Abstract
Among emerging zoonotic pathogens, mosquito-borne viruses (MBVs) circulate between vertebrate animals and mosquitoes and represent a serious threat to humans via spillover from enzootic cycles to the human community. Active surveillance of MBVs in their vectors is therefore essential to better understand and prevent spillover and emergence, especially at the human–animal interface. In this study, we assessed the presence of MBVs using molecular and phylogenetic methods in mosquitoes collected along an ecological gradient ranging from rural urbanized areas to highland forest areas in northern Thailand. We have detected the presence of insect specific flaviviruses in our samples, and the presence of the emerging zoonotic Tembusu virus (TMUV). Reported for the first time in 1955 in Malaysia, TMUV remained for a long time in the shadow of other flaviviruses such as dengue virus or the Japanese encephalitis virus. In this study, we identified two new TMUV strains belonging to cluster 3, which seems to be endemic in rural areas of Thailand and highlighted the genetic specificities of this Thai cluster. Our results show the active circulation of this emerging flavivirus in Thailand and the need for continuous investigation on this poorly known but threatening virus in Asia.
1. Introduction
Mosquito-borne viruses (MBVs) of zoonotic origins are responsible for multiple animal and human diseases worldwide and represent a large reservoir of viruses with emergence potential via spillover from their enzootic cycles. Tembusu virus (TMUV) is an emerging mosquito-borne flavivirus that belongs to the Ntaya serocomplex, including Ntaya virus, Bagaza virus and Israel Turkey virus (refer to the ICTV database—https://ictv.global/report/chapter/flaviviridae/flaviviridae/orthoflavivirus (accessed on 26 June 2023)). Similar to other flaviviruses, including DENV and JEV, TMUV is an enveloped, positive-sense single-stranded RNA virus with an approximately 11-kb genome (reviewed in [1]). Despite recent sporadic outbreaks, knowledge on TMUV ecology and biology remains fragmented, precluding a thorough evaluation of its emergence potential.
TMUV was first isolated in Malaysia in 1955 [2], before being reported in different surveys in Asia and Southeast Asia (SEA) including China, Malaysia, Taiwan and Thailand [3,4,5,6]. TMUV has been intermittently reported in wild and domestic birds and in trapped mosquitoes [7,8]. TMUV infects a wide variety of avian species such as ducks, geese, chickens, sparrows and pigeons [1]. Since 2000, new variants of TMUV have been reported to cause several outbreaks in poultry and birds. Symptoms include dramatic decreases in egg production, severe neurologic disorders and retarded growth [5,7]. Migration of wild birds close to poultry farms could allow transmission to domestic ducks, while retention of the virus in high-density duck-producing areas could facilitate the rapid spread of the disease. Because of the symptom severity in ducks and the economic importance of ducks, some reports named the new viral variant Duck-TMUV (DTMUV) [3]. Nonetheless, hereafter, we will use TMUV as a generic term to refer to all viruses belonging to the TMUV phylogenetic group. TMUVs are phylogenetically divided into two lineages: the “TMUV lineage” including the original viruses, and the “DTMUV lineage”. The DTMUV lineage is divided into three different clusters, named “TMUV cluster-1”, “TMUV cluster-2” (with sub-cluster a and b) and “TMUV cluster-3” [9]. Culex mosquitoes are likely the main vector of transmission, as TMUVs have been isolated from several Culex species such as Culex tritaeniorhynchus, Cx. Vishnui, and Cx. Gellidus [1,10]. In addition to vector transmission, vertical transmission and non-vector transmission in birds (by air droplet exposure or by close contact) are suspected [11,12,13].
Located in the heart of South East Asia, Thailand has tight interactions with surrounding countries, including China and Laos. Endemic transmission of numerous mosquito-borne flaviviruses such as JEV and DENV occurs in Thailand, and other arboviruses associated with diseases in humans [14,15]. Thailand is largely covered with forests and rural areas, with an increasing entanglement of rural and urban territories and a densification of urban areas. In Thailand, TMUV was isolated in mosquitoes from the rural parts of the country, including the provinces of Kamphaengphet [8], Chiang Mai [16] and Kanchanaburi [5,17]. TMUV strains were also detected in broiler and layer ducks from the provinces of Chonburi, Nakhom Pathom, Nakhon Ratchasima, Prachinburi and Signburi [5]. Such recurrent detection indicates a wide distribution of TMUV in Thailand. Accordingly, in 2013, TMUV outbreaks occurred throughout the year (August 2013–September 2014) and many farms were affected, leading to losses in the poultry industry. Alarmingly, seroconversion was detected in humans, irrespective of contact with ducks, suggesting a zoonotic emergence of TMUV [18]. However, the serological survey in humans was conducted with a limited number of samples, and the survey lacked methodological details. In this context, TMUV surveillance in animals and vectors is essential to prevent agro-economical losses and evaluate emergence in humans.
In this study, we conducted agnostic arbovirus surveillance in mosquitoes along an ecological gradient in the Thai northern province of Nan, which shares a border with Lao PDR. The sub-district of Saenthong in the province of Nan is divided into two geographical landscape types: an agricultural lowland, including low-density urbanized villages, and a highland with sparse villages and an agricultural zone embedded in the forest zone located close to the protected Nanthaburi National Park. This contrasted area is separated by a transition zone including agricultural areas with rice paddy fields and dwellings. These landscapes provided an ideal study area with low and high levels of human-impacted habitats to study the ecology of MBVs and their mosquito vectors. In the different landscapes we sampled, we detected TMUV and insect-specific flaviviruses in the transition area, and in the lowland as well as the forest. We further characterized the phylogeny of the new TMUV strains, revealing a potential endemic cluster in Thailand.
2. Materials and Methods
2.1. Mosquito Collections
Mosquitoes were collected in the province of Nan in the northern part of Thailand (Figure 1a). The survey area was located in the Saen Thong sub-district of the Tha Wang Pha district, a rural area localized in an ecological gradient between forests, paddy fields lowlands and peridomestic urban areas. The eight collection points were distributed along a transect covering eight villages and a forested area (three sessions) (Figure 1a,b). The research proposal, involving specimen collection in Nan province, was approved by the Faculty of Tropical Medicine, Mahidol University, under agreement number FTM ECF-033-00.
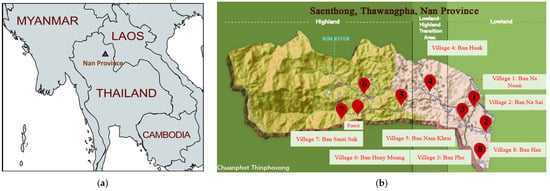
Figure 1.
Geographic localization of the study area. (a) Localization of Nan province in Thailand. (map created with mapchart.net) and (b) localization of the collection sites in the Saen Thong sub-district, Tha Wang Pha district (design credit to Chuanphot Thinphovong) on a schematic flat map reflecting the different areas.
Samples were collected using BG-sentinel traps combined with BG Lure (Biogents AG, Regensburg, Germany). BG-sentinel traps operated for 48 h, for day- and night-time sessions.
Mosquito specimens were transported in cold boxes containing frozen cold packs to the field laboratory, for sorting up to the species identification level. In cases in which species could not be determined, specimens were grouped according to their genus only, and noted “Genus” sp. Mosquitoes were sorted and pooled in a 15 mL tube according to genus, sex and collection site, and then stored frozen. The samples were then transported in a liquid nitrogen tank to the department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, where samples were identified up to the species level following the morphological identification keys outlined by Rattanarithikul, R. et al. [19,20,21]. Mosquitoes were identified on a chilled table set to −4 °C, then mosquitoes were pooled according to species, sex and collection site and stored in a −80 °C freezer.
Mosquito pools (1 to 15 specimens per pool) were made according to mosquito species, sex and collection location. Stainless steel beads (5 mm diameter) were added to tubes containing mosquitoes before homogenizing using a TissueLyzer (Qiagen, Hilden, Germany) at 50 cycles/s for 5 min in 500 µL of DMEM medium (Gibco, Waltham, MA, USA), complemented with 1% of penicillin/streptomycin solution (Gibco, Waltham, MA, USA) and 1× of Fungizone solution (Gibco, Waltham, MA, USA). After homogenization, an additional 1 mL of DMEM medium was added. Tubes were clarified using centrifugation at 13,000× g at 4 °C for 10 min. The supernatants were collected and filtered, using an 0.2 µm syringe filter (Sartorius, Bangkok, Thailand), into 1.5 mL tube and stored at −80 °C before RNA extraction.
2.2. RNA Extraction and Reverse Transcription
Viral RNA was extracted from the supernatant of mosquito homogenate using a NucleoSpin® virus kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol. Briefly, a 200 µL of homogenized sample was lysed in 5 µL of proteinase K and 200 µL lysis buffer containing guanidine hydrochloride. Carrier RNA was then added to the mixture, and the viral nucleic acid was then extracted and collected in an elution volume of 30 µL of RNase-free water. Purified RNA extracts were stored at −80 °C until virus screening using RT-PCR. Reverse transcription (RT) was performed using an M-MLV reverse transcriptase kit (Promega, Madison, WI, USA) on 14 µL of an RNA sample, following the manufacturer’s instructions. cDNA was stored at −20 °C until subsequent analyses.
2.3. Detection of Flaviviruses and Alphaviruses Using PCR
The pan-flavivirus primers [22] PFlav-fAAR (5′-TACAACATGATGGGAAAGAGAGAGAARAA-3′) and PFlav-rKR (5′-GTGTCCCAKCCRGCTGTGTCATC-3′) were used to amplify a 256 base pair(bp) region of the NS5 gene of Flaviviruses. PCR was performed using GoTaq G2 Master Mix (Promega, Charbonnières-les-Bains, France) and 2 µL of cDNA with the following parameters: 95 °C for 3 min, 45 cycles of 95 °C 15 s, 56 °C 15 s, 72 °C 20 s and 72 °C for 2 min. PCR products were visualized on 1.8% agarose gel. Amplicons were purified from gel using a PureLink Gel extraction kit (Thermo Fisher Scientific, Illkirch-Graffenstaden, France) and stored at −20 °C.
The pan-alphavirus primers [23] PanAlpha F2A forward primer (5′-ATGATGAARTCIGGIATGTTYYT-3′), and reverse primers R2A (5′-ATYTTIACTTCCATGTTCATCCA-3′), R3A (5′-ATYTTIACTTCCATRTTCARCCA-3′), R4A (5′-ATYTTIACTTCCATGTTGACCCA-3′) were used to amplify a 200-pb region of the nsP4 gene in the alphavirus genome. PCR was performed using GoTaq G2 Master Mix (Promega, Charbonnières-les-Bains, France) and 2 µL of cDNA with the following parameters: 95 °C for 3 min, 45 cycles of 95 °C 15 s, 54 °C 15 s, 72 °C 20 s and 72 °C for 2 min. PCR products were visualized on 1.8% agarose gel.
All purified amplicons obtained with pan-flavivirus- or pan-alphavirus-PCR were characterized using Sanger sequencing in both forward and reverse directions (Eurofins, Vergèze, France). Sequence identities were determined via BLAST alignment (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 26 June 2023).
2.4. TMUV Envelope Sequencing
A 1503-bp amplicon covering the entire TMUV envelope gene was amplified using the primers TMUV-E_F (5′-TTCAGCTGTCTGGGGATGCA-3′) and TMUV-E_R (5′-GGCATTGACATTTACTGCCA-3′). PCR amplification was conducted from 2 µL of cDNA using Q5 High Fidelity DNA Polymerase (NEB, Évry-Courcouronnes, France) and the following parameters: 98 °C for 1 min, 40 cycles of 98 °C 10 s, 60 °C 15 s, 72 °C 60 s and 72 °C for 2 min. PCR products were visualized on 1.5% agarose gel, and amplicons were gel-purified using a PureLink Gel extraction kit (Thermo Fisher Scientific, Illkirch-Graffenstaden, France) and stored at −20 °C. Purified amplicons were sequenced via Sanger sequencing in both forward and reverse directions (Eurofins, Vergèze, France).
2.5. Phylogenic Analysis
To characterize TMUV isolated from mosquito homogenates, sequences encoding TMUV Envelope were subjected to phylogenetic analysis, along with representative TMUV sequences obtained from the NCBI GenBank database (Table 1).

Table 1.
TMUV sequences used in the phylogenetic tree, including the two strains identified in this study.
Reference sequences were selected to cover all the diversity of TMUV strains and a broad range of geographical origins. All sequences were referenced into the phylogenetic tree in a format consisting of “accession number_country_year of isolation”. Multiple sequence alignments and edits were carried out using MEGA 11. Sequences were edited and sites that could not be unambiguously aligned were excluded from the analyses. Maximum likelihood trees were constructed using PhyML software [24,25] with the best-fit nucleotide substitution model (GTR+G) identified by Akaike’s information criterion (AIC). The bootstrap method was used to estimate the robustness of nodes with 1000 iterations. Phylogenetic trees were edited using FigTree v1.4.4 software. Sequences of Zika virus (ZIKV) (GenBank number: KY766069), JEV (GenBank number: NC001437) and West Nile virus (WNV) (GenBank number: NC009942) were used as the outgroup to provide a relative framework for analyzing the phylogenetic differences between viruses within the Ntaya complex. All sequences from this study have been deposited in the GenBank database, and their accession numbers are shown in Table 1 Amino acid sequences were aligned using the MEGA 11 program to identify specific amino acid variations in the envelop protein sequences of TMUV strains.
3. Results
3.1. Collection of Mosquitoes
Mosquitoes were collected in eight villages and in one forested area (over three sessions) from the 19th to 26th of July 2019 in the Saenthong sub-district of the province of Nan, Thailand (Figure 1b). The Saenthong sub-district is located in the rural district of Thawangpha. Samples were collected along an ecological gradient from lowland areas, including villages, farms, and agricultural areas (village 1, 2, 3 and 8), to highland areas wherein three villages (village 5, 6 and 7) are located, with small agricultural zones surrounded by a vast forest area. Village 4 is located in a transition zone between lowland and highland (Figure 1b).
A total of 596 mosquitoes were collected and homogenized in 116 pools (Table 2). genera total of 5 genera of Culicidae were reported, including 12 species. Some specimens were damaged during collection, making it impossible to identify the species. In these cases, specimens have been listed as “Genus” sp. (Table 2). Overall, the Culex genus was the most represented (75%, n = 436), with Cx. vishnui representing the most prevalent species (39.8%, n = 237). The Aedes genus represented 15.4% (n = 92), and Armigeres 8.9% (n = 53), while Mansonia and Toxorhynchites both represented 0.17% (n = 1). However, the mosquito genera largely varied depending on location. All Toxorhynchites (n = 1), 78.3% of Aedes (n = 72) and 50.9% of Armigeres (n = 27) mosquitoes were caught in the forested area, whereas only 1.1% of Culex (n = 5) were collected in this area. In contrast, Culex mosquitoes represented 74% (n = 431) of all mosquitoes collected in the villages. Furthermore, the majority of Culex mosquitoes (89%, n = 387) were collected in the lowland villages and in the transition zone to the highland (villages 1–4 and 8, Figure 1b).

Table 2.
Summary of mosquito species, number of mosquitoes, and pools tested in the study. Mosquitoes for which we only identified the Genus were recorded in the column indicated as “Genus sp.”.
3.2. Detection of Flaviviruses and Alphaviruses
A total of 116 pools of mosquito homogenates were tested for flaviviruses and alphaviruses (Table 2). Six pools were positive for flaviviruses, and none for alphaviruses. Positive PCR products were sequenced and sequences were identified using the NCBI BLAST® website. Out of six samples, one Aedes and one Culex genus pool contained sequences related to the Yunnan Culex flavivirus (YNCxFV), with a maximum identity of 80.68% and 81.31%, respectively (Table 3). The sequences of one pool of Aedes aegypti and one pool of Culex vishnui contained the sequence of Phlebotomus-associated flavivirus (PAFV) with a maximum identity of 98.07% and 98.78%, respectively (Table 3). The pools P#73 with Cx. vishnui and P#49 with Cx sp. were both collected in “Ban Huak” village 4, and contained a TMUV-like sequence, which we noted as P73_TH_2019 and P49_TH_2019, respectively. The identity score for the first two hits for P49_TH_2019 sequence was 97.36% (coverage = 99%) and 96.04% (coverage = 99%) to TMUV KAN2016 (GenBank access number: KX184310) and TMUV HNU-NX2-2019 (GenBank access number: OP186478), respectively (Table 3 and Table S1). The P73_TH_2019 sequence was similar, at 98.83% (coverage = 100%) with TMUV_ GX2021 (GenBank access number: OM240641) and 98.44% (coverage = 100%), with TMUV_ SD2021 (GenBank access number: OM240640) (Table 3 and Table S1). The first ten hits of each sample are visualized in the Supplementary Materials (Table S1). The alignment of the P73_TH_2019 with P49_TH_2019 shows a very high similarity (96%), suggesting close phylogenic history between the two TMUVs collected in two different mosquito-pools.

Table 3.
Detection and identification of viruses in the mosquito pools.
3.3. Phylogenetic Analysis of the TMUV Isolates
To phylogenetically characterize the two virus isolates from the pools P49_TH_2019 and P73_TH_2019, we analyzed the envelope sequences. Alignment with representatives of TMUVs’ phylogenetic diversity (Table 1) illustrated the distribution of TMUV strains in five distinctives clusters, and their relation to the Ntaya virus (Figure 2a,b) [1].
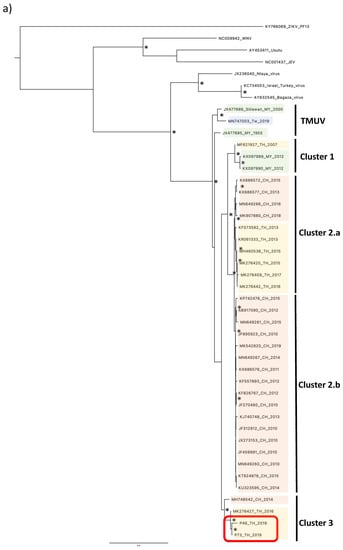
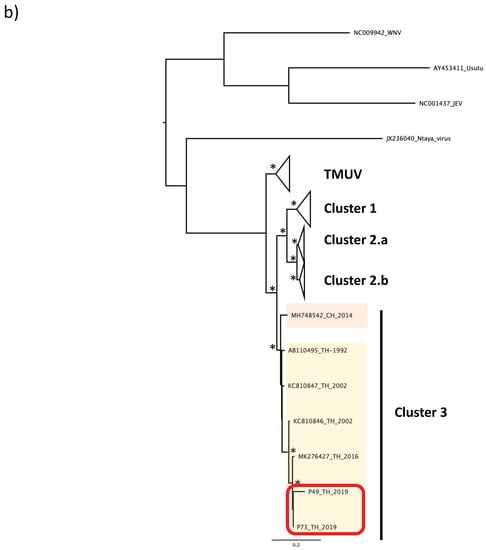
Figure 2.
Phylogenetic tree of TMUV isolates from Nan province. Maximum likelihood tree of TMUV envelope sequences, generated using the GTR+G substitution model. Bootstrap values higher than 0.85 are shown on branch nodes by an asterisk. Samples collected in this study are indicated by a red-colored rectangle. (a) Phylogenetic tree based on the full envelope gene sequence’s alignment. (b) Phylogenetic tree based on the partial envelope gene sequence’s alignment. Country acronyms are abbreviated as CH for China, MY for Malaysia, TW for Taiwan, and TH for Thailand. Strains from this study are marked in red.
A first group, named “TMUV”, includes the original strain isolated in 1955 in Malaysia and the MN747003 strain isolated in Taiwan in 2019. The other viruses are divided into four different clusters, named “cluster 1”, comprising strains isolated in Malaysia and Thailand, “cluster 2.a”, including strains isolated in Thailand and China, “cluster 2.b”, corresponding to strains isolated only in China, and “cluster 3”, including strains isolated in Thailand and China. P49_TH_2019 and P73_TH_2019 isolates from this study form a monophyletic lineage closely related to strains belonging to the cluster 3. The strains in cluster 3 were isolated in Thailand in 2016 and in China in 2014. Furthermore, we generated another phylogenetic tree by including the partial sequences of the envelope gene of three other TMUV strains isolated in Thailand in 1992 and 2002 [10,16]. All three Thai TMUV strains were clustered into the “cluster 3” with the isolates from this study (Figure 2b).
3.4. Identification of Envelope Amino Acid Modifications Specific to the TMUV Isolates from Nan Province
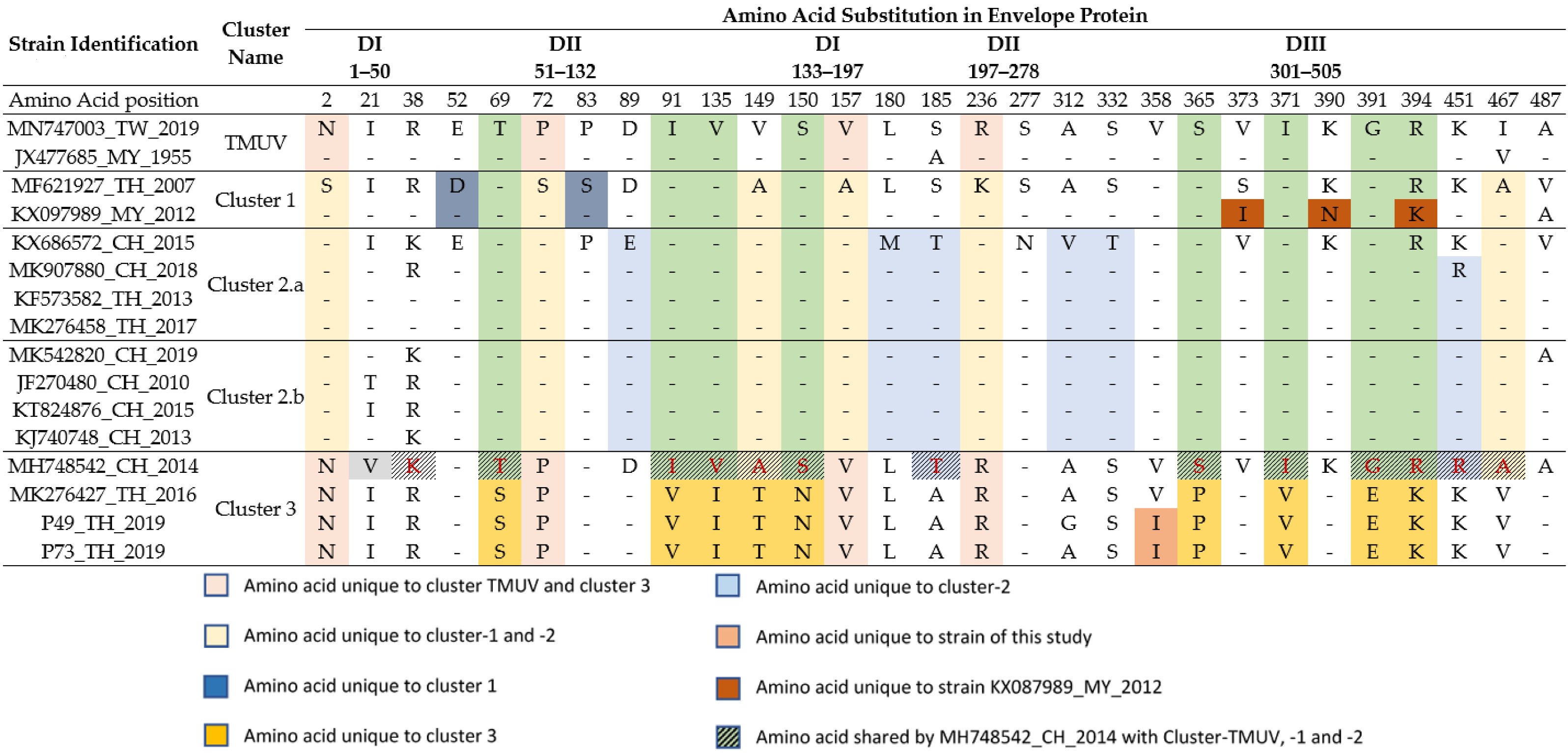
The genomic sequences of the P49_TH_2019 and P73_TH_2019 samples cover the entire coding sequence of the envelope protein (E protein), and were used to reveal amino acid differences from other strains belonging to every cluster of TMUV. The positions of amino acids with characteristic substitutions are shown in Table 4. All TMUV clusters have specific amino acid variations at certain positions, with unique patterns for every cluster (Table 4). The strains of cluster 1 carry specific amino acids on position 52 and 83, except for the strain KX097989, isolated in Malaysia in 2012, which has unique substitutions at position 373, 390 and 394. The strains of cluster 2 present unique amino acids on position 89, 180,185, 312, 332 and 451.

Table 4.
Cluster-specific amino acid substitution for the envelope among TMUV strains. The different domains of the envelope are indicated as DI to DIII.
The strains of cluster 3 have nine amino acids unique to this cluster, except for MH748542_CH2014 (Chinese isolate from 2014), and five other amino acids in common with the ancestral cluster “TMUV”. Positions 69, 91, 135, 149, 150, 365, 371, 391 and 394 have common amino acids for the strains MK276427-TH-2016 and P49_TH_2019, and P73_TH_2019 isolates. These amino acids are positioned in the DI (position 135; 149 and 150), DII (position 69) and DIII (position 365; 371; 391 and 394) domains. All TMUV strains present an Asn residue at the position 154; in addition, the strains MK276427_TH_2016, P49_TH_2019 and P73_TH_2019 present an S150N substitution. Finally, both P49_TH_2019 and P73_TH_2019 isolates have two unique substitutions at position 358 (V358I) of the domain III of the envelope protein.
4. Discussion
In this study, we report the investigation of more than 596 mosquitoes collected in 2019 in the Nan province of northern Thailand. Samples were collected along a transect covering an ecological gradient ranging from a sparsely urbanized rural area to dwellings clustered in a few villages surrounded by forests and highland. Although we screened for flaviviruses and alphaviruses, we only detected three different flaviviruses in six different mosquito pools. These flaviviruses included two insect-specific flaviviruses and two TMUV strains in two pools of Culex mosquitoes.
Composition of mosquito species varied along the ecological gradient depending on the type of landscape. Aedes albopictus was abundantly collected in the forest, although a few specimens were captured inside the villages. Our observations were as expected based on its reported peridomestic distribution [26], and were in accordance with previous Ae. albopictus collections in the rural and forested habitats of Thailand [27,28]. Additionally, Ae. albopictus was the most prevalent species in the forest sample site, although we could not identify all Aedes sp. due to sample damage. Aedes aegypti was strictly found in village 8, which corresponds to an urbanized rural area in the lowland. Aedes aegypti prefers urban zones in part because of its use of human-made containers as breeding sites [29]. Culex spp. were the most prevalent genera in the villages, likely in relation to the breeding conditions made available by agricultural activities. We identified Cx vishnui in all habitats (from forested to urban areas), as previously reported [30]. In addition to variations in landscape, environmental conditions including altitude can influence mosquito species’ composition and abundance [31]. Accordingly, we reported that 90% of Culex spp. were collected in the lowland villages and at the intersection of lowland and highland. Our study provides important information about mosquito species’ distribution in different ecological settings, which will be important when conducting spatial risk analyses for arbovirus circulation.
We were able to identify three different flaviviruses, including two insect-specific flaviviruses: the Phlebotomus-associated flavivirus (PAFV) and the Yunnan Culex flavivirus (YNCxFV). Interestingly, both viruses were detected in Culex and Aedes spp. These viruses were previously identified in other regions in Cx. gellidus, Cx. tritaeniorhynchus, Cx. vishnui and Cx. quinquefasciatus [32,33]. There is a growing interest in insect flaviviruses, as two of these have been shown to increase transmission of pathogenic flaviviruses [34], although the mechanism remains elusive. The wide distribution of insect-specific flaviviruses and their potential role in pathogenic virus transmission warrants further studies [35,36].
Importantly, we identified two isolates as belonging to the TMUV group. The isolate P73_TH_2019 was isolated from a pool of Cx. Vishnui, and the isolate P49_TH_2019 was isolated from Culex spp. in the same village located in the transition zone between the highlands and lowlands. Although the species of the pool of Culex spp. could not be identified at the species level, it is likely Cx. Vishnui, since this species was overwhelmingly present among the other identified mosquitoes at the same site. Previous studies looked at the ability of mosquito vectors to transmit TMUV, and observed that mosquitoes of the genus Culex were very competent [10]. Accordingly, in Malaysia, China, Thailand, and Taiwan, TMUV was detected in Cx. tritaeniorhynchus, Cx. vishnui, Cx. quinquefasciatus, Cx. annulus and Cx. pipiens [1]. Although the transmission capacity of Culex mosquitoes seems variable, and a source of discussion [17,37], Cx. tritaeniorhynchus was proposed as the principal vector [38]. In Thailand, TMUV infection in Cx. tritaeniorhynchus collected in paddy fields was reported in the vicinity of Kamphaeng Phet province both in 1982 and 2002 [8,10]. Culex vishnui and Cx. tritaeniorhynchus belong to the same subgroup, and are also considered major vectors of JEV.
After its first identification in 1955 in Malaysia, TMUV has only been reported in four different countries in Asia. In Thailand, TMUV has been detected mostly in association with large duck farms in the center of the country [9,39]. In both China and Thailand and in a wetland habitat for waterbirds in a suburban area of Taipei city in Taiwan, the strains isolated were related to cluster 2. In contrast, strains belonging to the cluster TMUV and cluster 1 were found in rural or forest areas in Malaysia and Taiwan [6]. In our study, we identified TMUV strains that belong to cluster 3 in a rural area in northern Thailand. The collection site was in a village of 360 inhabitants, surrounded by rice fields and forested areas downstream to a dam. Prior to the large outbreaks of the 2010s, associated with TMUV cluster 2, in China and Thailand, TMUV cluster 3 had already been identified in Thailand, in rural provinces in the west of the country [2,16]. Although Ninvilai et al. previously reported a cluster 3 TMUV being present on a large duck farm in central Thailand [9], it would seem that viruses affiliated with clusters TMUV, 1 and 3 are found preferentially in rural and forested areas. These locations, including wetlands or rice fields, are particularly favorable to the development of mosquitoes of the genus Culex, which are described as major vectors of TMUV, and are also areas with domestic and wild birdlife. Thus, these areas could be favorable for the maintenance and spread of TMUV. Further investigation should be carried out to elucidate the possible relationships between the type of viral strain and the kind of ecological area.
It is interesting to note that, similar to the strain P73_TH_2019 in our study, the strains isolated from mosquitoes in 1982 and 2002 in Thailand also belong to cluster 3. Although TMUV has been detected in large parts of the Thai territory, cluster 3 strains were mostly found in rural areas in the north-west and north parts of the country. Our phylogenetic analysis showed that the two new TMUV strains form a monophyletic group closely related to TMUV cluster 3. While they do display the specificities of recent TMUV strains, such as the presence of the S156P substitution in «loop 150» region of the envelope protein [40], Thai cluster 3 strains are phylogenetically closer to the TMUV cluster than to clusters 1 and 2. Previous studies have shown the important effect of amino acid substitution sequences in the viral envelope on the virulence and pathogenicity of TMUV [40,41,42]. Recently, Nivilai et al. showed the presence of unique residues in the envelope protein for strains belonging to cluster 3 [9]. The cluster 3 strains identified in our study possess unique amino acid substitutions that are partially different from those described by Ninvilai et al. We found the same residues in position 149, 150, 391 and 394, as previously described by Ninvilai et al., but we also found other amino acid substitutions in positions 69, 91, 135, 365 and 371. As with most flaviviruses, we found an Asn residue at position 154 for the two TMUV isolates identified in our study. The position of this Asn residue forms an N-x-(S/T) glycosylation motif that plays an important role in pathogenicity of flaviviruses [43,44]. In the cluster 3 strains, except for the strain MH748542_2014_Ch, we also found the presence of an Asn residue in position 150, resulting from an S150N substitution. However, this substitution does not lead to the formation of an N-x-(S/T) motif, thus suggesting the absence of glycosylation in this region. Moreover, a unique V358I substitution appears in both strains P49_TH_2019 and P73_TH_2019, whereas the substitution was not present in the cluster 3 strains MK276427_TH_2016 and MH748542_2014_Ch, or in strains isolated from other clusters. Differences in the envelope sequence may stem from selection in either the host or mosquito. Finally, the whole envelope sequence of the strains isolated in 1992 and 2002 is not available, and therefore we are unable to evaluate if the amino acid signature of the cluster 3 strains isolated in Thailand is recent or not.
We would like to propose that the cluster 3 strains circulate at a regional scale between Thailand and the south of China. The local maintenance of these strains could be explained by the presence of the virus in more isolated areas of Thailand, such as the province of Nan, which carry out less trading than the central regions of the country; these central regions are more densely populated and carry out more economic exchanges with other Asian countries. However, the low number of strains from cluster 3 does not provide sufficient hindsight on the evolution of TMUV in Thailand, and further studies should be conducted to evaluate the impact of the different TMUV clusters on its dissemination, the evolution of pathogenicity and TMUV emergence risk in humans.
In conclusion, we report the detection of TMUV in Culex mosquito populations in northern Thailand. Our phylogenetic analysis classified these isolates into TMUV cluster 3, and highlighted the genetic specificities of this cluster, providing insights into the diversity and evolution of TMUV. TMUV surveillance is particularly important in a context of global changes and the intensification of trade between China, Laos, and Thailand. The opening of new economic corridors and new trade routes in the Indo-Pacific region will have a major impact on the emergence of pathogens that were previously restricted to small geographical areas. It is therefore essential to maintain active surveillance for arbovirus emergence and spillover in areas in which there is strong interaction between wildlife, domestic animals, and human communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15071447/s1, Table S1: The first ten hits alignment of sample P49_TH_2019 and P73_TH_2019 were obtained using the NCBI BLAST® website.
Author Contributions
Conceptualization, R.H., R.E.M.V. and S.M.; methodology, R.H., R.E.M.V. and S.M.; formal analysis, R.H., D.M.R. and R.E.M.V.; investigation, R.H., D.M.R., R.E.M.V., A.K., K.C. and S.W.; resources, R.H. and A.Y.; writing—original draft preparation, R.H. and J.J.; writing—review and editing, R.H., R.E.M.V., S.M. and J.P.; visualization, R.H.; supervision, R.H., S.M. and R.E.M.V.; project administration, R.H., A.K., S.M. and K.C.; funding acquisition, R.H., R.E.M.V., A.Y., J.P., J.J., S.W., D.M. and D.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through (i) the French project INGENIOUS, supported by the Labex CEMEB and the I-SITE Excellence Program of the University of Montpellier, under the Investissements France 2030, granted to Dr. Rodolphe Hamel; (ii) the French project BILAO supported by FSPI OHSEA «One Health (Une seule Santé) en pratique en Asie du Sud-Est», a program supervised by IRD, CIRAD and CNRS and granted to Dr. Rodolphe Hamel; (iii) the French ANR Project FutureHealthSEA (grant number: ANR-17-CE35-0003-02) “Predictive scenarios of health in Southeast Asia: linking land use and climate changes to infectious diseases”, and supported by the Thailand International Cooperation Agency for the project “Innovative Animal Health” and National Research Council of Thailand, granted to Dr. Serge Morand; and (iiii) the ANR project V-DOSAGE (grant number: ANR-22-CE35-0012-03) granted to Dr. Julien Pompon. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. D.M. Rajonhson is a student enrolled in the international PhD program at the faculty of Tropical Medicine, Mahidol University, supported by the Organization for Women in Science for the Developing World (OWSD) and the Swedish International Development Cooperation Agency (SIDA).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our gratitude to the National Research Council of Thailand (NRCT) and the local administration in Nan province, including the Nan Provincial Public Health Office, Tha Wang Pha District Public Health Office, Tha Wang Pha Hospital and Saen Thong Subdistrict Health Promoting Hospital for supporting and facilitating research in the field. We thank Malee Tanita (Saen Thong Health Promoting Hospital), Chuanphot Thinphovong, Yossapong Paladsing and Phurin Makaew, the health village volunteers of Saen Thong, for their great help in carrying out the field study. Special thanks also go to Chuanphot Thinphovong of the Faculty of Veterinary Technology from Kasetsart University for the illustrative map of the field location. We also thank the International Join Unit (LMI) PRESTO (Protect-Detect-Stop), the representation of IRD in Thailand and the Japan Agency for Medical Research and Development (AMED) (JP22wm0325004s0503) for their help in the smooth running of this study in the field and in the lab.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hamel, R.; Phanitchat, T.; Wichit, S.; Morales Vargas, R.E.; Jaroenpool, J.; Diagne, C.T.; Pompon, J.; Missé, D. New Insights into the Biology of the Emerging Tembusu Virus. Pathogens 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- US Army Medical Research Unit (Malaya), Institute for Medical Research. Annual Report; Federation of Malaya: Kuala Lumpur, Malaysia, 1957; pp. 100–103.
- Cao, Z.; Zhang, C.; Liu, Y.; Ye, W.; Han, J.; Ma, G.; Zhang, D.; Xu, F.; Gao, X.; Tang, Y.; et al. Tembusu virus in ducks, China. Emerg. Infect. Dis. 2011, 17, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Homonnay, Z.G.; Kovács, E.W.; Bányai, K.; Albert, M.; Fehér, E.; Mató, T.; Tatár-Kis, T.; Palya, V. Tembusu-like flavivirus (Perak virus) as the cause of neurological disease outbreaks in young Pekin ducks. Avian Pathol. 2014, 43, 552–560. [Google Scholar] [CrossRef]
- Thontiravong, A.; Ninvilai, P.; Tunterak, W.; Nonthabenjawan, N.; Chaiyavong, S.; Angkabkingkaew, K.; Mungkundar, C.; Phuengpho, W.; Oraveerakul, K.; Amonsin, A. Tembusu-Related Flavivirus in Ducks, Thailand. Emerg. Infect. Dis. 2015, 21, 2164–2167. [Google Scholar] [CrossRef]
- Peng, S.H.; Su, C.L.; Chang, M.C.; Hu, H.C.; Yang, S.L.; Shu, P.Y. Genome Analysis of a Novel Tembusu Virus in Taiwan. Viruses 2020, 12, 567. [Google Scholar] [CrossRef]
- Kono, Y.; Tsukamoto, K.; Abd Hamid, M.; Darus, A.; Lian, T.C.; Sam, L.S.; Yok, C.N.; Di, K.B.; Lim, K.T.; Yamaguchi, S.; et al. Encephalitis and retarded growth of chicks caused by Sitiawan virus, a new isolate belonging to the genus Flavivirus. Am. J. Trop. Med. Hyg. 2000, 63, 94–101. [Google Scholar] [CrossRef]
- Leake, C.J.; Ussery, M.A.; Nisalak, A.; Hoke, C.H.; Andre, R.G.; Burke, D.S. Virus isolations from mosquitoes collected during the 1982 Japanese encephalitis epidemic in northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 831–837. [Google Scholar] [CrossRef]
- Ninvilai, P.; Tunterak, W.; Oraveerakul, K.; Amonsin, A.; Thontiravong, A. Genetic characterization of duck Tembusu virus in Thailand, 2015–2017: Identification of a novel cluster. Transbound. Emerg. Dis. 2019, 66, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- O’Guinn, M.L.; Turell, M.J.; Kengluecha, A.; Jaichapor, B.; Kankaew, P.; Miller, R.S.; Endy, T.P.; Jones, J.W.; Coleman, R.E.; Lee, J.S. Field detection of Tembusu virus in western Thailand by rt-PCR and vector competence determination of select culex mosquitoes for transmission of the virus. Am. J. Trop. Med. Hyg. 2013, 89, 1023–1028. [Google Scholar] [CrossRef]
- Tunterak, W.; Prakairungnamthip, D.; Ninvilai, P.; Tiawsirisup, S.; Oraveerakul, K.; Sasipreeyajan, J.; Amonsin, A.; Thontiravong, A. Patterns of duck Tembusu virus infection in ducks, Thailand: A serological study. Poult. Sci. 2021, 100, 537–542. [Google Scholar] [CrossRef]
- Ninvilai, P.; Limcharoen, B.; Tunterak, W.; Prakairungnamthip, D.; Oraveerakul, K.; Banlunara, W.; Thontiravong, A. Pathogenesis of Thai duck Tembusu virus in Cherry Valley ducks: The effect of age on susceptibility to infection. Vet. Microbiol. 2020, 243, 108636. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Liu, Q.; Wang, Y.; Li, G.; Teng, Q.; Zhang, Y.; Liu, S.; Li, Z. Airborne Transmission of a Novel Tembusu Virus in Ducks. J. Clin. Microbiol. 2015, 53, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Surasombatpattana, P.; Wichit, S.; Dauvé, A.; Donato, C.; Pompon, J.; Vijaykrishna, D.; Liegeois, F.; Vargas, R.M.; Luplertlop, N.; et al. Phylogenetic analysis revealed the co-circulation of four dengue virus serotypes in Southern Thailand. PLoS ONE 2019, 14, e0221179. [Google Scholar] [CrossRef] [PubMed]
- Raksakoon, C.; Potiwat, R. Current Arboviral Threats and Their Potential Vectors in Thailand. Pathogens 2021, 10, 80. [Google Scholar] [CrossRef]
- Pandey, B.D.; Karabatsos, N.; Cropp, B.; Tagaki, M.; Tsuda, Y.; Ichinose, A.; Igarashi, A. Identification of a flavivirus isolated from mosquitos in Chiang Mai Thailand. Southeast Asian J. Trop. Med. Public Health 1999, 30, 161–165. [Google Scholar]
- Nitatpattana, N.; Apiwatanason, C.; Nakgoi, K.; Sungvornyothin, S.; Pumchompol, J.; Wanlayaporn, D.; Chaiyo, K.; Siripholvat, V.; Yoksan, S.; Gonzalez, J.-P. Isolation of Tembusu virus from Culex quinquefasciatus in Kanchanaburi Province, Thailand. Southeast Asian J. Trop. Med. Public Health 2017, 48, 546–551. [Google Scholar]
- Pulmanausahakul, R.; Ketsuwan, K.; Jaimipuk, T.; Smith, D.R.; Auewarakul, P.; Songserm, T. Detection of antibodies to duck tembusu virus in human population with or without the history of contact with ducks. Transbound. Emerg. Dis. 2021, 69, 870–873. [Google Scholar] [CrossRef]
- Rattanarithikul, R.; Harbach, R.E.; Harrison, B.A.; Panthusiri, P.; Jones, J.W.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health 2005, 36 (Suppl. S2), 1–97. [Google Scholar]
- Rattanarithikul, R.; Harbach, R.E.; Harrison, B.A.; Panthusiri, P.; Coleman, R.E.; Richardson, J.H. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J. Trop. Med. Public Health 2010, 41 (Suppl. S1), 1–225. [Google Scholar]
- Rattanarithikul, R.; Harrison, B.A.; Panthusiri, P.; Peyton, E.L.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health 2006, 37 (Suppl. S1), 1–85. [Google Scholar]
- Vina-Rodriguez, A.; Sachse, K.; Ziegler, U.; Chaintoutis, S.C.; Keller, M.; Groschup, M.H.; Eiden, M. A Novel Pan-Flavivirus Detection and Identification Assay Based on RT-qPCR and Microarray. BioMed Res. Int. 2017, 2017, 4248756. [Google Scholar] [CrossRef]
- Giry, C.; Roquebert, B.; Li-Pat-Yuen, G.; Gasque, P.; Jaffar-Bandjee, M.C. Improved detection of genus-specific Alphavirus using a generic TaqMan® assay. BMC Microbiol. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Mendenhall, I.H.; Manuel, M.; Moorthy, M.; Lee, T.T.M.; Low, D.H.W.; Missé, D.; Gubler, D.J.; Ellis, B.R.; Ooi, E.E.; Pompon, J. Peridomestic Aedes malayensis and Aedes albopictus are capable vectors of arboviruses in cities. PLoS Negl. Trop. Dis. 2017, 11, e0005667. [Google Scholar] [CrossRef]
- Sumodan, P.K.; Vargas, R.M.; Pothikasikorn, J.; Sumanrote, A.; Lefait-Robin, R.; Dujardin, J.-P. Rubber plantations as a mosquito box amplification in South and Southeast Asia. In Socio-Ecological Dimensions of Infectious Diseases in Southeast Asia; Springer: Singapore, 2015; pp. 155–167. [Google Scholar]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagi, M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban–rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control Assoc. 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Akratanakul, P.; Nettanomsak, S.; Huntamai, S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health 2003, 34, 529–535. [Google Scholar]
- Maquart, P.O.; Chann, L.; Boyer, S. Culex vishnui (Diptera: Culicidae): An Overlooked Vector of Arboviruses in South-East Asia. J. Med. Entomol. 2022, 59, 1144–1153. [Google Scholar] [CrossRef]
- Eisen, L.; Bolling, B.G.; Blair, C.D.; Beaty, B.J.; Moore, C.G. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado Front Range. J. Med. Entomol. 2008, 45, 800–811. [Google Scholar] [CrossRef]
- Fang, Y.; Tambo, E.; Xue, J.B.; Zhang, Y.; Zhou, X.N.; Khater, E.I.M. Detection of DENV-2 and Insect-Specific Flaviviruses in Mosquitoes Collected From Jeddah, Saudi Arabia. Front. Cell. Infect. Microbiol. 2021, 11, 626368. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, W.; Xue, J.B.; Zhang, Y. Monitoring Mosquito-Borne Arbovirus in Various Insect Regions in China in 2018. Front. Cell. Infect. Microbiol. 2021, 11, 640993. [Google Scholar] [CrossRef]
- Olmo, R.P.; Todjro, Y.M.H.; Aguiar, E.R.G.R.; de Almeida, J.P.P.; Ferreira, F.V.; Armache, J.N.; de Faria, I.J.S.; Ferreira, A.G.A.; Amadou, S.C.G.; Silva, A.T.S.; et al. Mosquito vector competence for dengue is modulated by insect-specific viruses. Nat. Microbiol. 2023, 8, 135–149. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Atoni, E.; Zhang, B.; Yuan, Z. Mosquito-Associated Viruses in China. Virol. Sin. 2018, 33, 5–20. [Google Scholar] [CrossRef]
- Agboli, E.; Zahouli, J.B.Z.; Badolo, A.; Jöst, H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses 2021, 13, 891. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, T.; Jiang, Y.; Zhao, T.; Li, C.; Dong, Y.; Xing, D.; Qin, C. Potential Vector Competence of Mosquitoes to Transmit Baiyangdian Virus, a New Tembusu-Related Virus in China. Vector Borne Zoonotic Dis. 2020, 20, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Sanisuriwong, J.; Yurayart, N.; Thontiravong, A.; Tiawsirisup, S. Vector competence of Culex tritaeniorhynchus and Culex quinquefasciatus (Diptera: Culicidae) for duck Tembusu virus transmission. Acta Trop. 2021, 214, 105785. [Google Scholar] [CrossRef] [PubMed]
- Sanisuriwong, J.; Yurayart, N.; Thontiravong, A.; Tiawsirisup, S. Duck Tembusu virus detection and characterization from mosquitoes in duck farms, Thailand. Transbound. Emerg. Dis. 2020, 67, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, Y.; Wang, H.; Li, G.; Li, X.; Wang, B.; Su, X.; Wang, J.; Teng, Q.; Yang, J.; et al. A Single Mutation at Position 156 in the Envelope Protein of Tembusu Virus Is Responsible for Virus Tissue Tropism and Transmissibility in Ducks. J. Virol. 2018, 92, e00427-18. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, L.; Cao, Y.; Wang, J.; Yu, Z.; Sun, X.; Liu, F.; Li, Z.; Liu, P.; Su, J. Basic Amino Acid Substitution at Residue 367 of the Envelope Protein of Tembusu Virus Plays a Critical Role in Pathogenesis. J. Virol. 2020, 94, e02011-19. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Zhang, L.; Yu, Z.; Li, J.; Xie, W.; Su, J. Amino Acid Substitutions in NS5 Contribute Differentially to Tembusu Virus Attenuation in Ducklings and Cell Cultures. Viruses 2021, 13, 921. [Google Scholar] [CrossRef]
- Carbaugh, D.L.; Lazear, H.M. Flavivirus Envelope Protein Glycosylation: Impacts on Viral Infection and Pathogenesis. J. Virol. 2020, 94, 94. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Li, S.; Dong, F.; Zhang, Y.; Lin, Y.; Wang, J.; Zou, Z.; Zheng, A. N-glycosylation of Viral E Protein Is the Determinant for Vector Midgut Invasion by Flaviviruses. mBio 2018, 9, e00046-18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).