Novel Organogels from Mauritia flexuosa L.f and Caryodendron orinocense Karst.: A Topical Alternative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fatty Acid Profile
2.3. Physicochemical Characteristics
2.4. Preparation of Organogelators
2.5. Characterization of Organogel
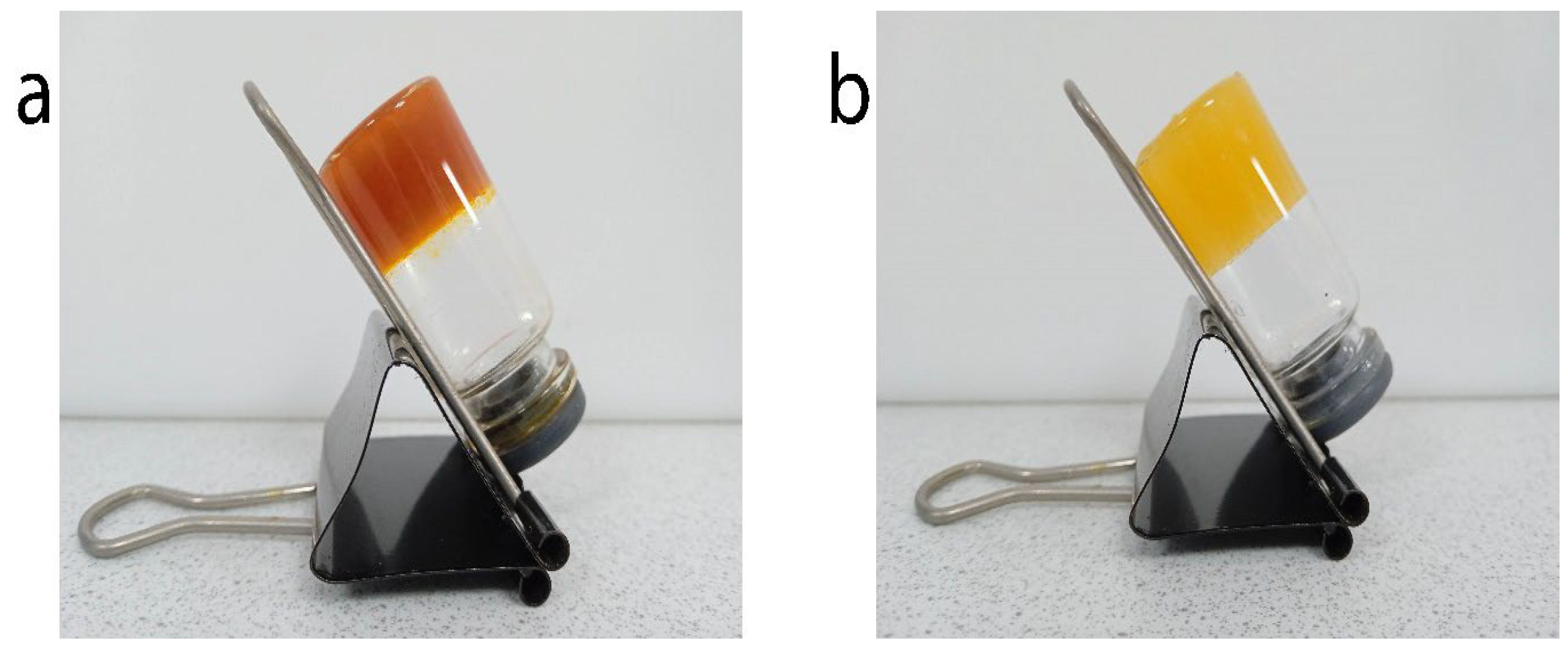
2.5.1. Evaluation of the Inverted Tube
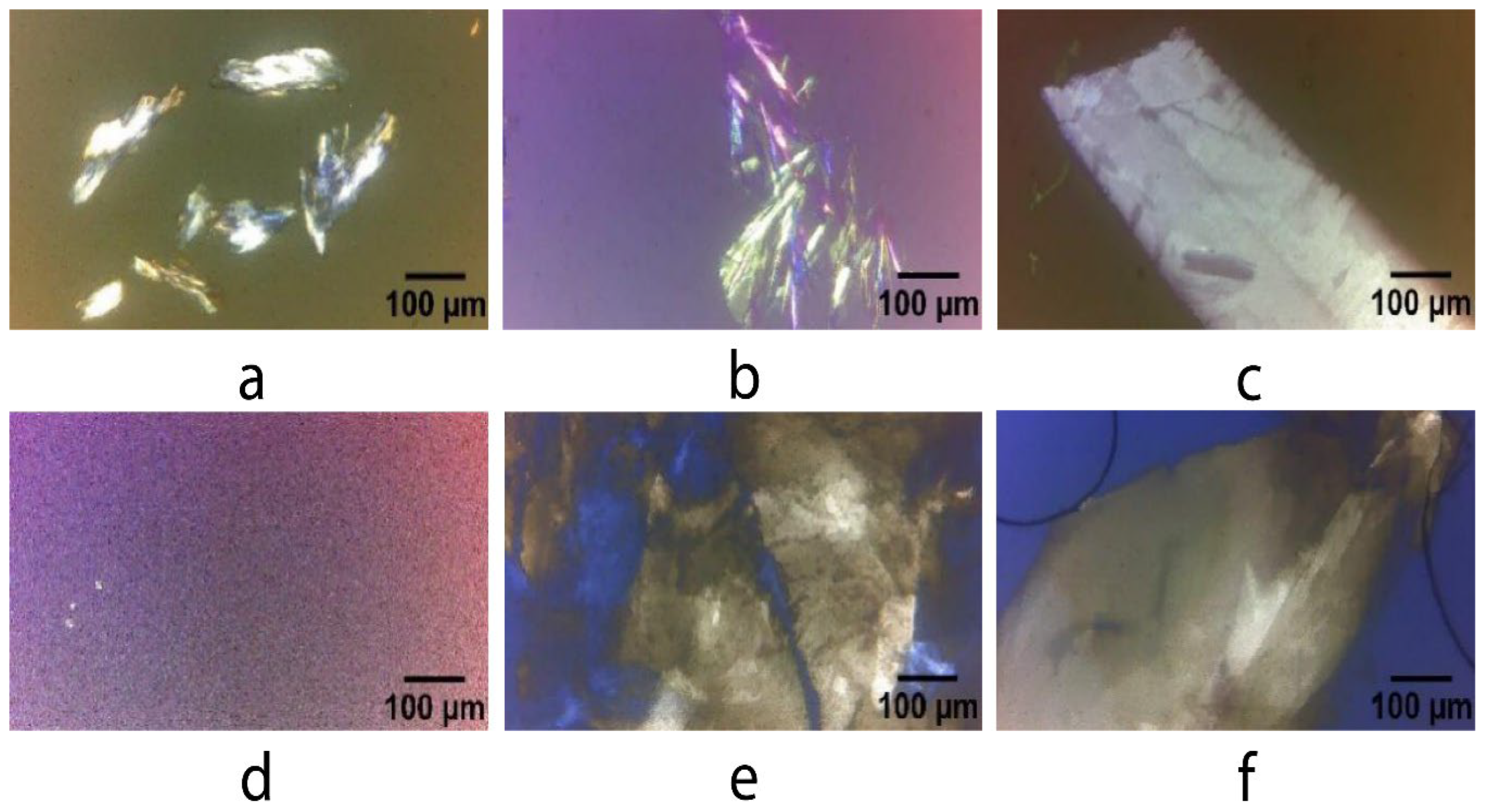
2.5.2. Microstructure and Appearance
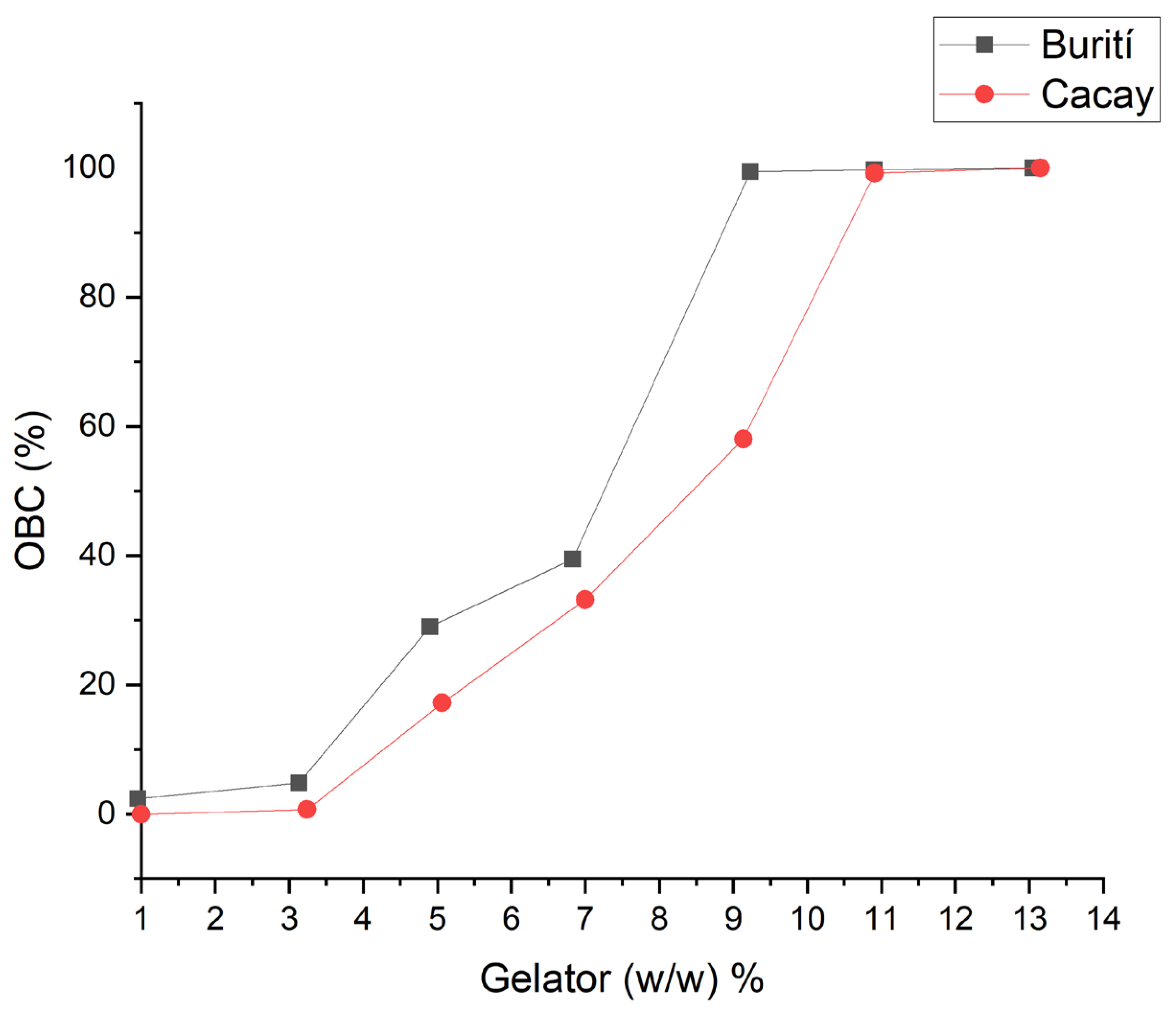
2.5.3. Oil Binding Capacity (OBC)
2.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.5. X-ray Diffraction
2.5.6. Crystallization Evolution
2.5.7. Thermal Analysis
2.5.8. Rheological Analysis
2.6. Statistical Analysis
3. Results
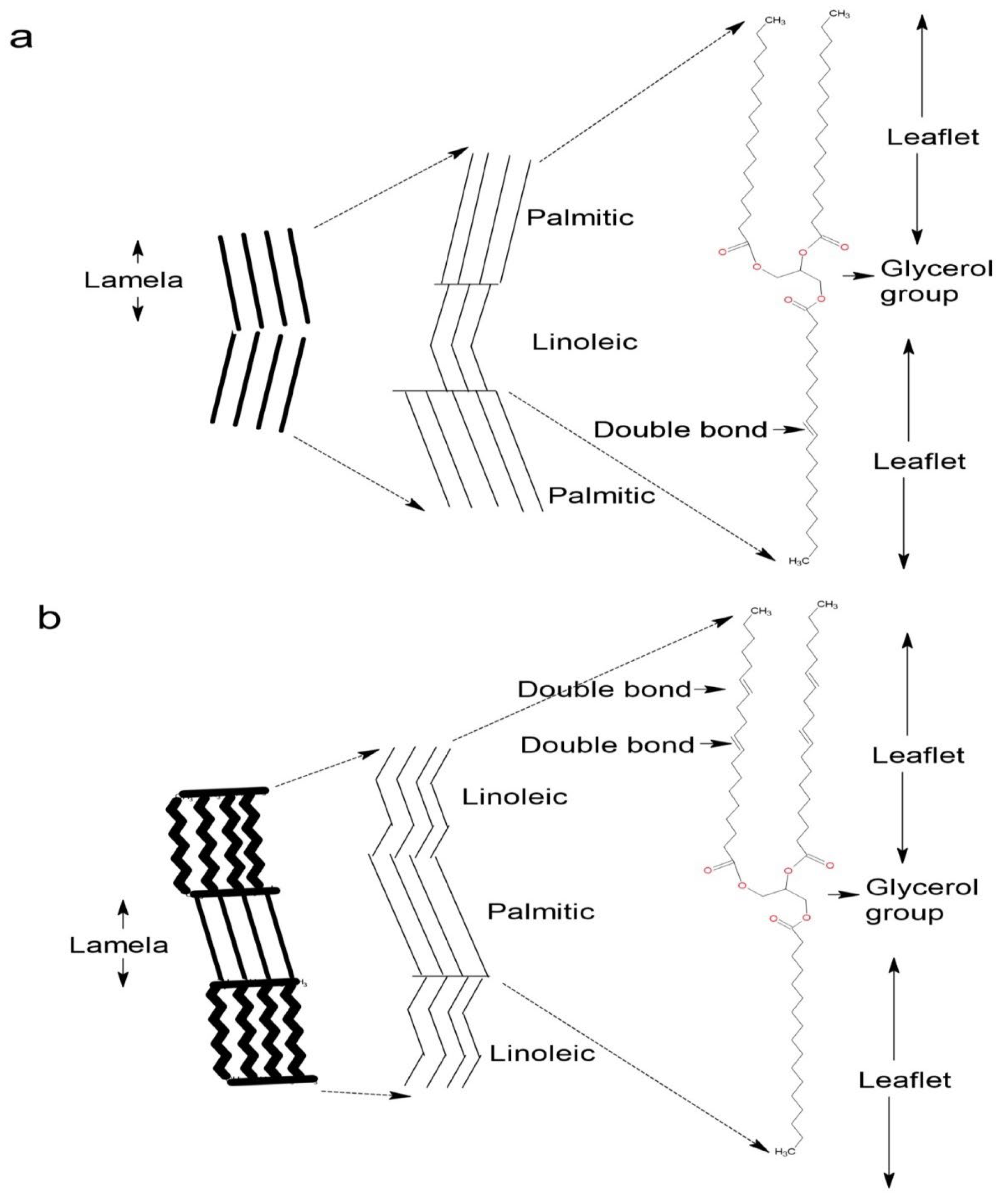
3.1. Analysis of Amazonian Oils
3.2. Obtaining Organogels
3.3. Polarized Light Microscopy Analysis
3.4. Oil-Binding Capacity (OBC)
3.5. FTIR Analysis
3.6. X-ray Diffraction
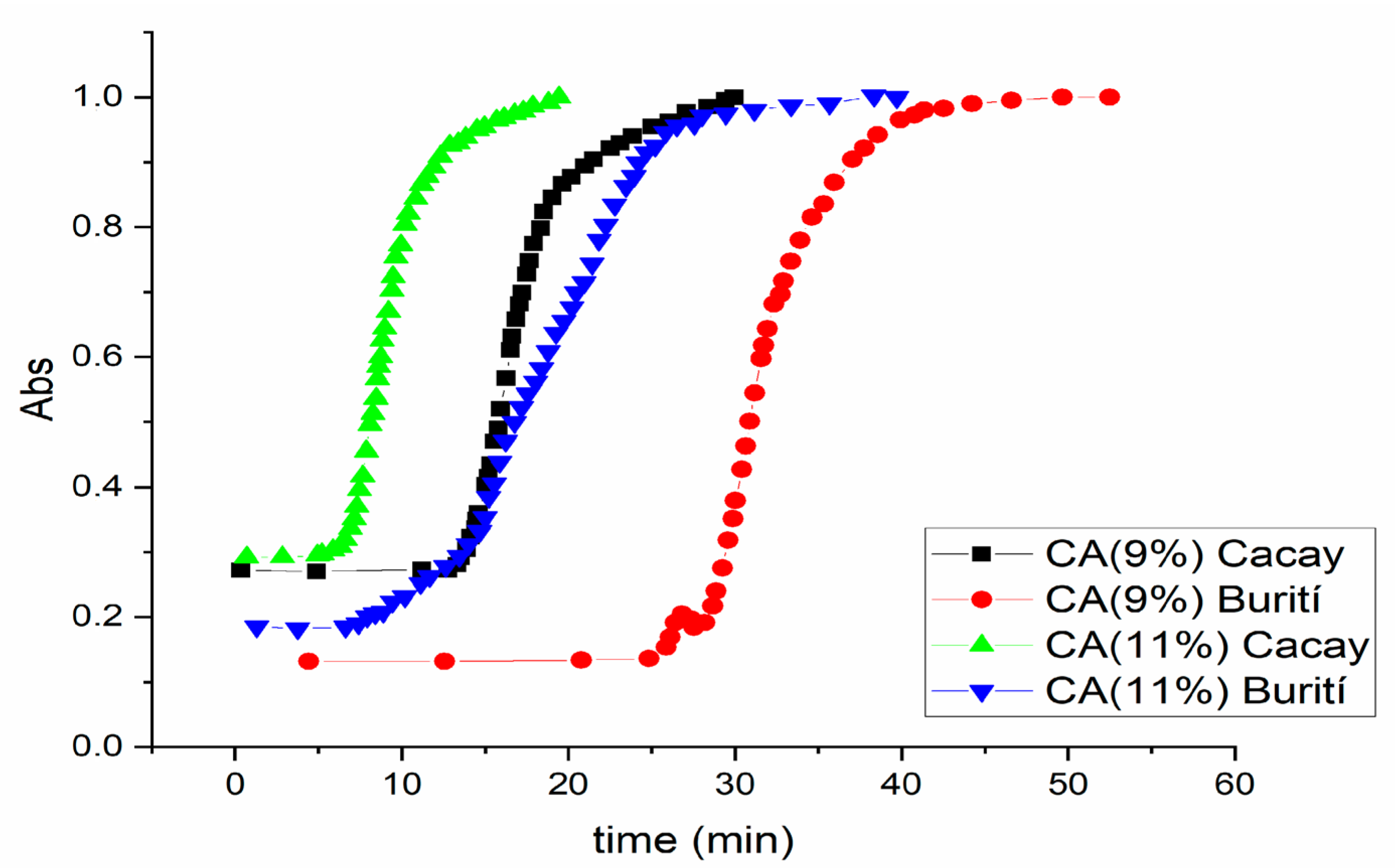
3.7. Crystallization Evolution
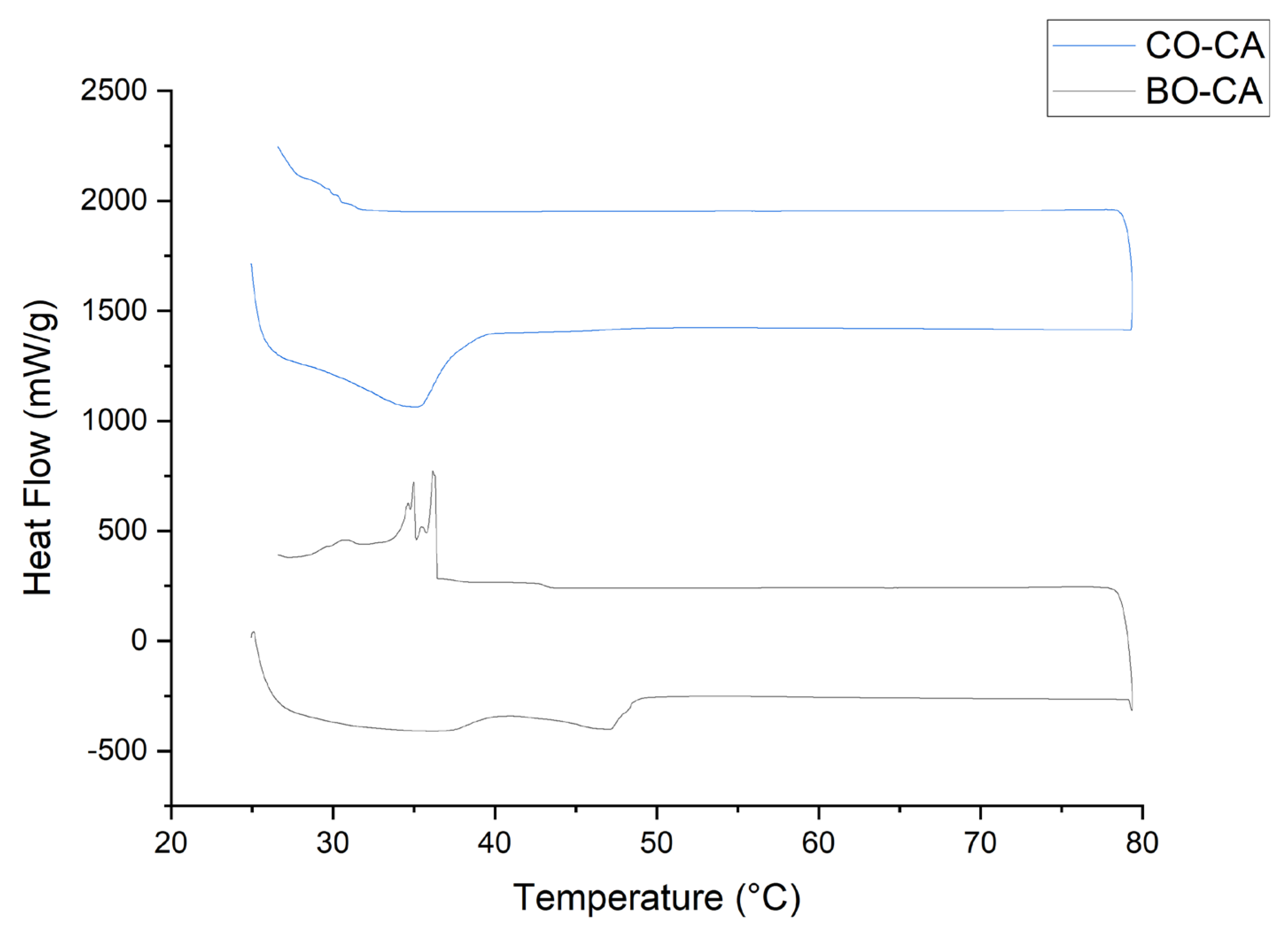
3.8. Thermal Analysis
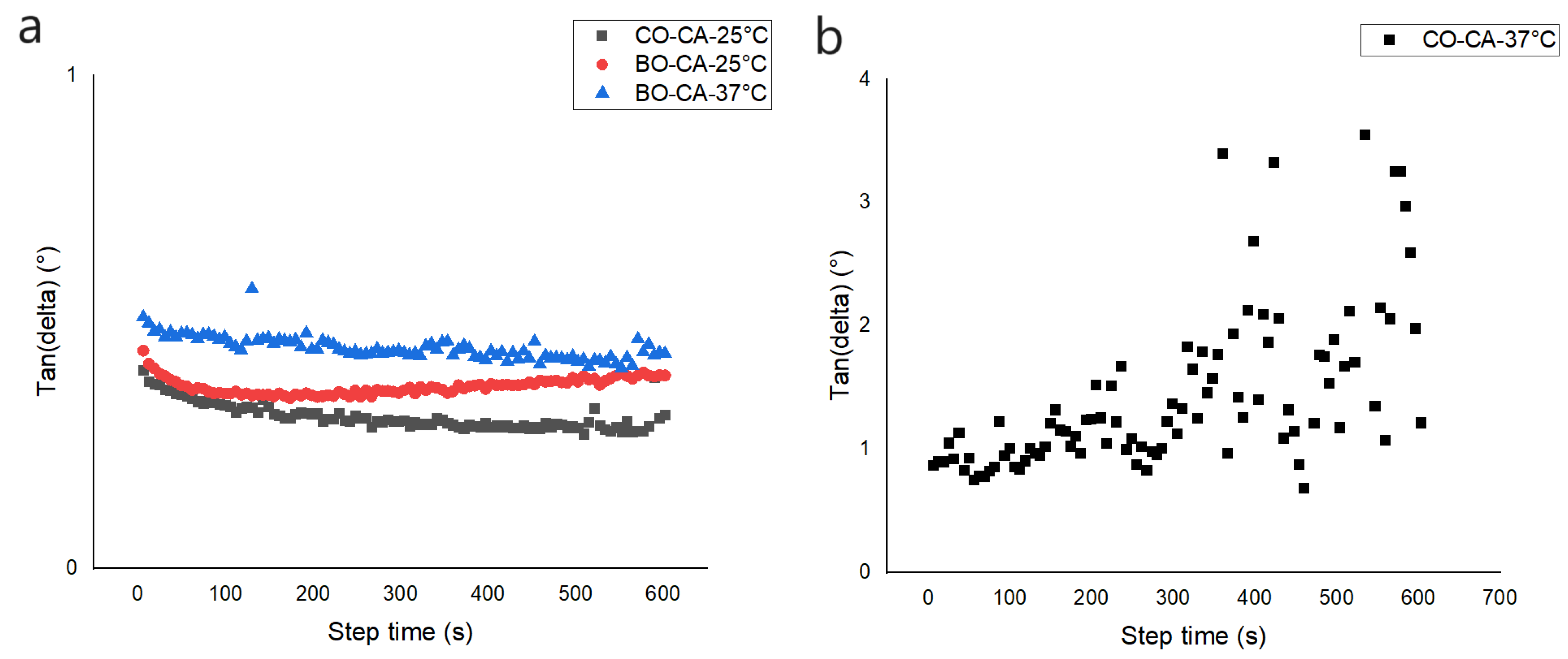
3.9. Rheological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive Compounds from Natural Resources against Skin Aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- César, F.C.; Maia Campos, P.M. Influence of Vegetable Oils in the Rheology, Texture Profile and Sensory Properties of Cosmetic Formulations Based on Organogel Texture and Sensory of Organogel Formulations. Int. J. Cosmet. Sci. 2021, 42, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main Features and Applications of Organogels in Cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, C.F.; Ugartondo, V.; Mitjans, M.; Rocha-Filho, P.A.; Vinardell, M.P. Low Cytotoxicity of Creams and Lotions Formulated with Buriti Oil (Mauritia Flexuosa) Assessed by the Neutral Red Release Test. Food Chem. Toxicol. 2008, 46, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Kandavilli, S.; Nair, V.; Panchagnula, R. Polymers in Transdermal Drug Delivery Systems. Pharm. Technol. 2002, 26, 62–81. [Google Scholar]
- Kirilov, P.; Le, C.A.K.; Denis, A.; Rabehi, H.; Rum, S.; Villa, C.; Haftek, M.; Pirot, F. Organogels for Cosmetic and Dermo-Cosmetic Applications: Classification, Preparation and Characterization of Organogel Formulations. Househ. Pers. Care Today 2015, 10, 16–21. [Google Scholar]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Tarun, G.; Ajay, B.; Bhawana, K.; Sunil, K.; Ravi, J. Organogels: Advanced and Novel Drug Delivery System. Int. Res. J. Pharm. 2011, 2, 15–21. [Google Scholar]
- Vintiloiu, A.; Leroux, J.C. Organogels and Their Use in Drug Delivery—A Review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible Oleogels for the Oral Delivery of Lipid Soluble Molecules: Composition and Structural Design Considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Mosquera Narvaez, L.E.; Ferreira, L.M.D.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef] [PubMed]
- Pernetti, M.; van Malssen, K.F.; Flöter, E.; Bot, A. Structuring of Edible Oils by Alternatives to Crystalline Fat. Curr. Opin. Colloid Interface Sci. 2007, 12, 221–231. [Google Scholar] [CrossRef]
- Patel, A.R. A Colloidal Gel Perspective for Understanding Oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organogels as Matrices for Controlled Drug Delivery: A Review on the Current State. Soft Mater. 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Oleochemical Sources: Basic Science, Processing and Applications of Oils. Biodiesel Sci. Technol. 2010, 3, 62–113. [Google Scholar] [CrossRef]
- Hidalgo, P.S.P.; Nunomura, R.D.C.S.; Nunomura, S.M. Plantas Oleaginosas Amazônicas: Química e Atividade Antioxidante de Patauá (Oenocarpus Bataua Mart.). Rev. Virtual De Quim. 2016, 8, 130–140. [Google Scholar] [CrossRef]
- Rezaire, A.; Robinson, J.C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Durand, P.; Prost, E.; Fils-Lycaon, B. Amazonian Palm Oenocarpus Bataua (“patawa”): Chemical and Biological Antioxidant Activity—Phytochemical Composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef]
- Scharfe, M.; Ahmane, Y.; Seilert, J.; Keim, J.; Flöter, E. On the Effect of Minor Oil Components on β-Sitosterol/γ-Oryzanol Oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800487. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, R.; Meirelles, A.J.A. Characterization of Oil Extracted from Buriti Fruit (Mauritia Flexuosa) Grown in the Brazilian Amazon Region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Coelho, M.A.Z.; Barreto, D.W. Production of Concentrated Natural Beta-Carotene from Buriti (Mauritia vinifera) Oil by Enzymatic Hydrolysis. Food Bioprod. Process. 2012, 90, 141–147. [Google Scholar] [CrossRef]
- Leão, K.M.M.; Reis, L.V.C.; Speranza, P.; Rodrigues, A.P.; Ribeiro, A.P.B.; Macedo, J.A.; Macedo, G.A. Physicochemical Characterization and Antimicrobial Activity in Novel Systems Containing Buriti Oil and Structured Lipids Nanoemulsions. Biotechnol. Rep. 2019, 24, e00365. [Google Scholar] [CrossRef]
- Barros, E.M.L.; de Sousa Lira, S.R.; Lemos, S.I.A.; Luis, T.; dos Santos Rizo, M. Estudo Do Creme de Buriti (Mauritia flexuosa L.) No Processo de Cicatrização. ConScientiae Saúde 2015, 13, 503–610. [Google Scholar] [CrossRef]
- De Alfaro, M.J.; de Padilla, F.C. Physico-Chemical Characteristics of the Barinas Nut (Caryodendron orinocense Karst. Euphorbiaceae) Crude Oil. Archivos Latinoam. Nutr. 1994, 44, 172–175. [Google Scholar]
- Radice, M.; Viafara, D.; Neill, D.; Asanza, M.; Sacchetti, G.; Guerrini, A.; Maietti, S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth Seed Oils. J. Oleo Sci. 2014, 1250, 1243–1250. [Google Scholar] [CrossRef]
- Lozano-Garzón, K.; Orduz-Díaz, L.L.; Guerrero-Perilla, C.; Quintero-Mendoza, W.; Carrillo, M.P.; Cardona-Jaramillo, J.E.C. Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential. Biomolecules 2023, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde; Agência Nacional de Vigilância Sanitária—Anvisa.Farmacopeia Brasileira 2019, v. 1, 6 ed. Available online: http://portal.anvisa.gov.br (accessed on 15 July 2022).
- Singh, V.K.; Pramanik, K.; Ray, S.S.; Pal, K. Development and Characterization of Sorbitan Monostearate and Sesame Oil-Based Organogels for Topical Delivery of Antimicrobials. AAPS PharmSciTech 2015, 16, 293–305. [Google Scholar] [CrossRef]
- Alongi, M.; Lucci, P.; Clodoveo, M.L.; Schena, F.P.; Calligaris, S. Oleogelation of Extra Virgin Olive Oil by Different Oleogelators Affects the Physical Properties and the Stability of Bioactive Compounds. Food Chem. 2022, 368, 130779. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Zhang, Q.; Ju, X.; Wang, Z.; He, R. Study of Monoglycerides Enriched with Unsaturated Fatty Acids at Sn-2 Position as Oleogelators for Oleogel Preparation. Food Chem. 2021, 354, 129534. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef]
- Naeli, M.H.; Milani, J.M.; Farmani, J.; Zargaraan, A. Development of Innovative Ethyl Cellulose-Hydroxypropyl Methylcellulose Biopolymer Oleogels as Low Saturation Fat Replacers: Physical, Rheological and Microstructural Characteristics. Int. J. Biol. Macromol. 2020, 156, 792–804. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.L.; Wei, Z.J. Characterization of Functional Chocolate Formulated Using Oleogels Derived from β-Sitosterol with γ-Oryzanol/Lecithin/Stearic Acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef]
- Zeng, C.; Wan, Z.; Xia, H.; Zhao, H.; Guo, S. Structure and Properties of Organogels Developed by Diosgenin in Canola Oil. Food Biophys. 2020, 15, 452–462. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yilmaz, E. Storage Stability of Cod Liver Oil Organogels Formed with Beeswax and Carnauba Wax. Int. J. Food Sci. Technol. 2015, 50, 404–412. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic Acid Based Oleogels: A Study on the Molecular, Thermal and Mechanical Properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef] [PubMed]
- De Pérez, R.M.N.; Alfaro, M.D.J.; Padilla, F.C. Evaluation of “nuez de Barinas” (Caryodendron orinocense) Oil for Possible Use in Cosmetic. Int. J. Cosmet. Sci. 1999, 21, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Aquino, J.D.S.; Pessoa, D.C.; Araújo, K.D.L.G.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.D.; Stamford, T.L.M. Refining of Buriti Oil (I) Originated from the Brazilian Cerrado: Physicochemical, Thermal-Oxidative and Nutritional Implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Cruz, M.B.; da Silva Oliveira, W.; Araújo, R.L.; Honório França, A.C.; Pertuzatti, P.B. Buriti (Mauritia flexuosa L.) Pulp Oil as an Immunomodulator against Enteropathogenic Escherichia Coli. Ind. Crops Prod. 2020, 149, 112330. [Google Scholar] [CrossRef]
- Rizza, M.A.; Wijayanti, W.; Hamidi, N.; Wardana, I.N.G. Role of Intermolecular Forces on the Contact Angle of Vegetable Oil Droplets during the Cooling Process. Sci. World J. 2018, 2018, 5283753. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and External Factors Affecting the Crystallization, Gelation and Applicability of Wax-Based Oleogels in Food Industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Sato, K. Crystallization of Lipids Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Crystallization Kinetics of Organogels Prepared by Rice Bran Wax and Vegetable Oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the Oil on Glyceryl Monostearate Based Oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Rice Bran Wax in Bulk and Organogels. J. Am. Oil Chem. Soc. 2009, 86, 1163–1173. [Google Scholar] [CrossRef]
- Blount, R.J.S.; Claro, R. Assessment of Natural Waxes as Stabilizers in Peanut Butter. Foods 2022, 11, 3127. [Google Scholar]
- O’briend, R.D. Fats Oils: Formulating and Processing for Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420061666. [Google Scholar]
- Smith, K.W.; Bhaggan, K.; Talbot, G.; Van Malssen, K.F. Crystallization of Fats: Influence of Minor Components and Additives. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 1085–1101. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Rogers, M.A. Activation Energy of Crystallization for Trihydroxystearin, Stearic Acid, and 12-Hydroxystearic Acid under Nonisothermal Cooling Conditions. Cryst. Growth Des. 2011, 11, 3593–3599. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M. Oleogels. In Polymeric Gels; Woodhead Publishing: Sawston, UK, 2018; pp. 231–249. [Google Scholar] [CrossRef]
- Derivatives, D.; Yu, Y.; Chu, N.; Pan, Q.; Zhou, M.; Qiao, S.; Zhao, Y. Solvent Effects on Gelation Behavior of the Organogelator Based on L-Phenylalanine. Materials 2019, 12, 1890. [Google Scholar]
- Daniel, J.; Rajasekharan, R. Organogelation of Plant Oils and Hydrocarbons by Long-Chain Saturated FA, Fatty Alcohols, Wax Esters, and Dicarboxylic Acids. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 417–421. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.F.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and Mechanical Properties of Organogels: Role of Oil and Gelator Molecular Structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Y.; Zhang, Y. Nucleation and Morphology of Monosodium Aluminate Hydrate from Concentrated Sodium Aluminate Solutions. Cryst. Growth Des. 2010, 10, 2–7. [Google Scholar] [CrossRef]
- Liu, X.Y. Gelation with Small Molecules: From Formation Mechanism to Nanostructure Architecture. Low Mol. Mass Gelator 2005, 256, 1–37. [Google Scholar]
- Tudorachi, N.; Mustata, F. Thermal Degradation and Evolved Gas Analysis of Some Vegetable Oils Using TG/FT-IR/MS Technique. J. Therm. Anal. Calorim. 2015, 119, 1703–1711. [Google Scholar] [CrossRef]
- Mehta, C.; Bhatt, G.; Kothiyal, P. A Review on Organogel for Skin Aging. Indian J. Pharm. Biol. Res. 2016, 4, 28–37. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P. Organogel-Based Lipstick Formulations: Application in Cosmetics. Gels 2021, 7, 97. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Li, S.; Brehm, M.; Bartschat, A.; Theato, P.; Levkin, P.A. Inherently UV Photodegradable Poly(Methacrylate) Gels. Adv. Funct. Mater. 2021, 31, 2105681. [Google Scholar] [CrossRef]
- Martinez, R.M.; Filho, P.L.O.; Gerbelli, B.B.; Magalhães, W.V.; Velasco, M.V.R.; da Silva Lannes, S.C.; de Oliveira, C.L.P.; Rosado, C.; Baby, A.R. Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels. Gels 2022, 8, 36. [Google Scholar] [CrossRef]
- Murdan, S. Organogels in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 489–505. [Google Scholar] [CrossRef]




| Fatty Acid | Cacay Oil | Buriti Oil |
|---|---|---|
| Palmitic (C16:0) (%) | 9.5 | 21.96 |
| Stearic (C18:0) (%) | 2.2 | 5.03 |
| Oleic (C18:1 cis9) (%) | 11.8 | 69.78 |
| Linoleic (C18:2 cis9,12) (%) | 75.1 | ND |
| Unsaturated fatty acid (UFA) | 86,9 | 69.78 |
| Saturated Fatty acid (SFA) | 11.7 | 26.99 |
| Monounsaturated fatty acid (MUFA) | 11.8 | 69.78 |
| Free acidity (%) | 4.583 ± 0.09 | 2.653 ± 0.05 |
| Saponification index (mgKOH/g) | 188.862 ± 8.605 | 190.405 +/− 2.412 |
| Refractive index (25 °C) | 1.464 ± 0.002 | 1.4629 ± 0.00005 |
| Viscosity (Cp) | 137.4 ± 0.00308 | 180.76 ± 0.0026 |
| Relative density (kg/m3) | 0.908 ± 0.001 | 0.905 ± 0.0004 |
| Sample | Image | Crystal Length Lc (µm) | Dimension Fractal (DB) | Lacunarity (A) |
|---|---|---|---|---|
| BO–CA-9% |  | 169.05 ± 1.7263 | 1.595 ± 0.0184 | 1.0116± 0.045 |
| CO–CA-9% | 226.20 ± 1.577 | 1.379 ± 0.0110 | 2.046± 0.026 |
| Sample | Type of Peaks | Peak Position (°2θ) | d-Spacing (Å) | FWHM of Peak (°2θ) | D (nm) |
|---|---|---|---|---|---|
| CO | Long spacing peaks | 5.99 | 14.74 | 0.23 | 59.48 |
| 7.15 | 12.36 | 0.21 | 69.43 | ||
| 7.88 | 11.21 | 0.24 | 56.94 | ||
| 9.56 | 9.24 | 334.07 | 0.02 | ||
| 9.85 | 8.97 | 0.49 | 20.30 | ||
| 11.90 | 7.43 | 0.20 | 80.59 | ||
| Short spacing peaks | 21.39 | 4.15 | 0.21 | 71.77 | |
| 24.10 | 3.69 | 0.34 | 33.09 | ||
| 24.68 | 3.60 | 0.19 | 90.40 | ||
| 43.89 | 2.06 | 0.16 | 134.81 | ||
| BO | Long spacing peaks | 7.09 | 12.45 | 0.26 | 48.04 |
| 9.56 | 9.24 | 0.47 | 21.41 | ||
| 11.90 | 7.43 | 0.22 | 65.80 | ||
| Short spacing peaks | 21.75 | 4.08 | 0.21 | 73.90 | |
| 24.65 | 3.61 | 0.25 | 54.48 | ||
| 43.86 | 2.06 | 0.22 | 67.72 |
| Oil | Concentration of the Gelator | Δt1(min) | Δt2 (min) | Δt3 (min) |
|---|---|---|---|---|
| Cacay oil | 9% | 13.53 | 6.11 | 10.36 |
| 11% | 6.28 | 6.58 | 5.84 | |
| Burití oil | 9% | 26.42 | 13.48 | 15.1 |
| 11% | 7.38 | 18.47 | 15.17 |
| Sample | Melting, Endotherm | Crystallization, Exotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| Tsm (°C) | Tfm (°C) | ΔHm (J/g) | ΔSm (J/g) | Tsc (°C) | Tfc (°C) | ΔHc (J/g) | ΔSc (J/g) | |
| Burití | 41.90 | 47.12 | 5.8101 | 0.0349 | 36.47 | 29.26 | 7.3085 | 0.2497 |
| Cacay | 26.63 | 35.23 | 8.6196 | 0.244 | 30.68 | 27.66 | 0.2221 | 0.0073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narvaez, L.E.M.; Carrillo, M.P.; Cardona-Jaramillo, J.E.C.; Vallejo, B.M.; Ferreira, L.M.d.M.C.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Novel Organogels from Mauritia flexuosa L.f and Caryodendron orinocense Karst.: A Topical Alternative. Pharmaceutics 2023, 15, 2681. https://doi.org/10.3390/pharmaceutics15122681
Narvaez LEM, Carrillo MP, Cardona-Jaramillo JEC, Vallejo BM, Ferreira LMdMC, Silva-Júnior JOC, Ribeiro-Costa RM. Novel Organogels from Mauritia flexuosa L.f and Caryodendron orinocense Karst.: A Topical Alternative. Pharmaceutics. 2023; 15(12):2681. https://doi.org/10.3390/pharmaceutics15122681
Chicago/Turabian StyleNarvaez, Luis Eduardo Mosquera, Marcela P. Carrillo, Juliana E. C. Cardona-Jaramillo, Bibiana Margarita Vallejo, Lindalva Maria de Meneses Costa Ferreira, José Otávio Carréra Silva-Júnior, and Roseane Maria Ribeiro-Costa. 2023. "Novel Organogels from Mauritia flexuosa L.f and Caryodendron orinocense Karst.: A Topical Alternative" Pharmaceutics 15, no. 12: 2681. https://doi.org/10.3390/pharmaceutics15122681
APA StyleNarvaez, L. E. M., Carrillo, M. P., Cardona-Jaramillo, J. E. C., Vallejo, B. M., Ferreira, L. M. d. M. C., Silva-Júnior, J. O. C., & Ribeiro-Costa, R. M. (2023). Novel Organogels from Mauritia flexuosa L.f and Caryodendron orinocense Karst.: A Topical Alternative. Pharmaceutics, 15(12), 2681. https://doi.org/10.3390/pharmaceutics15122681






