Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TM HPLC Determination
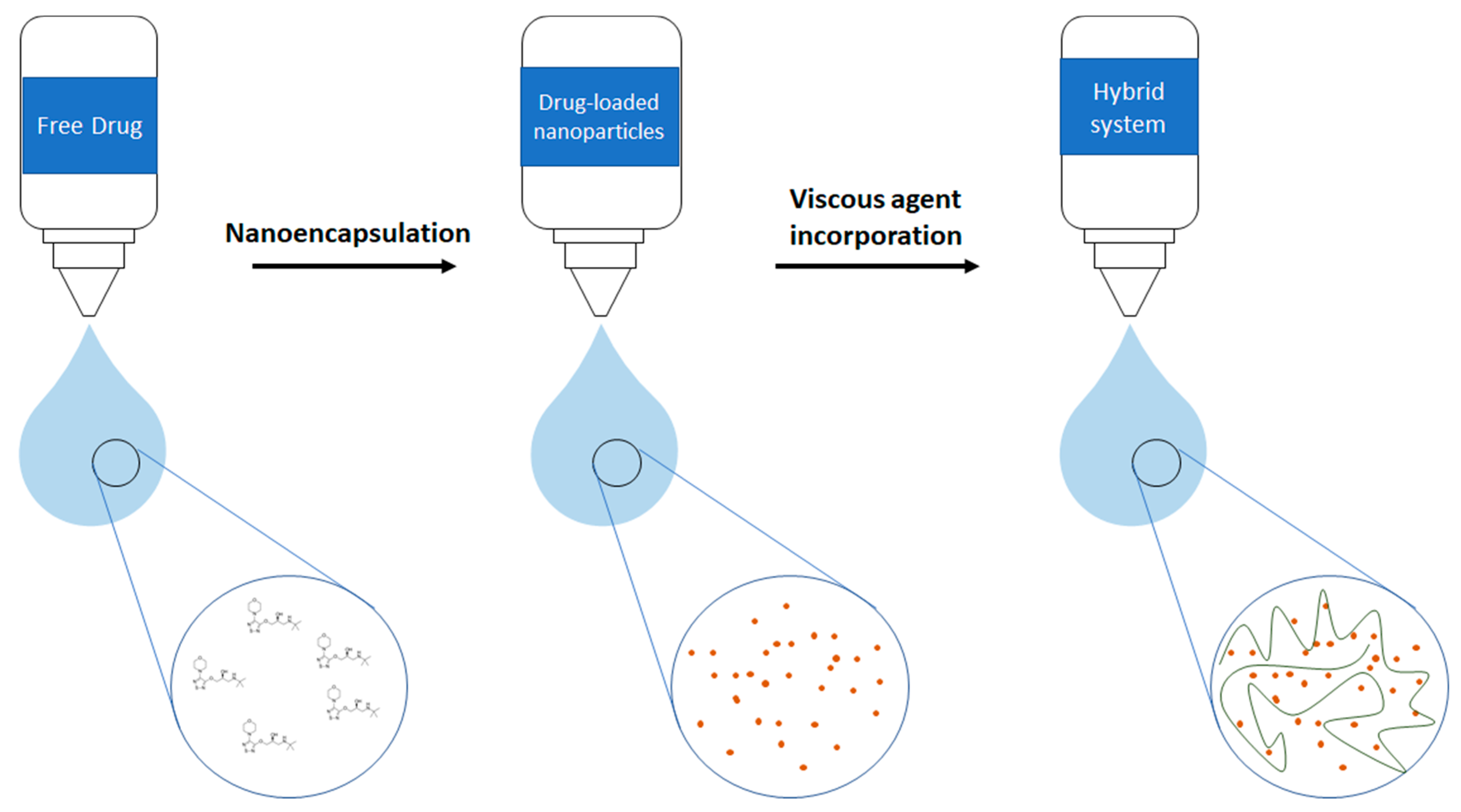
2.3. Elaboration of Gelatin Nanoparticles (GNPs) and Eye Drops Formulations
2.4. Measurement of Mean Particle Size, Particle Size Distribution, Zeta Potential and Polydispersity Index of the TM-GNP
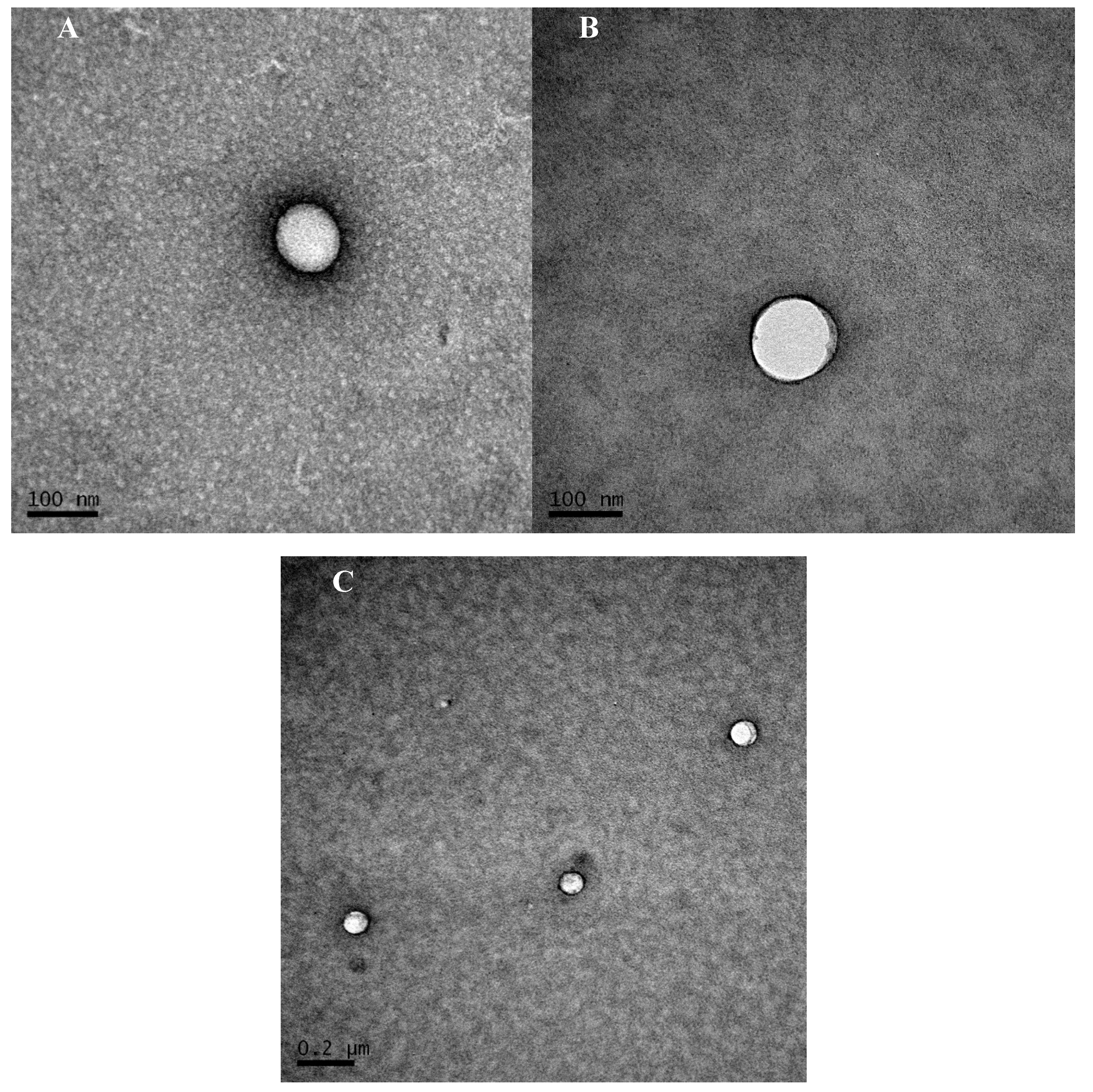
2.5. Transmission Electron Microscopy
2.6. Determination of TM Encapsulation Efficiency
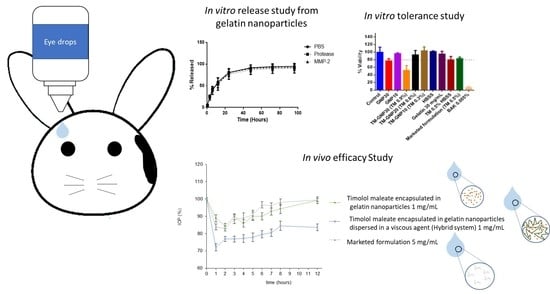
2.7. In Vitro Release Study
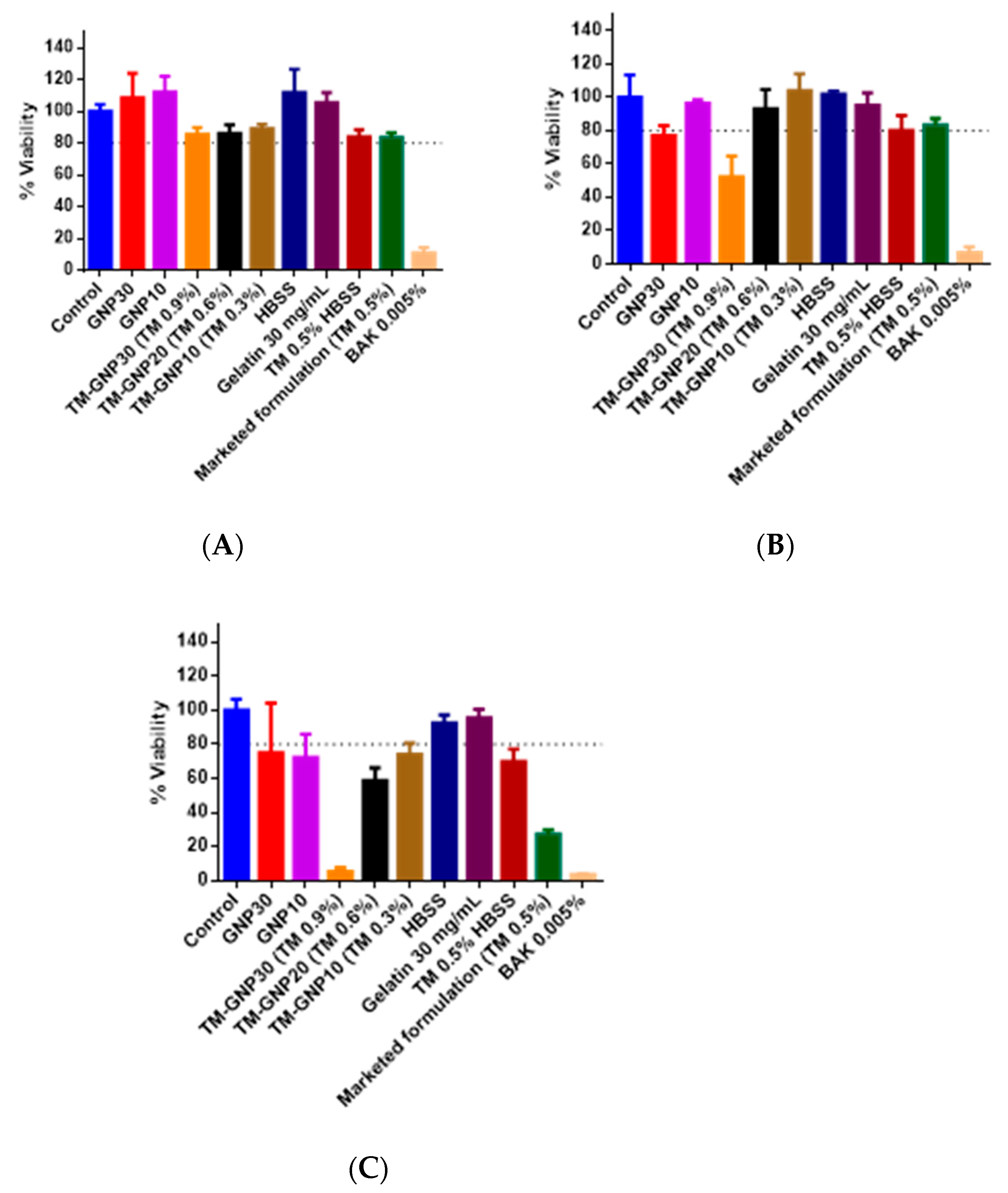
2.8. In Vitro Tolerance Studies
2.9. Animals
2.10. In Vivo Acute Tolerance Tests
2.11. In Vivo Efficacy Study
2.12. Statistical Analysis
3. Results
3.1. Timolol Maleate Loaded Gelatin Nanoparticles Characterization
3.2. In Vitro Release Studies
3.3. In Vitro and in Vivo Tolerance Studies
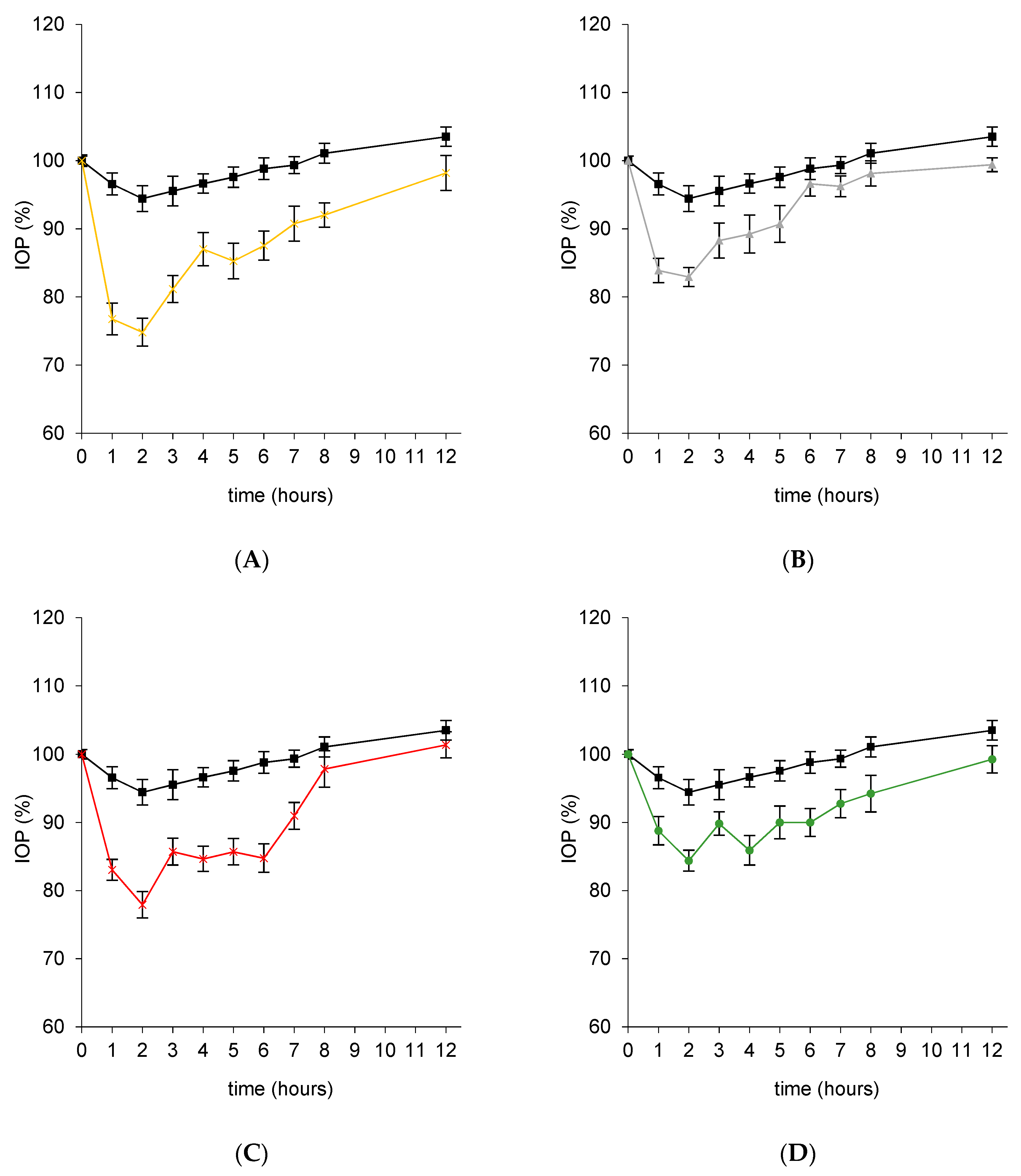
3.4. In Vivo Efficacy Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das Gupta, B.K. Glaucoma. J. Indian Med. Assoc. 1955, 25, 668–674. [Google Scholar]
- Davis, B.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Doozandeh, A.; Yazdani, S. Neuroprotection in Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 209–220. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Pharmacotherapy of Glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Chiou, G.C.Y.; Garg, L.C. Ocular Hypotensive Effects of Timolol in Cat Eyes. Arch. Ophthalmol. 1980, 98, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Martinez-De-La-Casa, J.M.; Rayward, O.; Saenz-Frances, F.; Santos-Bueso, E.; Mendez-Hernandez, C.; Herrero-Vanrell, R.; Feijoo, J.G.; García-Sánchez, J. Effects of corneal thickness on the intraocular penetration of travoprost 0.004%. Eye 2012, 26, 972–975. [Google Scholar] [CrossRef][Green Version]
- Ramsay, E.; Del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.-P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Goyal, G.; Garg, T.; Rath, G.; Goyal, A.K. Current nanotechnological strategies for treating glaucoma. Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 365–405. [Google Scholar] [CrossRef]
- Cohen, L.P.; Pasquale, L.R. Clinical Characteristics and Current Treatment of Glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017236. [Google Scholar] [CrossRef]
- Lowenthal, D.T.; Pitone, J.M.; Affrime, M.B.; Shirk, J.; Busby, P.; Kim, K.E.; Nancarrow, J.; Swartz, C.D.; Onesti, G. Timolol kinetics in chronic renal insufficiency. Clin. Pharmacol. Ther. 1978, 23, 606–615. [Google Scholar] [CrossRef]
- Waterman, H.; Evans, J.; Gray, T.; Henson, D.; Harper, R.A. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst. Rev. 2013, 4, CD006132. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.J.; Baumann, J.D.; Hetherington, J. Side effects of timolol. Surv. Ophthalmol. 1983, 28, 243–249. [Google Scholar] [CrossRef]
- Mäenpää, J.; Pelkonen, O. Cardiac safety of ophthalmic timolol. Expert Opin. Drug Saf. 2016, 15, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Vicario-De-La-Torre, M.; Guerrero, V.A.; Sánchez-Nieves, J.; Guzman-Navarro, M.; De La Mata, F.J.; Gómez, R.; Heras, B.D.L.; Argüeso, P.; Ponchel, G.; et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol. Pharm. 2016, 13, 2966–2976. [Google Scholar] [CrossRef]
- Gómez-Ballesteros, M.; Guerrero, V.A.; Parra, F.J.; Marinich, J.; Heras, B.D.L.; Martínez, I.T.M.; Vázquez-Lasa, B.; Del Barrio, J.S.R.; Herrero-Vanrell, R. Amphiphilic Acrylic Nanoparticles Containing the Poloxamer Star Bayfit® 10WF15 as Ophthalmic Drug Carriers. Polymer 2019, 11, 1213. [Google Scholar] [CrossRef]
- Katiyar, S.; Pandit, J.; Mondal, R.S.; Mishra, A.K.; Chuttani, K.; Aqil, M.; Ali, A.; Sultana, Y. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr. Polym. 2014, 102, 117–124. [Google Scholar] [CrossRef]
- Gautam, N.; Kesavan, K. Development of microemulsions for ocular delivery. Ther. Deliv. 2017, 8, 313–330. [Google Scholar] [CrossRef]
- Hui, A. Contact lenses for ophthalmic drug delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Guerrero, V.A.; Arranz-Romera, A.; Pérez, S.E.; Martínez, I.T.M.; Herrero-Vanrell, R. Microspheres as intraocular therapeutic tools in chronic diseases of the optic nerve and retina. Adv. Drug Deliv. Rev. 2018, 126, 127–144. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Figueiras, A.; Veiga, F. Improvements in Topical Ocular Drug Delivery Systems: Hydrogels and Contact Lenses. J. Pharm. Pharm. Sci. 2015, 18, 683–695. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; De La Torre, M.V.; Guerrero, V.A.; Barbosa-Alfaro, D.; Martínez, I.T.M.; Bravo-Osuna, I. Nano and microtechnologies for ophthalmic administration, an overview. J. Drug Deliv. Sci. Technol. 2013, 23, 75–102. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Gastrointest. Pharmacol. Ther. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, V.A.; Vicario-De-La-Torre, M.; Herrero-Vanrell, R.; Martínez, I.T.M.; Benítez-Del-Castillo, J.M.; Feijoo, J.G. Comparison of the In Vitro Tolerance and In Vivo Efficacy of Traditional Timolol Maleate Eye Drops versus New Formulations with Bioadhesive Polymers. Investig. Opthalmol. Vis. Sci. 2011, 52, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Greaves, J.L.; Wilson, C.G. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv. Drug Deliv. Rev. 1993, 11, 349–383. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2010, 11, 748–764. [Google Scholar] [CrossRef]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.W.; Tiffany, J.M.; Wilson, C.G.; Bron, A.J. Ocular Surface Residence Times of Artificial Tear Solutions. Cornea 1992, 11, 288–293. [Google Scholar] [CrossRef]
- Tighsazzadeh, M.; Mitchell, J.C.; Boateng, J. Development and evaluation of performance characteristics of timolol-loaded composite ocular films as potential delivery platforms for treatment of glaucoma. Int. J. Pharm. 2019, 566, 111–125. [Google Scholar] [CrossRef]
- Kouchak, M.; Mahmoodzadeh, M.; Farrahi, F. Designing of a pH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation. AAPS PharmSciTech 2019, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Thomas, C.R.; Byrne, M.E. Bringing comfort to the masses: A novel evaluation of comfort agent solution properties. Contact Lens Anterior Eye 2014, 37, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vadoothker, S.; Munir, W.M.; Saeedi, O. Ocular Surface Disease and Glaucoma Medications. Eye Contact Lens Sci. Clin. Pract. 2019, 45, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Gu, Y.; Cheng, Y.; Cao, F. Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2018, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, V.; Kandasamy, R.; Alagarsamy, S.; Palanisamy, R.; Natesan, S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int. J. Boil. Macromol. 2018, 110, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.F.; Almeida, A.; Gonçalves, L.M. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Elgawad, A.-E.H.; Soliman, O.A.-E.; Jablonski, M.M. Stability and Ocular Pharmacokinetics of Celecoxib-Loaded Nanoparticles Topical Ophthalmic Formulations. J. Pharm. Sci. 2016, 105, 3691–3701. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef]
- Warsi, M.H.; Anwar, M.; Garg, V.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: Pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf. B Biointerfaces 2014, 122, 423–431. [Google Scholar] [CrossRef]
- De La Fuente, M.; Raviña, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Alonso, M.J. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 100–117. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, M.; Seijo, B.; Alonso, M.J. Novel Hyaluronic Acid-Chitosan Nanoparticles for Ocular Gene Therapy. Investig. Opthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S.P. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: development, characterization, and evaluation. J. Drug Target. 2009, 18, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G.; Echave, M.C. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, M.; Shoichet, M.S. Click Chemistry Functionalized Polymeric Nanoparticles Target Corneal Epithelial Cells through RGD-Cell Surface Receptors. Bioconjugate Chem. 2009, 20, 87–94. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Leucuta, S.E. The kinetics of in vitro release and the pharmacokinetics of miotic response in rabbits of gelatin and albumin microspheres with pilocarpine. Int. J. Pharm. 1989, 54, 71–78. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur. J. Pharm. Biopharm. 2004, 57, 251–261. [Google Scholar] [CrossRef]
- Shokry, M.; Hathout, R.M.; Mansour, S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. Int. J. Pharm. 2018, 545, 229–239. [Google Scholar] [CrossRef]
- Mahor, A.; Prajapati, S.K.; Verma, A.; Gupta, R.; Iyer, A.K.; Kesharwani, P. Moxifloxacin loaded gelatin nanoparticles for ocular delivery: Formulation and in vitro, in vivo evaluation. J. Colloid Interface Sci. 2016, 483, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Contreras-Ruiz, L.; Párraga, J.E.; López-García, A.; Bello, R.; Diebold, Y.; Seijo, B.; Sanchez, A. Expression of MUC5AC in Ocular Surface Epithelial Cells Using Cationized Gelatin Nanoparticles. Mol. Pharm. 2011, 8, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, T.; Shoji, J.; Kanno, H.; Sawa, M. Gelatinase Expression in Ocular Surface Disorders. Jpn. J. Ophthalmol. 2004, 48, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Rao, A.; Padhy, D.; Sarangi, S.; Das, G.; Reddy, M.M.; Modak, R. Functional Activity of Matrix Metalloproteinases 2 and 9 in Tears of Patients with Glaucoma. Investig. Opthalmol. Vis. Sci. 2017, 58, 106. [Google Scholar] [CrossRef]
- Määttä, M.; Tervahartiala, T.; Harju, M.; Airaksinen, J.; Autio-Harmainen, H.; Sorsa, T. Matrix metalloproteinases and their tissue inhibitors in aqueous humor of patients with primary open-angle glaucoma, exfoliation syndrome, and exfoliation glaucoma. J. Glaucoma 2005, 14, 64–69. [Google Scholar]
- Boiero, C.; Allemandi, D.; Longhi, M.R.; Llabot, J.M. RP-HPLC method development for the simultaneous determination of timolol maleate and human serum albumin in albumin nanoparticles. J. Pharm. Biomed. Anal. 2015, 111, 186–189. [Google Scholar] [CrossRef]
- Kommareddy, S.; Amiji, M.M. Preparation and loading of gelatin nanoparticles. CSH Protoc. 2008, 2008, 4885. [Google Scholar] [CrossRef]
- Qin, Z.; Joo, J.; Gu, L.; Sailor, M.J. Porous Films: Size Control of Porous Silicon Nanoparticles by Electrochemical Perforation Etching (Part. Part. Syst. Charact. 2/2014). Part. Part. Syst. Charact. 2014, 31, 171. [Google Scholar] [CrossRef]
- Garcia-Valldecabres, M.; López-Alemany, A.; Refojo, M.F. pH stability of ophthalmic solutions. Optom. J. Am. Optom. Assoc. 2004, 75, 161–168. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, 095505. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbe, A.; Liang, H.; Pauly, A.; Baudouin, C. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Enríquez-De-Salamanca, A. Chitosan Nanoparticles as a Potential Drug Delivery System for the Ocular Surface: Toxicity, Uptake Mechanism and In Vivo Tolerance. Investig. Opthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Acar, D.; Martínez, I.T.M.; Gómez-Ballesteros, M.; Guzman-Navarro, M.; Benítez-Del-Castillo, J.M.; Herrero-Vanrell, R. Novel liposome-based and in situ gelling artificial tear formulation for dry eye disease treatment. Contact Lens Anterior Eye 2017, 41, 93–96. [Google Scholar] [CrossRef]
- Vicario-De-La-Torre, M.; Benítez-Del-Castillo, J.M.; Vico, E.; Guzman-Navarro, M.; Heras, B.D.L.; Herrero-Vanrell, R.; Martínez, I.T.M. Design and Characterization of an Ocular Topical Liposomal Preparation to Replenish the Lipids of the Tear Film. Investig. Opthalmol. Vis. Sci. 2014, 55, 7839–7847. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.-P. In Vitro Dissolution Profile Comparison—Statistics and Analysis of the Similarity Factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-based natural polymers as smart carriers for the controlled delivery of Timolol Maleate through the cornea for glaucoma. Int. J. Boil. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef]
- Hathout, R.M.; Omran, M.K. Gelatin-based particulate systems in ocular drug delivery. Pharm. Dev. Technol. 2015, 21, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Segtnan, V.H.; Isaksson, T. Temperature, sample and time dependent structural characteristics of gelatine gels studied by near infrared spectroscopy. Food Hydrocoll. 2004, 18, 1–11. [Google Scholar] [CrossRef]
- Kempka, A.P.; Souza, S.G.; De Souza, A.A.U.; Prestes, R.C.; Ogliari, D. Influence of bloom number and plastifiers on gelatin matrices produced for enzyme immobilization. Braz. J. Chem. Eng. 2014, 31, 95–108. [Google Scholar] [CrossRef][Green Version]
- Coester, K.L.C.J. Gelatin nanoparticles by two step desolvation a new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, M.G.; Ueda, H.; Yang, J.; Davda, J.; Labhasetwar, V.; Lee, V. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm. Res. 2004, 21, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Alonso, M.J.; Robinson, J.R.; Vila-Jato, J.L. Improved Ocular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef]
- Azarmi, S.; Huang, Y.; Chen, H.; A McQuarrie, S.; Abrams, D.; Roa, W.H.; Finlay, W.H.; Miller, G.G.; Löbenberg, R. Optimization of a two-step desolvation method for preparing gelatin nanoparticles and cell uptake studies in 143B osteosarcoma cancer cells. J. Pharm. Pharm. Sci. 2006, 9, 124–132. [Google Scholar]
- Sanzgiri, Y.D.; Maschi, S.; Crescenzi, V.; Callegaro, L.; Topp, E.M.; Stella, V.J. Gellan-based systems for ophthalmic sustained delivery of methylprednisolone. J. Control. Release 1993, 26, 195–201. [Google Scholar] [CrossRef]
- Cohen, S.; Lobel, E.; Trevgoda, A.; Peled, Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J. Control. Release 1997, 44, 201–208. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. The role of surface charge in the desolvation process of gelatin: implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef]
- De Saint Jean, M.; Debbasch, C.; Brignole, F.; Rat, P.; Warnet, J.M.; Baudouin, C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr. Eye Res. 2000, 20, 85–94. [Google Scholar] [CrossRef]
- Uusitalo, H.; Niño, J.; Tahvanainen, K.; Turjanmaa, V.; Ropo, A.; Tuominen, J.; Kähönen, M. Efficacy and systemic side-effects of topical 0.5% timolol aqueous solution and 0.1% timolol hydrogel. Acta Ophthalmol. Scand. 2005, 83, 723–728. [Google Scholar] [CrossRef]
- Korte, J.-M.; Kaila, T.; Saari, M.K. Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eyedrops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Araie, M.; Tomita, K.; Nagahara, M.; Tomidokoro, A. Effect of topical beta-blockers on tissue blood flow in the human optic nerve head. Curr. Eye Res. 1997, 16, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Amit, C.; Muralikumar, S.; Janaki, S.; Lakshmipathy, M.; Therese, K.L.; Vetrivel, U.; Padmanabhan, P.; Narayanan, J.; Therese, K.L. Designing and enhancing the antifungal activity of corneal specific cell penetrating peptide using gelatin hydrogel delivery system. Int. J. Nanomed. 2019, 14, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.R.; Meyer, L.M.; Schönfeld, C.-L. Effect of Viscous Agents on Corneal Density in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2015, 31, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Goto, T.; Ohashi, Y. Comparison of In Vivo Efficacy of Different Ocular Lubricants in Dry Eye Animal Models. Investig. Opthalmol. Vis. Sci. 2014, 55, 3454–3460. [Google Scholar] [CrossRef] [PubMed]


| Grade | Discomfort | Cornea | Conjunctiva | Discharge | Lids |
|---|---|---|---|---|---|
| 0 | No reaction | No alterations | No alterations | No discharge | No swelling |
| 1 | Blinking | Mild Opacity | Mild hyperemia/mild oedema | Mild discharge without moistened hair | Mild swelling |
| 2 | Enhanced blinking/intense tearing/vocalizations | Intense opacity | Intense hyperemia/intense oedema/haemorrhage | Intense discharge with moistened hair | Obvious swelling |
| Release Media | Initial Burst (0.5 h) | 24 h |
|---|---|---|
| PBS | 5.90 ± 1.04 | 80.46 ± 1.44% |
| PBS and Protease (60 ng/mL) | 4.88 ± 1.29% | 80.46 ± 9.17% |
| PBS and MMP-2 (60 ng/mL) | 4.50 ± 0.83% | 77.00 ± 11.87% |
| Formulation | Maximal % IOP Reduction | Maximal mmHg Reduction | tmax | Hypotensive Effect Duration (hours) | AUC (0–12) |
|---|---|---|---|---|---|
| Marketed formulation (TM 0.5%) | 21.11 ± 1.72 | 3.23 ± 0.26 | 1.5 | 8 | 83.77 ± 11.71 |
| TM-GNP16 (TM 0.5%) | 30.09 ± 1.85 | 4.33 ± 0.30 | 2.0 | 8 | 143.63 ± 14.16 |
| TM-GNP8 (TM 0.25%) | 26.39 ± 1.38 | 3.75 ± 0.23 | 2.0 | 8 | 124.37 ± 11.89 |
| TM-GNP3 (TM 0.1%) | 20.46 ± 1.41 | 2.98 ± 0.20 | 2.0 | 8 | 102.44 ± 13.47 |
| AUC (0–12) | Maximal mmHg Reduction % | Maximal mmHg Reduction | |
|---|---|---|---|
| TM-GNP3 (TM 0.1%) | p = 0.1581 | p = 0.9376 | p = 0.7339 |
| TM-GNP8 (TM 0.25%) | p = 0.0075 * | p = 0.0119 * | p = 0.0279 * |
| TM-GNP16 (TM 0.5%) | p = 0.0009 * | p = 0.0011 * | p = 0.0011 * |
| AUC (0–12) | Maximal mmHg Reduction % | Maximal mmHg Reduction | |
|---|---|---|---|
| TM-GNP3 (TM 0.1%) | p = 0.1581 | p = 0.9376 | p = 0.7339 |
| TM-GNP3 (TM 0.1%) + HPMC 0.3% | p < 0.0001 * | p = 0.0007 * | p = 0.0010 * |
| Formulation | Maximal % IOP Reduction | Maximal mmHg Reduction | tmax | Hypotensive Effect Duration (hours) | AUC (0–12) |
|---|---|---|---|---|---|
| Marketed formulation (TM 0.5%) | 21.11 ± 1.72 | 3.23 ± 0.26 | 1.5 | 8 | 83.77 ± 11.71 |
| TM-GNP3 (TM 0.1%) | 20.46 ± 1.41 | 2.98 ± 0.20 | 2.0 | 8 | 102.44 ± 13.47 |
| TM-GNP3 (TM 0.1%) + HPMC 0.3% | 29.64 ± 1.60 | 4.65 ± 0.24 | 1.0 | 12 | 213.85 ± 13.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. https://doi.org/10.3390/pharmaceutics12040306
Esteban-Pérez S, Andrés-Guerrero V, López-Cano JJ, Molina-Martínez I, Herrero-Vanrell R, Bravo-Osuna I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics. 2020; 12(4):306. https://doi.org/10.3390/pharmaceutics12040306
Chicago/Turabian StyleEsteban-Pérez, Sergio, Vanessa Andrés-Guerrero, José Javier López-Cano, Irene Molina-Martínez, Rocio Herrero-Vanrell, and Irene Bravo-Osuna. 2020. "Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents" Pharmaceutics 12, no. 4: 306. https://doi.org/10.3390/pharmaceutics12040306
APA StyleEsteban-Pérez, S., Andrés-Guerrero, V., López-Cano, J. J., Molina-Martínez, I., Herrero-Vanrell, R., & Bravo-Osuna, I. (2020). Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics, 12(4), 306. https://doi.org/10.3390/pharmaceutics12040306