Abstract
The increasing consumer demand for minimally processed foods (MPFs) has highlighted the need for innovative preservation methods that ensure both safety and quality. Among promising biocontrol tools, bacteriophages—viruses that selectively destroy bacteria—have gained significant attention. This review explores the dual role of bacteriophages in the food industry. On one hand, they offer a natural, highly specific, and environmentally friendly means of controlling both pathogenic and spoilage bacteria in MPFs, contributing to improved food safety, extended shelf life, and reduced reliance on antibiotics and chemical preservatives. Their use spans primary production, bio-sanitization, and biopreservation. On the other hand, phages pose significant risks in fermentation-based industries such as dairy, where they can disrupt starter cultures and impair production. This review also examines the regulatory, technological, and safety challenges involved in phage application, including concerns about antibiotic resistance gene transfer, the presence of endotoxins, and scale-up limitations. Ultimately, this paper argues that with proper strain selection and regulation, bacteriophages can become valuable allies in sustainable food systems, despite their potential drawbacks. The application of strictly virulent bacteriophages as part of “green biotechnology” could enhance food quality and improve consumer health safety. By implementing the “farm to fork” strategy, bacteriophages may contribute to the production of health-promoting and sustainable food.
1. Introduction
Malnutrition arises from deficiencies or poor absorption of essential nutrients, and it encompasses both undernutrition and overnutrition, including obesity—a major global cause of disease and mortality [,]. Diet is a key modifiable risk factor for various conditions, influencing blood pressure, glucose, cholesterol, and body weight [,]. Alongside poor dietary habits, smoking and inactivity significantly contribute to disease development [,]. Food processing also affects health outcomes [,]. The NOVA classification, introduced in 2009, categorizes foods based on processing level and purpose into four groups: (a) unprocessed and minimally processed foods; (b) processed culinary ingredients; (c) processed foods, and (d) ultra-processed foods [,,,]. Though endorsed by the World Health Organization (WHO), Food and Agriculture Organization (FAO), and Pan American Health Organization (PAHO), NOVA has faced criticism in the U.S. and lacks Food and Drug Administration (FDA) and United States Department of Agriculture (USDA) recognition [,].
Growing awareness of the link between diet and health has shifted consumer preferences toward fresh, minimally processed foods (MPFs) due to their role in preventing degenerative diseases such as heart disease and cancer [,,]. However, maintaining both safety and nutritional quality in MPFs remains a challenge. Traditional methods often compromise one for the other, prompting interest in biological solutions like bacteriophages—viruses that specifically target bacteria. This review explores their dual role as tools for enhancing safety and serving as potential disruptors in MPF systems.
2. Minimally Processed Food
The concept of “minimal processing” generally refers to using the least amount of processing needed to achieve the desired outcome []. It often emphasizes preserving nutritional and sensory qualities while reducing reliance on heat []. Modern definitions highlight techniques that ensure shelf life and meet consumer expectations for freshness and convenience []. The NOVA classification offers a distinct view, defining MPFs as natural items treated with basic preservation methods (e.g., drying, cooking, freezing) without added salt, sugar, fats, or other substances. These processes aim to retain the food’s natural state, aid storage, and ensure safety. Many foods classified in the first NOVA group reflect home-cooked meals and support a healthy diet when eaten in balanced combinations [,,]. Despite variations in how “minimally processed food” is interpreted, several common characteristics can be identified: (a) they retain the freshness of raw ingredients—such as texture, color, taste, and aroma—due to gentle heat treatment and preservation methods; (b) they maintain nutrients that are sensitive to processing, particularly vitamins, provitamins, phytonutrients, and minerals; (c) they often involve combined preservation methods that incorporate mild processing techniques alongside physicochemical or biological approaches; (d) packaging involves modified conditions and materials specifically tailored to the product; and (e) they require refrigeration throughout the production and distribution process to maintain quality and safety [,,].
2.1. Microbiological Hazards in Minimally Processed Food Products
The interest in and demand for fresh, natural foods have been steadily increasing for many years, with consumers seeking low-processed products that offer high nutritional value [,]. This growing preference for minimally processed and functional foods presents a significant challenge for food technologists. Producing safe, MPFs with an adequate shelf life is complex and requires the development of innovative, non-thermal processing methods within the food industry [,,].
MPFs can be broadly categorized into two main groups: (a) plant-based products, such as fruits and vegetables, and (b) animal-based products, including meat, fish, and seafood. In addition to these primary categories, the expanding market for low-processed foods also encompasses other product types, such as whole-grain foods, ready-to-eat meals, and items preserved using advanced techniques like cook–chill, cook–freeze, and sous-vide methods [,].
Minimally processed technologies often involve mechanical operations such as cutting or peeling, which can cause tissue damage and the release of cell contents at injury sites [,]. The leakage of cell juices during processing increases water activity in the product, creating favorable conditions for microbial growth [,]. Specifically, latex secreted from cut lettuce surfaces can stimulate the proliferation of enterohemorrhagic Escherichia coli O157:H7 (EHEC), a verotoxin-producing bacterium []. The elevated microbial load in MPF products often stems from the high level of microorganisms present in the raw materials. In addition to monitoring pathogenic bacteria that pose a risk to consumer health, it is equally important to address the presence of saprophytic bacteria. Their rapid growth can lead to a decline in product quality and a shortened shelf life [,].
2.2. Methods of Preserving Minimally Processed Foods
To guarantee the safety and high quality of minimally processed foods, the food industry is advancing various preservation techniques. Common methods include thermal and non-thermal processing, refrigerated storage, innovative packaging solutions (modified atmosphere packaging, controlled atmosphere packaging, edible coatings), the application of natural antimicrobials or chemicals, and the use of hurdle technology [,,].
In recent years, there has been a growing shift away from physical and chemical food preservation methods in favor of biological approaches. Among the most promising biocontrol agents for extending the shelf life of fresh-cut fruits and vegetables are lactic acid bacteria (LAB) [,], which have demonstrated antibacterial activity against pathogens such as Escherichia coli, Listeria monocytogenes, Salmonella Typhimurium, and Shigella sonnei []. Both Gram-positive and Gram-negative bacteria produce bacteriocins—protein-based compounds with bactericidal or bacteriostatic properties [,]. Unlike antibiotics, which are produced during the dying phase of microbial growth (secondary metabolites), bacteriocins are synthesized during the stationary phase as primary metabolites []. These compounds target bacterial cytoplasmic membranes, disrupting proton movement, which leads to ion leakage, a loss of ATP, the release of genetic material (RNA or DNA), and ultimately cell death [].
Another promising biological preservation method involves the use of bacterio-phages—highly specific, strictly lytic viruses that target bacteria. The application of phage-based biopreparations for food biopreservation is discussed in Section 4.4.
Figure 1 illustrates the primary physical, chemical, and biological methods employed in preserving MPF products.
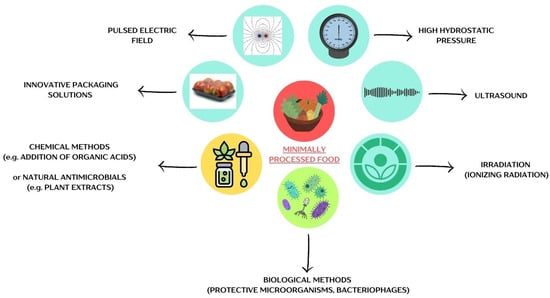
Figure 1.
Methods of preserving minimally processed foods. The figure was prepared in Canva.
3. Food as a Carrier of Antibiotic-Resistant Bacteria
The antibiotic era began in the 20th century with Alexander Fleming’s discovery of penicillin in 1928 [,]. The period from 1950 to 1970 is often referred to as the “golden age” of antibiotic discovery, during which half of the antibiotics we use today were identified. However, since then, no new classes of antibiotics have been discovered [,]. Unfortunately, poor antibiotic stewardship (AMS) has contributed to the onset of what is now known as the post-antibiotic era [,,]. Antibiotics are becoming less effective in treating bacterial infections, and the rise of antibiotic resistance in microorganisms is closely linked to the presence of antibiotic resistance genes (ARGs) in the environment [,]. These resistance genes can be transferred to pathogenic bacteria through horizontal gene transfer (HGT) [,]. Conjugation, one of the primary mechanisms for spreading ARGs, bypasses the usual genetic barriers to gene transfer [,]. Some conjugative modules even enable resistance plasmids to be exchanged between unrelated bacterial species []. Numerous studies highlight that food is a significant reservoir of ARGs (Figure 2).
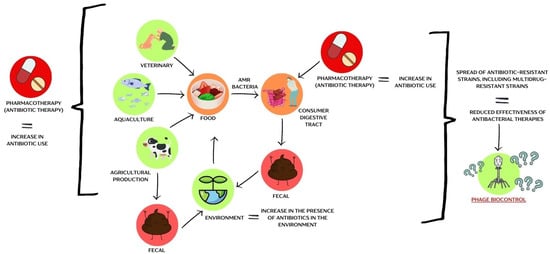
Figure 2.
A schematic representation of the problem of antibiotic-resistant bacteria within the food chain. The figure was prepared in Canva.
The rapid spread of ARGs is mainly driven by excessive antibiotic use in agriculture and HGT [,]. For instance, streptomycin use in the 1950s to control Erwinia amylovora in fruit trees led to resistance development; it remains approved in some countries outside the EU, including Israel, Canada, Mexico, and New Zealand []. Agricultural antibiotic use far exceeds medical use—about 80% of U.S. antibiotic sales are for farming []. Resistant bacteria from treated animals are excreted into the environment via feces, contaminating manure-fertilized fields and aquatic ecosystems []. ARGs have since been found in meat and minimally processed plant-based foods. The dairy industry also contributes to environmental ARG spread [,].
Inappropriate human antibiotic use—such as prescribing for viral infections or improper dosage—further worsens resistance [,,]. This has led to increased prevalence of MDR strains and reduced antibiotic effectiveness [,]. Notably, methicillin-resistant Staphylococcus aureus (MRSA) causes more annual deaths in the U.S. than emphysema, Parkinson’s, or AIDS [,].
In response, the EU banned antibiotics as growth promoters in 2006 [,], and resistance now ranks among the top public health priorities in EU programs []. Member states must develop national action plans. In Poland, the National Antibiotic Protection Programme, now partly integrated into the National Health Programme (2021–2025), addresses this issue [].
Eliminating all drug resistance genes is not feasible, as some have other functions unrelated to antimicrobial resistance (AMR). Therefore, efforts should focus on controlling the emergence, selection, and spread of these genes, particularly in bacteria that interact with humans, animals, and plants. Researchers are intensively working on methods to prevent HGT. Another promising approach involves combining principles from developmental biology, evolution, and ecology to combat AMR spread []. Increasing attention is also being given to the use of bacteriophages as a potential solution to fighting MDR strains (discussed in Section 4) [,,].
3.1. Antibiotics and Mechanisms of Their Action
Antibacterial drugs are a broad group of compounds. The original definition of “antibiotics” referred to natural, secondary metabolites produced by microorganisms with antibacterial properties, while chemotherapeutics were considered synthetic chemical compounds that did not have a natural equivalent. Today, the term “antibiotics” encompasses all antibacterial drugs, including both naturally derived and synthetic chemotherapeutic agents [,]. Antibiotics with similar molecular structures tend to have similar efficacy, toxicity, and potential side effects [,]. Figure 3 illustrates the classification of antibiotics based on how they act on or within bacterial cells.
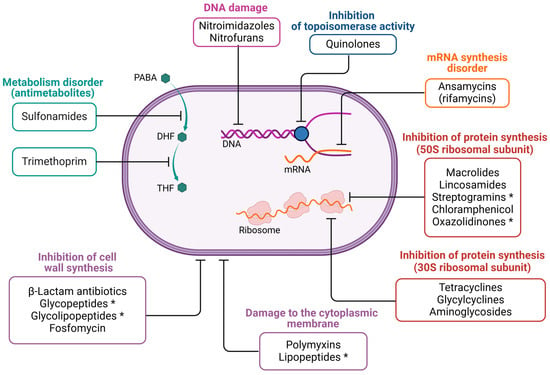
Figure 3.
The mechanism of action of selected drug groups. The figure was created in BioRender. Wójcicki, M. (2025) https://BioRender.com/r74m836, accessed on 27 May 2025 (license no.: KO27XLU1BJ). Symbols in the figure: PABA—p-aminobenzoic acid; DHF—dihydrofolate; THF—tetrahydrofolate. The asterisk (*) indicates antibiotics that are active only against Gram-positive bacteria.
3.2. Antibiotic Resistance and Mechanisms of Bacterial Resistance
The development of new antibiotics has driven the emergence of drug-resistant bacterial strains. Acquired resistance arises from genomic changes, either through spontaneous mutations (e.g., DNA polymerase errors) or via transposable elements. HGT also facilitates resistance spread through conjugation, transformation, and transduction. Key contributors include mobile genetic elements (e.g., plasmids, phages, integrons), recombination processes, and selective pressure []. Definitions of acquired antibiotic resistance were established by experts from the European Centre for Disease Prevention and Control (ECDC) in Stockholm and the US Centers for Disease Control and Prevention (CDC):
- i.
- MDR (multidrug resistance): the strain shows resistance to at least one antibiotic from three or more different classes of antibacterial drugs;
- ii.
- XDR (extensively drug resistance): the strain is resistant to at least one antibiotic from all but a maximum of two classes of antibiotics used to treat infections caused by the microorganism;
- iii.
- PDR (pandrug resistance): the strain is resistant to all available, approved antibiotics from all categories used to treat infections caused by the specific microorganism species [,,,].
Over time, bacteria have evolved various mechanisms to resist antibiotics, including (a) target site alteration; (b) reduced membrane permeability; (c) drug-inactivating enzymes; (d) efflux pumps; (e) alternative pathways or enzymes; and (f) protective proteins shielding drug targets [,].
3.3. Natural Resistance of Bacteria to Antibiotics
In addition to acquired resistance, bacteria also exhibit natural resistance, which is commonly observed within a particular family, genus, or species. This type of resistance is not linked to prior antibiotic exposure and does not involve HGT. Natural resistance arises from (a) the absence of a target site for the drug within the bacterial cell, (b) the presence of structures that block the drug from reaching its target, such as lipopolysaccharides in the outer membrane of Gram-negative bacteria, or (c) the activity of specific resistance mechanisms, where effectors like enzymes or membrane transporters are encoded by chromosomal genes characteristic of the bacterial species [,,].
4. Bacteriophages as a Natural Method of Biocontrol of Bacterial Microbiota in Food
The growing prevalence of MDR strains and the increasing frequency of infections caused by these bacteria have prompted the search for alternative antibacterial agents to traditional antibiotics [,]. One potential solution is the use of bacteriophages—natural enemies of bacteria. Phage therapy is not a new concept, as it was practiced even before the discovery of antibiotics [,,]. For many years, experimental, targeted phage therapy has been carried out in Poland at the Phage Therapy Unit of the Medical Center of the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences (HIIET PAS) in Wroclaw [,,].
The rising demand for minimally processed food, where maintaining structure and nutritional value is essential, has led the food industry to explore new, non-invasive preservation methods [,]. The use of bacteriophages throughout the food production chain—such as directly eliminating bacteria from food surfaces and processing equipment in contact with the product—could help improve the microbiological safety of minimally processed food [,,,]. Since bacteriophages are obligate bacterial parasites, they naturally coexist with the food microbiota and could serve as natural biological agents targeting their bacterial hosts [,,].
4.1. Phage Particle Structure
Bacteriophages (phages), like eukaryotic viruses, lack the ability to amplify independently and do not have a functional metabolism or cellular structure [,]. Despite this, phages undergo evolutionary processes and exhibit variability. Their genome is composed of nucleic acids, which may include configurations not commonly found in cellular genomes, such as double-stranded RNA (dsRNA) [,]. The unique characteristics of phages place them at the intersection of living and non-living matter []. Virus classification typically relies on analyzing virion morphology through transmission electron microscopy (TEM) and genome sequencing methods [,]. The International Committee on Taxonomy of Viruses (ICTV) is responsible for the taxonomic classification of viruses, including bacteriophages [,]. However, there are no universal criteria for defining the genus or species of individual viruses. The ICTV adopts a polythetic species concept, where a species is identified by a set of common features, some of which may be absent in certain members of the species []. According to ICTV guidelines, subfamilies are only created when they provide additional hierarchical information []. A genus is defined as a group of viruses with over 70% nucleotide sequence similarity, distinct from other viral types, while a species is a monophyletic group of viruses distinguishable by multiple criteria. A species is the lowest taxonomic level recognized by the ICTV [].
The primary demarcation criterium for defining bacteriophage species is a genome sequence similarity of at least 95%, meaning that two viruses within the same species can differ by no more than 5% in nucleotide sequence alignment []. In 2020, the ICTV adopted a binomial naming system for virus species, where the genus name and species epithet together create a unique species name [,]. The Bacterial Viruses Subcommittee (BVS) implemented a fully open binomial format, allowing research groups and authors of taxonomic proposals to determine the final species name format []. Classifying bacteriophages based on experimental data is both time-consuming and labor-intensive, which has led to the exploration of machine learning as an appealing alternative [,,]. New tools for supporting bacteriophage classification include applications like INPHARED, GRAViTy, vConTact2, virClust, PhaGCN2, and PhageAI [,,,,].
Phage particles are composed of genetic material in the form of either single-stranded or double-stranded DNA or RNA, encased in structural proteins that form a capsid. The capsid can have a helical (filamentous or rod-like; such as phage M13), isometric (icosahedral or spherical, such as phage ΦX174), or icosahedral shape, often connected to a tail, forming a complex (tailed) structure [,,]. The phage genome contains all the necessary information for replication within the bacterial host cell, the synthesis of phage proteins, and the assembly of progeny phages capable of infecting other bacterial cells []. The protein capsid is highly resistant to most external environmental factors, safeguarding the genetic material inside [,]. Many phage nucleocapsids are surrounded by an additional lipid membrane, which is a fragment of the host cytoplasmic membrane, forming an envelope connected to the capsid by matrix proteins. In contrast, non-enveloped (naked) viruses lack this lipid membrane [,].
Typical examples of phages with complex structures are virions belonging to the Caudoviricetes class, which includes both bacterial and archaeal viruses containing a double-stranded DNA (dsDNA) genome [,,]. In 2022, the BVS recognized the Caudoviricetes class, removing the Caudovirales order, including previous morphological Myoviridae, Podoviridae, and Siphoviridae families, and introducing a binomial system for species naming []. Research on the classification of newly discovered bacteriophage sequences is ongoing, and as of 30 April 2025, the Caudoviricetes class includes 11 orders, 105 families, 132 subfamilies, 1680 genera, and 5799 species of bacterial and archaeal viruses [].
4.2. Bacteriophage Replication Cycles
The first step in phage infection is the adsorption of virions to the surface of the bacterial host cell [,]. This process varies depending on the type of bacteria. In Gram-negative bacteria, phage attachment occurs in two stages. Initially, the phage binds reversibly to lipopolysaccharides (LPSs) that contain the polymannose O antigen, and then it attaches permanently to the ferrichrome transporter [,,]. Ferrichromes are siderophores, compounds that bacteria secrete to capture iron ions from the environment []. For Gram-positive bacteria, phage attachment involves the binding of the phage to specific sugar receptors in the bacterial cell wall. These receptors may include glucose, galactose, rhamnose, glucosamine, or galactosamine [,]. In some cases, the interaction between the phage and bacterial host may require the involvement of bacterial phage infection proteins (PIPs) or membrane proteins [,].
Environmental factors, as well as the physiological and genetic traits of the bacterial host, can lead to variations in phage replication pathways []. Phage replication strategies exist on a spectrum, from highly productive infections that result in the release of new virions to persistent nonproductive infections that do not generate new phages but instead spread prophage genomes within the bacterial population (transferred from parent to daughter cells) [,]. The two most well-known and studied phage replication pathways are the lytic and lysogenic cycles [,]. A variation of the lysogenic cycle is the carrier state, also known as the pseudolysogenic cycle []. Another form of phage–host interaction is chronic infection [,]. Figure 4 illustrates the differences between these various phage infection strategies.
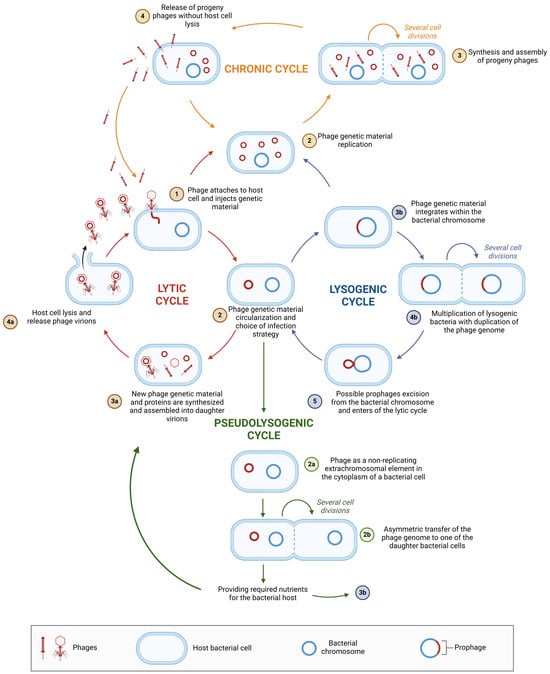
Figure 4.
Possible phage–bacterial host interactions. The figure was created in BioRender. Wójcicki, M. (2025) https://BioRender.com/r74m836, accessed on 27 May 2025 (license number: FR27XLSFWZ).
4.3. Benefits and Risks of Using Bacteriophages
Phage therapy refers to the use of phages and/or their enzymes to treat bacterial infections. This approach offers several advantages over antibiotic therapy [,]. However, despite its benefits, phage application also carries certain risks that must be considered when selecting phages for the development of effective biocontrol preparations.
Bacteriophages, as viruses that infect prokaryotic cells, can replicate only within bacterial hosts, making them harmless to human, animal, and other eukaryotic cells [,]. They are a natural part of the human gut microbiome [,,] and have also been found in blood, the genitourinary tract, the respiratory system, the skin, and cerebrospinal fluid [,]. The gut microbiota is a complex ecosystem containing trillions of microbial cells, including bacteria, archaea, eukaryotes, and viruses. It plays a crucial role in human health, contributing to the biosynthesis of vitamins, amino acids, and short-chain fatty acids (SCFAs), supporting immune system development, and providing protection against pathogens [,]. Disruptions in the gut microbiome have been linked to various diseases, such as inflammatory bowel disease, obesity, diabetes, and neurological disorders [,]. Bacteriophages are the most abundant component of the gut microbiota, with an estimated 1015 phage particles in the human gastrointestinal tract. They significantly influence the composition, function, and modulation of the gut microbiome [,]. Each individual has a unique “phageome”, with only about 5% of phages forming a core common to all humans []. The structure and function of the gut phage community are shaped by multiple factors, including environmental influences, gut microbiota composition, and host immune responses [,,,]. Phages and bacteria exhibit a distinct distribution within the intestines. Temperate phages and numerous bacteria are found closer to the intestinal lumen, while deeper layers of the mucosa contain lytic phages and fewer bacteria resistant to phage infection [,,]. Despite their high morphological, structural, and genetic diversity, intestinal phages share certain characteristics. Most are non-enveloped and possess DNA genomes, though some RNA phages are present, likely originating from ingested plant-based food [,]. A promising emerging approach involves using lytic phages as probiotics or dietary supplements to target and eliminate harmful bacteria. The key distinction between bacterial probiotics and lytic phage-based probiotics, known as phagobiotics, lies in their mechanisms of action. While bacterial probiotics utilize nonpathogenic bacteria to inhibit the colonization and pathogenicity of harmful bacteria, phagobiotics employ lytic phages to selectively eliminate specific pathogens while preserving the beneficial microbial community. Due to their high specificity, phagobiotics are expected to have minimal impact on the overall microbiota and may even act synergistically with traditional probiotics []. Numerous studies have explored potential interactions between bacteriophages and eukaryotic cells, demonstrating their influence on biochemical and physiological processes [,]. Although phages do not trigger acute immune responses, they can affect tissue and organ function. Research has shown that phages can enter breast epithelial cells via endocytosis and reach the nucleus, suggesting a role in transcytosis across epithelial barriers [,]. Additionally, phages have been found to modulate gene expression in eukaryotic cells [,]. The therapeutic effects of phage preparations may extend beyond their bactericidal activity, as they can also stimulate immune system cells and enhance proliferation in response to mitogens []. Phage lysates, which contain bacterial remnants following lysis—such as endotoxins—can also influence immune responses [,]. Studies indicate that phages may exhibit immunomodulatory properties by affecting phagocyte functions, including phagocytosis, reactive oxygen species (ROS) production, and T lymphocyte proliferation [,,]. Additionally, they possess immunogenic properties, stimulating the production of specific antibodies against phage antigens. Bacteriophages interact with innate immune cells, inducing phagocytosis and influencing antimicrobial protein levels, as well as modulating cytokine production by other immune cells. Due to their nucleoprotein structure, phages are recognized by immune system cells, leading to their neutralization and clearance from the body []. Furthermore, they can influence the adaptive immune response by stimulating the production of antiphage antibodies [,].
One of the key advantages of bacteriophages is their ability to increase their dose automatically. As self-amplifying entities, phages replicate in the presence of their bacterial hosts, accumulating precisely where they are needed [,]. A significant benefit of phage therapy is its effectiveness against multidrug-resistant bacteria, helping to control infections caused by resistant strains [,]. Additionally, bacteriophages through enzymes encoded in their genomes can penetrate and disrupt bacterial biofilms, improving access for other antibacterial agents [,,]. Phages are highly specific to their bacterial targets, which can be both an advantage and a limitation. Their precise targeting makes them valuable for therapeutic applications and biocontrol in the agri-food industry. However, to ensure broad efficacy, phage-based treatments should consist of cocktails containing multiple phages with a polyvalent specificity [,]. On the other hand, this strain specificity prevents phages from harming beneficial bacteria used in biotechnological and food industries, such as dairy starter cultures [,]. Using phage cocktails or polyvalent phages (capable of infecting multiple bacterial strains) may introduce complex pharmacological interactions. In some cases, these interactions can lead to antagonistic effects, reducing phage efficacy and resulting in bacteriostatic rather than bactericidal activity [].
For both therapeutic applications and the development of biopreparations for the agri-food industry and bioremediation, only virulent phages should be identified and selected [,]. This is because temperate phages, which integrate into the bacterial genome during the lysogenic cycle, can facilitate HGT, including the spread of antibiotic resistance genes [,,,]. Prophages often encode virulence factors within pathogenicity islands (PAIs), a subset of genome islands (GEIs), and lysogenic conversion can contribute to the pathogenicity of certain bacteria [,]. A notable example is Vibrio cholerae, where virulence is driven by transducing phages CTXΦ and VPIΦ, known as choleraphages, which carry virulence-related genetic elements in their genomes [,]. The virulence cassette within these phages contains four key genes that contribute to bacterial pathogenicity: (a) ctxAB—encodes cholera toxin (CT), a cytotoxic AB-type enterotoxin. Its A subunit induces intracellular cAMP (cyclic adenosine monophosphate) production, disrupting ion transport and causing severe fluid loss; (b) zot—encodes the zonula occludens toxin (ZOT), which disrupts intercellular junctions, increasing intestinal permeability; (c) ace—encodes the accessory cholera enterotoxin (ACE), which contributes to fluid accumulation in the intestine; and (d) cep—encodes fimbriae, aiding bacterial colonization of the small intestine [,,].
Over the course of evolution, bacteria have developed various defense mechanisms against bacteriophage infections, acting at different stages of the infection process [,]. One such mechanism is adsorption inhibition, which prevents phage attachment by physically masking receptors, altering their structure, or eliminating them entirely. The masking process often involves the synthesis of extracellular polysaccharides, which form an additional protective layer that blocks phage recognition sites. Additionally, spontaneous mutations in bacterial genes responsible for receptor synthesis or transport can lead to the loss or malfunction of these receptors, making bacterial cells resistant to phage adsorption [,]. Other bacterial defense strategies include blocking phage DNA injection through the superinfection exclusion (Sie) system, which prevents foreign phage genomes from entering the cytoplasm []. Another important mechanism is abortive infection (Abi), which operates within the cytoplasm to disrupt various stages of phage replication, ultimately leading to the programmed cell death (apoptosis) of infected bacteria. This self-sacrificial strategy prevents the spread of phages within the bacterial population, ensuring the survival of uninfected cells [,,,,]. Additionally, phages themselves can encode membrane-bound Sie proteins which protect against the entry of genetic material from competing lytic phages []. The restriction–modification (R-M) system is another bacterial defense mechanism that involves the recognition and destruction of foreign DNA through endonucleolytic digestion. Bacterial DNA is protected from degradation by specific methylation modifications introduced by enzymes with methyltransferase activity [,,]. A more advanced bacterial immune defense mechanism is the CRISPR-Cas system (clustered regularly interspaced short palindromic repeats), which enables bacteria to recognize and degrade foreign genetic material. This system consists of short DNA fragments (spacers) homologous to phage genomes, separated by palindromic sequences within the bacterial genome. The associated Cas proteins degrade the invading phage DNA through a three-stage process: (1) adaptation—the bacterium incorporates a new spacer sequence from the phage genome into its CRISPR array; (2) expression—the CRISPR array is transcribed into CRISPR RNA (crRNA), which contains a single spacer sequence; and (3) interference—the crRNA associates with effector proteins that scan invading DNA for complementarity. If a match is found, the phage genome is degraded by Cas nucleases, preventing bacterial lysis [,,,,]. Other bacterial defense systems include the DISARM (defense island system associated with restriction–modification), which consists of five genes encoding a DNA methyltransferase, DrmA helicase, DrmB (a protein of unknown function), phospholipase D domain nuclease (DrmC), and an accessory protein. This system functions by methylating bacterial DNA while restricting and modifying invading phage genomes [,,]. Similarly, the BREX (bacteriophage exclusion) system prevents phage replication and integration through bacterial DNA methylation [,]. Recent discoveries have revealed additional bacterial antiphage defense systems, though their exact molecular mechanisms remain unknown and require further research [,,].
Bacteriophages and bacteria are engaged in a continuous evolutionary arms race, each adapting to outcompete the other for survival. Phages evolve to overcome bacterial defense mechanisms by recognizing and developing alternatives to altered bacterial receptors, allowing them to effectively adhere to host cells []. When bacteria modify their receptors, phages counteract by producing polysaccharide-degrading enzymes, such as lyases and hydrolases, to break through bacterial defenses []. Phages can also bypass bacterial modification-dependent systems and deploy their own anti-CRISPR mechanisms to evade acquired bacterial resistance []. Additionally, they can escape CRISPR-Cas defenses through mutations in protospacer-adjacent motif (PAM) sequences, preventing recognition and cleavage by bacterial nucleases []. Some phages have evolved protective genomic modifications, such as glucosylated hydroxymethylcytosine (HMC) or methylated DNA, which shield them from bacterial restriction enzymes. For example, Staphylococcus phage K can eliminate Sau3A restriction sites on both DNA strands to evade degradation [,]. Similarly, bacteriophage T4 protects itself by glucosylating HMC residues, making its genome resistant to bacterial restriction–modification systems []. Phages can also produce proteins that inhibit bacterial defenses. For instance, some phages synthesize a 76-amino-acid restriction nuclease inhibitor (IPI*), which binds to bacterial GmrS and GmrD proteins, preventing phage DNA digestion []. Mutations in nucleotide metabolism genes allow phages to escape the Abi system, particularly the AbiQ mechanism [,]. To counteract bacterial toxin–antitoxin (TA) systems, bacteriophage ΦTE produces pseudoantitoxin RNA (pseudo-ToxI), which mimics bacterial antitoxins and neutralizes apoptosis-associated defense responses [].
4.4. Invisible Guardians: The Role of Bacteriophages in Food Safety
Phages can be applied across three key sectors of the agri-food industry: (a) primary production, including animal farming and crop cultivation; (b) bio-sanitization, primarily in manufacturing facilities to prevent biofilm formation on equipment surfaces; and (c) biopreservation, which focuses on extending the microbiological shelf life of food by inhibiting the growth of both pathogenic and spoilage bacteria [,].
Given the potential risks associated with bacteriophage applications, phage preparations should comply with strict safety criteria: (a) they should be classified as organisms generally recognized as safe (GRAS); (b) they must be strictly lytic (virulent); (c) for pathogenic bacterial biocontrol, they should be capable of replicating in nonpathogenic host cells; and (d) their genomes must be free of toxin- and antibiotic resistance-related genes [,]. Additionally, bacteriophages used in biopreparations should exhibit a broad bacterial host range, high stability under various storage conditions (resistance to pH fluctuations, temperature changes, and gastrointestinal enzymes), and the ability to replicate efficiently, producing high phage titers [,,,].
4.4.1. Primary Production
Bacterial phytopathogens significantly reduce crop yields and thus pose a threat to food supply []. The use of bacteriophages is considered a potential biocontrol measure in sustainable primary production. Phage preparations specifically targeting major bacterial plant pathogens (including Xanthomonas, Pseudomonas, Erwinia, or Pectobacterium) in field cultivation could be applied in the form of sprays or surface treatments [,]. Unlike pesticides, phages as biodegradable particles do not leave harmful chemical residues in the environment [,]. Compared to antibiotics or copper-based sprays, they also exert lower selective pressure for resistance []. Phages have already been tested or applied to combat diseases such as bacterial fruit blotch, goss wilt, soft rot and blackleg, fire blight, bacterial canker, bacterial leaf blight, or pepper bacterial spot []. However, regulatory hurdles and the need for cost-effective large-scale production still limit their widespread adoption. Despite these challenges, some commercial phage products, such as AgriPhage in the USA, have been registered as biopesticides by the U.S. Environmental Protection Agency and are being marketed. AgriPhage phage cocktails combat bacterial spot and speck disease in tomatoes and peppers (“AgriPhage”), tomato bacterial canker (“AgriPhage-CMM”), fire blight in apple and pear (“AgriPhage-Fire blight”), and citrus canker (“Agriphage-Citrus canker”) [,]. Another preparation available in the US is “Xylphi-PD”, which targets Pierce’s disease of grape (related to Xylella fastidiosa infection) []. In Europe, only a very limited number of commercial phage bioproducts are available. The Scottish company APS Biocontrol sells the post-harvest food processing aid “BioLyse-PB”, which prevents Pectobacterium infections in potato tubers, while the Hungarian company Enviroinvest has received authorization for local sales of a phage cocktail targeting E. amylovora (“Erwiphage Plus”) [,]. However, to date, the EFSA has not registered any phage-based product as a plant protection agent or biopesticide.
Phages may also find application in targeted approaches to controlling bacterial infections such as Salmonella, E. coli, and Clostridium in livestock. By reducing the bacterial load and preventing disease outbreaks, phages can help improve survival rates, growth performance, and feed efficiency, which are key in breeding programs [,,,]. In addition, phages can selectively modulate the gut microbiome, promoting beneficial bacteria that enhance nutrient absorption and immune function, thereby indirectly supporting better reproductive outcomes. An important advantage of using phages is the reduction in antibiotic use, which helps lower the risk of developing antibiotic-resistant bacteria—a major global issue in both human and veterinary medicine. Phages may also play a role in improving reproductive health by treating infections in the reproductive system, potentially increasing fertility rates in breeding animals [,]. Current research focuses on developing phage-based feed additives, sprays, and modified phages with a broader host range or enhanced activity, as well as integrating phages into comprehensive herd health management strategies [,]. The Polish biotechnology company Proteon Pharmaceuticals specializes in developing commercial bacteriophage preparations aimed at improving animal health, reducing antibiotic use, and supporting sustainable livestock farming and aquaculture. It offers feed additives for poultry targeted at combating Salmonella (BAFASAL® and BAFASAL® + G) and avian pathogenic E. coli (BAFACOL®). Additionally, it produces products for aquaculture. The BAFADOR® preparation, targeting Aeromonas and Pseudomonas, aims to maintain gut microbiota balance, support fish welfare, and increase the economic profitability of farming. Proteon Pharmaceuticals’ products are commercially available in selected markets in Southeast Asia, South America, and Africa [,,,,].
4.4.2. Bio-Sanitization
Phages are becoming innovative tools in the bio-sanitation of food production lines. Unlike traditional chemical disinfectants, phages are highly specific, targeting only host bacteria without disrupting beneficial microbial communities or leaving chemical residues on equipment or surfaces [,]. This makes them particularly valuable when used against persistent bacterial pathogens such as L. monocytogenes, Salmonella spp., and E. coli, which can form biofilms on production line surfaces and resist conventional cleaning methods [,,,]. Phage-based bio-sanitation involves directly applying phage preparations onto food contact surfaces and production equipment [,,]. These preparations can penetrate biofilms and effectively reduce bacterial loads, enhancing food safety while complying with regulatory and environmental standards. Moreover, phages are generally considered safe, making them a viable addition to integrated sanitation protocols in the food industry [,]. This approach enhances food safety, supports clean-label requirements, and may help combat antimicrobial resistance. Ongoing research aims to optimize phage application methods, formulations, and combinations with other sanitation strategies to improve efficacy and minimize the risk of bacterial resistance. As demand grows for safer, more sustainable food production, phage bio-sanitation stands out as an innovative tool for maintaining hygiene and ensuring the microbiological safety of food products [,,].
4.4.3. Biopreservation
In the food industry, commercial phage preparations have emerged as promising biocontrol tools to enhance food safety by targeting foodborne bacterial pathogens such as L. monocytogenes, Salmonella spp., E. coli O157:H7, and Campylobacter spp. [,,]. Information on commercially available phage preparations for food biocontrol and legal aspects is presented in Section 4.7.
Research institutions worldwide are exploring the potential of phage biopreparations as a means of preserving MPF products. Recent selected studies on the preservation of plant-based MPF products conducted over the past five years are summarized in Table 1.

Table 1.
Biocontrol of saprophytic and pathogenic bacteria in minimally processed plant-based food products based on single phages and/or their cocktails.
4.5. The Dark Side of Phages: Challenges in Food Production and Fermentation
Bacteriophages may find applications in many branches of the agri-food industry. However, in the dairy industry, bacteriophages pose a serious problem because they can cause lysis of the starter culture, leading to slowed fermentation or complete inhibition of microbial growth [,,]. This, in turn, leads to a decrease in the quality of the final product or its spoilage [,]. It is estimated that about 70% of technological process disturbances in the dairy industry (in the production of curd and rennet cheeses) are related to phage infections. The biotechnological processes carried out in the plant are susceptible to infections because the raw material is not sterile. The use of the same starter culture (bacterial inoculum) further supplies host cells for phage development []. The primary sources of bacteriophages in the dairy industry are raw milk and starter cultures, while secondary contamination can occur via inadequate hygiene practices by employees (e.g., contaminated clothing) or production and ventilation equipment. Many phages retain their activity during pasteurization and spray drying processes. Starter cultures, particularly in the case of Lactococcus spp. strains, may in turn contain prophages capable of induction. Especially sensitive is Lactococcus lactis subsp. lactis var. diacetylactis, which is responsible for shaping the sensory characteristics of many dairy products []. Raw milk delivered to dairies may contain up to 104 PFU mL−1 of bacteriophages. The phage titer in whey can reach up to 109 PFU mL−1, making it the main secondary source of bacteriophages in the dairy plant. Aerosols generated from whey can lead to air contamination at levels up to 108 PFU m−3 [].
Phage detection in dairy production relies on monitoring acidification and performing lysogeny tests. In addition, qPCR or PCR methods (with a detection limit of 103 PFU mL−1) are employed, as well as flow cytometry, which requires prior removal of lipid droplets from the samples [,]. Compliance with hygiene standards in production, the use of starter culture rotation, and the acquisition of phage-resistant strains reduce the risk of phage infections. Modern phage infection control methods include the use of phage-resistant starter culture strains free of prophages, the use of lyophilized or frozen concentrated starter cultures (DVI/DVS), and early addition of rennet, which, in combination with elevated temperature, limits the absorption of certain phages. The possibility of controlled selection of phage-resistant strains may be provided by the use of the CRISPR/Cas system []. Media that inhibit phage replication and aseptic inoculation systems are also used, along with separation of the CIP (clean-in-place) systems between starter culture preparation and production areas, and the use of HEPA filters. Destruction of phages with chemical agents can involve treatment with 0.05% sodium hypochlorite or potassium permanganate solutions for 5 min at room temperature [].
4.6. Risks and Implications of Contaminants in Phage-Based Preparations
The application of bacteriophages in the food industry has gained increasing interest due to their specificity and effectiveness in controlling foodborne pathogens. However, the production and use of phage-based preparations are associated with several safety concerns, primarily related to the presence of contaminants originating from the bacterial host or the culture medium [,,,,]. One of the most critical risks involves endotoxins, particularly LPS, which are components of the outer membrane of Gram-negative bacteria frequently used as host strains in phage amplification. These endotoxins are known for their pyrogenic and immunostimulatory properties and can pose significant health risks if not properly removed, even when phages are applied externally to food products [,].
In addition to endotoxins, there is a risk of contamination with exotoxins, which are toxic proteins secreted by certain bacterial hosts during growth. These substances, if retained in crude lysates, may contribute to toxicity or allergenicity. Furthermore, bacterial cell debris, host DNA, enzymes, and residual proteins may persist through insufficient purification processes and negatively impact the safety or regulatory compliance of the final product [,,]. Residues from nutrient-rich culture media, such as peptones or yeast extract, may also remain in the preparation, potentially affecting product stability or triggering unwanted reactions. Another concern is the inadvertent inclusion of non-target or temperate bacteriophages, which could facilitate HGT or unpredictably disrupt microbial communities [,].
Scaling up bacteriophage production for food industry use also presents significant technological challenges [,]. In upstream processes, the selection of suitable host strains is crucial; these strains must be nonpathogenic, genetically stable, and incapable of producing harmful byproducts. Controlled bacterial lysis is essential to ensure efficient phage release while minimizing the liberation of cellular contaminants. Downstream processing, which includes purification, concentration, and formulation, is often complex and costly. Techniques such as ultrafiltration, chromatography, and ultracentrifugation are employed to reduce endotoxin levels and remove debris, but these methods can reduce phage viability and overall yield [,,].
Concentration and stabilization of phage preparations to reach high titers (typically 109–1011 PFU mL−1) without compromising their infectivity is another critical hurdle []. The formulation must also ensure long-term stability under various environmental conditions, particularly for industrial food applications where shelf life and robustness are key. In addition, the absence of standardized production protocols leads to variability in product quality, making regulatory approval and batch-to-batch consistency more difficult to achieve [].
These limitations have important implications for the food industry. Contaminants, even in trace amounts, may affect regulatory acceptance by bodies such as the FDA or EFSA and could result in delays, recalls, or loss of consumer trust. Moreover, the high costs of advanced purification technologies and the need for rigorous quality control present economic barriers to large-scale implementation.
In summary, while bacteriophages offer a promising and natural alternative for pathogen control in food systems, their safe and effective use requires overcoming substantial technological and regulatory challenges. Addressing contamination risks and optimizing large-scale production processes will be essential steps toward integrating phage-based solutions into mainstream food safety practices.
4.7. Legal Aspects Related to the Use of Bacteriophages in the Agri-Food Industry
Currently, only a few centers in the EU use bacteriophages for therapeutic purposes, the leading ones being the Phage Therapy Unit of the Medical Centre of the HIIET PAS in Wroclaw [,,] and the Queen Astrid Military Hospital in Brussels [,]. Despite extensive research on bacteriophage biology and their effects on the human immune system, phage therapy in Poland remains an experimental approach, with no government funding to support its clinical use [,]. In the agri-food sector, the use of phages as antibacterial agents is also highly restricted. While there have been successful applications of bacteriophages in food biocontrol, their incorporation into food products is only allowed in certain countries, with regulations applying exclusively to specific commercial phage-based products []. Within the EU, phage preparations have not yet been approved for direct contact with food [,,]. However, several non-EU countries, including the United States, Brazil, Israel, Canada, Switzerland, Australia, and New Zealand, permit the use of phages in the food industry. Commercial biotechnology companies offer phage-based biopreparations targeted at food safety, with many of these products receiving FDA approval. Notable FDA-approved phage preparations include ListexTM P100, Secure Shield E1, EcoShieldTM, ListShieldTM, ShigaShieldTM, and SalmoFreshTM, and USDA-approved products include Ecolicide®, SalmoFreshTM, and Finalyse® [,,]. Ten commercial phage preparations have earned GRAS status from the FDA, ensuring that their endotoxin content is below 250 endotoxin units per milliliter []. For phage therapy solutions administered intravenously, the permissible endotoxin level is set at 5 EU per kilogram of body weight per hour [,]. Given the empirical nature of phage dosing, further research is necessary to fully understand the pharmacokinetics of phage biopreparations []. Some commercial phage biopreparations are Kosher- and Halal-certified and are approved for use in organic food production []. The Polish company Proteon Pharmaceuticals S.A. (Łódź, Poland) also develops and markets phage biopreparations for use as feed additives in animal husbandry []. One of their products, Bafasal®, containing four strictly lytic bacteriophages specific to S. enterica serovar Gallinarum strain B/00111, has received a positive opinion from the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), at the request of the European Commission. Bafasal® is designed to be used as a zootechnical additive in drinking water and complementary liquid feed for all bird species [,].
In 2016, the European Food Safety Authority (EFSA) published a report [] confirming the toxicological safety and efficacy of Listex™ P100 by Micreos Food Safety, while recommending further studies to assess the preparation’s effectiveness. In the United States, when Listex™ P100 (targeting L. monocytogenes) is used on ready-to-eat products, meat, and poultry, the manufacturer is not required to include information about the preparation on the food label, provided permissible doses are followed. The approved limit is up to 109 PFU g−1, with a maximum of 50 ppm of potassium lactate (as Listex™ P100 contains potassium lactate) [,]. While Listex™ P100 is a single-phage preparation (utilizing phage P100), another commercially available biopreparation for eliminating L. monocytogenes is ListShield™ produced by Intralytix, Inc. This product contains six bacteriophages: LIST–36, LMSP–25, LMTA–34, LMTA–57, LMTA–94, and LMTA–148. ListShield™ can be used as a food biopreservative and for food processing in products like fish and shellfish, fresh and processed fruits, vegetables, and dairy products at a concentration of 106 PFU g−1 [,].
In October 2023, the European Medicines Agency (EMA) published the “Guideline on quality, safety and efficacy of veterinary medicinal products specifically designed for phage therapy” [], while in August 2024, the EFSA issued the “EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain” [], which opens up the possibility of using phage biopreparations in the agri-food industry in the future.
5. Conclusions
Bacteriophages play a dual role in the food industry—acting as both beneficial agents and potential threats. On the positive side, their usefulness is most evident in ensuring food safety, especially for minimally processed plant- and animal-based products. Phages applied in biocontrol, bio-sanitation, and biopreservation effectively combat pathogenic and spoilage bacteria, helping to extend shelf life and reduce the need for chemical preservatives. Moreover, thanks to their high specificity, they do not harm beneficial microorganisms and can target antibiotic-resistant strains, making them a valuable tool in the fight against antimicrobial resistance.
However, phages also have a dark side. In fermentation industries, particularly in dairy production, their presence poses a serious threat. By infecting starter cultures, phages can slow down or completely halt fermentation processes, resulting in reduced product quality or spoilage. Many dairy plants must invest in costly measures to limit the risk of phage contamination in production lines. Additionally, not all phages are suitable for industrial applications—temperate phages carry the risk of transferring antibiotic resistance or virulence genes, so only strictly lytic phages, which lead to complete bacterial cell lysis, should be used for biocontrol purposes.
In summary, bacteriophages can be both friends and foes of the food industry. With proper selection, quality control, and advanced purification technologies, their potential as natural, effective biocontrol agents clearly outweighs the risks. As a result, they may become an important part of strategies to enhance food safety, reduce chemical use, and address the global challenge of antibiotic resistance.
Author Contributions
Conceptualization, M.W.; data curation, M.W.; writing—original draft preparation, M.W.; writing—review and editing, M.W., B.S., A.G. and E.J.-M.; visualization, M.W.; supervision, B.S., A.G. and E.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abi | abortive infection system |
| ACE | accessory cholera enterotoxin |
| AIDS | acquired immunodeficiency syndrome |
| AMR | antimicrobial resistance |
| AMS | antibiotic stewardship |
| ARG | antibiotic resistance gene |
| BREX | bacteriophage exclusion |
| BVS | Bacterial Viruses Subcommittee |
| cAMP | cyclic adenosine monophosphate |
| Cas | CRISPR-associated genes |
| CDC | Centers for Disease Control and Prevention |
| CFU | colony-forming unit |
| CIP | clean-in-place |
| CRISPR | clustered, regularly interspaced short palindromic repeats |
| CT | cholera toxin |
| DHF | dihydrofolate |
| DISARM | defense island system associated with restriction modification |
| dsDNA | double-stranded deoxyribonucleic acid |
| dsRNA | double-stranded ribonucleic acid |
| ECDC | European Centre for Disease Prevention and Control |
| EFSA | European Food Safety Authority |
| EHEC | enterohemorrhagic Escherichia coli |
| EMA | European Medicines Agency |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| FDA | Food and Drug Administration |
| FEEDAP | Panel on Additives and Products or Substances used in Animal Feed |
| GEI | genome island |
| GRAS | generally recognized as safe |
| HGT | horizontal gene transfer |
| HHP | high hydrostatic pressure |
| HIIET PAS | Ludwik Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences |
| HMC | glucosylated hydroxymethylcytosine |
| ICTV | International Committee on Taxonomy of Viruses |
| LAB | lactic acid bacteria |
| LPS | lipopolysaccharide |
| MDR | multidrug-resistant, multidrug resistance |
| MPF | minimally processed food |
| MRSA | methicillin-resistant Staphylococcus aureus |
| PABA | p–aminobenzoic acid |
| PAHO | Pan American Health Organization |
| PAI | pathogenicity island |
| PAM | protospacer-adjacent motif |
| PDR | pandrug resistance |
| PFU | plaque-forming unit |
| PIP | phage infection protein |
| qPCR | quantitative polymerase chain reaction |
| R-M | restriction–modification |
| ROS | reactive oxygen species |
| SCFA | short-chain fatty acid |
| Sie | superinfection exclusion mechanism |
| TA | toxin-antitoxin |
| TEM | transmission electron microscopy |
| THF | tetrahydrofolate |
| TNB | total number of bacteria |
| USDA | United States Department of Agriculture |
| WHO | World Health Organization |
| XDR | extensively drug resistance |
| ZOT | zonula occludens toxin |
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age–sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [PubMed]
- Vandevijvere, S.; De Ridder, K.; Fiolet, T.; Bel, S.; Tafforeau, J. Consumption of ultra–processed food products and diet quality among children, adolescents and adults in Belgium. Eur. J. Nutr. 2019, 58, 3267–3278. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra–processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity—Modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.V. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr. Diabetes Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra–processed foods and cardiovascular diseases: Potential mechanisms of action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef]
- Petrus, R.R.; do Amaral Sobral, P.J.; Tadini, C.C.; Gonçalves, C.B. The NOVA classification system: A critical perspective in food science. Trends Food Sci. Tech. 2021, 116, 603–608. [Google Scholar] [CrossRef]
- Katidi, A.; Xanthopoulou, S.; Vlassopoulos, A.; Noutsos, S.; Priftis, K.; Kapsokefalou, M. Food allergens in ultra–processed foods according to the NOVA classification system: A Greek branded food level analysis. Nutrients 2023, 15, 2767. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN decade of nutrition, the NOVA food classification and the trouble with ultra–processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- De Corato, U. Improving the shelf–life and quality of fresh and minimally–processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Meserole, L. Health foods in anti–aging therapy: Reducers of physiological decline and degenerative diseases. In Advances in Phytomedicine, 1st ed.; Iwu, M.M., Wootton, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 173–196. [Google Scholar]
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer perception and choice of minimally processed vegetables and packaged fruits. Food Qual. Prefer. 2004, 15, 259–270. [Google Scholar] [CrossRef]
- Knorr, D.; Watzke, H. Food processing at a crossroad. Front. Nutr. 2019, 6, 85. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Machado, P.P. Ultra–Processed Foods, Diet Quality, and Health Using the Nova Classification System; FAO: Rome, Italy, 2019; pp. 1–48. [Google Scholar]
- Sarker, A.; Grift, T.E. Bioactive properties and potential applications of Aloe vera gel edible coating on fresh and minimally processed fruits and vegetables: A review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Castro, I.R.R.D.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef]
- Bansal, V.; Siddiqui, M.W.; Rahman, M.S. Minimally processed foods: Overview. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience, 1st ed.; Siddiqui, M., Rahman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 1–15. [Google Scholar]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Chakraborty, I.; Ayala-Zavala, J.F.; Dhua, R.S. Advances in minimal processing of fruits and vegetables: A review. J. Sci. Ind. Res. 2011, 70, 823–834. [Google Scholar]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Tech. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Yu, J.; Zhao, L.; Wang, K.; Tao, Y.; Renard, C.M.G.C.; Hu, Z. Trends and challenges on fruit and vegetable processing: Insights into sustainable, traceable, precise, healthy, intelligent, personalized and local innovative food products. Trends Food Sci. Tech. 2022, 125, 12–25. [Google Scholar] [CrossRef]
- Al-Khusaibi, M.; Al-Habsi, N.; Shafiur Rahman, M. Traditional Foods, 1st ed.; Al-Khusaibi, M., Al-Habsi, N., Shafiur Rahman, M., Eds.; Food Engineering Series; Springer: Cham, Switzerland, 2019; Volume 1, pp. 9–35. [Google Scholar]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot water treatment to alleviate chilling injury and enhance ascorbate-glutathione cycle in sweet pepper fruit during postharvest cold storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality–related enzymes in fruit and vegetable products: Effects of novel food processing technologies, part 1: High–pressure processing. Crit. Rev. Food Sci. Nutr. 2014, 54, 24–63. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Volume 1, pp. 323–355. [Google Scholar]
- George, A.S.; Simko, I.; Brandl, M.T. Escherichia coli O157:H7 multiplication in the latex of diverse lettuce genotypes is negatively correlated with plant peroxidase activity. Int. J. Food Microbiol. 2025, 431, 111095. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, X.; Liu, Y.; Hu, J.; Yang, C.; Zhao, X. The effects of ultrasound on the growth, nutritional quality and microbiological quality of sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- Shymialevich, D.; Wójcicki, M.; Wardaszka, A.; Świder, O.; Sokołowska, B.; Błażejak, S. Application of lytic bacteriophages and their enzymes to reduce saprophytic bacteria isolated from minimally processed plant–based food products—In vitro studies. Viruses 2023, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.M.; Borges, F.; Foligné, B. Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: A multifaceted functional health perspective. Front. Microbiol. 2018, 9, 2899. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio–control agents to improve the safety, shelf–life and quality of minimally processed fruits and vegetables. Trends Food Sci. Tech. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Sharma, V.; Ranveer, R.C.; Jain, N.; Aseri, G.K. Bacteriocins: Production, different strategies of purification and applications. Int. J. Res. Pharm. Sci. 2019, 10, 1808–1817. [Google Scholar] [CrossRef]
- Barbosa, A.A.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, E.; Chauvier, A.; Romero, R.A.; Liu, Y.; Ray, S.; Walter, N.G. Riboswitches as therapeutic targets: Promise of a new era of antibiotics. Expert Opin. Ther. Tar. 2023, 27, 433–445. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Pusparajah, P.; Goh, B.-H.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Unveiling the impact of antibiotics and alternative methods for animal husbandry: A review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Ilia, A.; Mpekoulis, G.; Papadopoulou, C. Antimicrobial resistance in bacterial pathogens and detection of carbapenemases in Klebsiella pneumoniae isolates from hospital wastewater. Antibiotics 2019, 8, 85. [Google Scholar] [CrossRef]
- Chandra, P.; Unnikrishnan, M.K.; Vandana, K.E.; Mukhopadhyay, C.; Dinesh Acharya, U.; Surulivel Rajan, M.; Rajesh, V. Antimicrobial resistance and the post antibiotic era: Better late than never effort. Expert. Opin. Drug Saf. 2021, 20, 1375–1390. [Google Scholar] [CrossRef]
- Priyadharsini, R.P. Antibiotic resistance: What is there in past, present and future? J. Young Pharm. 2019, 11, 333. [Google Scholar] [CrossRef]
- Kose, A.; Colak, C. Knowledge and awareness of physicians about rational antibiotic use and antimicrobial resistance before and after graduation: A cross–sectional study conducted in Malatya Province in Turkey. Infect. Drug Resist. 2021, 14, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
- Godziszewska, J.; Guzek, D.; Głąbski, K.; Wierzbicka, A. Mobilna antybiotykooporność—O rozprzestrzenianiu się genów determinujących oporność bakterii poprzez produkty spożywcze [Mobile antibiotic resistance—The spread of genes determining the resistance of bacteria through food products]. Postep. Hig. Med. Dosw. 2016, 70, 803–810. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Pruden, A.; Alvarez, P.J.J.; Aga, D.; Bürgmann, H.; Li, X.D.; Manaia, C.M.; Nambi, I.; Wigginton, K.; Zhang, T.; et al. Toward a comprehensive strategy to mitigate dissemination of environmental sources of antibiotic resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. [Google Scholar] [CrossRef] [PubMed]
- Vinayamohan, P.G.; Pellissery, A.J.; Venkitanarayanan, K. Role of horizontal gene transfer in the dissemination of antimicrobial resistance in food animal production. Curr. Opin. Food Sci. 2022, 47, 100882. [Google Scholar] [CrossRef]
- Mole, B. MRSA: Farming up trouble. Nature 2013, 499, 398–400. [Google Scholar] [CrossRef]
- Wang, N.; Yang, X.; Jiao, S.; Zhang, J.; Ye, B.; Gao, S. Sulfonamide–resistant bacteria and their resistance genes in soils fertilized with manures from Jiangsu Province, Southeastern China. PLoS ONE 2014, 9, e112626. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef]
- Bisht, R.; Katiyar, A.; Singh, R.; Mittal, P. Antibiotic resistance—A global issue of concern. Asian J. Pharm. Clin. Res. 2009, 2, 34–39. [Google Scholar]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Chaudhary, A.S. A review of global initiatives to fight antibiotic resistance and recent antibiotics’ discovery. Acta Pharm. Sin. B 2016, 6, 552–556. [Google Scholar] [CrossRef]
- Habib, F.; Roy, D.; Nity, M. Effect of promoter region sequence variations in relation to antibiotic resistance of methicillin–resistant Staphylococcus aureus. J. Med. Clin. Res. Rev. 2021, 5, 1–7. [Google Scholar] [CrossRef]
- Bhat, R.A.H.; Altinok, I. Antimicrobial resistance (AMR) and alternative strategies for combating AMR in aquaculture. Turk. J. Fish. Aquat. Sci. 2023, 23, TRJFAS24068. [Google Scholar] [CrossRef]
- Oluwafemi, R.A.; Olawale, I.; Alagbe, J.O. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition—A review. Res. Agric. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Cuong, N.V.; Kiet, B.T.; Hien, V.B.; Truong, B.D.; Phu, D.H.; Thwaites, G.; Choisy, M.; Carrique-Mas, J. Antimicrobial use through consumption of medicated feeds in chicken flocks in the Mekong Delta of Vietnam: A three–year study before a ban on antimicrobial growth promoters. PLoS ONE 2021, 16, e0250082. [Google Scholar] [CrossRef]
- Council of the European Union. Programme of Community Action in the Field of Public Health (2003 to 2008). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=LEGISSUM:c11503b (accessed on 20 February 2025).
- Narodowy Program Ochrony Antybiotyków [National Antibiotic Protection Program]. Available online: https://antybiotyki.edu.pl/ (accessed on 20 February 2025).
- Taati Moghadam, M.; Khoshbayan, A.; Chegini, Z.; Farahani, I.; Shariati, A. Bacteriophages, a new therapeutic solution for inhibiting multidrug–resistant bacteria causing wound infection: Lesson from animal models and clinical trials. Drug Des. Dev. Ther. 2020, 14, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Broncano-Lavado, A.; Santamaría-Corral, G.; Esteban, J.; García-Quintanilla, M. Advances in bacteriophage therapy against relevant multidrug–resistant pathogens. Antibiotics 2021, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Nikolich, M.P.; Filippov, A.A. bacteriophage therapy: Developments and directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef]
- Hyldgaard, M. Mechanisms of Action, Resistance, and Stress Adaptation. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 735–784. [Google Scholar]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Bitew, Z.; Amare, M. Recent reports on electrochemical determination of selected antibiotics in pharmaceutical formulations: A mini review. Electrochem. Commun. 2020, 121, 106863. [Google Scholar] [CrossRef]
- Al-Hasani, H.M.; Al-Rubaye, D.S.; Abdelhameed, A. The emergence of multidrug–resistant (MDR), extensively drug–resistant (XDR), and pandrug–resistant (PDR) in Iraqi clinical isolates of Escherichia coli. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 469–482. [Google Scholar]
- Rafailidis, P.I.; Kofteridis, D. Proposed amendments regarding the definitions of multidrug–resistant and extensively drug–resistant bacteria. Expert Rev. Anti Infect. Ther. 2022, 20, 139–146. [Google Scholar] [CrossRef]
- Alkofide, H.; Alhammad, A.M.; Alruwaili, A.; Aldemerdash, A.; Almangour, T.A.; Alsuwayegh, A.; Almoqbel, D.; Albati, A.; Alsaud, A.; Enani, M. Multidrug–resistant and extensively drug–resistant Enterobacteriaceae: Prevalence, treatments, and outcomes—A retrospective cohort study. Infect. Drug Resist. 2020, 13, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.H.; Al-Harmoosh, R.A. Mechanisms of antibiotics resistance in bacteria. Syst. Rev. Pharm. 2020, 11, 817–823. [Google Scholar]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of antibiotic resistance in important Gram–positive and Gram–negative pathogens and novel antibiotic solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Odonkor, S.T.; Addo, K.K. Bacteria resistance to antibiotics: Recent trends and challenges. Int. J. Biol. Med. Res. 2011, 2, 1204–1210. [Google Scholar]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef]
- Thandar, M.; Lood, R.; Winer, B.Y.; Deutsch, D.R.; Euler, C.W.; Fischetti, V.A. Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug–resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 2671–2679. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi–drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef]
- Makumi, A.; Mhone, A.L.; Odaba, J.; Guantai, L.; Svitek, N. Phages for Africa: The potential benefit and challenges of phage therapy for the livestock sector in Sub–Saharan Africa. Antibiotics 2021, 10, 1085. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage therapy in Poland—A centennial journey to the first ethically approved treatment facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Rostkowska, O.M.; Międzybrodzki, R.; Miszewska-Szyszkowska, D.; Górski, A.; Durlik, M. Treatment of recurrent urinary tract infections in a 60–year–old kidney transplant recipient. The use of phage therapy. Transpl. Infect. Dis. 2021, 23, e13391. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.; Casey, E.; van Sinderen, D. The impact and applications of phages in the food industry and agriculture. Viruses 2020, 12, 210. [Google Scholar] [CrossRef]
- Gientka, I.; Wójcicki, M.; Żuwalski, A.W.; Błażejak, S. Use of phage cocktail for improving the overall microbiological quality of sprouts—Two methods of application. Appl. Microbiol. 2021, 1, 289–303. [Google Scholar] [CrossRef]
- Xu, Y. Phage and phage lysins: New era of bio–preservatives and food safety agents. J. Food Sci. 2021, 86, 3349–3373. [Google Scholar] [CrossRef]
- Wójcik, E.A.; Stańczyk, M.; Wojtasik, A.; Kowalska, J.D.; Nowakowska, M.; Łukasiak, M.; Bartnicka, M.; Kazimierczak, J.; Dastych, J. Comprehensive evaluation of the safety and efficacy of BAFASAL® bacteriophage preparation for the reduction of Salmonella in the food chain. Viruses 2020, 12, 742. [Google Scholar] [CrossRef]
- Kuek, M.; McLean, S.K.; Palombo, E.A. Application of bacteriophages in food production and their potential as biocontrol agents in the organic farming industry. Biol. Control 2022, 165, 104817. [Google Scholar] [CrossRef]
- Wójcicki, M.; Błażejak, S.; Gientka, I.; Brzezicka, K. The concept of using bacteriophages to improve the microbiological quality of minimally processed foods. Acta Sci. Pol. Technol. Aliment. 2019, 18, 373–383. [Google Scholar]
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; De Antoni, L.; Beccari, T.; et al. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Biomed. 2020, 91, e2020024. [Google Scholar]
- Holtappels, D.; Alfenas-Zerbini, P.; Koskella, B. Drivers and consequences of bacteriophage host range. FEMS Microbiol. Rev. 2023, 47, fuad038. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Jørgensen, J.S.; Lamont, R.F. The contribution of bacteriophages to the aetiology and treatment of the bacterial vaginosis syndrome. Fac. Rev. 2022, 11, 8. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Naturae 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Kurilovich, E.; Geva-Zatorsky, N. Effects of bacteriophages on gut microbiome functionality. Gut Microbes 2025, 17, 2481178. [Google Scholar] [CrossRef]
- Brussow, H.; Canchaya, C.; Hardt, W.D. Phages and the evolution of bacterial pathogens: From genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Superio, J.; Galindo-Villegas, J.; Acosta, F. Phage therapy as a focused management strategy in aquaculture. Int. J. Mol. Sci. 2021, 22, 10436. [Google Scholar] [CrossRef]
- Siddell, S.G.; Smith, D.B.; Adriaenssens, E.; Alfenas-Zerbini, P.; Dutilh, B.E.; Garcia, M.L.; Junglen, S.; Krupovic, M.; Kuhn, J.H.; Lambert, A.J.; et al. Virus taxonomy and the role of the International Committee on Taxonomy of Viruses (ICTV). J. Gen. Virol. 2023, 104, 001840. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.; Ackermann, H.W.; Calisher, C.H.; Dietzgen, R.G.; Horzinek, M.C.; Keil, G.M.; Mahy, B.W.J.; Martelli, G.P.; Murphy, F.A.; Pringle, C.; et al. Virus species polemics: 14 senior virologists oppose a proposed change to the ICTV definition of virus species. Arch. Virol. 2013, 158, 1115–1119. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Brister, J.R. How to name and classify your phage: An informal guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2017, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Siddell, S.G.; Walker, P.J.; Lefkowitz, E.J.; Mushegian, A.R.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Junglen, S.; Knowles, N.J.; Kropinski, A.M.; et al. Binomial nomenclature for virus species: A consultation. Arch. Virol. 2020, 165, 519–525. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Mushegian, A.R.; Walker, P.J.; Lefkowitz, E.J.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dutilh, B.E.; García, M.L.; Junglen, S.; et al. Differentiating between viruses and virus species by writing their names correctly. Arch. Virol. 2022, 167, 1231–1234. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of morphology–based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef]
- Nami, Y.; Imeni, N.; Panahi, B. Application of machine learning in bacteriophage research. BMC Microbiol. 2021, 21, 193. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Zhao, Q. Recent advances on the machine learning methods in identifying phage virion proteins. Curr. Bioinform. 2020, 15, 657–661. [Google Scholar] [CrossRef]
- Lood, C.; Boeckaerts, D.; Stock, M.; De Baets, B.; Lavigne, R.; van Noort, V.; Briers, Y. Digital phagograms: Predicting phage infectivity through a multilayer machine learning approach. Curr. Opin. Virol. 2022, 52, 174–181. [Google Scholar] [CrossRef]
- Boeckaerts, D.; Stock, M.; De Baets, B.; Briers, Y. Identification of phage receptor–binding protein sequences with hidden Markov models and an extreme gradient boosting classifier. Viruses 2022, 14, 1329. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Martínez, J.S.; Camelo Valera, L.C.; Chica Cardenas, L.A.; Forero-Junco, L.; López-Leal, G.; Moreno-Gallego, J.L.; Rangel-Pineros, G.; Reyes, A. Computational tools for the analysis of uncultivated phage genomes. Microbiol. Mol. Biol. Rev. 2022, 86, e00004-21. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Yuan, W.G.; Shang, J.; Shi, Y.H.; Yang, L.L.; Liu, M.; Zhu, P.; Jin, T.; Sun, Y.; Yuan, L.H. Virus classification for viral genomic fragments using PhaGCN2. Brief. Bioinform. 2023, 24, bbac505. [Google Scholar] [CrossRef]
- Cebeci, A.; Türe, M.; Alemdağ, M.; Altinok, I. Whole genome sequence of a novel bacteriophage APT65 infecting Aeromonas hydrophila. PHAGE 2023, 4, 46–50. [Google Scholar] [CrossRef]
- Tynecki, P.; Guziński, A.; Kazimierczak, J.; Jadczuk, M.; Dastych, J.; Onisko, A. PhageAI—Bacteriophage life cycle recognition with machine learning and natural language processing. bioRXiv 2020. bioRXiv:198606. [Google Scholar]
- Verheust, C.; Pauwels, K.; Mahillon, J.; Helinski, D.R.; Herman, P. Contained use of bacteriophages: Risk assessment and biosafety recommendations. Appl. Biosaf. 2010, 15, 32–44. [Google Scholar] [CrossRef]
- Sanz-Gaitero, M.; Seoane-Blanco, M.; van Raaij, M.J. Structure and function of bacteriophages. In Bacteriophages, 1st ed.; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer: Cham, Switzerland, 2021; Volume 1, pp. 19–91. [Google Scholar]
- Aksyuk, A.A.; Rossmann, M.G. Bacteriophage assembly. Viruses 2011, 3, 172–203. [Google Scholar] [CrossRef]
- Chaitanya, K.V. Structure and organization of virus genomes. In Genome and Genomics: From Archaea to Eukaryotes, 1st ed.; Springer: Singapore, 2019; Volume 1, pp. 1–30. [Google Scholar]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Orlova, E. Bacteriophages and their structural organisation. In Bacteriophages, 1st ed.; Kurtboke, I., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 3–30. [Google Scholar]
- Lauman, P.; Dennis, J.J. Advances in phage therapy: Targeting the Burkholderia cepacia complex. Viruses 2021, 13, 1331. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Freidy, S.; Hanafin, P.O.; Braunstein, M.; Rao, G.G. Reapproaching old treatments: Considerations for PK/PD studies on phage therapy for bacterial respiratory infections. Clin. Pharmacol. Ther. 2021, 109, 1443–1456. [Google Scholar] [CrossRef]
- Liu, Y.; Demina, T.A.; Roux, S.; Aiewsakun, P.; Kazlauskas, D.; Simmonds, P.; Prangishvili, D.; Oksanen, H.M.; Krupovic, M. Diversity, taxonomy, and evolution of archaeal viruses of the class Caudoviricetes. PLoS Biol. 2021, 19, e3001442. [Google Scholar] [CrossRef]
- Dutilh, B.E.; Varsani, A.; Tong, Y.; Simmonds, P.; Sabanadzovic, S.; Rubino, L.; Roux, S.; Muñoz, A.R.; Lood, C.; Lefkowitz, E.J.; et al. Perspective on taxonomic classification of uncultivated viruses. Curr. Opin. Virol. 2021, 51, 207–215. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses. Available online: https://ictv.global/ (accessed on 30 April 2025).
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; de Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A hundred years of bacteriophages: Can phages replace antibiotics in agriculture and aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.-L.; Jin, M. The life cycle transitions of temperate phages: Regulating factors and potential ecological implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef]
- Leprince, A.; Mahillon, J. Phage adsorption to Gram–positive bacteria. Viruses 2023, 15, 196. [Google Scholar] [CrossRef]
- Dupont, K.; Janzen, T.; Vogensen, F.K.; Josephsen, J.; Stuer-Lauridsen, B. Identification of Lactococcus lactis genes required for bacteriophage adsorption. Appl. Environ. Microb. 2004, 70, 5825–5832. [Google Scholar] [CrossRef]
- Yamaki, S.; Yamazaki, K.; Kawai, Y. Broad host range bacteriophage, EscoHU1, infecting Escherichia coli O157:H7 and Salmonella enterica: Characterization, comparative genomics, and applications in food safety. Int. J. Food Microbiol. 2022, 372, 109680. [Google Scholar] [CrossRef]
- Golonka, R.; Yeoh, B.S.; Vijay-Kumar, M. The iron tug–of–war between bacterial siderophores and innate immunity. J. Innate Immun. 2019, 11, 249–262. [Google Scholar] [CrossRef]
- Dunne, M.; Hupfeld, M.; Klumpp, J.; Loessner, M.J. Molecular basis of bacterial host interactions by Gram–positive targeting bacteriophages. Viruses 2018, 10, 397. [Google Scholar] [CrossRef]
- Stone, E.; Campbell, K.; Grant, I.; McAuliffe, O. Understanding and exploiting phage–host interactions. Viruses 2019, 11, 567. [Google Scholar] [CrossRef]
- Millen, A.M.; Romero, D.A. Genetic determinants of lactococcal c2 viruses for host infection and their role in phage evolution. J. Gen. Virol. 2016, 97, 1998–2007. [Google Scholar] [CrossRef]
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef]
- Correa, A.M.S.; Howard-Varona, C.; Coy, S.R.; Buchan, A.; Sullivan, M.B.; Weitz, J.S. Revisiting the rules of life for viruses of microorganisms. Nat. Rev. Microbiol. 2021, 19, 501–513. [Google Scholar] [CrossRef]
- Mäntynen, S.; Laanto, E.; Oksanen, H.M.; Poranen, M.M.; Díaz-Muñoz, S.L. Black box of phage–bacterium interactions: Exploring alternative phage infection strategies. Open Biol. 2021, 11, 210188. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage therapy in the resistance era: Where do we stand and where are we going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the immune system. Ann. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Samtlebe, M.; Denis, S.; Chalancon, S.; Atamer, Z.; Wagner, N.; Neve, H.; Franz, C.; Schmidt, H.; Blanquet-Diot, S.; Hinrichs, J. Bacteriophages as modulator for the human gut microbiota: Release from dairy food systems and survival in a dynamic human gastrointestinal model. LWT Food Sci. Technol. 2018, 91, 235–241. [Google Scholar] [CrossRef]
- Blanco-Picazo, P.; Fernández-Orth, D.; Brown-Jaque, M.; Miró, E.; Espinal, P.; Rodríguez-Rubio, L.; Muniesa, M.; Navarro, F. Unravelling the consequences of the bacteriophages in human samples. Sci. Rep. 2020, 10, 6737. [Google Scholar] [CrossRef]
- Podlacha, M.; Grabowski, Ł.; Kosznik-Kwaśnicka, K.; Zdrojewska, K.; Stasiłojć, M.; Węgrzyn, G.; Węgrzyn, A. Interactions of bacteriophages with animal and human organisms—Safety issues in the light of phage therapy. Int. J. Mol. Sci. 2021, 22, 8937. [Google Scholar] [CrossRef]
- Średnicka, P.; Roszko, M.Ł.; Popowski, D.; Kowalczyk, M.; Wójcicki, M.; Emanowicz, P.; Szczepańska, M.; Kotyrba, D.; Juszczuk-Kubiak, E. Effect of in vitro cultivation on human gut microbiota composition using 16S rDNA amplicon sequencing and metabolomics approach. Sci. Rep. 2023, 13, 3026. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes. 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef]
- Rowan-Nash, A.D.; Korry, B.J.; Mylonakis, E.; Belenky, P. Cross–domain and viral interactions in the microbiome. Microbiol. Mol. Biol. Rev. 2019, 83, 10–1128. [Google Scholar] [CrossRef]
- Hsu, C.L.; Duan, Y.; Fouts, D.E.; Schnabl, B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J. Hepatol. 2021, 75, 1465–1475. [Google Scholar] [CrossRef]
- Manrique, P.; Dills, M.; Young, M.J. The human gut phage community and its implications for health and disease. Viruses 2017, 9, 141. [Google Scholar] [CrossRef]
- Rybicka, I.; Kaźmierczak, Z. The human phageome: Niche-specific distribution of bacteriophages and their clinical implications. Appl. Environ. Microbiol. 2025, e0178824. [Google Scholar] [CrossRef]
- Townsend, E.M.; Kelly, L.; Muscatt, G.; Box, J.D.; Hargraves, N.; Lilley, D.; Jameson, E. The human gut phageome: Origins and roles in the human gut microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 643214. [Google Scholar] [CrossRef]
- Tariq, M.A.; Everest, F.L.C.; Cowley, L.A.; De Soyza, A.; Holt, G.S.; Bridge, S.H.; Perry, A.; Perry, J.D.; Bourke, S.J.; Cummings, S.P.; et al. A metagenomic approach to characterize temperate bacteriophage populations from Cystic Fibrosis and non–Cystic Fibrosis bronchiectasis patients. Front. Microbiol. 2015, 18, 97. [Google Scholar] [CrossRef]
- Aleshkin, A.V.; Ershova, O.N.; Volozhantsev, N.V.; Svetoch, E.A.; Popova, A.V.; Rubalskii, E.O.; Borzilov, A.I.; Aleshkin, V.A.; Afanas’ev, S.S.; Karaulov, A.V.; et al. Phagebiotics in treatment and prophylaxis of healthcare–associated infections. Bacteriophage 2016, 6, e1251379. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Owczarek, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Łodej, N.; Górski, A. Phage–phagocyte interactions and their implications for phage application as therapeutics. Viruses 2017, 9, 150. [Google Scholar] [CrossRef]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef]
- Zimecki, M.; Weber-Dąbrowska, B.; Łusiak-Szelachowska, M.; Mulczyk, M.; Boratyński, J.; Poźniak, G.; Syper, D.; Górski, A. Bacteriophages provide regulatory signals in mitogen–induced murine splenocyte proliferation. Cell. Mol. Biol. Lett. 2003, 8, 699–711. [Google Scholar]
- Shuwen, H.; Kefeng, D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes 2022, 14, 2113717. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Jończyk-Matysiak, E.; Węgrzyn, A.; Węgrzyn, G.; Górski, A. Temperate bacteriophages—The powerful indirect modulators of eukaryotic cells and immune functions. Viruses 2021, 13, 1013. [Google Scholar] [CrossRef]
- Jaglan, A.B.; Anand, T.; Verma, R.; Vashisth, M.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Tripathi, B.N. Tracking the phage trends: A comprehensive review of applications in therapy and food production. Front. Microbiol. 2022, 13, 993990. [Google Scholar] [CrossRef]
- Rai, S.; Kaur, B.; Singh, P.; Singh, A.; Benjakul, S.; Vijay Kumar Reddy, S.; Nagar, V.; Tyagi, A. Perspectives on phage therapy for health management in aquaculture. Aquacult. Int. 2023, 32, 1349–1393. [Google Scholar] [CrossRef]
- Pradal, I.; Casado, A.; del Rio, B.; Rodriguez-Lucas, C.; Fernandez, M.; Alvarez, M.A.; Ladero, V. Enterococcus faecium bacteriophage vB_EfaH_163, a new member of the Herelleviridae family, reduces the mortality associated with an E. faecium vanR clinical isolate in a Galleria mellonella animal model. Viruses 2023, 15, 179. [Google Scholar] [CrossRef]
- Arumugam, S.N.; Manohar, P.; Sukumaran, S.; Sadagopan, S.; Loh, B.; Leptihn, S.; Nachimuthu, R. Antibacterial efficacy of lytic phages against multidrug–resistant Pseudomonas aeruginosa infections in bacteraemia mice models. BMC Microbiol. 2022, 22, 187. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial biofilm destruction: A focused review on the recent use of phage–based strategies with other antibiofilm agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against pathogenic bacterial biofilms and biofilm–based infections: A review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef]
- Blasco, L.; Bleriot, I.; González de Aledo, M.; Fernández-García, L.; Pacios, O.; Oliveira, H.; López, M.; Ortiz-Cartagena, C.; Fernández-Cuenca, F.; Pascual, Á.; et al. Development of an anti—Acinetobacter baumannii biofilm phage cocktail: Genomic adaptation to the host. Antimicrob. Agents Chemother. 2022, 66, e0192321. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.P. European regulatory aspects of phage therapy: Magistral phage preparations. Curr. Opin. Virol. 2021, 52, 24–29. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Han, K.; Wang, L.; Cao, Y.; Ma, D.; Wang, X. A polyvalent broad–spectrum Escherichia phage Tequatrovirus EP01 capable of controlling Salmonella and Escherichia coli contamination in foods. Viruses 2022, 14, 286. [Google Scholar] [CrossRef]
- Ortiz Charneco, G.; de Waal, P.P.; van Rijswijck, I.M.; van Peij, N.N.; van Sinderen, D.; Mahony, J. Bacteriophages in the dairy industry: A problem solved? Annu. Rev. Food Sci. Technol. 2023, 14, 367–385. [Google Scholar] [CrossRef]
- Olejnik-Schmidt, A.; Pietrzak, B.; Kawacka, I.; Malak, K.; Wawrzyniak, W.; Schmidt, M. A simple method for assessing diversity and dynamics of microbial community: Comparison of dairy phages from industrial and spontaneous fermentation. Appl. Sci. 2021, 11, 8915. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Islam, M.S.; Yan, T.; Zhou, Y.; Liang, L.; Connerton, I.F.; Deng, K.; Li, J. Application of a novel phage vB_SalS–LPSTLL for the biological control of Salmonella in foods. Food Res. Int. 2021, 147, 110492. [Google Scholar] [CrossRef]
- Cristobal-Cueto, P.; García-Quintanilla, A.; Esteban, J.; García-Quintanilla, M. Phages in food industry biocontrol and bioremediation. Antibiotics 2021, 10, 786. [Google Scholar] [CrossRef]
- Chen, Q.; Dharmaraj, T.; Cai, P.C.; Burgener, E.B.; Haddock, N.L.; Spakowitz, A.J.; Bollyky, P.L. Bacteriophage and bacterial susceptibility, resistance, and tolerance to antibiotics. Pharmaceutics 2022, 14, 1425. [Google Scholar] [CrossRef]
- Pfeifer, E.; Bonnin, A.R.; Rocha, E.P. Phage–plasmids spread antibiotic resistance genes through infection and lysogenic conversion. mBio 2022, 13, e0185122. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.-T.; Wu, V.C.H. The role of temperate phages in bacterial pathogenicity. Microorganisms 2023, 11, 541. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Ren, J.; Liang, L.; Li, J.; Niu, S.; Wu, X.; Zhao, Y.; Gao, S.; Yan, F.; et al. RAP44 phage integrase–guided 50K genomic island integration in Riemerella anatipestifer. Front. Vet. Sci. 2022, 9, 961354. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Hui, J.G.; Kjelleberg, S.; Rakonjac, J.; McDougald, D.; Rice, S.A. Big things in small packages: The genetics of filamentous phage and effects on fitness of their host. FEMS Microbiol. Rev. 2015, 39, 465–487. [Google Scholar] [CrossRef]
- Khouadja, S.; Roque, A.; Gonzalez, M.; Furones, D. Vibrio pathogenicity island and phage CTX genes in Vibrio alginolyticus isolated from different aquatic environments. J. Water Health 2022, 20, 1469–1478. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Z.; Ren, S.; Alali, W.; Chen, L. Rapid and visualized detection of virulence–related genes of Vibrio cholerae in water and aquatic products by loop–mediated isothermal amplification. J. Food Prot. 2022, 85, 44–53. [Google Scholar] [CrossRef]
- Faruque, S.M.; Mekalanos, J.J. Phage–bacterial interactions in the evolution of toxigenic Vibrio cholerae. Virulence 2012, 3, 556–565. [Google Scholar] [CrossRef]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage therapy: A different approach to fight bacterial infections. Biol. Targets Ther. 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Azam, A.H.; Tanji, Y. Bacteriophage–host arm race: An update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 2121–2131. [Google Scholar] [CrossRef]
- Jariah, R.O.A.; Hakim, M.S. Interaction of phages, bacteria, and the human immune system: Evolutionary changes in phage therapy. Rev. Med. Virol. 2019, 29, e2055. [Google Scholar] [CrossRef]
- Isaev, A.B.; Musharova, O.S.; Severinov, K.V. Microbial arsenal of antiviral defenses—Part I. Biochemistry 2021, 86, 319–337. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawooud, A.; Rezk, N.; Makky, S.; Safwat, A.; Richards, P.J.; El-Shibiny, A. How to train your phage: The recent efforts in phage training. Biologics 2021, 1, 70–88. [Google Scholar] [CrossRef]
- Kelly, A.; Arrowsmith, T.J.; Went, S.C.; Blower, T.R. Toxin–antitoxin systems as mediators of phage defence and the implications for abortive infection. Cur. Opin. Microbiol. 2023, 73, 102293. [Google Scholar] [CrossRef]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Birkholz, N.; Jackson, S.A.; Fagerlund, R.D.; Fineran, P.C. A mobile restriction–modification system provides phage defence and resolves an epigenetic conflict with an antagonistic endonuclease. Nucleic Acids Res. 2022, 50, 3348–3361. [Google Scholar] [CrossRef]
- Eriksen, R.S.; Malhotra, N.; Seshasayee, A.S.N.; Sneppen, K.; Krishna, S. Emergence of networks of shared restriction–modification systems in phage–bacteria ecosystems. J. Biosci. 2022, 47, 38. [Google Scholar] [CrossRef]
- Ambroa, A.; Blasco, L.; Lopez, M.; Pacios, O.; Bleriot, I.; Fernandez-Garcia, L.; Gonzalez de Aledo, M.; Ortiz-Cartagena, C.; Millard, A.; Tomas, M. Genomic analysis of molecular bacterial mechanisms of resistance to phage infection. Front. Microbiol. 2021, 12, 784949. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Steens, J.A.; Staals, R.H.J.; Westra, E.R.; van Houte, S. Coevolution between bacterial CRISPR–Cas systems and their bacteriophages. Cell Host Microbe 2021, 29, 715–725. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, H.; Cui, Y.; Cong, L.; Zhang, D. Application of different types of CRISPR/Cas–based systems in bacteria. Microb. Cell Fact. 2020, 19, 172. [Google Scholar] [CrossRef]
- Nussenzweig, P.M.; Marraffini, L.A. Molecular mechanisms of CRISPR–Cas immunity in bacteria. Annu. Rev. Genet. 2020, 54, 93–120. [Google Scholar] [CrossRef]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM is a widespread bacterial defence system with broad anti–phage activities. Nat. Microbiol. 2018, 3, 90–98. [Google Scholar] [CrossRef]
- Bravo, J.P.; Aparicio-Maldonado, C.; Nobrega, F.L.; Brouns, S.J.; Taylor, D.W. Structural basis for broad anti–phage immunity by DISARM. Nat. Commun. 2022, 13, 2987. [Google Scholar] [CrossRef]
- Morgan, T.; Rezende, R.R.d.; Lima, T.T.M.; Souza, F.d.O.; Alfenas-Zerbini, P. Genomic analysis unveils the pervasiveness and diversity of prophages infecting Erwinia species. Pathogens 2023, 12, 44. [Google Scholar] [CrossRef]
- Tesson, F.; Bernheim, A. Synergy and regulation of antiphage systems: Toward the existence of a bacterial immune system? Curr. Opin. Microbiol. 2023, 71, 102238. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, Z.; Zhu, Z.; Zhang, J.; Wu, Q.; Xue, L.; Wang, J.; Ding, Y. Recent advances in phage defense systems and potential overcoming strategies. Biotechnol. Adv. 2023, 65, 108152. [Google Scholar] [CrossRef]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and function of phage encoded depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef]
- Sun, C.L.; Barrangou, R.; Thomas, B.C.; Horvath, P.; Fremaux, C.; Banfield, J.F. Phage mutations in response to CRISPR diversification in a bacterial population. Environ. Microbiol. 2013, 15, 463–470. [Google Scholar] [CrossRef]
- Rostøl, J.T.; Marraffini, L. (Ph)ighting phages: How bacteria resist their parasites. Cell Host Microbe 2019, 25, 184–194. [Google Scholar] [CrossRef]
- Kȩsik-Szeloch, A.; Drulis-Kawa, Z.; Weber-Dąbrowska, B.; Kassner, J.; Majkowska-Skrobek, G.; Augustyniak, D.; Łusiak-Szelachowska, M.; Zaczek, M.; Górski, A.; Kropinski, A.M. Characterising the biology of novel lytic bacteriophages infecting multidrug resistant Klebsiella pneumoniae. Virol. J. 2013, 10, 100. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Rifat, D.; Wright, N.T.; Varney, K.M.; Weber, D.J.; Black, L.W. Restriction endonuclease inhibitor IPI* of bacteriophage T4: A novel structure for a dedicated target. J. Mol. Biol. 2008, 375, 720–734. [Google Scholar] [CrossRef]
- Samson, J.E.; Magadán, A.H.; Sabri, M.; Moineau, S. Revenge of the phages: Defeating bacterial defences. Nat. Rev. Microbiol. 2013, 11, 675–687. [Google Scholar] [CrossRef]
- Blower, T.R.; Evans, T.J.; Przybilski, R.; Fineran, P.C.; Salmond, G.P.C. Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet. 2012, 8, e1003023. [Google Scholar] [CrossRef]
- Połaska, M.; Sokołowska, B. Bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef]
- Goodridge, L.D.; Bisha, B. Phage–based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 2011, 1, 130–137. [Google Scholar] [CrossRef]
- Abraha, H.B.; Kim, K.P.; Sbhatu, D.B. Bacteriophages for detection and control of foodborne bacterial pathogens—The case of Bacillus cereus and their phages. J. Food Saf. 2021, 43, e12906. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. The perfect bacteriophage for therapeutic applications—A quick guide. Antibiotics 2019, 8, 126. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A.J.F. Bacteriophage procurement for therapeutic purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef]
- Vázquez, R.; Díez-Martínez, R.; Domingo-Calap, P.; García, P.; Gutiérrez, D.; Muniesa, M.; Ruiz-Ruigómez, M.; Sanjuán, R.; Tomás, M.; Tormo-Mas, M.Á.; et al. Essential topics for the regulatory consideration of phages as clinically valuable therapeutic agents: A perspective from Spain. Microorganisms 2022, 10, 717. [Google Scholar] [CrossRef]
- Bumunang, E.W.; Zaheer, R.; Niu, D.; Narvaez-Bravo, C.; Alexander, T.; McAllister, T.A.; Stanford, K. Bacteriophages for the targeted control of foodborne pathogens. Foods 2023, 12, 2734. [Google Scholar] [CrossRef]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr. Opin. Biotechnol. 2021, 68, 60–71. [Google Scholar] [CrossRef]
- Farooq, T.; Hussain, M.D.; Shakeel, M.T.; Tariqjaveed, M.; Aslam, M.N.; Naqvi, S.A.H.; Amjad, R.; Tang, Y.; She, X.; He, Z. Deploying Viruses against Phytobacteria: Potential Use of Phage Cocktails as a Multifaceted Approach to Combat Resistant Bacterial Plant Pathogens. Viruses 2022, 14, 171. [Google Scholar] [CrossRef]
- Frampton, R.A.; Pitman, A.R.; Fineran, P.C. Advances in bacteriophage-mediated control of plant pathogens. Int. J. Microbiol. 2012, 2012, 326452. [Google Scholar] [CrossRef]
- Liu, S.; Quek, S.Y.; Huang, K. An Ecofriendly Nature-Inspired Microcarrier for Enhancing Delivery, Stability, and Biocidal Efficacy of Phage-Based Biopesticides. Small 2024, 20, 2403465. [Google Scholar] [CrossRef]
- Siyanbola, K.F.; Ejiohuo, O.; Ade-adekunle, O.A.; Adekunle, F.O.; Onyeaka, H.; Furr, C.-L.L.; Hodges, F.E.; Carvalho, P.; Oladipo, E.K. Bacteriophages: Sustainable and Effective Solution for Climate-Resilient Agriculture. Sustain. Microbiol. 2024, 1, qvae025. [Google Scholar] [CrossRef]
- Kumar, P.; Minnatullah, M.; Dhakar, M.; Parihar, P.; Choudhary, M.K.; Rao, B.S.; Mehta, Y.H.; Meena, M.; Yadav, A. Harnessing Bacteriophages for Sustainable Plant Disease Management: A Review of their Potential and Market Prospects. J. Adv. Biol. Biotechnol. 2025, 28, 708–728. [Google Scholar] [CrossRef]
- Yang, L.; Zhong, W.; Tang, T.; He, M.; Zhang, T.; Zhou, B.; Yin, Y.; Guo, J.; Gao, Z. Phage-Based Biocontrol Strategies and Application in Aquatic Animal Disease Prevention and Control. Rev. Aquac. 2025, 17, e70024. [Google Scholar] [CrossRef]
- Jordá, J.; Lorenzo-Rebenaque, L.; Montoro-Dasi, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry. Animals 2023, 13, 3826. [Google Scholar] [CrossRef]
- Berry, E.D.; Wells, J.E. Reducing foodborne pathogen persistence and transmission in animal production environments: Challenges and opportunities. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef]
- Doyle, M.P.; Erickson, M.C. Opportunities for mitigating pathogen contamination during on-farm food production. Int. J. Food Microbiol. 2012, 152, 54–74. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phage Therapy in Livestock and Companion Animals. Antibiotics 2021, 10, 559. [Google Scholar] [CrossRef]
- Appiah, M.O.; Wang, J.; Lu, W. Microflora in the Reproductive Tract of Cattle: A Review. Agriculture 2020, 10, 232. [Google Scholar] [CrossRef]
- Islam, M.R.; Martinez-Soto, C.E.; Lin, J.T.; Khursigara, C.M.; Barbut, S.; Anany, H. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 2021, 63, 3097–3129. [Google Scholar] [CrossRef]
- Fokas, R.; Giormezis, N.; Vantarakis, A. Synergistic Approaches to Foodborne Pathogen Control: A Narrative Review of Essential Oils and Bacteriophages. Foods 2025, 14, 1508. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Alsayeqh, A.F.; Aqib, A.I. Role of Bacteriophages for Optimized Health and Production of Poultry. Animals 2022, 12, 3378. [Google Scholar] [CrossRef]
- Wahab, A.A.-E.; Basiouni, S.; El-Seedi, H.R.; Ahmed, M.F.E.; Bielke, L.R.; Hargis, B.; Tellez-Isaias, G.; Eisenreich, W.; Lehnherr, H.; Kittler, S.; et al. An overview of the use of bacteriophages in the poultry industry: Successes, challenges, and possibilities for overcoming breakdowns. Front. Microbiol. 2023, 14, 1136638. [Google Scholar]
- Pereira, C.; Duarte, J.; Costa, P.; Braz, M.; Almeida, A. Bacteriophages in the Control of Aeromonas sp. in Aquaculture Systems: An Integrative View. Antibiotics 2022, 11, 163. [Google Scholar] [CrossRef]
- Sonne, M.; Ghayal, N.; Ahiwale, S. Therapeutic application of bacteriophages against Aeromonas spp. mediated diseases in aquaculture: A critical review. IOSR J. Biotechnol. Biochem. 2023, 9, 42–53. [Google Scholar]
- Proteon Pharmaceuticals. Available online: https://www.proteonpharma.com/ (accessed on 30 April 2025).
- Sevilla-Navarro, S.; Torres-Boncompte, J.; Garcia-Llorens, J.; Bernabéu-Gimeno, M.; Domingo-Calap, P.; Catalá-Gregori, P. Fighting Salmonella Infantis: Bacteriophage-driven cleaning and disinfection strategies for broiler farms. Front. Microbiol. 2024, 15, 1401479. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.-J. Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yuan, L.; Chen, C.; Zhang, Y.; Yang, Z. Bacteriophages: A weapon against mixed-species biofilms in the food processing environment. J. Appl. Microbiol. 2022, 133, 2107–2121. [Google Scholar] [CrossRef]
- Bhandare, S.; Goodridge, L. Bacteriophages as bio-sanitizers in food production and healthcare settings. In Bacteriophages; Harper, D., Abedon, S., Burrowes, B., McConville, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 769–788. [Google Scholar]
- Sulakvelidze, A. Using Lytic Bacteriophages to Eliminate or Significantly Reduce Contamination of Food by Foodborne Bacterial Pathogens. J. Sci. Food Agric. 2013, 93, 3137–3146. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kajla, P.; Lather, D.; Chaudhary, N.; Dangi, P.; Singh, P.; Pandiselvam, R. Bacteriophages: A potential game changer in food processing industry. Crit. Rev. Biotechnol. 2024, 44, 1325–1349. [Google Scholar] [CrossRef]
- Álvarez, B.; Biosca, E.G. Harnessing the Activity of Lytic Bacteriophages to Foster the Sustainable Development Goals and the “One Health” Strategy. Viruses 2025, 17, 549. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef]
- Ngene, A.C.; Aguiyi, J.C.; Uzal, U.; Egbere, J.O.; Onyimba, I.A.; Umera, A.E.; Nnadi, N.E. Bacteriophages as Bio-control agent against Food-Borne Pathogen E. coli O157: H7. IOSR J. Pharm. Biol. Sci. 2020, 15, 23–36. [Google Scholar]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a phage cocktail as a potential biocontrol agent against saprophytic bacteria in ready–to–eat plant–based food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef]
- Wójcicki, M.; Żuwalski, A.W.; Świder, O.; Gientka, I.; Shymialevich, D.; Błażejak, S. The use of bacteriophages against saprophytic mesophilic bacteria in minimally processed food. Acta Sci. Pol. Technol. Aliment. 2021, 20, 473–484. [Google Scholar]
- Kuek, M.; McLean, S.K.; Palombo, E.A. Control of Escherichia coli in Fresh-Cut Mixed Vegetables Using a Combination of Bacteriophage and Carvacrol. Antibiotics 2023, 12, 1579. [Google Scholar] [CrossRef]
- Pintor-Cora, A.; Carpintero, A.; Alegría, Á.; Giannis, A.; Lopez-Díaz, T.-M.; Santos, J.A.; Rodríguez-Calleja, J.M. A Novel Bacteriophage Targeting mcr-9 Enterobacter kobei with Potential Application in Fresh Leafy Greens. Appl. Microbiol. 2025, 5, 25. [Google Scholar] [CrossRef]
- Shymialevich, D.; Wójcicki, M.; Błażejak, S. Wykorzystanie fagów litycznych do ograniczenia liczby pałeczek Salmonella w roślinnej matrycy żywnościowej [Using lytic phages to reduce the number of Salmonella rods in plant food matrix]. Zywn. Nauk. Technol. Ja. 2021, 28, 61–77. [Google Scholar]
- Wójcicki, M.; Swider, O.; Średnicka, P.; Shymialevich, D.; Ilczuk, T.; Koperski, Ł.; Cieslak, H.; Sokołowska, B.; Juszczuk-Kubiak, E. Newly Isolated Virulent Salmophages for Biocontrol of Multidrug-Resistant Salmonella in Ready-to-Eat Plant-Based Food. Int. J. Mol. Sci. 2023, 24, 10134. [Google Scholar] [CrossRef]
- Wong, C.W.Y.; Wang, S. Efficacy of Repeated Applications of Bacteriophages on Salmonella enterica-Infected Alfalfa Sprouts during Germination. Pathogens 2022, 11, 1156. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, S.; Na, J.B.; Kim, Y.B.; Lee, K.M.; Park, S.Y.; Kim, J.H. Characterization of Broad Spectrum Bacteriophage vB ESM-pEJ01 and Its Antimicrobial Efficacy Against Shiga Toxin-Producing Escherichia coli in Green Juice. Microorganisms 2025, 13, 103. [Google Scholar] [CrossRef]
- Lu, Y.T.; Ma, Y.; Wong, C.W.; Wang, S. Characterization and application of bacteriophages for the biocontrol of Shiga-toxin producing Escherichia coli in Romaine lettuce. Food Control 2022, 140, 109109. [Google Scholar] [CrossRef]
- Byun, K.-H.; Han, S.H.; Choi, M.W.; Park, S.H.; Ha, S.-D. Isolation, Characterization, and Application of Bacteriophages to Reduce and Inhibit Listeria monocytogenes in Celery and Enoki Mushroom. Food Control 2022, 135, 108826. [Google Scholar] [CrossRef]
- Stone, E.; Lhomet, A.; Neve, H.; Grant, I.R.; Campbell, K.; McAuliffe, O. Isolation and Characterization of Listeria monocytogenes Phage vB_LmoH_P61, a Phage With Biocontrol Potential on Different Food Matrices. Front. Sustain. Food Syst. 2020, 4, 521645. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, M.; Wang, Y.; Yang, Z.; Ye, M.; Wu, L.; Bao, H.; Pang, M.; Zhou, Y.; Wang, R. Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready-to-eat food. Food Control 2020, 108, 106830. [Google Scholar] [CrossRef]
- Wójcicki, M.; Średnicka, P.; Zapaśnik, A.; Emanowicz, P. Risk Assessment of Foodborne and Waterborne Viruses, Detection Methods, and Possibilities of Eradication during Non-thermal Food Processing. Food Biotechnol. Agric. Sci. 2024, 78, 47–55. [Google Scholar]
- Raza, S.; Bończak, B.; Atamas, N.; Karpińska, A.; Ratajczyk, T.; Łoś, M.; Hołyst, R.; Paczesny, J. The activity of indigo carmine against bacteriophages: An edible antiphage agent. Appl. Microbiol. Biotechnol. 2025, 109, 24. [Google Scholar] [CrossRef]
- Fernández, L.; Escobedo, S.; Gutiérrez, D.; Portilla, S.; Martínez, B.; García, P.; Rodríguez, A. Bacteriophages in the Dairy Environment: From Enemies to Allies. Antibiotics 2017, 6, 27. [Google Scholar] [CrossRef]
- Szczepankowska, A.K.; Górecki, R.K.; Kołakowski, P.; Bardowski, J.K. Lactic acid bacteria resistance to bacteriophage and prevention techniques to lower phage contamination in dairy fermentation. In Lactic Acid Bacteria—R&D for Food, Health and Livestock Purposes, 1st ed.; Marcelino Kongo, J., Ed.; InTech: Rijeka, Croatia, 2013; pp. 23–71. [Google Scholar]
- Pujato, S.; Quiberoni, A.; Mercanti, D. Bacteriophages on dairy foods. J. Appl. Microbiol. 2018, 126, 14–30. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Starter cultures. In Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2017; pp. 121–183. [Google Scholar]
- Zalewska-Piątek, B. Phage Therapy—Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Szermer-Olearnik, B.; Boratyński, J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS ONE 2015, 10, e0122672. [Google Scholar] [CrossRef]
- Hietala, V.; Horsma-Heikkinen, J.; Carron, A.; Skurnik, M.; Kiljunen, S. The removal of endo- and enterotoxins from bacteriophage preparations. Front. Microbiol. 2019, 10, 1674. [Google Scholar] [CrossRef]
- Abdelsattar, A.; Dawoud, A.; Makky, S.; Nofal, R.; Aziz, R.; El-Shibiny, A. Bacteriophages: From isolation to application. Curr. Pharm. Biotechnol. 2022, 23, 337–360. [Google Scholar] [CrossRef]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.B.; Bishoyi, A.K. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Marks, T.; Sharp, R. Bacteriophages and biotechnology: A review. J. Chem. Technol. Biotechnol. 2000, 75, 6–17. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Merabishvili, M.; Vergauwen, B.; Lavigne, R.; Mario Vaneechoutte, M. A comparative study of different strategies for removal of endotoxins from bacteriophage preparations. J. Microbiol. Methods 2017, 132, 153–159. [Google Scholar] [CrossRef]
- Ali, J.; Rafiq, Q.; Ratcliffe, E. A Scaled-down Model for the Translation of Bacteriophage Culture to Manufacturing Scale. Biotechnol. Bioeng. 2019, 116, 972–984. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Onsea, J.; Fintan Moriarty, T.; Pruidze, N.; Nadareishvili, L.; Dadiani, M.; Kutateladze, M.; Eliava, G. Bacteriophage therapy for human musculoskeletal and skin/soft tissue infections. Clin. Microbiol. Infect. 2023, 29, 695–701. [Google Scholar] [CrossRef]
- Liang, S.; Qi, Y.; Yu, H.; Sun, W.; Raza, S.H.A.; Alkhorayef, N.; Alkhalil, S.S.; Salama, E.E.A.; Zhang, L. Bacteriophage therapy as an application for bacterial infection in China. Antibiotics 2023, 12, 417. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]
- Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.-P. Processing phage therapy requests in a Brussels military hospital: Lessons identified. Viruses 2019, 11, 265. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Kłak, M.; Bubak, B.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Zaczek, M.; Fortuna, W.; Rogóz, P.; Letkiewicz, S.; et al. The effect of bacteriophage preparations on intracellular killing of bacteria by phagocytes. J. Immunol. Res. 2015, 2015, 482863. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of phage–based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of bacteriophages in the agro–food sector: A long way toward approval. Front. Cell. Infect. Microbiol. 2018, 8, 296. [Google Scholar] [CrossRef]
- Osei, E.K.; Mahony, J.; Kenny, J.G. From farm to fork: Streptococcus suis as a model for the development of novel phage–based biocontrol agents. Viruses 2022, 14, 1996. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage application for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the future with bacteriophages in agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef]
- Jakobsen, R.R.; Trinh, J.T.; Bomholtz, L.; Brok-Lauridsen, S.K.; Sulakvelidze, A.; Nielsen, D.S. A bacteriophage cocktail significantly reduces Listeria monocytogenes without deleterious impact on the commensal gut microbiota under simulated gastrointestinal conditions. Viruses 2022, 14, 190. [Google Scholar] [CrossRef]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef]
- Schooley, R.T. Exploring bacteriophage therapy for drug–resistant bacterial infections. Top. Antivir. Med. 2023, 31, 23. [Google Scholar]
- Cobián Güemes, A.G.; Le, T.; Rojas, M.I.; Jacobson, N.E.; Villela, H.; McNair, K.; Hung, S.-H.; Han, L.; Boling, L.; Octavio, J.C.; et al. Compounding Achromobacter phages for therapeutic applications. Viruses 2023, 15, 1665. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of a feed additive consisting on the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 infecting Salmonella Gallinarum B/00111 (Bafasal®) for all avian species (Proteon Pharmaceuticals SA). EFSA J. 2021, 19, e06534. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of a feed additive consisting of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 (Bafasal®) for all poultry (Proteon Pharmaceuticals S.A.). EFSA J. 2024, 22, e9132. [Google Scholar]
- EFSA. Evaluation of the safety and efficacy of ListexTM P100 for reduction of pathogens on different ready–to–eat (RTE) food products. EFSA Panel on Biological Hazards. EFSA J. 2016, 14, e04565. [Google Scholar]
- U.S. Food & Drug Administration GRAS Notice No. 198. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=198 (accessed on 20 February 2025).
- U.S. Food & Drug Administration GRAS Notice No. 218. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=218 (accessed on 20 February 2025).
- U.S. Food & Drug Administration GRAS Notice No. 528. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=528 (accessed on 20 February 2025).
- European Medicines Agency. Guideline on Quality, Safety and Efficacy of Veterinary Medicinal Products Specifically Designed for Phage Therapy; 13 October 2023, EMA/CVMP/NTWP/32862/2022; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- European Food Safety Authority (EFSA). EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2024, 22, e8912. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).