1. Introduction
Antimicrobial resistance (AMR) is among the biggest challenges in modern healthcare. The increasing ineffectiveness of broad spectrum antibiotics, but more importantly also of last-resort antibiotics towards several bacterial pathogens, causes a large number of deaths every year. In the EU, more than 35,000 patients die from AMR-related infections every year, a number comparable to the combined death rate caused by influenza, tuberculosis, and acquired immunodeficiency syndrome (AIDS) [
1]. Worldwide, the number of deaths in the context of AMR is estimated to be more than 700,000 per year and is predicted to rise according to the World Health Organization (WHO) to more than 10 million by 2050 [
2].
One of the main causes for such an increase in mortality is the limited options for the efficient treatment of antimicrobial-resistant pathogens and also a lack of rapid diagnosis. This results in common nosocomial pathogens, including
Enterococcus faecium,
Staphylococcus aureus,
Klebsiella pneumoniae,
Acinetobacter baumannii,
Pseudomonas aeruginosa, and
Enterobacter spp. (known as the ESKAPE group), becoming drastically increasingly multidrug resistant within the last years [
3]. Among the ESKAPE pathogens,
Pseudomonas aeruginosa, which is also listed as a critical priority 1 pathogen in the WHO’s priority list for the research and development of new antibiotics [
4], is one of most common pathogens causing life-threatening respiratory tract infections, for example, in human immunodeficiency virus (HIV) -infected [
5] and CF [
6] patients. Other common infections caused by this pathogen are diabetic foot, urinary tract, bloodstream, and skin and soft tissue infections [
7,
8,
9]. A big challenge in medicine is the targeted treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR)
Pseudomonas aeruginosa that requires susceptibility screening for the available drug classes to circumvent resistance mechanisms [
10]. Additionally, tolerance mechanisms due to strong biofilm formation capacity can exacerbate recalcitrant bacterial infections [
11].
To treat MDR bacteria, aPDT has been investigated. Due to its fast and efficient bactericidal effect and its lower risk of resistance build-up to a variety of different bacterial pathogens [
12], aPDT has been shown to be a promising alternative for the treatment of microbial infections. The principle of aPDT is based on the excitation of PS molecules with light to locally generate reactive oxygen species (ROS), including
1O
2, at the target site. Since the early 1990s, many different systems have been investigated for aPDT, ranging from novel photosensitizers over photoactive surfaces and different kinds of nanoparticles, fibers, and nanocarriers of various origins [
12,
13,
14].
One of the main advantages of aPDT is the localized on-demand treatment of infections caused by MDR bacteria. It has been shown that aPDT possesses a very low risk of contributing to resistance build-up, since the killing of bacteria is based on the action of ROS, which addresses multiple targets in the bacterial cell and hence impedes the resistance build-up [
12]. For many common MDR bacteria, aPDT has already been proven to be a strategy to avoid resistance build-up [
15,
16].
However, many combinatory approaches for aPDT usually only reach a log reduction factor (LRF) < 3. For instance, methylene blue (MB) -loaded electrospun nanofibers showed an LRF of 3 [
17], while MB or erythrosine B encapsulated in PCL fibers showed an LRF of only 1 [
18]. Inhibitory (not bactericidal) effects were reported for MB in chitosan-loaded silver nanoparticles [
19]; PLA nanofibers loaded with indocynine green and curcumin showed a combined LRF > 4 [
20]. Various studies have been carried out to improve the efficiency of aPDT using higher irradiation doses or higher PS concentrations to achieve higher LRF values [
21,
22,
23].
Furthermore, different metal-based PSs have also been investigated for aPDT effects for various applications. In 2022, de Siqueira et al. reviewed phthalocyanines containing aluminum, cobalt, gallium, silicon, and zinc as PSs in different nanocarriers, such as liposomes, for studying aPDT against bacteria, fungi, viruses, and protozoans [
24]. However, many known PSs used for aPDT show only limited biocompatibility, limited solubility in aqueous or biological media, dark toxicity, and reduced photoactivity in the aggregated state, which leads to difficulties in applications [
25,
26].
The use of Ru complexes as PSs offers several advantages compared with other PSs. Ru(II) -based complexes have been extensively studied for PDT effects against fatal cancers and some lead complexes undergo phase II clinical studies for PDT treatment of bladder cancer [
27,
28]. Despite this, challenges with the solubility and stability in aqueous media remain to be improved to achieve optimal performance in medical applications.
One method to overcome such limitations is adapted from the drug delivery of water-insoluble drugs. Among the carriers established for encapsulation approaches are polymeric micelles, polymersomes, liposomes, and different kinds of nanoparticles [
29]. Important for such systems is the ability to remain in circulation without passively releasing the payload until reaching the infection site and then releasing the cargo on demand. One method to improve the pharmacokinetics and stabilized circulation within the body is the PEGylation of the nanocarriers, as used in cancer therapy [
30,
31].
Thus, in this current study, a PEG-based polymer was chosen for the encapsulation of a ruthenium-based photosensitizer in polymeric nanocarriers to increase the solubility and biocompatibility of the PS. Further, the enzyme–labile polymer PLA as the hydrophobic block affords the possibility to potentially release the photosensitizer on demand. In the presence of proteinases, produced by
Pseudomonas aeruginosa in larger concentrations at the infection site [
32], PLA is enzymatically cleaved. Corresponding PLA block copolymers have been previously reported for the encapsulation of signaling molecules and their triggered release in the presence of, e.g., the model enzyme proteinase K [
33,
34]. In this current study, this strategy was adapted and applied to the encapsulation of a potent Ru-based photosensitizer that was reported to show an aPDT effect against planktonic cultures of
Pseudomonas aeruginosa and
Staphylococcus aureus strains [
12,
35,
36]. In particular, the effectiveness of aPDT against
Pseudomonas aeruginosa was studied for PEG
114-
block-PLA
x micelles and vesicles (polymersomes).
3. Results
For the study of the encapsulation and release and the aPDT efficiency of the PS RuPhen3, a series of PEG-
b-PLA block copolymers with different lengths of PLA block was synthesized. As reported in the literature, micelles are typically formed by PEG-
b-PLA polymers with shorter PLA chains and a mass fraction of PLA below 0.5. The morphology of the assemblies depends nevertheless on the polymer composition and experimental conditions of the assembly process [
37,
38,
39,
40]. However, micellar PEG-
b-PLA structures with PLA fractions above 0.5 and polymersomes with PLA fractions below 0.5 have also been reported [
41,
42,
43]. Thus, polymers with a PLA fraction of more than 0.80 are expected to form polymersomes, while PLA fractions close to 0.5 might lead to formation of either polymersomes or micelles, or even mixtures, depending on experimental conditions.
3.1. Synthesis and Characterization of PEG114-b-PLA143 and PEG114-b-PLA499
A library of amphiphilic PEG
114-
b-PLA polymers with nominal block lengths between 50 and 500 units was synthesized via ring-opening polymerization of cis-lactide according to
Figure 1, using the macroinitiator MeO-PEG
114-OH for PLA, with yields of 82–87%. The synthesized polymers were characterized using
1H-NMR spectroscopy and TGA to determine the average number of PLA repeat units and to verify the composition.
The
1H-NMR spectra showed the characteristic proton signals for mPEG
114 and PLA and the residues of the used monomer (cis-lactide), as shown in
Figures S1–S7 (Supporting Information). Due to the known unreliability of the gel permeation chromatography (GPC) analysis for this polymer, TGA was used to determine the weight fraction of PEG and PLA of the synthesized polymers. Our GPC results in THF and water differed from the
1H-NMR spectroscopy and TGA results, which is in line with previous reports in the literature for comparable systems, especially for polymers with larger PLA blocks, due to the amphiphilic nature and solubility differences in various solvents [
44,
45,
46]. The TGA traces showed two distinctive mass losses at decomposition temperatures of PLA (150–250 °C) and PEG (330–400 °C), which allowed one to roughly estimate the PEG/PLA ratio, as seen in
Figures S8 and S9 and
Table 1. The values for the mass fractions of PLA were converted into the mass average degree of polymerization. These values were found to be consistent with the values for the number average degree of polymerization obtained from
1H-NMR. The degree of polymerization obtained by TGA was in both cases to within a few % identical to the degree of polymerization determined by
1H-NMR (
Table 1). The experimentally determined degree of polymerization also matched the nominal values of both polymers to within ~5%.
From the panel of block copolymers, we chose PEG114-b-PLA143 and PEG114-b-PLA499 for further investigations, since these polymers can be expected to form micelles and polymersomes, respectively, considering the mass fraction of PLA (see above).
3.2. Encapsulation of RuPhen3 Photosensitizer into PEG114-b-PLA143 and PEG114-b-PLA499 Nanocarriers
PEG
114-
b-PLA
143 and PEG
114-
b-PLA
499 were used for the formation of polymersomes and micelles to encapsulate the PS via the solvent shift method, as mentioned in
Section 2.2.2.
The dispersions were characterized by DLS analysis to determine the mean hydrodynamic diameter of formed assemblies, respectively, as shown in
Figures S9–S12 and
Table 2. The size distributions by number obtained by DLS showed for all samples a monomodal distribution with a mean hydrodynamic diameter of close to 100 nm. Furthermore, the diameter of loaded nanocontainers was in all cases larger than non-loaded micelles and polymersomes. The values for the hydrodynamic radii are summarized in
Table 2 and are shown in
Figures S9–S12.
The mean hydrodynamic diameter increased upon the addition of RuPhen3 during the self-assembly for the observed polymers. However, the influence seemed to be larger on the PEG114-b-PLA143 assemblies. The increase in Ruphen3 amounts during formation of assemblies did not show a significant change in mean hydrodynamic diameter of the respective assembly. Repetitions of self-assembly experiments in the presence of RuPhen3 with the polymers under the same conditions showed similar results regarding size distribution and loading efficiency.
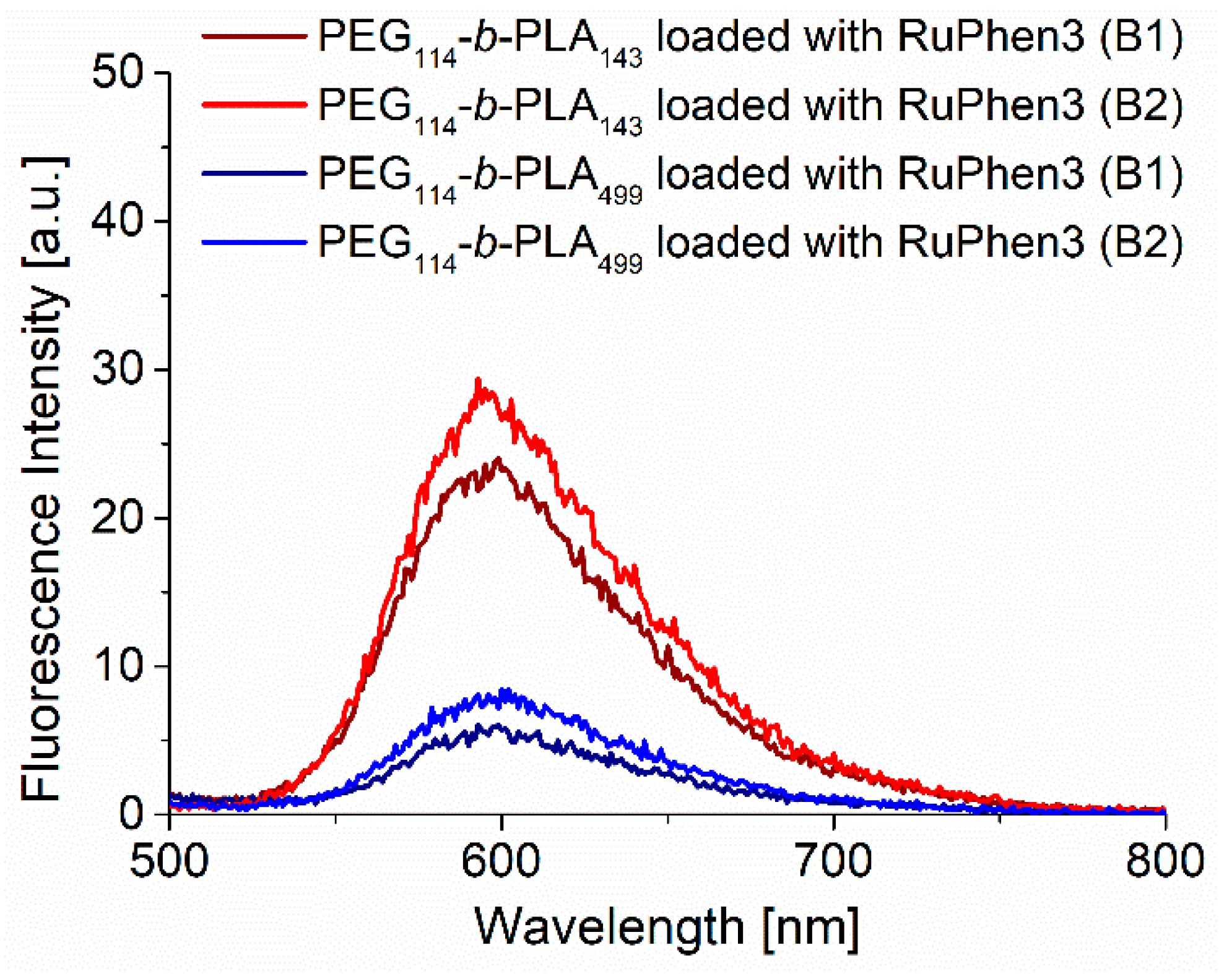
The concentration of RuPhen3 in the dispersions was determined by fluorescence spectroscopy, as described in Experimental
Section 2.2.3, using a calibration curve (
Figure S14). The fluorescence spectra (
Figure 2) of RuPhen3 encapsulated in PEG
114-
b-PLA
143 micelles showed a slight blue shift of around 2 nm compared with RuPhen3 encapsulated in PEG
114-
b-PLA
499 vesicles and RuPhen3 in the aqueous solution (
Figure S14). This blue shift might indicate different local environments of the photosensitizer inside the different assemblies. It was also shown that the fluorescence emission was higher for RuPhen3 encapsulated in PEG
114-
b-PLA
143 assemblies than for PEG
114-
b-PLA
499 assemblies. The doubling of the concentration of RuPhen3 during the formation of micelles and vesicles led to an increase in the RuPhen3 concentration in the final dispersion of 6% and 14%, respectively. For samples without RuPhen3, no fluorescence emission was observed.
The apparent RuPhen3 concentrations indicated a higher loading, and consequently a higher encapsulation efficiency, for the PEG
114-
b-PLA
143 micelles compared with the PEG
114-
b-PLA
499 polymersomes. The estimated concentrations of RuPhen3 from fluorescence spectroscopy and the determined mass concentrations of assemblies are summarized in
Table 3. Due to the potential quenching of RuPhen3 or energy transfer processes, these estimates may lead to underestimating the overall RuPhen3 concentration.
The nanocontainer dispersions showed a mass concentration of polymeric assemblies between 1.5 and 2.0 mg/mL, depending on the loading and the used polymer. The mass concentration of loaded polymersome and micelle dispersions was in all cases significantly higher, ranging from 1.8 to 1.9 mg/mL, compared with non-loaded assemblies, which exhibited values between 1.4 and 1.5 mg/mL. This mass increase was only in part due to the encapsulated RuPhen3, which would constitute a mass difference of around 0.8 mg/mL, assuming 100% of added RuPhen3 to be encapsulated. However, the mass increase was not proportional to the determined RuPhen3 concentrations of around 54.0 and 15.5 µM (B2) for PEG
114-b-PLA
143 and PEG
114-b-PLA
499 respectively, which would cause a mass difference of less than 0.1 mg/mL, but was similar for all RuPhen3-loaded samples (1.8 to 1.9 mg/mL). The higher mass concentration under identical conditions could further be attributed to the formation of larger assemblies for RuPhen3-loaded nanocarriers (B1 and B2) compared with non-loaded batches, as shown in
Table 2 (
Figures S9, S10 and S12).
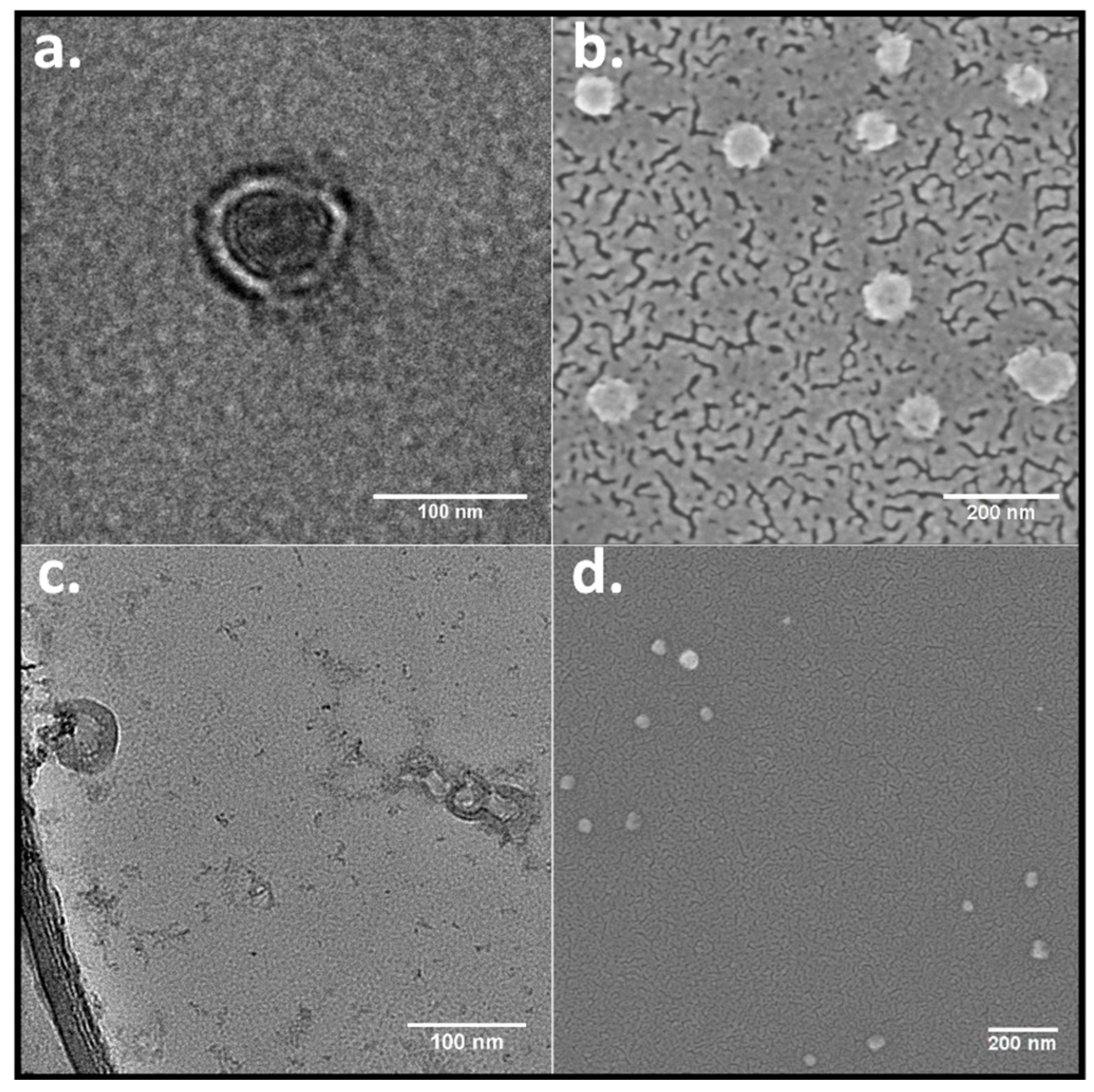
To confirm the morphology of the RuPhen3-loaded nanocontainers, TEM and FE-SEM microscopy images were acquired. The PEG
114-
b-PLA
143 assemblies appeared in the TEM images (
Figure 3a) as filled particles, consistent with block copolymer micelles, whereas PEG
114-
b-PLA
499 formed assemblies with clearly discernible walls around inner circular areas that exhibited light contrast [
47]. The appearance was consistent with the morphology of polymersomes. An estimate for the wall thickness afforded a mean value of 17 ± 4 nm, determined from the data of several single polymersomes, as shown in
Figure 3c, by equating the difference of the outer and the inner diameter of the polymersomes. These values showed to be similar to the wall thickness for similar PEG-
b-PLA polymersomes determined in the literature [
48], stating a wall thickness of 21 ± 4 nm determined from TEM images. For this purpose, the polymersome wall thickness was measured several times at different positions for each polymersome. Consistent with all these observations, the FE-SEM images show assemblies that were predominantly spherical in shape for both polymers (
Figure 3b,d).
The overall concentration of encapsulated RuPhen3 was further used to roughly estimate the amount of encapsulated RuPhen3 per nanocarrier, considering the mass concentration and average hydrodynamic diameter. The maximum amount of RuPhen3 that could be encapsulated per nanocarrier was estimated, considering the mass concentration, total amount of encapsulated RuPhen3, average hydrodynamic diameter, and approximate wall thickness of polymersomes (17 ± 4 nm) estimated from TEM micrographs, as shown in
Figure 3. The volume for micelles and shell volume for vesicles, where RuPhen3 was expected to be located due to its better solubility in hydrophobic environments, was calculated using the hydrodynamic diameter determined by DLS, assuming that all nanocarriers were spherical in shape. Furthermore, the number of micelles and vesicles per volume unit were estimated considering the mass concentration of nanocarriers in dispersion and average hydrodynamic diameter determined by DLS. The density of the polymer was assumed to be around 1.2 g/cm
3, with respect to the density of PEG stated by the supplier and PLA polymers [
49]. This estimation indicated that larger amounts of RuPhen3 were encapsulated in the internal volume of micelles (~7 × 10
3 molecules per micelle, 35 mM) in comparison with vesicles with approximately 1 × 10
3 molecules per polymersome (10 mM), where RuPhen3 was expected to be localized in the shell volume. These values were an order of magnitude higher than concentrations determined for similar ruthenium-based photosensitizers inside PLGA nanoparticles developed for potential use in photodynamic therapy, as described by Bœuf et al. [
50].
3.3. Increasing the Number Concentration of Loaded Micelles and Polymersomes
The concentrations of RuPhen3 in the polymersome dispersions (15.5 μM) were potentially too low to show a sufficient reduction in bacteria in incubation and irradiation experiments, since these experiments required further dilution of the dispersion. As previously reported, RuPhen3 and comparable complexes showed to be effective down to concentrations of 10 µM [
16]. Preliminary experiments with neat RuPhen3 also confirmed this and showed that RuPhen3 concentrations of <10 μM were no longer affecting the bacterial growth. Hence, the concentration of micelles and polymersomes was increased by upconcentrating the dispersions of micelles and vesicles. To this end, the dispersions were centrifuged. The supernatant volume was reduced by the removal of the medium, and the pellet of sedimented nanocarriers was redispersed in a reduced volume of dispersant. This led to an increase in the mass concentration and concentration of encapsulated PS by a factor of 10.
To obtain sufficient volumes of loaded micelle and polymersome dispersions for different incubation and irradiation experiments, the batch size was increased fourfold, as described in Experimental
Section 2.2.2 (B3). The increase in batch size led to a reduction in concentration of encapsulated RuPhen3 of around 60–70% from 54.0 ± 1.8 μM to 16.0 ± 0.2 μM for the micelles. Furthermore, a broadening of the size distribution was observed, while the mean hydrodynamic diameter was nearly constant. By contrast, the encapsulation efficiency of PS and size distribution of polymersomes for the PEG
114-
b-PLA
499 polymer were not affected significantly by the upscaling.
The number concentration of micelles or polymersomes, and thus the concentration of encapsulated PS in the final dispersions, was increased 10-fold via reducing the mass of dispersant (water) proportionally. The concentrations of PS and average hydrodynamic diameters and mass concentrations of the micelles and polymersomes are summarized in
Table 4. The size distribution of micelles and polymersomes was not markedly affected upon removal of the dispersant volume.
To prove that repetitive centrifugation did not affect the size distribution drastically, DLS was applied, and the mass concentration was determined after 10 centrifugation, supernatant exchange, and redispersion cycles. The mean hydrodynamic diameter did not change in this process, as shown in
Table 5. The mass concentration of PEG
114-
b-PLA
499 assemblies was measured before and after 3-, 5-, 7-, and 10-times centrifugation, solvent exchange, and redispersion, and remained constant at 1.9 ± 0.1 mg/mL.
3.4. Enzymatic Release of PS from Polymeric Micelles and Polymersomes
The enzymatically triggered release of PS was achieved using the model enzyme proteinase K, which was expressed among others by the fungus
Tritirachium album. This type of proteinase can hydrolyze peptide and ester bonds, such as the bonds present in PLA polymers. In line with earlier reports [
33,
34], proteinase K was expected to destabilize micelles and polymersomes containing PLA polymers, resulting in the release of encapsulated RuPhen3. The enzymatic attack should enable the released photosensitizer to diffuse to the targeted bacteria, leading to more efficient eradication of the bacteria by aPDT.
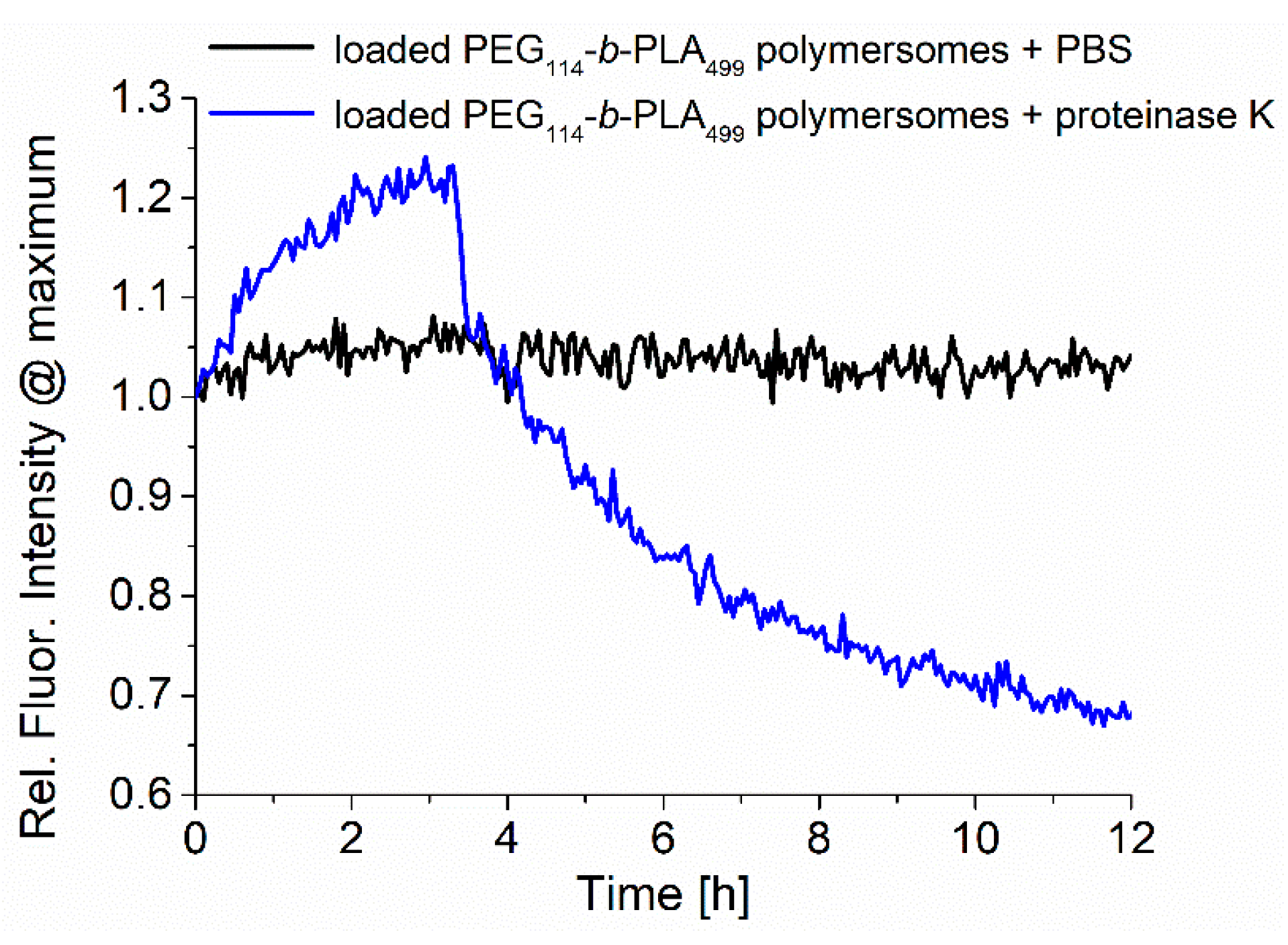
The samples were analyzed during enzymatic release by recording the fluorescence emission, as shown in
Figure 4. The normalized fluorescence spectra of RuPhen3-loaded PEG
114-
b-PLA
499 polymersomes after the addition of proteinase K in PBS (5.0 mg/mL) and neat PBS as a control were recorded for 12 h, and the maximum intensity at ~600 nm was plotted as a function time. While the fluorescence of RuPhen3 in the absence of proteinase K remained constant over time with few minor fluctuations, the presence of proteinase K led to a significant initial increase, followed by a rapid drop in fluorescence emission intensity. After 12 h, sedimentation of the polymer and PS was observed inside the cuvette, implying that the degradation of polymersomes due to the enzyme cleaving of PLA repetition units under the formation of lactic acid caused the polymersomes to disintegrate. When the sediments were redispersed, the fluorescence emission reached a level comparable to the maximum fluorescence emission observed after approximately 3 h after the addition of proteinase K. The increasing fluorescence could be caused by the release of the photosensitizer from the polymersomes and reduced quenching, caused initially by the high local concentration of PS inside the polymersomes.
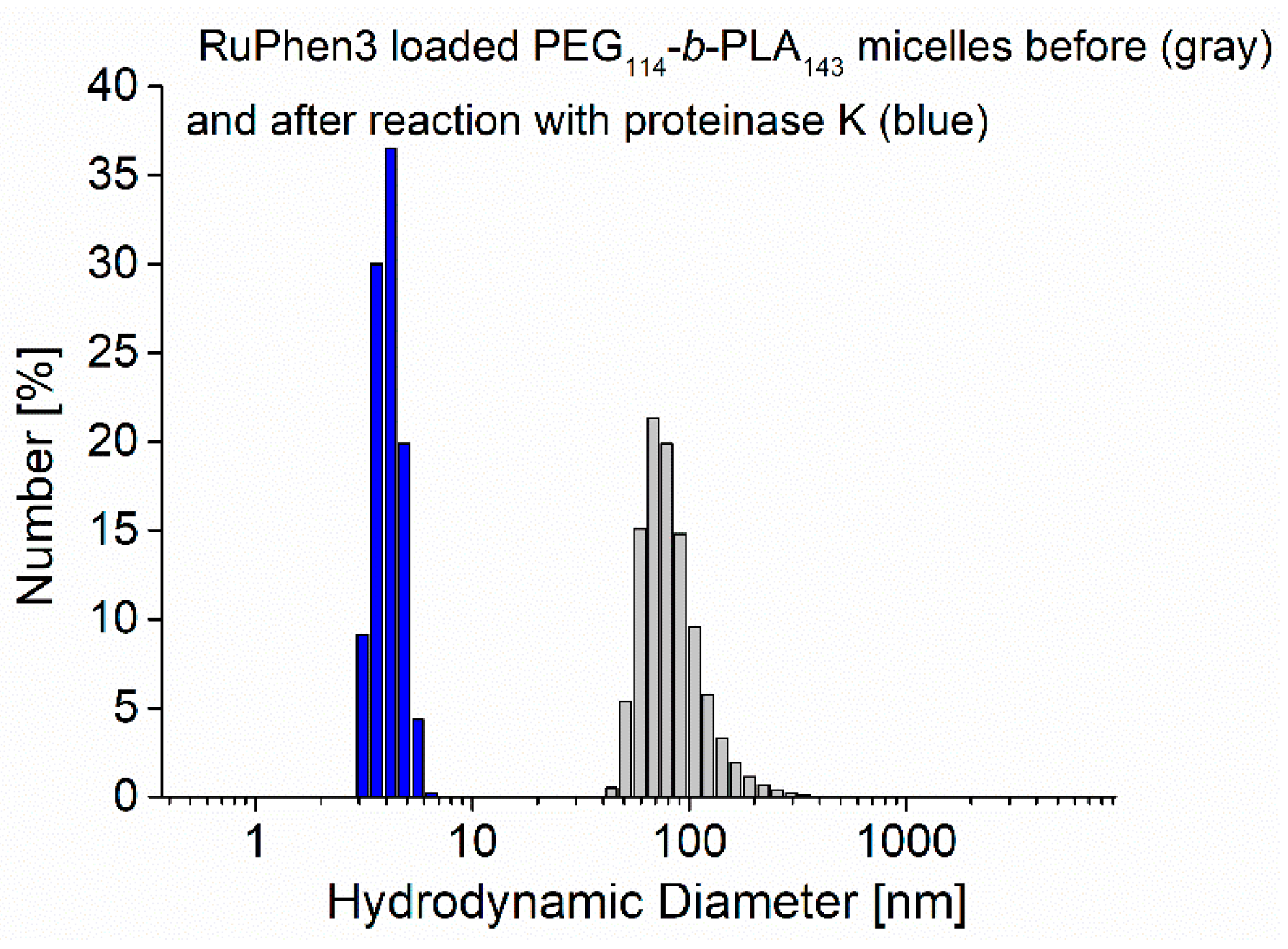
Additionally, the size distribution of the assemblies was analyzed with DLS before and 24 h after enzymatic degradation. The size distributions showed a strong shift from an initial mean hydrodynamic diameter of 73 ± 22 nm to lower values of the hydrodynamic diameter of 4 ± 1 nm, as shown in
Figure 5, which was consistent with comparable systems reported in the literature [
33,
34].
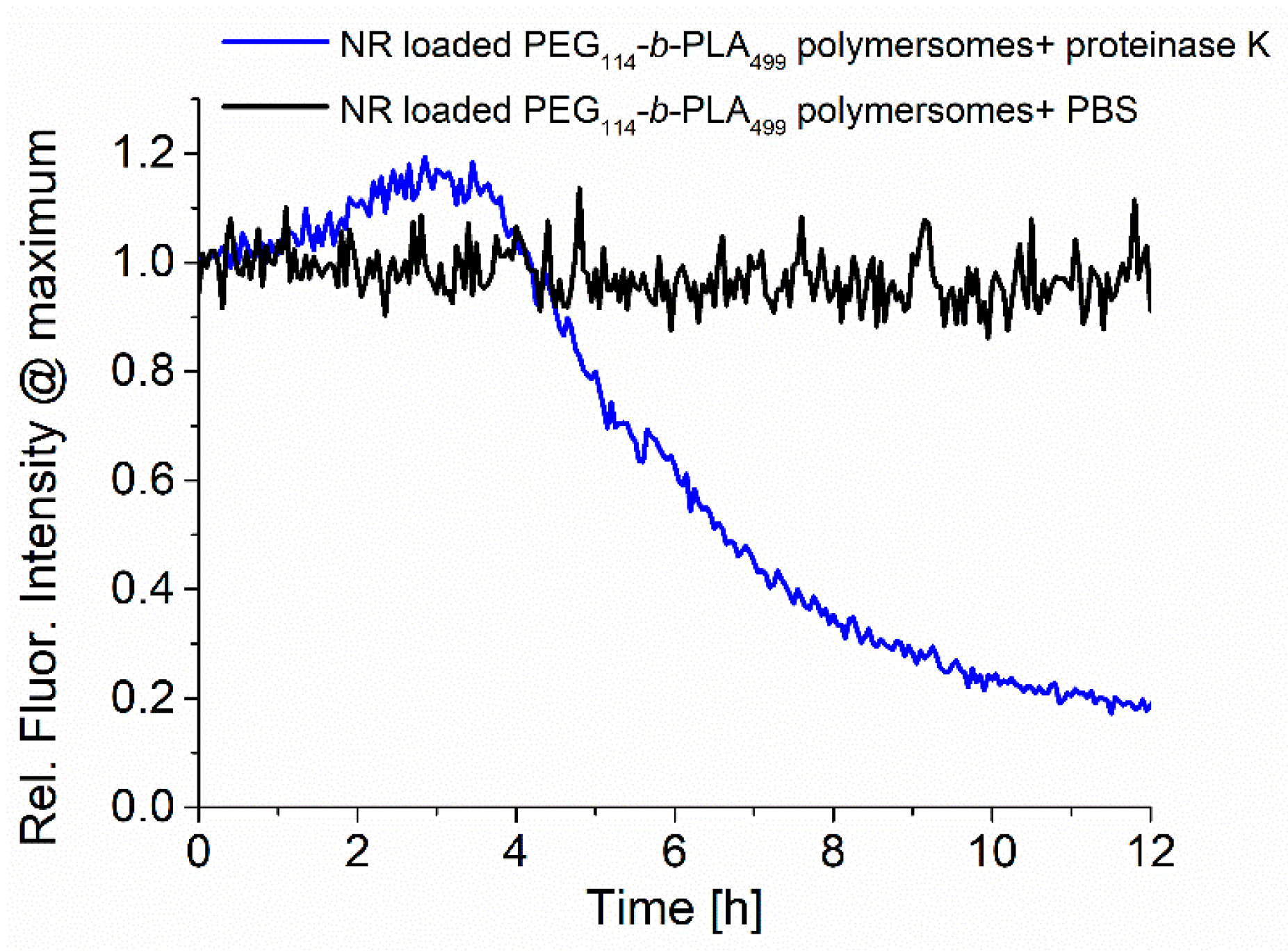
A similar behaviour could be observed for the enzymatic degradation of PEG
114-
b-PLA
499 polymersomes loaded with the model dye Nile Red, as shown in
Figure 6. The change in fluorescence emission around 620 nm showed a comparable increase within the first 3 h, followed by a rapid decrease due to the precipitation of insoluble Nile Red and polymer upon degradation of the polymersomes, as reported in the literature [
33,
34].
3.5. Detection of Singlet Oxygen Production
The well-known SOSG probe was used to detect the singlet oxygen production in both irradiated and non-irradiated samples [
51]. The fluorescence intensity of the probe signal after a certain irradiation time was measured. Fluorescence was only observed in the presence of a photosensitizer that produced
1O
2 species, which were then detected by the probe [
51].
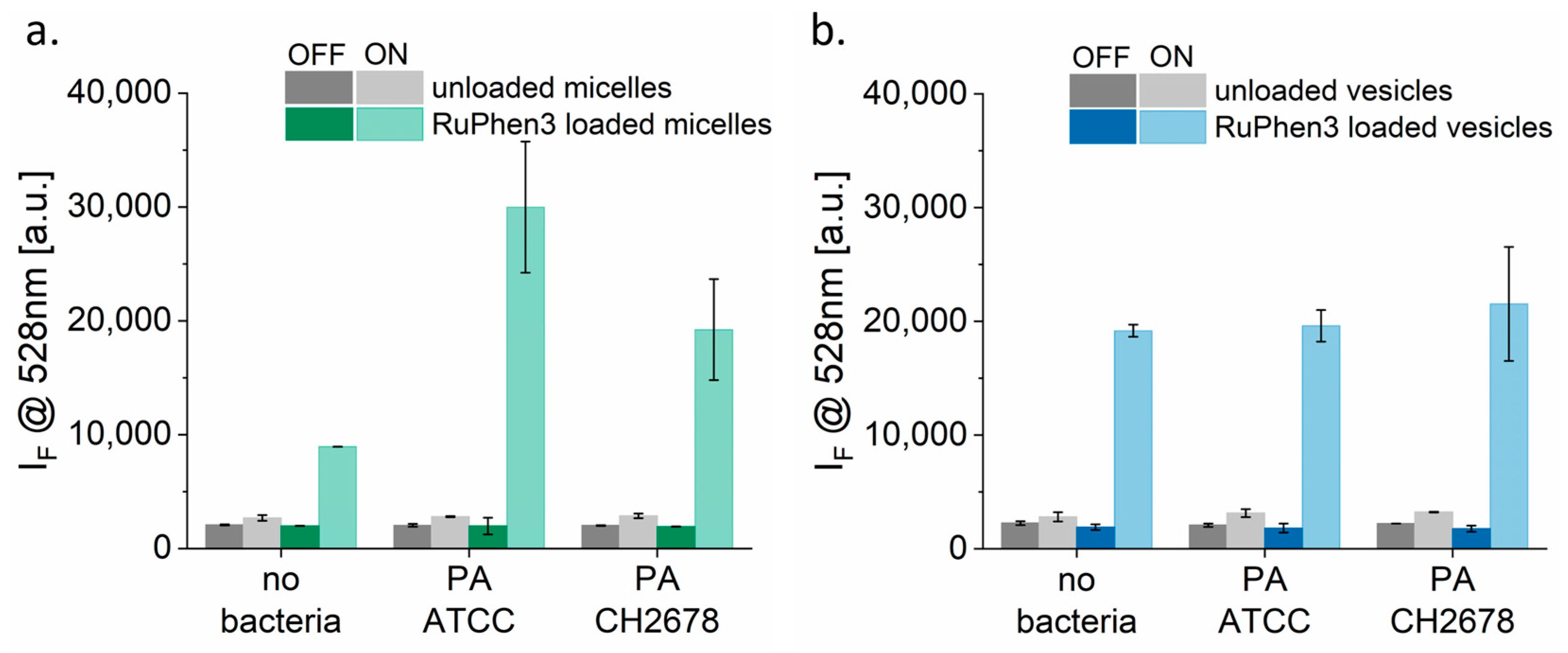
Figure 7 shows the SOSG probe signal of unloaded and RuPhen3-loaded micelles and vesicles incubated with and without bacterial strains PA ATCC and PA CH2678 in 0.9% saline. The concentrations of the RuPhen3-loaded micelles (165.1 ± 15.4 µM) and vesicles (186.9 ± 13.1 µM) given in
Table 4 were used for the experiments. For both the SOSG testing and aPDT experiments, the loaded micelles and vesicles were mixed in 1:1 ratio with the studied different mediums to have their final concentrations to be ~half of the undiluted loaded micelles and vesicles.
For unloaded micelles (
Figure 7a) and vesicles (
Figure 7b), a slight increase in probe signal was observed after irradiation. This increase corresponded to the signal originating from the SOSG probe itself, which underwent photochemistry to a fluorescent product through a different mechanism [
51]. The non-irradiated RuPhen3-loaded micelles and vesicles did not show any significant change in fluorescence intensity.
By contrast, a strong increase in fluorescence intensity was observed for irradiated RuPhen3-loaded micelles and vesicles. For loaded micelles, a significantly higher probe signal was observed for samples incubated with bacteria compared with samples incubated in 0.9% NaCl (i.e., without bacteria). The fluorescence of the probe also differed among the two bacterial strains. However, the probe signal did not vary for loaded vesicles measured with and without bacteria in sterile water or among the two strains. These observations indicate that the bacteria may affect the micelles differently compared with the polymersomes.
For the controls, the probe itself (diluted in tris buffer, pH 7.5) and both bacterial strains (in 0.9% saline) were also irradiated in the presence of SOSG (
Figure S18a). After irradiation, a small increase in fluorescence intensity was observed in all cases that corresponded to the mentioned side reaction of SOSG probe during irradiation [
51]. In addition, a significant increase in probe signal was observed after the irradiation, when neat RuPhen3 was incubated without and in the presence of the bacterial strains, at the same concentrations as both the micelles and vesicles (
Figure S18b). A higher probe signal was observed when RuPhen3 was irradiated and incubated in bacterial suspensions compared with the bacteria-free solution. As the intensity was lower in the absence of any polymer, this indicated that the probe may partition into the nanocontainers’ PEG corona, which in turn may alter the reacted probes’ quantum yield.
3.6. aPDT Effect on Planktonic Bacteria
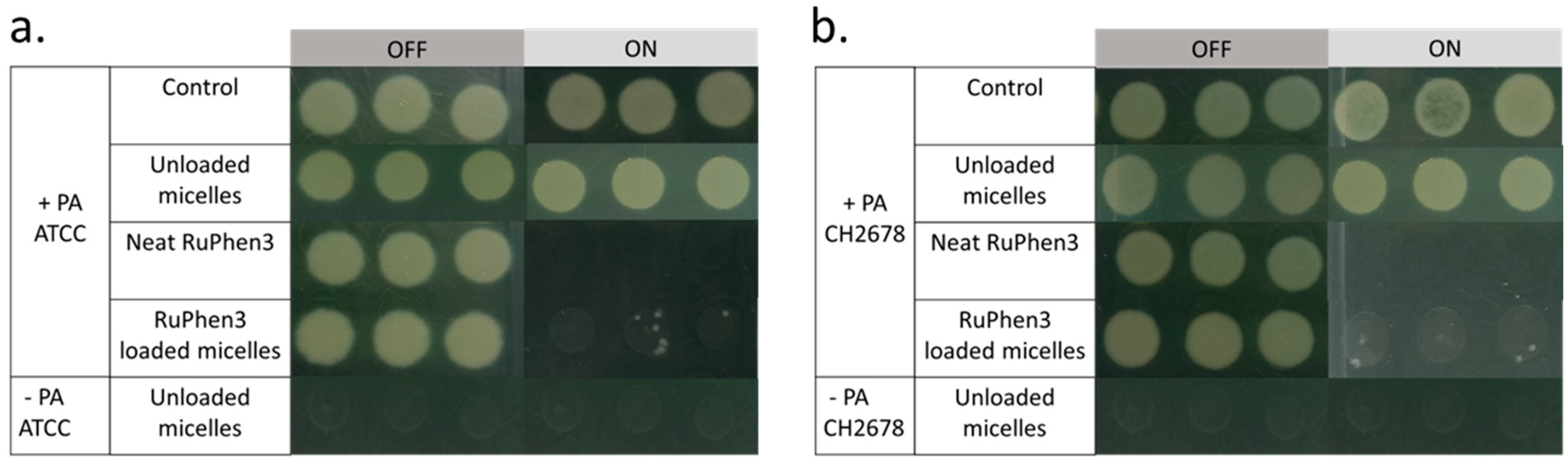
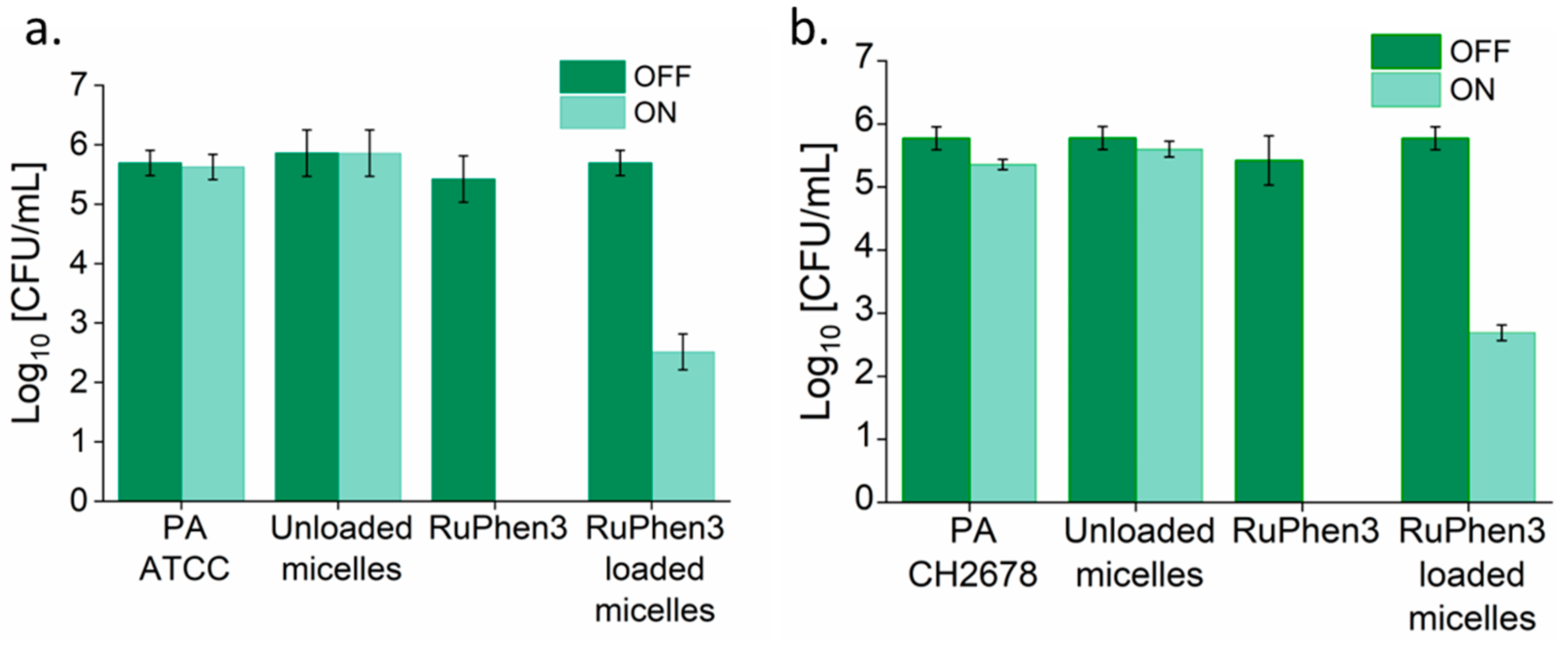
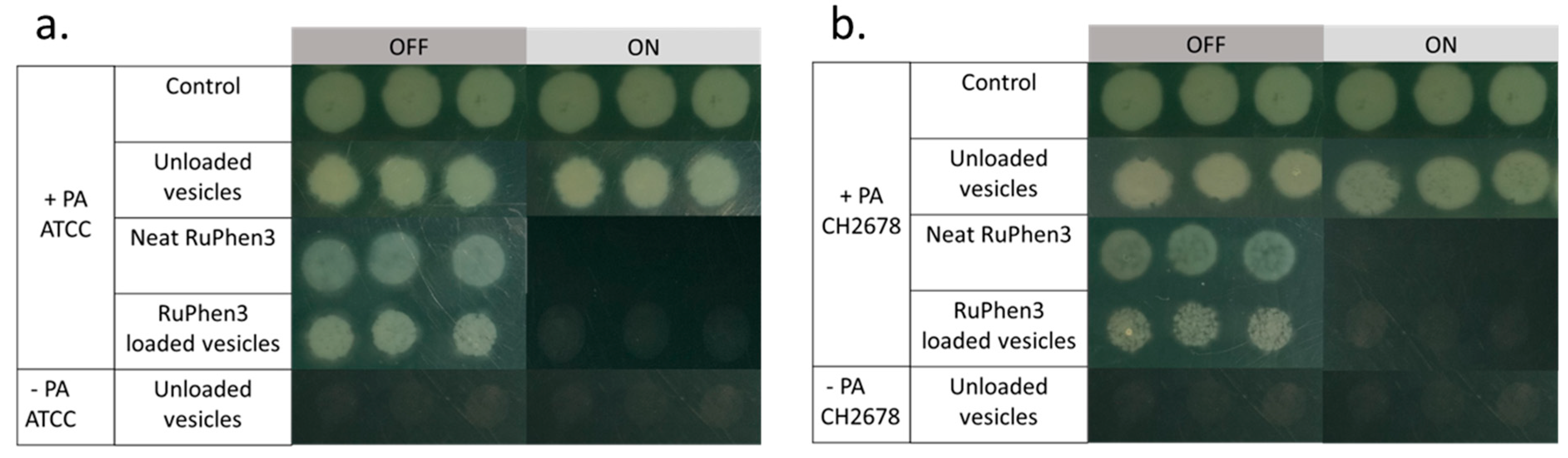
To investigate the aPDT effect of the RuPhen3-loaded micelles and vesicles on PA ATCC and PA CH2678, the nanocarriers were added to the respective bacterial suspensions and were subjected to irradiation following incubation for 15 min. Afterwards, serial dilutions of each sample were performed and three times 5 µL of each dilution was plated to count the CFU/mL for both the irradiated and non-irradiated samples. For comparison, the aPDT effect of neat RuPhen3 was also investigated. The spotting results of the bacteria colonies of RuPhen3-loaded micelles for non-irradiated and irradiated samples incubated with both bacteria strains are shown in
Figure 8. No effect of irradiation was observed for the bacteria controls and unloaded micelles incubated with bacteria suspension. After irradiation, neat RuPhen3 showed no visible colonies, while, for RuPhen3-loaded micelles, very few colonies were visible. The faint background color of RuPhen3-loaded micelles after irradiation was very similar to that of spotted unloaded micelles. No dark toxicity was obvious.
Analysis of the serial dilutions afforded the log
10 CFU/mL values of the RuPhen3-loaded micelles incubated with each bacterial suspension (
Figure 9).
The non-irradiated samples showed similar CFUs compared to the corresponding bacteria controls. Neat RuPhen3 showed no countable bacteria after the irradiation, while the RuPhen3-loaded micelles showed a > 3 log10 CFU/mL for each bacterial strain after the irradiation.
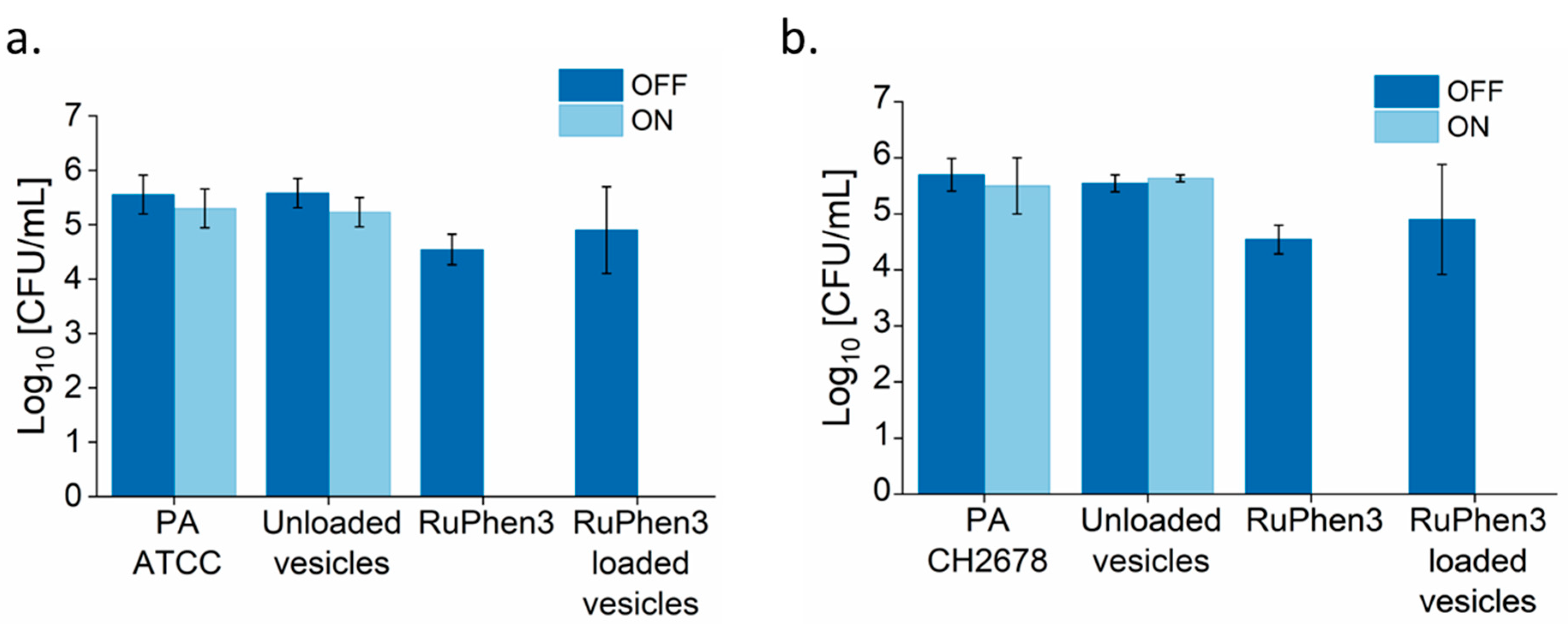
Similarly, the aPDT effect was investigated for RuPhen3-loaded vesicles.
Figure 10 shows the spotting results of bacterial colonies for non-irradiated and irradiated samples. After the irradiation, there were no colonies observed for both the neat RuPhen3 and RuPhen3-loaded vesicles. Unloaded vesicles were also plated and resulted in very faint but discernible spots that were attributed to the scattering of light due to the dried vesicles. Hence, the very faint spots observed in
Figure 10 for irradiated vesicle samples were attributed to the vesicles themselves.
The dilutions were analyzed and the log
10 CFU/mL values of the RuPhen3-loaded vesicles were then calculated (
Figure 11). No changes in CFU/mL were observed for the non-irradiated samples. Both for neat RuPhen3 and RuPhen3-loaded vesicles, no countable CFUs were observed after the irradiation.
The LRF values were then calculated by comparing each sample to respective non-irradiated bacterial suspensions. While calculating the LRF values, the LOD values of the biological experiments (67 CFU/mL) were also explicitly considered, which meant that the LRF values reported here were lower bounds.
Table 6 summarizes the resulting LRFs of the RuPhen3-loaded micelles and vesicles. For the non-irradiated RuPhen3-loaded micelles and vesicles, an LRF < 0.8 was observed. For irradiated samples, LRF values > 3 were observed for loaded micelles, while LRF values > 4.7 were observed for RuPhen3-loaded vesicles. The RuPhen3-loaded vesicle formulations thus showed a much higher bacteria inactivation capacity compared with the RuPhen3-loaded micelles.
As controls, the LRF values for non-irradiated and irradiated neat RuPhen3 and unloaded micelles and vesicles incubated with respective bacterial suspensions are shown in
Table 7. For non-irradiated and irradiated unloaded micelles and vesicles, < 0.5 LRF was observed. The non-irradiated neat RuPhen3 showed ~2 LRF, while the irradiated one showed LRF values of >4.
4. Discussion
PEG
114-
b-PLA block copolymers with different PLA block lengths were synthesized and characterized using
1H-NMR and TGA (
Figures S1–S8 and
Table S1). Micelles and vesicles were formed via the solvent shift method with PEG
114-
b-PLA
143 and PEG
114-
b-PLA
499, respectively, and yielded nanocarriers with average hydrodynamic diameters below 100 nm. The assignment to micelles and vesicles was carried out for RuPhen3-loaded assemblies on the basis of TEM and FE-SEM data (
Figure 3), in accordance with the literature of unloaded assemblies [
47]. The TEM images showed spherical micelles for the PEG
114-
b-PLA
143 polymer assemblies, while, for the PEG
114-
b-PLA
499 polymer, spherical but hollow polymersome-like structures were observed. This influence of the block length of PLA on the morphology of the assemblies is well known in the literature and has attributed to a change in packing parameters, which depend on the length and volume of the hydrophobic chain and the optimal area of the hydrophilic head group for amphiphilic molecules [
52]. RuPhen3 could be successfully encapsulated into micelles and polymers with total concentrations up to 54.0 μM that were already sufficient for antimicrobial effects upon irradiation (
Table 7). The increase in RuPhen3 amounts did not lead to a proportional increase in encapsulated RuPhen3 in the dispersions but only a minor increase upon reduction of encapsulation efficiency. However, the batch size could be increased, and assemblies upconcentrated in dispersion by increasing the mass concentration and number density upon centrifugation and reduction of dispersant to further increase the overall RuPhen3 concentration.
Via fluorescence spectroscopy, it was confirmed that the PEG
114-
b-PLA
143 micelles and PEG
114-
b-PLA
499 polymersomes were affected by the presence of the enzyme proteinase K in the same way that has previously been reported for PEG-
b-PLA block copolymers (
Figure 4 and
Figure 6) [
33,
34]. The enzyme-triggered PLA degradation facilitated the release of the encapsulated RuPhen3. The release coincided with an initial increase in fluorescence, attributed to a dilution of the PS and hence dequenching upon release (
Figure 4). The subsequent rapid decrease in fluorescence intensity was attributed to the precipitation of polymer together with the PS in the enzyme buffer, which was qualitatively similar for the light-off reporter dye Nile Red (
Figure 6). In comparison to other enzyme-triggered systems used for aPDT, which showed either enzyme-triggered release of PS from PEG-
b-PCL [
53] or of signaling dyes to detect bacterial enzymes and bacteria using PEG-
b-PLA or PEG-
b-PCL polymers [
33,
34], this current approach system could be further developed for improved simultaneous detection and treatment of bacterial infections towards a theranostic device, since it combines the properties of an enzyme-sensing fluorescent probe with a high aPDT potential, as shown for
Pseudomonas aeruginosa.
The singlet oxygen species produced by RuPhen3 from loaded nanocarriers were detected by measuring the fluorescence intensity of the SOSG probe. RuPhen3 complexes absorbed photons and were excited from the ground state to a short-lived excited state, which could then be converted into a longer-lived triplet excited state via inter-system crossing. From this state, reactive oxygen species could be produced by either electron transfer or energy transfer, which occurred simultaneously [
35]. These singlet oxygen species then reacted with the anthracene unit of the SOSG probe (non-fluorescent) to form its endoperoxide form (
Figure S19), which showed green fluorescence with an emission maximum at 525 nm when excited at 504 nm. The SOSG probe itself was observed to show an increase in fluorescence intensity, even in the absence of any photosensitizer. This observation has also been reported in the literature and has been attributed to different conversion mechanisms of the SOSG probe that contributed to the final probe signal [
51,
54,
55]. In case of unloaded micelles and vesicles, no significant change in the SOSG probe signal was observed for irradiated and non-irradiated samples. For RuPhen3-loaded micelles and vesicles, the SOSG probe signal increased significantly after the irradiation, indicating
1O
2 production.
Moreover, for irradiated RuPhen3-loaded micelles, the SOSG probe signal increased when incubated with bacterial suspensions (
Figure 7a). By contrast, for RuPhen3-loaded vesicles, no significant change in probe signal was observed for samples incubated with or without bacterial suspensions (
Figure 7b). The detection of
1O
2 species depended on various factors, including the concentration of the PS and the local oxygen concentration. In addition, the lifetime of
1O
2 and its diffusion rate to reach the probe were important, which both changed according to the surrounding media (viscosity, presence of other reactants, etc.).
The aPDT effect of RuPhen3-loaded micelles and vesicles against
Pseudomonas aeruginosa, both lab strain and clinical CF isolate, was very pronounced according to the results summarized in
Table 6. For RuPhen3-loaded micelles, the agar spotting method revealed that there were only a few countable colonies in the non-diluted sample (
Figure 8), equating to an at least 3-log reduction of the bacteria after irradiation (see LRF,
Table 7). This means that a bactericidal effect was observed, which was defined as the inactivation of 99.9% of the initially viable (here, colony forming) bacteria. For RuPhen3-loaded vesicles, no visible colonies could be identified after irradiation for either bacterial strain (
Figure 10), referring to an LFR > 4.7 when calculating the reduction of CFU/mL in each individual experiment compared with the control (
Table 6). So, higher PDI efficiency was observed for RuPhen3-loaded vesicles in the formulations used compared with RuPhen3-loaded micelles and even with neat RuPhen3 (
Table 7). The mechanisms for this improvement via the nanocarrier require further investigation. One may hypothesize that, apart from stabilizing the PS itself, the nanocarriers may affect the interaction of RuPhen3 with the bacterial cell wall, which depends both on the bacterial pathogen and its wall structure (Gram-positive or Gram-negative) and the surrounding media [
27,
36].
When comparing the SOSG probe signal intensities of RuPhen3-loaded micelles and vesicles incubated with bacterial suspensions, higher SOSG signal intensities were observed for loaded micelles compared with loaded vesicles after irradiation, while the aPDT results showed the opposite trend. Higher LRFs were observed for loaded vesicles compared with micelles. Therefore, it was not a priori valid to directly correlate the SOSG probe signal with the aPDT efficiency of the systems. Several other factors are required to be considered, such as the interaction of bacteria with the nanocarriers, the availability of the produced singlet oxygen species to reach the bacteria [
35], and various effects that reduce the quantum yields of the reporter probe.
As a final goal with high medical impact, aPDT should achieve a complete eradication of bacteria in order to avoid the recolonization of infected tissue, wound dressings, etc. Although RuPhen3-loaded micelles also resulted in a clear bactericidal effect with an LRF > 3, only the RuPhen3-loaded vesicle formulations afforded a complete killing in this study. This effect even exceeded the photodynamic inactivation (PDI) efficiency of the neat RuPhen3 (
Table 6 and
Table 7). Hence, the encapsulation of RuPhen3 in vesicles may enhance the PDI efficiency for planktonic
Pseudomonas aeruginosa. A similar effect was observed by Mirzahosseinipour et al., who compared the aPDT effect of curcumin and curcumin encapsulated in silica nanoparticles [
56]. They observed a further reduction in the CFU/mL of planktonic bacteria for curcumin-encapsulated silica nanoparticles compared with neat curcumin. The curcumin silica nanoparticles were shown to improve the activity of curcumin due to good uptake and its controlled release.
The RuPhen3-loaded PEG
114-
b-PLA
143 micelles showed similar aPDT efficiency of 99.9% inactivation (3-log reduction) compared with the comparable system of lipase-sensitive PEG-
b-PCL micelles reported by Guo et al. [
53]. However, the RuPhen3-loaded PEG
114-
b-PLA
499 polymersomes showed an antimicrobial effect with a >4.7-log reduction, which was comparable, but they required less light intensity compared with reports about similar polymeric nanocarrier systems. Microparticles irradiated with red light power of 9.28 mW/cm
2 for 30 min or other types of inorganic nanoparticles, e.g., gallium-substituted silver nanoparticles irradiated with nearly 50 times higher light intensity but only 30s irradiation of blue light as used in this study, were previously reported [
57,
58].
The slightly higher log-reduction factor observed for polymersomes with encapsulated RuPhen3 compared with neat RuPhen3 might be explained by the improved solubility of RuPhen3 upon encapsulation and thus a higher local concentration, which would be enhanced if an enzymatically triggered release of RuPhen3 from the polymersomes occurred. In the absence of bacteria, the release was shown here to be triggered by a model enzyme, as shown in
Figure 4 and
Figure 6. It can also be envisioned that high local concentrations of RuPhen3 in polymersomes result in higher concentrations of
1O
2 upon irradiation if the polymersomes reside close to the bacteria.
The herewith-reported systems could be further improved by the use of more efficient ruthenium-based photosensitizers, as reported in the recent literature [
16,
35,
59,
60], or photosensitizers that have been shown to be efficient against various bacteria, e.g., methylene blue or rose bengal. Also, PS molecules, which are excited at longer wavelengths, could be used for encapsulation, since these PSs might be easier to excite within biological media or tissues due to the more pronounced penetration depths of light.