African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells
Abstract
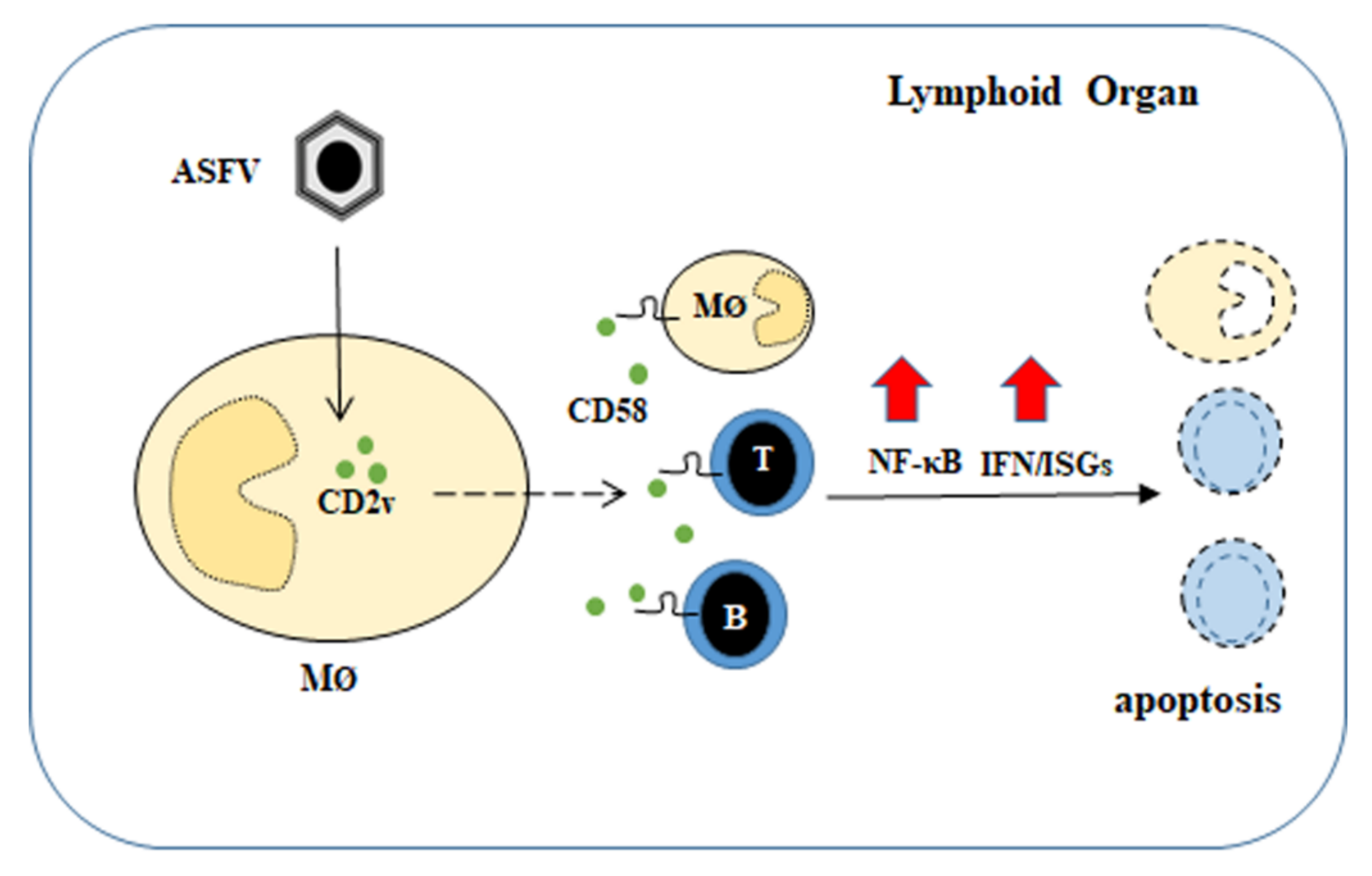
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Plasmids and Transfection
2.3. Hemadsorption Assay
2.4. CD2v Purification and Quantification
2.5. Monoclonal Antibodies against CD2v
2.6. Western Blot
2.7. Immunoprecipitation
2.8. CD58 siRNA Knockdown
2.9. Real-Time PCR
2.10. NF-κB-p65 Nuclear Translocation Assay
2.11. Interferon Bioassay
2.12. Flow Cytometry
2.13. Coimmunoprecipitation
2.14. CD2v–CD58 Colocalization Assay
2.15. TUNEL Assay
2.16. Statistics
3. Results
3.1. ASFV CD2v Localizes in the Perinuclear Region, Cytoplasm and Cell Membrane of PK15 Cells and Is Present in the Culture Supernatant
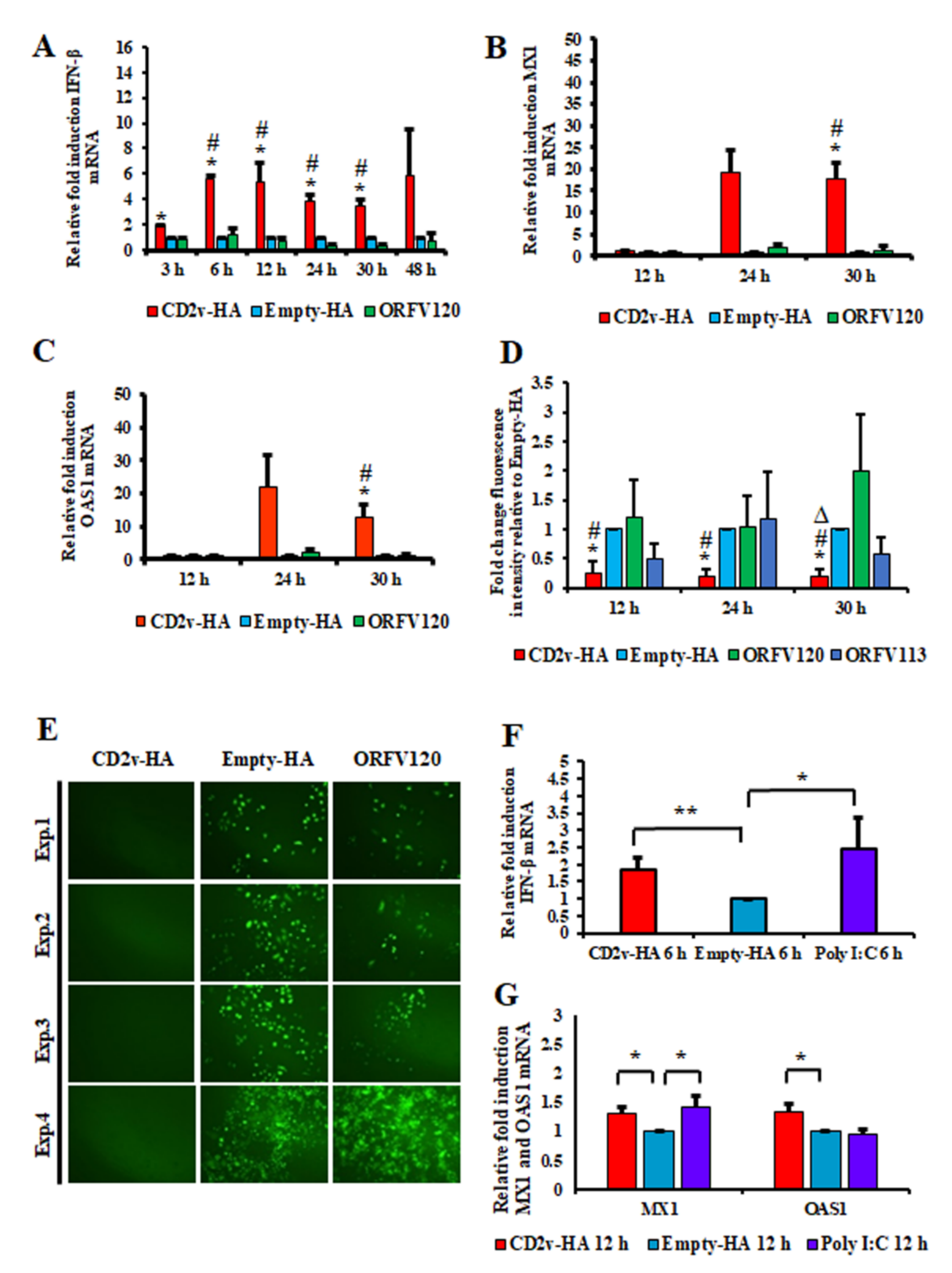
3.2. Expression of ASFV CD2v Induces IFN-β and ISG Transcription in PK15 Cells and Swine Macrophages
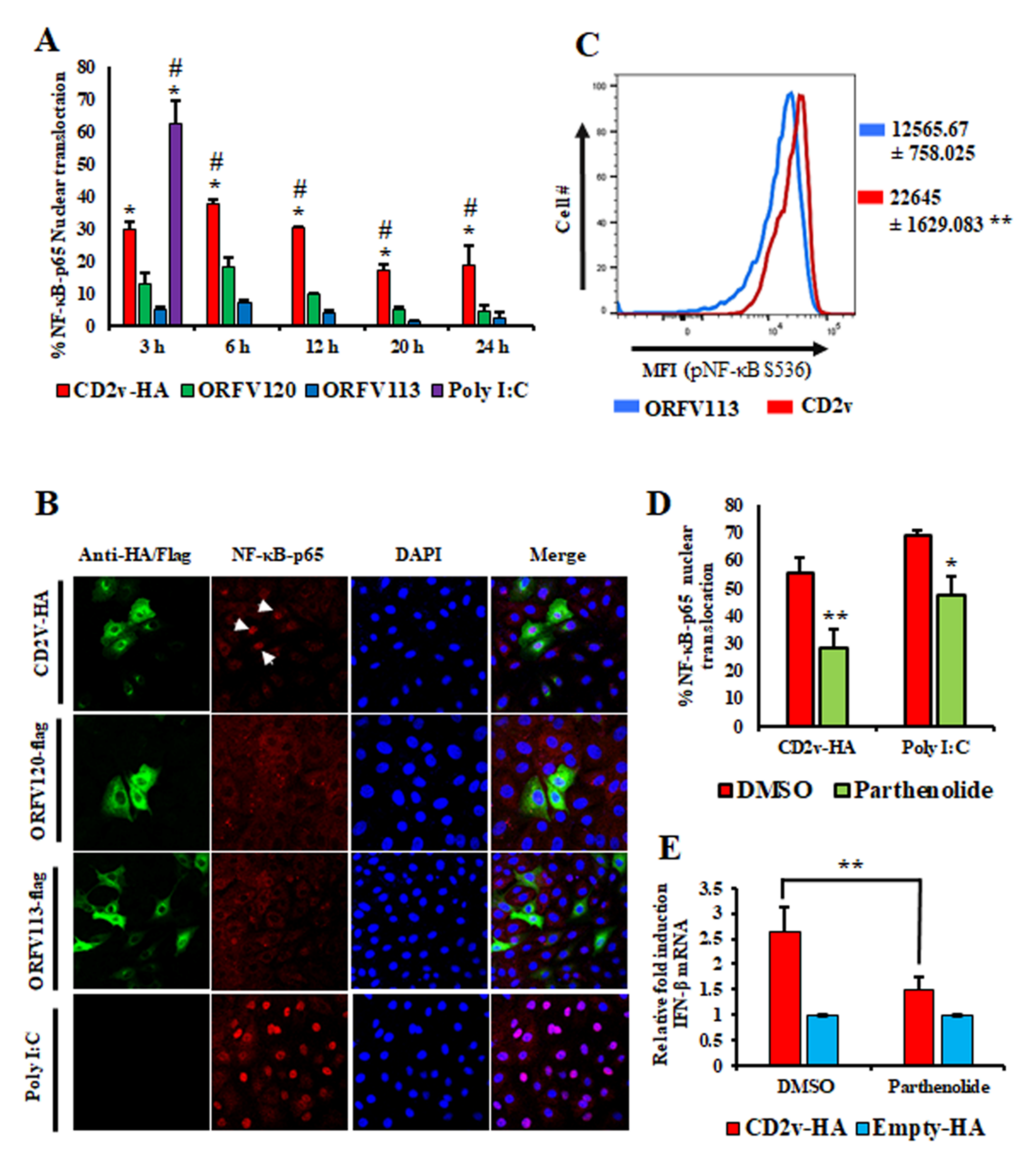
3.3. Induction of IFN-β by ASFV CD2v Is Dependent on NF-κB Activation
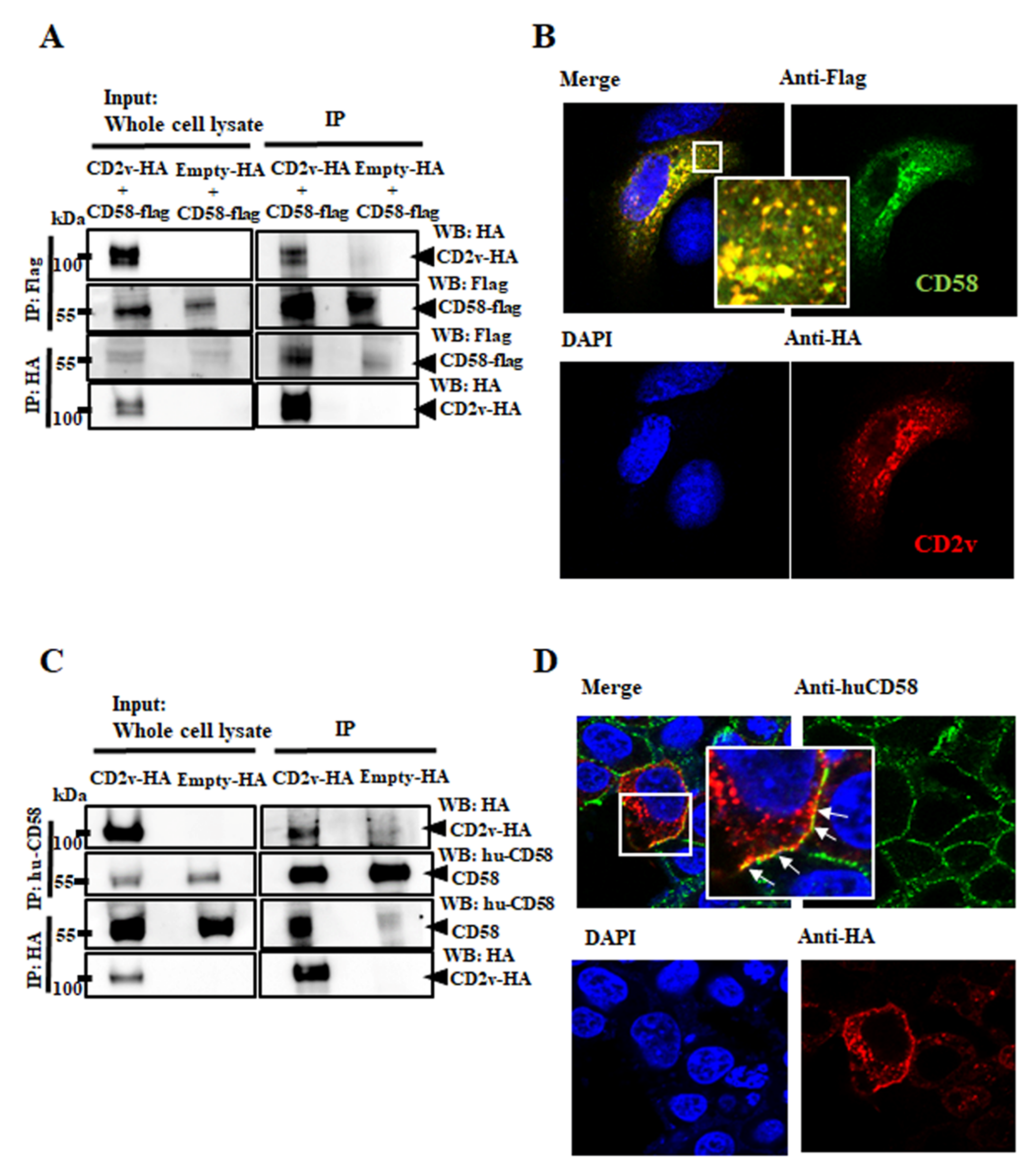
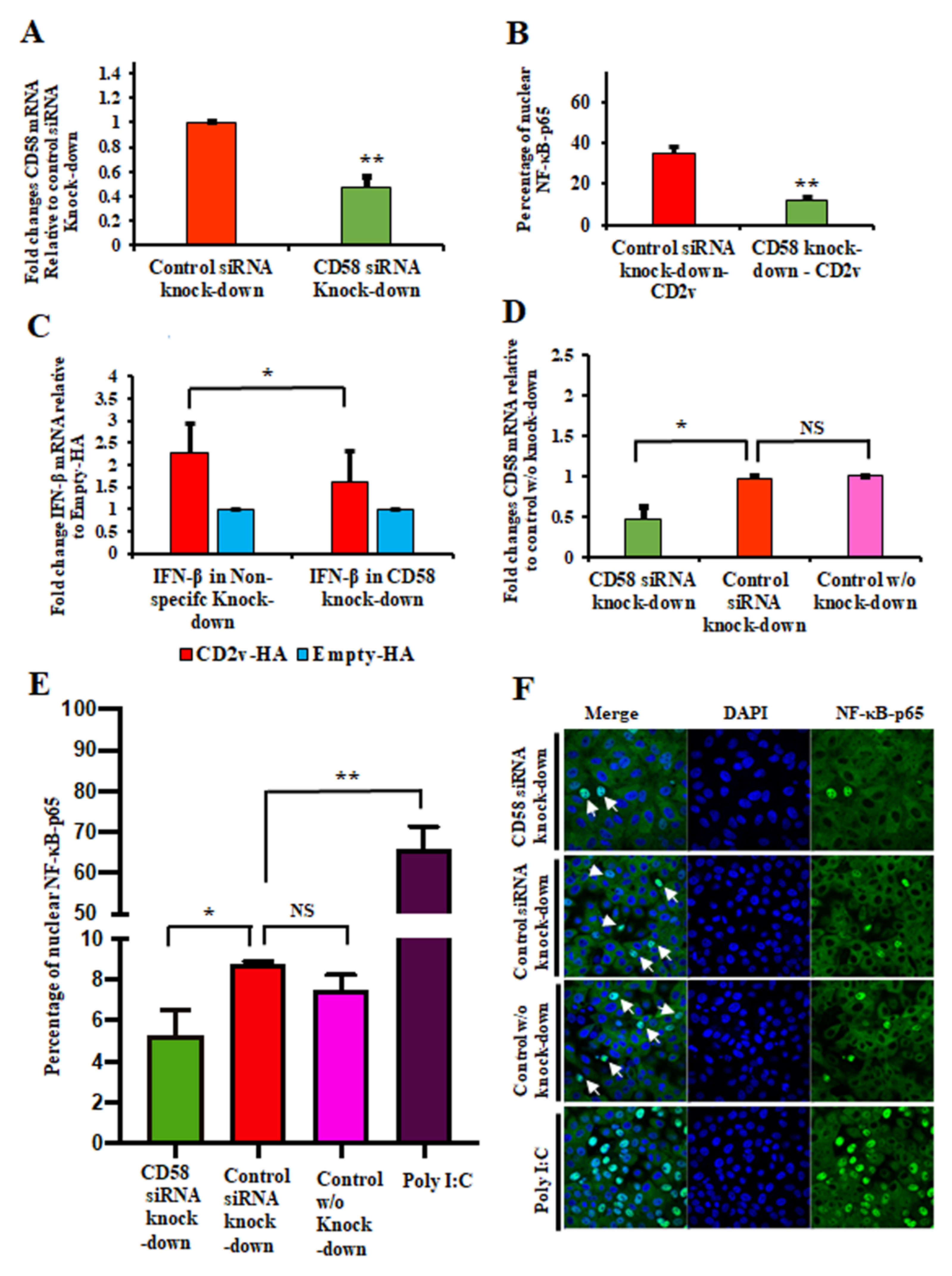
3.4. CD2v–CD58 Interaction Mediates NF-κB Activation and IFN-β Transcription
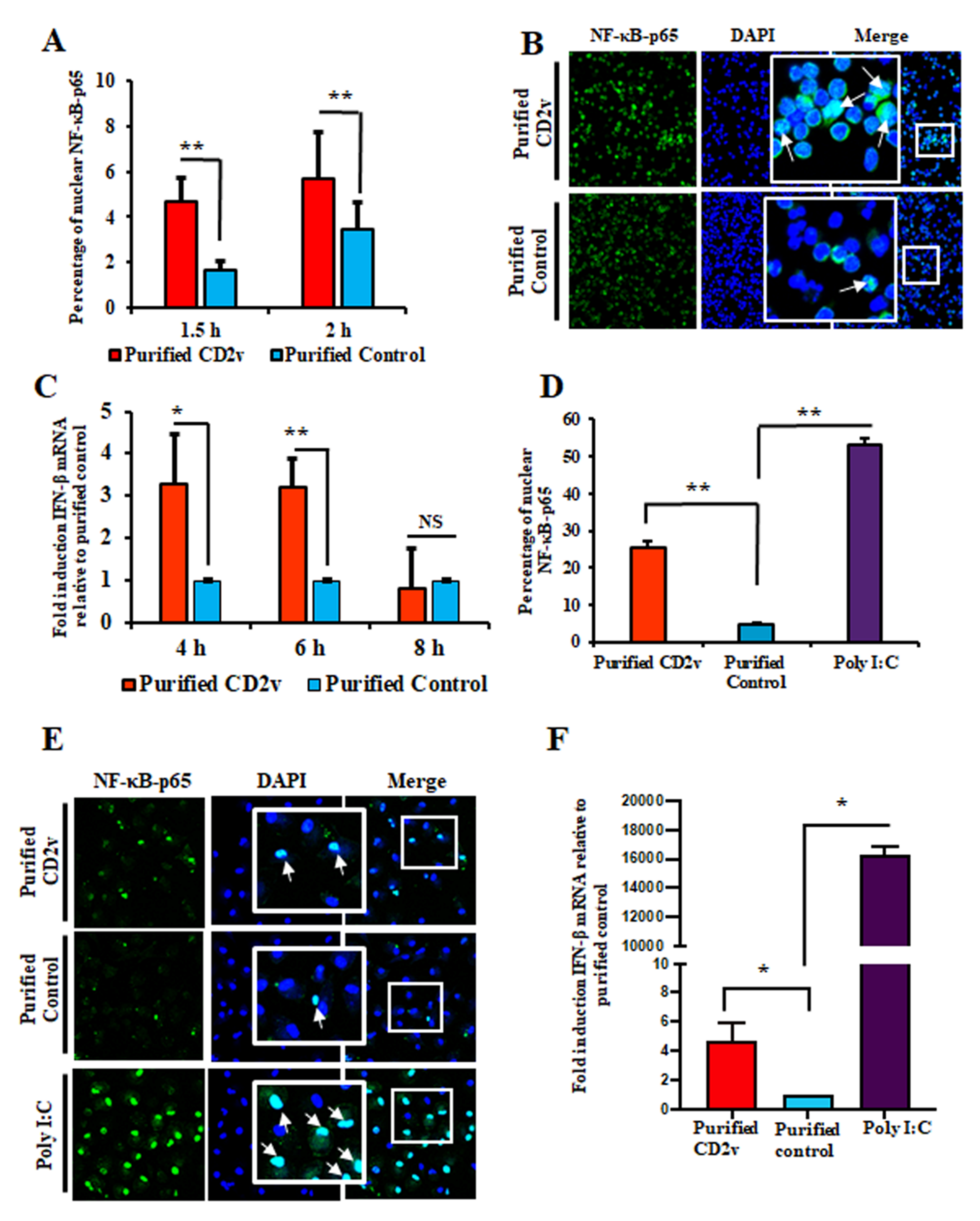
3.5. Purified CD2v Induces NF-κB-p65 Nuclear Translocation and IFN-β Transcription in Swine PBMCs and Macrophage Cultures
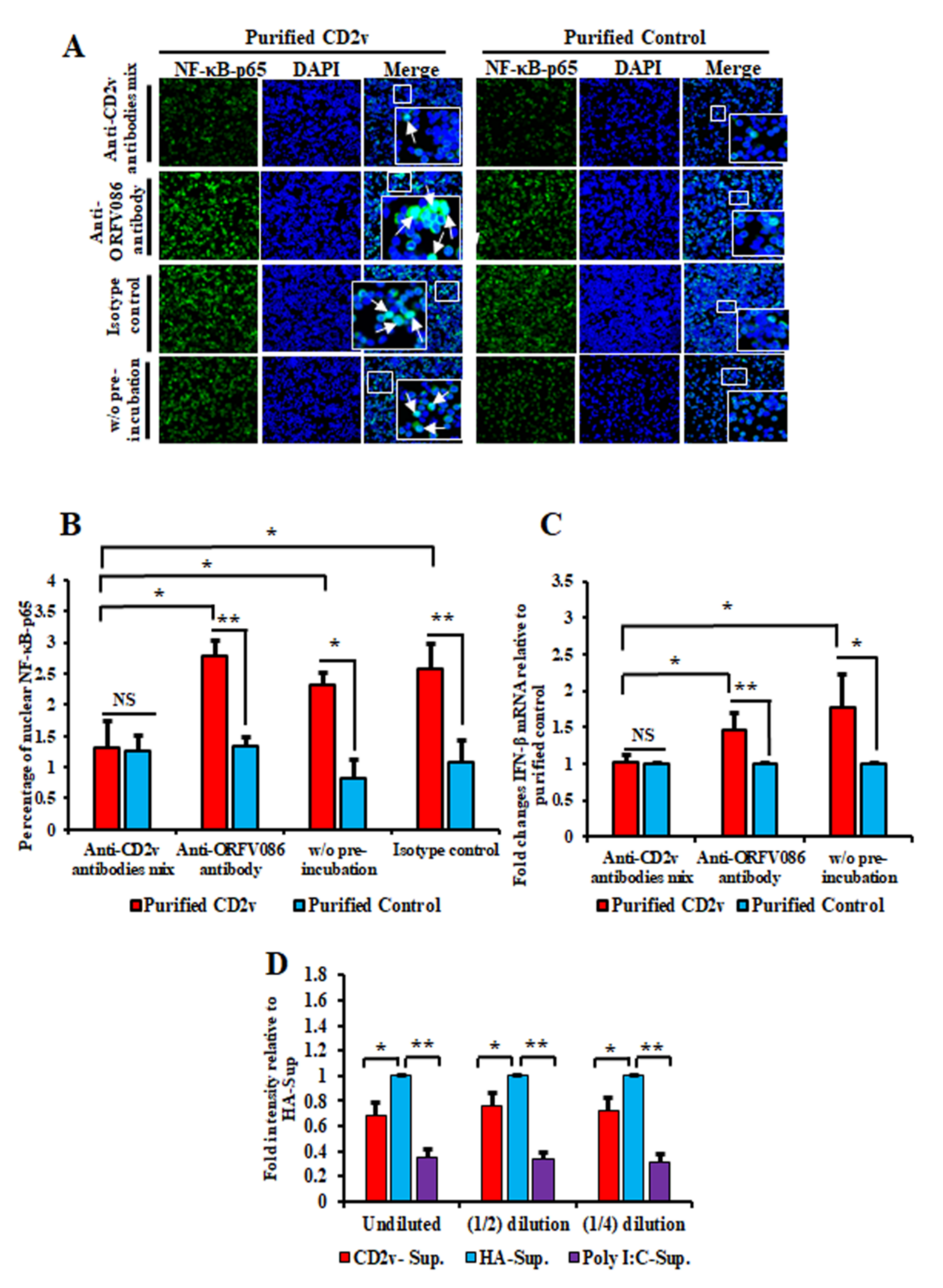
3.6. Antibodies against ASFV CD2v Inhibit CD2v-Induced NF-κB Activation and IFN-β Transcription in Swine PBMC Cultures
3.7. Supernatants from CD2v-Treated Swine PBMCs Exhibit Antiviral Activity
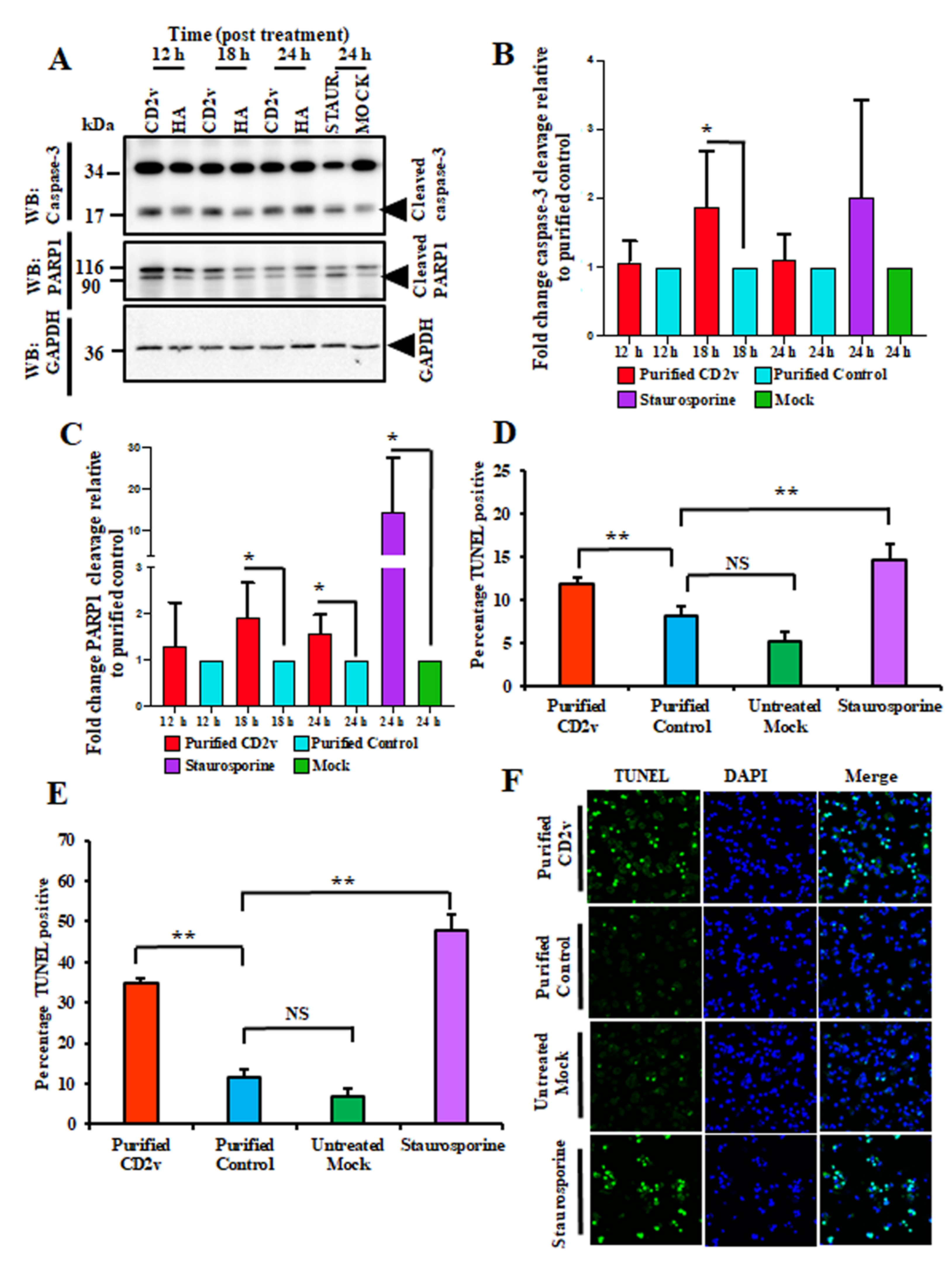
3.8. CD2v Induces Apoptosis in Swine PBMCs and Macrophage Cultures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulman, E.R.; Delhon, G.; Ku, B.; Rock, D.L. Asfarviruses. In Lesser Known Big DNA Viruses. In Current Topics in Microbiology and Immunology; Van Etten, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Montgomery, R.E. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. 1921, 34, 159–191. [Google Scholar] [CrossRef] [Green Version]
- Detray, D. Persistence of viremia and immunity in African swine fever. Am. J. Vet. Res. 1957, 18, 811–816. [Google Scholar]
- Oura, C.A.; Powell, P.P.; Anderson, E.; Parkhouse, R.M. The pathogenesis of African swine fever in the resistant bushpig. J. Gen. Virol. 1998, 79, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Villamandos, J.C.; Bautista, M.J.; Sanchez-Cordon, P.J.; Carrasco, L. Pathology of African swine fever: The role of monocyte-macrophage. Virus Res. 2013, 173, 140–149. [Google Scholar] [CrossRef]
- Moulton, J.; Coggins, L. Comparison of lesions in acute and chronic African swine fever. Cornell Vet. 1968, 58, 364–388. [Google Scholar] [PubMed]
- Carrasco, L.; Chacon-M de Lara, F.; Martin de las Mulas, J.; Gomez-Villamandos, J.C.; Perez, J.; Wilkinson, P.J.; Sierra, M.A. Apoptosis in lymph nodes in acute African swine fever. J. Comp. Pathol. 1996, 115, 415–428. [Google Scholar] [CrossRef]
- Salguero, F.J.; Sanchez-Cordon, P.J.; Nunez, A.; Fernandez de Marco, M.; Gomez-Villamandos, J.C. Proinflammatory cytokines induce lymphocyte apoptosis in acute African swine fever infection. J. Comp. Pathol. 2005, 132, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villamandos, J.C.; Hervas, J.; Mendez, A.; Carrasco, L.; De las Mulas, J.M.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. Experimental African swine fever: Apoptosis of lymphocytes and virus replication in other cells. J. Gen. Virol. 1995, 76, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Gomez del Moral, M.; Ortuno, E.; Fernandez-Zapatero, P.; Alonso, F.; Alonso, C.; Ezquerra, A.; Dominguez, J. African swine fever virus infection induces tumor necrosis factor alpha production: Implications in pathogenesis. J. Virol. 1999, 73, 2173–2180. [Google Scholar] [CrossRef] [Green Version]
- Salguero, F.J.; Ruiz-Villamor, E.; Bautista, M.J.; Sanchez-Cordon, P.J.; Carrasco, L.; Gomez-Villamandos, J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: Expression of cytokines. Vet. Immunol. Immunopathol. 2002, 90, 11–22. [Google Scholar] [CrossRef]
- Hume, D. The many faces of macrophage activation. Front. Immunol. 2015, 6, 370. [Google Scholar] [CrossRef] [Green Version]
- Gil, S.; Sepulveda, N.; Albina, E.; Leitao, A.; Martins, C. The low-virulent African swine fever virus (ASFV/NH/P68) induces enhanced expression and production of relevant regulatory cytokines (IFNα, TNFα and IL12p40) on porcine macrophages in comparison to the highly virulent ASFV/L60. Arch. Virol. 2008, 153, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Hopwood, P.; Abrams, C.C.; Downing, A.; Murray, F.; Talbot, R.; Archibald, A.; Lowden, S.; Dixon, L.K. Macrophage transcriptional responses following in vitro infection with a highly virulent African swine fever virus isolate. J. Virol. 2006, 80, 10514–10521. [Google Scholar] [CrossRef] [Green Version]
- Portugal, R.; Leitao, A.; Martins, C. Apoptosis in porcine macrophages infected in vitro with African swine fever virus (ASFV) strains with different virulence. Arch. Virol. 2009, 154, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Dei Giudici, S.; Oggiano, A. Infection, modulation and responses of antigen-presenting cells to African swine fever viruses. Virus Res. 2018, 258, 73–80. [Google Scholar] [CrossRef]
- Gonzalez, S.; Mendoza, C.; Sanchez-Vizcaino, J.M.; Alonso, F. Inhibitory effect of African swine fever virus on lectin-dependent swine lymphocyte proliferation. Vet. Immunol. Immunopathol. 1990, 26, 71–80. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Yanez, R.J.; Almazan, F.; Vinuela, E.; Rodriguez, J.F. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J. Virol. 1993, 67, 5312–5320. [Google Scholar] [CrossRef] [Green Version]
- Borca, M.V.; Kutish, G.F.; Afonso, C.L.; Irusta, P.; Carrillo, C.; Brun, A.; Sussman, M.; Rock, D.L. An African Swine Fever Virus Gene with Similarity to the T-Lymphocyte Surface Antigen CD2 Mediates Hemadsorption. Virology 1994, 199, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Goatley, L.C.; Dixon, L.K. Processing and Localization of the African Swine Fever Virus CD2v Transmembrane Protein. J. Virol. 2011, 85, 3294–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borca, M.V.; Carrillo, C.; Zsak, L.; Laegreid, W.W.; Kutish, G.F.; Neilan, J.G.; Burrage, T.G.; Rock, D.L. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 1998, 72, 2881–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteagudo, P.L.; Lacasta, A.; Lopez, E.; Bosch, L.; Collado, J.; Pina-pedrero, S.; Correa-fiz, F.; Accensi, F. A71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Zsak, L.; Delhon, G.; Diel, D.G.; Shobogorov, N.M.; Morgunov, Y.P.; Morgunov, S.Y.; Kutish, G.F.; et al. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J. Gen. Virol. 2016, 97, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.A.G.; Tcherepanov, V.; Upton, C.; Dixon, L.K. Comparison of the genome sequences of non-pathogenic and pathogenic African swine fever virus isolates. J. Gen. Virol. 2008, 89, 397–408. [Google Scholar] [CrossRef]
- Borca, M.V.; O’Donnell, V.; Holinka, L.G.; Risatti, G.R.; Ramirez-Medina, E.; Vuono, E.A.; Shi, J.; Pruitt, S.; Rai, A.; Silva, E.; et al. Deletion of CD2-like gene from the genome of African swine fever virus strain Georgia does not attenuate virulence in swine. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Duarte, M.M.; Boinas, F.; Hutchings, G.; Dixon, L.K. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology 2009, 393, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiboeker, S.B.; Burrage, T.G.; Scoles, G.A.; Fish, D.; Rock, D.L. African swine fever virus infection in the argasid host, Ornithodoros porcinus porcinus. J. Virol. 1998, 72, 1711–1724. [Google Scholar] [CrossRef] [Green Version]
- Bockenstedt, L.K.; Goldsmith, M.A.; Dustin, M.; Olive, D.; Springer, T.A.; Weiss, A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J. Immunol. 1988, 141, 1904–1911. [Google Scholar] [PubMed]
- Satiimul, T. Accessory Cell-Dependent T-Cell Activation via Involvement of CD2-LFA-3 Interaetions. Scand. J. Immunol. 1988, 28, 277–284. [Google Scholar]
- Karmann, K.; Hughes, C.C.W.; Fanslow, W.C.; Pober, J.S. Endothelial cells augment the expression of CD40 ligand on newly activated human CD4+T cells through a CD2/LFA-3 signaling pathway. Eur. J. Immunol. 1996, 26, 610–617. [Google Scholar] [CrossRef]
- Diaz-Sanchez, D.; Chegini, S.; Zhang, K.; Saxon, A. CD58 (LFA-3) stimulation provides a signal for human isotype switching and IgE production distinct from CD40. J. Immunol. 2018, 153, 10–20. [Google Scholar]
- King, P.D.; Sadra, A.; Han, A.; Liu, X.R.; Sunder-Plassmann, R.; Reinherz, E.L.; Dupont, B. CD2 signaling in T cells involves tyrosine phosphorylation and activation of the Tec family kinase, EMT/ITK/TSK. Int. Immunol. 1996, 8, 1707–1714. [Google Scholar] [CrossRef]
- Lin, H.; Hutchcroft, J.E.; Andoniou, C.E.; Kamoun, M.; Band, H.; Bierer, B.E. Association of p59 fyn with the T Lymphocyte Costimulatory Receptor CD2. Binding of the Fyn Src homology (SH)3 domain is regulated by the Fyn SH2 domain. J. Biol. Chem. 1998, 273, 19914–19921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Guiner, S.; Le Drean, E.; Labarriere, N.; Fonteneau, J.F.; Viret, C.; Diez, E.; Jotereau, F. LFA-3 co-stimulates cytokine secretion by cytotoxic T lymphocytes by providing a TCR-independent activation signal. Eur. J. Immunol. 1998, 28, 1322–1331. [Google Scholar] [CrossRef]
- Meinl, E.; Lengenfelder, D.; Blank, N.; Pirzer, R.; Barata, L.; Hivroz, C. Differential Requirement of ZAP-70 for CD2-Mediated Activation Pathways of Mature Human T Cells. J. Immunol. 2000, 165, 3578–3583. [Google Scholar] [CrossRef] [Green Version]
- Bagnasco, M.; Nunes, J.; Lopez, M.; Cerdan, C.; Pierres, A.; Mawas, C.; Olive, D. T cell activation via the CD2 molecule is associated with protein kinase C translocation from the cytosol to the plasma membrane. Eur. J. Immunol. 1989, 19, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Skanland, S.S.; Moltu, K.; Berge, T.; Aandahl, E.M.; Tasken, K. T-cell co-stimulation through the CD2 and CD28 co-receptors induces distinct signalling responses. Biochem. J. 2014, 460, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.A.; Galeota, J.; Osorio, F.A.; Husmann, R.J.; Schnitzlein, W.M.; Zuckermann, F.A. Gradual development of the interferon-γ response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 2003, 309, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Parida, R.; Choi, I.S.; Peterson, D.A.; Pattnaik, A.K.; Laegreid, W.; Zuckermann, F.A.; Osorio, F.A. Location of T-cell epitopes in nonstructural proteins 9 and 10 of type-II porcine reproductive and respiratory syndrome virus. Virus Res. 2012, 169, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Rock, D.L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34. 5 gene. J. Virol. 1996, 70, 8865–8871. [Google Scholar] [CrossRef] [Green Version]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef]
- Diel, D.G.; Delhon, G.; Luo, S.; Flores, E.F.; Rock, D.L. A Novel Inhibitor of the NF-κB Signaling Pathway Encoded by the Parapoxvirus Orf Virus. J. Virol. 2010, 84, 3962–3973. [Google Scholar] [CrossRef] [Green Version]
- Malogolovkin, A.; Burmakina, G.; Tulman, E.R.; Delhon, G.; Diel, D.G.; Salnikov, N.; Kutish, G.F.; Kolbasov, D.; Rock, D.L. African swine fever virus CD2v and C-type lectin gene loci mediate serological specificity. J. Gen. Virol. 2015, 96, 866–873. [Google Scholar] [CrossRef]
- Kay-Jackson, P.C.; Goatley, L.C.; Cox, L.; Miskin, J.E.; Parkhouse, R.M.; Wienands, J.; Dixon, L.K. The CD2v protein of African swine fever virus interacts with the actin-binding adaptor protein SH3P7. J. Gen. Virol. 2004, 85, 119–130. [Google Scholar] [CrossRef]
- Ruiz-Gonzalvo, F.; Coll, J.M. Characterization of a soluble hemagglutinin induced in African swine fever virus-infected cells. Virology 1993, 196, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Maniatis, T. The mechanism of transcriptional synergy of an in vitro assembled interferon-β enhanceosome. Mol. Cell 1997, 1, 119–129. [Google Scholar] [CrossRef]
- Lenardo, M.J.; Fan, C.M.; Maniatis, T.; Baltimore, D. The involvement of NF-κB in β-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 1989, 57, 287–294. [Google Scholar] [CrossRef]
- Thanos, D.; Maniatis, T. Identification of the rel family members required for virus induction of the human beta interferon gene. Mol. Cell. Biol. 1995, 15, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Suemori, H.; Hata, N.; Asagiri, M.; Ogasawara, K.; Nakao, K.; Nakaya, T.; Katsuki, M.; Noguchi, S.; Tanaka, N.; et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 2000, 13, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Takaoka, A.; Taniguchi, T. Type I Inteferon Gene Induction by the Interferon Regulatory Factor Family of Transcription Factors. Immunity 2006, 25, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Ibanez, F.; Ortega, A.; Brun, A.; Escribano, J.M.; Alonso, C. Apoptosis: A mechanism of cell killing and lymphoid organ impairment during acute African swine fever virus infection. J. Gen. Virol. 1996, 77, 2209–2219. [Google Scholar] [CrossRef]
- Fensterl, V.; Sen, G.C. Interferons and viral infections. BioFactors 2009, 35, 14–20. [Google Scholar] [CrossRef]
- Fensterl, V.; Chattopadhyay, S.; Sen, G.C. No Love Lost Between Viruses and Interferons. Annu. Rev. Virol. 2015, 2, 549–572. [Google Scholar] [CrossRef]
- Yen, J.H.; Ganea, D. Interferon β induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood 2009, 114, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedoni, S.; Olianas, M.C.; Onali, P. Interferon-β induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK–STAT signaling and down-regulation of PI3K/Akt pathway. J. Neurochem. 2010, 115, 1421–1433. [Google Scholar] [CrossRef]
- Makowska, A.; Wahab, L.; Braunschweig, T.; Kapetanakis, N.I.; Vokuhl, C.; Denecke, B.; Shen, L.; Busson, P.; Kontny, U. Interferon beta induces apoptosis in nasopharyngeal carcinoma cells via the TRAIL-signaling pathway. Oncotarget 2018, 9, 14228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Silverman, R.H. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J. Biol. Chem. 1995, 270, 4133–4137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, J.; Wood, K.A.; Youle, R.J. The 2-5A system in viral infection and apoptosis. Biomed. Pharmacother. 1998, 52, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Castelli, J.C.; Hassel, B.A.; Maran, A.; Paranjape, J.; Hewitt, J.A.; Li, X.L.; Hsu, Y.T.; Silverman, R.H.; Youle, R.J. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ. 1998, 5, 313–320. [Google Scholar] [CrossRef]
- Diaz-Guerra, M.; Rivas, C.; Esteban, M. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology 1997, 236, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; He, S.; Minassian, A.; Li, J.; Feng, P. Recent advances on viral manipulation of NF-κB signaling pathway. Curr. Opin. Virol. 2015, 15, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, M.; Zheng, H.; Muster, T.; Palese, P.; Beg, A.A.; Garcia-Sastre, A. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 2000, 74, 11566–11573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanos, D.; Maniatis, T. Virus induction of human IFNβ gene expression requires the assembly of an enhanceosome. Cell 1995, 83, 1091–1100. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, V.L.; Almeida, S.C.P.; Soares, H.R.; Crespo, A.; Marshall-Clarke, S.; Parkhouse, R.M. A novel TLR3 inhibitor encoded by African swine fever virus (ASFV). Arch. Virol. 2011, 156, 597–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, S.; Ventura, S.; Parkhouse, R.M. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 2013, 173, 87–100. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Wu, Y.; Chen, H.; Zhang, S.; Li, J.; Xin, T.; Jia, H.; Hou, S.; Jiang, Y.; et al. Inhibition of cGAS-STING-TBK1 signaling pathway by DP96R of ASFV China 2018/1. Biochem. Biophys. Res. Commun. 2018, 506, 437–443. [Google Scholar] [CrossRef]
- Revilla, Y.; Callejo, M.; Rodrı, J.M.; Culebras, E.; Nogal, L.; Salas, L.; Vin, E.; Fresno, M. Inhibition of nuclear factor kappaB activation by a virus-encoded IkappaB-like protein. J. Biol. Chem. 1998, 273, 5405–5411. [Google Scholar] [CrossRef] [Green Version]
- Afonso, C.L.; Piccone, M.E.; Zaffuto, K.M.; Neilan, J.; Kutish, G.F.; Lu, Z.; Balinsky, C.A.; Gibb, T.R.; Bean, T.J.; Zsak, L.; et al. African swine fever virus multigene family 360 and 530 genes affect host interferon response. J. Virol. 2004, 78, 1858–1864. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Belmonte, R.; Perez-Nunez, D.; Pittau, M.; Richt, J.A.; Revilla, Y. African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway. J. Virol. 2019, 93, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Portugal, R.; Leitao, A.; Martins, C. Modulation of type I interferon signaling by African swine fever virus (ASFV) of different virulence L60 and NHV in macrophage host cells. Vet. Microbiol. 2018, 216, 132–141. [Google Scholar] [CrossRef]
- Reis, A.L.; Abrams, C.C.; Goatley, L.C.; Netherton, C.; Chapman, D.G.; Sanchez-Cordon, P.; Dixon, L.K. Deletion of African swine fever virus interferon inhibitors from the genome of a virulent isolate reduces virulence in domestic pigs and induces a protective response. Vaccine 2016, 34, 4698–4705. [Google Scholar] [CrossRef] [Green Version]
- Karalyan, Z.; Zakaryan, H.; Sargsyan, K.; Voskanyan, H.; Arzumanyan, H.; Avagyan, H.; Karalova, E. Interferon status and white blood cells during infection with African swine fever virus in vivo. Vet. Immunol. Immunopathol. 2012, 145, 551–555. [Google Scholar] [CrossRef]
- Golding, J.P.; Goatley, L.; Goodbourn, S.; Dixon, L.K.; Taylor, G.; Netherton, C.L. Sensitivity of African swine fever virus to type I interferon is linked to genes within multigene families 360 and 505. Virology 2016, 493, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itzhaky, D.; Raz, N.; Hollander, N. The glycosylphosphatidylinositol-anchored form and the transmembrane form of CD58 associate with protein kinases. J. Immunol. 1998, 160, 4361–4366. [Google Scholar]
- Ariel, O.; Kukulansky, T.; Raz, N.; Hollander, N. Distinct membrane localization and kinase association of the two isoforms of CD58. Cell. Signal. 2004, 16, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Ariel, O.; Levi, Y.; Hollander, N. Signal transduction by CD58: The transmembrane isoform transmits signals outside lipid rafts independently of the GPI-anchored isoform. Cell. Signal. 2009, 21, 1100–1108. [Google Scholar] [CrossRef]
- Le, P.T.; Vollger, L.W.; Haynes, B.F.; Singer, K.H. Ligand binding to the LFA-3 cell adhesion molecule induces IL-1 production by human thymic epithelial cells. J. Immunol. 1990, 144, 4541–4547. [Google Scholar] [PubMed]
- Carrera, A.C.; Rincon, M.; Sanchez-Madrid, F.; Lopez-Botet, M.; de Landaźuri, M.O. Triggering of co-mitogenic signals in T cell proliferation by anti-LFA-1 (CD18, CD11a), LFA-3, and CD7 monoclonal antibodies. J. Immunol. 1988, 141, 1919–1924. [Google Scholar]
- Webb, D.S.; Shimizu, Y.; Van Seventer, G.A.; Shaw, S.; Gerrard, T.L. LFA-3, CD44, and CD45: Physiologic triggers of human monocyte TNF and IL-1 release. Science 1990, 249, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yang, S.; Liao, Q. CD58 Immunobiology at a Glance. Front. Immunol. 2021, 12, 2212. [Google Scholar]
- Deckert, M.; Kubar, J.; Zoccola, D.; Bernard-Pomier, G.; Angelisova, P.; Horejsi, V.; Bernard, A. CD59 molecule: A second ligand for CD2 in T cell adhesion. Eur. J. Immunol. 1992, 22, 2943–2947. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, M.S.; Mouhtouris, E.; Vaughan, H.A.; Warren, H.S.; Parish, C.R. CD48 is a low affinity ligand for human CD2. J. Immunol. 1993, 151, 4606–4613. [Google Scholar]
- Van Der Merwe, P.A.; McPherson, D.C.; Brown, M.H.; Barclay, A.N.; Cyster, J.G.; Williams, A.F.; Davis, S.J. The NH2-terminal domain of rat CD2 binds rat CD48 with a low affinity and binding does not require glycosylation of CD2. Eur. J. Immunol. 1993, 23, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Menu, E.; Bothwell, A.L.; Sims, P.J.; Bierer, B.E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science 1992, 256, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Menu, E.; Tsai, B.C.; Bothwell, A.L.; Sims, P.J.; Bierer, B.E. CD59 costimulation of T cell activation. CD58 dependence and requirement for glycosylation. J. Immunol. 1994, 153, 2444–2456. [Google Scholar]
- Arulanandam, A.R.; Moingeon, P.; Concino, M.F.; Recny, M.A.; Kato, K.; Yagita, H.; Koyasu, S.; Reinherz, E.L. A soluble multimeric recombinant CD2 protein identifies CD48 as a low affinity ligand for human CD2: Divergence of CD2 ligands during the evolution of humans and mice. J. Exp. Med. 1993, 177, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.W.; Williams, A.F.; Kingsmore, S.F.; Seldin, M.F. Structure, expression, and genetic linkage of the mouse BCM1 (OX45 or Blast-1) antigen. Evidence for genetic duplication giving rise to the BCM1 region on mouse chromosome 1 and the CD2/LFA3 region on mouse chromosome 3. J. Exp. Med. 1990, 171, 2115–2130. [Google Scholar] [CrossRef] [Green Version]
- Barbalat, R.; Lau, L.; Locksley, R.M.; Barton, G.M. Toll-like receptor 2 on inflammatory monocytes induces type i interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009, 10, 1200–1209. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Hornung, V. Tlr2 joins the interferon gang. Nat. Immunol. 2009, 10, 1139–1141. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.; Jiang, Z.; Kunz, S.; Janssen, E.; Mols, J.; Hoebe, K.; Bahram, S.; Oldstone, M.B.A.; Beutler, B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 2007, 362, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, X.; Cao, Y.; Li, S.; Shao, J.; Sun, S.; Guo, H.; Yin, S. Identification of a new cell-penetrating peptide derived from the african swine fever virus CD2v protein. Drug Deliv. 2021, 28, 957–962. [Google Scholar] [CrossRef]
- Perez-Nunez, D.; Garcia-Urdiales, E.; Martinez-Bonet, M.; Nogal, M.L.; Barroso, S.; Revilla, Y.; Madrid, R. CD2v interacts with Adaptor Protein AP-1 during African swine fever infection. PLoS ONE 2015, 10, e0123714. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, C.; Mwaengo, D.M.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martin, E.; Kasiti, J.; Bishop, R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312. [Google Scholar] [CrossRef]
- Carlson, J.; O’Donnell, V.; Alfano, M.; Velazquez Salinas, L.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Higgs, S.; Borca, M.V. Association of the host immune response with protection using a live attenuated African swine fever virus model. Viruses 2016, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; McVicar, J.W.; Mebus, C.A. African swine fever convalescent sows: Subsequent pregnancy and the effect of colostral antibody on challenge inoculation of their pigs. Am. J. Vet. Res. 1984, 45, 1361–1366. [Google Scholar]
- Schlafer, D.H.; Mebus, C.A.; McVicar, J.W. African swine fever in neonatal pigs: Passively acquired protection from colostrum or serum of recovered pigs. Am. J. Vet. Res. 1984, 45, 1367–1372. [Google Scholar]
- Onisk, D.V.; Borca, M.V.; Kutish, S.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef]
- Ruiz-Gonzalvo, F.; Rodriguez, F.; Escribano, J.M. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 1996, 218, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaulagain, S.; Delhon, G.A.; Khatiwada, S.; Rock, D.L. African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells. Viruses 2021, 13, 1480. https://doi.org/10.3390/v13081480
Chaulagain S, Delhon GA, Khatiwada S, Rock DL. African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells. Viruses. 2021; 13(8):1480. https://doi.org/10.3390/v13081480
Chicago/Turabian StyleChaulagain, Sabal, Gustavo A. Delhon, Sushil Khatiwada, and Daniel L. Rock. 2021. "African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells" Viruses 13, no. 8: 1480. https://doi.org/10.3390/v13081480
APA StyleChaulagain, S., Delhon, G. A., Khatiwada, S., & Rock, D. L. (2021). African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells. Viruses, 13(8), 1480. https://doi.org/10.3390/v13081480




