Impact of Influenza on Children in a Referral Hospital in Mexico City: Clinical Burden and Predictors of Mechanical Ventilation
Abstract
1. Introduction
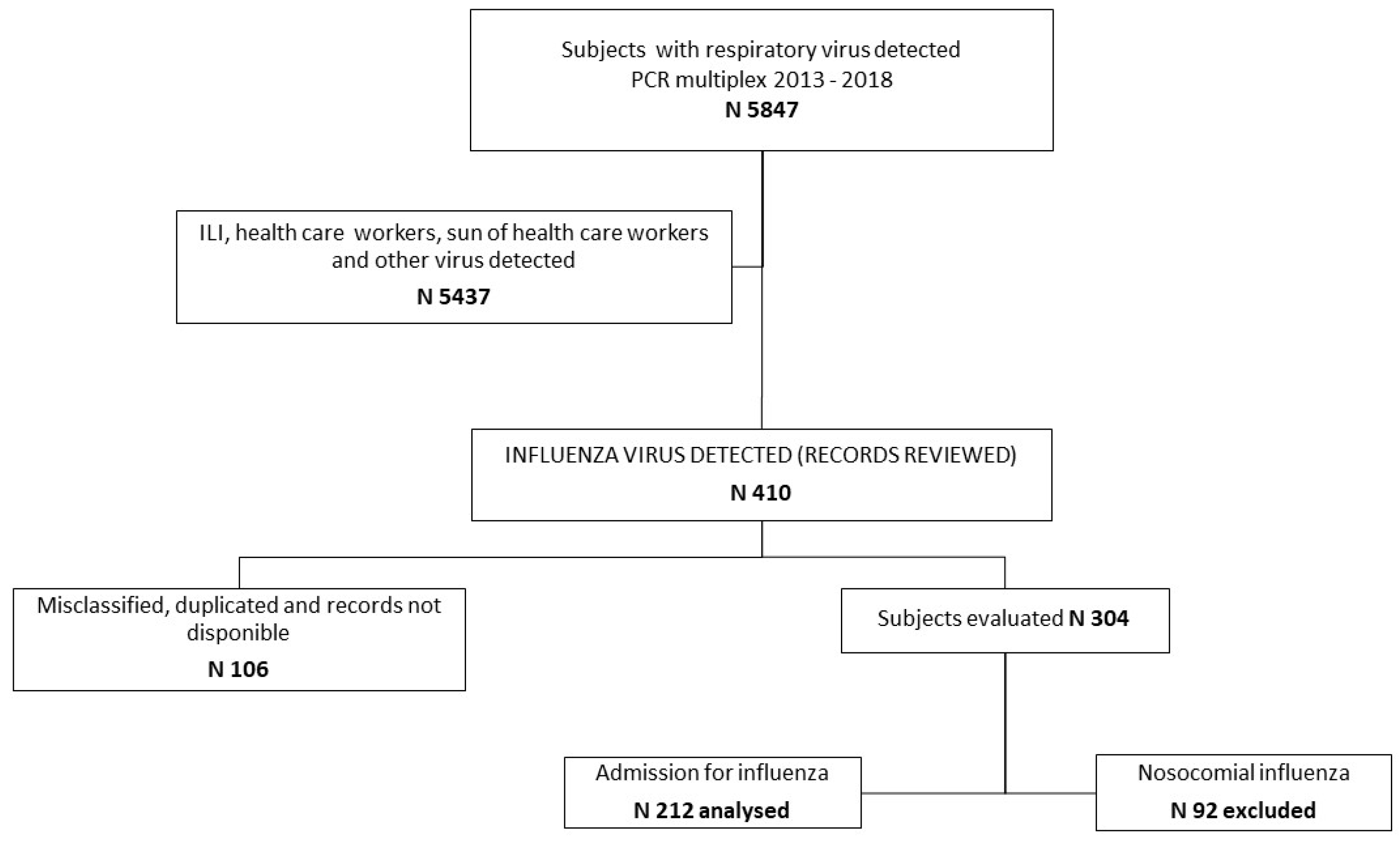
2. Materials and Methods
2.1. Study Population and Definitions
2.2. Microbiology
2.3. Ethics Statement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Influenza (Seasonal). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 26 December 2024).
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.A.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Lafond, K.E.; Nair, H.; Rasooly, M.H.; Valente, F.; Booy, R.; Rahman, M.; Kitsutani, P.; Yu, H.; Guzman, G.; Coulibaly, D.; et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Med. 2016, 13, e1001977. [Google Scholar] [CrossRef]
- Chong, C.Y.; Yung, C.F.; Gan, C.; Thio, S.T.; Tan, N.W.; Tee, N.W.; Lin, C.; Lin, R.T.; Thoon, K.C. The burden and clinical manifestation of hospitalized influenza among different pediatric age-groups in the tropics. Influenza Other Respir. Viruses 2020, 14, 46–54. [Google Scholar] [CrossRef]
- Neuzil, K.M.; Mellen, B.G.; Wright, P.F.; Mitchel, E.F., Jr.; Griffin, M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000, 342, 225–231. [Google Scholar] [CrossRef]
- Guzman Holst, A.; Gilberto Gomez, L.; Yolanda Cervantes Apolinar, M.; Huerta, G. 1667. Influenza A and B Co-Circulation and Burden: A 2018–2019 Influenza Season Analysis Using the National Active Surveillance Database in Mexico. Open Forum Infect. Dis. 2019, 6 (Suppl. S2), S609–S610. [Google Scholar] [CrossRef]
- Secretaría de Salud de la Ciudad de México. Informe Semanal de Vigilancia Epidemiológica: Influenza, Semana 1 del 2024; Dirección de Epidemiología y Medicina Preventiva: Ciudad de México, Mexico, 2024. [Google Scholar]
- Gentile, A.; Paget, J.; Bellei, N.; Torres, J.P.; Vazquez, C.; Laguna-Torres, V.A.; Plotkin, S. Influenza in Latin America: A report from the Global Influenza Initiative (GII). Vaccine 2019, 37, 2670–2678. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases. Recommendations for Prevention and Control of Influenza in Children, 2023–2024. Pediatrics 2023, 152, e2023063773. [Google Scholar] [CrossRef]
- Sikora, A.; Zahra, F. Nosocomial Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559312/ (accessed on 27 April 2023).
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Health Organ. 2018, 96, 122–128. [Google Scholar] [CrossRef]
- World Health Organization. Implementing the Integrated Sentinel Surveillance of Influenza and Other Respiratory Viruses of Epidemic and Pandemic Potential by the Global Influenza Surveillance and Response System: Standards and Operational Guidance. World Health Organization: Geneva, Switzerland, 2024. Available online: https://iris.who.int/handle/10665/379678 (accessed on 2 January 2025).
- CONAVE. Lineamientos Para la Vigilancia Epidemiológica de Influenza. 2012, pp. 1–33. Available online: https://www.gob.mx/cms/uploads/attachment/file/20769/lineamientos_para_la_vigilancia_de_influenza.pdf (accessed on 2 January 2025).
- Walter, J.M.; Corbridge, T.C.; Singer, B.D. Invasive Mechanical Ventilation. South. Med. J. 2018, 111, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Tsai, C.L.; Chang, J.; Hsu, T.C.; Lin, S.; Lee, C.C. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2018, 24, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Burden of Influenza. Available online: https://www.who.int/news-room/feature-stories/detail/the-burden-of-influenza (accessed on 30 March 2024).
- Potdar, V.; Vijay, N.; Mukhopadhyay, L.; Aggarwal, N.; Bhardwaj, S.D.; Choudhary, M.L.; Gupta, N.; Kaur, H.; Narayan, J.; Kumar, P.; et al. Pan-India influenza-like illness (ILI) and Severe acute respiratory infection (SARI) surveillance: Epidemiological, clinical and genomic analysis. Front. Public Health 2023, 11, 1218292. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Esquivel, J.; Mendoza-Cano, O.; Trujillo, X.; Huerta, M.; Ríos-Silva, M.; Lugo-Radillo, A.; Benites-Godínez, V.; Bricio-Barrios, J.A.; Ríos-Bracamontes, E.F.; Cárdenas-Rojas, M.I.; et al. Evaluating the performance of WHO and CDC case definitions for influenza-like illness in diagnosing influenza during the 2022–2023 flu season in Mexico. Public Health 2023, 222, 175–177. [Google Scholar] [CrossRef]
- Jané, M.; Vidal, M.J.; Soldevila, N.; Romero, A.; Martínez, A.; Torner, N.; Godoy, P.; Launes, C.; Rius, C.; Marcos, M.A.; et al. Epidemiological and clinical characteristics of children hospitalized due to influenza A and B in the south of Europe, 2010–2016. Sci. Rep. 2019, 9, 12853. [Google Scholar] [CrossRef]
- Launes, C.; García-García, J.J.; Martínez-Planas, A.; Moraga, F.; Soldevila, N.; Astigarraga, I.; Arístegui, J.; Korta, J.; Quintana, J.M.; Torner, N.; et al. Clinical features of influenza disease in admitted children during the first postpandemic season and risk factors for hospitalization: A multicentre Spanish experience. Clin. Microbiol. Infect. 2013, 19, E157–E162. [Google Scholar] [CrossRef]
- Böncüoğlu, E.; Kıymet, E.; Çağlar, İ.; Tahta, N.; Bayram, N.; Ayhan, F.Y.; Genel, F.; Ecevit, Ç.Ö.; Apa, H.; Tanju, Çelik; et al. Influenza-related hospitalizations due to acute lower respiratory tract infections in a tertiary care children’s hospital in Turkey. J. Clin. Virol. 2020, 128, 104355. [Google Scholar] [CrossRef]
- Romero-Feregrino, R.; Romero-Cabello, R.; Rodríguez-León, M.A.; Rocha-Rocha, V.M.; Romero-Feregrino, R.; Muñoz-Cordero, B. Report of the Influenza Vaccination Program in Mexico (2006–2022) and Proposals for Its Improvement. Vaccines 2023, 11, 1686. [Google Scholar] [CrossRef]
- Organizacion Panamericana de Salud. Influenza Vaccine Coverage. Available online: http://ais.paho.org/imm/InfluenzaCoverageMap.asp (accessed on 26 December 2024).
- Jules, A.; Grijalva, C.G.; Zhu, Y.; Talbot, H.K.; Williams, J.V.; Poehling, K.A.; Chaves, S.S.; Edwards, K.M.; Schaffner, W.; Shay, D.K.; et al. Influenza-related hospitalization and ED visits in children less than 5 years: 2000–2011. Pediatrics 2015, 135, e66–e74. [Google Scholar] [CrossRef]
- Díaz-García, R.S.; Sánchez-Gómez, A.; López-Zambrano, M.A.; Esteban-Vasallo, M.D.; Cañellas Llabrés, S.; Gutiérrez Rodríguez, M.; Lasheras Carbajo, M.D. Vaccination against influenza: Coverage and adherence in children under 15 years with high-risk medical conditions in the Community of Madrid. An. Pediatr. 2023, 98, 3–11. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Feldstein, L.R.; Novak, T.; Weiss, S.L.; Coates, B.M.; Schuster, J.E.; Schwarz, A.J.; Maddux, A.B.; et al. Vaccine Effectiveness Against Life-Threatening Influenza Illness in US Children. Clin. Infect. Dis. 2022, 75, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Lezama, J.A.; Saucedo-Martínez, R.; Betancourt-Cravioto, M.; Alfaro-Cortes, M.M.; Bahena-González, R.I.; Tapia-Conyer, R. Influenza in the school-aged population in Mexico: Burden of disease and cost-effectiveness of vaccination in children. BMC Infect. Dis. 2020, 20, 240. [Google Scholar] [CrossRef]
- Reyes-Lopez, A.; Moreno-Espinosa, S.; Hernandez-Olivares, Y.O.; Rodolfo Norberto, J.J. Economic issues of Severe Acute Respiratory Infections for influenza in Mexican children attended in a tertiary public hospital. PLoS ONE 2022, 17, e0273923. [Google Scholar] [CrossRef] [PubMed]
- Gamiño-Arroyo, A.E.; Del Carmen Guerra de Blas, P. Impact of influenza vaccination on hospitalised patients in South America. Lancet Infect. Dis. 2023, 23, 136–138. [Google Scholar] [CrossRef] [PubMed]
- El Guerche-Séblain, C.; Etcheto, A.; Parmentier, F.; Afshar, M.; Macias, A.E.; Puentes, E.; Gresset-Bourgeois, V.; Akcay, M.; Petitjean, A.; Coudeville, L. Hospital admissions with influenza and impact of age and comorbidities on severe clinical outcomes in Brazil and Mexico. PLoS ONE 2022, 17, e0273837. [Google Scholar] [CrossRef]
- Kamidani, S.; Garg, S.; Rolfes, M.A.; Campbell, A.P.; Cummings, C.N.; Haston, J.C.; Openo, K.P.; Fawcett, E.; Chai, S.J.; Herlihy, R.; et al. Epidemiology, Clinical Characteristics, and Outcomes of Influenza-Associated Hospitalizations in US Children Over 9 Seasons Following the 2009 H1N1 Pandemic. Clin. Infect. Dis. 2022, 75, 1930–1939. [Google Scholar] [CrossRef]
- Chaudhari, P.P.; Monuteaux, M.C.; Pannaraj, P.S.; Khemani, R.G.; Bachur, R.G. Age-Stratified Risk of Critical Illness in Young Children Presenting to the Emergency Department with Suspected Influenza. J. Pediatr. 2019, 215, 132–138. [Google Scholar] [CrossRef]
- WHO. FluNet—Charts. Available online: https://worldhealthorg.shinyapps.io/flunetchart/ (accessed on 2 January 2025).
- Thielen, B.K.; Friedlander, H.; Bistodeau, S.; Shu, B.; Lynch, B.; Martin, K.; Bye, E.; Como-Sabetti, K.; Boxrud, D.; Strain, A.K.; et al. Detection of Influenza C Viruses Among Outpatients and Patients Hospitalized for Severe Acute Respiratory Infection, Minnesota, 2013–2016. Clin. Infect. Dis. 2017, 66, 1092–1098. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.; Noh, J.Y.; Song, J.Y.; Cheong, H.J.; Lee, J.H.; Woo, S.I.; Kim, W.J. Identification of influenza C virus in young South Korean children, from October 2013 to September 2016. J. Clin. Virol. 2019, 115, 47–52. [Google Scholar] [CrossRef]
- Njouom, R.; Monamele, G.C.; Ermetal, B.; Tchatchouang, S.; Moyo-Tetang, S.; McCauley, J.W.; Daniels, R.S. Detection of Influenza C Virus Infection among Hospitalized Patients, Cameroon. Emerg. Infect. Dis. 2019, 25, 607–609. [Google Scholar] [CrossRef]
- Global Influenza Programme (GIP), WHO. Review of global influenza circulation, late 2019 to 2020, and the impact of the COVID-19 pandemic on influenza circulation. Wkly. Epidemiol. Rec. 2021, 96, 241–264. [Google Scholar]
- Laris-González, A.; Avilés-Robles, M.; Domínguez-Barrera, C.; Parra-Ortega, I.; Sánchez-Huerta, J.L.; Ojeda-Diezbarroso, K.; Bonilla-Pellegrini, S.; Olivar-López, V.; Chávez-López, A.; Jiménez-Juárez, R. Influenza vs. COVID-19: Comparison of Clinical Characteristics and Outcomes in Pediatric Patients in Mexico City. Front. Pediatr. 2021, 9, 676611. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.N.; O’Halloran, A.C.; Azenkot, T.; Reingold, A.; Alden, N.B.; Meek, J.I.; Anderson, E.J.; Ryan, P.A.; Kim, S.; McMahon, M.; et al. Hospital-acquired influenza in the United States, FluSurv-NET, 2011–2012 through 2018–2019. Infect. Control. Hosp. Epidemiol. 2022, 43, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Scotta, M.C.; Mattiello, R.; Marostica, P.J.; Jones, M.H.; Martins, L.G.; Fischer, G.B. Risk factors for need of mechanical ventilation in children with influenza A(H1N1)pdm09. J. Pediatr. 2013, 89, 444–449. [Google Scholar] [CrossRef]
- Eriksson, C.O.; Graham, D.A.; Uyeki, T.M.; Randolph, A.G. Risk factors for mechanical ventilation in U.S. children hospitalized with seasonal influenza and 2009 pandemic influenza A*. Pediatr. Crit. Care Med. 2012, 13, 625–631. [Google Scholar] [CrossRef]
- Norman, D.A.; Cheng, A.C.; Macartney, K.K.; Moore, H.C.; Danchin, M.; Seale, H.; McRae, J.; Clark, J.E.; Marshall, H.S.; Buttery, J.; et al. Influenza hospitalizations in Australian children 2010–2019: The impact of medical comorbidities on outcomes, vaccine coverage, and effectiveness. Influenza Other Respir. Viruses 2022, 16, 316–327. [Google Scholar] [CrossRef]
- Ghimire, L.V.; Chou, F.S.; Moon-Grady, A.J. Impact of congenital heart disease on outcomes among pediatric patients hospitalized for influenza infection. BMC Pediatr. 2020, 20, 450. [Google Scholar] [CrossRef]
- Gao, Y.; Guyatt, G.; Uyeki, T.M.; Liu, M.; Chen, Y.; Zhao, Y.; Shen, Y.; Xu, J.; Zheng, Q.; Li, Z.; et al. Antivirals for treatment of severe influenza: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2024, 404, 753–763. [Google Scholar] [CrossRef]
- Tuckerman, J.; Misan, S.; Crawford, N.W.; Marshall, H.S. Influenza in Children with Special Risk Medical Conditions: A Systematic Review and Meta-analysis. Pediatr. Infect. Dis. J. 2019, 38, 912–919. [Google Scholar] [CrossRef]
| Variable | All Patients (n= 212) | Under 1 Year (n = 29) | 1 to 4 Years (n = 84) | 5 to 9 Years (n = 61) | 10 to 18 Years (n = 38) | p Value |
|---|---|---|---|---|---|---|
| Female, n (%) | 99 (46.7) | 14 (48.3) | 33 (39.3) | 30 (49.2) | 22 (57.9) | 0.269 |
| Comorbidities, n (%) | 198 (93.4) | 26 (89.7) | 77 (91.7) | 58 (95.1) | 37 (97.4) | 0.517 |
| Pulmonary disease, n (%) | 30 (15.2) | 7 (26.9) | 17 (22.1) | 4 (6.9) | 2 (5.4) | 0.014 |
| Allergic disease, n (%) | 17 (8.6) | 0 (0.0) | 7 (9.1) | 9 (15.5) | 1 (2.7) | 0.057 |
| Endocrinologic disease, n (%) | 17 (8.6) | 3 (11.5) | 6 (7.8) | 3 (5.2) | 5 (13.5) | 0.461 |
| Immunodeficiencies, n (%) | 66 (33.3) | 1 (3.8) | 18 (23.4) | 26 (44.8) | 21 (56.8) | <0.001 |
| Renal disease, n (%) | 18 (9.1) | 1 (3.8) | 8 (10.4) | 4 (6.9) | 5 (13.5) | 0.513 |
| Rheumatologic disease, n (%) | 8 (4.0) | 0 (0.0) | 0 (0.0) | 2 (3.) | 6 (16.2) | <0.001 |
| Gastrointestinal disease, n (%) | 20 (10.1) | 5 (19.2) | 7 (9.1) | 5 (8.6) | 3 (8.1) | 0.526 |
| Hematologic disease, n (%) | 7 (3.5) | 1 (3.8) | 0 (0.0) | 2 (3.4) | 4 (10.8) | 0.014 |
| Congenital malformations | 40 (20.2) | 10 (38.5) | 21 (27.3) | 8 (13.8) | 1 (2.7) | 0.001 |
| Cancer, n (%) | 59 (27.8) | 0 (0.00) | 18 (21.4) | 24 (39.3) | 17 (44.7) | < 0.001 |
| Cardiovascular disease, n (%) | 37 (17.5) | 14 (48.3) | 20 (23.8) | 3 (4.9) | 0 (0.0) | < 0.001 |
| Vaccine vs. influenza, n (%) | 13 (6.1) | 1 (3.5) | 7 (8.3) | 3 (4.9) | 2 (5.3) | 0.163 |
| Influenza vaccine not registered, n (%) | 96 (45.3) | 10 (34.5) | 31 (36.9) | 35 (57.4) | 20 (52.6) | 0.163 |
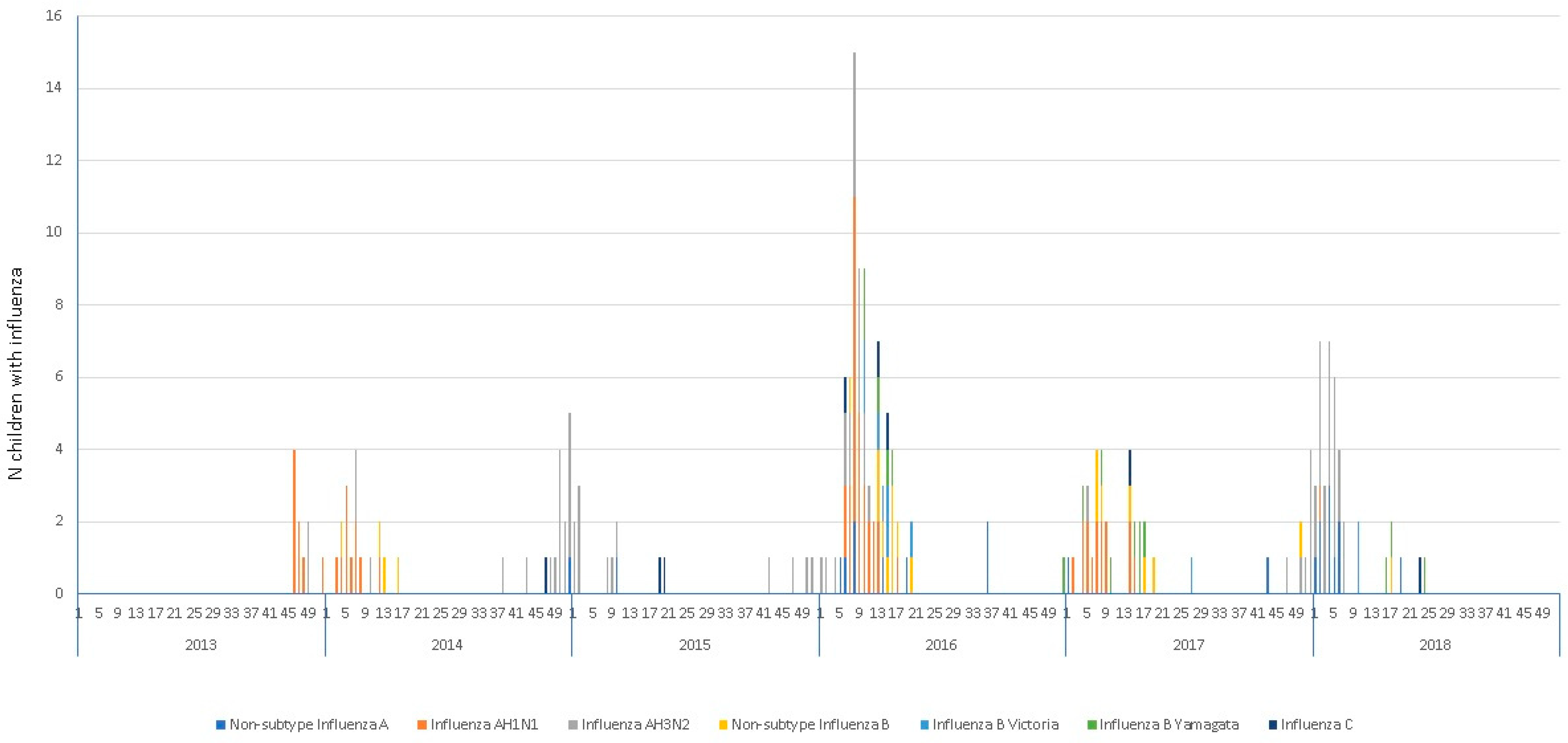
| Nonsubtype influenza A, n (%) | 21 (9.9) | 4 (13.8) | 8 (9.5) | 4 (6.6) | 5 (13.2) | 0.564 |
| Influenza AH1N1, n (%) | 61 (28.8) | 12 (41.4) | 35 (41.7) | 10 (16.4) | 4 (10.5) | < 0.001 |
| Influenza AH3N2, n (%) | 72 (33.9) | 2 (6.9) | 26 (30.9) | 28 (45.9) | 16 (42.1) | 0.001 |
| Influenza B, n (%) | 50 (23.6) | 7 (24.1) | 14 (16.7) | 16 (16.2) | 13 (34.2) | 0.311 |
| Influenza C, n (%) | 9 (4.2) | 2 (6.9) | 1 (1.2) | 4 (6.6) | 2 (5.3) | 0.199 |
| Viral Coinfection, n (%) | 54 (25.5) | 13 (44.8) | 18 (21.4) | 12 (19.7) | 11 (28.9) | 0.061 |
| Coinfection Viruses | Under 1 Year (n = 13) | 1 to 4 Years (n = 18) | 5 to 9 Years (n = 12) | 10–18 Years (n = 11) |
|---|---|---|---|---|
| Influenza A—Influenza AH1N1 | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza A—Influenza B Victoria | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza A—RSVB | 3 (10.34) | 2 (2.38) | 0 (0.00) | 2 (5.26) |
| Influenza A—RSVA | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza A—Rhinovirus | 1 (3.45) | 1 (1.19) | 0 (0.00) | 1 (2.63) |
| Influenza A—Metapneumovirus B | 1 (3.45) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1—RSVA | 3 (10.34) | 2 (2.38) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1—Parainfluenza 3 | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1—Metapneumovirus B | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH3N2—RSVA | 0 (0.00) | 1 (1.19) | 1 (1.64) | 0 (0.00) |
| Influenza AH3N2—RSVB | 0 (0.00) | 3 (3.57) | 2 (3.28) | 1 (2.83) |
| Influenza AH3N2—Parainfluenza 4 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.63) |
| Influenza AH3N2—Rhinovirus | 0 (0.00) | 1 (1.19) | 0 (0.00) | 1 (2.63) |
| Influenza AH3N2—Bocavirus | 0 (0.00) | 1 (1.19) | 1 (1.64) | 1 (2.63) |
| Influenza AH3N2—Adenovirus | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.63) |
| Influenza B—RSVA | 1 (3.45) | 1 (1.19) | 1 (1.64) | 0 (0.00) |
| Influenza B—RSVB | 1 (3.45) | 1 (1.19) | 1 (1.64) | 0 (0.00) |
| Influenza B—Parainfluenza 3 | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza B—Rhinovirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza B—Metapneumovirus B | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza B—Bocavirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 1 (2.63) |
| Influenza B—Coronavirus 229E | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.63) |
| Influenza B Yamagata—RSVB | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza Yamagata—Rhinovirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza B Victoria—Parainfluenza 1 | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza B Victoria—Metapneumovirus B | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza B Victoria—Coronavirus NL63 | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza B Victoria—Adenovirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza C—RSVB | 0 (0.00) | 1 (1.19) | 1 (1.64) | 0 (0.00) |
| Influenza C—Parainfluenza 3 | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza C—Parainfluenza 4 | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1—RSVA—RSVB | 2 (6.90) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1—RSVA—Parainfluenza 3 | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH1N1 -RSVA—Metapneumovirus B | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH3N2—RSVA—RSVB | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza AH3N2 -RSVB—Rhinovirus | 0 (0.00) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza AH3N2 -Rhinovirus—Bocavirus | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.63) |
| Influenza B—RSVA—RSVB | 1 (3.45) | 1 (1.19) | 0 (0.00) | 0 (0.00) |
| Influenza B—RSVA—Metapneumovirus B | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| Influenza B—Rhinovirus—Bocavirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Influenza B Victoria—Coronavirus NL63—Adenovirus | 1 (3.45) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Variable | Under 1 Year (n = 29) | 1 to 4 Years (n = 84) | 5 to 9 Years (n = 61) | 10 to 18 Years (n = 38) | p Value |
|---|---|---|---|---|---|
| Fever, n (%) | 23 (79.3) | 72 (85.7) | 58 (95.1) | 34 (89.5) | 0.119 |
| Cough, n (%) | 23 (79.3) | 69 (82.1) | 55 (90.2) | 31 (81.6) | 0.412 |
| Irritability, n (%) | 11 (37.9) | 14 (16.7) | 2 (3.3) | 2 (5.3) | <0.001 |
| General discomfort, n (%) | 6 (20.7) | 12 (14.3) | 14 (22.9) | 5 (13.2) | 0.468 |
| Respiratory distress, n (%) | 20 (68.9) | 55 (65.5) | 18 (29.5) | 6 (15.8) | <0.001 |
| Myalgia, n (%) | NA | 2 (2.4) | 6 (9.8) | 2 (5.3) | 0.131 |
| Odynophagia, n (%) | 1 (3.5) | 4 (4.8) | 8 (13.1) | 5 (13.5) | 0.156 |
| Fatigue, n (%) | 3 (10.3) | 11 (13.1) | 8 (13.1) | 4 (10.5) | 0.981 |
| Conjunctival redness, n (%) | 0 (zero) | 6 (7.1) | 4 (6.6) | 3 (7.9) | 0.542 |
| Sneeze, n (%) | 0 (zero) | 6 (7.1) | 3 (4.9) | 3 (7.9) | 0.509 |
| Headache, n (%) | 2 (6.9) | 3 (3.6) | 11 (18.0) | 11 (28.9) | 0.001 |
| Nausea or vomiting, n (%) | 9 (31.0) | 15 (17.9) | 11 (18.0) | 4 (10.5) | 0.212 |
| Diarrhea, n (%) | 5 (17.2) | 13 (15.5) | 3 (4.9) | 1 (2.6) | 0.036 |
| Laboratory tests | |||||
| Leukocytes, mean (SD) | 12,600 (7682) | 9000 (6392) | 6149 (4618) | 7039 (8871) | 0.3128 |
| Lymphocyte percentage, mean (SD) | 36.11 (16.76) | 31.49 (18.69) | 28.27 (18.05) | 28.44 (23.96) | 0.3128 |
| Neutrophil percentage, mean (SD) | 49.06 (18.80) | 51.71 (20.46) | 54.38 (25.03) | 59.62 (22.97) | 0.9860 |
| Platelets, mean (SD) | 288,481 (144,607) | 244,228 (159,478) | 224,377 (134,048) | 186,921 (125,779) | 0.9860 |
| Outcomes | |||||
| Admitted to PICU, n (%) | 5 (17.2) | 15 (17.9) | 6 (9.8) | 10 (26.3) | 0.297 |
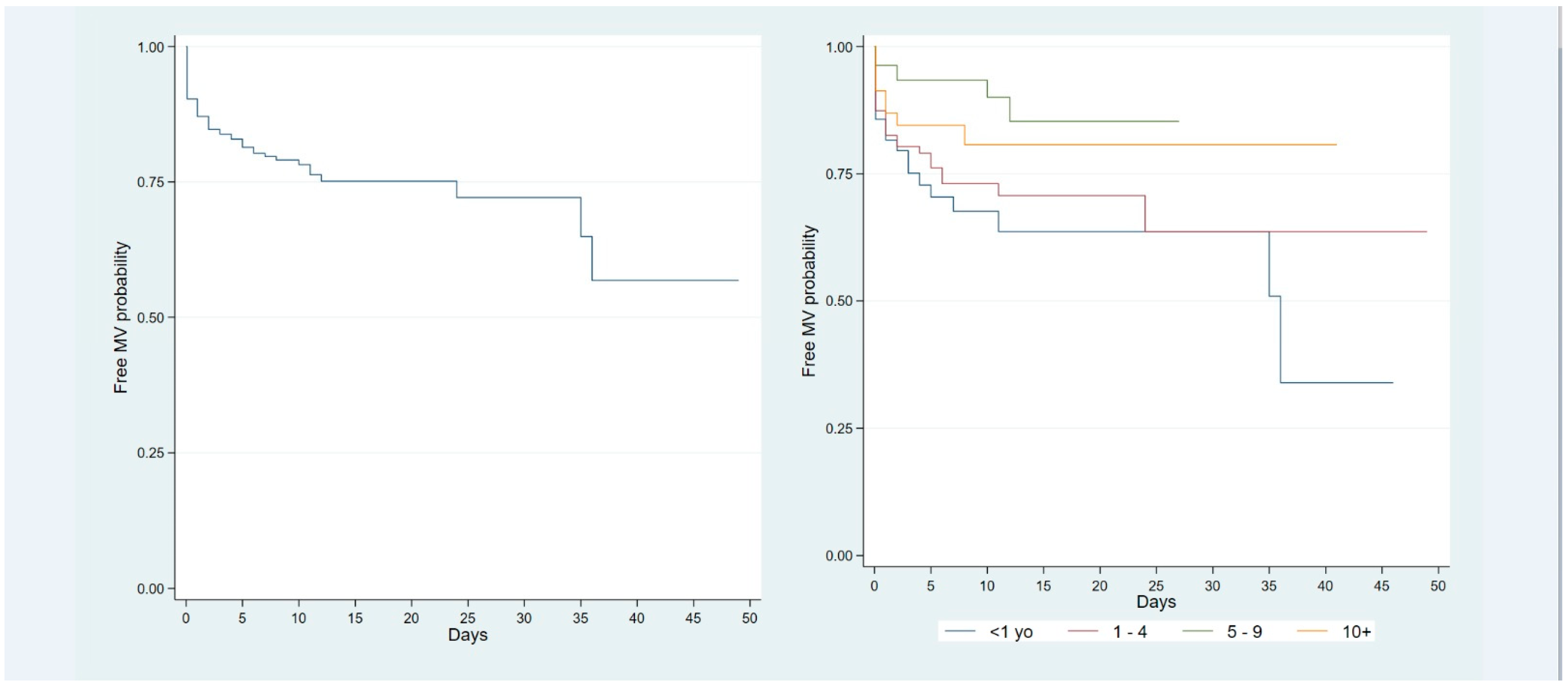
| Mechanical ventilation, n (%) | 7 (24.1) | 25 (29.8) | 3 (4.9) | 10 (26.3) | <0.001 |
| Deaths, n (%) | 3 (10.3) | 2 (2.4) | 1 (1.6) | 0 (zero) | 0.135 |
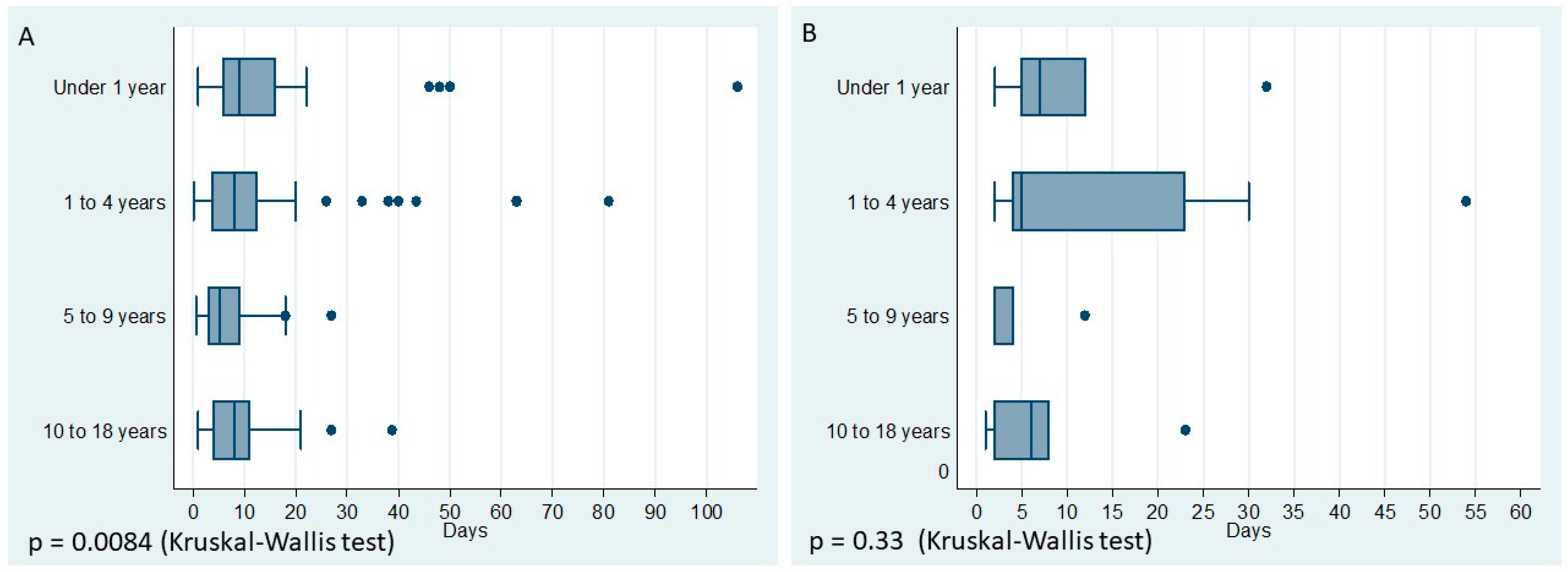
| Length of stay in a hospital, days (IQR 25th–75th) | 25 (6–16) | 8 (3.66–12.81) | 5.22 (3–9) | 8 (4.5–12.5). | |
| Length of stay in a PICU, days (IQR 25th–75th) | 8 (5–12) | 5 (4–23) | 2 (2–4) | 6 (3–8) | |
| Predictors | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Sex | 2.22 | 0.98–5.06 | 0.057 |
| Age groups (ref: 5–9) | |||
| <1 years old | 0.89 | 0.19–4.18 | 0.89 |
| 1–4 | 2.16 | 0.60–7.84 | 0.24 |
| ≥10 years | 4.38 | 0.85–22.67 | 0.78 |
| Influenza season (ref: 2012–2013) | |||
| 2013–2014 | 0.12 | 0.01–1.22 | 0.073 |
| 2014–2015 | 0.05 | 0.002–0.67 | 0.024 |
| 2015–2016 | 0.09 | 0.008–0.99 | 0.049 |
| 2016–2017 | 0.12 | 0.009–1.36 | 0.086 |
| 2017–2018 | 0.02 | 0.0006–0.33 | 0.008 |
| Hypotension | 3.27 | 1.30–8.24 | 0.012 |
| Paradoxical breathing | 6.79 | 2.49–18.46 | <0.001 |
| Nosocomial Infection | 2.78 | 1.10–7.01 | 0.03 |
| Immunodeficiency | 0.34 | 0.08–1.42 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Juárez, R.N.; Moreno-Espinosa, S.; Reyes-Lopez, A.; Parra-Ortega, I.; Laris-Gonzalez, A.; De la Rosa-Zamboni, D.; Guerra-de-Blas, P.; Gamiño-Arroyo, A.E. Impact of Influenza on Children in a Referral Hospital in Mexico City: Clinical Burden and Predictors of Mechanical Ventilation. Viruses 2025, 17, 771. https://doi.org/10.3390/v17060771
Jiménez-Juárez RN, Moreno-Espinosa S, Reyes-Lopez A, Parra-Ortega I, Laris-Gonzalez A, De la Rosa-Zamboni D, Guerra-de-Blas P, Gamiño-Arroyo AE. Impact of Influenza on Children in a Referral Hospital in Mexico City: Clinical Burden and Predictors of Mechanical Ventilation. Viruses. 2025; 17(6):771. https://doi.org/10.3390/v17060771
Chicago/Turabian StyleJiménez-Juárez, Rodolfo Norberto, Sarbelio Moreno-Espinosa, Alfonso Reyes-Lopez, Israel Parra-Ortega, Almudena Laris-Gonzalez, Daniela De la Rosa-Zamboni, Paola Guerra-de-Blas, and Ana Estela Gamiño-Arroyo. 2025. "Impact of Influenza on Children in a Referral Hospital in Mexico City: Clinical Burden and Predictors of Mechanical Ventilation" Viruses 17, no. 6: 771. https://doi.org/10.3390/v17060771
APA StyleJiménez-Juárez, R. N., Moreno-Espinosa, S., Reyes-Lopez, A., Parra-Ortega, I., Laris-Gonzalez, A., De la Rosa-Zamboni, D., Guerra-de-Blas, P., & Gamiño-Arroyo, A. E. (2025). Impact of Influenza on Children in a Referral Hospital in Mexico City: Clinical Burden and Predictors of Mechanical Ventilation. Viruses, 17(6), 771. https://doi.org/10.3390/v17060771






