The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Sample Preparation
2.3. Evaluation of Virucidal Activity of HIDROX and HT Solutions Against SARS-CoV-2
2.4. Western Blotting
2.5. Real-Time RT-PCR
2.6. Evaluation of Virucidal Activity of HIDROX-Containing Cream against SARS-CoV-2
2.7. Statistical Analysis
3. Results
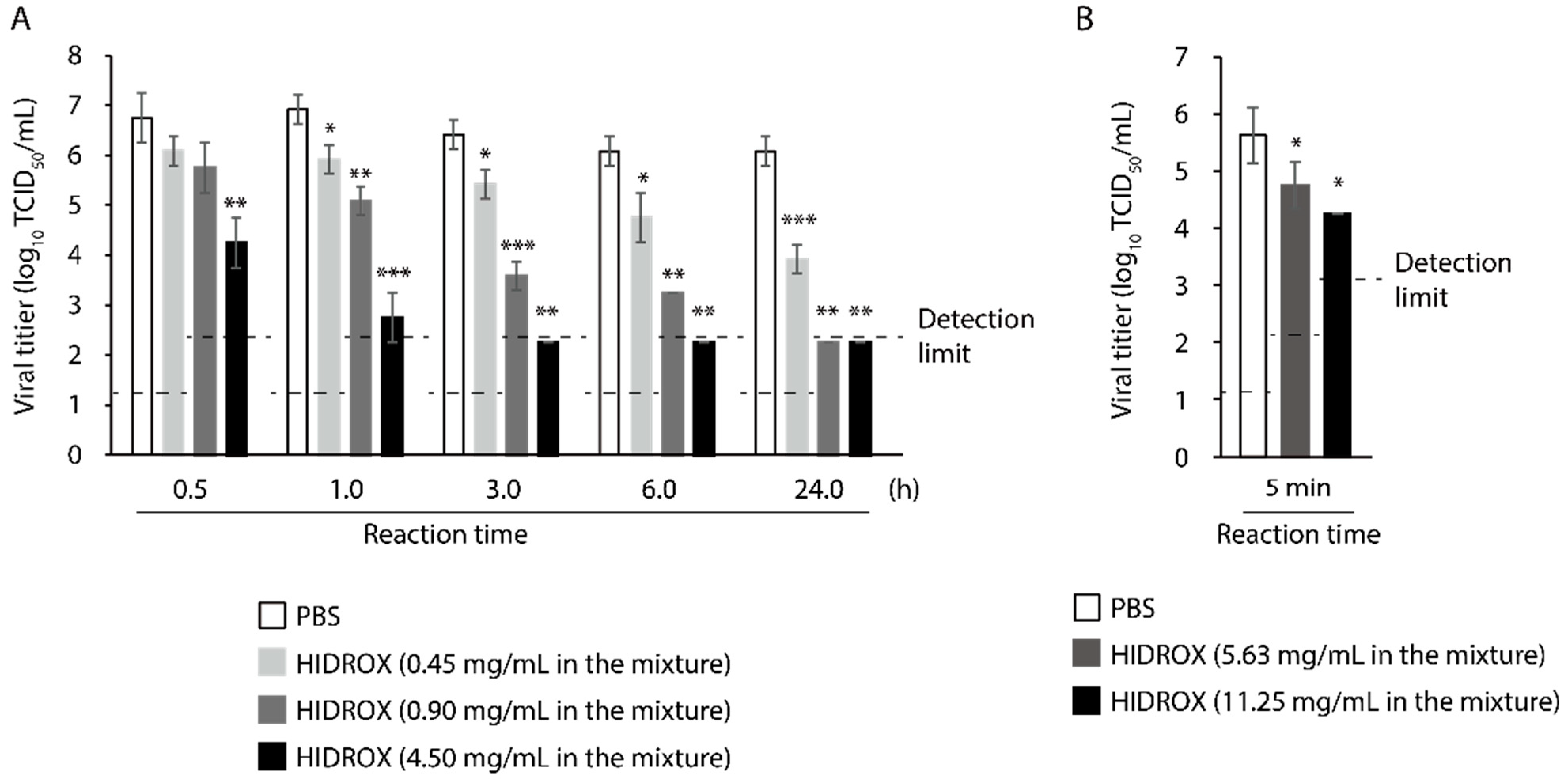
3.1. Evaluation of Time- and Concentration-Dependent Virucidal Activity of HIDROX against SARS-CoV-2
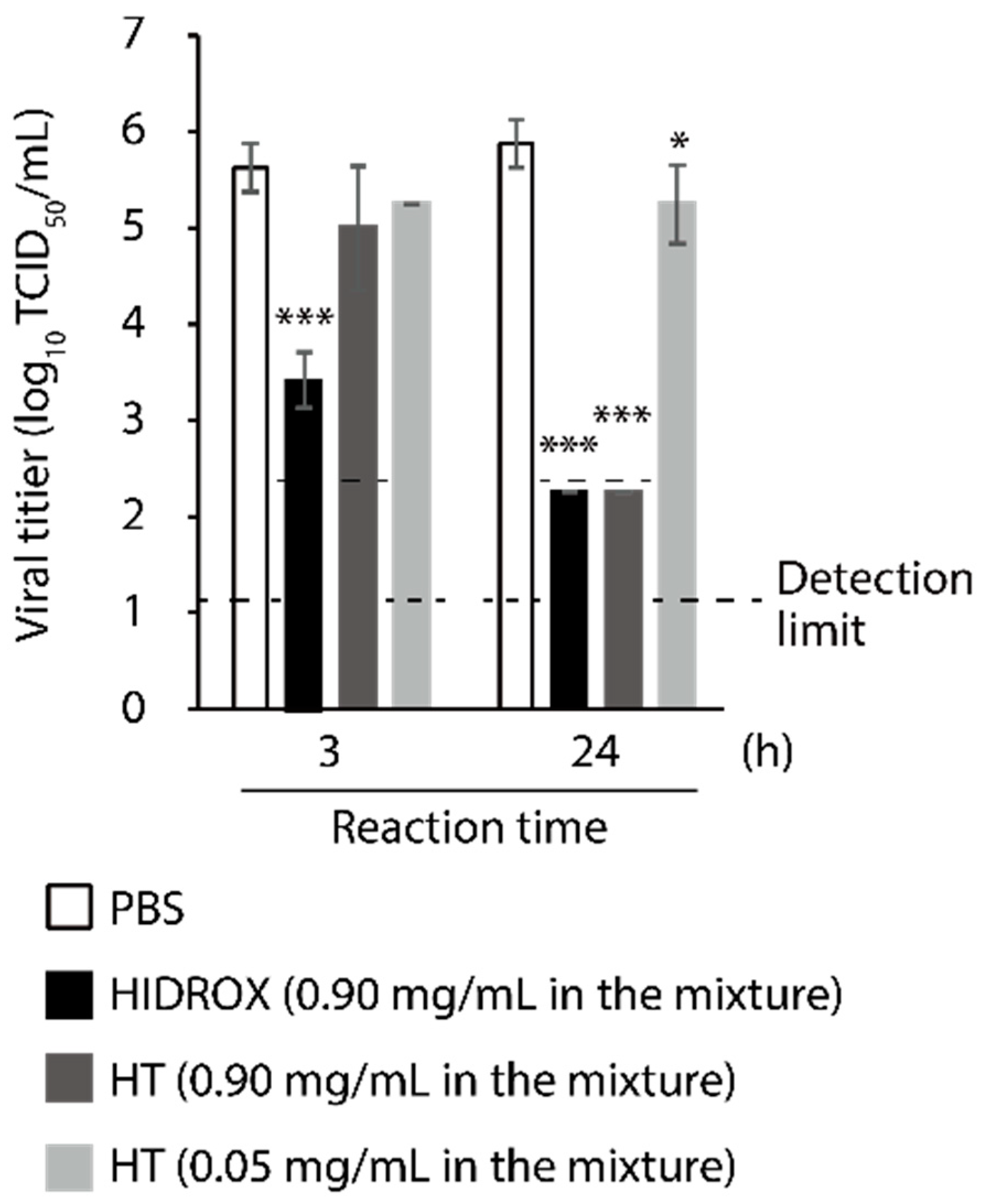
3.2. Comparison of Virucidal Activity of HIDROX and HT against SARS-CoV-2
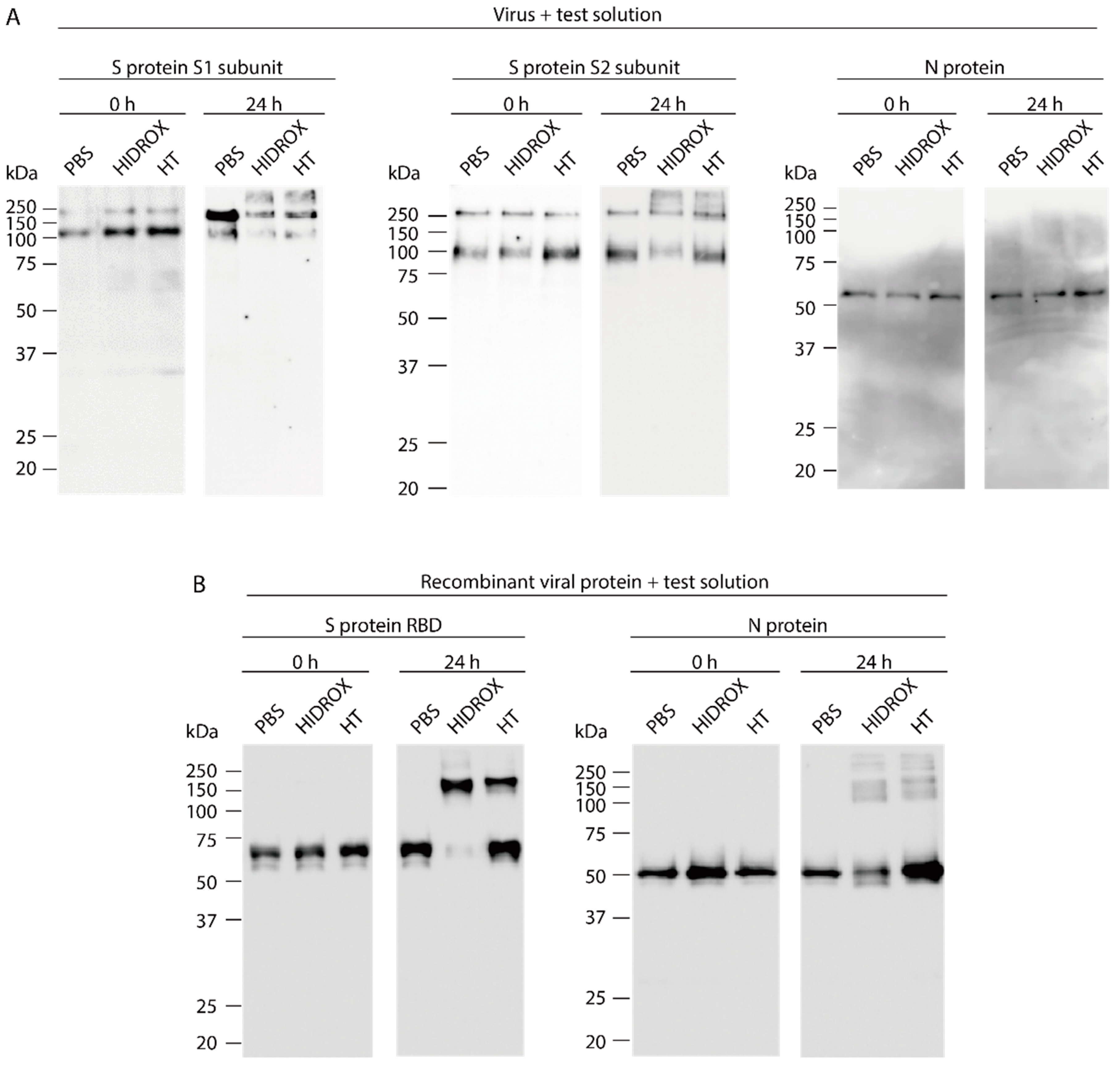
3.3. Evaluating the Impact of HIDROX and HT on SARS-CoV-2 Structural Proteins
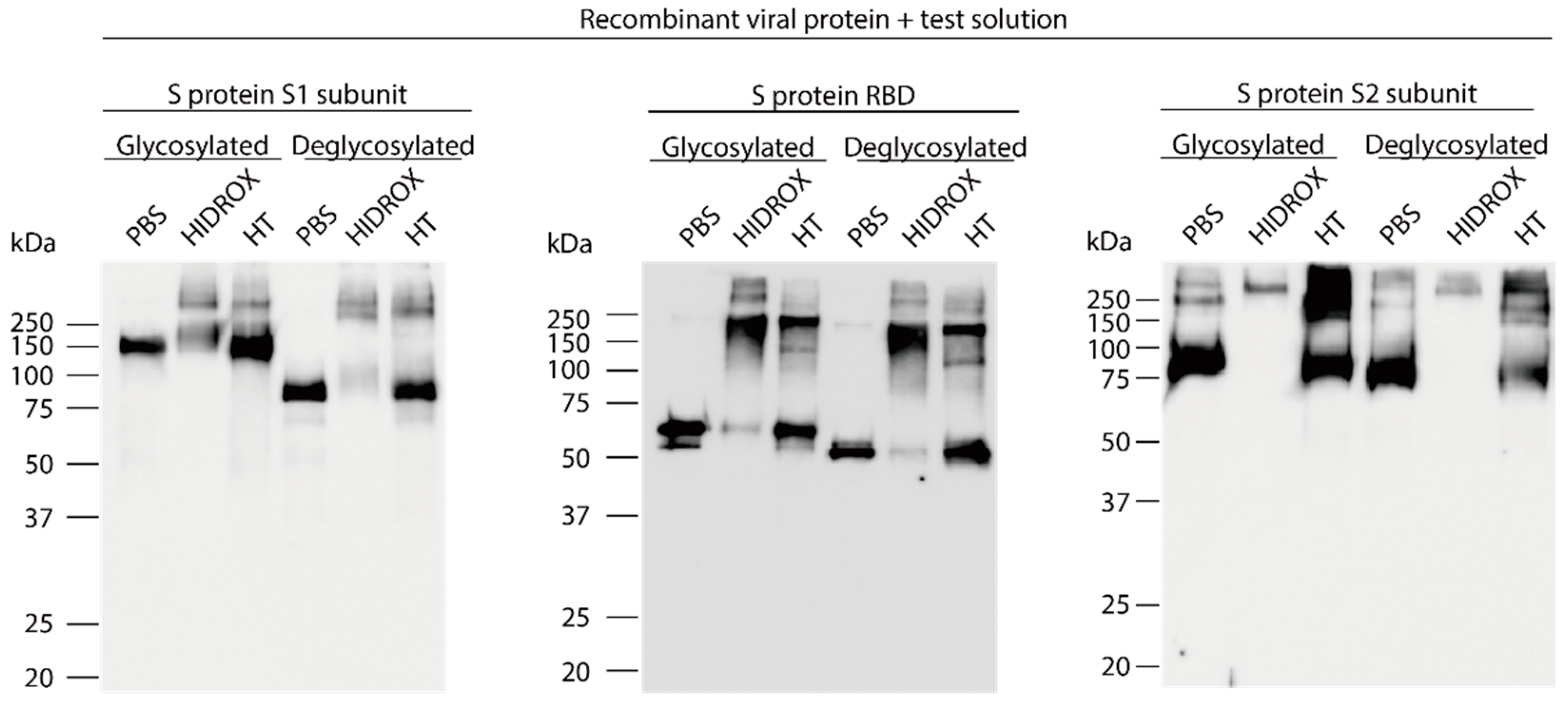
3.4. Evaluation of the Interaction of HIDROX or HT with Carbohydrate Chains Expressed on S Proteins
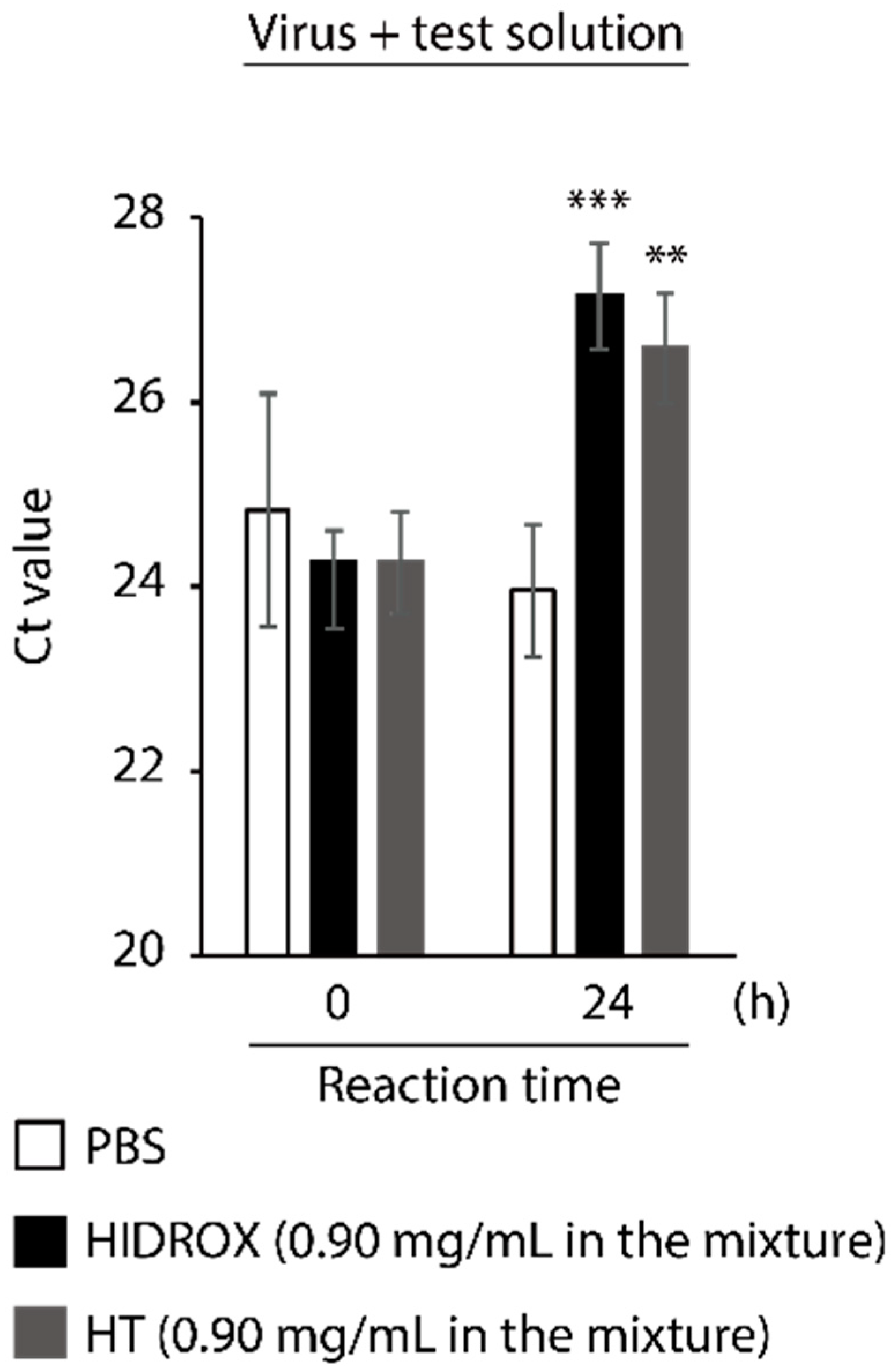
3.5. Evaluating the Impact of HIDROX and HT on SARS-CoV-2 Genome
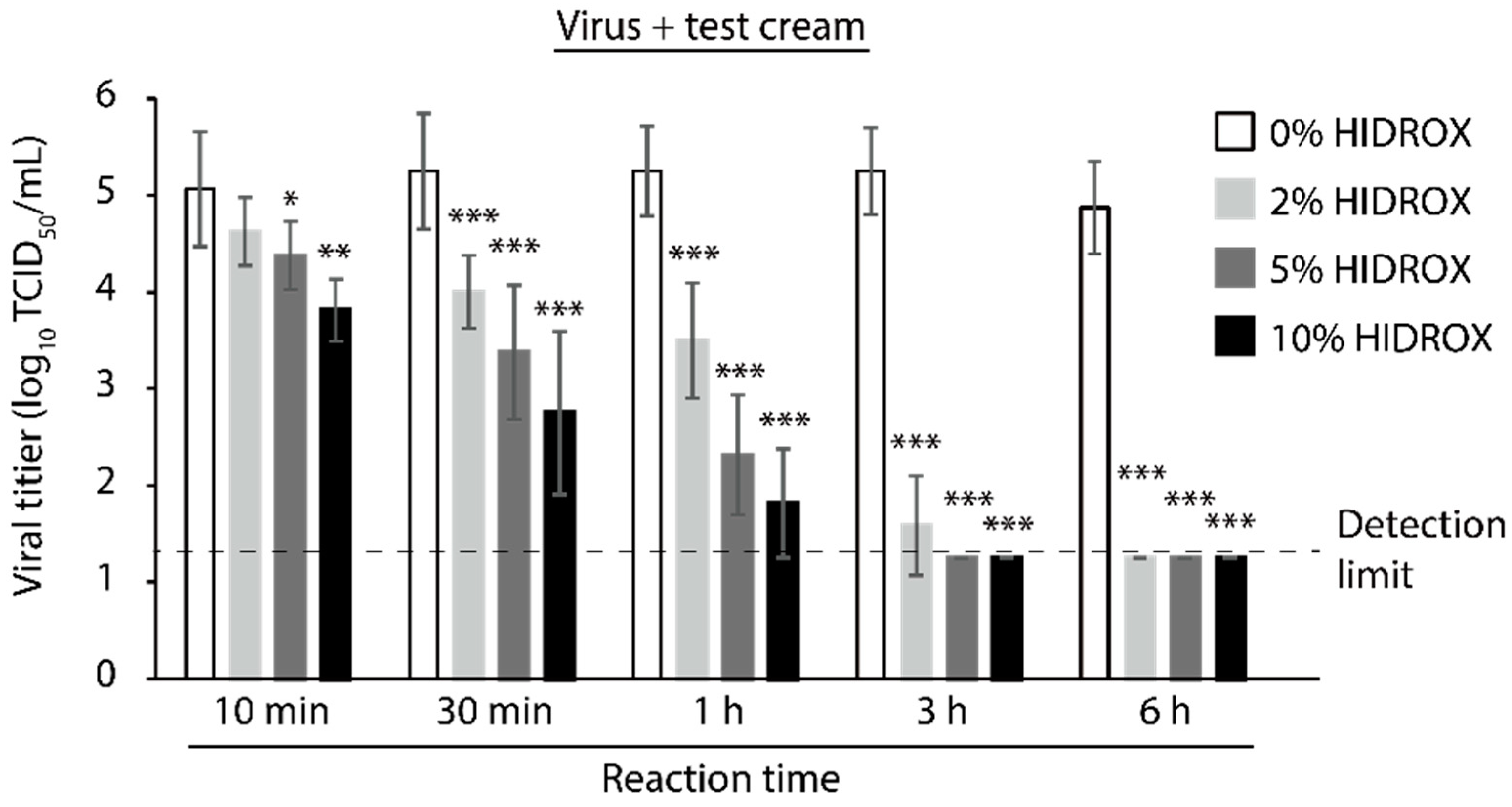
3.6. Evaluating the Time- and Concentration-Dependent Virucidal Activity of HIDROX-Containing Cream against SARS-CoV-2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 1 December 2020).
- World Health Organization. Covid-19 Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 25 January 2021).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA J. Am. Med. Assoc. 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA J. Am. Med. Assoc. 2020, 324, 1317–1329. [Google Scholar] [CrossRef]
- Dequin, P.F.; Heming, N.; Meziani, F.; Plantefève, G.; Voiriot, G.; Badié, J.; François, B.; Aubron, C.; Ricard, J.D.; Ehrmann, S.; et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2020, 324, 1298–1306. [Google Scholar] [CrossRef]
- Takeda, Y.; Murata, T.; Jamsransuren, D.; Suganuma, K.; Kazami, Y.; Batkhuu, J.; Badral, D.; Ogawa, H. Saxifraga spinulosa-derived components rapidly inactivate multiple viruses including SARS-CoV-2. Viruses 2020, 12, 699. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S. High Content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti—SARS—CoV—2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Runfeng, L.; Yunlong, H.; Jicheng, H.; Weiqi, P.; Qinhai, M.; Yongxia, S.; Chufang, L.; Jin, Z.; Zhenhua, J.; Haiming, J.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharm. Res. 2020, 156, 104761. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K.; Kumar, M.; Atri, P. Antiviral activity of plant polyphenols. J. Pharm. Res. 2012, 5, 2402–2412. [Google Scholar]
- Soni, M.G.; Burdock, G.A.; Christian, M.S.; Bitler, C.M.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M.; et al. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antivir. Res. 2009, 83, 35–44. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Beltrán, M.; Obregón-Calderón, P.; García-Pérez, J.; De La Torre, H.E.; González, N.; Pérez-Olmeda, M.; Auñón, D.; Capa, L.; Gómez-Acebo, E.; et al. Hydroxytyrosol: A new class of microbicide displaying broad anti-HIV-1 activity. AIDS 2016, 30, 2767–2776. [Google Scholar] [CrossRef]
- Christian, M.S.; Sharper, V.A.; Hoberman, A.M.; Seng, J.E.; Fu, L.; Covell, D.; Diener, R.M.; Bitler, C.M.; Crea, R. The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug Chem. Toxicol. 2004, 27, 309–330. [Google Scholar] [CrossRef]
- Bitler, C.M.; Viale, T.M.; Damaj, B.; Crea, R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J. Nutr. 2005, 135, 1475–1479. [Google Scholar] [CrossRef]
- Crea, R.; Liu, S.; Zhu, H.; Yang, Y.; Pontoniere, P. Validation of neuroprotective action of a commercially available formulation of olive polyphenols in a zebra-fish model vis-a-vis pure hydroxytyrosol. J. Agric. Sci. Technol. 2017, 1, 22–26. [Google Scholar]
- Di Rosa, G.; Brunetti, G.; Scuto, M.; Salinaro, A.T.; Calabrese, E.J.; Crea, R.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan enhancement by olive polyphenols in C. elegans wild type and parkinson’s models. Int. J. Mol. Sci. 2020, 21, 3893. [Google Scholar] [CrossRef]
- Ng, S.F.; Tan, L.S.; Buang, F. Transdermal anti-inflammatory activity of bilayer film containing olive compound hydroxytyrosol: Physical assessment, in vivo dermal safety and efficacy study in Freund’s adjuvant-induced arthritic rat model. Drug Dev. Ind. Pharm. 2017, 43, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bitler, C.M.; Matt, K.; Irving, M.; Hook, G.; Yusen, J.; Eagar, F.; Kirschner, K.; Walker, B.; Crea, R. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr. Res. 2007, 27, 470–477. [Google Scholar] [CrossRef]
- Nao, N.; Sato, K.; Yamagishi, J.; Tahara, M.; Nakatsu, Y.; Seki, F.; Katoh, H.; Ohnuma, A.; Shirogane, Y.; Hayashi, M.; et al. Consensus and variations in cell line specificity among human metapneumovirus strains. PLoS ONE 2019, 14, e0215822. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of genetic diagnostic methods for detection for novel coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020, 73, 304–307. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, K.; Zhang, X.; Chen, J.; Jiang, Z.; Jiang, J.; Ji, F.; Ouyang, X.; Li, L. Mass spectrometry analysis of newly emerging coronavirus HCoV-19 spike protein and human ACE2 reveals camouflaging glycans and unique post-translational modifications. Engineering 2020, in press. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, D.W.; Zhang, J.Z.H.; Huang, P.L.; Huang, P.L.; Lee-Huang, S. Computational study of bindings of olive leaf extract (OLE) to HIV-1 fusion protein gp41. FEBS Lett. 2007, 581, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. Fusion [corrected] inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 872–878. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part II. Integrase inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 879–884. [Google Scholar] [CrossRef]
- Brudzynski, K.; Alvarez, L.M. Polyphenol-protein complexes and their consequences for the redox activity, structure and function of honey. A current view and new hypothesis—A review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Furukawa, A.; Oikawa, S.; Murata, M.; Hiraku, Y.; Kawanishi, S. (-)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem. Pharm. 2003, 66, 1769–1778. [Google Scholar] [CrossRef]
- Amoros, M.; Simōes, C.M.O.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Knox, Y.M.; Hayashi, K.; Suzutani, T.; Ogasawara, M.; Yoshida, I.; Shiina, R.; Tsukui, A.; Terahara, N.; Azuma, M. Activity of anthocyanins from fruit extract of Ribes nigrum L. against influenza A and B viruses. Acta Virol. 2001, 45, 209–215. [Google Scholar] [PubMed]
- Bulotta, S.; Oliverio, M.; Russo, D.; Procopio, A. Biological activity of oleuropein and its derivatives. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Merillo, J.-M., Eds.; Springer: Heidelberg, Germany, 2013; pp. 3605–3638. [Google Scholar]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.W.; Bae, J.Y.; Heo, J.; Kim, D.; Han, S.Z.; Park, M.S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef]
- Ou, C.; Shi, N.; Yang, Q.; Zhang, Y.; Wu, Z.; Wang, B.; Compans, R.W.; He, C. Protocatechuic acid, a novel active substance against avian influenza virus H9N2 infection. PLoS ONE 2014, 9, e111004. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Wong, V.W.Y.; Cowling, B.J.; Aiello, A.E. Hand hygiene and risk of influenza virus infections in the community: A systematic review and meta-analysis. Epidemiol. Infect. 2014, 142, 922–932. [Google Scholar] [CrossRef]
- Ran, L.; Chen, X.; Wang, Y.; Wu, W.; Zhang, L.; Tan, X. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin. Infect. Dis. 2020, 71, 2218–2221. [Google Scholar] [CrossRef]
- Larson, E.; Girard, R.; Pessoa-Silva, C.L.; Boyce, J.; Donaldson, L.; Pittet, D. Skin reactions related to hand hygiene and selection of hand hygiene products. Am. J. Infect. Control. 2006, 34, 627–635. [Google Scholar] [CrossRef]
- Chan, V.W.M.; So, S.Y.C.; Chen, J.H.K.; Yip, C.C.Y.; Chan, K.H.; Chu, H.; Chung, T.W.H.; Sridhar, S.; To, K.K.W.; Chan, J.F.W.; et al. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect. Control. Hosp. Epidemiol. 2020, 41, 1258–1265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Jamsransuren, D.; Matsuda, S.; Crea, R.; Ogawa, H. The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application. Viruses 2021, 13, 232. https://doi.org/10.3390/v13020232
Takeda Y, Jamsransuren D, Matsuda S, Crea R, Ogawa H. The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application. Viruses. 2021; 13(2):232. https://doi.org/10.3390/v13020232
Chicago/Turabian StyleTakeda, Yohei, Dulamjav Jamsransuren, Sachiko Matsuda, Roberto Crea, and Haruko Ogawa. 2021. "The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application" Viruses 13, no. 2: 232. https://doi.org/10.3390/v13020232
APA StyleTakeda, Y., Jamsransuren, D., Matsuda, S., Crea, R., & Ogawa, H. (2021). The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application. Viruses, 13(2), 232. https://doi.org/10.3390/v13020232





