Abstract
Nanotechnology is an emerging field of science that is widely used in medical sciences. However, it has limited uses in monogastric farm animal as well as fish and poultry nutrition. There are some works that have been done on curcumin and curcumin nanoparticles as pharmaceutics in animal nutrition. However, studies have shown that ingestion of curcumin or curcumin nanoparticles does not benefit the animal health much due to their lower bioavailability, which may result because of low absorption, quick metabolism and speedy elimination of curcumin from the animal body. For these reasons, advanced formulations of curcumin are needed. Curcumin nanospheres is a newly evolved field of nanobiotechnology which may have beneficial effects in terms of growth increment, anti-microbial, anti-inflammatory and neuroprotective effects on animal and fish health by means of nanosphere forms that are biodegradable and biocompatible. Thus, this review aims to highlight the potential application of curcumin, curcumin nanoparticles and curcumin nanospheres in the field of monogastric farm animal, poultry and fish nutrition. We do believe that the review provides the perceptual vision for the future development of curcumin, curcumin nanoparticles and curcumin nanospheres and their applications in monogastric farm animal, poultry and fish nutrition.
1. Introduction
Biotechnology and nanotechnology are considered as the 21st century’s most emerging and advanced technologies. The term nanotechnology was first coined by Norio Taniguchi in 1974 [1]. Nanotechnology (from the Latin nanus, meaning the dwarf) can be defined as the understanding, design, development and application of materials and devices whose least functional makeup is on a nanometer scale (~1 to 100 nm) or one billionth of a meter (10−9) [2]. In brief, nanotechnology deals with the conversion of larger molecules to nanometer size and it changes the physico-chemical nature of the cell matrix in terms of human or animal welfare. The changes may occur based on solubility, absorption, the transporting system, excretion and antagonistic behavior of the nanomaterials [3]. Nanobiotechnology can be an indispensable tool to monitor the individual cell development at the individual molecule level [4]. Nevertheless, one of the most important and extensive uses of nanobiotechnology is the area of human medicine [5], where it provides nanomaterials with the controlled drug delivery system for cancer remediation [6], nutrient utilization [7], hormonal controls [8] and gene therapy [9]. In case of animal welfare, the technology can be utilized to improve growth [10,11] and meat quality [11], reproductive performance [12,13], immunity enhancement [14] as well as disease resistance [10], antioxidant activity [15,16,17], and intestinal function [16] including food preservation [18]. Nanomaterials can be classified into four categories: metals, polymers, natural compounds and nanostructured materials [19]. Metals are usually mineral nanoparticles such as gold [20], copper [21], selenium [22], palladium [23] etc. However, the main problem of using metal nanomaterial is its non-biodegradability [19]. Polymers of nanomaterials are pieces of nanometer-sized polymers which are biodegradable and biocompatible and can be utilized very well [24]. The biocompatibility is the most important concern in nanobiotechnology in order to have low or no toxic effects on the organism [25,26,27,28]. Nanoparticles that come from natural sources are known as natural compounds (natural polymers or proteins) which are highly biodegradable, biocompatible and distributable in the body [19]. Nanostructured materials are the conjugations of lipid or protein with nanoparticles such as phospholipid-based nanoparticles (curcumin nanospheres) or phosphoprotein-based nanoparticles (casein micelles). From a nutritional point of view, natural and nanostructured nanoparticles have great advantages in terms of nutrient delivery by means of encapsulation or adhesion of the nanomaterials. However, natural or nanostructured nanoparticles may be toxic in high doses if they are not properly adjusted in the organisms. In animal nutrition, nanotechnology has been discussed regarding the nature of food and its taste, texture, processing time, thermal susceptibility, stability, safety and bioavailability of nutrients as well as the feeding of animals [29]. Nutrients can be delivered in the form of nanoparticles or in aided forms such as nanocapsules and emulsifications as well as nanosphere forms.
In recent years, phytogenic natural compounds are widely used in animal feeds as antimicrobial growth promoters (AGPs) to replace the antibiotics in monogastric animal [30,31], poultry [11] or aquaculture [32] feed since the indiscriminate use of antibiotics in animal feed may favor the emergence of antibiotic-resistant strains of bacteria [11] and their accumulation in edible tissues [33]. In fact, the European Union (EU) has already banned AGPs in animal feeds, and the Food and Drug Administration (FDA) of the USA is going to restrict the use of AGPs in animal feeds [34,35]. For animal disease resistance and growth enhancement, currently researchers are putting emphasis on the use of natural growth promoters and non-antibiotic growth promoters (NGPs) in terms of improvement of the gastrointestinal tract (GIT) through the incorporation of various feed additives, for example, curcumin and its derivatives which have antimicrobial, antioxidant, anti-inflammatory, appetite increasing, immune-modulatory and gastroprotectve effects on animal health [11].
Turmeric (Curcuma longa L.), a tropical rhizomatous herb of the Zingiberaceae family, is regarded as the “golden spice”, used as curry powder in South and South-East Asian cuisine [11,36]. Turmeric has a medicinal value in human and animal health [37]. The dried turmeric rhizome consists of 3–6% terpenes and terpenoids, 6–8% protein, 6–10% fat, 60–70% carbohydrate and 3–6% fiber [38]. Curcumin or diferuloylmethane [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a hydrophobic and polyphenolic compound extracted from the perennial herb, Curcuma longa [39,40,41]. Curcumin, is a yellow pigment, found in turmeric at 3–6% with bioactive properties [37]. The melting point of curcumin ranges from 176 to 177 °C [42]. Commercial curcumin usually has 77% diferuloylmethane, 17% dimethoxy-curcumin and 6% bisdemethoxycurcumin [43]. Curcumin has a relatively low toxicity and is generally regarded as safe (GRAS) which defined as the chemical or substance added to food is considered as safe by experts, and so is exempted from the usual Federal Food, Drug, and Cosmetics Act food additive tolerance requirements by the United States Food and Drug Administration (FDA) [44]. For many years, curcumin has been used in Indian ayurvedic remedies and Chinese medicines [44,45]. Despite the beneficial effects of curcumin, it has some limitations such as low water solubility (hydrophobic), an unstable chemical structure, being rapidly metabolized but poorly absorbed in the body as well as its utilization or bioavailability differing depending on the species and sex [41]. Likewise, Wahlstrom and Blennow [46] reported that oral administration of curcumin (1 g/kg) to rats resulted in a huge excretion of curcumin (about 75%) in the feces with a small amount in the urine and blood plasma. However, Patil et al. [47] postulated that the piperine in black pepper could enhance the bioavailability of curcumin by 20-fold and CYP3A4 in cytochrome P450, p-Glycoprotein and uridine diphosphate (UDP)-glucuronosyltransferase (UGT), the enzyme responsible for glucuronosylation, increased the solubility of curcumin.
Curcumin in the form of a nanoparticle (hereafter, curcumin nanoparticle) is a widely reported form for enhancing the bioavailability and solubility of lipophilic curcumin [10,48]. Kurita and Makino [49] as well as Hani and Shivakumar [50] reported that the solubility and absorption rate of nanocurcumin is higher than the normal curcumin form, respectively. Furthermore, curcumin nanoparticles can be more bioavailable and deposited more highly than the normal curcumin in comparison of the tissues of the Sprague-Dawley rat model [51,52]. However, no form of curcumin or its closely related analogues poses the properties required for a good drug or additive candidate in terms of chemical stability, high water solubility, potent and selective target activity, high bioavailability, broad tissue distribution, stable metabolism and low toxicity [53].
Due to the problems of water insolubility and low bioavailability of curcumin or curcumin nanoparticles, it has been reported that biodistribution and bioavailability of curcumin or curcumin nanoparticles would be increased by encapsulation processes such as nanoemulsions, liposomes, micelles, polymeric micro or nanoparticles, phospholipid complexes and hydrogels which showed the potential for efficient drug delivery systems that minimize the delay and reduce the vulnerability of diseases in organisms [44,54,55]. Prasad et al. [37] postulated that an increased amount and length of curcumin in blood circulation could enhance the tissue deposition and biological activity in animals. Furthermore, Mohamed et al. [56] opined that hydrogen-bromide-treated curcumin can be exclusively used as a source of antioxidant in bioactive food materials. As an organic curcumin nanocarrier, liposomal curcumin is considered as the best way of improving bioavailability of curcumin in cellular level [57] and the commodities based on liposomal formulations are being marketed for different dietary applications of curcumin [58]. In addition, in case of inorganic nano formulations, mesoporous silica nanoparticles (MSN) are the most used nanosystems for improving the bioavailability of poorly water soluble drugs [59,60,61,62].
Recently, there are a significant number of research works have been conducted on curcumin or curcumin nanoparticles alone or in combination with other additives in the diet of monogastric farm animals and poultry as well as fish (Figure 1) [14,31,63,64,65,66,67,68,69]. Most of the studies dealt with growth enhancement, improvement of reproductive health and metabolism, antioxidants and immunomodulatory effects of curcumin or curcumin nanoparticles. However, a scarce number of articles were reported regarding the nanospheric forms [70,71]. This review article discuss the recent advancement of curcumin research based on nutritional studies in monogastric animals such as pig, poultry and fish. We hope that the review article will benefit the scientific communities for the further development of curcumin research in nutritional science.
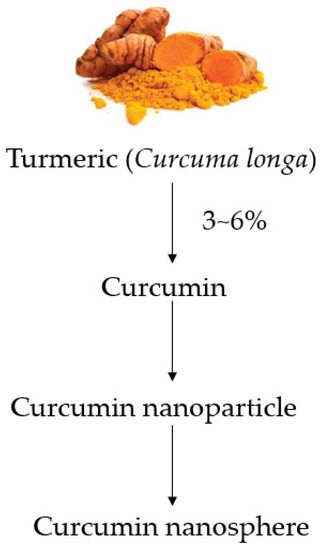
Figure 1.
Outline of the production process from turmeric to curcumin nanospheres.
2. Curcumin or Nanocurcumin in Monogastric Farm Animal, Poultry and Fish Nutrition
Indiscriminate use of antibiotics in monogastric farm animal and poultry farming as well as in aquaculture has caused the emergence of new pathogenic strains. These consequences have warranted the development of safe and alternative growth promoters and immunity enhancers in farm animal, poultry and fish production. Monogastric farm animals like swine, poultry and fish are usually reared in intensive production systems which may ultimately affect their health status through different types of diseases and secondary infections and ultimately reduce their production performance [72,73,74,75,76]. Use of phytogenic additives in animal, bird and fish feed is a centuries-old practice. With vast potential areas of application, nanotechnology is still in its infancy for animal feed and nutritional studies [4]. The most common health-enhancing prophylaxis is nutritional strengthening of the gastrointestinal system in animals [77]. In this section we discuss the use of curcumin or turmeric (as the source of curcumin), and nanocurcumin based on the nutritional aspects related to the growth, reproductive capacity, digestibility, stress response, immune functions and histopathology as well as disease distance in different age stages of swine, rabbit, poultry and fish (Table 1, Table 2, Table 3 and Table 4).

Table 1.
Effects of dietary turmeric and curcumin in swine nutrition.

Table 2.
Effects of dietary turmeric, curcumin and nanocurcumin in poultry nutrition.

Table 3.
Effects of dietary turmeric and curcumin in rabbit nutrition.

Table 4.
Effects of dietary turmeric and curcumin in fish nutrition.
2.1. Dietary Turmeric/Curcumin in Swine Nutrition
In swine, the weanling period is the most crucial time from a nutritional perspective because at this stage young piglets are quickly shifted from sow’s milk to a dry- and low-digestible-starch-based diet which may ultimately affect their digestive and immune systems and finally cause morbidity or mortality in pigs [78]. The consequences may be due to distinctive changes in gastrointestinal tract (GIT) architecture, changes in autochthonous bacteria in the gut which deteriorate the small-intestinal barrier function and absorption of potential nutrients [79,80]. In the weanling period, low feed intake and feed efficiency, body weight loss and diarrhea are common phenomena in piglets. In farm levels, nearly 50% growth retardation may occur at the weaning stage of pigs [80]. To overcome this problem and to increase the post-weaning growth of pigs, farmers usually depend on some commercial antibiotics in the diets of the weanling pigs. However, the indiscriminate use of therapeutic antibiotics in farm animals may create the development of antibiotic-resistant strains of bacteria which may be harmful for the animal as well as the consumers in turn. Different countries of the world are now thinking to minimize or completely eliminate the inclusion of antibiotics in the diet for farm animals. To increase the production and health status of pigs, it is imperative to find out the alternatives to antibiotics which can be used either singly or in combination that are able to overcome stunted growth as well as the problems in the digestive system of weanling pigs. Different dietary components depending on their solubility, digestibility, viscous-forming ability and acid-buffering ability can potentiate or stimulate the gut health of pigs by preventing proliferation of pathogenic bacteria [78]. In this regard, dietary turmeric or curcumin have shown their positive effects in swine nutrition [31,68,69,81,82,83,84,85,86]. Maneewan et al. [81] reported that dietary turmeric can induce nutrient digestibility, blood parameters and intestinal gut health in nursery piglets when they offered 0.10% and 0.20% turmeric in the diets. In another reports, Liu [82,83] postulated that dietary turmeric oleoresin or in combination with capsicum oleoresin and garlic botanical could enhance the weight gain and gut mucosa health by alleviating diarrhea and reducing inflammation caused by E. coli bacteria. Dietary curcumin extracted from the turmeric rhizome also proved its promising effects as an immune stimulant in weaned piglets. As an antibiotic replacer and potential additive, dietary curcumin at 300 or 400 mg/kg of diet has shown its effectiveness in replacing quinocetone by means of enhancing growth performance, jejunal mucosal barrier integrity in the intestine and stimulating the immune system of weaned piglets challenged with enterotoxigenic E. coli [31]. But, Ilsley et al. [84] reported that dietary curcumin (200 mg/kg) had no effect on the growth and immune system of weaned piglets in relation to quillaja saponin. Intrauterine growth retardation (IUGR) which is defined as the growth below the 10th percentile for gestational age, has adverse effects on animals as demonstrated in pigs in terms of stunted growth, fatty liver and insulin resistance [68,69]. However, these conditions can be attenuated by the supplementation of curcumin in the diets of IUGR piglets. In addition, dietary supplementation of curcumin at 200 mg/kg can alleviate the jejunum damage through the Nrf2/Keap1 pathway in the intestine of IUGR growing pigs. Curcumin with other phytogenic additives such as resveratrol has shown combined effects in replacing dietary antibiotics in pigs. Resveratrol, like curcumin, has potential anti-inflammatory, bacteria-regulating and immune-promoting effects [85]. In a study, dietary curcumin (300 mg/kg) in combination with resveratrol (300 mg/kg) showed regulatory effects on gut microbiota, down-regulating the Toll-like-receptor 4 mRNA (TLR4) signaling pathway, reducing the intestinal pathology and enhancing the immunity by secreting immunoglobulin in weaned piglets.
Piglets are often subjected to long road transportation. This consequence may cause different physical problems such as very high or low temperature, motion, long fasting, dehydration as well as a crowding problem. Dietary curcumin has shown neurological effects during pigs’ transportation. Wei et al. [86] postulated that dietary curcumin can increase the brain hippocampal nitric oxide (NO) production and reduce brain-derived neurotrophic factor (BDNF) gene expression which in turn could protect from subacute stress in pigs.
2.2. Dietary Turmeric/Curcumin in Poultry Nutrition
As the world human population is increasing, the demand for poultry meat also increasing. In poultry industry, birds are usually raise in extremely crowded condition which causes poor hygiene and stunted growth in chickens. To solve this problem, the antibiotics are extensively use to enhance chicks growth and health status. Due to the abuse of antibiotics and antimicrobial resistance matter, some phytogenic compounds especially turmeric or curcumin could be used to reduce the overwhelming dependence on antibiotics as well as increase the production performance of poultry industry [73]. A numerous studies have been conducted on the efficacy of turmeric or curcumin in poultry nutrition. In poultry industry, necrotic enteritis (NE) and avian coccidiosis are commons among the most important infectious diseases which resulted extensive mortality and substantive economic losses world-wide [87]. In a study, Lee et al. [87] demonstrated that dietary supplementation of turmeric oleoresin (4 mg/kg) and capsicum oleoresin (4 mg/kg) extracts could enhance the growth performance, reduce the gut lesions as well as serum levels of Clostridium perfringens (etiological agent of NE) toxins which resulted from molecular and cellular immune changes and finally showed their resistance against avian NE. In addition, dietary turmeric powder (TP) in single (7 g/kg) or in combination with neem and vitamin E have shown to increase growth, hematology, body composition, meat quality and serum biochemical parameters in broiler chicken [88,89]. However, Qasem et al. [90] reported that dietary turmeric powder (20 g/kg) did not improve the growth and immunity in broiler chickens. In case of Wenchang broiler chickens, dietary turmeric extract (100 to 300 mg/kg) could enhance the growth performance, breast muscle content and reduce the fat content in abdomen of chicks [91]. Moreover, Akhavan-Salamat et al. [92] demonstrated that dietary turmeric rhizome powder (TRP) or betaine (Bet) alone or in combination could improve the hematology and immune responses but TRP is more effective than Bet in terms of tolerance to heat stress in male Ross chicks. In laying hens, dietary TP showed no effects on body or egg weight and production at higher level (4% TP/kg), however, low level of TP (2% TP/kg) could increase egg production and quality slightly in Hisex laying hens [93]. Mirbod et al. [94] reported that increasing dietary turmeric or Curcuma longa rhizome powder (CRP) could enhance the egg quality in terms of improving egg shell thickness and hardness but abating cholesterol level in egg yolk in Leghorn laying hens. Nowadays, quail raising is getting much attention to meet the protein demand in addition to chickens. Dietary turmeric has shown its positive effects in quail rearing [12,95,96]. Turmeric (0.5%) with exogenous commercial enzyme, Panzyme (0.1%) has proven to increase growth performance and immune status in Japanese quail. Furthermore, dietary supplementation of turmeric in parent stock of Japanese quail may increase the egg quality in terms of higher vitellogenin, high density lipoprotein (HDL), vitamin A, B12, white egg protein and decrease the low density lipoprotein (LDL), total fat contents in eggs, in addition, increase the carcass content and antioxidant activity in female offsprings.
Nevertheless, curcumin extracted from turmeric has beneficial effects on poultry nutrition. Curcumin and turmerones extracted from lipophilic turmeric have shown their combined effect on the increment of growth, antioxidant and antimicrobial activities in broiler chickens [11]. Kim et al. [97] reported that dietary curcumin can be coccidiosis resistant challenged with Eimeria by means increasing body weight, and reducing fecal oocysts, gut lesions as well as increasing body immunity in Ross broiler chickens. On the other hand, Galli et al. [14,66] demonstrated curcumin (30 and 50 mg/kg) alone can reduce the lipid peroxidation and increase the antioxidant level in eggs of laying hens or curcumin combined with microencapsulated phytogenics (carvacrol, thymol and cinnamaldehyde improve the growth, flesh quality and unsaturated fatty acids in broiler chicks. Curcuminoids (222 mg/kg) in feeds showed protective effects on aflatoxin B1 (1 mg/kg) toxicity in male Ross broiler chicks [15] or curcuminoids (60 mg/kg) with tuna oil could increase the breast fillet and inhibit the auto-oxidation in thigh meat in slow growing chickens [98]. Rajput et al. [99,100] reported that supplementation of curcumin at 200 mg/kg can improve the growth, fat metabolism and intestinal integrity, whereas; curcumin (150 mg/kg) with lutein (150 mg/kg) could enhance the freshness of meat and antioxidant capacity in Arbor Acres broiler chickens. In addition, dietary curcumin alone (50 and 100 mg/kg) could improve the meat quality and oxidant stability in muscle as well as activate the glutathione (GSH) related enzymes and detoxify the Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens [17,101,102]. Xie et al. [65] explained that curcumin has important role in reducing fat deposition in abdomen by decreasing liver and blood lipid contents, and lipogenic gene expressions. Thermal or temperature and feed-borne aflatoxin B1 (AFB1) effects are the most crucial events in poultry industry. The supplementation of dietary curcumin can attenuate the AFB1 toxicity and resistance to heat stress in broiler and laying hens in terms of different attributes such as growth increment, as well as inhibition of hepatoxicity, CYP2A6 enzyme-mediated bioactivation of AFB1, enhancement antioxidant biomarkers, blood chemistry and immune related gene expressions [103,104,105,106]. In case of Japanese quail, Sahin et al. [107] reported that high ambient temperature (34 °C) causes oxidative stress in quail, however, dietary curcumin supplementation (200 or 400 mg/kg) could ameliorate the problem by increasing body weight, feed intake and reducing serum malondialdehyde (MDA) and heat shock protein 70 (HSP70) as well as modulating Nrf2/HO-1 pathway in quail. Ruan et al. [16,108] postulated that supplementation of curcumin (200 to 800 mg/kg) could enhance the growth, serum GSH, jujunal mucosa and villus surface in intestine and reduce MDA, interleukin-1β, tumor necrosis factor-α as well as detoxify ochratoxin A-related gene expressions in ducklings.
Use of curcumin nanoparticle or nanocurcumin in animal nutrition is a very recent event. In poultry nutrition, Rahmani et al. [10,109] reported that male broiler chicks (Ross 308) had decreased weight gain and high feed conversion ratio, and increased blood partial pressure of carbon dioxide (pCO2) and hematocrit at cold temperature (13–15 °C), however, when the chicks were offered feed with curcumin or nanocurcumin, the body weight and villus surface in intestine increased but MDA and caecal Escherichia coli decreased in broiler chicken at cold stressed condition. In another report, Marchiori et al. [12] demonstrated that thermal stress in quail negatively affects performance, however, dietary curcumin (30 mg/kg) and nanoencapsulated curcumin (10 mg/kg) could effectively enhance the quail health in terms of performance production, egg quality like antioxidant capacity of egg yolk, reduce feed conversion, lower thiobarbituric acid reactive substances (TBARS) and higher monounsaturated fatty acids (MUFA) in egg yolk of quail at cold stress condition (1 to 17 °C). Interestingly, in this experiment, dose of nanoencapsulated curcumin (10 mg/kg) was three time lesser than free curcumin (30 mg/kg) which showed potential effect using nanotechnological tool.
2.3. Dietary Turmeric and Curcumin in Rabbit Nutrition
Rabbit has a great importance like poultry as well as a high delicacy as a human food. However, rabbits are also very much prone to coccidiosis which is the most common digestive disease in rabbits. Due to coccidiosis, huge intestinal damage can occur in rabbits which may cause reduced body weight and nutrient-absorption capacity. As an endoparasite, Eimeria spp. is the most prevalent in the digestive system of farm rabbits [30]. There are few research works which have been conducted on dietary turmeric or curcumin in rabbit ailments [30,110]. Cervantes-Valencia et al. [30], for the first time, reported on the use of dietary curcumin as a natural anticoccidial alternative in adult rabbits. The results showed the positive effect of aqueous extract of curcumin on the excretion of oocysts of Eimeria spp. in New Zealand white rabbits such as reducing the number of oocysts and their concentration of eggs per gram of feces at the dose of 40 mg curcumin /kg body weight. However, Basavaraj et al. [110] found no beneficial effects of dietary turmeric rhizome powder (TRP) either at 0.15% or at 0.30% in the diets on blood biochemical and meat characteristics of broiler rabbits reared under summer stress conditions.
2.4. Dietary Turmeric and Curcumin in Fish Nutrition
Aquaculture is considered as one of the fastest growing food producing sectors world-wide, serving a vital role in mitigating the problem of animal-protein demand, especially in the third-world countries [75]. In comparison to farm animals or poultry, fish is a better source of high quality protein, micronutrients (vitamins and minerals) and essential fatty acids, especially polyunsaturated fatty acids (linoleic acid, LA; α-linolenic acid, ALA; eicosapentaenoic acid, EPA; docosahexaenoic acid, DHA; and arachidonic acid, ARA) [72,111]. However, due to the rapid horizontal and vertical expansion of aquaculture as well as a land shortage problem, most of aquaculture is practiced under intensive and stressful farming system which causes emerging and infectious disease outbreaks [112]. In this regard, fish farmers mostly use antibiotics and chemicals to enhance the fish health status and productivity. However, unauthorized and extensive use of antibiotics in aquaculture may lead to the emergence of antibiotic-resistant bacteria which results in environmental hazards, bacteriological changes and accumulation of antibiotic residuals in fish tissues that have public health concerns [33]. To overcome the problem of unethical use of antibiotics in aquaculture, many researchers have proposed the use of herbal medicines, especially turmeric or curcumin, as antibiotic replacers in the aquaculture industry (Table 4). In a very recent report, Rajabiesterabadi [113] postulated that dietary turmeric at 10 mg/kg could ameliorate the harmful effect of copper in terms of enhancing the antioxidant parameters such as superoxide dismutase (SOD), lysozyme, catalase (CAT), glutathione peroxidase (GSH-Px) and bactericidal activity, as well as decreasing liver cytokine’s gene expressions such as tumor necrosis factor-alpha (TNF-α) and interleukins like IL1-b and IL10 in common carp. Dietary curcumin (2%) can also enhance the growth and hematological values, and antioxidant as well as immune responses in rainbow trout challenged with Aeromonas salmonicida subsp. achromogenes [114]. Baldissera et al. [63] reported that a 100% disease resistance against Streptococcus agalactiae in silver catfish with high appetite can be achieved when fish were offered dietary curcumin (150 mg/kg). Tilapia is one of the most cultured fish species in the world. However, there is also disease problems and health issues in tilapia aquaculture. Mahmud et al. [64] demonstrated that dietary curcumin supplementation (50 or 100 mg/kg diet) can improve the growth (increased final weight, daily weight gain, specific growth rate), feed utilization (low feed conversion ratio, high protein efficiency ratio), antioxidant status (high catalase, and low GSH and MDA activities), immune responses (increased lysozyme activity, total immunoglobulins like IgG and IgM levels) as well as disease resistance in tilapia challenged with A. hydrophila. Likewise, some other studies reported that dietary curcumin supplementation in percentage inclusion (0.5%, 1% or 2%) or amount inclusion in the diet (100–200 mg/kg) could enhance the growth, feed efficiency, hematology, glycogenesis, serum total protein and bactericidal activity, liver heat shock protein and disease resistance against Vibrio alginolyticus in Nile tilapia [32,115,116]. Furthermore, El-Barbary et al. [117,118] postulated that tilapia fish with intraperitoneal injection of AFB1 (6 mg/kg body weight) can be ameliorated by dietary garlic which showed better performance than curcumin, however, the combination of garlic and curcumin (10 mg/kg) had more benefits than high concentrations (20 mg/kg) of garlic or curcumin against aflatoxicosis. In case of freshwater climbing perch fish (Anabas testudineus), Manju et al. [119,120,121] explained that dietary curcumin (0.5% or 1%) or curcumin analogue as salicylcurcumin (0.5%) have protective effects against lipid peroxidation and DNA damage as well as increase the growth, survival rate and disease resistance in A. testudineus. Dietary curcumin can enhance the liver health status in fish. In a study, curcumin supplementation (0.5% and 1%) showed the hepatoprotective effects in Jian carp on carbon tetrachloride (CCl4)-induced liver damage (low aspartate transaminase (AST), alanine transaminase (ALT), hepatocyte degeneration, MDA in liver) in terms of enhanced antioxidative activities (SOD, antioxidant capacity, GSH in liver) and inhibiting related cytokine expressions (TNF-α, IL-1β) via the NF-kB signaling pathway [122]. Jiang et al. [123] reported that crucian carp offered with dietary curcumin (5 mg/kg) could improve the growth (increase final weight, feed efficiency, hepatopancreas weight), digestibility (high trypsin and lipase activities), gastrointestinal (GIT) antioxidant activities (high SOD, CAT, glutathione reductase (GR), GSH, GSH-Px, glutathione-S-transferase (GST) activities) and upregulation of GIT-related mRNA gene expressions (trypsin, lipase, Na+, K+-ATPase (NKA), alkaline phosphatase (AKP), gamma-glutamyl transpeptidase (γGT), creatine kinase (CK), SOD1, CAT, GSH-Px, GST, and GR genes). To date, there has been no research work conducted on curcumin nanoparticles in fish nutrition and most of the studies dealt with the extremely high dosage of curcumin in fish which seemed to be effective. However, for the sense of cost-effective and more scientific usage, we need to minimize the amount of curcumin in fish feed as an alternative to antibiotics. Therefore, it is imperative to find out correct nanoformulations of curcumin in aquafeeds to produce disease-free fish and a higher production of the aquaculture species.
3. Curcumin Nanospheres in Monogastric Farm Animal, Poultry and Fish Nutrition
Natural products especially from plant sources always have a significant and promising role in pharmaceutics discovery in terms of therapeutic effects and biological properties. Curcumin as a natural therapeutic agent consists of two symmetric o-methoxy phenolic groups attached to α and β -unsaturated β–diketone which have potential as photophysical and photochemical properties [124].
Priyadarsini [58] reported that β–diketone groups can chelate ions with formation of curcumin-metal complexes and reduce metal toxicity in living organisms. However, due to the poor solubility, poor pharmacokinetic profile and lack of proper bioavailability problems, it is needed to improve and find out a more efficient way to deliver the curcumin nanoparticles in a site-specific manner with highest bioavailability. In this regard, nanotechnological incorporation of curcumin using different nanocarriers may enhance efficacy and ensure a targeted and sustained release of curcumin [125]. Moreover, curcumin has great potential as natural pigment which may increase the feed intake in animals and due to its antioxidant properties it can produce quality and healthy animals for human consumption, which is particularly important [126]. Eight types of nanocarriers have been proposed which can be used in nanobiotechnology as smart drug delivery systems (SDDSs) (Figure 2): carbon nanotubes, dendrimers, micelles, liposomes, super paramagnetic iron oxide nanoparticles (SPION), gold nanoparticles, quantum dot and mesoporous silica nanoparticles (MSN) [127]. In vitro and in vivo studies recommend that nanocurcumin loaded with a nanocarrier has more health-span-promoting factors than traditional or native curcumin where water solubility and bioavailability of curcumin can be significantly improved through nanoencapsulation with lipid, polymeric nanoparticles, nanogels and dendrimers, and conjugating to metal oxide nanoparticles [125].
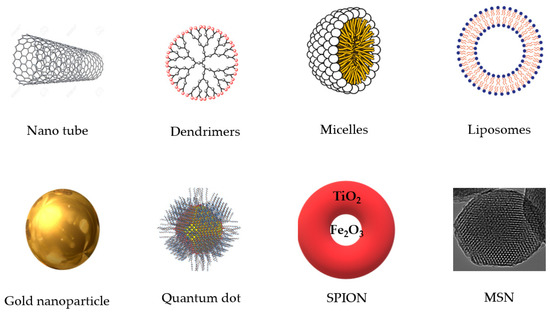
Figure 2.
Eight different types of important nanocarriers. SPION: super paramagnetic iron oxide nanoparticles; MSN: mesoporous silica nanoparticles.
The effectiveness of nanodelivery systems depend on the mode of loading and release of pharmaceutic agents, long life-span as well as high therapeutic efficiency with minimum side-effects [48]. Recently, our research group developed a nanosphere loaded with curcumin which indicates curcumin nanosphere is a functional agent that can manipulate pathogenic and Gram-negative bacterium, Vibrio vulnificus in GIT cell deaths [128]. This concept can be further utilized in gastro-enteritis diseases in animals to reduce the overwhelming dependence on antibiotics. In addition, Suwannateep et al. [129] reported that mucoadhesive curcumin nanospheres showed 48–49% of curcumin loading and easy adherence to stomach mucosa as well as release of curcumin in blood circulation of mice model. The application of curcumin nanoparticles with a nanocarrier is not seen yet in animal nutrition or nutritional diseases, as most of the works are related to therapeutic application in human diseases, especially in Alzheimer’s disease in which curcumin pigments exhibit unique charge and bonding characteristics that facilitate penetration into the blood–brain barrier and polymerization of β-amyloid in terms of anti-Alzheimer activity [130,131], neurodegenerative disease in central nervous system or skin cancers in the form of polymer-curcumin conjugate micelles, eucalyptol microemulsions, selenium nanoparticles encapsulated PLGA nanospheres with curcumin, nanosuspension-based curcumin etc. [132,133,134,135,136]. Dende et al. [137] reported that nanocurcumin is superior to native curcumin in preventing degenerative changes in terms of cerebral malaria in mice. The experimental study showed that a nanoformulated curcumin (PLGA-curcumin) has better therapeutic index than native curcumin in preventing the onset of neurological symptoms and delaying the death of mice in experimental cerebral malaria. The result of the experiment revealed that a single oral dose of 5 mg PLGA-curcumin containing 350μg of curcumin resulted in 3- to 4-fold higher concentration and prolonged presence of curcumin in the brain than that obtained with 5mg of native curcumin, indicating better bioavailability and more effective nature of PLGA-curcumin. Recently, Baldissera and his group [71,138,139] reported the potential effect of dietary nerolidol nanospheres at 0.5 or 1.0 mL/kg diet in Nile tilapia. The results demonstrated that nerolidol nanospheres supplementation improved the growth, survival, antioxidant activities and fillet fatty acids profile and attenuated/reduced hepatic cellular damage, impaired bioenergetics, microbial contents and neuronal damage as well as disease resistance against the pathogenic Gram-negative bacterium, Streptococcus agalactiae in Nile tilapia. For the first time, the studies showed the benefits of nanobiotechnology in terms of fish health and meat quality as well as the field of animal nutrition. In another study, the same group of researchers have shown that the dietary curcumin-loaded nanocapsule, Eudragit L-100 polymer, enhanced the anti-inflammatory and antioxidant capacity in dairy sheep, where the doses were 10 times lower than the free curcumin [69]. There was no evidence to report the effects of nanospheres or curcumin nanosphere in monogastric farm animal or poultry nutrition.
4. Conclusions and Future Outlook
During the last decades, people have witnessed the immense popularity of nanotechnology and the potential improvement of phytogenic drugs in terms of their encapsulation to protect from enzymatic degradation, increase solubility, minimize toxicity, act on target basis with prolonged and controlled release, change the mode of pharmacokinetics and the pharmacodynamics of plant-based pharmaceutics and their overall application for mankind. Curcumin and its derivatives have potential with antioxidant, anti-inflammatory, and antimicrobial actions in applied animal science. It has safe, non-toxic and inexpensive but outstanding functional properties which make it more attractive to researchers in comparison to other phytogenic compounds. However, research works are still going on as it has some limitations due to its lipophilic and hydrophobic nature. So, there will be a great scope to use nanotechnological tools for the advancement of curcumin research in animal science. Furthermore, even though curcumin nanoparticles decrease the doses of curcumin, the nanocurcumin form may be toxic and limited in its target delivery system. Therefore, it is imperative to study the toxicokinetics of nanocurcumin in order to minimize the nanocurcumin toxicity as well as effectively deliver the curcumin in target organs of animals. From the perspective of animal nutritional biotechnology, curcumin can be more efficiently utilized using different nanocarriers especially in nanospheric form which may help to ensure effective and target-based controlled release of curcumin in different animal organs. These approaches will be helpful to improve the growth and reproductive health status, immune responses and disease resistance in terms of understanding the digestion, metabolism, immune and excretion pathways of monogastric farm animals, poultry and fishes in a more scientific way as well as to replace antibiotics on farm levels.
Funding
This work was supported by the Korea Research Fellowship Program (grant No. 2019H1D3A1A01101555) for postdoctoral research to Mohammad Moniruzzaman (M.M.) and by the Basic Science Research Program (grant No. 2017R1A2B2007741) to Taesun Min (T.M.) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning. This work was also supported by the 2020 Scientific Promotion Program funded by the Jeju National University to Taesun Min (T.M.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toniguchi, N.; Arakawa, C.; Kobayashi, T. On the basic concept of nano-technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; Volume 2, pp. 18–23. [Google Scholar]
- Silva, G.A. Introduction to nanotechnology and its application to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Gopi, M.; Pearlin, B.; Kumar, R.D.; Shanmathy, M.; Prabakar, G. Role of nanoparticles in animal and poultry nutrition: Modes of action and applications in formulating feed additives and food processing. Int. J. Pharmacol. 2017, 13, 724–731. [Google Scholar] [CrossRef]
- Thulasi, A.; Rajendran, D.; Jash, S.; Selvaraju, S.; Jose, V.L.; Velusamy, S.; Mathivanan, S. Nanobiotechnology in Animal Nutrition. In Animal Nutrition and Reproductive Physiology (Recent Concepts), 1st ed.; Sampath, K.T., Ghosh, J., Eds.; Nanobiotechnology in Animal Nutrition; Satish Serial Publishing House: New Delhi, India, 2013; pp. 499–516. [Google Scholar]
- Uniyal, S.; Dutta, N.; Raza, M.; Jaiswal, S.K.; Sahoo, J.K.; Ashwin, K. Application of Nano Minerals in the Field of Animal Nutrition: A Review. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 4–8. [Google Scholar]
- Sinha, R.; Kim, G.; Nie, S.; Shin, D. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Srinivas, P.R.; Clifford, A.J.; Lee, S.C.; Philbert, M.A.; Hettich, R.L. New technologies for nutrition research. J. Nutr. 2004, 134, 681–685. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; Mezzalira, G.; Guterres, S.S. Development of nanocapsule suspensions and nanocapsule spray-dried powders containing melatonin. J. Braz. Chem. Soc. 2006, 17, 562–569. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Rahmani, M.; Golian, A.; Kermanshahi, H.; Bassami, M.R. Effects of curcumin and nanocurcumin on growth performance, blood gas indices and ascites mortalities of broiler chickens reared under normal and cold stress conditions. Italian J. Anim. Sci. 2017, 16, 438–446. [Google Scholar] [CrossRef]
- Johannah, N.M.; Joseph, A.; Maliakel, B.; Krishnakumar, I.M. Dietary addition of a standardized extract of turmeric (TurmaFEEDTM) improves growth performance and carcass quality of broilers. J. Anim. Sci. Tech. 2018, 60, 8. [Google Scholar]
- Marchiori, M.S.; Oliveira, R.C.; Souza, C.F.; Baldissera, M.D.; Ribeiro, Q.M.; Wagner, R.; Gundel, S.S.; Ourique, A.F.; Kirinus, J.K.; Stefani, L.M.; et al. Curcumin in the diet of quail in cold stress improves performance and egg quality. Anim. Feed Sci. Tech. 2019, 254, 144–157. [Google Scholar] [CrossRef]
- Al-Kassie, G.A.M.; Mohseen, A.M.; Abd-Al-Jaleel, R.A. Modification of productive performance and physiological aspects of broilers on the addition of a mixture of cumin and turmeric to the diet. Res. Opin. Anim. Vet. Sci. 2011, 1, 31–34. [Google Scholar]
- Galli, G.M.; Da Silva, A.S.; Biazus, A.H.; Reis, J.H.; Boiago, M.M.; Topazio, J.P.; Migliorini, M.J.; Guarda, N.S.; Moresco, R.N.; Ourique, A.F.; et al. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res. Vet. Sci. 2018, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Gowda, N.K.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Antioxidant efficacy of curcuminoids from Turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009, 102, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 2019, 13, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Lu, C.; Bai, K.; Zhang, L.; Wang, T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015, 63, 3880–3886. [Google Scholar] [CrossRef]
- Dar, A.H.; Rashid, N.; Majid, I.; Hussain, S.; Dar, M.A. Nanotechnology interventions in aquaculture and seafood preservation. Crit. Rev. Food Sci. Nutr. 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotech. 2017, 8, 26. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Sierra-Ávila, R.; Carlos Alberto Ávila-Orta, C.A.; Jiménez-Regalado, E.; Bello, A.M.; González-Morones, P.; Cadenas-Pliego, G. Oxidation of Copper Nanoparticles Protected with Different Coatings and Stored under Ambient Conditions. J. Nannomat. 2018, 2018, 9512768. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of Selenium Nanoparticles from Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Sudip Mondal, S.; Junghwan Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Oh, J.M.; Choy, J.H. Biocompatible nanoparticles intercalated with anticancer drug for target delivery: Pharmacokinetic and biodistribution study. J. Nanosci. Nanotechnol. 2010, 10, 2913–2916. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Ichikawa, S.; Iwamoto, S.; Watanabe, J. Formation of biocompatible nanoparticles by self-assembly of enzymatic hydrolysates of chitosan and carboxymethyl cellulose. Biosci. Biotechnol. Biochem. 2005, 69, 1637–1642. [Google Scholar] [CrossRef]
- Taylor, T.M.; Davidson, P.M. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Konkol, D.; Wojnarowski, K. The Use of Nanominerals in Animal Nutrition as a Way to Improve the Composition and Quality of Animal Products. J. Chem. 2018, 2018, 5927058. [Google Scholar] [CrossRef]
- Cervantes-Valencia, M.E.; Alcala-Canto, Y.; Salem, A.Z.M.; Kholif, A.E.; Ducoing-Watty, A.M.; Bernad-Bernad, M.J.; Gutiérrez-Olvera, C. Influence of curcumin (Curcuma longa) as a natural anticoccidial alternative in adult rabbits: First results. Ital. J. Anim. Sci. 2015, 14, 3838. [Google Scholar] [CrossRef]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic E. coli. Int. Immunopharmacol. 2015, 27, 46–52. [Google Scholar] [CrossRef]
- Sruthi, M.V.; Nair, A.B.; Arun, D.; Thushara, V.V.; Sheeja, C.C.; Vijayasree, A.S.; Oommen, O.V.; Divya, L. Dietary curcumin influences leptin, growth hormone and hepatic growth factors in Tilapia (Oreochromis mossambicus). Aquaculture 2018, 496, 105–111. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- EU. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Brussels. 2005. Available online: http://europa.eu/rapid/press-release_IP-05-1687_en.htm (accessed on 6 March 2020).
- FDA, Center for Veterinary Medicine. FDA Reminds Retail Establishments of Upcoming Changes to the Use of Antibiotics in Food Animals; FDA, Center for Veterinary Medicine: Rockville, Maryland, 2016. Available online: https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm507355.htm (accessed on 6 March 2020).
- Samarasinghe, K.; Wenk, C.; Silva, K.F.S.T.; Gunasekera, J.M.D.M. Turmeric (Curcuma longa) Root Powder and Mannanoligosaccharides as Alternatives to Antibiotics in Broiler Chicken Diets. Asian-Aust. J. Anim. Sci. 2003, 16, 1495–1500. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma Longa Linn). Food Chem. Toxicol. 2010, 48, 1026–1031. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Coll. Inter. Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotech. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhatt, I.B.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K.; Natarajan, C.; Seeram, N.; Shishodia, S. Curcumin biological and medicinal properties. In Turmeric: The Genus Curcuma; Ravindran, P.N., Nirmal Babu, K., Sivaraman, K., Eds.; CRC Press: New York, NY, USA, 2007; pp. 297–368. [Google Scholar]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1978, 43, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Das, S.; Balasubramanian, K. Quantum Chemical and Docking Insights into Bioavailability Enhancement of Curcumin by Piperine in Pepper. J. Phys. Chem. A 2016, 120, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-Applied Curcumin for Different Diseases Therapy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Makino, Y. Novel curcumin oral delivery systems. Anticancer Res. 2013, 33, 2807–2821. [Google Scholar]
- Hani, U.; Shivakumar, H.G. Solubility enhancement and delivery systems of curcumin a herbal medicine: A review. Curr. Drug Deliv. 2014, 11, 792–804. [Google Scholar] [CrossRef]
- Ma, Z.; Shayeganpour, A.; Brocks, D.R.; Lavasanifar, A.; Samuel, J. High-performance liquid chromatography analysis of curcuminin rat plasma: Application to pharmacokinetics of polymericmicellar formulation of curcumin. Biomed. Chromatogr. 2007, 21, 546–552. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Sobh, R.A.; Mohamed, W.S.; Moustafa, A.B.; Nasr, H.E. Encapsulation of Curcumin and Curcumin Derivative in Polymeric Nanospheres. Polymer-Plastics Tech. Eng. 2015, 54, 1457–1467. [Google Scholar] [CrossRef]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Shishtawy, R.M.; Al-Bar, O.A.M.; Al-Najada, A.R. Chemical modification of curcumin: Solubility and antioxidant capacity. Int. J. Food Prop. 2017, 20, 718–724. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Pandey, R.; Priyadarsini, K.I. Transport of liposomal and albumin loaded curcumin to living cells: An absorption and fluorescence spectroscopic study. Biochim. Biophys. Acta (Gen.) 2006, 1760, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.K.; Tomar, G.B.; Dhumale, V.A.; Zinjarde, S.; Sharma, R.B.; Datar, S. Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J. Agric. Food Chem. 2013, 61, 9632–9637. [Google Scholar] [CrossRef]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Enhancement of phototoxicity of curcumin in human oral cancer cells using silica nanoparticles as delivery vehicle. Lasers Med. Sci. 2014, 29, 645–652. [Google Scholar] [CrossRef]
- Ma’Mani, L.; Nikzad, S.; Kheiri-Manjili, H.; Al-Musawi, S.; Saeedi, M.; Askaralou, S. Curcumin-loaded guanidine functionalized PEGylated mesoporous silica nanoparticles KIT-6: Practical strategy for the breast cancer therapy. Eur. J. Med. Chem. 2014, 83, 646–654. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Descovi, S.N.; Machado, V.S.; Santos, R.C.V.; Baldisserotto, B. Efficacy of dietary curcumin supplementation as bactericidal for silver catfish against Streptococcus agalactiae. Microb. Pathog. 2018, 116, 237–240. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Al-Sagheera, A.A.; Redab, F.M.; Mahgoub, S.A.; Ayyata, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2018, 98, 422–429. [Google Scholar] [CrossRef]
- Galli, G.M.; Gerbet, R.R.; Griss, L.G.; Fortuoso, B.F.; Petrolli, T.G.; Boiago, M.M.; Souza, C.F.; Matheus, D.; Baldissera, M.D.; Mesadri, J.; et al. Combination of herbal components (curcumin, carvacrol, thymol, cinnamaldehyde) in broiler chicken feed: Impacts on response parameters, performance, fatty acid profiles, meat quality and control of coccidia and bacteria. Microb. Pathol. 2020, 139, 103916. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.; da Rocha, B.A.; Francisco, C.R.L.; Miranda, C.G.; Santos, P.D.F.; de Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Goncalves, O.H.; Bersani-Amado, C.A. Evaluation of the in vivo acute antiinflammatory response of curcumin loaded nanoparticles. Food. Funct. 2018, 24, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Zhao, W.; Shen, M.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Effect of Curcumin on Growth Performance, Inflammation, Insulin level, and Lipid Metabolism in Weaned Piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.; Zhang, J.; Han, H.; Wu, J.; Gan, Z.; Wei, C.; Zhang, L.; Wang, C.; Wang, T. Curcumin Alleviates IUGR Jejunum Damage by Increasing Antioxidant Capacity through Nrf2/Keap1 Pathway in Growing Pigs. Animals 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Jaguezeski, A.M.; Gündel, S.S.; Favarin, F.R.; Gündel, A.; Souza, C.F.; Baldissera, M.D.; Cazarotto, C.C.; Volpato, A.; Fortuoso, B.F.; Ourique, A.F. Low-dose curcumin-loaded Eudragit L-100-nanocapsules in the diet of dairy sheep increases antioxidant levels and reduces lipid peroxidation in milk. J. Food Eng. 2019, 43, e12942. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Velho, M.C.; Klein, B.; Abbad, L.B.; Ourique, A.F.; Wagner, R.; Da Silva, A.S.; Baldisserotto, B. Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: Benefits of nanotechnology for fish health and meat quality. Aquaculture 2020, 516, 734635. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Al-Yasiry, A.R.M.; Kiczorowski, P.; Winiarska-Mieczan, A. The natural feed additives as immunostimulants in monogastric animal nutrition—A review. Ann. Anim. Sci. 2017, 17, 605–625. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos, L.; Paredes, J.C.; Carlosama-Yépez, M.; Moreno, C.; Ruales, P. Effects of turmeric rhizome powder and curcumin on poultry production. A review. J. Anim. Feed Sci. 2017, 26, 293–302. [Google Scholar] [CrossRef]
- Seven, P.T.; Seven, I.; Baykalir, B.G.; Mutlu, S.I.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Italian J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquacult. 2019, 1–18. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. British J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.D.; Grace, D.; Sones, K. Current drivers and future directions of global livestock disease dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 20871–20877. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Hansen, C.F.; Mullana, B.P.; Pluske, J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012, 173, 3–16. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotech. 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Maneewan, C.; Yamauchi, K.E.; Mekbungwan, A.; Maneewan, B.; Siri, S. Effects of turmeric (Curcuma longa Linnaeus) on growth performance, nutrient digestibility, hematological values, and intestinal histology in nursery pigs. J. Swine Health Prod. 2012, 20, 231–240. [Google Scholar]
- Liu, Y.; Song, M.; Che, T.M.; Almeida, J.A.; Lee, J.J.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 2013, 91, 5294–5306. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Anim. Sci. 2014, 92, 3426–3440. [Google Scholar] [CrossRef]
- Ilsley, S.E.; Miller, H.M.; Kamel, C. Effects of dietary quillaja saponin and curcumin on the performance and immune status of weaned piglets. J. Anim. Sci. 2005, 83, 82–88. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and Resveratrol Regulate Intestinal Bacteria and Alleviate Intestinal Inflammation in Weaned Piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef]
- Wei, S.; Xu, H.; Xia, D.; Zhao, R. Curcumin attenuates the effects of transport stress on serum cortisol concentration, hippocampal NO production, and BDNF expression in the pig. Domestic Anim. Endocrinol. 2010, 39, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lillehoj, E.P.; Min, W.; Bravo, D.M. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 2013, 110, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.H. Effect of Turmeric (Curcuma longa) powder on growth performance, carcass traits, meat quality and serum biochemical parameters in broilers. J. Adv. Biomed. Path. Res. 2013, 3, 25–32. [Google Scholar]
- Nayaka, H.B.S.; Umakantha, B.; Ruban, S.W.; Murthy, H.N.N.; Narayanaswamy, H.D. Performance and hematological parameters of broilers fed neem, turmeric, vitamin e and their combinations. Emir. J. Food Agric. 2013, 25, 483–488. [Google Scholar] [CrossRef]
- Qasem, M.A.A.; Alhajj, M.S.; Ger El Nabi, A.R.; Al-Mufarrej, S.I. Effect of turmeric powder as a dietary supplement on performance indicators and immune responses in broiler chickens. J. Anim. Vet. Adv. 2015, 14, 30–35. [Google Scholar]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of dietary supplementation with turmericrhi zome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 2015, 14, 344–349. [Google Scholar] [CrossRef]
- Akhavan-Salamat, H.; Ghasemi, H.A. Alleviation of chronic heat stress in broilers by dietary supplementation of betaine and turmeric rhizome powder: Dynamics of performance, leukocyte profile, humoral immunity, and antioxidant status. Trop. Anim. Health Prod. 2016, 48, 181–188. [Google Scholar] [CrossRef]
- Hassan, S.M. Effects of adding different dietary levels of turmeric (Curcuma longa Linn) powder on productive performance and egg quality of laying hens. Int. J. Poult. Sci. 2016, 15, 156–160. [Google Scholar] [CrossRef][Green Version]
- Mirbod, M.; Mahdavi, A.H.; Samie, A.H.; Mehri, M. Effects of Curcuma longa rhizome powder on egg quality, performance, and some physiological indices of laying hens fed different levels of metabolizable energy. J. Sci. Food Agric. 2017, 97, 1286–1294. [Google Scholar] [CrossRef]
- Kilany, O.E.; Mahmoud, M.M. Turmeric and exogenous enzyme supplementation improve growth performance and immune status of Japanese quail. Worlds Vet. J. 2014, 4, 20–29. [Google Scholar] [CrossRef]
- Saraswati, T.R.; Tana, S. Physiological condition of first female and male offspring of Japanese quail (Coturnix japonica) whose parents were supplemented by turmeric powder. J. World Poult. Res. 2016, 6, 59–65. [Google Scholar]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.T.T.; Molee, W.; Khempaka, S.; Paraksa, N. Supplementation with curcuminoids and tuna oil influenced skin yellowness, carcass composition, oxidation status, and meat fatty acids of slow-growing chickens. Poult. Sci. 2017, 97, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.; Muhammad, N.; Yan, R.; Zhong, X.; Wang, T. Effect of dietary supplementation of curcumin on growth performance, intestinal morphology and nutrients utilization of broiler chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar] [CrossRef]
- Rajput, R.; Ali, S.; Naeem, M.; Khan, M.A.; Wang, T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on pigmentation, oxidative stability and quality of meat from broiler chickens affected by a coccidiosis challenge. Br. Poult. Sci. 2014, 55, 501–509. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, K.; Su, W.; Wang, A.; Zhang, L.; Huang, K.; Wang, T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018, 97, 1209–1219. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, K.W.; He, J.; Niu, Y.; Lu, Y.; Zhang, L.; Wang, T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018, 96, 867–879. [Google Scholar] [CrossRef]
- Salah, A.S.; Mahmoud, M.A.; Ahmed-Farid, O.A.; El-Tarabany, M.S. Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. J. Therm. Biol. 2019, 84, 259–265. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Qi, M.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Curcumin Prevents Aflatoxin B1 Hepatoxicity by Inhibition of Cytochrome P450 Isozymes in Chick Liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin Successfully Inhibited the Computationally Identified CYP2A6 Enzyme-Mediated Bioactivation of Aflatoxin B1 in Arbor Acres broiler. Front. Pharmacol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Nawab, A.; Li, G.; Liu, W.; Lan, R.; Wu, J.; Zhao, Y.; Kang, K.; Kieser, B.; Sun, C.; Tang, S.; et al. Effect of Dietary Curcumin on the Antioxidant Status of Laying Hens under High-Temperature Conditions. Braz. J. Poult. Sci. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, Z.; Tuzcu, M.; Sahin, N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012, 50, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Zhu, Y.W.; Fouad, A.M.; Yan, S.J.; Chen, W.; Zhang, Y.N.; Xia, W.G.; Wang, S.; Jiang, S.Q.; Yang, L.; et al. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes. Poult. Sci. 2019, 98, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Golian, A.; Kermanshahi, H.; Bassami, M.R. Effects of curcumin or nanocurcumin on blood biochemical parameters, intestinal morphology and microbial population of broiler chickens reared under normal and cold stress conditions. J. Appl. Anim. Res. 2018, 46, 200–209. [Google Scholar] [CrossRef]
- Basavaraj, M.; Nagabhushana, V.; Prakash, N.; Mallikarjunappa, S.; Appannavar, M.M.; Wagmare, P. Effect of dietary supplementation of Pulvis Curcuma longa on the voluntary feed intake, nutrient digestibility and growth performance of broiler rabbits under summer stress. Vet. World 2010, 3, 369–372. [Google Scholar]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as functional ingredients: Focus on Mediterranean fish aquaculture. Rev. Aquacult. 2018, 10, 800–832. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Khanongnuch, C.; Kanpiengjai, A.; Unban, K.; Kim, V.V.; Srichaiyo, S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 491, 94–100. [Google Scholar] [CrossRef]
- Rajabiesterabadi, H.; Hoseini, S.M.; Fazelan, Z.; Hoseinifar, S.H.; Doan, H.V. Effects of dietary turmeric administration on stress, immune, antioxidant and inflammatory responses of common carp (Cyprinus carpio) during copper exposure. Aquacult. Nutr. 2020, 1–11. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; İspir, U.; Ural, M.S. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. Achromogenes. Fish. Shell. Immun. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Elgendy, M.; Hakim, A.; Ibrahim, T.; Soliman, W.; Ali, S. Immunomodulatory effects of curcumin on nile tilapia, Oreochromis niloticus and its antimicrobial properties against Vibrio alginolyticus. J. Fish. Aquat. Sci. 2016, 11, 206–215. [Google Scholar] [CrossRef][Green Version]
- Cui, H.; Liu, B.; Ge, X.; Xieb, J.; Xub, P.; Miao, L.; Sun, S.; Liaoa, Y.; Chen, R.; Ren, M.; et al. Effects of dietary curcumin on growth performance, biochemical parameters, HSP70 gene expression and resistance to Streptococcus iniae of juvenile Gift Tilapia, Oreochromis niloticus. Isr. J. Aquacult. Bamidgeh 2017, 66, 986–996. [Google Scholar]
- El-Barbary, M.I. Detoxification and antioxidant effects of garlic and curcumin in Oreochromis niloticus injected with aflatoxin B1 with reference to gene expression of glutathione peroxidase (GPx) by RT-PCR. Fish. Physiol. Biochem. 2016, 42, 617–629. [Google Scholar] [CrossRef] [PubMed]
- El-Barbary, M.I. Impact of garlic and curcumin on the hepatic histology and cytochrome P450 gene expression of aflatoxicosis Oreochromis niloticus using RT-PCR. Turk. J. Fish. Aquat. Sci. 2018, 18, 405–415. [Google Scholar]
- Manju, M.; Akbarsha, M.A.; Oommen, O.V. In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol. Biochem. 2012, 38, 309–318. [Google Scholar] [CrossRef]
- Manju, M.; Vijayasree, A.S.; Akbarsha, M.A.; Oommen, O.V. Protective effect of dietary curcumin in Anabas testudineus (Bloch) with a special note on DNA fragmentation assay on hepatocytes and micronucleus assay on erythrocytes in vivo. Fish. Physiol. Biochem. 2013, 39, 1323–1330. [Google Scholar] [CrossRef]
- Manju, M.; Sherin, T.G.; Rajasekharan, K.N.; Oommen, O.V. Curcumin analogue inhibits lipid peroxidation in a freshwater teleost, Anabas testudineus (Bloch)-an in vitro and in vivo study. Fish. Physiol. Biochem. 2009, 35, 413–420. [Google Scholar] [CrossRef]
- Cao, L.; Ding, W.; Du, J.; Jia, R.; Liu, Y.; Zhao, C.; Shen, Y.; Yin, G. Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinus carpio var. Jian) with CCl 4-induced liver damage. Fish. Shell. Immunol. 2015, 43, 150–157. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, X.; Zhou, X.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, Y. Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 2016, 463, 174–180. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Uyanik, C. Biological evaluation of bioavailable amphiphilic polymeric conjugate based-on natural products: Diosgenin and curcumin. Int. J. Poly. Mat. Poly. Biol. 2020, 69, 73–84. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef]
- Faehnrich, B.; Lukas, B.; Humer, E.; Zebeli, Q. Phytogenic pigments in animal nutrition: Potentials and risks. J. Sci. Food Agric. 2016, 96, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.M.; Kim, D.W.; Min, T.S.; Lee, S.J. Nanosphere Loaded with Curcumin Inhibits the Gastrointestinal Cell Death Signaling Pathway Induced by the Foodborne Pathogen Vibrio vulnificus. Cells 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Suwannateep, N.; Banlunara, W.; Wanichwecharungruang, S.P.; Chiablaem, K.; Lirdprapamongkol, K.; Svasti, J. Mucoadhesive curcumin nanospheres: Biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J. Contr. Rel. 2011, 151, 176–182. [Google Scholar] [CrossRef]
- Balasubramanian, K. Molecular Orbital Basis for Yellow Curry Spice Curcumin’s Prevention of Alzheimer’s Disease. J. Agric. Food Chem. 2006, 54, 3512–3520. [Google Scholar] [CrossRef]
- Balasubramanian, K. Quantum chemical insights into Alzheimer’s disease: Curcumin’s chelation with Cu(II), Zn(II), and Pd(II) as a mechanism for its prevention. Int. J. Quant. Chem. 2016, 116, 1107–1119. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, S.; Kong, D.; Gao, X.; Zhao, Y.; Wang, Z. Biodegradable Polymer-Curcumin Conjugate Micelles Enhance the Loading and Delivery of Low-Potency Curcumin. Pharm. Res. 2012, 29, 3512–3525. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, F.Y. Development and Characterization of Eucalyptol Microemulsions for Topic Delivery of Curcumin. Chem. Pharm. Bull. 2011, 59, 172–178. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Yongang Li, Y.; Li, Z. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larraneta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; da Silva, A.S.; Velho, M.C.; Ourique, A.F.; Baldisserotto, B. Benefits of nanotechnology: Dietary supplementation with nerolidol-loaded nanospheres increases survival rates, reduces bacterial loads and prevents oxidative damage in brains of Nile tilapia experimentally infected by Streptococcus agalactiae. Microb. Pathol. 2020, 141, 103989. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Velho, M.C.; Bassotto, V.A.; Ourique, A.F.; da Silva, A.S.; Baldisserotto, B. Nanospheres as a technological alternative to suppress hepatic cellular damage and impaired bioenergetics caused by nerolidol in Nile tilapia (Oreochromis niloticus). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 751–759. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).