Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines
Abstract
1. Introduction
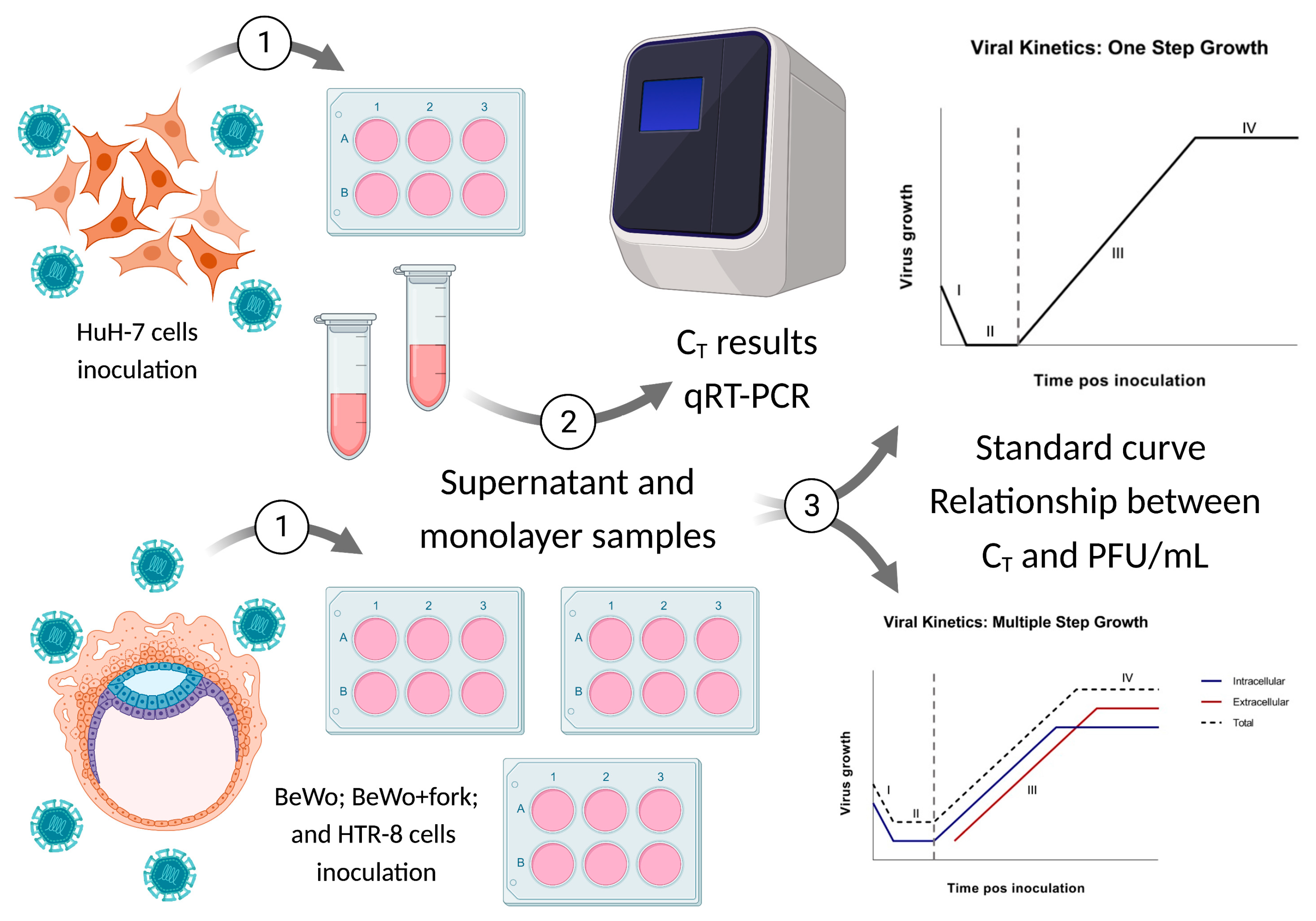
2. Materials and Methods
2.1. Viral Isolation and Formation of Viral Stocks
2.2. Cells Cultures
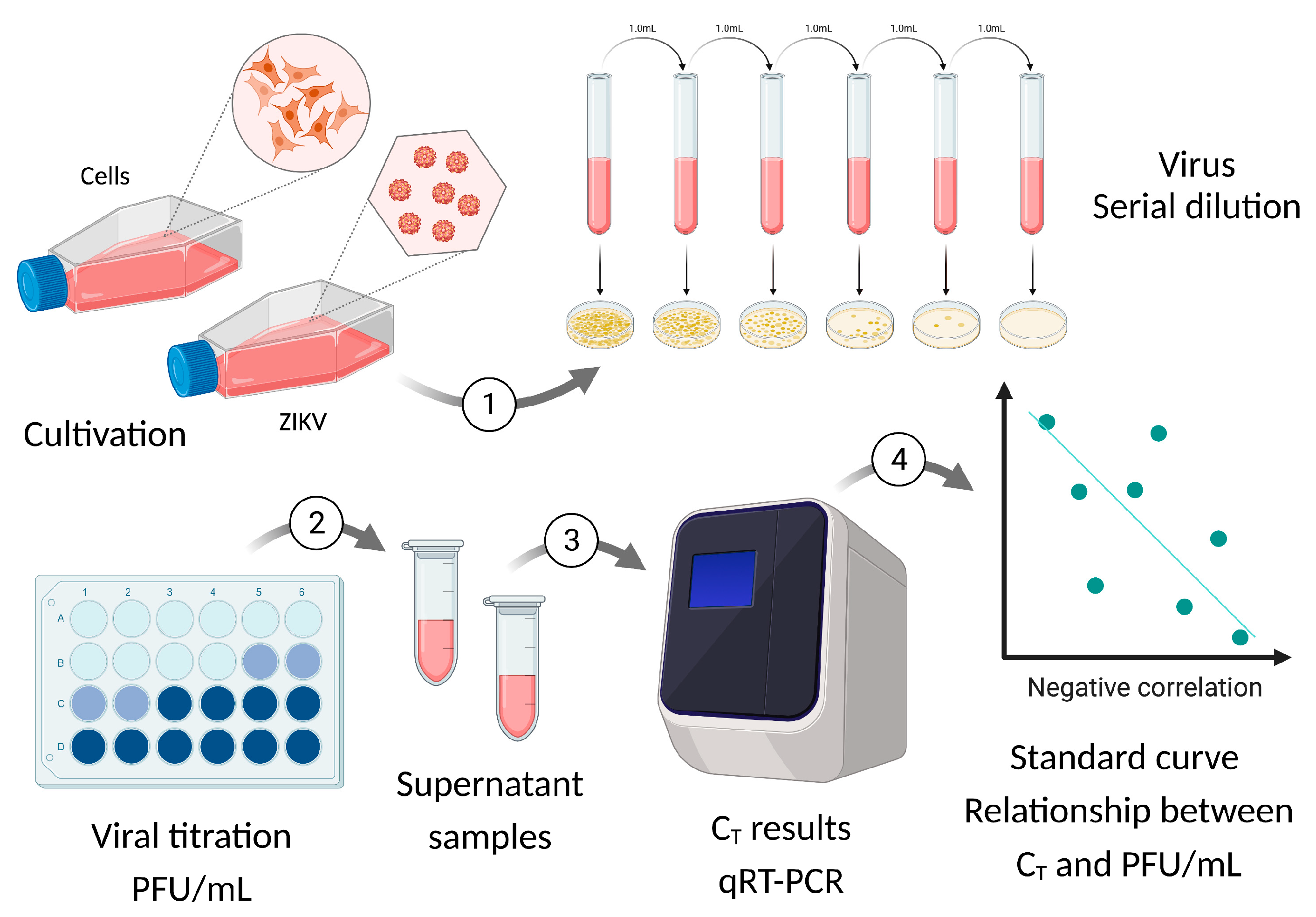
2.3. Standard Curve for PFU/mL Determination
2.4. Viral Kinetics (Step Growth Curve)
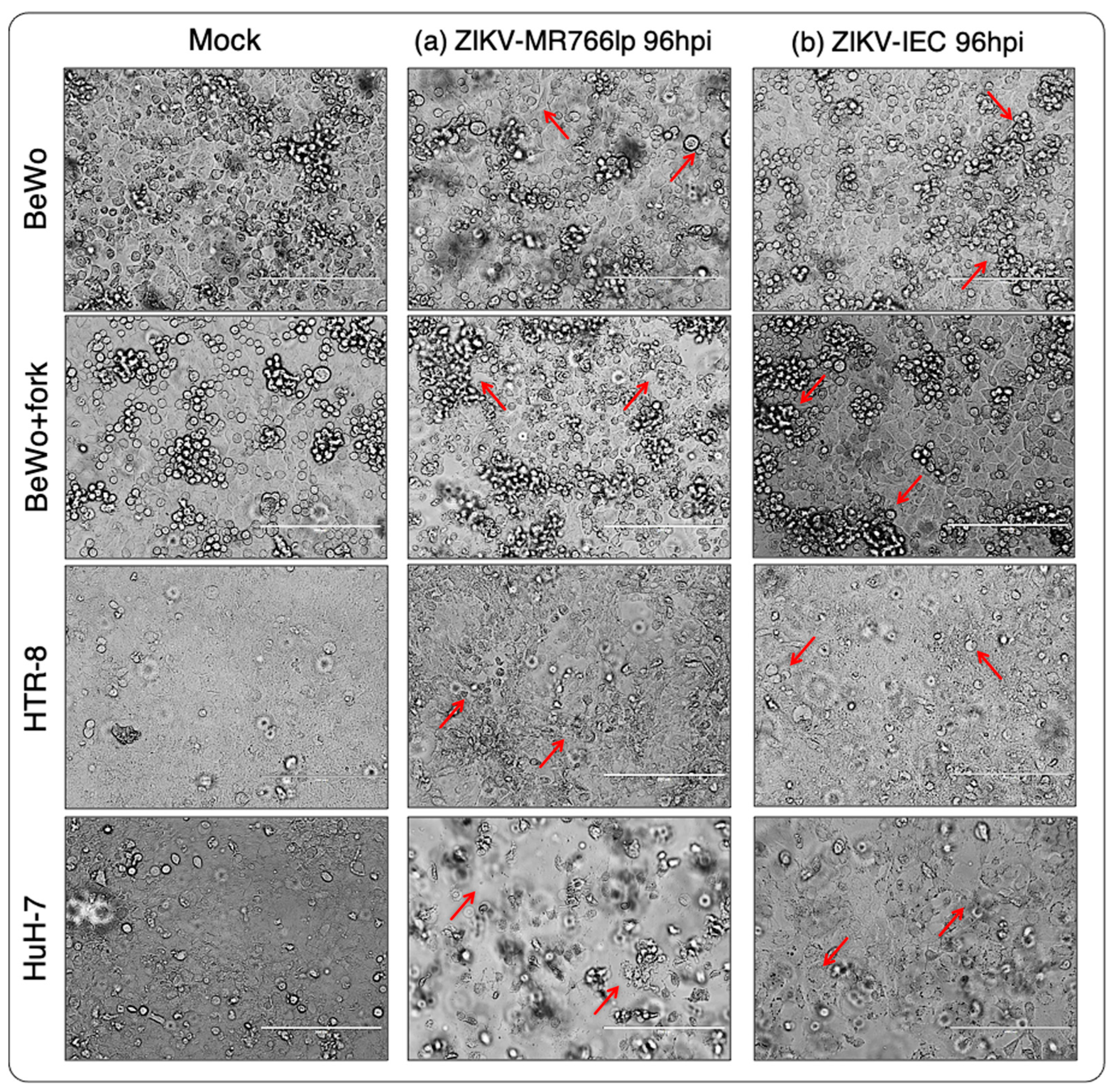
3. Results
3.1. Inoculation of ZIKV-MR766lp (a)
3.2. Inoculation of ZIKV-IEC-Paraíba (b)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. BeWo Treated with Forskolin
Appendix B. Adaptation of qRT-PCR Performed by Lanciotti et al. 2008 [31]
Appendix C. Viral Kinetics
Appendix D. Standard Curve|Direct Relation between CT and PFU/mL
Appendix E. Standard Curve|Calculation of Direct Relation between CT and PFU/mL
Appendix F. Conversion of CT Values into PFU/mL of the Viral Kinetics Graph
| Strain/Replicate (PFU/mL) | First | Second | Third | Average |
| ZIKV-MR766 low passage | 1.57 × 108 | 2.05 × 108 | 1.43 × 108 | 1.68 × 108 |
| ZIKV-IEC-Paraíba | 1.30 × 106 | 1.40 × 106 | 1.68 × 106 | 1.46 × 108 |
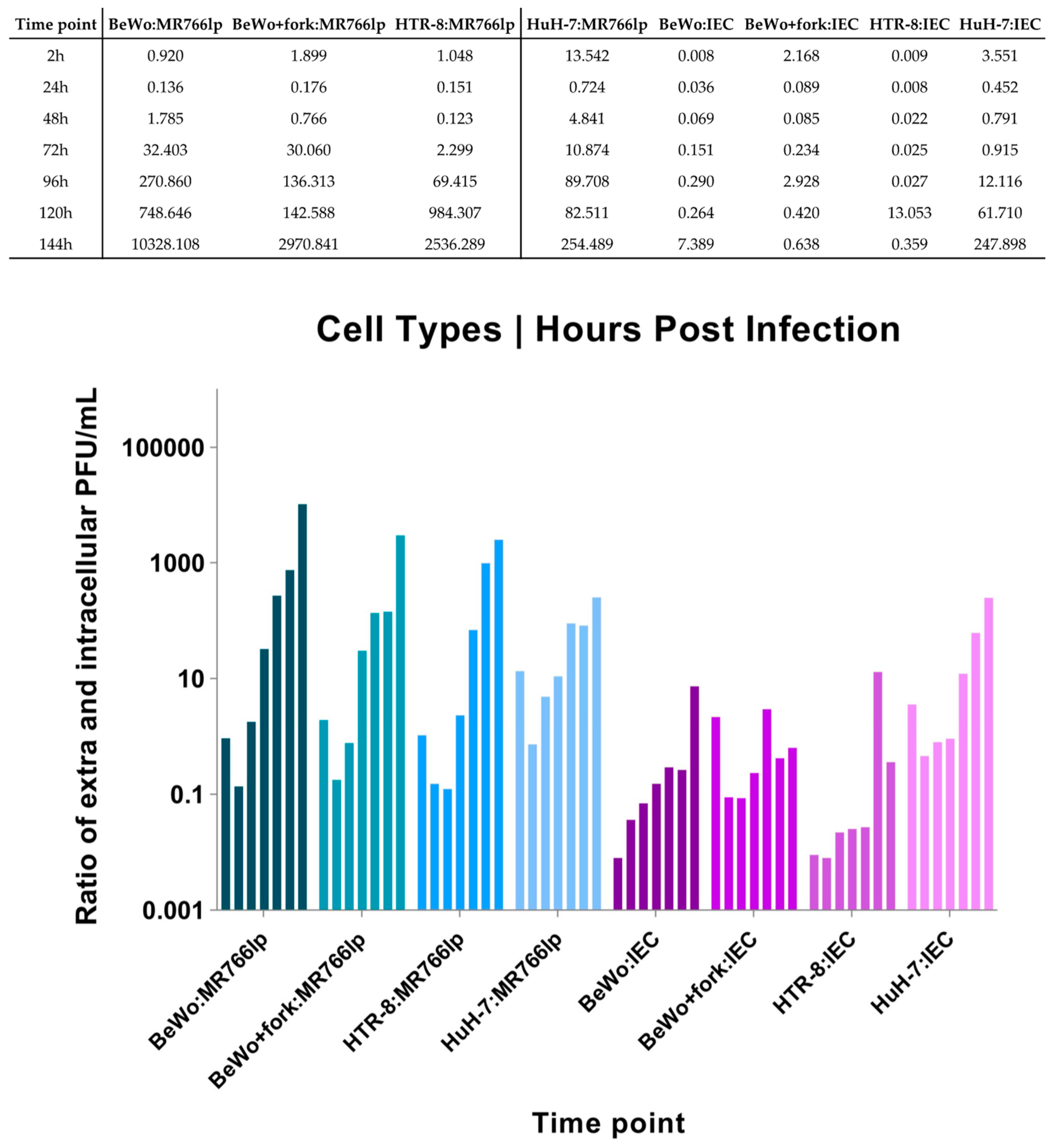
Appendix G. Calculation of the Extra- and Intracellular Ratio
Appendix H. Figure of Standard Curves

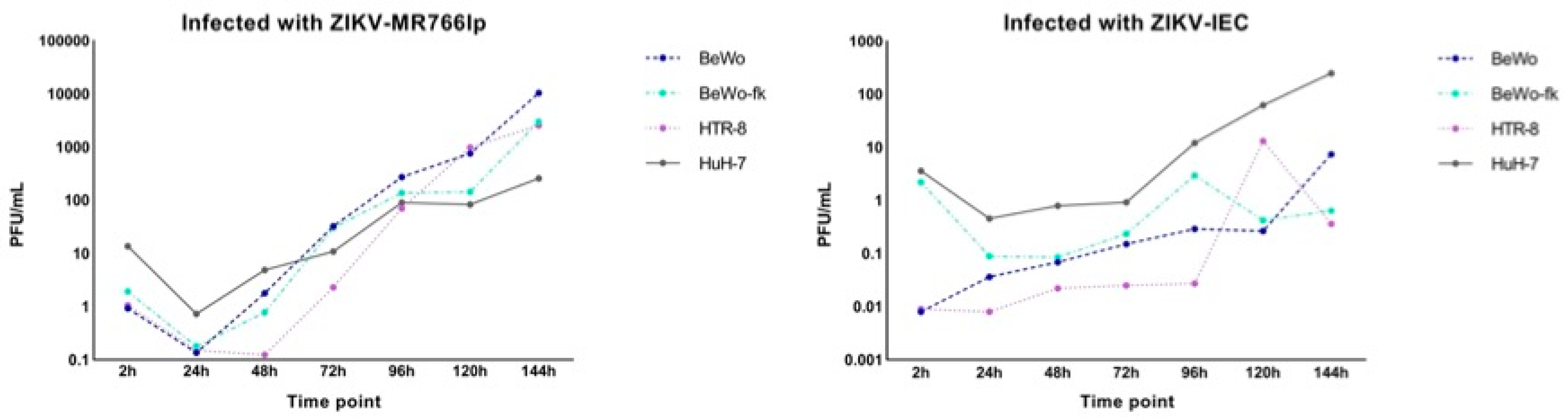
Appendix I. Figure of Complete Viral Kinetics
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (II). Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 521–534. [Google Scholar] [CrossRef]
- Dick, G.W. Epidemiological notes on some viruses isolated in Uganda (Yellow fever, Rift Valley fever, Bwamba fever, West Nile, Mengo, Semliki Forest, Bunyamwera, Ntaya, Uganda S and Zika viruses). Trans. R. Soc. Trop. Med. Hyg. 1953, 47, 13–48. [Google Scholar] [CrossRef]
- Simpson, D.I. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef]
- Olson, J.G.; Ksiazek, T.G. Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Posen, H.J.; Keystone, J.S.; Gubbay, J.B.; Morris, S.K. Epidemiology of Zika virus, 1947–2007. BMJ Glob. Health 2016, 1, e000087. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Masmejan, S.; Musso, D.; Vouga, M.; Pomar, L.; Dashraath, P.; Stojanov, M.; Panchaud, A.; Baud, D. Zika virus. Pathogens 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika virus infection—After the pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Pardy, R.D.; Richer, M.J. Zika virus Pathogenesis: From Early Case Reports to Epidemics. Viruses 2019, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.B.; Bhatnagar, J.; de Oliveira Ramos, A.M.; Davi, H.P.; Iglezias, S.D.; Kanamura, C.T.; Keating, M.K.; Hale, G.; Silva-Flannery, L.; Muehlenbachs, A.; et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet 2016, 388, 898–904. [Google Scholar] [CrossRef]
- Miner, J.J.; Diamond, M.S. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, K.; De Souza, L.J.; Salomão, N.G.; Machado, L.N.; Pereira, P.G.; Portari, E.A.; Basílio-de-Oliveira, R.; Dos Santos, F.B.; Neves, L.D.; Morgade, L.F.; et al. Zika induces human placental damage and inflammation. Front. Immunol. 2020, 1, 2146. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Xu, Y.; He, Y.; Momben-Abolfath, S.; Vertrees, D.; Li, X.; Norton, M.G.; Struble, E.B. Zika virus Infection and Antibody Neutralization in FcRn Expressing Placenta and Engineered Cell Lines. Vaccines 2022, 10, 2059. [Google Scholar] [CrossRef]
- Cao, B.; Diamond, M.S.; Mysorekar, I.U. Maternal-fetal transmission of Zika virus: Routes and signals for infection. J. Interferon Cytokine Res. 2017, 37, 287–294. [Google Scholar] [CrossRef]
- Mysorekar, I.U.; Diamond, M.S. Modeling Zika virus infection in pregnancy. N. Engl. J. Med. 2016, 375, 481–484. [Google Scholar] [CrossRef]
- Carrera, J.; Trenerry, A.M.; Simmons, C.P.; Mackenzie, J.M. Flavivirus replication kinetics in early-term placental cell lines with different differentiation pathways. Virol. J. 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Ellis, E.L.; Delbruck, M. The growth of bacteriophage. J. Gen. Physiol. 1939, 22, 365–384. [Google Scholar] [CrossRef]
- Keogh, B.P. Adsorption, latent period and burst size of phages of some strains of lactic streptococci. J. Dairy Res. 1973, 40, 303–309. [Google Scholar] [CrossRef]
- Falke, D. Virologia; E.P.U. Springer EDUSP: São Paulo, Brasil, 1979; pp. 13–34. [Google Scholar]
- Burleson, F.G.; Chamber, T.M.; Widebrauk, D. Virology: A Laboratory Manual; Academic Press, Inc.: San Diego, CA, USA, 1992; pp. 100–106. [Google Scholar]
- Mautner, L.; Hoyos, M.; Dangel, A.; Berger, C.; Ehrhardt, A.; Baiker, A. Replication kinetics and infectivity of SARS-CoV-2 variants of concern in common cell culture models. Virol. J. 2022, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo, R.D.; Kraemer, M.U.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Aubry, F.; Jacobs, S.; Darmuzey, M.; Lequime, S.; Delang, L.; Fontaine, A.; Jupatanakul, N.; Miot, E.F.; Dabo, S.; Manet, C.; et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 2021, 12, 916. [Google Scholar] [CrossRef]
- Timenetsky, J.; Santos, L.M.; Buzinhani, M.; Mettifogo, E. Detection of multiple mycoplasma infection in cell cultures by PCR. Braz. J. Med. Biol. Res. 2006, 39, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Orendi, K.; Gauster, M.; Moser, G.; Meiri, H.; Huppertz, B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010, 40, 759–766. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Harris, E.; Pereira, L. Zika virus replicates in proliferating cells in explants from first-trimester human placentas, potential sites for dissemination of infection. J. Infect. Dis. 2018, 217, 1202–1213. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Barresi, M.J.F. Developmental Biology, 11th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2016; pp. 143–180. [Google Scholar]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Balaraman, V.; Schust, D.J.; Ezashi, T.; Roberts, R.M.; Franz, A.W. African and Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS ONE 2018, 13, e0200086. [Google Scholar] [CrossRef] [PubMed]
- León-Juárez, M.; Martínez-Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Cheng, M.; Ge, N.; Shu, J.; Xu, Z.; Su, X.; Kou, Z.; Tong, Y.; Qin, C.; et al. A single nonsynonymous mutation on ZIKV E protein-coding sequences leads to markedly increased neurovirulence in vivo. Virol. Sin. 2022, 37, 115–126. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.Y.; Liu, Z.Y.; Zhang, F.; Zhu, X.L.; Yu, J.Y.; Ji, X.; Xu, Y.P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef] [PubMed]
- King, E.L.; Irigoyen, N. Zika virus and Neuropathogenesis: The Unanswered Question of Which Strain Is More Prone to Causing Microcephaly and Other Neurological Defects. Front. Cell. Neurosci. 2021, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef]
- Adibi, J.J.; Marques, E.T., Jr.; Cartus, A.; Beigi, R.H. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 2016, 387, 1587–1590. [Google Scholar] [CrossRef]
- Meaney-Delman, D.; Oduyebo, T.; Polen, K.N.; White, J.L.; Bingham, A.M.; Slavinski, S.A.; Heberlein-Larson, L.; St George, K.; Rakeman, J.L.; Hills, S.; et al. Prolonged detection of Zika virus RNA in pregnant women. Obstet. Gynecol. 2016, 128, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Suy, A.; Sulleiro, E.; Rodó, C.; Vázquez, É.; Bocanegra, C.; Molina, I.; Esperalba, J.; Sánchez-Seco, M.P.; Boix, H.; Pumarola, T.; et al. Prolonged Zika virus viremia during pregnancy. N. Engl. J. Med. 2016, 375, 2611–2613. [Google Scholar] [CrossRef]
- Muthuraj, P.G.; Sahoo, P.K.; Kraus, M.; Bruett, T.; Annamalai, A.S.; Pattnaik, A.; Pattnaik, A.K.; Byrareddy, S.N.; Natarajan, S.K. Zika virus infection induces endoplasmic reticulum stress and apoptosis in placental trophoblasts. Cell Death Discov. 2021, 7, 24. [Google Scholar] [CrossRef]
- Da Silva, S.; Martins, D.O.S.; Jardim, A.C.G. A review of the ongoing research on Zika virus treatment. Viruses 2018, 10, 255. [Google Scholar] [CrossRef]
- Saiz, J.C.; Oya, N.J.D.; Blázquez, A.B.; Escribano-Romero, E.; Martín-Acebes, M.A. Host-directed antivirals: A realistic alternative to fight Zika virus. Viruses 2018, 10, 453. [Google Scholar] [CrossRef]
- Giraldo, M.I.; Gonzalez-Orozco, M.; Rajsbaum, R. Pathogenesis of Zika virus Infection. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 181–203. [Google Scholar] [CrossRef]
- Pena, L.J.; Guarines, K.M.; Silva, A.J.D.; Leal, L.R.S.; Félix, D.M.; Silva, A.; de Oliveira, S.A.; Ayres, C.F.J.; Júnior, A.S.; de Freitas, A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Msheik, H.; Azar, J.; El Sabeh, M.; Abou-Kheir, W.; Daoud, G. HTR-8/SVneo: A model for epithelial to mesenchymal transition in the human placenta. Placenta 2020, 90, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Soares, M.J.; Audus, K.L. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am. J. Physiol. Cell Physiol. 1997, 273, C1596–C1604. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.D.; Costa, A.; Prieto-Oliveira, P.; Andreata-Santos, R.; Peter, C.M.; Zanotto, P.M.A.; Janini, L.M.R. Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines. Viruses 2023, 15, 1446. https://doi.org/10.3390/v15071446
Barbosa MD, Costa A, Prieto-Oliveira P, Andreata-Santos R, Peter CM, Zanotto PMA, Janini LMR. Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines. Viruses. 2023; 15(7):1446. https://doi.org/10.3390/v15071446
Chicago/Turabian StyleBarbosa, Márcia Duarte, Anderson Costa, Paula Prieto-Oliveira, Robert Andreata-Santos, Cristina M. Peter, Paolo M. A. Zanotto, and Luiz Mario Ramos Janini. 2023. "Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines" Viruses 15, no. 7: 1446. https://doi.org/10.3390/v15071446
APA StyleBarbosa, M. D., Costa, A., Prieto-Oliveira, P., Andreata-Santos, R., Peter, C. M., Zanotto, P. M. A., & Janini, L. M. R. (2023). Proposal of Model for Evaluation of Viral Kinetics of African/Asian/Brazilian—Zika virus Strains (Step Growth Curve) in Trophoblastic Cell Lines. Viruses, 15(7), 1446. https://doi.org/10.3390/v15071446





