In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex
Abstract
:1. Introduction
2. Results
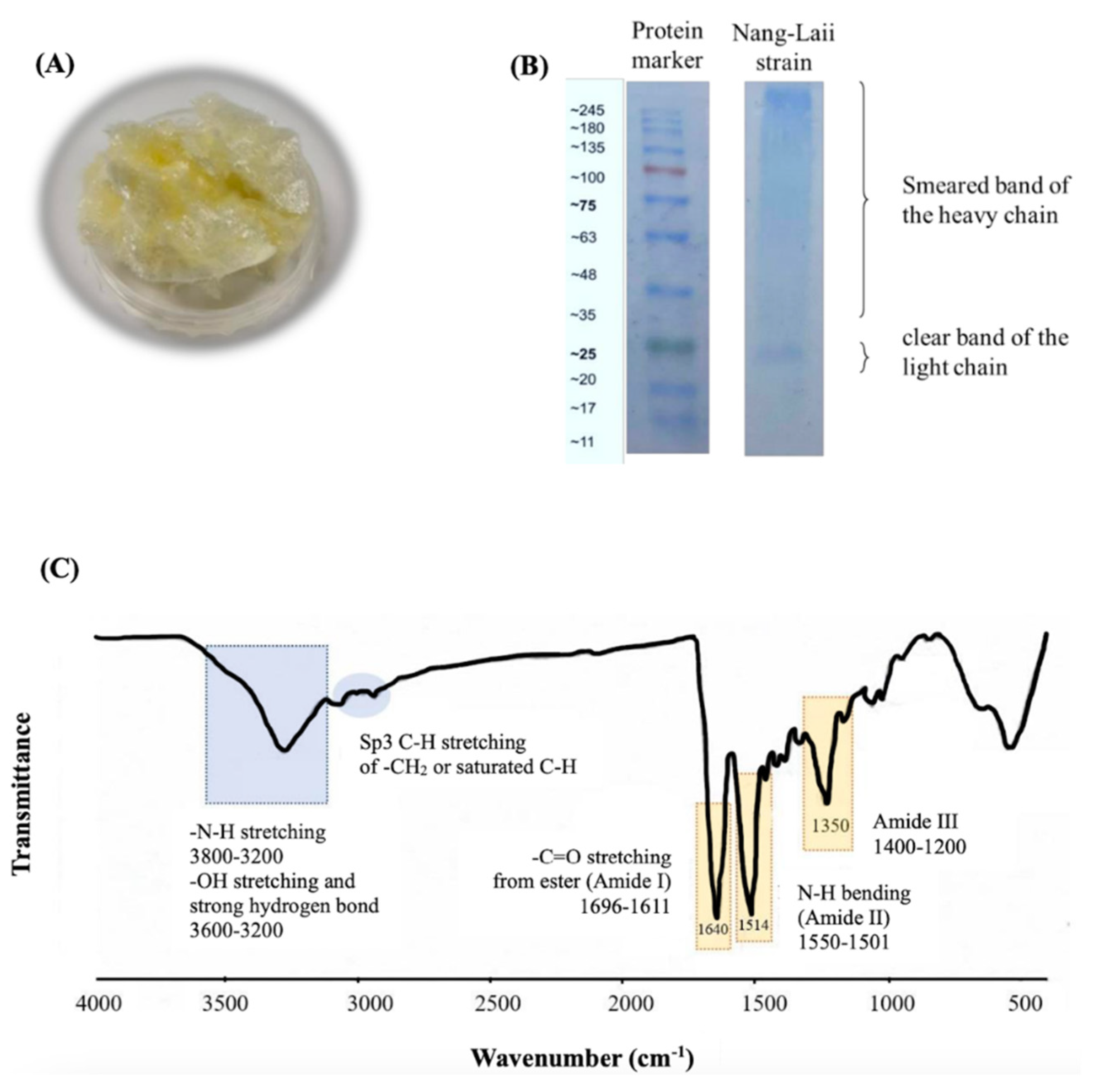
2.1. Characteristics of the Isolated Fibroin
2.2. Characteristics of the CBD/HP-β-CD Inclusion Complex
2.3. Characteristics of the Fibroin Film Containing the CBD/HP-β-CD Complex
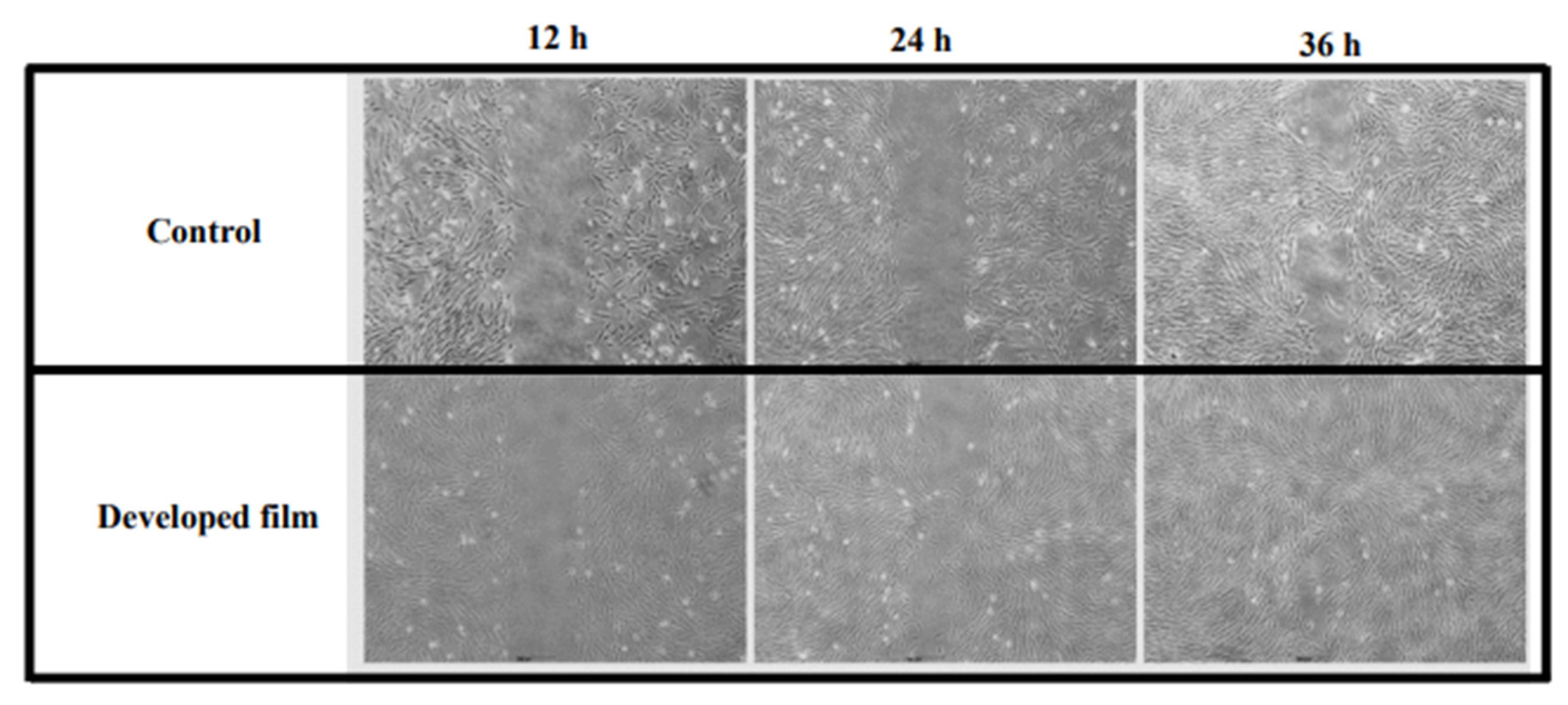
2.4. Wound Healing Potential of the Developed Film (Fibroin Film Containing the CBD/HP-β-CD Complex)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Silk Fibroin
4.3. Characterization of the Isolated Fibroin
4.3.1. Determination of the Protein Content
4.3.2. Determination of the Molecular Weight Pattern
4.3.3. Chemical Functional Groups
4.4. Preparation of the CBD/HP-β-CD Inclusion Complex
4.5. Characterization of the CBD/HP-β-CD Inclusion Complex
4.5.1. The CBD Content in the Complex
4.5.2. Chemical Functional Groups and Intermolecular Bonding
4.5.3. Surface Morphology
4.5.4. Crystallinity
4.6. Fabrication of the Fibroin Film Containing the CBD/HP-β-CD Complex (The Developed Film)
4.7. Characterization of the Developed Film
4.7.1. Determination of the CBD Content in the Film
4.7.2. Surface Morphology
4.7.3. Mechanical Properties
4.7.4. Chemical Functional Groups and Intermolecular Bonding
4.7.5. Crystallinity
4.8. Determination of Wound Healing Potential of the Developed Film through Cell-Based Assays
4.8.1. Preparation of the Film-Free Supernatants
4.8.2. Culture of Normal Human Dermal Fibroblast (NHDF)
4.8.3. Cytocompatibility Test
4.8.4. Cell Cycle Analysis
4.8.5. Immunofluorescence of Vascular Epidermal Growth Factor (VEGF)
4.8.6. Wound Healing Test
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Moravvej, H.; Hosseinzadeh, S.; Ebrahim, M.; Bayat, H.; Kazemi, B.; Bandehpour, M.; Rostami, E.; Rahimpour, A.; Moosavian, H. Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: In vitro and in vivo studies. Sci. Rep. 2022, 12, 18529. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenesis activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The impact of prolonged inflammation on wound healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.; Smola, H.; Hartmenn, B.; Malchau, G.; Wegner, R.; Krieg, T.; Smola-Hess, S. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials 2008, 29, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cai, E.; Xiang, Y.; Zhang, C.; Ge, X.; Wang, J.; Lan, Y.; Xu, H.; Hu, R.; Shen, J. An immunomodulatoey hydrogel by hyperthermia-assisted self-cascade glucose depletion and ROS scavenging for diabetic foot ulcer wound therapeutics. Adv. Mater. 2023, 2306632. [Google Scholar] [CrossRef] [PubMed]
- Phimnuan, P.; Dirand, Z.; Tissot, M.; Worasakwutiphong, S.; Sittichokechaiwut, A.; Grandmottet, F.; Viyoch, J.; Viennet, C. Beneficial effects of a blended firoin/aloe gel extract film on biomolecular mechanism(s) via the MAPK/ERK pathway relating to diabetic wound healing. ACS Omega 2023, 8, 6813–6824. [Google Scholar] [CrossRef]
- Phimnuan, P.; Worasakwutiphong, S.; Sittichokechaiwut, A.; Grandmottet, F.; Nakyai, W.; Luangpraditkun, K.; Viennet, C.; Viyoch, J. Physicochemical and biological activities of the gamma-irradiated blended fibroin/aloe gel film. ScienceAsia 2022, 48, 278–286. [Google Scholar] [CrossRef]
- Watchararot, T.; Prasongchean, W.; Thongnuek, P. Angiogenic property of silk fibroin scaffolds with adipose-derived stem cells on chick chorioallantoic membrane. R. Soc. Open Sci. 2021, 8, 20161. [Google Scholar] [CrossRef]
- Liu, J.; Xie, X.; Wang, T.; Chen, H.; Fu, Y.; Cheng, X.; Wu, J.; Li, G.; Liu, C.; Liimatainen, H.; et al. Promotion of wound healing using nanoporous silk fibroin sponges. ACS Appl. Mater. Interfaces 2023, 15, 10. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded silk fibroin/sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104 Pt A, 969–997. [Google Scholar] [CrossRef]
- He, S.; Shi, D.; Han, Z.; Dong, Z.; Xie, Y.; Zhang, F.; Zeng, W.; Yi, Q. Heparinized silk fibroin hydrogels loading FGF1 promote the wound healing in rate with full-thickness skin excision. Biomed. Eng. Online 2019, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate-based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Zhao, R.; Li, J.-Y.; Li, S.-S.; Liu, M.; Wang, M.; Zhang, M.-Z.; Dong, W.-W.; Jiang, S.-K.; Zhang, M.; et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur. J. Pharmocol. 2016, 786, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Stolzke, T.; Krieg, F.; Peng, T.; Zhang, H.; Häusler, O.; Brandenbusch, C. Hydroxylpropyl-β-cyclodextrin as Potential Excipient to Prevent Stress-Induced Aggregation in Liquid Protein Formulations. Molecules 2022, 27, 5094. [Google Scholar] [CrossRef]
- Inpanya, P.; Faikrua, A.; Ounaroon, A.; Sittichokechaiwut, A.; Viyoch, J. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, J.; Niu, L.; Wang, Y.; Cheng, Z.; Sun, X.; Li, M. High molecular weight silk fibroin prepared by papain degumming. Polymers 2020, 12, 2105. [Google Scholar] [CrossRef]
- Worasakwutiphong, S.; Termwattanaphakdee, T.; Kamolhan, T.; Phimnuan, P.; Sittichokechaiwut, A.; Viyoch, J. Evaluation of the safety and healing potential of a fibroin-aloe gel film for the treatment of diabetic foot ulcers. J. Wound Care 2021, 30, 1020–1028. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.; Guo, M.; Liu, J.; Chen, X.; Guo, R.; Xu, Y.; Zhang, Q.; Liu, Y.; Guo, H.; et al. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J. Drug Deliv. Sci. Technol. 2019, 51, 337–344. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Patel, M.; Hirlekar, R. Multicomponent cyclodextrin system for improvement of solubility and dissolution rate of poorly water soluble drug. Asian J. Pharm. Sci. 2019, 14, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Sun, Q.; Tang, P.; Zhao, L.; Li, Q.; Liu, Y.; Li, H. Characterization and antioxidant activity of the complexes of tertiary butylhydroquinone with β-cyclodextrin and its derivatives. Food Chem. 2018, 260, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Triamchaisri, N.; Toochinda, P.; Lawtrakul, L. Structural investigation of beta-cyclodextrin complexes with cannabidiol and delta-9-tetrahydrocannabinol in 1:1 and 2:1 host-guest stoichiometry: Molecular dicking and density functional calculation. Int. J. Mol. Sci. 2023, 24, 1525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Zhu, Z. Study on the blends of silk fibroin and sodium alginate: Hydrogen bond formation, strudture and properties. Polymer 2019, 163, 144–153. [Google Scholar] [CrossRef]
- Gao, Q.; Shao, Z.; Sun, Y.; Lin, H.; Zhou, P.; Yu, T. Comples formation of silk fibroin with poly(acrylic acid). Polym. J. 2000, 32, 269–274. [Google Scholar] [CrossRef]
- Viyoch, J.; Sudedmark, T.; Srema, W.; Suwongkrua, W. Development of hydrogel patch for controlled release of alpha-hydroxy acid contained in tamarind fruit pulp extract. Int. J. Cosmet. Sci. 2005, 27, 89–99. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Kovalchuk, O.; Kovalch, I. Phytocannabinoids stimulate rejuvenation and prevent cellular senescence in human dermal fibroblasts. Cells 2022, 11, 3939. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and anti-inflammatory effect of cannabidiol contributes to the decreased lipid peroxidation of keratinocytes of rat skin exposed to UV radiation. Oxid. Med. Cell. Longev. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020, 11, 714. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Xiao, L.; Li, S.; Liao, X.; Liu, H. Autocrine and paracrine effects of vascular endothelial cells promote cutaneous wound healing. Biomed. Res. Int. 2021, 2021, 6695663. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cantelmo, A.R.; Cattaneo, M.G.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L. /M.; Noonan, D.M.; et al. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br. J. Pharmacol. 2012, 167, 1218–1231. [Google Scholar] [CrossRef]
- Geskovski, N.; Stefkov, G.; Gigopulu, O.; Stefov, S.; Huck, C.W.; Makreski, P. Mid-infrared spectroscopy as process analytical technology tool for estimation of THC and CBD content in Cannabis flowers and extracts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119422. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinsang, T.; Charoensit, P.; Phimnuan, P.; Luangpraditkun, K.; Ross, G.M.; Viennet, C.; Ross, S.; Viyoch, J. In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex. Pharmaceutics 2023, 15, 2682. https://doi.org/10.3390/pharmaceutics15122682
Klinsang T, Charoensit P, Phimnuan P, Luangpraditkun K, Ross GM, Viennet C, Ross S, Viyoch J. In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex. Pharmaceutics. 2023; 15(12):2682. https://doi.org/10.3390/pharmaceutics15122682
Chicago/Turabian StyleKlinsang, Thamonphat, Pensri Charoensit, Preeyawass Phimnuan, Kunlathida Luangpraditkun, Gareth M. Ross, Céline Viennet, Sukunya Ross, and Jarupa Viyoch. 2023. "In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex" Pharmaceutics 15, no. 12: 2682. https://doi.org/10.3390/pharmaceutics15122682
APA StyleKlinsang, T., Charoensit, P., Phimnuan, P., Luangpraditkun, K., Ross, G. M., Viennet, C., Ross, S., & Viyoch, J. (2023). In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-cyclodextrin Complex. Pharmaceutics, 15(12), 2682. https://doi.org/10.3390/pharmaceutics15122682





