Development of a Deer Tick Virus Infection Model in C3H/HeJ Mice to Mimic Human Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus
2.3. Animals
2.4. RNA Extractions and qRT PCR
2.5. Histology
2.6. RNA In Situ Hybridization
3. Results
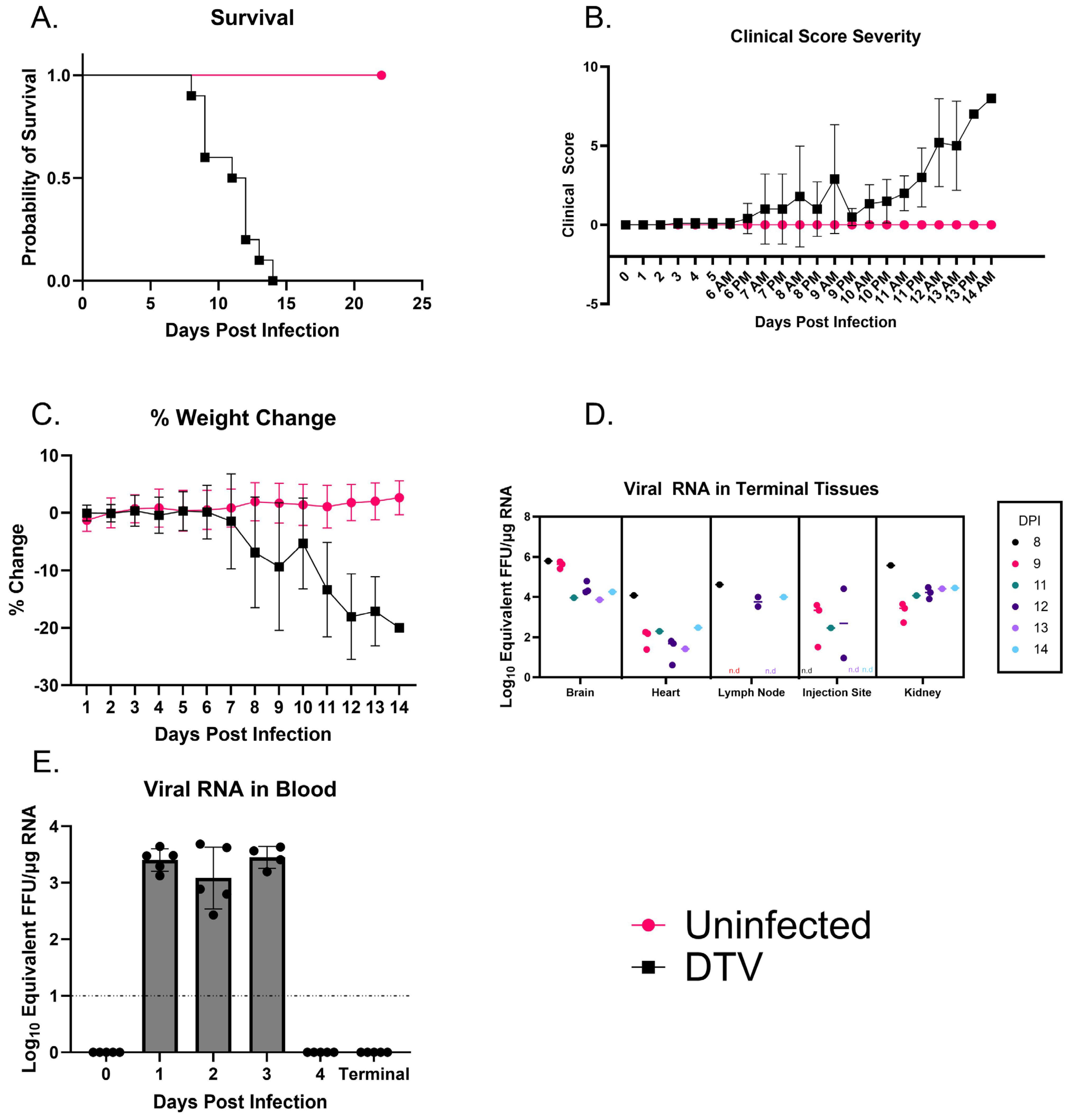
3.1. Clinical Observations Following Inoculation of DTV-Spooner
3.2. Moribund C3H/HeJ Mice Show Consistent Viral Titers Across Major Organs
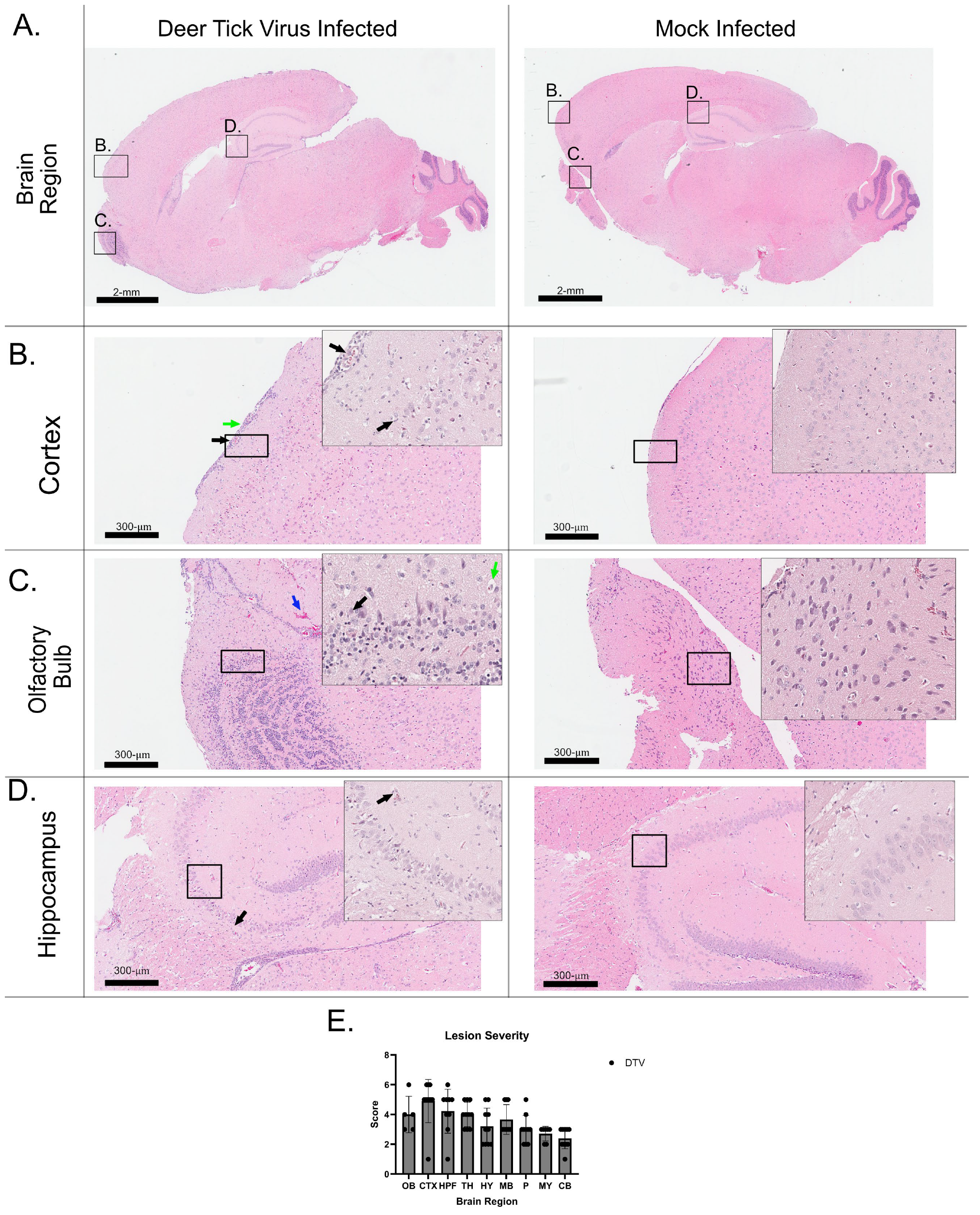
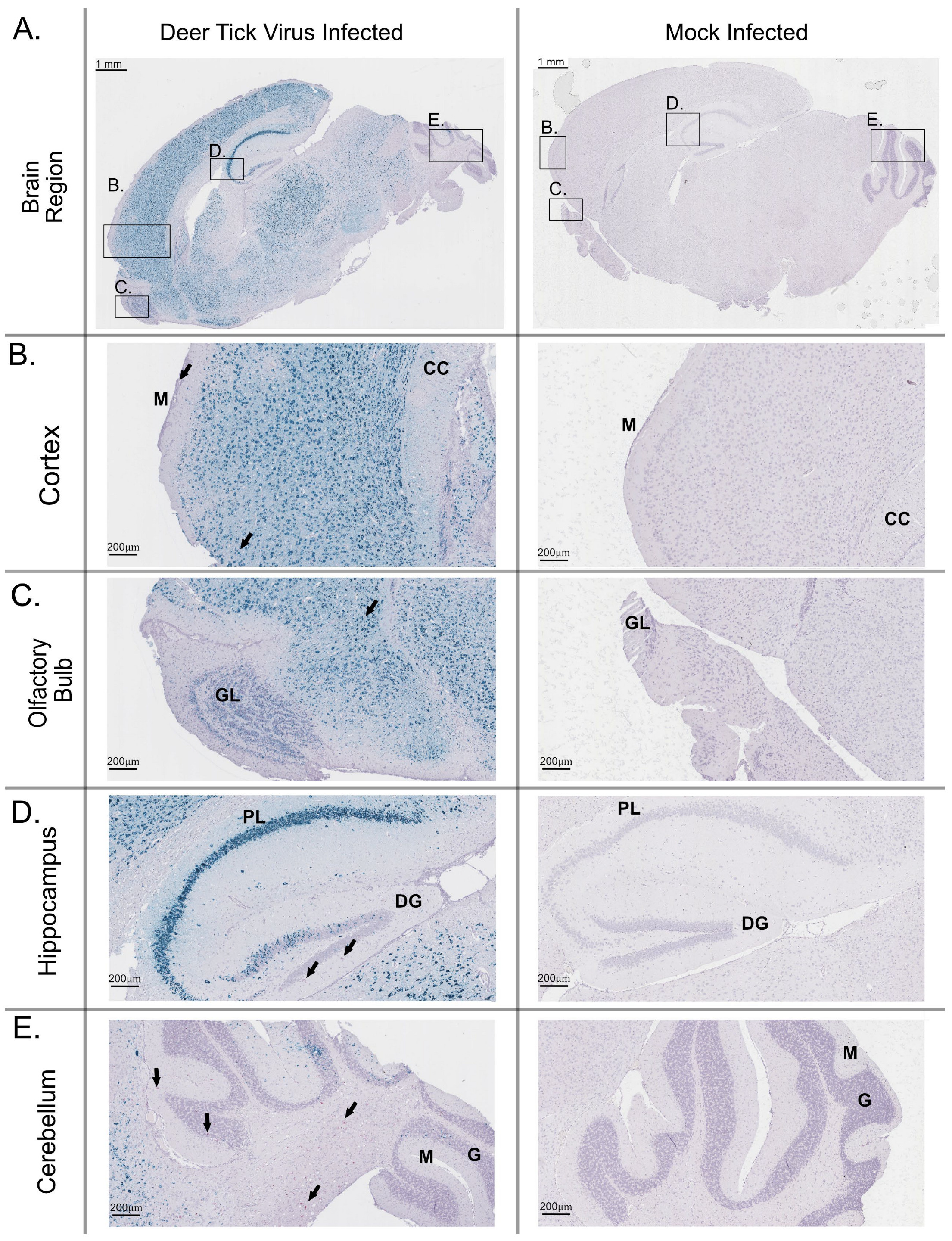
3.3. Histology and Neuropathology of DTV in the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| POWV | Powassan Virus |
| DTV | Deer Tick Virus |
| FDA | Food and Drug Administration |
| ABSL3 | Animal Biosafety Level 3 |
| DMEM | Dulbecco’s Modified Essential Media |
| FFU | Focus Forming Units |
| qRTPCR | Quantitative Real-Time Polymerase Chain Reaction |
| FFPE | Formalin Fixed Paraffin Embedded |
| H&E | Hematoxylin and Eosin |
| RNAish | RNA-In Situ Hybridization |
| dpi | Days Post Infection |
References
- CDC. Tickborne Disease Surveillance Data Summary | CDC. Available online: https://www.cdc.gov/ticks/data-research/facts-stats/tickborne-disease-surveillance-data-summary.html?CDC_AAref_Val=https://www.cdc.gov/ticks/data-summary/index.html (accessed on 26 October 2022).
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks; Oxford University Press: Oxford, UK, 2013; Volume 2, ISBN 978-0-19-937928-6. [Google Scholar]
- Kazimírová, M.; Thangamani, S.; Bartíková, P.; Hermance, M.; Holíková, V.; Štibrániová, I.; Nuttall, P.A. Tick-Borne Viruses and Biological Processes at the Tick-Host-Virus Interface. Front. Cell. Infect. Microbiol. 2017, 7, 339. [Google Scholar] [CrossRef]
- Hermance, M.E.; Thangamani, S. Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector-Borne Zoonotic Dis. 2017, 17, 453–462. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.M.; Donohue, W.L. Powassan Virus: Isolation of Virus from a Fatal Case of Encephalitis. Can. Med. Assoc. J. 1959, 80, 708–711. [Google Scholar]
- Historic Data (2004–2022) | Powassan | CDC. Available online: https://www.cdc.gov/powassan/data-maps/index.html (accessed on 2 April 2024).
- Telford, S.R.; Armstrong, P.M.; Katavolos, P.; Foppa, I.; Garcia, A.S.; Wilson, M.L.; Spielman, A. A New Tick-Borne Encephalitis-like Virus Infecting New England Deer Ticks, Ixodes dammini. Emerg. Infect. Dis. 1997, 3, 165–170. [Google Scholar] [CrossRef]
- Mclean, D.M.; Best, J.M.; Mahalingam, S.; Chernesky, M.A.; Wilson, W.E. POWASSAN VIRUS: SUMMER INFECTION CYCLE, 1964. Can. Med. Assoc. J. 1964, 91, 1360–1362. [Google Scholar]
- Mclean, D.M.; Larke, R.P. Powassan and Silverwater Viruses: Ecology of Two Ontario Arboviruses. Can. Med. Assoc. J. 1963, 88, 182–185. [Google Scholar]
- Kuno, G.; Artsob, H.; Karabatsos, N.; Tsuchiya, K.R.; Chang, G.J. Genomic Sequencing of Deer Tick Virus and Phylogeny of Powassan-Related Viruses of North America. Am. J. Trop. Med. Hyg. 2001, 65, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Beasley, D.W.C.; Suderman, M.T.; Holbrook, M.R.; Barrett, A.D.T. Nucleotide Sequencing and Serological Evidence That the Recently Recognized Deer Tick Virus is a Genotype of Powassan Virus. Virus Res. 2001, 79, 81–89. [Google Scholar] [CrossRef]
- Reynolds, E.S.; Hart, C.E.; Nelson, J.T.; Marzullo, B.J.; Esterly, A.T.; Paine, D.N.; Crooker, J.; Massa, P.T.; Thangamani, S. Comparative Pathogenesis of Two Lineages of Powassan Virus Reveals Distinct Clinical Outcome, Neuropathology, and Inflammation. BioRxiv 2024. [Google Scholar] [CrossRef]
- Danielová, V.; Holubová, J.; Pejcoch, M.; Daniel, M. Potential Significance of Transovarial Transmission in the Circulation of Tick-Borne Encephalitis Virus. Folia Parasitol. 2002, 49, 323–325. [Google Scholar] [CrossRef]
- Hermance, M.E.; Thangamani, S. Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease. J. Virol. 2015, 89, 7852–7860. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Schauber, E.M.; Gertz, S.J.; Maple, W.T.; Ostfeld, R.S. Coinfection of Blacklegged Ticks (Acari: Ixodidae) in Dutchess County, New York, with the Agents of Lyme Disease and Human Granulocytic Ehrlichiosis. J. Med. Entomol. 1998, 35, 901–903. [Google Scholar] [CrossRef]
- Piesman, J.; Mather, T.N.; Telford, S.R.; Spielman, A. Concurrent Borrelia burgdorferi and Babesia microti Infection in Nymphal Ixodes dammini. J. Clin. Microbiol. 1986, 24, 446–447. [Google Scholar] [CrossRef]
- Akeson, E.C.; Donahue, L.R.; Beamer, W.G.; Shultz, K.L.; Ackert-Bicknell, C.; Rosen, C.J.; Corrigan, J.; Davisson, M.T. Chromosomal Inversion Discovered in C3H/HeJ Mice. Genomics 2006, 87, 311–313. [Google Scholar] [CrossRef][Green Version]
- Cheers, C.; McKenzie, I.F. Resistance and Susceptibility of Mice to Bacterial Infection: Genetics of Listeriosis. Infect. Immun. 1978, 19, 755–762. [Google Scholar] [CrossRef]
- Akoolo, L.; Djokic, V.; Rocha, S.C.; Parveen, N. Pathogenesis of Borrelia burgdorferi and Babesia microti in TLR4-Competent and TLR4-dysfunctional C3H Mice. Cell. Microbiol. 2021, 23, e13350. [Google Scholar] [CrossRef]
- Porcelli, S.; Heckmann, A.; Lagrée, A.-C.; Galon, C.; Moutailler, S.; Deshuillers, P.L. Exploring the Susceptibility of C3H Mice to Tick-Borne Encephalitis Virus Infection: Implications for Co-Infection Models and Understanding of the Disease. Viruses 2023, 15, 2270. [Google Scholar] [CrossRef]
- Porcelli, S.; Heckmann, A.; Deshuillers, P.L.; Wu-Chuang, A.; Galon, C.; Mateos-Hernandez, L.; Rakotobe, S.; Canini, L.; Rego, R.O.M.; Simo, L.; et al. Co-Infection Dynamics of B. afzelii and TBEV in C3H Mice: Insights and Implications for Future Research. Infect. Immun. 2024, 92, e00249-24. [Google Scholar] [CrossRef]
- Hart, C.E.; Middleton, F.A.; Thangamani, S. Infection with Borrelia burgdorferi Increases the Replication and Dissemination of Coinfecting Powassan Virus in Ixodes scapularis Ticks. Viruses 2022, 14, 1584. [Google Scholar] [CrossRef]
- Rossi, S.L.; Nasar, F.; Cardosa, J.; Mayer, S.V.; Tesh, R.B.; Hanley, K.A.; Weaver, S.C.; Vasilakis, N. Genetic and Phenotypic Characterization of Sylvatic Dengue Virus Type 4 Strains. Virology 2012, 423, 58–67. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Hermance, M.E.; Hart, C.E.; Esterly, A.T.; Reynolds, E.S.; Bhaskar, J.R.; Thangamani, S. Development of a Small Animal Model for Deer Tick Virus Pathogenesis Mimicking Human Clinical Outcome. PLoS Negl. Trop. Dis. 2020, 14, e0008359. [Google Scholar] [CrossRef]
- Hermance, M.E.; Thangamani, S. Utilization of RNA in Situ Hybridization to Understand the Cellular Localization of Powassan Virus RNA at the Tick-Virus-Host Interface. Front. Cell. Infect. Microbiol. 2020, 10, 172. [Google Scholar] [CrossRef]
- Baylis, M. Potential Impact of Climate Change on Emerging Vector-Borne and Other Infections in the UK. Environ. Health 2017, 16, 112. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, C.D. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- CDC. Geographic Distribution of Ticks That Bite Humans | CDC. Available online: https://www.cdc.gov/ticks/geographic_distribution.html (accessed on 14 October 2022).
- Mladinich, M.C.; Himmler, G.E.; Conde, J.N.; Gorbunova, E.E.; Schutt, W.R.; Sarkar, S.; Tsirka, S.-A.E.; Kim, H.K.; Mackow, E.R. Age-Dependent Powassan Virus Lethality is Linked to Glial Cell Activation and Divergent Neuroinflammatory Cytokine Responses in a Murine Model. J. Virol. 2024, 98, e00560-24. [Google Scholar] [CrossRef]
- Ebel, G.D. Update on Powassan Virus: Emergence of a North American Tick-Borne Flavivirus. Annu. Rev. Entomol. 2010, 55, 95–110. [Google Scholar] [CrossRef]
- Santos, R.I.; Hermance, M.E.; Reynolds, E.S.; Thangamani, S. Salivary Gland Extract from the Deer Tick, Ixodes scapularis, Facilitates Neuroinvasion by Powassan Virus in BALB/c Mice. Sci. Rep. 2021, 11, 20873. [Google Scholar] [CrossRef]
- Santos, R.I.; Hermance, M.E.; Gelman, B.B.; Thangamani, S. Spinal Cord Ventral Horns and Lymphoid Organ Involvement in Powassan Virus Infection in a Mouse Model. Viruses 2016, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, Y.; Schoenfeld, R.; Griffiths, M.; Eichwald, E.; Araneo, B.; Weis, J.J. Evidence for B-Lymphocyte Mitogen Activity in Borrelia burgdorferi-Infected Mice. Infect. Immun. 1992, 60, 3033–3041. [Google Scholar] [CrossRef]
- Helble, J.D.; Walsh, M.J.; McCarthy, J.E.; Smith, N.P.; Tirard, A.J.; Arnold, B.Y.; Villani, A.-C.; Hu, L.T. Single-Cell RNA Sequencing of Murine Ankle Joints over Time Reveals Distinct Transcriptional Changes Following Borrelia burgdorferi Infection. iScience 2023, 26, 108217. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Degree of Parameter | Possible Score |
|---|---|---|
| Appearance | Normal (smooth coat, eyes/nose clear), weight loss < 4.99% | 0 |
| Reduced grooming, weight loss between 5 and 9.99%, slightly ruffled | 1 | |
| Ruffled coat, ocular/nasal discharge, eye(s) partially closed, warm to touch, weight loss between 10 and 19.99% | 2 | |
| No grooming, eye(s) closed, hunched posture, pale, cold to touch, weight loss > 20% total body weight * | 3 | |
| Neurological Signs of Disease | Normal | 0 |
| Weak grip, reduced limb usage | 1 | |
| Paresis, ataxia, tremors, head tilt | 2 | |
| Total paralysis *, seizures *, unresponsive *, loss of righting reflex | 3 | |
| Provoked Behavior | Normal | 0 |
| Subdued but normal when stimulated | 1 | |
| Subdued even when stimulated, lethargic | 2 | |
| Unresponsive when stimulated *, moribund *, prostrate * | 3 | |
| Respiration | Normal | 0 |
| Rapid, shallow | 1 | |
| Diaphragmatic, labored | 2 | |
| Gasping * | 3 | |
| Cumulative Score | Score of 0–5 = No intervention | |
| Score of >6 = More frequent monitoring | ||
| Score of 8–10 = Contact PI and/or DLAR Veterinarian | ||
| Score of ≥11 = Euthanasia |
| Sample ID | OB | CTX | HPF | TH | HY | MB | P | MY | CB | Meningitis/Encephalitis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purkinje Cells | Granule Cells | Molecular Cells | ||||||||||
| 2001 | NP | + | - | NP | NP | + MG, PC, | + MG | NP | + NN | + NN | ++ | ME |
| MG, | NN | MG | ||||||||||
| PC, | ||||||||||||
| NN | ||||||||||||
| 2002 | + | ++ | ++ | + MG, | ++ | + MG, PC | + MG | + MG, PC | - | + NN | + MG | ME |
| MG, NN | MG, | MG, | PC, | MG, | ||||||||
| PC, | PC, | NN | PC, | |||||||||
| NN | NN | NN | ||||||||||
| 2003 | ++ | +++ MG, | ++ | ++ | ++ | + + | + | NP | + NN | + NN | + MG | ME |
| MG, NN | PC, | MG, | MG, | MG, | MG, | MG, | ||||||
| NN | PC, | PC, | PC, | PC, | PC, | |||||||
| NN | NN | NN | NN | NN | ||||||||
| 2004 | + | ++ | ++ | ++ | ++ | ++ | + | + MG, PC | - | + | + MG | ME |
| MG, PC | MG, PC, NN | MG, PC, NN | MG, PC, NN | MG, PC | MG, PC, NN | MG, PC | NN | |||||
| 2005 | NP | ++ | + MG, PC | + MG, PC | + MG, PC | + MG, PC | + | + MG, PC | - | + NN | + MG | ME |
| MG, | MG, PC | |||||||||||
| PC, | ||||||||||||
| NN | ||||||||||||
| 2006 | NP | +++ MG, | +++ MG, | ++ | ++ | + + | ++ | + MG, PC | + NN | + NN | + MG | ME |
| PC, | PC, | MG, | MG, PC | MG, | MG, | |||||||
| NN | NN | PC, | PC, | PC, | ||||||||
| NN | NN | NN | ||||||||||
| 2007 | - | ++ | ++ | + MG, PC | + MG, PC | + MG, PC | + | + MG | + NN | + NN | ++ MG | ME |
| MG, | MG, PC | MG, PC | ||||||||||
| PC, | ||||||||||||
| NN | ||||||||||||
| 2008 | - | +++ | - | ++ | + MG | + | + | + MG, PC | + NN | + NN | + MG | ME |
| MG, | MG, PC | MG, PC | MG, PC | |||||||||
| PC, | ||||||||||||
| NN | ||||||||||||
| 2009 | NP | +++ | ++ | ++ | + MG, PC | + MG | + | + MG, PC | - | - | + MG | ME |
| MG, | MG, | MG, | MG, PC | |||||||||
| PC, | PC, | PC, | ||||||||||
| NN | NN | NN | ||||||||||
| 2010 | NP | ++ | + MG, PC, | ++ | + MG | + | + MG | NP | - | + NN | + MG | ME |
| MG, | NN | MG, PC | MG, PC | |||||||||
| PC, | ||||||||||||
| NN | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paine, D.N.; Reynolds, E.S.; Hart, C.E.; Crooker, J.; Thangamani, S. Development of a Deer Tick Virus Infection Model in C3H/HeJ Mice to Mimic Human Clinical Outcomes. Viruses 2025, 17, 1092. https://doi.org/10.3390/v17081092
Paine DN, Reynolds ES, Hart CE, Crooker J, Thangamani S. Development of a Deer Tick Virus Infection Model in C3H/HeJ Mice to Mimic Human Clinical Outcomes. Viruses. 2025; 17(8):1092. https://doi.org/10.3390/v17081092
Chicago/Turabian StylePaine, Dakota N., Erin S. Reynolds, Charles E. Hart, Jessica Crooker, and Saravanan Thangamani. 2025. "Development of a Deer Tick Virus Infection Model in C3H/HeJ Mice to Mimic Human Clinical Outcomes" Viruses 17, no. 8: 1092. https://doi.org/10.3390/v17081092
APA StylePaine, D. N., Reynolds, E. S., Hart, C. E., Crooker, J., & Thangamani, S. (2025). Development of a Deer Tick Virus Infection Model in C3H/HeJ Mice to Mimic Human Clinical Outcomes. Viruses, 17(8), 1092. https://doi.org/10.3390/v17081092





