgrowth. J. Periodontol. 2006, 77, 922–928. [Google Scholar] [CrossRef]
- 102. Lestari, W.; Irfanita, N.; Haris, M.S.; Lin, G.S.S.; Jaswir, I.; Darnis, D.S.; Ruziantee, N.; Mazlan, N.; Idrus, E.; Amir, L.R.; et al. Advancements and applications of gelatin-based scaffolds in dental engineering: A narrative review. Odontology 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Hutomo, D.I.; Deandra, F.A.; Ketherin, K.; García-Gareta, E.; Bachtiar, E.W.; Amir, L.; Tadjoedin, F.M.; Widaryono, A.; Haerani, N.; Lessang, R.; et al. The Effect of Carbodiimide Crosslinkers on Gelatin Hydrogel as a Potential Biomaterial for Gingival Tissue Regeneration. Gels 2024, 10, 674. [Google Scholar] [CrossRef]
- López-Domínguez, S.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Ríos-Arana, J.V.; Saucedo Acuña, R.A.; Cuevas-González, M.V.; Zaragoza-Contreras, E.A.; Tovar Carrillo, K.L. An Evaluation of Cellulose Hydrogels Derived from tequilana Weber Bagasse for the Regeneration of Gingival Connective Tissue in Lagomorphs. Gels 2025, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.T.; Scanlon, C.S.; Soehren, S.; Matsuo, M.; Kapila, Y.L. Implications of cultured periodontal ligament cells for the clinical and experimental setting: A review. Arch. Oral Biol. 2011, 56, 933–943. [Google Scholar] [CrossRef]
- Nagy, K.; Láng, O.; Láng, J.; Perczel-Kovách, K.; Gyulai-Gaál, S.; Kádár, K.; Kőhidai, L.; Varga, G. A novel hydrogel scaffold for periodontal ligament stem cells. Interv. Med. Appl. Sci. 2018, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Gao, L.; Zhang, W. Hydrogels in Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 7337–7351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, D.; Tu, H.; Cao, M.; Li, M.; Peng, L.; Yang, J. Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases. Gels 2023, 9, 146. [Google Scholar] [CrossRef]
- Sultan, N.; Camilleri, J.; Scheven, B.A. Biocompatibility and antimicrobial effect of demineralised dentin matrix hydrogel for dental pulp preservation. Odontology 2025, 113, 585–597. [Google Scholar] [CrossRef]
- Osman, M.; Sharmin, Z.; Suchy, S.; Gao, F.; Kaminski, A.; Mitchell, J.C.; Sigar, I.M.; Carrilho, M.R. Bioinspired smart dentin ECM-chitosan hydrogels for dentin-pulp complex regeneration. J. Dent. 2025, 105811. [Google Scholar] [CrossRef]
- Sun, H.; Luan, J.; Dong, S. Hydrogels promote periodontal regeneration. Front. Bioeng. Biotechnol. 2024, 12, 1411494. [Google Scholar] [CrossRef]
- Santos, M.S.; dos Santos, A.B.; Carvalho, M.S. New Insights in Hydrogels for Periodontal Regeneration. J. Funct. Biomatter. 2023, 14, 545. [Google Scholar] [CrossRef]
- Khademi, R.; Kharaziha, M. Recent advances in hydrogel-based platforms for periodontal tissue regeneration. Curr. Opin. Biomed. Eng. 2025, 35, 100615. [Google Scholar] [CrossRef]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- Tan, N.; Sabalic-Schoener, M.; Nguyen, L.; D’Aiuto, F. β-Tricalcium Phosphate-Loaded Chitosan-Based Thermosensitive Hydrogel for Periodontal Regeneration. Polymers 2023, 15, 4146. [Google Scholar] [CrossRef]
- Roldan, L.; Montoya, C.; Solanki, V.; Cai, K.Q.; Yang, M.; Correa, S.; Orrego, S. A Novel Injectable Piezoelectric Hydrogel for Periodontal Disease Treatment. ACS Appl. Mater. Interfaces 2023, 15, 43441–43454. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Sepe, F.; Gianfreda, F.; Condò, R.; Cerroni, L.; Calarco, A.; Peluso, G. Resveratrol-Loaded Solid Lipid Nanoparticles Reinforced Hyaluronic Hydrogel: Multitarget Strategy for the Treatment of Diabetes-Related Periodontitis. Biomedicines 2025, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Liang, C.; Qu, X.; Zou, X.; Du, S.; Zhang, Q.; Wang, L. Role of Berberine Thermosensitive Hydrogel in Periodontitis via PI3K/AKT Pathway In Vitro. Int. J. Mol. Sci. 2023, 24, 6364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Xue, P.; Zhang, J. Collagenase-Responsive Hydrogel Loaded with GSK2606414 Nanoparticles for Periodontitis Treatment through Inhibiting Inflammation-Induced Expression of PERK of Periodontal Ligament Stem Cells. Pharmaceutics 2023, 15, 2503. [Google Scholar] [CrossRef]
- Kevci, M.; Lauridsen, E.; Andersson, L. Risk of Healing Complications Following Alveolar Process Fractures in the Primary Dentition: A Retrospective Clinical Cohort Study. Dent Traumatol. 2025, 41, 29–36. [Google Scholar] [CrossRef]
- Dipalma, G.; Marinelli, G.; Fiore, A.; Balestriere, L.; Carone, C.; Buongiorno, S.; Inchingolo, F.; Minervini, G.; Palermo, A.; Inchingolo, A.M.; et al. The Evolving Role of Stem Cells in Oral Health and Regeneration: A Systematic Review. Surgeries 2025, 6, 65. [Google Scholar] [CrossRef]
- Marian, D.; Toro, G.; D’Amico, G.; Trotta, M.C.; D’Amico, M.; Petre, A.; Lile, I.; Hermenean, A.; Fratila, A. Challenges and Innovations in Alveolar Bone Regeneration: A Narrative Review on Materials, Techniques, Clinical Outcomes, and Future Directions. Medicina 2025, 61, 20. [Google Scholar] [CrossRef] [PubMed]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef]
- Iviglia, G.; Cassinelli, C.; Torre, E.; Baino, F.; Morra, M. Novel bioceramic-reinforced hydrogel for alveolar bone regeneration. Acta Biomater. 2016, 44, 97–109. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.-C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Yu, W.; Hu, L.; Wei, Y.; Xue, C.; Liu, Y.; Xie, H. Advances of novel hydrogels in the healing process of alveolar sockets. Biomater. Adv. 2025, 173, 214280. [Google Scholar] [CrossRef] [PubMed]
- Neovius, E.; Lemberger, M.; Skogh, A.C.D.; Hilborn, J.; Engstrand, T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 37–42. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, Q.; Zhang, H.; Zhao, N.; Liao, L. PF127 Hydrogel-Based Delivery of Exosomal CTNNB1 from Mesenchymal Stem Cells Induces Osteogenic Differentiation during the Repair of Alveolar Bone Defects. Nanomaterials 2023, 13, 1083. [Google Scholar] [CrossRef]
- Fratila, D.N.; Virvescu, D.I.; Luchian, I.; Hancianu, M.; Baciu, E.R.; Butnaru, O.; Budala, D.G. Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels 2024, 10, 661. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast Encapsulation in Gelatin Methacryloyl (GelMA) versus Collagen Hydrogel as Substrates for Oral Mucosa Tissue Engineering. J. Oral Biol. Craniofacial Res. 2020, 10, 573–577. [Google Scholar] [CrossRef]
- Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, P.K.; Jayaramu, R.A.; Boregowda, S.S.; Eshwar, S.; Suresh, N.V.; Abu Lila, A.S.; Moin, A.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels 2023, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Budala, D.G.; Martu, M.-A.; Maftei, G.-A.; Diaconu-Popa, D.A.; Danila, V.; Luchian, I. The Role of Natural Compounds in Optimizing Contemporary Dental Treatment—Current Status and Future Trends. J. Funct. Biomater. 2023, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Muntean, A.; Sarosi, C.; Petean, I.; Cuc, S.; Carpa, R.; Chis, I.A.; Ilea, A.; Delean, A.G.; Moldovan, M. Developing Bioactive Hydrogels with Peptides for Dental Application. Biomedicines 2024, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Chakraborty, A.; Sandhu, G.; Naim, S.; Nowotny, E.B.; Moradian-Oldak, J. Amelogenin Peptide-Chitosan Hydrogel for Biomimetic Enamel Regrowth. Front. Dent. Med. 2021, 2, 697544. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Pintado, M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef]
- Gloria-Garza, M.A.; Reyna-Martínez, G.R.; Jiménez-Salas, Z.; Campos-Góngora, E.; Kačániová, M.; Aguirre-Cavazos, D.E.; Bautista-Villarreal, M.; Leos-Rivas, C.; Elizondo-Luevano, J.H. Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants 2025, 14, 1390. [Google Scholar] [CrossRef]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.; Silva, M.; Reis, V. Chitosan-properties and applications in dentistry. Adv. Tissue Eng. Regen. Med. 2017, 2, 00035. [Google Scholar] [CrossRef]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wu, Y.; Xu, L. Recent advancements in hydrogels as novel tissue engineering scaffolds for dental pulp regeneration. Int. J. Biol. Macromol. 2024, 264, 130708. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, J.; Chopra, H.; Zhang, Z.; Dubey, N.; Dissanayaka, W.L.; Nor, J.E.; Boini, M.C. Injectable Tissue-Specific Hydrogel System for Pulp–Dentin Regeneration. J. Dent. Res. 2024, 103(4), 398–408. [Google Scholar] [CrossRef] [PubMed]
- Im, G.-B.; Lin, R.-Z. Bioengineering for vascularization: Trends and directions of photocrosslinkable gelatin methacrylate hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 1053491. [Google Scholar] [CrossRef]
- Vargas-Alfredo, N.; Munar-Bestard, M.; Ramis, J.M.; Monjo, M. Synthesis and Modification of Gelatin Methacryloyl (GelMA) with Antibacterial Quaternary Groups and Its Potential for Periodontal Applications. Gels 2022, 8, 630. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res. 2016, 96, 192–199. [Google Scholar] [CrossRef]
- Piglionico, S.S.; Pons, C.; Romieu, O.; Cuisinier, F.; Levallois, B.; Panayotov, I.V. In vitro, ex vivo, and in vivo models for dental pulp regeneration. J. Mater. Sci. Mater. Med. 2023, 34, 15. [Google Scholar] [CrossRef]
- Piglionico, S.S.; Varga, B.; Pall, O.; Romieu, O.; Gergely, C.; Cuisinier, F.; Levalloisa, B.; Panayotov, I.V. Biomechanical characterization of a fibrinogen–blood hydrogel for human dental pulp regeneration. Biomater. Sci. 2023, 11, 6919. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2024, 36, e2210707. [Google Scholar] [CrossRef] [PubMed]
- Dinh, L.; Hwang, S.-J.; Yan, B. Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery. Pharmaceutics 2025, 17, 897. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Li, T.; Zhang, M.; Wang, J.; Wang, T.; Yao, Y.; Zhang, X.; Zhang, C.; Zhang, N. Thermosensitive Hydrogel Co-loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy. AAPS J. 2016, 18, 146–155. [Google Scholar] [CrossRef]
- Pandya, K.; Abbinayah, D.; Selvakumar, D.; Jayakumar, N. Efficacy of topical Curcuma longa in the healing of extraction sockets: A split-mouth clinical trial. Dent. Res. J. 2023, 20, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J. Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels 2023, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Echazú, M.I.A.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C 2017, 81, 588–596. [Google Scholar] [CrossRef]
- Nidhi, P.; Dev, K.; Negi, P.; Sourirajan, A. Development and evaluation of hydrogel formulation comprising essential oil of Mentha longifolia L. for oral candidiasis. Adv. Tradit. Med. (ADTM) 2023, 23, 777–787. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Ge, X.; Qi, X.; Xiang, Y.; Shi, Y.; Li, Y.; Pan, Y.; Wang, Y.; Ru, Y.; et al. Ferric Iron/Shikonin Nanoparticle-Embedded Hydrogels with Robust Adhesion and Healing Functions for Treating Oral Ulcers in Diabetes. Adv. Sci. 2024, 11, e2405463. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhuge, P.; Qi, X.; Ge, X.X.; Xiang, J.; Xu, H.; Cai, E.; Lan, Y.; Chen, X.; Li, Y.; et al. A cuttlefish ink nanoparticle-reinforced biopolymer hydrogel with robust adhesive and immunomodulatory features for treating oral ulcers in diabetes. Bioact. Mater. 2024, 39, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Yang, X.; Zhang, Y.; Liang, Y.; Chen, X.; Qiu, X.; Chen, X. Advancements in GelMA bioactive hydrogels: Strategies for infection control and bone tissue regeneration. Theranostics 2025, 15, 460–493. [Google Scholar] [CrossRef] [PubMed]



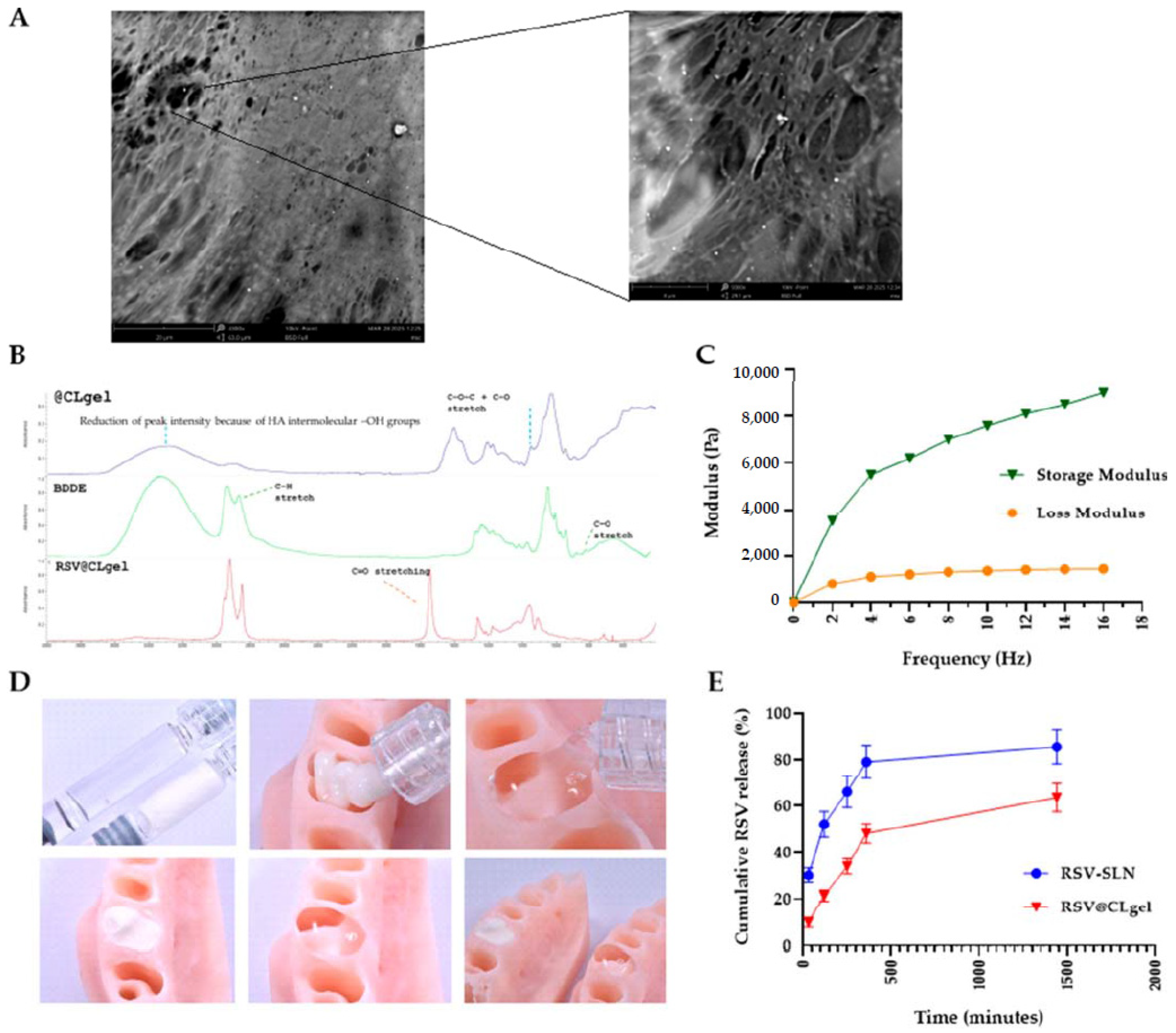

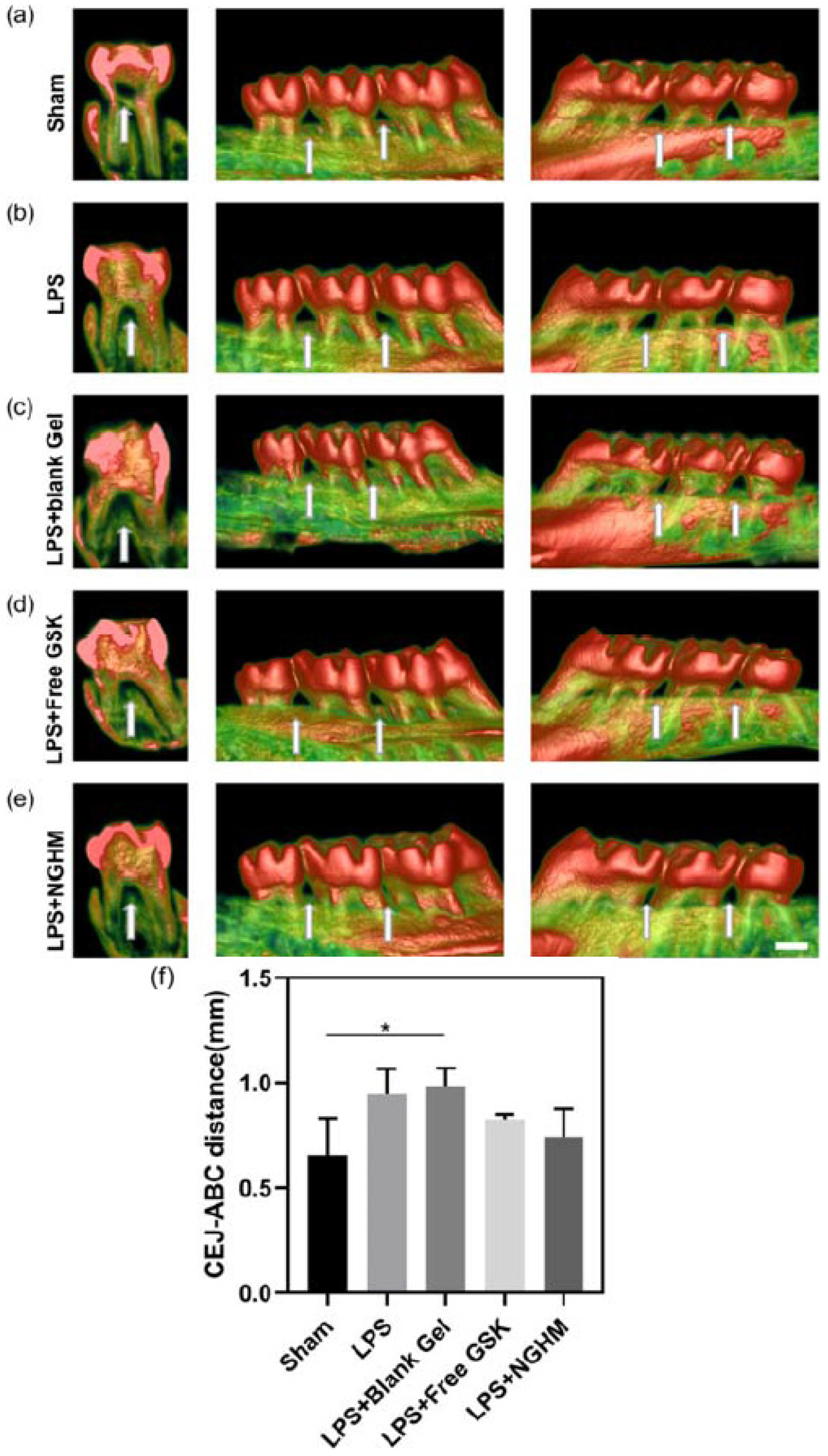
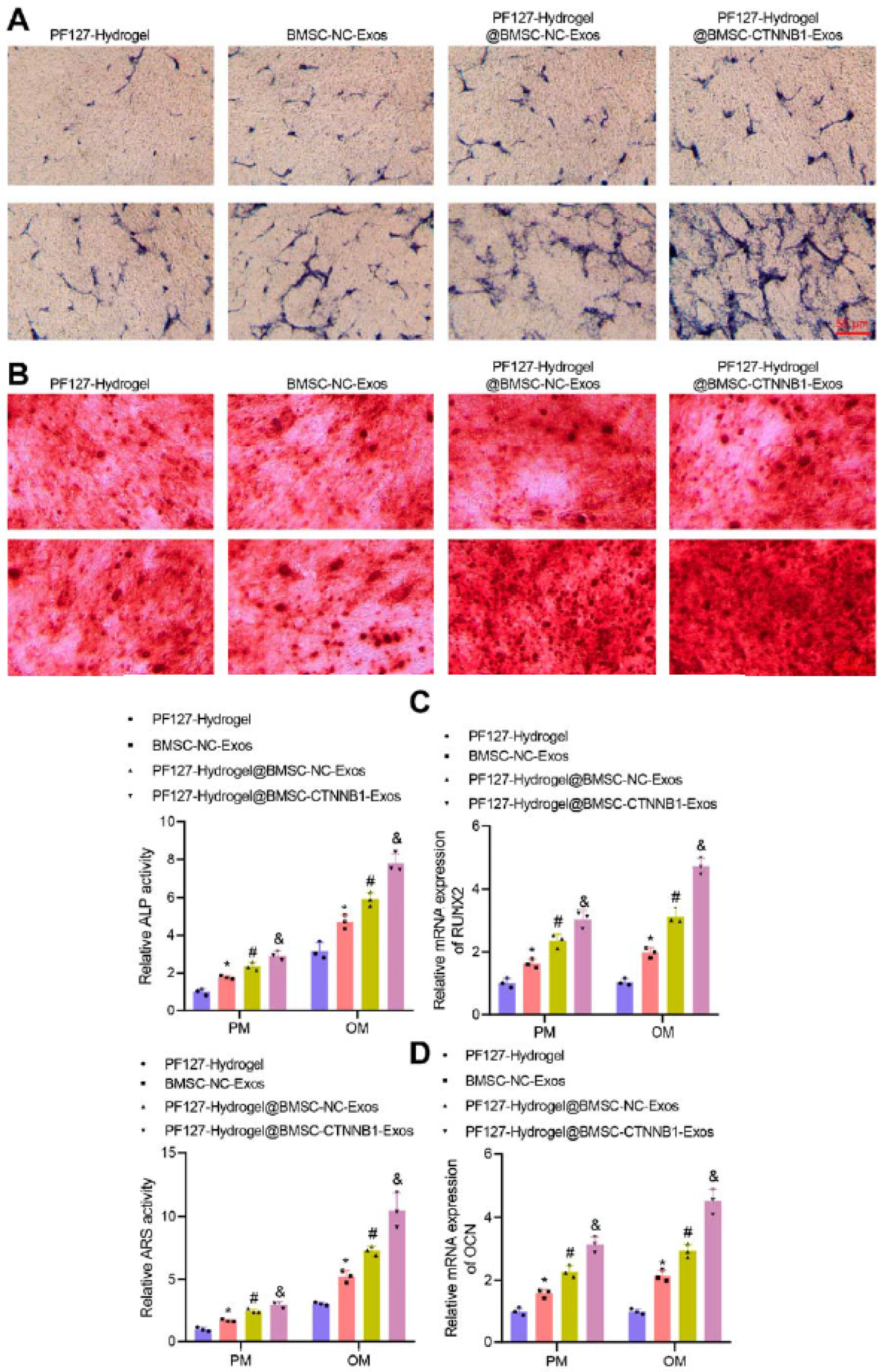
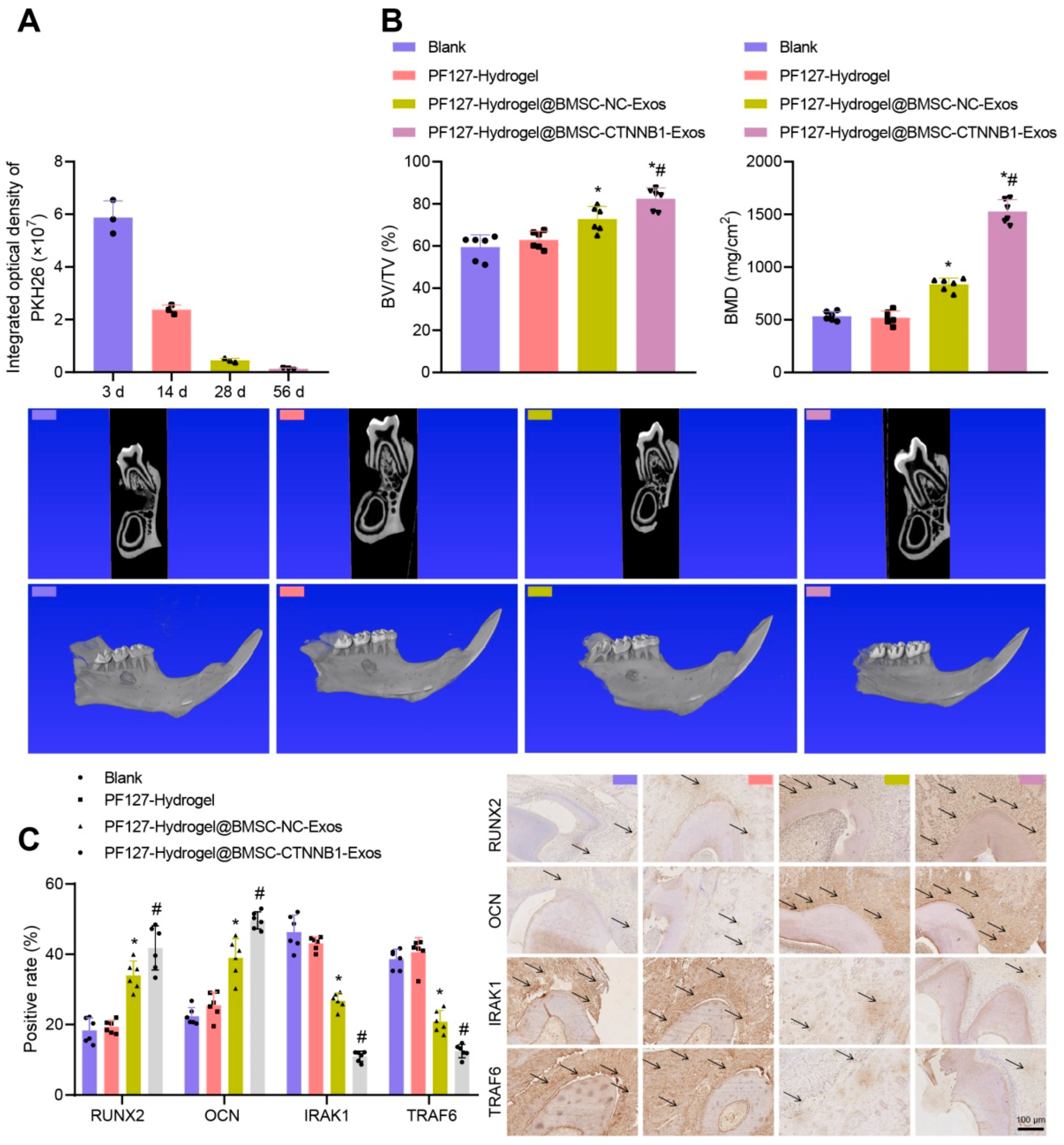
| Natural hydrogels | Advantages | Disadvantages | Applications |
| Biocompatibility | Poor mechanical properties | Wound healing (collagen, fibrin, chitin, chitosan, HA, pullulan) | |
| Biodegradability | High water content | Wound dressing (albumin, silk fibroin, wheat gluten, alginate, cellulose) | |
| Typically, inexpensive | Source-induced batch variability | Drug delivery (gelatin, albumin, sericin, soy protein isolate, chitosan, alginate, carrageenan, cellulose, starch, dextran, pullulan) | |
| Derived from substances found within the ECM in vivo | Poor stability over a long period of time | 3D scaffolds (silk fibroin, gelatin, soy protein isolate, dextran, xanthan gum, cellulose, chitin | |
| Can be modified to include binding sites or alter stiffness | May lack reproducibility | ||
| Promotes cell adhesion, proliferation and growth | Variable solubility in water Sensitive to environment and pH | ||
| Synthetic hydrogels | Customizable composition | Large excess of water | Wound Healing (as dressings) |
| Modifiable stiffness | May have cytotoxic effect | Drug delivery systems: -Targeted release (encapsulated antibacterial agents, fluoride, or other therapeutic compounds) for localized treatment of oral infections, caries, and periodontitis -Controlled release (to release drugs slowly and in response to stimuli, such as pH or temperature changes) | |
| High Reproducibility | Requires addition of binding sites to allow cells to adhere | Tissue scaffolds in periodontal or dental pulp regeneration | |
| High durability, Better processability, and tunable characteristics | Lower cytocompatibility, can be biologically inert, more costly to produce, and may pose toxicity risks if they release harmful byproducts upon degradation. | -Orthodontic tooth movement regulation -Enamel and dentin remineralization |
| Hydrogel | Advantages | Constraints |
|---|---|---|
| Collagen | Excellent biocompatibility; mimics natural ECM; supports cell adhesion and proliferation | Weak mechanical strength; fast degradation; immunogenicity (bovine sources) |
| Gelatin | Thermo-responsive; cost-effective; easy to modify | Poor mechanical properties; enzymatic degradation in vivo |
| Chitosan | Antibacterial; hemostatic; promotes osteogenesis | Limited solubility at physiological pH; low elasticity |
| Alginate | Easy gelation (ionic crosslinking); non-immunogenic | Poor cell adhesion; brittle mechanical properties |
| Hyaluronic Acid (HA) | Promotes cell migration and angiogenesis; highly hydrophilic | Rapid degradation; weak mechanical strength |
| Fibrin | Autologous source; supports angiogenesis and cell migration | Fast degradation; poor mechanical integrity |
| Hydrogels | Composition and Origin | Biocompatibility and Bioactivity | Mechanical Properties and Stability | Degradation and Biodegradability | Processability and Functionalization |
|---|---|---|---|---|---|
| Natural | Derived from biological sources such as proteins (e.g., gelatin, collagen) or polysaccharides (e.g., alginate, chitosan, hyaluronic acid) | -Highly biocompatible -Intrinsic bioactivity (e.g., cell adhesion, enzymatic degradation) | -Poor mechanical strength and sensitivity to environmental conditions (e.g., temperature, pH) | Degradable through enzymatic or hydrolytic pathways | -Limited processability -Functionalization is possible but complex |
| Synthetic | Engineered from polymers like polyethylene glycol (PEG), polyvinyl alcohol (PVA), or polyacrylamide | -Generally inert -Non-immunogenic | -Tunable and superior mechanical properties, -Durability, -Elasticity, -Responsiveness | -Controllable degradation -Non-degradable, toxic residues | -Highly processable -Precise functionalization for drug delivery, sensing, or mechanical tuning. |
| Hybrid | Blend natural and synthetic polymers to combine the bioactivity of natural materials with the tunability of synthetic ones | Balance between natural components (bioactivity), and synthetic (stability and strength) | -Improved mechanical performance -Biofunctionality | Enable tunable degradation rates | Ease engineered for specific applications |
| Tissue Type | Main Function | Major Causes of Damage | Regeneration Focus |
|---|---|---|---|
| Gingival tissue | Barrier, esthetics | Periodontitis, trauma, surgery | Epithelial barrier, vascularization, contour |
| Periodontal ligament | Tooth anchorage, shock absorption | Periodontitis, trauma | Fiber orientation, cementum-PDL-bone interface |
| Alveolar bone | Tooth support | Periodontitis, trauma, extraction | Bone volume, density, vascularization |
| Oral mucosa | Protective lining | Burns, ulcers, surgery | Rapid coverage, elasticity, keratinization |
| Dental pulp | Sensory, dentinogenesis | Caries, trauma | Angiogenesis, neurogenesis, odontogenesis |
| Dentin | Structural support | Caries, abrasion, fracture | Odontoblast stimulation, mineral deposition |
| Application Area | Common Hydrogel Types | Functional Role |
|---|---|---|
| Periodontal regeneration | Collagen, chitosan, alginate | Scaffold for PDLSCs, growth factor delivery, fiber orientation |
| Bone/alveolar ridge regeneration | Alginate, gelatin, collagen | Osteoconduction, osteoinduction, injectable defect filling |
| Soft tissue repair | Collagen, hyaluronic acid | Moisture retention, fibroblast/keratinocyte proliferation |
| Dental pulp regeneration | Collagen, GelMA | DPSC delivery, angiogenesis, odontogenesis |
| Drug/growth factor delivery | Chitosan, gelatin, alginate | Sustained local release, targeted therapy |
| Wound healing, antiinflammatory | Chitosan, Aloe Vera gel, alginate | Moist healing, infection control, inflammation modulation |
| Challenge | Primary factor | Impact on Application |
|---|---|---|
| Mechanical weaknesses | Low tensile or compressive strength; bioactive compounds -induced matrix softening | Limits used in load-bearing oral sites |
| Immunogenicity and biodegradation control | Allergic reactions; unpredictable degradation in vivo | Risk of inflammation, scaffold mismatch |
| Scaling up and translation | Raw material variability; stability issues | Hinders reproducibility and market readiness |
| Regulatory and ethical barriers | Device–drug combination regulations; sustainability concerns | Slows approval, raises compliance demands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, M.; Popa, M.; Calderón Moreno, J.M. Next-Generation Natural Hydrogels in Oral Tissue Engineering. Pharmaceutics 2025, 17, 1256. https://doi.org/10.3390/pharmaceutics17101256
Chelu M, Popa M, Calderón Moreno JM. Next-Generation Natural Hydrogels in Oral Tissue Engineering. Pharmaceutics. 2025; 17(10):1256. https://doi.org/10.3390/pharmaceutics17101256
Chicago/Turabian StyleChelu, Mariana, Monica Popa, and José María Calderón Moreno. 2025. "Next-Generation Natural Hydrogels in Oral Tissue Engineering" Pharmaceutics 17, no. 10: 1256. https://doi.org/10.3390/pharmaceutics17101256
APA StyleChelu, M., Popa, M., & Calderón Moreno, J. M. (2025). Next-Generation Natural Hydrogels in Oral Tissue Engineering. Pharmaceutics, 17(10), 1256. https://doi.org/10.3390/pharmaceutics17101256








