Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review
Abstract
1. Mushrooms and Their Compounds
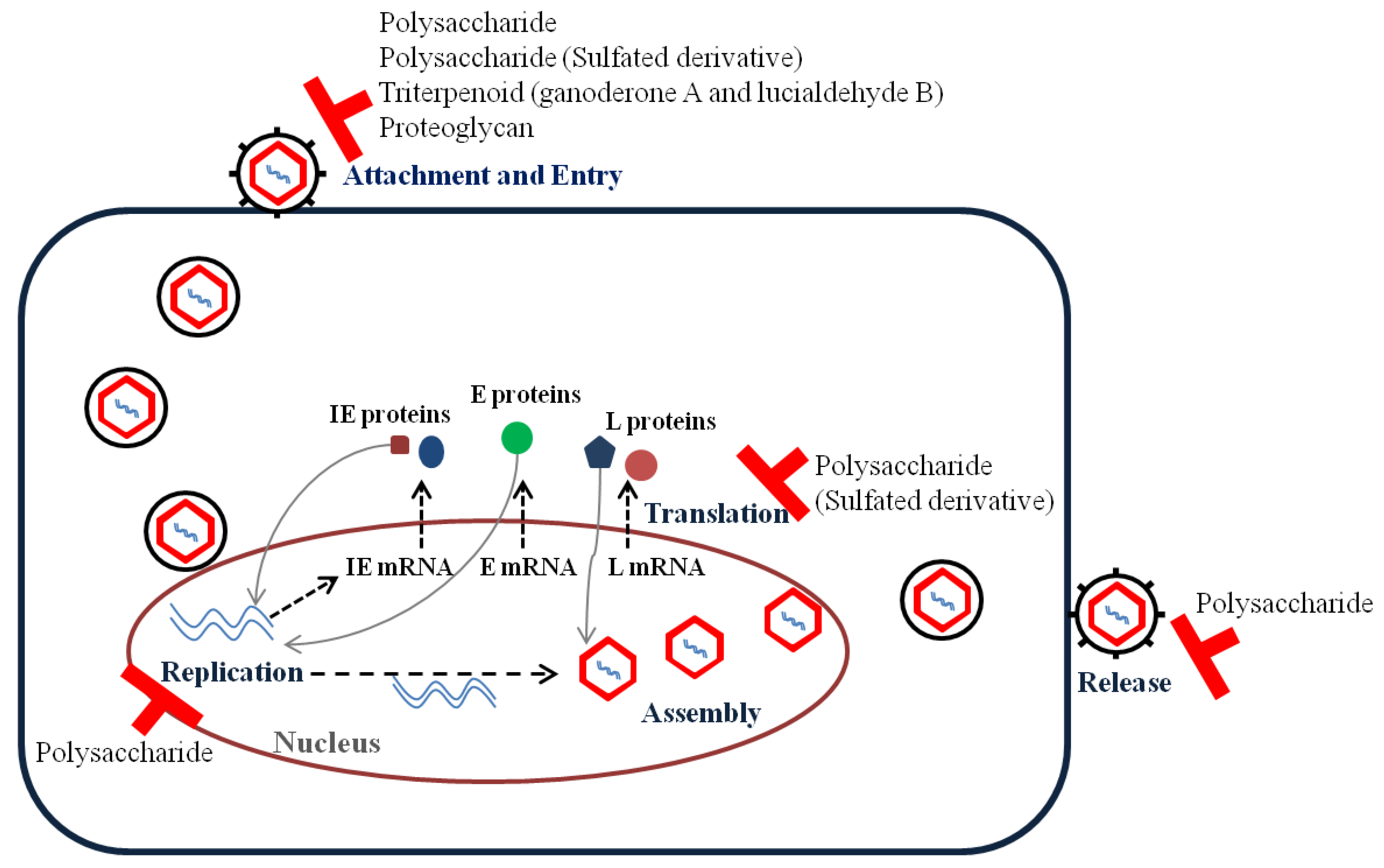
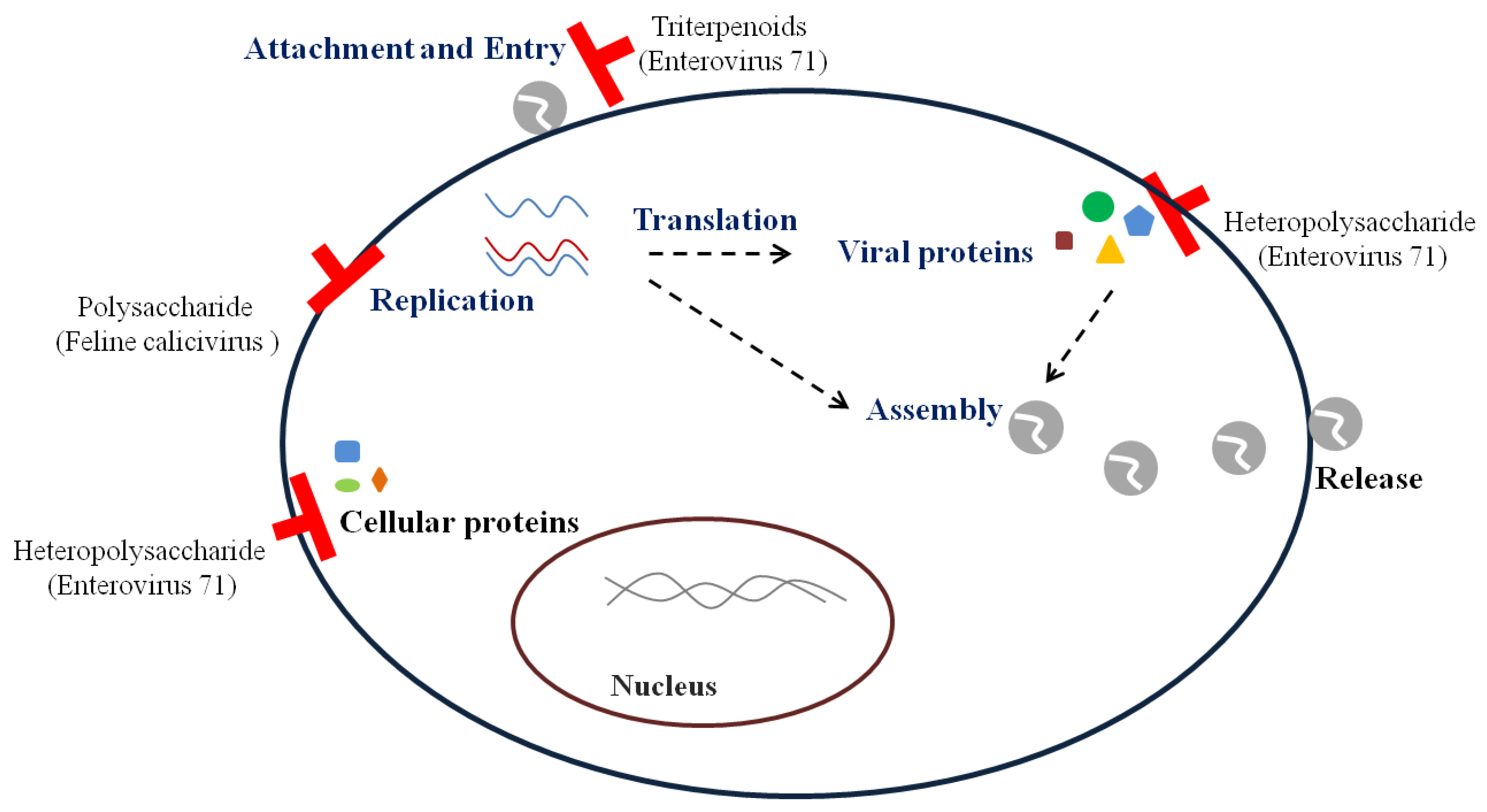
2. Replication Steps of Viruses and Antiviral Targets
3. Antiviral Activity of Mushroom Compounds against Viruses
3.1. Enveloped DNA Viruses
Herpes Simplex Viruses
3.2. Enveloped RNA Viruses
3.2.1. Influenza Viruses
3.2.2. Human Immunodeficiency Virus
3.2.3. Hepatitis C Virus
3.3. Non-Enveloped RNA Viruses
3.3.1. Norovirus
3.3.2. Enterovirus 71
3.3.3. Poliovirus and Coxsackievirus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, P.C. Mushrooms as Functional Foods; Cheung, P.C.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rahi, D.K.; Malik, D. Diversity of mushrooms and their metabolites of nutraceutical and therapeutic significance. J. Mycol. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Carter, J.; Saunders, V.A. Virology: Principles and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Menéndez-Arias, L.; Álvarez, M. Antiretroviral therapy and drug resistance in human immunodeficiency virus type 2 infection. Antivir. Res. 2014, 102, 70–86. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chen, I.T.; Wu, K.S.; Tseng, Y.T.; Sy, C.L.; Chen, J.K.; Lee, S.S.J.; Chen, Y.S. High rate of HIV-1 drug resistance in treatment failure patients in Taiwan, 2009–2014. Infect. Drug resist. 2017, 10, 343. [Google Scholar] [CrossRef]
- Omotani, S.; Ishizaka, T.; Inoue, M.; Nishida, K.; Yasui, Y.; Hatsuda, Y.; Mukai, J.; Myotoku, M. Drug-induced lung disease adverse effect with Ledipasvir Acetonate/Sofosbuvir. JPHCS 2020, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Breakefield, X.O.; Fraefel, C. HSV-1-based vectors for gene therapy of neurological diseases and brain tumors: Part I. HSV-1 structure, replication and pathogenesis. Neoplasia 1999, 1, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Muszalska, I.; Sobczak, A.; Dadej, A.; Tomczak, S.; Jelińska, A. Hepatitis C–New drugs and treatment prospects. Eur. J. Med. Chem. 2019, 165, 225–249. [Google Scholar] [CrossRef]
- Santoyo, S.; Ramírez-Anguiano, A.C.; Aldars-García, L.; Reglero, G.; Soler-Rivas, C. Antiviral activities of Boletus edulis, Pleurotus ostreatus and Lentinus edodes extracts and polysaccharide fractions against Herpes simplex virus type 1. J. Food Nutr. Res. 2012, 51, 225–235. [Google Scholar]
- Lee, S.M.; Kim, S.M.; Lee, Y.H.; Kim, W.J.; Park, J.K.; Park, Y.I.; Jang, W.J.; Shin, H.; Synytsya, A. Macromolecules isolated from Phellinus pini fruiting body: Chemical characterization and antiviral activity. Macromol. Res. 2010, 18, 602–609. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral Terpenoid Constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.; Ali, N.A.; Jansen, R.; Wegner, U.; Mentel, R.; Lindequist, U. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia 2003, 74, 177–180. [Google Scholar] [CrossRef]
- Yan, N.; He, F.; Piraino, F.F.; Xiang, H.; Chen, J.; Wang, Y.; Liu, X. Antiviral activity of a cloned peptide RC28 isolated from the higher basidiomycetes mushroom Rozites caperata in a mouse model of HSV-1 keratitis. Int. J. Med. Mushrooms 2015, 17, 819–828. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Cardozo, F.T.G.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.M.; de Mendonça, M.M.; Simões, C.M.O. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, F.; Larsen, I.V.; Carballo, E.V.; Jose, G.; Stern, R.A.; Brummel, R.C.; Camelini, C.M.; Rossi, M.J.; Simões, C.; Brandt, C.R. In vivo anti-herpes simplex virus activity of a sulfated derivative of Agaricus brasiliensis mycelial polysaccharide. Antimicrob. Agents Chemother. 2013, 57, 2541–2549. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Ye, L.; Yang, X.; Timani, K.A.; Zheng, Y.; Wang, Y. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J. Ethnopharmacol. 2004, 95, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Zhao, Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. BMB Rep. 2005, 38, 34–40. [Google Scholar] [CrossRef]
- Ilyicheva, T.N.; Teplyakova, T.V.; Svyatchenko, S.V.; Asbaganov, S.V.; Zmitrovich, I.V.; Vlasenko, A.V. Antiviral activity of total polysaccharide fraction of water and ethanol extracts of Pleurotus pulmonarius against the influenza A virus. Curr. Res. Environ. Appl. Mycol. J. Fungal Biol. 2020, 10, 224–235. [Google Scholar]
- Ohta, Y.; Lee, J.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Lee, M.; Lee, S.W.; Lee, I.; Seo, G.; Choi, H.J.; Yun, B. Neuraminidase inhibitors from the fermentation broth of Phellinus linteus. Mycobiology 2014, 42, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Suzuki, C.; Shimomura, E.; Maeda, H.; Fujil, T.; Ishida, N. Antiviral and interferon-inducing activities of a new peptidomannan, KS-2, extracted from culture mycelia of Lentinus edodes. J. Antibiot. 1979, 32, 1336–1345. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.; Choi, H.J.; Yun, B. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef]
- Song, A.; Sun, X.; Kong, C.; Zhao, C.; Qin, D.; Huang, F.; Yang, S. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch. Virol. 2014, 159, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Adotey, G.; Quarcoo, A.; Holliday, J.; Fofie, S.; Saaka, B. Effect of immunomodulating and antiviral agent of medicinal mushrooms (immune assist 24/7 TM) on CD4 T-lymphocyte counts of HIV-infected patients. Int. J. Med. Mushrooms 2011, 13, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Ng, T.B.; Li, L.; Fang, J.C.; Jiang, Y.; Wen, T.Y.; Qiao, W.T.; Li, N.; Liu, F. Isolation of a polysaccharide with antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse transcriptase inhibitory activities from the fruiting bodies of the abalone mushroom Pleurotus abalonus. J. Pharm. Pharmacol. 2011, 63, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Ng, T.B. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sci. 1997, 60, PL383–PL387. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001, 67, 669–672. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta-Gen. Subjects 2008, 1780, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochem. Biophys. Res. Commun. 2000, 276, 587–593. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.X.; Ng, T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochem. Biophys. Res. Commun. 2004, 315, 450–454. [Google Scholar] [CrossRef]
- Lv, H.; Kong, Y.; Yao, Q.; Zhang, B.; Leng, F.; Bian, H.; Balzarini, J.; Van Damme, E.; Bao, J. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine 2009, 16, 198–205. [Google Scholar] [CrossRef] [PubMed]
- M EL-Fakharany, E.; M Haroun, B.; Ng, T.; M Redwan, E. Oyster mushroom laccase inhibits hepatitis C virus entry into peripheral blood cells and hepatoma cells. Protein Peptide Lett. 2010, 17, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tejedor, D.; Claveria-Gimeno, R.; Velazquez-Campoy, A.; Abian, O.; Palomo, J.M. Tyrosinase from mushroom Agaricus bisporus as an inhibitor of the Hepatitis C virus. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Inhibition of murine norovirus and feline calicivirus by edible herbal extracts. Food Environ. Virol. 2017, 9, 35–44. [Google Scholar] [CrossRef]
- Tian, J.; Hu, X.; Liu, D.; Wu, H.; Qu, L. Identification of Inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. Int. J. Biol. Macromol. 2017, 95, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Faccin, L.C.; Benati, F.; Rincao, V.P.; Mantovani, M.S.; Soares, S.A.; Gonzaga, M.L.; Nozawa, C.; Carvalho Linhares, R.E. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007, 45, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Rincão, V.P.; Yamamoto, K.A.; Ricardo, N.M.P.S.; Soares, S.A.; Meirelles, L.D.P.; Nozawa, C.; Linhares, R.E.C. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef]
- Nishiyama, Y. Herpesvirus genes: Molecular basis of viral replication and pathogenicity. Nagoya J. Med. Sci. 1996, 59, 107–120. [Google Scholar] [PubMed]
- Birkmann, A.; Zimmermann, H. HSV antivirals–current and future treatment options. Curr. Opin. Virol. 2016, 18, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Morita, H.; Ogawa, R.; Nakamura, K.; Fujisaki, S.; Shirakura, M.; Kuwahara, T.; Kishida, N.; Watanabe, S.; Odagiri, T. Susceptibility of influenza viruses to the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Front. Microbiol. 2018, 9, 3026. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.B.; Chisari, F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. USA 2005, 102, 2561–2566. [Google Scholar] [CrossRef]
- Kotwal, G.; Cannon, J.L. Environmental persistence and transfer of enteric viruses. Curr. Opin. Virol. 2014, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Baert, L.; Uyttendaele, M. Inactivation of food-borne viruses using natural biochemical substances. Food Microbiol. 2013, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). Available online: https://talk.ictvonline.org/ (accessed on 6 November 2020).
- Abzug, M.J. The enteroviruses: Problems in need of treatments. J. Infect. 2014, 68, S108–S114. [Google Scholar] [CrossRef] [PubMed]
| Viruses | Mushrooms | Compounds | Targets or Effects | References | |
|---|---|---|---|---|---|
| Enveloped DNA Viruses | |||||
| Herpes simplex virus type 1 (HSV-1) | dsDNA | Boletus edulis, Pleurotus ostreatus, and Lentinus edodes | Polysaccharide fraction | Pre- and post-treatment effect | [9] |
| HSV-1 | Phellinus pini | Polysaccharide | Simultaneous-treatment effect | [10] | |
| HSV-1 | Ganoderma pfeifferi | Triterpenoid (ganoderone A and lucialdehyde B) | Pre-treatment effect | [11] | |
| HSV-1 | G. pfeifferi | Triterpenoid (ganodermadiol) | Protection of cells | [12] | |
| HSV-1 | Rozites caperata | Peptide RC28 | Keratitis (in vivo) | [13] | |
| HSV-1 and HSV-2 | Agaricus brasiliensis | Polysaccharide (Sulfated derivative) | Viral attachment and penetration, cell-to-cell spread, and the expression of ICP27, UL42, gB, and gD proteins | [14] | |
| HSV-1 and HSV-2 | A. brasiliensis | Polysaccharide (Sulfated derivative) | Cutaneous and genital infection (in vivo) | [15] | |
| HSV-1 and HSV-2 | G. lucidum | Proteoglycan | Pre- and co-treatment effect | [16,17] | |
| Viruses | Mushrooms | Compounds | Targets or Effects | References | |
|---|---|---|---|---|---|
| Enveloped RNA viruses | |||||
| Influenza A virus (H1N1pdm09) | Pleurotus pulmonarius | Polysaccharide fraction | Post-treatment effect | [18] | |
| Influenza A virus (H1N1) | Segmented (-)ssRNA | Cordyceps militaris | Acidic Polysaccharide | Decreased viral titer in bronchoalveolar and lung, increased TNF-α and IFN-γ in mice, and enhanced NO, iNOS, IL-1β, IL-6, IL-10, and TNF-α in cells | [19] |
| Influenza A virus (H1N1 and WS/33) | Phellinus linteus | Inotilone and 4-(3,4-dihydroxyphenyl)-3-buten-2-one | Neuraminidase (NA) Simultaneous-treatment effect | [20] | |
| Influenza A virus (H2N2) | Lentinus edodes | Peptidomannan | Decreased viral titer and lung consolidation in lung tissue, elaborated IFN level in serum, and increased survival in mice | [21] | |
| Influenza A virus (H1N1, H5N1, and H3N2) | P. baumii | Polyphenols (hispidin, hypholomine B, inoscavin A, davallialactone, and phelligridin D) | NA | [22] | |
| Influenza A virus (H5N1) | P. ignarius | Sesquiterpenoid | NA | [23] | |
| Sesquiterpenoid Pyrone Polyphenols | Post-treatment effect | ||||
| Influenza A virus | Ganoderma pfeifferi | Triterpenoid (ganodermadiol and lucidadiol) Triterpene (applanoxidic acid G) | Protection of cells | [12] | |
| Human immunodeficiency virus (HIV) | (+)ssRNA | Immune Assist 24/7™(L. edodes, G. frondosa, G. lucidum, Trametes versicolor, C. sinensis, and A. brasiliensis) | Multiple polysaccharide and heteropolysaccharide | Increased CD4+ T-lymphocyte | [24] |
| HIV-1 | P. abalonus | Polysaccharide | Reverse transcriptase | [25] | |
| HIV-1 | Coriolus versicolor | Polysaccharopeptide | Interaction of HIV-l gp120 with CD4, reverse transcriptase, and glycohydrolase | [26] | |
| HIV-1 | C. versicolor | Polysaccharopeptide (modified with chlorosulfonic acid) | Reverse transcriptase and glycohydrolase | [27] | |
| HIV-1 | Agaricus bisporus | Lectin | Reverse transcriptase | ||
| HIV-1 | P. citrinopileatus | Lectin | Reverse transcriptase | [28] | |
| HIV-1 | L. edodes | Lentin | Reverse transcriptase | [29] | |
| HIV | P. ostreatus | Ubiquitin-like Protein | Reverse transcriptase | [30] | |
| HIV-1 | Russula paludosa | Peptide | Reverse transcriptase | [31] | |
| HIV-1 | Tricholoma giganteum | Laccase | Reverse transcriptase | [32] | |
| HIV-1 | P. nebrodensis | Nebrodeolysin | HIV-induced syncytia formation in cells | [33] | |
| Hepatitis C virus (HCV) | (+)ssRNA | P. ostreatus | Laccase | Pre-, co-, post- treatment effect | [34] |
| HCV | A. bisporus | Tyrosinases | NS3, NS4A, and NS5A | [35] | |
| Viruses | Mushrooms | Compounds | Targets or Effects | References | |
|---|---|---|---|---|---|
| Non-enveloped RNA Viruses | |||||
| Murine norovirus Feline calicivirus (FCV) | (+)ssRNA | Inonotus obliquus | - | Pre-treatment effect | [36] |
| FCV | I. obliquus | Polysaccharide | Pre-, co-, post-, and simultaneous-treatment effect Viral replication | [37] | |
| Enterovirus 71 (EV71) | (+)ssRNA | Grifola frondosa | Heteropolysaccharide | Pre-, simultaneous-, and post- treatment effect VP1, caspase-3, and IκBα | [38] |
| EV71 | Ganoderma lucidum | Triterpenoids | Pre- and co- treatment effect Adsorption | [39] | |
| Poliovirus type 1 (PV-1) | (+)ssRNA | Agaricus brasiliensis | Polysaccharide | Simultaneous-treatment effect | [40] |
| PV-1 | Lentinula edodes | Polysaccharide | Simultaneous-treatment effect | [41] | |
| Coxsackie virus B3 | (+)ssRNA | Phellinus pini | Polysaccharide | Simultaneous-treatment effect | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. https://doi.org/10.3390/v13020350
Seo DJ, Choi C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses. 2021; 13(2):350. https://doi.org/10.3390/v13020350
Chicago/Turabian StyleSeo, Dong Joo, and Changsun Choi. 2021. "Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review" Viruses 13, no. 2: 350. https://doi.org/10.3390/v13020350
APA StyleSeo, D. J., & Choi, C. (2021). Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses, 13(2), 350. https://doi.org/10.3390/v13020350







