Concern for Highly Pathogenic Avian Influenza Spillover into Cetaceans
Abstract
1. Introduction
2. Materials and Methods
2.1. Review of IAV Cases Diagnosed in Cetaceans
2.2. Analysis of Mammalian Adaptation Molecular Markers in Cetacean IAV Isolates
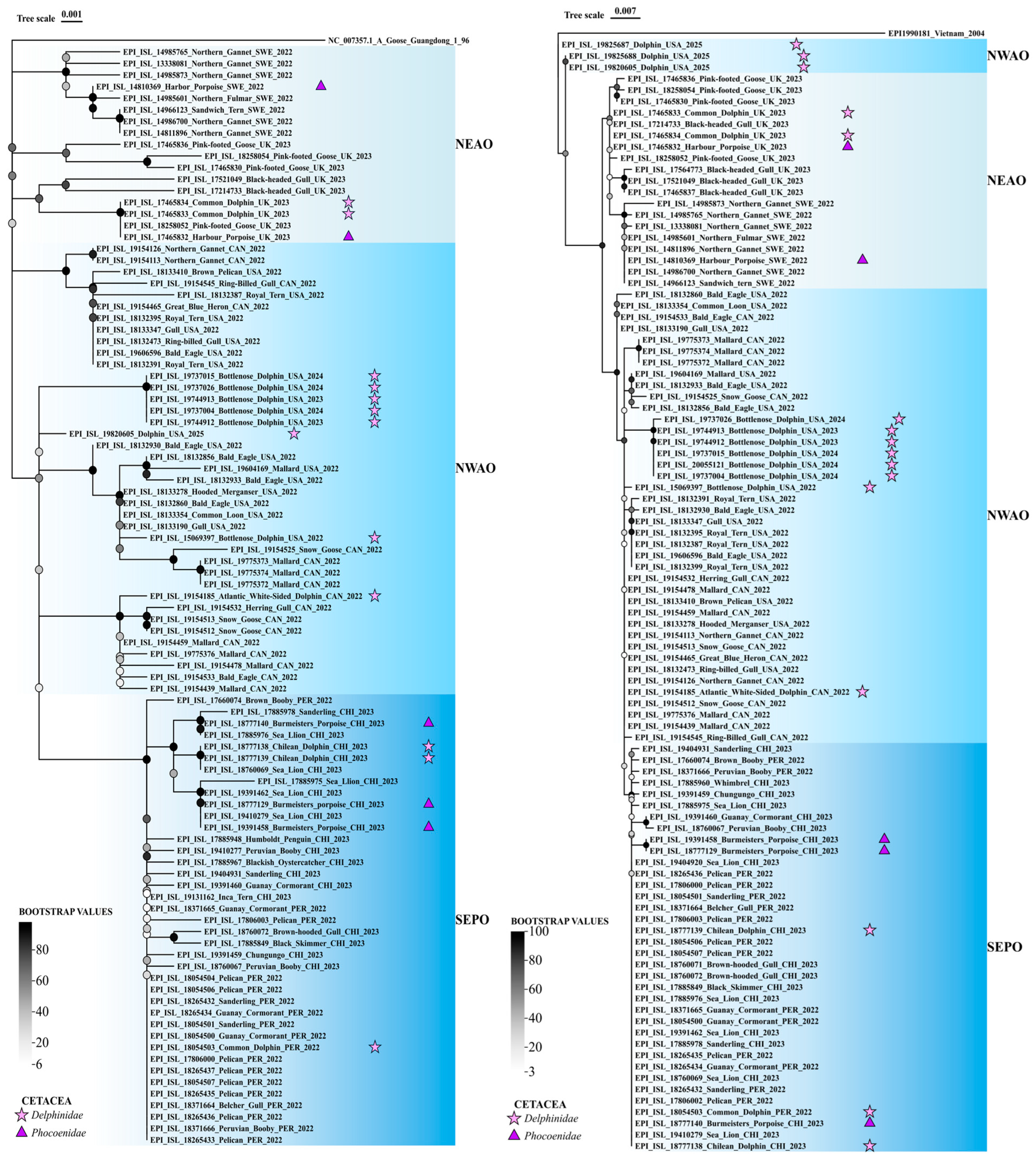
2.3. Phylogenetic Analysis of PB2 and HA Sequences in Cetacean and Marine Host Species
3. Results
3.1. The Number of Cases of IAV Infection Reported in Cetaceans Is Increasing
3.2. IAV H5N1 Isolated from Cetaceans Have Mammal Adaptation Molecular Markers
3.3. Phylogenetic Analysis of PB2 and HA Supports Cross-Species Transmission of IAV H5N1 to Cetaceans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IAV | Influenza A virus |
| AI | Avian influenza |
| ANP32 | Acidic nuclear phosphoprotein 32 |
| ECDC | European Centre for Disease Prevention and Control |
| EFSA | European Food Safety Authority |
| GISAID | Global initiative on sharing all influenza data |
| HA | Hemagglutinin |
| HPAI | High pathogenic avian influenza |
| HPAIV | High pathogenic avian influenza virus |
| LPAI | Low pathogenic avian influenza |
| LPAIV | Low pathogenic avian influenza virus |
| M1 | Matrix protein 1 |
| MEGA | Molecular Evolutionary Genetic Analysis |
| NA | Neuraminidase |
| NCBI | National Centre for Biotechnology Information |
| NEAO | North-East Atlantic Ocean |
| Neu5Ac | N-Acetylneuraminic acid |
| Neu5Gc | N-Glycolylneuraminic acid |
| NP | Nucleoprotein |
| NWAO | North-West Atlantic Ocean |
| PA | Polymerase acidic protein |
| PB1 | Polymerase basic protein 1 |
| PB2 | Polymerase basic protein 2 |
| SEPO | South-East Pacific Ocean |
| vRNP | Viral ribonucleoprotein |
| WoRMS | World Register of Marine Species |
References
- Fusaro, A.; Zecchin, B.; Giussani, E.; Palumbo, E.; Agüero-García, M.; Bachofen, C.; Bálint, Á.; Banihashem, F.; Banyard, A.C.; Beerens, N.; et al. High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe-Why trends of virus evolution are more difficult to predict. Virus Evol. 2024, 10, veae027. [Google Scholar] [CrossRef]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef]
- Kilbourne, E.D. Taxonomy and Comparative Virology of the Influenza Viruses. Influenza; Springer: Boston, MA, USA, 1987; pp. 25–32. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H. Daniels Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Chauhanl, R.P.; Gordonl, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef]
- Xie, R.; Edwards, K.M.; Wille, M.; Wei, X.; Wong, S.S.; Zanin, M.; El-Shesheny, R.; Ducatez, M.; Poon, L.L.M.; Kayali, G.; et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 2023, 622, 810–817. [Google Scholar] [CrossRef]
- Griffin, E.F.; Tompkins, S.M. Fitness Determinants of Influenza A Viruses. Viruses 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.; Iqbal, M. The Influenza A Virus Replication Cycle: A Comprehensive Review. Viruses 2024, 16, 316. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic Acid Species as a Determinant of the Host Range of Influenza A Viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.H.; Sharif, S. Immune responses to avian influenza viruses in chickens. Viruses 2025, 603, 110405. [Google Scholar] [CrossRef]
- Nao, N.; Yamagishi, J.; Miyamoto, H.; Igarashi, M.; Manzoor, R.; Ohnuma, A.; Tsuda, Y.; Furuyama, W.; Shigeno, A.; Kajihara, M.; et al. Genetic predisposition to acquire a polybasic cleavage site for highly pathogenic avian influenza virus hemagglutinin. mBio 2017, 8, e02298-16. [Google Scholar] [CrossRef]
- França, M.S.; Brown, J.D. Influenza Pathobiology and Pathogenesis in Avian Species. In Influenza Pathogenesis and Control–Volume I.; Compans, R.W., Oldstone, M.A.B., Eds.; Springer: Cham, Switzerland, 2014; pp. 221–242. [Google Scholar] [CrossRef]
- Sacristán, C.; Ewbank, A.C.; Ibáñez Porras, P.; Pérez-Ramírez, E.; de la Torre, A.; Briones, V.; Iglesias, I. Novel Epidemiologic Features of High Pathogenicity Avian Influenza Virus A H5N1 2.3.3.4b Panzootic: A Review. Transbound. Emerg. Dis. 2024, 2024, 5322378. [Google Scholar] [CrossRef]
- Plaza, P.I.; Gamarra-Toledo, V.; Rodríguez, E.J.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg. Infect. Dis. 2024, 30, 444–452. [Google Scholar] [CrossRef]
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.L.; Dezordi, F.Z.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: Implications for bird–mammal transmission in South America. Virus Evol. 2024, 10, veae031. [Google Scholar] [CrossRef]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A (H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill. 2023, 28, 2300001. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Reinhart, K.; Couture, A.; Kniss, K.; Davis, C.T.; Kirby, M.K.; Murray, E.L.; Zhu, S.; Kraushaar, V.; Wadford, D.A.; et al. Highly pathogenic avian influenza A (H5N1) virus infections in humans. N. Engl. J. Med. 2025, 392, 843–854. [Google Scholar] [CrossRef]
- Uhart, M.M.; Vanstreels, R.E.T.; Nelson, M.I.; Olivera, V.; Campagna, J.; Zavattieri, V.; Lemey, P.; Campagne, C.; Falabella, V.; Rimondi, A. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat. Commun. 2024, 15, 9516. [Google Scholar] [CrossRef]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef]
- Suttie, A.; Deng, Y.M.; Greenhill, A.R.; Dussart, P.; Horwood, P.F.; Karlsson, E.A. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes 2019, 55, 739–768. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Boklund, A.; Dippel, S.; Dórea, F.; Figuerola, J.; Herskin, M.S.; Michel, V.; Miranda Chueca, M.A.; Nannoni, E.; Nielsen, S.S.; et al. Preparedness, prevention and control related to zoonotic avian influenza. EFSA J. 2025, 23, e9191. [Google Scholar] [CrossRef] [PubMed]
- Vigil, K.; Wu, H.; Aw, T.G. A systematic review on global zoonotic virus-associated mortality events in marine mammals. One Health 2024, 19, 100872. [Google Scholar] [CrossRef] [PubMed]
- Lvov, D.K.; Zdanov, V.M.; Sazonov, A.A.; Braude, N.A.; Vladimrtceva, E.A.; Agafonova, L.V.; Skljanskaja, E.I.; Kaverin, N.V.; Reznik, V.I.; Pysina, T.V.; et al. Comparison of influenza viruses isolated from man and from whales. Bull. World Health Organ. 1978, 56, 923–930. [Google Scholar]
- Hinshaw, V.S.; Bean, W.J.; Geraci, J.; Fiorelli, P.; Early, G.; Webster, R.G. Characterization of Two Influenza A Viruses from a Pilot Whale. J. Virol. 1986, 58, 655–656. [Google Scholar] [CrossRef]
- Ohishi, K.; Maruyama, T.; Ninomiya, A.; Kida, H.; Zenitani, R.; Bando, T.; Fujise, Y.; Nakamatsu, K.; Miyazaki, N.; Boltunov, A.N. Serologic investigation of influenza A virus infection in cetaceans from the western North Pacific and the Southern oceans. Mar. Mammal Sci. 2006, 22, 214–221. [Google Scholar] [CrossRef]
- Nymo, I.H.; Siebert, U.; Baechlein, C.; Postel, A.; Breines, E.M.; Lydersen, C.; Kovacs, K.M.; Tryland, M. Pathogen Exposure in White Whales (Delphinapterus leucas) in Svalbard, Norway. Pathogens 2023, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.; Clavijo, A.; Boughen, J.A. Serologic Evidence of Influenza A Infection in Marine Mammals of Arctic Canada. J. Wildl. Dis. 2001, 37, 820–825. [Google Scholar] [CrossRef]
- Groth, M.; Lange, J.; Kanrai, P.; Pleschka, S.; Scholtissek, C.; Krumbholz, A.; Platzer, M.; Sauerbrei, A.; Zell, R. The genome of an influenza virus from a pilot whale: Relation to influenza viruses of gulls and marine mammals. Infection. Genet. Evol. 2014, 24, 183–186. [Google Scholar] [CrossRef]
- Harvey, J.A.; Mullinax, J.M.; Runge, M.C.; Prosser, D.J. The changing dynamics of highly pathogenic avian influenza H5N1: Next steps for management & science in North America. Biol. Conserv. 2023, 282, 110041. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Highly Pathogenic Avian Influenza–Wildlife Dashboard; CFIA: Ottawa, ON, Canada, 2024. Available online: https://cfia-ncr.maps.arcgis.com/apps/dashboards/89c779e98cdf492c899df23e1c38fdbc (accessed on 15 October 2025).
- U.S. Geological Survey. WHISPers–Wildlife Health Information Sharing Partnership–Event Reporting System; U.S. Geological Survey: Reston, VA, USA, 2019. Available online: https://whispers.usgs.gov/home (accessed on 15 October 2025).
- Murawski, A.; Fabrizio, T.; Ossiboff, R.; Kackos, C.; Jeevan, T.; Jones, J.C.; Kandeil, A.; Walker, D.; Turner, J.C.M.; Patton, C.; et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Commun. Biol. 2024, 7, 476. [Google Scholar] [CrossRef]
- Thorsson, E.; Zohari, S.; Roos, A.; Banihashem, F.; Bröjer, C.; Neimanis, A. HighlyPathogenic Avian Influenza A (H5N1) virus in a Harbor Porpoise, Sweden. Emerg. Infect. Dis. 2023, 29, 852–855. [Google Scholar] [CrossRef]
- Servicio Nacional de Pesca y Acuicultura. Sernapesca Reports That Two Chilean Dolphins Tested Positive for Avian Influenza; Sernapesca: Valparaíso, Chile, 2023. Available online: https://www.sernapesca.cl/noticias/sernapesca-informa-que-dos-delfines-chilenos-dieron-positivo-gripe-aviar/ (accessed on 15 October 2025).
- García-Cegarra, A.M.; Hall, A.; Martínez-López, E. Bycatch and pollution are the main threats for Burmeister’s porpoises inhabiting a high-industrialized bay in the Humboldt Current System. Environ. Res. 2024, 251, 118621. [Google Scholar] [CrossRef]
- Department for Environment, Food & Rural Affairs/Animal and Plant Health Agency. Confirmed Findings of Influenza of Avian Origin in Non-Avian Wildlife; DEFRA&APHA: London, UK, 2022. Available online: https://www.gov.uk/government/publications/bird-flu-avian-influenza-findings-in-non-avian-wildlife/confirmed-findings-of-influenza-of-avian-origin-in-non-avian-wildlife (accessed on 15 October 2025).
- U.S. Department of Agriculture, Animal and Plant Health Inspection Service. Detections of Highly Pathogenic Avian Influenza in Mammals; USDA APHIS: Washington, DC, USA, 2025. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (accessed on 15 October 2025).
- Shu, Y.; McCauley, J. GISAID: Global initiative on sharing all influenza data–from vision to reality. Eurosurveillance 2017, 22, 30494. [Google Scholar] [CrossRef]
- Pugliares, K.R.; Bogomolni, A.; Touhey, K.M.; Herzig, S.M.; Harry, C.T.; Moore, M.J. Marine Mammal Necropsy: An introductory guide for stranding responders and field biologists. Woods Hole Oceanogr. Inst. 2007, 6, 133. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; de Guise, S.; Di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Practical Guide for Authorised Field Responders: Highly Pathogenic Avian Influenza (HPAI) in Marine Mammals. 2024. Available online: https://www.woah.org/app/uploads/2024/02/woah-practicalguide-forauthorisedfieldresponders-hpaimarinemammals-feb24.pdf (accessed on 15 October 2025).
- Pubmed. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 15 October 2025).
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Muloz Guajardo, I.; Chuzhakina, K.; et al. Avian influenza overview June–September 2022. EFSA J. 2022, 20, e07597. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Aznar, I.; Muñoz Guajardo, I.; et al. Avian influenza overview September–December 2022. EFSA J. 2023, 21, e07786. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Terregino, C.; et al. Avian influenza overview December 2022–March 2023. EFSA J. 2023, 21, e07917. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinaviciute, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Terregino, C.; Broglia, A.; et al. Avian influenza overview March–April 2023. EFSA J. 2023, 21, e08039. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Melidou, A.; Mirinavičiūtė, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Terregino, C.; et al. Avian influenza overview April–June 2023. EFSA J. 2023, 21, e08191. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Staubach, C.; Terregino, C.; Baldinelli, F.; Rusinà, A.; et al. Avian influenza overview June–September 2023. EFSA J. 2023, 21, e8328. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Terregino, C.; Willgert, K.; et al. Avian influenza overview September–December 2023. EFSA J. 2023, 21, e8539. [Google Scholar] [CrossRef]
- Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Stahl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; et al. Avian influenza overview December 2023–March 2024. EFSA J. 2024, 22, e8754. [Google Scholar] [CrossRef]
- Alexakis, L.; Fusaro, A.; Kuiken, T.; Mirinavičiūtė, G.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; Delacourt, R.; et al. Avian influenza overview March–June 2024. EFSA J. 2024, 22, e8930. [Google Scholar] [CrossRef]
- Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Stahl, K.; Staubach, C.; Svartström, O.; Terregino, C.; et al. Avian influenza overview June–September 2024. EFSA J. 2024, 22, e9057. [Google Scholar] [CrossRef]
- Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Stahl, K.; Staubach, C.; Svarström, O.; Terregino, C.; et al. Avian influenza overview September–December 2024. EFSA J. 2025, 23, e9204. [Google Scholar] [CrossRef]
- Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Stahl, K.; Staubach, C.; Svartström, O.; Terregino, C.; et al. Avian influenza overview December 2024–March 2025. EFSA J. 2025, 23, e9352. [Google Scholar] [CrossRef]
- Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Stahl, K.; Staubach, C.; Svarström, O.; et al. Avian influenza overview March–June 2025. EFSA J. 2025, 23, e9520. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Bird & Mammal Species Affected by H5Nx HPAI; FAO: Rome, Italy, 2025; Available online: https://www.fao.org/animal-health/situation-updates/global-aiv-with-zoonotic-potential/bird-species-affected-by-h5nx-hpai/en (accessed on 15 October 2025).
- Esri. ArcGIS Desktop Software, Version 3.4.3; Environmental Research Institute: Redlands, CA, USA, 2023. Available online: https://pro.arcgis.com (accessed on 20 November 2025).
- Cooper, P.; Landrum, M.; Mizrachi, I.; Weisemann, J. Entrez Sequences Quick Start; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK44863/ (accessed on 15 August 2025).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species; Flanders Marine Institute: Oostende, Belgium; Available online: https://www.marinespecies.org (accessed on 11 August 2025).
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Thi Hoang, D.; Chernomor, O.; von Haeseler, A.; Quang Minh, B.; Sy Vinh, L.; Rosenberg, M.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Aguilar, A.; Raga, J.A. The Striped Dolphin Epizootic in the Mediterranean Sea. Ambio 1993, 22, 524–528. Available online: https://www.jstor.org/stable/4314142 (accessed on 1 November 2025).
- Geraci, J.R.; St. Aubin, D.J.; Barker, I.K.; Webster, R.G.; Hinshaw, V.S.; Bean, W.J.; Ruhnke, H.L.; Prescott, J.H.; Early, G.; Baker, A.S.; et al. Mass mortality of harbor seals: Pneumonia associated with Influenza A virus. Science 1982, 215, 1129–1131. [Google Scholar] [CrossRef]
- Capelastegui, F.; Goldhill, D.H. H5N1 2.3.4.4b: A review of mammalian adaptations and risk of pandemic emergence. J. Gen. Virol. 2025, 106, 002109. [Google Scholar] [CrossRef]
- Gadzhiev, A.; Petherbridge, G.; Sharshov, K.; Sobolev, I.; Alekseev, A.; Gulyaeva, M.; Litvinov, K.; Boltunov, I.; Teymurov, A.; Zhigalin, A.; et al. Pinnipeds and avian influenza: A global timeline and review of research on the impact of highly pathogenic avian influenza on pinniped populations with particular reference to the endangered Caspian seal (Pusa caspica). Front. Cell. Infect. Microbiol. 2024, 14, 1325977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pu, J. Influence of Host Sialic Acid Receptors Structure on the Host Specificity of Influenza Viruses. Viruses 2022, 14, 2141. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009, 5, e1000709. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, B.; Zhou, H.; Suguitan, A.L.; Cheng, X.; Subbarao, K.; Kemble, G.; Jin, H. Glycosylation at 158N of the Hemagglutinin Protein and Receptor Binding Specificity Synergistically Affect the Antigenicity and Immunogenicity of a Live Attenuated H5N1 A/Vietnam/1203/2004 Vaccine Virus in Ferrets. J. Virol. 2010, 84, 6570–6577. [Google Scholar] [CrossRef]
- Toda, N.; Seno, N. Sialic acid in the keratan sulfate fraction from whale cartilage. Biochim. Biophys. Acta (BBA) Gen. Subj. 1970, 208, 227–235. [Google Scholar] [CrossRef]
- Ramis, A.J.; van Riel, D.; van de Bildt, M.W.G.; Osterhaus, A.; Kuiken, T. Influenza A and B virus attachment to respiratory tract in marine mammals. Emerg. Infect. Dis. 2012, 18, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Giotis, E.S.; Moncorgé, O.; Frise, R.; Mistry, B.; James, J.; Morisson, M.; Iqbal, M.; Vignal, A.; Skinner, M.A.; et al. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature 2016, 529, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Sheppard, C.M.; Lister, M.G.; Staller, E.; Frise, R.; Swann, O.C.; Goldhill, D.H.; Long, J.S.; Barclay, W.S. Mammalian ANP32A and ANP32B Proteins Drive Differential Polymerase Adaptations in Avian Influenza Virus. J. Virol. 2023, 97, e00213-23. [Google Scholar] [CrossRef]
- Carrique, L.; Fan, H.; Walker, A.P.; Keown, J.R.; Sharps, J.; Staller, E.; Barclay, W.S.; Fodor, E.; Grimes, J.M. Host ANP32A mediates the assembly of the influenza virus replicase. Nature 2020, 587, 638–643. [Google Scholar] [CrossRef]
- Pardo-Roa, C.; Nelson, M.I.; Ariyama, N.; Aguayo, C.; Almonacid, L.I.; Gonzalez-Reiche, A.S.; Muñoz, G.; Ulloa, M.; Ávila, C.; Navarro, C.; et al. Cross-species and mammal-to-mammal transmission of clade 2.3.4.4b highly pathogenic avian influenza A/H5N1 with PB2 adaptations. Nat. Commun. 2025, 16, 2232. [Google Scholar] [CrossRef] [PubMed]
- Sediri, H.; Thiele, S.; Schwalm, F.; Gabriel, G.; Klenk, H.D. PB2 subunit of avian influenza virus subtype H9N2: A pandemic risk factor. J. Gen. Virol. 2016, 97, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Bakshi, S.; Lytras, S.; Zakaria, M.K.; Swingler, S.; Worrell, J.C.; Herder, V.; Hargrave, K.E.; Varjak, M.; Cameron-Ruiz, N.; et al. BTN3A3 evasion promotes the zoonotic potential of influenza A viruses. Nature 2023, 619, 338–347. [Google Scholar] [CrossRef]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol. Spectr. 2023, 11, e02867-22. [Google Scholar] [CrossRef]
- Cui, P.; Zeng, X.; Li, X.; Li, Y.; Shi, J.; Zhao, C.; Qu, Z.; Wang, Y.; Guo, J.; Gu, W.; et al. Genetic and biological characteristics of the globally circulating H5N8 avian influenza viruses and the protective efficacy offered by the poultry vaccine currently used in China. Sci. China Life Sci. 2022, 65, 795–808. [Google Scholar] [CrossRef]
- Kuiken, T.; Vanstreels, R.E.T.; Banyard, A.; Begeman, L.; Breed, A.; Dewar, M.; Fijn, R.; Pereira Serafini, P.; Uhart, M.; Wille, M. Emergence, spread, and impact of high-pathogenicity avian influenza H5 in wild birds and mammals of South America and Antarctica. Conserv. Biol. 2025, 31, e70052. [Google Scholar] [CrossRef]
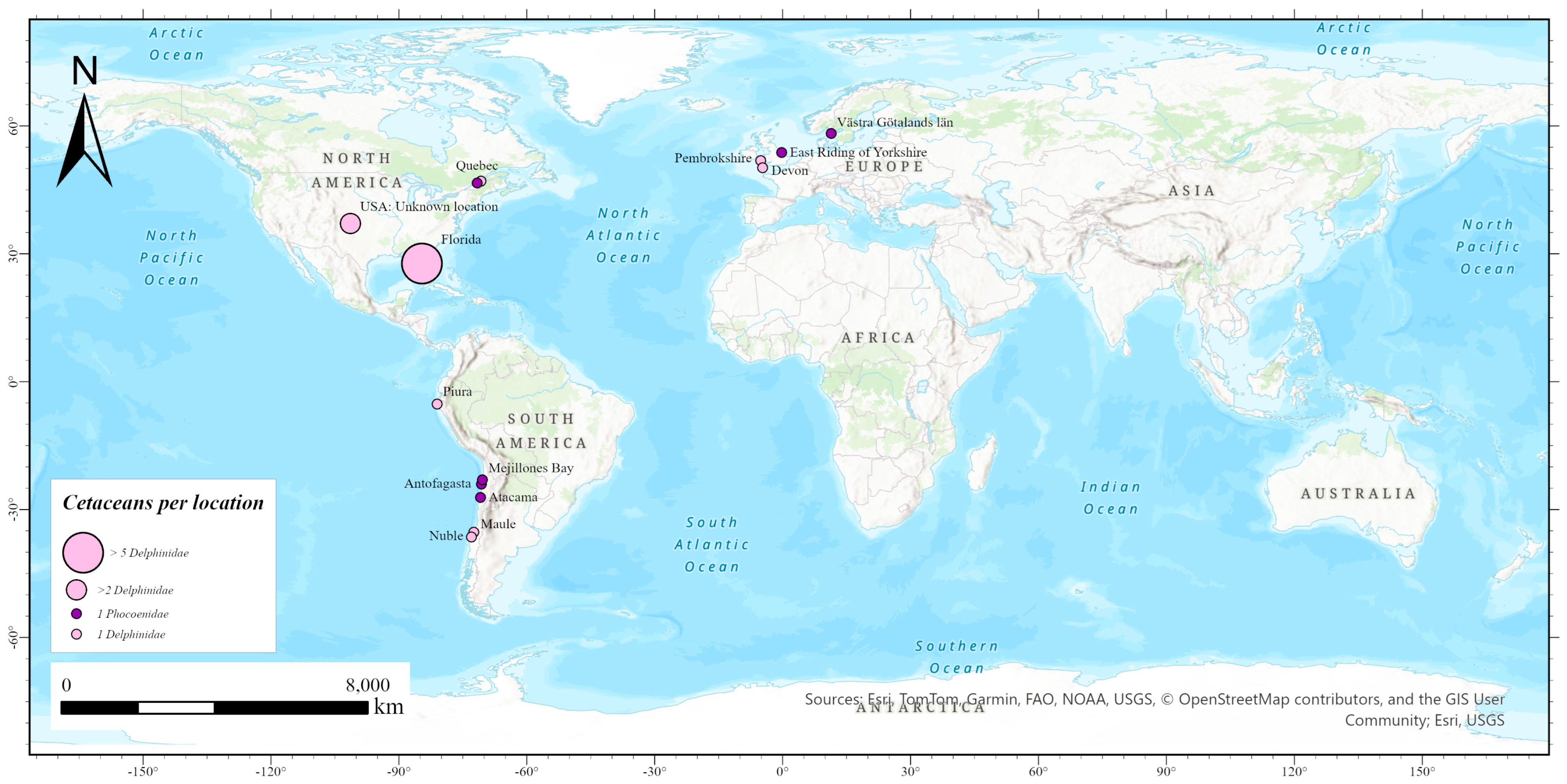
| Year | Subtype | Host | Family | Species | Location | Sample d | Reference |
|---|---|---|---|---|---|---|---|
| 1975/76 | H1N3 | Common minke whale b | Balaenopteridae | --- | South Pacific | Lung and liver | [24] |
| 1984 | H13N2 and H13N9 | 1 Long-finned pilot whale | Delphinidae | Globicephala melas | Coast of New England | Lung and hilar node | [25,29] |
| 1990–1991 | HXNX | 5 Beluga whales | Monodontidae | Delphinapterus leucas | Southeast Baffin Island (Canada) | Serum | [28] |
| 2000–2001 | HXNX a | 2 Dall’s porpoises | Phocoenidae | Phocoenoides dalli | Western north Pacific | Serum | [26] |
| HXNX a | 7 Common minke whales | Balaenopteridae | Balaenoptera acutorostrata | Western north Pacific | n.i. | ||
| 2001–2006 | HXNX | Beluga whales b | Monodontidae | Delphinapterus leucas | Van Mijenfjorden, Svalbard (Norway) | Blood | [27] |
| 2022 | H5N1 | 1 Bottlenose dolphin c | Delphinidae | Tursiops truncatus | Dixie County (Florida, USA) | Ocular conjunctival, rectal, spiracle swabs | [30,31,32,33] |
| H5N1 | 1 Harbour porpoise | Phocoenidae | Phocoena phocoena | Quebec (Canada) | Lung, bronchus, brain, kidney, liver, spleen, intestine, muscle and blubber | [30,31] | |
| H5N1 | 1 Atlantic white-sided dolphin | Delphinidae | Lagenorhynchus acutus | Quebec (Canada) | |||
| H5N1 | 1 Common dolphin | Delphinidae | Delphinus delphis | Piura (Peru) | n.i. | [20] | |
| H5N1 | 1 Harbour porpoise | Delphinidae | Phocoena phocoena | West Coast of Sweden | n.i. | [34] | |
| 2023 | H5N1 | 2 Chilean dolphins | Delphinidae | Cephalorhynchus eutropia | Maule y Nuble (Chile) | n.i. | [35] |
| H5N1 | 2 Burmeister’s porpoises | Phocoenidae | Phocoena spinipinnis | Antofagasta and Atacama (Chile) | n.i. | ||
| H5N1 | 1 Burmeister’s porpoise | Phocoenidae | Phocoena spinipinnis | Mejillones Bay (Chile) | Serum | [36] | |
| H5N1 | 2 Common dolphin | Delphinidae | Delphinus delphis | Devon (England) and Pembrokeshire (Wales) | n.i. | [37] | |
| H5N1 | 1 Harbour porpoise | Phocoenidae | Phocoena phocoena | East Riding of Yorkshire (England) | n.i. | ||
| H5N1 | 2 Bottlenose dolphins | Delphinidae | Tursiops truncatus | Florida (USA) | n.i. | [38,39] | |
| 2024 | H5N1 | 4 Bottlenose dolphins | Delphinidae | Tursiops truncatus | Florida (USA) | n.i. | |
| 2025 | H5N1 | 3 Bottlenose dolphins | Delphinidae | Tursiops truncatus | Florida (USA) | n.i. | [39] |
| Viral Protein | ||||||
|---|---|---|---|---|---|---|
| HA b | PB2 | NP | ||||
| IAV H5N1 clade 2.4.4.4b cetacean isolates a | 156A | 588V | 591K | 627K | 701N | 52H |
| A/Goose/Guangdong/1/96 | A | Q | E | D | Y | |
| A/Vietnam/1203/2004 | K | |||||
| EPI_ISL_13338081_Northern_Gannet_SWE_2022 | A | A | Q | E | D | H |
| EPI_ISL_14810369_Harbour_Porpoise_SWE_2022 | A | - | - | - | - | H |
| EPI_ISL_15069397_Bottlenose_Dolphin_USA_2022 | A | - | - | - | - | - |
| EPI_ISL_19154185_Atlantic_white-sided_Dolphin_CAN_2022 | A | - | - | - | - | - |
| EPI_ISL_18054503_Common_Dolphin_PER_2022 | A | - | - | - | - | - |
| EPI_ISL_17465832_Harbour_Porpoise_UK_2023 | A | - | - | - | - | - |
| EPI_ISL_17465833_Common_Dolphin_UK_2023 | A | - | - | - | - | - |
| EPI_ISL_17465834_Common_Dolphin_UK_2023 | A | - | - | - | - | - |
| EPI_ISL_18777140_Burmeisters_Porpoise_CHI_2023 | A | - | - | - | N | - |
| EPI_ISL_18777139_Chilean_Dolphin_CHI_2023 | A | - | - | - | N | - |
| EPI_ISL_18777129_ Burmeisters_Porpoise _CHI_2023 | A | - | K | - | N | - |
| EPI_ISL_19391458_Burmeisters_Porpoise_CHI_2023 | A | - | K | - | N | - |
| EPI_ISL_18777138_Chilean_Dolphin_CHI_2023 | A | - | - | - | N | - |
| EPI_ISL_19744912_Bottlenose_Dolphin_USA_2023 | A | - | - | K | - | - |
| EPI_ISL_19744913_Bottlenose_Dolphin_USA_2023 | A | - | - | K | - | - |
| EPI_ISL_20055121_Bottlenose_Dolphin_USA_2024 | A | - | - | - | - | - |
| EPI_ISL_19737004_Bottlenose_Dolphin_USA_2024 | A | - | - | K | - | - |
| EPI_ISL_19737015_Bottlenose_Dolphin_USA_2024 | A | - | - | K | - | - |
| EPI_ISL_19737026_Bottlenose_Dolphin_USA_2024 | A | - | - | K | - | - |
| EPI_ISL_19820605_Dolphin_USA_2025 | A | - | - | - | - | - |
| EPI_ISL_19825687_Dolphin_USA_2025 | A | V | - | - | - | - |
| EPI_ISL_19825688_Dolphin_USA_2025 | A | ns c | ns c | ns c | ns c | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sánchez, T.; Báez, J.C.; Johnstone, C. Concern for Highly Pathogenic Avian Influenza Spillover into Cetaceans. Viruses 2025, 17, 1536. https://doi.org/10.3390/v17121536
Pérez-Sánchez T, Báez JC, Johnstone C. Concern for Highly Pathogenic Avian Influenza Spillover into Cetaceans. Viruses. 2025; 17(12):1536. https://doi.org/10.3390/v17121536
Chicago/Turabian StylePérez-Sánchez, Teresa, José Carlos Báez, and Carolina Johnstone. 2025. "Concern for Highly Pathogenic Avian Influenza Spillover into Cetaceans" Viruses 17, no. 12: 1536. https://doi.org/10.3390/v17121536
APA StylePérez-Sánchez, T., Báez, J. C., & Johnstone, C. (2025). Concern for Highly Pathogenic Avian Influenza Spillover into Cetaceans. Viruses, 17(12), 1536. https://doi.org/10.3390/v17121536







