Abstract
Influenza reporter viruses are essential for studying viral infection dynamics and assessing antiviral drug efficacy. However, insertion of exogenous reporter genes can impair both viral replication and reporter expression, limiting the development of these systems. In this study, CE1 compensatory mutation (G3A/C8U) was introduced into the 3′ non-coding region of the NS segment of influenza A/Puerto Rico/8/1934 using reverse genetics, generating the recombinant reporter virus H1N1-PR8-NSCE1-mCherry. Compared with H1N1-PR8-NSWT-mCherry, H1N1-PR8-NSCE1-mCherry produced approximately 2.7-fold more infectious particles. CE1 compensatory mutation partially restored impaired replication kinetics in vitro, as evidenced by higher titers of H1N1-PR8-NSCE1-mCherry at 48 h post-infection in MDCK cells. Additionally, H1N1-PR8-NSCE1-mCherry maintained the intact mCherry gene insertion and high viral titers during serial passaging. Additionally, a real-time, non-invasive in vivo imaging of influenza A viruses was established using H1N1-PR8-NSCE1-mCherry. A significant correlation was observed between lung fluorescence intensity and viral load, indicating that fluorescence signals serve as a reliable indicator of lung viral load in infected mice. Finally, utility of this model for in vivo drug screening was confirmed by antiviral drug oseltamivir phosphate. Above all, H1N1-PR8-NSCE1-mCherry provides a tool for visualizing influenza A virus infection and evaluating antiviral drug efficacy.
1. Introduction
Influenza A virus (IAV) is an enveloped virus of the Orthomyxoviridae family. Its genome comprises eight single-stranded, negative-sense RNA segments that encode hemagglutinin (HA), neuraminidase (NA), polymerase (PB2, PB1 and PA), nucleoprotein (NP), matrix protein (M) and nonstructural protein (NS) []. As a pathogen that causes seasonal epidemics and occasional pandemics in humans, IAV remains a major global public health threat [,,,]. While multiple FDA-approved antiviral drugs are available, including M2 ion channel blockers, neuraminidase inhibitors and cap-dependent endonuclease inhibitors, their extended use has facilitated the development of drug-resistant variants of IAV [,]. Furthermore, conventional antiviral screening typically relies on endpoint assays or destructive sampling, which precludes real-time, dynamic monitoring of therapeutic interventions. Consequently, accurate real-time monitoring systems for IAV may be essential for evaluating the effectiveness of antiviral therapies [,].
Recombinant IAVs expressing reporter genes provide the dynamic detection of viral infections and quantitative assessment of viral replication. For in vivo imaging, bioluminescent reporter gene imaging enables evaluations of intrinsic biological processes in live organisms, such as Firefly luciferase [], Renilla/Gaussia luciferases [] and NanoLuc []. Bioluminescent reporter systems generate light through enzyme-substrate reactions without requiring external excitation light and therefore typically offer high detection sensitivity with low background noise. Among these, NanoLuc shows particularly strong performance in deep-tissue imaging, combining high signal intensity with very low background, and its sensitivity is significantly higher than that of most conventional luciferases [,]. However, bioluminescent reporter systems typically emit monochromatic light, which limits their utility for multichannel or multiplex labeling. Furthermore, luciferase substrates must be administered via invasive routes such as injection, increasing animal discomfort, and their relatively high cost substantially raises overall experimental expenses. Compared with bioluminescent reporter systems, fluorescent protein-based imaging systems (e.g., GFP [] and mCherry []) do not require substrate administration, simplify experimental workflows and enable truly non-invasive longitudinal imaging. Furthermore, fluorescent protein-based imaging systems can be readily combined with other imaging modalities to achieve multicolor visualization of diverse biological parameters. Among these, mCherry is widely used for live-animal imaging because its longer emission wavelength reduces tissue autofluorescence [,].
In influenza reporter viruses, mCherry gene is most commonly engineered into the NS segment, typically as an NS1 fusion protein or as an additional open reading frame immediately adjacent to nuclear export protein (NEP) []. By contrast, insertions of mCherry gene into the polymerase PA segment are less frequent []. However, published studies indicate that introducing fluorescent protein reporter genes into IAV impairs viral replication, compromises viral genetic stability, and leads to loss of reporter genes or mutations during serial passage or in vivo replication [,]. Consequently, improving the replication efficiency and enhancing the expression of reporter genes are critical for the development of in vivo imaging models of IAV and the assessment of antiviral drugs.
Non-coding regions (NCRs) play essential roles in the viral life cycle of IAV by regulating viral replication and transcription to maintain balanced gene expression and support efficient viral propagation [,,,]. Furthermore, sequence and structural variations in NCRs significantly influence viral adaptability and replication efficiency [,]. In recombinant IAVs expressing bioluminescent reporter genes, Zhao et al. introduced two mutations in the NS 3′-NCR, CE1 (G3A/C8U) and CE2 (G3A/U5C/C8U), to enhance vRNA replication and transcription and thereby restore replication–transcription balance following reporter gene insertions. CE1 mutation provides a moderate compensatory enhancement suitable for small-to-medium inserts, whereas CE2 mutation offers a stronger compensatory effect that can rescue and stably propagate large inserts but may overcompensate for small inserts [].
Therefore, mCherry gene was inserted into the NS segment of influenza A/Puerto Rico/8/1934 (H1N1-PR8), and CE1 mutation was introduced to generate H1N1-PR8-NSCE1-mCherry in this study. Using H1N1-PR8-NSCE1-mCherry, a real-time, non-invasive in vivo imaging platform was established to monitor IAV infection dynamics in mouse models. Furthermore, sensitivity and reliability of in vivo imaging platforms for antiviral drug screening were demonstrated by oseltamivir phosphate.
2. Materials and Methods
2.1. Cell Culture
Human embryonic kidney 293T cells and Madin-Darby canine kidney (MDCK) cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Procell, Wuhan, China) containing 10% fetal bovine serum (Vivacell, Shanghai, China), 100 U/mL penicillin (Gibco, Carlsbad, CA, USA) and 100 μg/mL streptomycin (Gibco, Carlsbad, CA, USA). All cells were maintained at 37°C and 5% CO2. For viral infection, MDCK cells were incubated in influenza viruses isolated serum free medium (Yocon, Beijing, China) containing 1.5 μg/mL TPCK-treated trypsin (Sigma, St. Louis, MO, USA).
2.2. Plasmid Construction
Plasmids (pDZ-PR8-PB2, pDZ-PR8-PB1, pDZ-PR8-PA, pDZ-PR8-HA, pDZ-PR8-NA, pDZ-PR8-M, pDZ-PR8-NP and pPOLI-NSCE1-Gluc) containing genome of H1N1-PR8 were provided by Dr. Ruikun Du. Plasmid pcDNA3.1-PGK-mCherry was provided by Dr. Fujun Qin. All primer sequences used in this study were listed in Supplementary Table S1.
NS vector sequences containing CE1 mutation were amplified from pPOLI-NSCE1-Gluc using primers pPOLI-NS-F and pPOLI-NS-R. mCherry gene fragment was amplified from pcDNA3.1-PGK-mCherry using primers mCherry-F and mCherry-R. Plasmid pPOLI-PR8-NSCE1-mCherry was constructed by homologous recombination of NS vector sequences and mCherry gene fragments using ClonExpress Ultra One Step Cloning Kit V2 (Vazyme, Nanjing, China).
To generate plasmid pPOLI-PR8-NSWT-mCherry containing the wild-type NCR, nucleotides (T3C and A8G) of the NCR in pPOLI-PR8-NSCE1-mCherry were mutated using Mut Express II Fast Mutagenesis Kit V2 (Vazyme, Nanjing, China) with primers 3′ NCR-NS-F and 3′ NCR-NS-R.
Recombinant plasmids were transformed into DH5α competent cells (TSingKe, Beijing, China) according to the manufacturer’s protocol. Positive clones were screened by colony PCR and verified by DNA sequencing (TSingKe, Beijing, China).
2.3. Rescue and Amplification of Recombinant Reporter Viruses
293T cells (1 × 106 cells/well) were seeded in 6-well plates and transfected upon reaching 50–60% confluency. Plasmid pPOLI-PR8-NSWT-mCherry or pPOLI-PR8-NSCE1-mCherry and seven other plasmids of H1N1-PR8 (pDZ-PR8-PB2, PB1, PA, HA, NA, M, NP; 500 ng) were co-transfected into 293T cells. MDCK cells (5 × 105 cells/well) and TPCK-treated trypsin were added at 12 h post-transfection (hpt) and 36 hpt, respectively. Supernatants were collected at 48 hpt and inoculated 9-day-old specific pathogen-free (SPF) chicken embryos to amplify viruses.
2.4. Virus Growth Kinetics and In Vitro Fluorescence Detection
MDCK cells (5 × 105 cells/well) in 6-well plates were infected with H1N1-PR8, H1N1-PR8-NSWT-mCherry or H1N1-PR8-NSCE1-mCherry at an MOI of 0.001 for 2 h at 37 °C. Then, supernatants were removed and influenza viruses isolated serum free medium containing TPCK-treated trypsin was added, followed by incubation at 37 °C. At the indicated times after infection, red fluorescent signals were monitored using a fluorescence microscope with a fixed 40 ms exposure time. Further, supernatants were collected for 50% tissue culture infectious dose (TCID50) assay.
2.5. TCID50 Assay
MDCK cells (5 × 103 cells/well) in 96-well plates were infected with 10-fold serial dilution of viruses for 48 h at 37 °C, and then cytopathic effects (CPE) were recorded. TCID50 was calculated using the Reed–Muench method.
2.6. Genome Genetic Stability Analysis
To evaluate genetic stability of the inserted mCherry gene, a serial passage experiment of H1N1-PR8-NSCE1-mCherry was performed in chicken embryos. Briefly, 6.32 × 103 TCID50 H1N1-PR8-NSCE1-mCherry was inoculated into 9-day-old SPF chicken embryo. Allantoic fluids were harvested at 48 h post-inoculation and used for TCID50 assay and RT-PCR.
2.7. RT-PCR
Viral RNA was extracted from allantoic fluids using TIANamp Viral RNA Kit (TianGen, Beijing, China) following the manufacturer’s protocol. mCherry gene fragment was amplified using primers (mCherry-F and mCherry-R) and HiScript® II One Step RT-PCR Kit (Vazyme, Nanjing, China). PCR products were analyzed by agarose gel electrophoresis.
2.8. Determination of Median Lethal Dose (LD50)
To evaluate viral pathogenicity, 18–20 g male BALB/c mice were anesthetized with isoflurane, and intranasally inoculated with 20 μL 10-fold serial dilutions of either H1N1-PR8 or H1N1-PR8-NSCE1-mCherry. Body weight and survival were recorded daily for 14 days post-inoculation. Mice were euthanized when body weight loss exceeded 25%. LD50 was determined using nonlinear regression.
2.9. Establishment of a Robust Live Imaging Animal Model for IAV
To establish a live imaging model for IAV, male C57BL/6J mice (18–20 g) were randomly assigned by body weight to control or model groups. Under isoflurane anesthesia, mice in the control group received 20 μL saline intranasally, while mice in the model group were inoculated intranasally with 20 μL H1N1-PR8-NSCE1-mCherry (1 × 104 TCID50). Mice were weighed daily for 14 days post-inoculation, and mCherry fluorescence signals were monitored at predefined time points using the Xenogen IVIS 200 imaging system (PerkinElmer, Waltham, USA) under isoflurane anesthesia. Data were analyzed using Living Image software (version 4.4).
2.10. Correlation Analysis Between Fluorescence Intensity and Viral Load
To investigate the correlation between fluorescence intensity and viral load, male C57BL/6J mice (18-20 g) were intranasally inoculated with 20 μL H1N1-PR8-NSCE1-mCherry (1 × 104 TCID50) under isoflurane anesthesia. At predefined time points, mice were anesthetized with isoflurane and mCherry fluorescence signals were monitored using the Xenogen IVIS 200 imaging system. Then, mice were euthanized and lung tissues were excised for in vitro mCherry fluorescence imaging using the Xenogen IVIS 200 imaging system. Viral load in lung tissues was quantified by qPCR.
2.11. qPCR
Total RNA was extracted from lung tissues using the RNAprep Pure Tissue Kit (TianGen, Beijing, China), then reverse-transcribed to cDNA using the FastKing RT Kit with gDNase (TianGen, Beijing, China). qPCR was performed using SYBR Green reagents (TianGen, Beijing, China). Plasmid pDZ-PR8-M was served as the quantification standard. Viral load was quantified via a standard curve by converting CT values to viral genome copy numbers.
2.12. Oseltamivir Intervention
For antiviral assessment, male C57BL/6J mice (18-20 g) were randomly assigned by body weight to control, model and oseltamivir groups. Under isoflurane anesthesia, mice in the control group received 20 μL saline intranasally, whereas mice in the model and oseltamivir groups were inoculated intranasally with 20 μL H1N1-PR8-NSCE1-mCherry (1 × 104 TCID50). Mice in the oseltamivir group were administered by oral gavage at 19.5 mg/kg oseltamivir phosphate. Mice in the control and model groups received distilled water. Treatment was administered once daily for 5 days, starting 1-day post-inoculation. mCherry fluorescence signals were monitored at predefined time points using the Xenogen IVIS 200 imaging system to assess antiviral efficacy.
2.13. Statistical Analysis
Data were expressed as mean ± SEM. Student’s t-test was used to determine statistical significance between two groups. Comparisons among three groups were performed by one-way ANOVA. Statistical significance was set at p < 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
3. Results
3.1. Generation of Recombinant IAV-Expressing mCherry
To generate recombinant IAV-expressing mCherry, mCherry coding sequences were inserted at nucleotide position 716 of NS segment (NSWT-mCherry; Figure 1A). In addition, PTV-1 2A peptide was used to facilitate co-translational separation of mCherry and NEP. Linker peptides (GSG) were inserted between NS1 and mCherry, as well as between mCherry and PTV-1 2A. To eliminate potential detrimental effects of mCherry insertion on viral replication, CE1 compensatory mutation (G3A/C8U) was introduced into the 3′-NCR of NS segment (NSCE1-mCherry; Figure 1A).
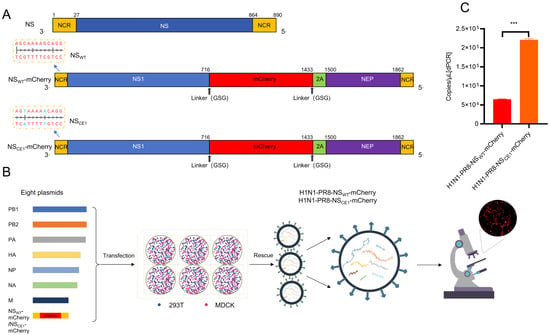
Figure 1.
Generation of H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry. (A) Schematic diagram of the natural and modified NS segment. NCR (yellow box), NS1 (blue box), NEP (purple box), mCherry (red box) and PTV-1 2A (green box) were shown. The introduced mutations at nucleotide positions 3 and 8 in the NCR were highlighted in blue. (B) Generation of reporter viruses by reverse genetics. (C) Quantification of viral gene copy numbers for reporter viruses by digital PCR. Data was presented as mean ± SEM of 2 independent replicates. *** p < 0.001, students’ t test.
Subsequently, recombinant influenza reporter viruses H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry were generated using the eight-plasmid reverse genetics system. Results showed that distinct red fluorescence signals were observed in MDCK cells at 24 hpt, which provided evidence for successful mCherry expression and virus rescue (Figure 1B). Then, supernatants were collected at 48 hpt and viral genome copy numbers were quantified by digital PCR. Results demonstrated that H1N1-PR8-NSCE1-mCherry exhibited significantly higher viral titers in the supernatants compared to H1N1-PR8-NSWT-mCherry. This finding suggested that CE1 mutation mitigated the detrimental effects of mCherry gene insertion on viral replication and enhanced viral production efficiency during the initial rescue phase (Figure 1C).
3.2. Characterization of H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry
To systematically evaluate the effects of mCherry gene insertion and CE1 mutation on IAV biology, mCherry expression and replication efficiency of H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry were compared. Results showed that H1N1-PR8-NSCE1-mCherry-infected cells exhibited significantly higher fluorescence intensity than H1N1-PR8-NSWT-mCherry-infected cells at all time points examined (Figure 2A), which indicated that CE1 mutation substantially enhanced mCherry expression. Although both recombinant viruses had the lower replication capacity than wild-type virus (H1N1-PR8), H1N1-PR8-NSCE1-mCherry exhibited higher replication efficiency than H1N1-PR8-NSWT-mCherry, reaching the titer of 108 TCID50/mL at 60 h post-infection (hpi), suggesting that CE1 mutation partially restored viral replication capacity compromised by mCherry gene insertion (Figure 2B).
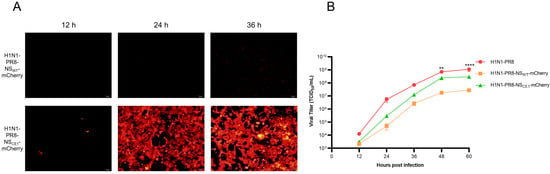
Figure 2.
mCherry expression and growth kinetics of H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry. (A) Red fluorescence signals of MDCK cells infected with H1N1-PR8-NSWT-mCherry or H1N1-PR8-NSCE1-mCherry. Scale bar: 100 μm. (B) Growth kinetics of H1N1-PR8, H1N1-PR8-NSWT-mCherry and H1N1-PR8-NSCE1-mCherry. Data was presented as mean ± SEM of 3 independent replicates. H1N1-PR8 vs. H1N1-PR8-NSCE1-mCherry: ** p < 0.01, **** p < 0.0001, one-way ANOVA.
Given that H1N1-PR8-NSCE1-mCherry demonstrated significantly superior virus rescue efficiency, mCherry expression, and replication capacity in vitro compared to H1N1-PR8-NSWT-mCherry, it was selected as the optimized reporter virus and used for subsequent investigations.
3.3. Genetic Stability and Pathogenicity of H1N1-PR8-NSCE1-mCherry
To evaluate genetic stability of H1N1-PR8-NSCE1-mCherry, serial passages of H1N1-PR8-NSCE1-mCherry in chicken embryos were performed. Agarose gel electrophoresis confirmed the stable maintenance of mCherry gene without deletion in all passaged viruses (Figure 3A). Furthermore, the high viral titers were detected throughout all passaged viruses, indicating that CE1 mutation retained stable replicative capacity during serial propagation (Figure 3B). Taken together, these results demonstrated the stability of H1N1-PR8-NSCE1-mCherry over at least five consecutive passages.
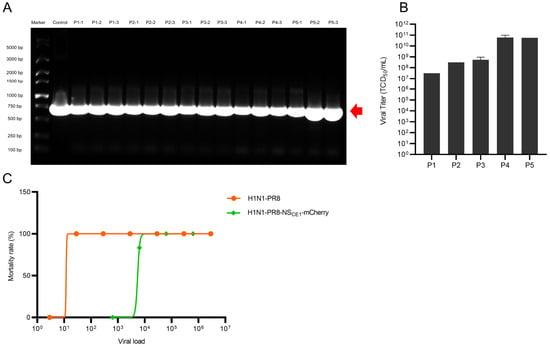
Figure 3.
Genetic stability and pathogenicity of H1N1-PR8-NSCE1-mCherry. (A) PCR analysis of mCherry gene insertion in passaged H1N1-PR8-NSCE1-mCherry. The red arrow indicated the position of band representing mCherry gene. (B) Viral titer per generation. Data was presented as mean ± SEM of 3 independent replicates. (C) LD50 of H1N1-PR8 and H1N1-PR8-NSCE1-mCherry. n = 6.
Subsequently, pathogenicity of H1N1-PR8-NSCE1-mCherry was assessed. H1N1-PR8 demonstrated higher virulence, which caused significant weight loss and mortality in mice at low doses (LD50 = 11.87 TCID50). In contrast, H1N1-PR8-NSCE1-mCherry showed a LD50 of 5443 TCID50, indicating that mCherry gene insertion significantly attenuated viral virulence (Figure 3C). Nevertheless, H1N1-PR8-NSCE1-mCherry still caused notable weight loss and lethality of mice (Figure S1).
3.4. Establishment of a Robust Live Imaging Animal Model Using H1N1-PR8-NSCE1-mCherry
To dynamically monitor the spatiotemporal distribution and replication of IAV in vivo, an imaging model using H1N1-PR8-NSCE1-mCherry was established in mice. Initial attempts using BALB/c mice failed to detect fluorescent signals, likely due to limited tissue penetration of mCherry fluorescence and interference from the white fur background. Consequently, male C57BL/6J mice were used to established imaging model via intranasal inoculation with H1N1-PR8-NSCE1-mCherry (Figure 4A). Results indicated that mCherry fluorescent signals were first detected in the nasal region at 2 days post-infection (dpi), and subsequently detected in the lungs at 3 dpi. Fluorescence intensity in lung showed progressive increase at 3–6 dpi, reaching the plateau phase with sustained high levels during 7–9 dpi. The fluorescent intensity then began to decline, with signals diminishing to levels comparable to the control group at 12 dpi (Figure 4B and C). Concurrently, mice infected with H1N1-PR8-NSCE1-mCherry exhibited body weight loss patterns consistent with viral replication (Figure 4D).
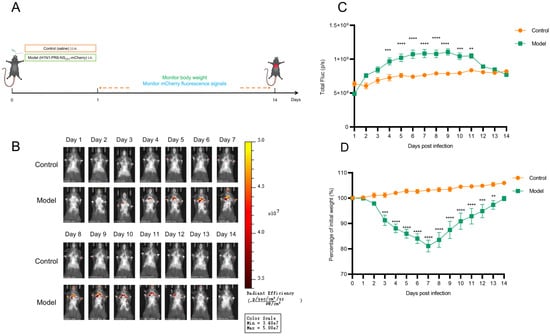
Figure 4.
In vivo visualization of H1N1-PR8-NSCE1-mCherry in C57BL/6J mice. (A) Schematic illustration of the experimental design. (B) Representative in vivo fluorescence images of mice in each group. (C) Longitudinal changes in lung fluorescence intensity of mice in each group. n = 6, data was presented as mean ± SEM. ** p < 0.01, *** p < 0.001, **** p < 0.0001, students’ t test. (D) Body weight changes in mice in each group. n= 6, data was presented as mean ± SEM. ** p < 0.01, *** p < 0.001, **** p < 0.0001, students’ t test.
Subsequently, correlation analysis between fluorescence intensity and viral load in mice lungs was quantified. Results demonstrated a significant association between fluorescence intensity and viral load in vivo (R2 = 0.48, p < 0.0001; Figure 5). In addition, correlation was stronger between fluorescence intensity and viral load in vitro (R2 = 0.69, p < 0.0001; Figure S2). These findings demonstrated that the H1N1-PR8-NSCE1-mCherry-based imaging model reflected viral replication dynamics in vivo and provided a method for real-time monitoring of IAV infection.
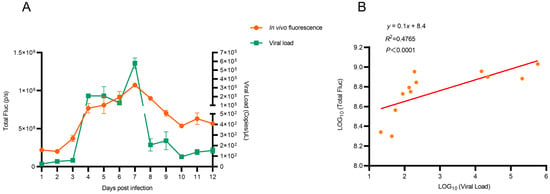
Figure 5.
Correlation between in vivo fluorescence intensity and viral load. (A) In vivo fluorescence signals and viral load in lungs. n= 3, data was presented as mean ± SEM. (B) Correlation between in vivo fluorescence intensity and viral load.
3.5. Application of H1N1-PR8-NSCE1-mCherry-Based Imaging Model for Antiviral Drug Screening
To validate H1N1-PR8-NSCE1-mCherry-based imaging model as a platform for antiviral drug evaluation, oseltamivir, which was a neuraminidase inhibitor that blocked viral particle release from infected cells, was selected as a representative compound (Figure 6A). Results indicated that model group exhibited significant weight loss, while the oseltamivir group showed attenuated weight loss (Figure 6B). These findings demonstrated that oseltamivir phosphate treatment significantly ameliorates clinical manifestations in mice infected with H1N1-PR8-NSCE1-mCherry. Additionally, oseltamivir phosphate treatment significantly reduced fluorescence intensity in lung at a clinically relevant dose (Figure 6C,D). These results indicated that the H1N1-PR8-NSCE1-mCherry-based imaging model provides an accurate method for assessing antiviral efficacy in vivo.
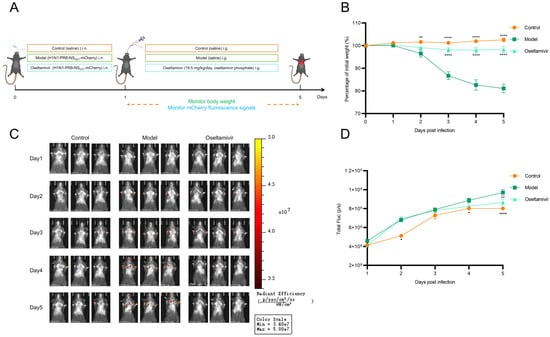
Figure 6.
Application of the H1N1-PR8-NSCE1-mCherry in antiviral drug research. (A) Schematic illustration of the experimental design. (B) Body weight changes in mice in each group. n = 6, data was presented as mean ± SEM. Control vs. model: ** p < 0.01, **** p < 0.0001; oseltamivir vs. model: **** p < 0.0001, one-way ANOVA (C) Representative in vivo fluorescence images of mice in each group. (D) Quantification of fluorescence intensity of mice in each group. n = 6, data was presented as mean ± SEM. Control vs. model: * p < 0.05, **** p < 0.0001; oseltamivir vs. model: ** p < 0.01, one-way ANOVA.
4. Discussion
Influenza reporter viruses are essential for probing infection dynamics and evaluating antiviral efficacy, because they enable real-time visualization of viral replication and dissemination. However, most recombinant reporter viruses exhibit attenuated replication capacity, which restrict their utility and reliability in vivo [,,]. Therefore, improving the replication capacity of influenza reporter viruses and enhancing the expression of reporter genes remain major challenges in the field.
Recently, studies of influenza reporter viruses expressing fluorescent proteins have increased markedly. Common fluorescent proteins for in vivo imaging include GFP and mCherry. Among these, mCherry is most frequently used for live animal imaging, owing to its longer emission wavelength and lower autofluorescence background [,]. Nogales et al. used reverse genetics to generate a recombinant reporter virus ΔNS1-mCherry, in which mCherry gene replaced the PR8 NS1 coding sequences (28-690 AA) []. In addition, Bu et al. used A/Swine/Guangdong/GLW/2018 (H1N1) as the parental strain and generated GLW/18-MA-mCherry by inserting mCherry gene with PTV-1 2A at the PA C-terminus []. However, both ΔNS1-mCherry and GLW/18-MA-mCherry replicated less efficiently than wild-type virus. Consistent with these reports, H1N1-PR8-NSWT-mCherry also exhibited reduced replication efficiency (Figure 2B).
Several strategies have been employed to mitigate the reduction in viral replication associated with reporter genes insertion in recombinant influenza viruses. These include codon optimization of reporter genes, careful selection of genomic segment used for reporter genes insertion (e.g., PA, PB1 or NS) and balancing of promoter-driven replication and transcription through modifications in the NCRs [,,]. To improve the replication capacity of influenza viruses expressing mCherry, a balance compensation strategy was adopted to construct H1N1-PR8-NSCE1-mCherry by introducing CE1 mutation into 3′-NCR of NS segment in this study. Compared with H1N1-PR8-NSWT-mCherry, CE1 mutation mitigated the mCherry gene insertion-associated replication defects and H1N1-PR8-NSCE1-mCherry showed a higher rescue rate (Figure 1C). Meanwhile, peak titers of H1N1-PR8-NSCE1-mCherry were approximately 10.79-fold higher than H1N1-PR8-NSWT-mCherry and about 3.78-fold lower than H1N1-PR8 in MDCK cells (Figure 2B). In parallel, CE1 mutation enhanced mCherry expression in vitro, producing stronger and earlier mCherry fluorescence signals. H1N1-PR8-NSCE1-mCherry produced detectable fluorescence signal at 12 hpi, whereas H1N1-PR8-NSWT-mCherry showed only a faint red signal even at 36 hpi (Figure 2A). Overall, H1N1-PR8-NSCE1-mCherry, which carries the CE1 mutation, shows greater replicative capacity and stronger mCherry expression than H1N1-PR8-NSWT-mCherry.
The reason for the greater replicative capacity and stronger mCherry expression of H1N1-PR8-NSCE1-mCherry may be that the CE1 mutation (G3A/C8U) altered terminal 3′-5′ base pairing, making viral RNA (vRNA)promoter more likely to form and stably maintain the pan-handle conformation. Pan-handle conformation enhances the recognition efficiency of RNA-dependent RNA polymerase, thereby promoting the initiation of vRNA replication and transcription and consequently compensating for the loss of viral replication capacity caused by the insertion of mCherry gene [,,]. In addition to restoring the replication–transcription balance, we hypothesize that CE1-enhanced promoter activity in the modified NS segment may also impact innate immune regulation. Increased promoter activity could elevate NS1 expression, thereby suppressing type I interferon and interferon-stimulated gene (ISG) expression. At the same time, alterations in the stability of the 3′-NCR pan-handle structure may influence how vRNA was recognized by cytoplasmic pattern recognition receptors and affect the production of aberrant viral RNAs (such as defective interfering RNAs), which in turn modulate innate immune sensing [,,]. Consistent with this, Zhao et al. showed that introducing CE1 or CE2 (G3A/U5C/C8U) mutations into 3′-NCR of NS gene in recombinant influenza viruses expressing Gluc could enhance viral RNA synthesis and help reporter viruses re-establish a replication–transcription balance that more closely resembles the physiological state []. CE2 mutation produces a stronger promoter-enhancing effect than CE1 mutation and is therefore a promising candidate to compensate larger exogenous gene insertions. Therefore, CE2 mutation was introduced into 3′-NCR to compare which CE2 or CE1 more effectively enhances mCherry expression in future. Given the conserved nature of 3′-NCR sequences, the balance compensation strategy may be applicable to other influenza virus subtypes (e.g., H3N2, H5N1 or H7N9). However, effectiveness of balance compensation strategy beyond the PR8 background requires experimental validation.
During model development, ΔNS1-mCherry failed to support fluorescence imaging in vivo, whereas GLW/18-MA-mCherry enabled real-time non-invasive fluorescence imaging. Based on H1N1-PR8-NSCE1-mCherry, we established an in vivo visualization model for IAV that exhibited a stable replication window, with peak fluorescence at 9 dpi. However, unlike GLW/18-MA-mCherry, H1N1-PR8-NSCE1-mCherry did not produce detectable fluorescence signals in BALB/c mice. Several factors may account for this discrepancy: (1) Differences in inoculum doses: H1N1-PR8-NSCE1-mCherry was administered at 1 × 104 TCID50 (≈7 × 103 PFU), whereas GLW/18-MA-mCherry was given at 1 × 105 PFU. The lower inoculum of H1N1-PR8-NSCE1-mCherry may have produced weaker fluorescence signals, reducing detectability under the imaging conditions used. (2) Differences between imaging platforms: GLW/18-MA-mCherry and H1N1-PR8-NSCE1-mCherry were imaged using the PerkinElmer IVIS Lumina III and Xenogen IVIS 200, respectively. Variations in system sensitivity, filter sets and optical calibration between the two platforms may have affected signal detectability. (3) Sex-related differences in murine models: Female and male BALB/c mice were infected with GLW/18-MA-mCherry and H1N1-PR8-NSCE1-mCherry, respectively. Sex-dependent biological factors are likely to underlie the observed variability in imaging outcomes. Innate and adaptive immunity (for example, interferon signaling and recruitment of macrophages and neutrophils) were modulated by sex hormones, which can lead to changes in viral replication kinetics and thereby alter the timing and magnitude of mCherry expression.
Despite its utility, use of mCherry as a reporter gene for in vivo imaging has inherent limitations. Fluorescence signals of mCherry, while superior to GFP, have limited tissue penetration depth due to absorption and scattering by biological tissues []. This likely contributed to our initial failure to detect signals in BALB/c mice and limits the sensitivity for detecting deep-seated or low-level infections. Furthermore, sensitivity of mCherry was lower compared to near-infrared fluorescent proteins or luciferase-based bioluminescence systems []. To address these issues, future works should (1) use red-shifted or near-infrared reporters (e.g., iRFP) to improve tissue penetration, (2) optimize imaging hardware and acquisition settings to increase signal-to-noise ratio, (3) apply basic spectral unmixing or denoising and (4) enhance fluorescence signals by optimizing the expression of fluorescent proteins.
Using oseltamivir phosphate as a positive control, feasibility and reliability of the H1N1-PR8-NSCE1-mCherry-based mice model for visualizing antiviral drug efficacy were assessed in this study. The efficacy of other antiviral agents (e.g., polymerase cap-dependent endonuclease inhibitor baloxavir marboxil and M2 ion channel blocker amantadine) and traditional Chinese medicines that were used in this model should be explored in future studies. Moreover, combining this model with other fluorescent proteins or bioluminescent reporters could enable simultaneous detection of diverse biological signals, further expanding its applicability in antiviral drug research [,].
5. Conclusions
In summary, the mCherry-expressing influenza reporter virus H1N1-PR8-NSCE1-mCherry was constructed by introducing CE1 mutation (G3A/C8U) in this study. Compared with the uncompensated control H1N1-PR8-NSWT-mCherry, H1N1-PR8-NSCE1-mCherry exhibits enhanced viral replication efficiency and mCherry expression levels in vitro. Meanwhile, H1N1-PR8-NSCE1-mCherry was used to establish an in vivo visualization model of IAV for real-time viral infection monitoring and antiviral drug efficacy evaluation. These results suggested that CE1 mutation represents a promising strategy for generating influenza reporter viruses with enhanced viral replication efficiency and reporter gene expression, expanding its application prospects in monitoring influenza virus infection dynamics and assessing drug efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17121537/s1, Figure S1: Pathogenicity of H1N1-PR8 and H1N1-PR8-NSCE1-mCherry in BALB/c mice; Figure S2: Correlation between in vitro fluorescence intensity and viral load; Table S1: Primers.
Author Contributions
Conceptualization, Y.Y. and X.L.; Data curation, Z.L. and X.L.; Formal analysis, R.D. and S.C.; Funding acquisition, J.Y., Q.S., R.R. and Y.Y.; Investigation, Z.L. and M.L.; Resources, R.D.; Supervision, R.R., Y.Y. and X.L.; Visualization, D.Y. and W.Z.; Writing—original draft, Z.L.; Writing—review and editing, J.Y., Q.S. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant Number: 82274397, 82505208 and 82574901), the 2023 Annual Jinan City-University Integration Development Strategy Project (Grant Number: JNSX2023054), Shandong Province Technological Innovation Guidance Program (Grant Number: YDZX2023044), Shandong University of Traditional Chinese Medicine Research Fund (Grant Number: KY2024Z04), Key Discipline Construction Project of High-level Traditional Chinese Medicine by National Administration of Traditional Chinese Medicine (Grant Number: ZYYZDXK-2023119), China Postdoctoral Science Foundation (Grant Number: 2025M773975) and Shandong Postdoctoral Science Foundation (Grant Number: SDCX-ZG-202503079).
Institutional Review Board Statement
This ethics application (SDUTCM20240520101, approved on 20 May 2024) was approved by the Institutional Animal Care and Use Committee of Shandong University of Traditional Chinese Medicine.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
Authors Wanhui Zhou and Shijuan Cheng were employed by the company Shandong Wohua Pharmaceutical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FBS | Fetal bovine serum |
| GFP | GFP Green fluorescent protein |
| GLuc | Gaussia luciferase |
| HA | Hemagglutinin |
| IVIS | In vivo imaging system |
| LD50 | Median lethal dose |
| M | Matrix protein |
| MOI | Multiplicity of infection |
| NA | Neuraminidase |
| NCR | Non-coding region |
| NEP | Nuclear export protein |
| NP | Nucleoprotein |
| NS | Nonstructural protein |
| NS1 | Nonstructural protein 1 |
| PA | Polymerase acidic protein |
| PB2 | Polymerase basic 2 protein |
| PBS | Phosphate-buffer saline |
| PCR | Polymerase chain Reaction |
| PTV | Porcine teschovirus |
| TCID50 | Median tissue culture infective dose |
References
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Liang, G.-D.; Chen, Z.-X.; Zhang, K.; Liang, J.-L.; Jiang, L.-R.; Yang, S.-Z.; Jiang, F.; Liu, S.-W.; Yang, J. A small molecule compound targeting hemagglutinin inhibits influenza A virus and exhibits broad-spectrum antiviral activity. Acta Pharmacol. Sin. 2024, 45, 2380–2393. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Musharrafieh, R.; Yin, H.; Wang, J. Focusing on the Influenza Virus Polymerase Complex: Recent Progress in Drug Discovery and Assay Development. Curr. Med. Chem. 2019, 26, 2243–2263. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wu, P.; Peng, Z.; Wang, X.; Chen, T.; Wong, J.Y.T.; Yang, J.; Bond, H.S.; Wang, L.; et al. Influenza-associated excess respiratory mortality in China, 2010–2015: A population-based study. Lancet Public Health 2019, 4, e473–e481. [Google Scholar] [CrossRef]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Hause, B.; Stigger-Rosser, E.; Darnell, D.; Corzo, C.; Juleen, K.; Simonson, R.; Brockwell-Staats, C.; Rubrum, A.; Wang, D.; et al. Multiple Reassortment between Pandemic (H1N1) 2009 and Endemic Influenza Viruses in Pigs, United States. Emerg. Infect. Dis. 2011, 17, 1624–1629. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, X.; Li, P.; Chen, Z.; Zhang, C.; Manicassamy, B.; Rong, L.; Cui, Q.; Du, R. Expanding the tolerance of segmented Influenza A Virus genome using a balance compensation strategy. PLoS Pathog. 2022, 18, e1010756. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, Y.; Lin, M.Z.; Ronald, J.A. Brightening up Biology: Advances in Luciferase Systems for in Vivo Imaging. ACS Chem. Biol. 2021, 16, 2707–2718. [Google Scholar] [CrossRef]
- Tran, V.; Moser, L.A.; Poole, D.S.; Mehle, A. Highly Sensitive Real-Time In Vivo Imaging of an Influenza Reporter Virus Reveals Dynamics of Replication and Spread. J. Virol. 2013, 87, 13321–13329. [Google Scholar] [CrossRef]
- Barre, R.S.; Escobedo, R.A.; Castro, E.M.; Gazi, M.; Castro, J.D.; Cupic, A.; Bayoumi, M.; Jackson, N.; Ye, C.; Nogales, A.; et al. A human H5N1 influenza virus expressing bioluminescence for evaluating viral infection and identifying therapeutic interventions. iScience 2025, 28, 113402. [Google Scholar] [CrossRef] [PubMed]
- Barre, R.S.; Mostafa, A.; Chiem, K.; Pearl, R.L.; Platt, R.N.; Cupic, A.; Anderson, T.J.C.; Knaus, U.G.; Albrecht, R.A.; García-Sastre, A.; et al. Bioluminescent reporter influenza A viruses to track viral infections. Microbiol. Spectr. 2025, 13, e0215025. [Google Scholar] [CrossRef]
- Shaner, N.C.; Lambert, G.G.; Chammas, A.; Ni, Y.; Cranfill, P.J.; Baird, M.A.; Sell, B.R.; Allen, J.R.; Day, R.N.; Israelsson, M.; et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 2013, 10, 407–409. [Google Scholar] [CrossRef]
- Nogales, A.; Schotsaert, M.; Rathnasinghe, R.; DeDiego, M.L.; García-Sastre, A.; Martinez-Sobrido, L. Replication-Competent ΔNS1 Influenza A Viruses Expressing Reporter Genes. Viruses 2021, 13, 698. [Google Scholar] [CrossRef]
- Cloin, B.M.C.; De Zitter, E.; Salas, D.; Gielen, V.; Folkers, G.E.; Mikhaylova, M.; Bergeler, M.; Krajnik, B.; Harvey, J.; Hoogenraad, C.C.; et al. Efficient switching of mCherry fluorescence using chemical caging. Proc. Natl. Acad. Sci. USA 2017, 114, 7013–7018. [Google Scholar] [CrossRef] [PubMed]
- Viotti, M.; Nowotschin, S.; Hadjantonakis, A.K. Afp::mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm. Genesis 2011, 49, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Reuther, P.; Göpfert, K.; Dudek, A.H.; Heiner, M.; Herold, S.; Schwemmle, M. Generation of a variety of stable Influenza A reporter viruses by genetic engineering of the NS gene segment. Sci. Rep. 2015, 5, 11346. [Google Scholar] [CrossRef]
- Bu, L.; Chen, B.; Xing, L.; Cai, X.; Liang, S.; Zhang, L.; Wang, X.; Song, W. Generation of a pdmH1N1 2018 Influenza A Reporter Virus Carrying a mCherry Fluorescent Protein in the PA Segment. Front. Cell Infect. Microbiol. 2022, 11, 827790. [Google Scholar] [CrossRef]
- Breen, M.; Nogales, A.; Baker, S.F.; Martínez-Sobrido, L. Replication-Competent Influenza A Viruses Expressing Reporter Genes. Viruses 2016, 8, 179. [Google Scholar] [CrossRef]
- Martinez-Sobrido, L.; Nogales, A. Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment. Int. J. Mol. Sci. 2024, 25, 10584. [Google Scholar] [CrossRef]
- Pflug, A.; Lukarska, M.; Resa-Infante, P.; Reich, S.; Cusack, S. Structural insights into RNA synthesis by the influenza virus transcription-replication machine. Virus Res. 2017, 234, 103–117. [Google Scholar] [CrossRef]
- Tagawa, T.; Serquiña, A.; Kook, I.; Ziegelbauer, J. Viral non-coding RNAs: Stealth strategies in the tug-of-war between humans and herpesviruses. Semin. Cell Dev. Biol. 2021, 111, 135–147. [Google Scholar] [CrossRef]
- Ye, L.; Ambi, U.B.; Olguin-Nava, M.; Gribling-Burrer, A.-S.; Ahmad, S.; Bohn, P.; Weber, M.M.; Smyth, R.P. RNA Structures and Their Role in Selective Genome Packaging. Viruses 2021, 13, 1788. [Google Scholar] [CrossRef]
- Girard, J.; Jakob, C.; Toews, L.K.; Fuchs, J.; Pohlmann, A.; Franzke, K.; Kolesnikova, L.; Jeney, C.; Beer, M.; Bron, P.; et al. Disruption of influenza virus packaging signals results in various misassembled genome complexes. J. Virol. 2023, 97, e0107623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, M.; Zheng, Q.; Gao, R.; Liu, X. Packaging signal of influenza A virus. Virol J. 2021, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Dumm, R.E.; Heaton, N.S. The Development and Use of Reporter Influenza B Viruses. Viruses 2019, 11, 736. [Google Scholar] [CrossRef]
- Ellis, D.; Lederhofer, J.; Acton, O.J.; Tsybovsky, Y.; Kephart, S.; Yap, C.; Gillespie, R.A.; Creanga, A.; Olshefsky, A.; Stephens, T.; et al. Structure-based design of stabilized recombinant influenza neuraminidase tetramers. Nat. Commun. 2022, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Yamada, S.; da Silva Lopes, T.J.; Dutta, J.; Khan, Z.; Kriti, D.; van Bakel, H.; Kawaoka, Y. Influenza Virus Polymerase Mutation Stabilizes a Foreign Gene Inserted into the Virus Genome by Enhancing the Transcription/Replication Efficiency of the Modified Segment. mBio 2019, 10, e01794-19. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Parada, G.E.; Hemberg, M. Secondary structures in RNA synthesis, splicing and translation. Comput. Struct. Biotechnol. J. 2022, 20, 2871–2884. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C.; Poiret, S.; Salomé Desnoulez, S.; Foligné, B.; Lafont, F.; Daniel, C. Study of the persistence and dynamics of recombinant mCherry-producing Yarrowia lipolytica strains in the mouse intestine using fluorescence imaging. Microb. Biotechnol. 2022, 16, 618–631. [Google Scholar] [CrossRef]
- Deng, H.; Cao, H.; Wang, Y.; Li, J.; Dai, J.; Li, L.-F.; Qiu, H.-J.; Li, S. Viral replication organelles: The highly complex and programmed replication machinery. Front. Microbiol. 2024, 15, 1450060. [Google Scholar] [CrossRef]
- Kleinehr, J.; Bojarzyn, C.R.; Schöfbänker, M.; Daniel, K.; Ludwig, S.; Hrincius, E.R. Metabolic interference impairs influenza A virus replication by dampening vRNA synthesis. npj Viruses 2025, 3, 22. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Gerasimova, Y.; Nanduri, R.; Lama Tamang, P.; Kandala, M.; Kelesidis, T. The Role of Inflammation in the Pathogenesis of Viral Respiratory Infections. Microorganisms 2024, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.P.; Bailey, K.; Lewis, P.J. Stage-specific fluorescence intensity of GFP and mCherry during sporulation In Bacillus Subtilis. BMC Res. Notes 2010, 3, 303. [Google Scholar] [CrossRef] [PubMed]
- Oliinyk, O.S.; Ma, C.; Pletnev, S.; Baloban, M.; Taboada, C.; Sheng, H.; Yao, J.; Verkhusha, V.V. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat. Methods 2023, 20, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.-S.; Li, X.-H.; Zhang, S.; Zeng, D.-D.; Cai, Y.-R.; Peng, D.-X.; Jiang, T.; Shi, J.-P.; Li, J. Screening for anti-influenza virus compounds from traditional Mongolian medicine by GFP-based reporter virus. Front. Cell Infect. Microbiol. 2024, 14, 1431979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).