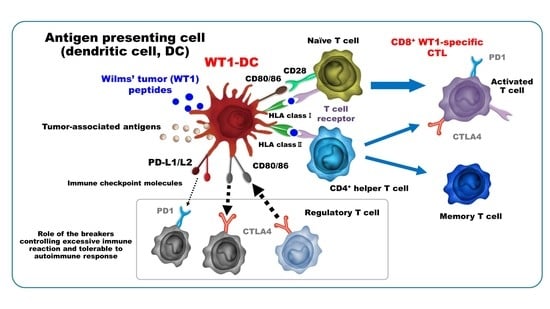
Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and DC Preparation
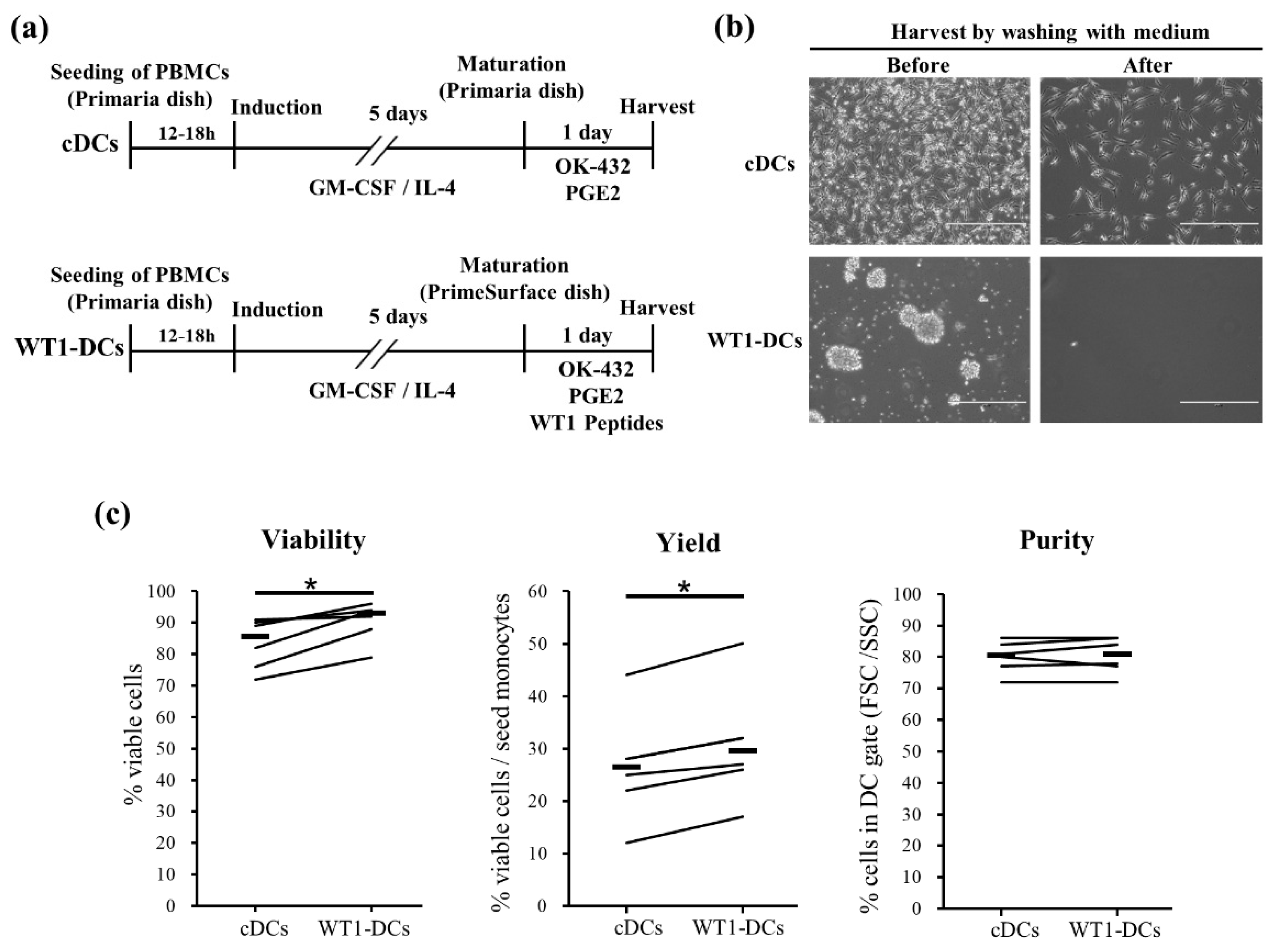
2.1.1. New Approach to Manufacture a DC Vaccine
2.1.2. DC Generation
2.2. Functional Analyses on the Obtained mDCs
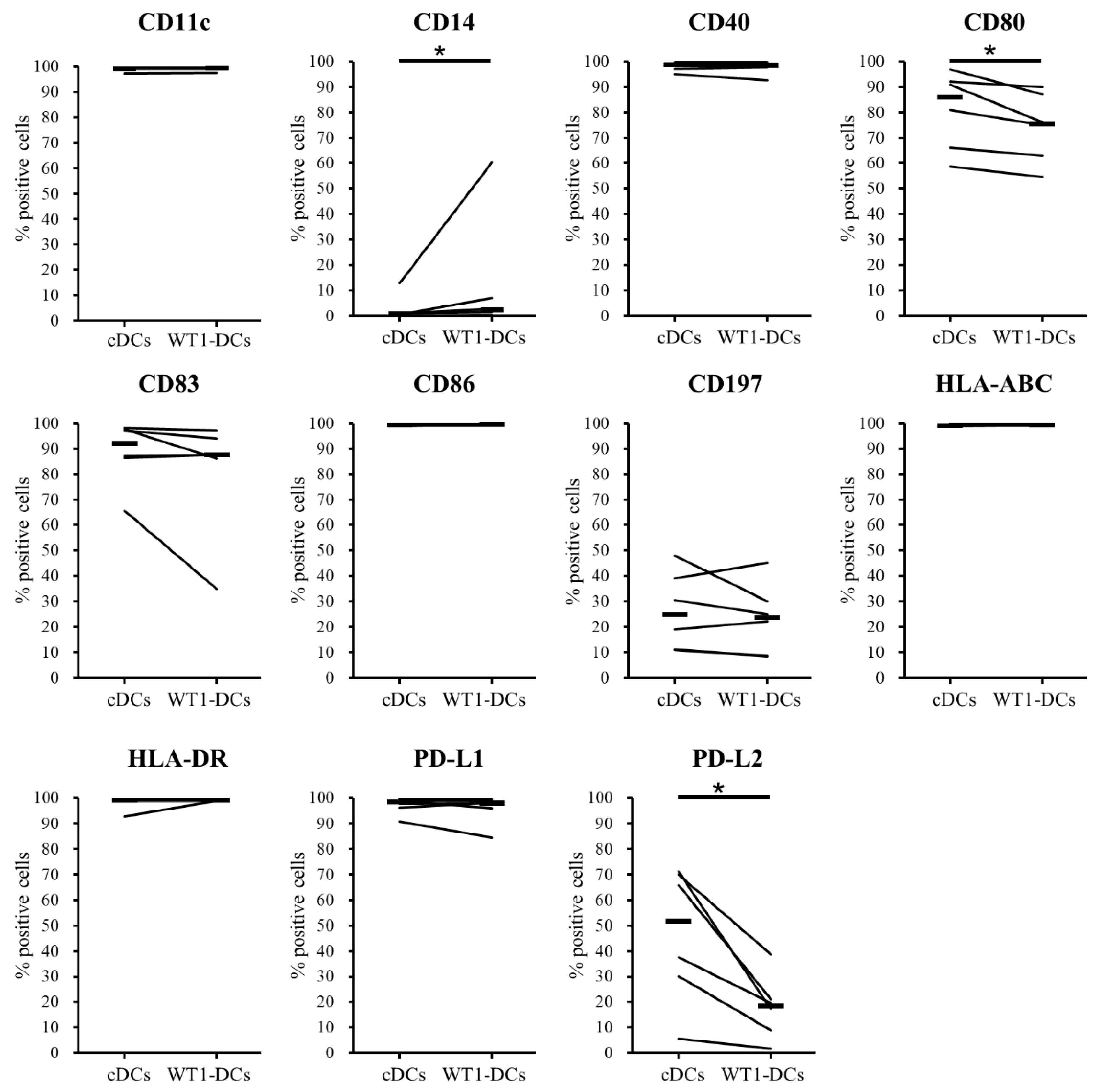
2.2.1. Phenotyping of DCs
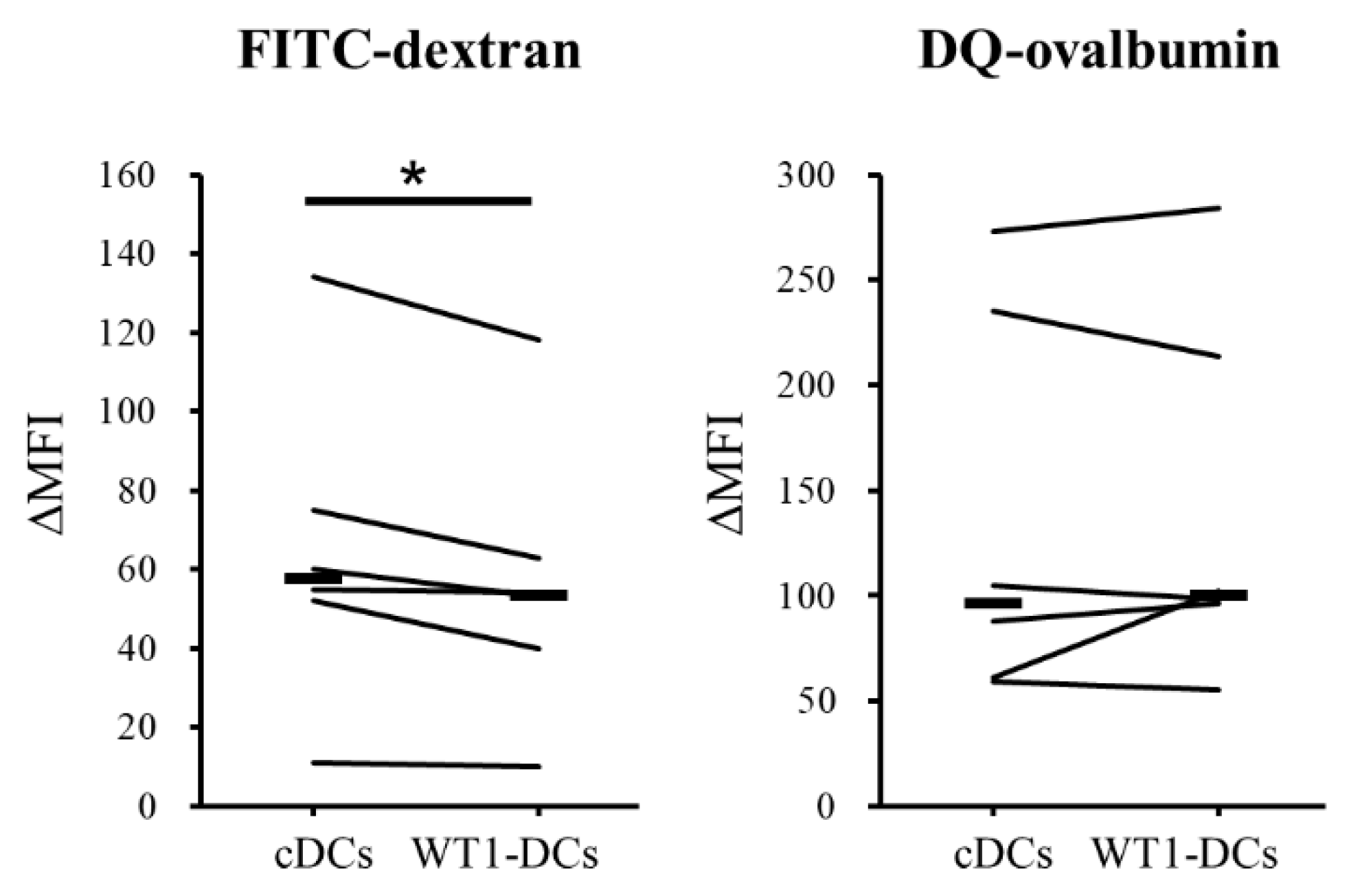
2.2.2. Pinocytotic and Phagocytic Assay
2.2.3. Measurement of Cytokine Production
2.2.4. CTL Induction in Vitro
2.3. WT1-DC Administration
2.3.1. Patients
2.3.2. WT1-DC Administration
2.3.3. Immune Monitoring for WT1-CTLs
2.3.4. Shipping of WT1-DCs
2.4. Statistical Analysis
3. Results
3.1. WT1-DCs Show Remarkable Cluster and Increase in Viability and Recovery of DC/Monocyte Ratio Compared to Conventional DCs (cDCs)
3.2. Comparison of Phenotypes of WT1-DCs and cDCs
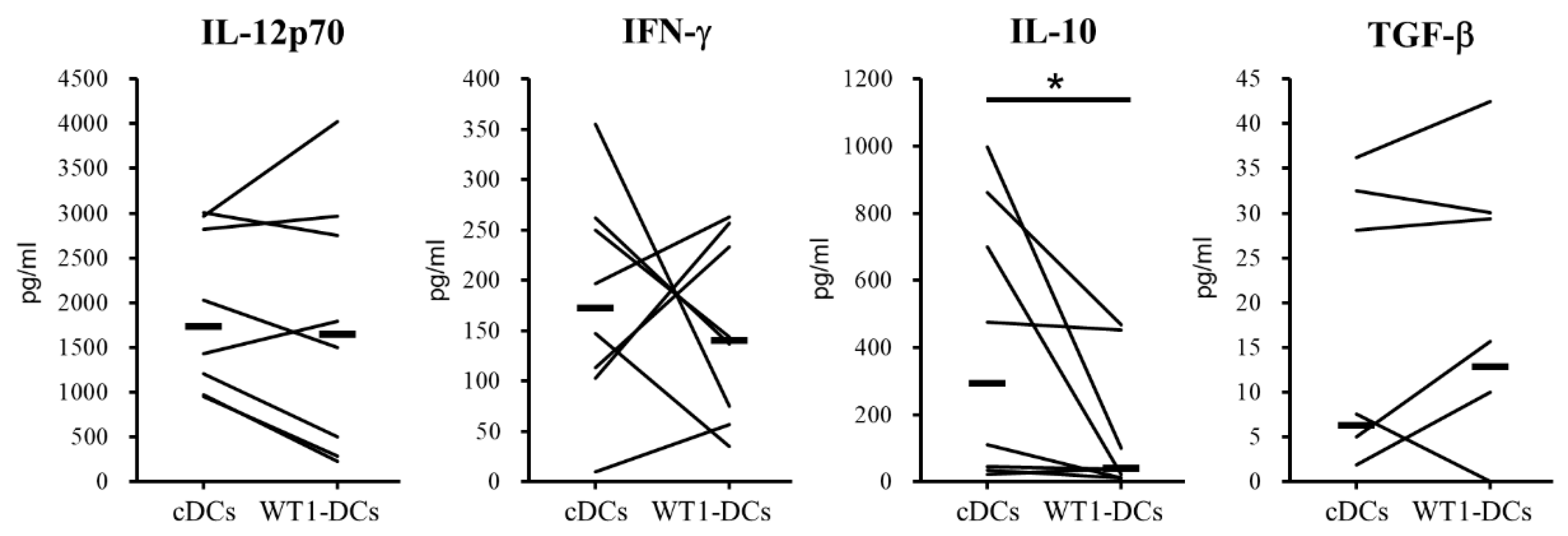
3.3. WT1-DCs Have Abilities of Lower Pinocytosis and IL-10 Production Compared with cDCs
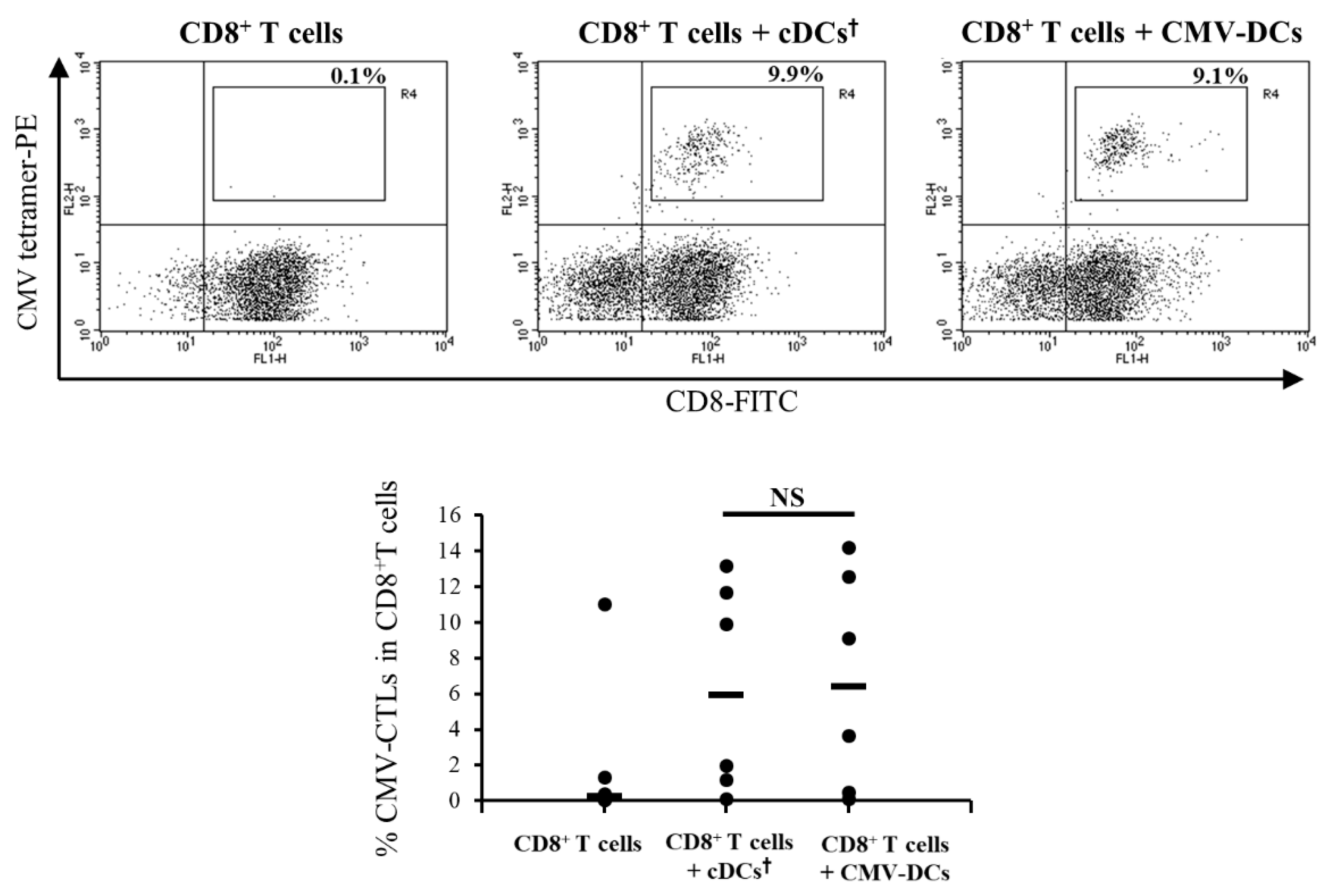
3.4. Antigen-Presentation Ability of DCs Pre-Pulsed with CMV Peptide in Low-Adhesion Culture Maturation (CMV-DCs) are Similar to cDCs Post-Pulsed with CMV Peptide
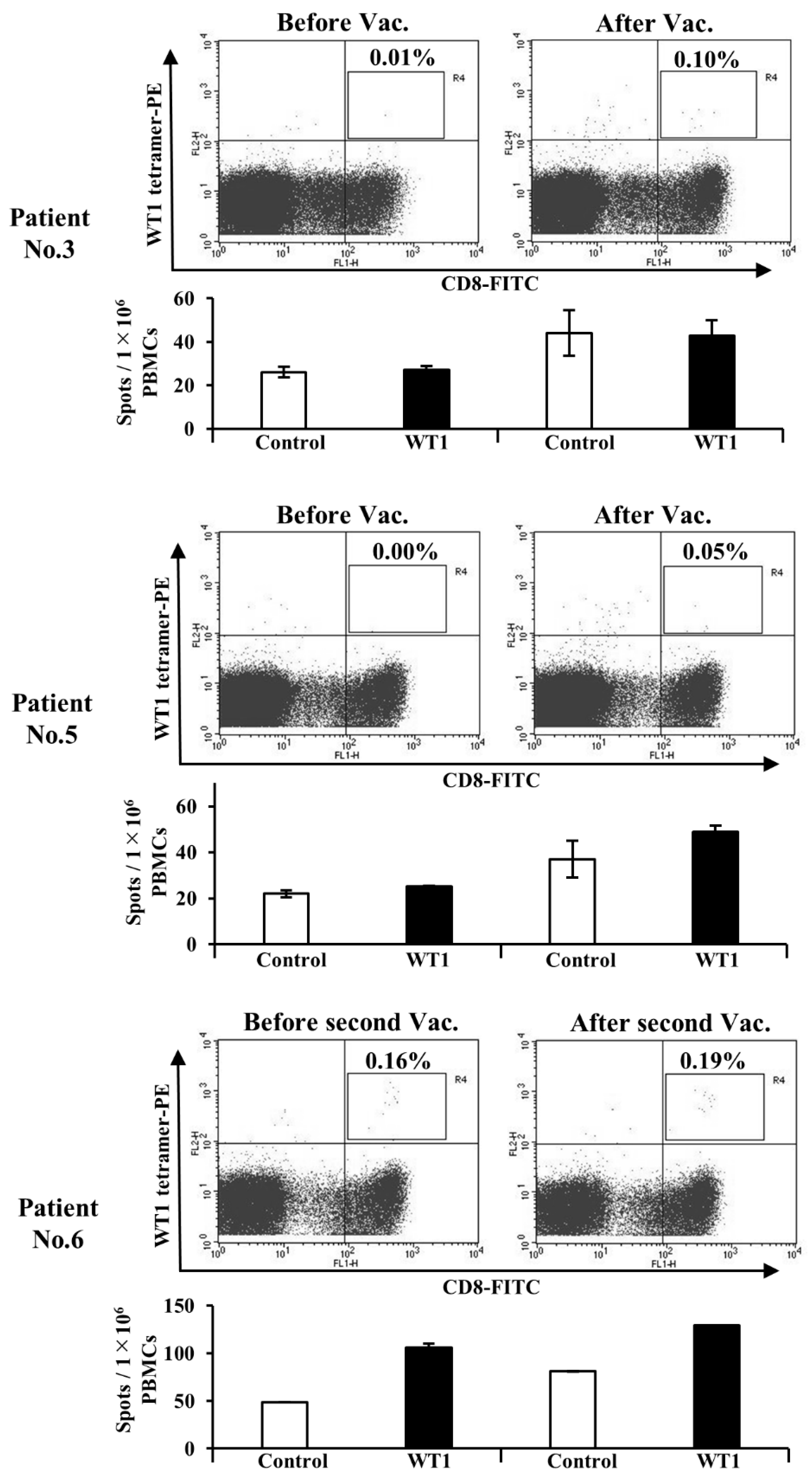
3.5. Administration of WT1-DCs Induces WT1-Specific CTLs in Patients with Cancer
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus Ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Calvo Tardón, M.; Allard, M.; Dutoit, V.; Dietrich, P.Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G.; Russo, V.; Maccalli, C.; Parolini, D.; Rizzo, N.; Maio, M. Peptide-based vaccines for cancer therapy. Hum. Vaccines Immunother. 2014, 10, 3175–3178. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Dupuis, L.; Ganne, V. Adjuvants designed for veterinary and human vaccines. Vaccine 2001, 19, 2666–2672. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; Medina, M.A.; Huang, X.F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W.; et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [PubMed]
- Steinman, R.M. Decisions About Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, P.; Merrick, A.E.; West, E.; O’Donnell, D.; Selby, P.; Vile, R.; Melcher, A.A. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J. Immunother. 2008, 31, 620–632. [Google Scholar] [CrossRef]
- Cho, D.Y.; Yang, W.K.; Lee, H.C.; Hsu, D.M.; Lin, H.L.; Lin, S.Z.; Chen, C.C.; Harn, H.J.; Liu, C.L.; Lee, W.Y.; et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: A phase II clinical trial. World Neurosurg. 2012, 77, 736–744. [Google Scholar] [CrossRef]
- Reyes, D.; Salazar, L.; Espinoza, E.; Pereda, C.; Castellón, E.; Valdevenito, R.; Huidobro, C.; Inés Becker, M.; Lladser, A.; López, M.N.; et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br. J. Cancer 2013, 109, 1488–1497. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Sakai, K.; Shimodaira, S.; Maejima, S.; Udagawa, N.; Sano, K.; Higuchi, Y.; Koya, T.; Ochiai, T.; Koide, M.; Uehara, S.; et al. Dendritic cell-based immunotherapy targeting Wilms’ tumor 1 in patients with recurrent malignant glioma. J. Neurosurg. 2015, 123, 989–997. [Google Scholar] [CrossRef]
- Shimodaira, S.; Sano, K.; Hirabayashi, K.; Koya, T.; Higuchi, Y.; Mizuno, Y.; Yamaoka, N.; Yuzawa, M.; Kobayashi, T.; Ito, K.; et al. Dendritic cell-based adjuvant vaccination targeting wilms’tumor 1 in patients with advanced colorectal cancer. Vaccines 2015, 3, 1004–1018. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimodaira, S.; Nagai, K.; Ogasawara, M.; Takahashi, H.; Abe, H.; Tanii, M.; Okamoto, M.; Tsujitani, S.-I.; Yusa, S.; et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: A multicenter analysis. Cancer Immunol. Immunother. 2014, 63, 797–806. [Google Scholar] [CrossRef]
- Koido, S.; Homma, S.; Okamoto, M.; Takakura, K.; Mori, M.; Yoshizaki, S.; Tsukinaga, S.; Odahara, S.; Koyama, S.; Imazu, H.; et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II- restricted epitopes for pancreatic cancer. Clin. Cancer Res. 2014, 20, 4228–4239. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shimodaira, S.; Ogasawara, M.; Ota, S.; Kobayashi, M.; Abe, H.; Morita, Y.; Nagai, K.; Tsujitani, S.; Okamoto, M.; et al. Lung adenocarcinoma may be a more susceptive subtype to a dendritic cell-based cancer vaccine than other subtypes of non-small cell lung cancers: A multicenter retrospective analysis. Cancer Immunol. Immunother. 2016, 65, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yanagisawa, R.; Yoshikawa, K.; Higuchi, Y.; Koya, T.; Yoshizawa, K.; Tanaka, M.; Sakashita, K.; Kobayashi, T.; Kurata, T.; et al. Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: A case report and review of the literature. Cytotherapy 2015, 17, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S.; Hirabayashi, K.; Yanagisawa, R.; Higuchi, Y.; Sano, K.; Koizumi, T. Dendritic cell-based cancer. In Wilms Tumor; van den Heuvel-Eibrink, M.M., Ed.; Codon Publications: Brisbane, Australia, 2016. [Google Scholar]
- Shimodaira, S.; Yanagisawa, S.; Koya, T.; Hirabayashi, K.; Higuchi, Y.; Sakamoto, T.; Togi, M.; Kato, J.; Kobayashi, T.; Koizumi, T.; et al. In Vivo Administration of Recombinant Human Granulocyte Colony-Stimulating Factor Increases the Immune Effectiveness of Dendritic Cell-Based Cancer Vaccination. Vaccines 2019, 7, 120. [Google Scholar] [CrossRef]
- Figdor, C.G.; De Vries, I.J.M.; Lesterhuis, W.J.; Melief, C.J.M. Dendritic cell immunotherapy: Mapping the way. Nat. Med. 2004, 10, 475–480. [Google Scholar] [CrossRef]
- Okamoto, M.; Oshikawa, T.; Tano, T.; Ohe, G.; Furuichi, S.; Nishikawa, H.; Ahmed, S.U.; Akashi, S.; Miyake, K.; Takeuchi, O.; et al. Involvement of Toll-like receptor 4 signaling in interferon-gamma production and antitumor effect by streptococcal agent OK-432. J. Nat. Cancer Inst. 2003, 95, 316–326. [Google Scholar] [CrossRef][Green Version]
- Oshikawa, T.; Okamoto, M.; Tano, T.; Sasai, A.; Kan, S.; Moriya, Y.; Ryoma, Y.; Saito, M.; Akira, S.; Sato, M. Antitumor effect of OK-432-derived DNA: One of the active constituents of OK-432, a streptococcal immunotherapeutic agent. J. Immunother. 2006, 29, 143–150. [Google Scholar] [CrossRef]
- Hill, K.S.; Errington, F.; Steele, L.P.; Merrick, A.; Morgan, R.; Selby, P.J.; Georgopoulos, N.T.; O’Donnell, D.M.; Melcher, A.A. OK432-Activated Human Dendritic Cells Kill Tumor Cells via CD40/CD40 Ligand Interactions. J. Immunol. 2008, 181, 3108–3115. [Google Scholar] [CrossRef]
- Nakahara, S.; Tsunoda, T.; Baba, T.; Asabe, S.; Tahara, H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 2003, 63, 4112–4118. [Google Scholar]
- Sato, M.; Takayama, T.; Tanaka, H.; Konishi, J.; Suzuki, T.; Kaiga, T.; Tahara, H. Generation of mature dendritic cells fully capable of T helper type 1 polarization OK-432 combined with prostaglandin E2. Cancer Sci. 2003, 94, 1091–1098. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.; Hsu, C.; Ming, C.; He, Y.; Zhang, J.; Wei, L.; Zhou, P.; Wang, C.Y.; Yang, J.; et al. Discrimination of the heterogeneity of bone marrow-derived dendritic cells. Mol. Med. Rep. 2017, 16, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Mc Duffie, Y.; Boehm, H.; Martinez, A.; Spatz, J.P.; Appel, S. Surface-mediated priming during in vitro generation of monocyte-derived dendritic cells. Scand. J. Immunol. 2015, 81, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, B.; Berger, T.G.; Maczek, C.; Röder, C.; Schreiner, D.; Hirsch, U.; Haendle, I.; Leisgang, W.; Glaser, A.; Kuss, O.; et al. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods 2000, 245, 15–29. [Google Scholar] [CrossRef]
- Szmania, S.; Yi, Q.; Cottler-Fox, M.; Rosen, N.A.; Freeman, J.; Kordsmeier, B.J.; Moreno, A.; Shi, J.; Barlogie, B.; Tricot, G.; et al. Clinical-grade myeloma Ag pre-loaded DC vaccines retain potency after cryopreservation. Cytotherapy 2005, 7, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Miller, E.; Spadaccia, M.; Vengco, I.; Hasan, F.; Bhardwaj, N. Preparation of tumor antigen-loaded mature dendritic cells for immunotherapy. J. Vis. Exp. 2013, 78, e50085. [Google Scholar] [CrossRef]
- Shimodaira, S.; Hirabayashi, K.; Kobayashi, T.; Higuchi, Y.; Yokokawa, K. Future Prospective of Cancer Vaccination Technology in Japan. Pharm. Reg. Aff. 2015, 4. [Google Scholar] [CrossRef]
- Higuchi, Y.; Koya, T.; Yuzawa, M.; Yamaoka, N.; Mizuno, Y.; Yoshizawa, K.; Hirabayashi, K.; Kobayashi, T.; Sano, K.; Shimodaira, S. Enzyme-Linked Immunosorbent Spot Assay for the Detection of Wilms’ Tumor 1-Specific T Cells Induced by Dendritic Cell Vaccination. Biomedicines 2015, 3, 304–315. [Google Scholar] [CrossRef]
- Švajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: An adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kimura, K.; Horiguchi, I.; Sakai, Y. Size-dependent hepatic differentiation of human induced pluripotent stem cells spheroid in suspension culture. Regen. Ther. 2019, 12, 66–73. [Google Scholar] [CrossRef]
- Agrawal, S.; Agrawal, A.; Doughty, B.; Gerwitz, A.; Blenis, J.; Van Dyke, T.; Pulendran, B. Cutting edge: Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 2003, 171, 4984–4989. [Google Scholar] [CrossRef]
- Dillon, S.; Agrawal, A.; Van Dyke, T.; Landreth, G.; McCauley, L.; Koh, A.; Maliszewski, C.; Akira, S.; Pulendran, B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 2004, 172, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Sutmuller, R.; Hermann, C.; Van der Graaf, C.A.A.; Van der Meer, J.W.M.; van Krieken, J.H.; Hartung, T.; Adema, G.; Kullberg, B.J. Toll-Like Receptor 2 Suppresses Immunity against Candida albicans through Induction of IL-10 and Regulatory T Cells. J. Immunol. 2004, 172, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Coronel, E.; Camacho-Sandoval, R.; Bonifaz, L.C.; López-Vidal, Y. PD-L2 induction on dendritic cells exposed to Mycobacterium avium downregulates BCG-specific T cell response. Tuberculosis 2011, 91, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Dorfman, D.M.; Ma, F.-R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Hobo, W.; Maas, F.; Adisty, N.; De Witte, T.; Schaap, N.; Van Der Voort, R.; Dolstra, H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8 + T cells. Blood 2010, 116, 4501–4511. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Koizumi, T.; Koya, T.; Sano, K.; Koido, S.; Nagai, K.; Kobayashi, M.; Okamoto, M.; Sugiyama, H.; Shimodaira, S. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018, 38, 2217–2225. [Google Scholar]
- Fujimoto, T.; Donnel, M.A.O.; Duds, B.; Szilvasi, A.; Chen, X.; Mai, M. Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state. J. Immunol. 1997, 158, 5619–5626. [Google Scholar]
- Ryoma, Y.; Moriya, Y.; Okamoto, M.; Kanaya, I.; Saito, M.; Sato, M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004, 24, 3295–3301. [Google Scholar]
- Hirayama, M.; Nishikawa, H.; Nagata, Y.; Tsuji, T.; Kato, T.; Kageyama, S.; Ueda, S.; Sugiyama, D.; Hori, S.; Sakaguchi, S.; et al. Overcoming regulatory T-cell suppression by a lyophilized preparation of Streptococcus pyogenes. Eur. J. Immunol. 2013, 43, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S. Induction of Antigen-Specific Cytotoxic T Lymphocytes by Chemoradiotherapy in Patients Receiving Wilms’ Tumor 1-Targetted Dendritic Cell Vaccinations for Pancreatic Cancer. Omi. J. Radiol. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Shitara, K.; Nishikawa, H. Regulatory T cells: A potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 2016, 1417, 104–115. [Google Scholar] [CrossRef] [PubMed]
| Patient No. | Age (Years) | Sex | Disease | Pre-DC Vaccination Status | ||
|---|---|---|---|---|---|---|
| Therapy | Stage (Operation) | Chemotherapy | ||||
| 3 | 60 | M | Gastric cancer | post operation; post chemotherapy | II | S1 |
| 4 | 57 | M | Salivary gland cancer | post operation; post radiation; post chemotherapy | IV | CDDP, Trastuzumab |
| 5 | 58 | M | Gastric cancer | post operation; during chemotherapy | IV | XELOX |
| 6 | 68 | M | Gastric cancer | post chemotherapy; post operation; DC vaccination | IA | non |
| Patient | HLA Typing | DC Vaccination | Immunological Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | A | DR | DQ | Status (Stage) | Total No. of DCs (×107) | Combination chemotherapy | ELISPOT * | Tetramer | |||
| 3 | 2402 | - | 0405 | 0901 | 0201 | - | CR | 12 | non | negative | positive |
| 4 | 0301 | 2402 | 0403 | 1301 | 0201 | - | IV | 8 | non | negative | negative |
| 5 | 2402 | 2601 | 0901 | 1101 | 0201 | - | IV | 9 | XELOX | negative | positive |
| 6 | 1101 | 2402 | 0405 | - | 0501 | - | IV | 7 | non | positive | positive |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koya, T.; Date, I.; Kawaguchi, H.; Watanabe, A.; Sakamoto, T.; Togi, M.; Kato, T., Jr.; Yoshida, K.; Kojima, S.; Yanagisawa, R.; et al. Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination. Pharmaceutics 2020, 12, 305. https://doi.org/10.3390/pharmaceutics12040305
Koya T, Date I, Kawaguchi H, Watanabe A, Sakamoto T, Togi M, Kato T Jr., Yoshida K, Kojima S, Yanagisawa R, et al. Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination. Pharmaceutics. 2020; 12(4):305. https://doi.org/10.3390/pharmaceutics12040305
Chicago/Turabian StyleKoya, Terutsugu, Ippei Date, Haruhiko Kawaguchi, Asuka Watanabe, Takuya Sakamoto, Misa Togi, Tomohisa Kato, Jr., Kenichi Yoshida, Shunsuke Kojima, Ryu Yanagisawa, and et al. 2020. "Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination" Pharmaceutics 12, no. 4: 305. https://doi.org/10.3390/pharmaceutics12040305
APA StyleKoya, T., Date, I., Kawaguchi, H., Watanabe, A., Sakamoto, T., Togi, M., Kato, T., Jr., Yoshida, K., Kojima, S., Yanagisawa, R., Koido, S., Sugiyama, H., & Shimodaira, S. (2020). Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination. Pharmaceutics, 12(4), 305. https://doi.org/10.3390/pharmaceutics12040305