Recent Updates on Research Models and Tools to Study Virus–Host Interactions at the Placenta
Abstract
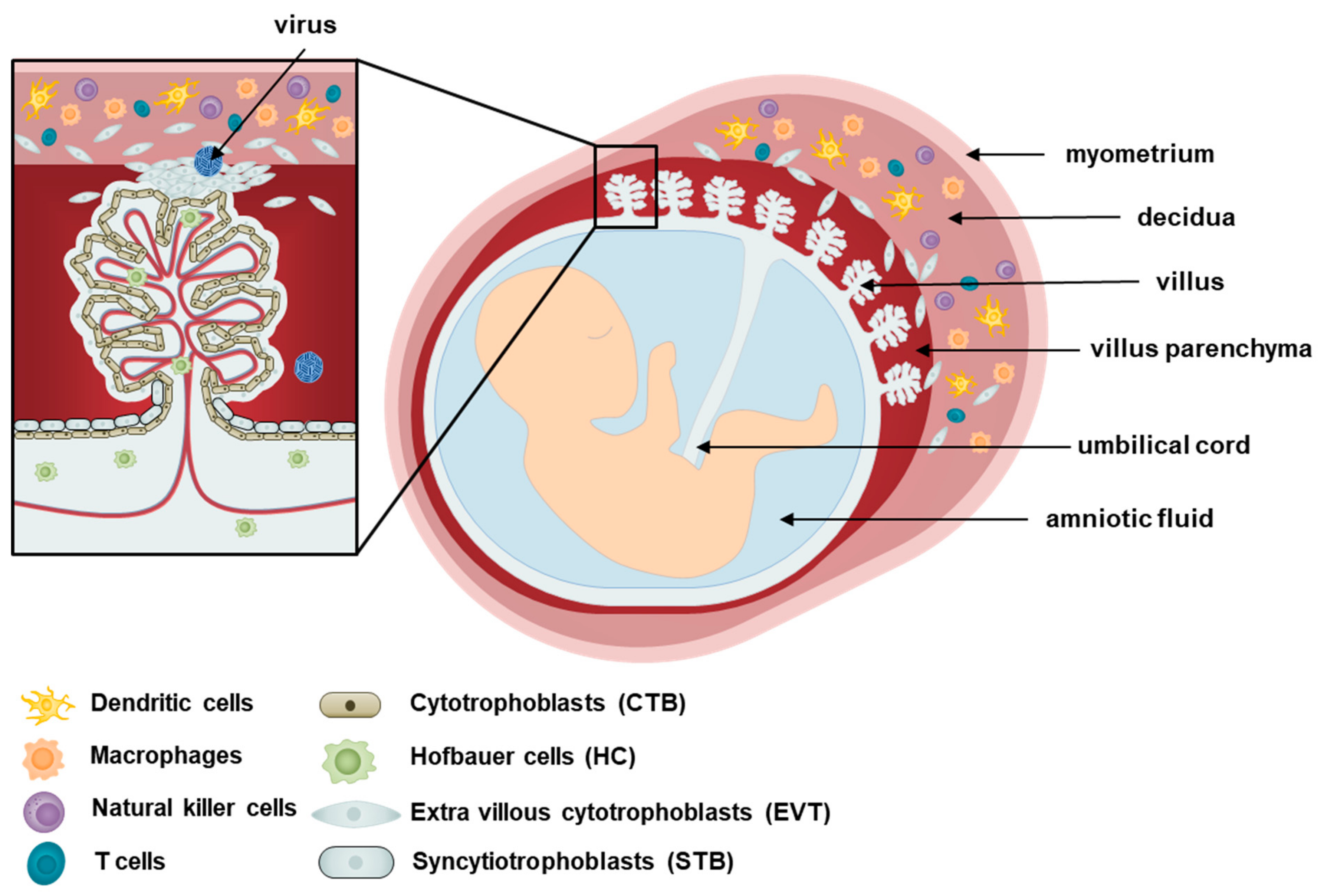
1. Introduction
2. Cellular Components of the Placenta
3. Viral Infections during Pregnancy
4. Research Models to Investigate Placental Pathogenesis
4.1. Pregnant Animal Model
4.2. Placental Explants and Placenta-Derived Primary Cell Models
4.3. 3D Cell Culture and Organoid Models
5. Current Progress and Future Perspectives of Studying Maternal–Fetal Interface
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Kaufmann, P. Pathology of the Human Placenta, 3rd ed.; Springer: New York, NY, USA, 1995. [Google Scholar]
- Kliman, H.J. Trophoblast to human placenta. In Encyclopedia of Reproduction; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.J.T.L. Association between Zika virus and microcephaly in French Polynesia, 2013–15: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Dudley, D.M.; Van, K.R.; Coffey, L.L.; Ardeshir, A.; Keesler, R.I.; Bliss-Moreau, E.; Grigsby, P.L.; Steinbach, R.J.; Hirsch, A.J.; MacAllister, R.P. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med. 2018, 24, 1104–1107. [Google Scholar] [CrossRef]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.-S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef]
- Leóón-Juárez, M.; Martínez–Castillo, M.; González-García, M.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75, 7. [Google Scholar]
- Sarah, A.; Robertson, M.G.P.; Joan, S.H. Knobil and Neill’s Physiology of Reproduction, 4th ed; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Wang, Y.Z.S. Chapter 4, Cell Types of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Castellucci, M.; Kaufmann, P. Basic structure of the villous trees. In Pathology of the Human Placenta; Springer Verlag: Berlin/Heidelberg, Germany, 2006; pp. 50–120. [Google Scholar]
- Pereira, L. Congenital viral infection: Traversing the uterine-placental interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Nonvillous parts and trophoblast invasion. In Pathology of the Human Placenta; Springer: New York, NY, USA, 2012; pp. 157–240. [Google Scholar]
- Loewendorf, A.I.; Nguyen, T.A.; Yesayan, M.N.; Kahn, D.A. Normal human pregnancy results in maternal immune activation in the periphery and at the uteroplacental interface. PLoS ONE 2014, 9, e96723. [Google Scholar] [CrossRef]
- Comiskey, M.; Warner, C.M.; Schust, D.J. MHC molecules of the preimplantation embryo and trophoblast. In Immunology of Pregnancy; Springer: Berlin/Heidelberg, Germany, 2006; pp. 130–147. [Google Scholar]
- Redman, C.; McMichael, A.; Stirrat, G.; Sunderland, C.; Ting, A. Class 1 major histocompatibility complex antigens on human extra-villous trophoblast. Immunology 1984, 52, 457. [Google Scholar]
- Ouyang, Y.; Mouillet, J.-F.; Coyne, C.B.; Sadovsky, Y. Placenta-specific microRNAs in exosomes–good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Bayer, A.; Chu, T.; Tyurin, V.A.; Kagan, V.E.; Morelli, A.E.; Coyne, C.B.; Sadovsky, Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta 2016, 47, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Golos, T. Hofbauer cells: Their role in healthy and complicated pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.; Reynard, J.; Krishnan, E.; Racela, L. Immunobiology of the human placenta: II. Localization of macrophages, in vivo bound IgG and C3. Cell. Immunol. 1978, 35, 205–216. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Khan, S.; Katabuchi, H.; Araki, M.; Nishimura, R.; Okamura, H. Human villous macrophage-conditioned media enhance human trophoblast growth and differentiation in vitro. Biol. Reprod. 2000, 62, 1075–1083. [Google Scholar] [CrossRef]
- Wetzka, B.; Clark, D.; Charnock-Jones, D.; Zahradnik, H.; Smith, S. Isolation of macrophages (Hofbauer cells) from human term placenta and their prostaglandin E2 and thromboxane production. Hum. Reprod. (Oxf. Engl.) 1997, 12, 847–852. [Google Scholar] [CrossRef]
- Castellucci, M.; Kaufmann, P. A three-dimensional study of the normal human placental villous core: II. Stromal architecture. Placenta 1982, 3, 269–285. [Google Scholar] [CrossRef]
- Ingman, K.; Cookson, V.; Jones, C.; Aplin, J. Characterisation of Hofbauer cells in first and second trimester placenta: Incidence, phenotype, survival in vitro and motility. Placenta 2010, 31, 535–544. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Takahashi, K.; Igura, K.; Zhang, X.; Mitsuru, A.; Takahashi, T.A. Effects of osteogenic induction on mesenchymal cells from fetal and maternal parts of human placenta. Cell Transplant. 2004, 13, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Igura, K.; Zhang, X.; Takahashi, K.; Mitsuru, A.; Yamaguchi, S.; Takahashi, T. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 2004, 6, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.; Al Jumah, M.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; AlTalabani, A.; Knawy, B. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. Rep. 2013, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Li, G.; Wang, G.; Wang, F.; Lin, Y. Human placenta-derived mesenchymal stem cells suppress T cell proliferation and support the culture expansion of cord blood CD34+ cells: A comparison with human bone marrow-derived mesenchymal stem cells. Tissue Cell 2013, 45, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Trowsdale, J.; Betz, A.G. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006, 7, 241. [Google Scholar] [CrossRef]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef]
- Kinney, J.S.; Kumar, M.L. Should we expand the TORCH complex?: A description of clinical and diagnostic aspects of selected old and new agents. Clin. Perinatol. 1988, 15, 727–744. [Google Scholar] [CrossRef]
- Schwartz, D.A. The origins and emergence of Zika virus, the newest TORCH infection: what’s old is new again. Arch. Pathol. Lab. Med. 2016, 141, 18–25. [Google Scholar] [CrossRef]
- Saraswathy, T.; Rozainanee, M.; Asshikin, R.N.; Zainah, S. Congenital rubella syndrome: A review of laboratory data from 2002 to 2011. Southeast Asian J. Trop. Med. Public Health 2013, 44, 429–435. [Google Scholar] [PubMed]
- Lazar, M.; Perelygina, L.; Martines, R.; Greer, P.; Paddock, C.D.; Peltecu, G.; Lupulescu, E.; Icenogle, J.; Zaki, S.R. Immunolocalization and distribution of rubella antigen in fatal congenital rubella syndrome. EBioMedicine 2016, 3, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Bauer, M.; Neumann, J.; Lüdde, A.; Rennert, P.; Friedrich, N.; Claus, C.; Perelygina, L.; Mankertz, A. Gene expression profiling of rubella virus infected primary endothelial cells of fetal and adult origin. Virol. J. 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Bowden, D.S. Rubella virus replication and links to teratogenicity. Clin. Microbiol. Rev. 2000, 13, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.; Howley, P.; Griffin, D.; Lamb, R.; Martin, M.; Roizman, B.; Straus, S. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 1001–1100. [Google Scholar]
- Töndury, G.; Smith, D.W. Fetal rubella pathology. J. Pediatrics 1966, 68, 867–879. [Google Scholar] [CrossRef]
- Anzivino, E.; Fioriti, D.; Mischitelli, M.; Bellizzi, A.; Barucca, V.; Chiarini, F.; Pietropaolo, V. Herpes simplex virus infection in pregnancy and in neonate: Status of art of epidemiology, diagnosis, therapy and prevention. Virol. J. 2009, 6, 40. [Google Scholar] [CrossRef]
- Mustonen, K.; Mustakangas, P.; Valanne, L.; Haltia, M.; Koskiniemi, M. Congenital varicella-zoster virus infection after maternal subclinical infection: Clinical and neuropathological findings. J. Perinatol. 2001, 21, 141. [Google Scholar] [CrossRef][Green Version]
- Nikkels, A.F.; Delbecque, K.; Pierard, G.E.; Wienkotter, B.; Schalasta, G.; Enders, M. Distribution of varicella-zoster virus DNA and gene products in tissues of a first-trimester varicella-infected fetus. J. Infect. Dis. 2005, 191, 540–545. [Google Scholar] [CrossRef][Green Version]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Emery, V.C.; Lazzarotto, T. Cytomegalovirus in pregnancy and the neonate. F1000 Res. 2017, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Enders, G.; Daiminger, A.; Bäder, U.; Exler, S.; Enders, M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol. 2011, 52, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Daiminger, A.; Bäder, U.; Enders, G. Pre--and periconceptional primary cytomegalovirus infection: Risk of vertical transmission and congenital disease. Bjog: Int. J. Obstet. Gynaecol. 2005, 112, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Pass, R.F.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Stagno, S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J. Clin. Virol. 2006, 35, 216–220. [Google Scholar] [CrossRef]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: Implications for transmission and pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef]
- Axelsson, C.; Bondestam, K.; Frisk, G.; Bergström, S.; Diderholm, H. Coxsackie B virus infections in women with miscarriage. J. Med Virol. 1993, 39, 282–285. [Google Scholar] [CrossRef]
- Brown, G.C.; Evans, T.N. Serologic evidence of Coxsackievirus etiology of congenital heart disease. JAMA 1967, 199, 183–187. [Google Scholar] [CrossRef]
- Bendig, J.W.; Franklin, O.M.; Hebden, A.K.; Backhouse, P.J.; Clewley, J.P.; Goldman, A.P.; Piggott, N. Coxsackievirus B3 sequences in the blood of a neonate with congenital myocarditis, plus serological evidence of maternal infection. J. Med. Virol. 2003, 70, 606–609. [Google Scholar] [CrossRef]
- Genen, L.; Nuovo, G.J.; Krilov, L.; Davis, J.M. Correlation of in situ detection of infectious agents in the placenta with neonatal outcome. J. Pediatrics 2004, 144, 316–320. [Google Scholar] [CrossRef]
- Konstantinidou, A.; Anninos, H.; Spanakis, N.; Kotsiakis, X.; Syridou, G.; Tsakris, A.; Patsouris, E. Transplacental infection of Coxsackievirus B3 pathological findings in the fetus. J. Med. Virol. 2007, 79, 754–757. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, J.W.; Hwang, J.Y.; Lee, K.M.; Shim, H.M.; Bae, Y.K.; Paik, S.S.; Park, H. Coxsackievirus B infection is highly related with missed abortion in Korea. Yonsei Med. J. 2014, 55, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Asher, D.R.; Cerny, A.M.; Weiler, S.R.; Horner, J.W.; Keeler, M.L.; Neptune, M.A.; Jones, S.N.; Bronson, R.T.; DePinho, R.A.; Finberg, R.W. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis 2005, 42, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dorner, A.A.; Wegmann, F.; Butz, S.; Wolburg-Buchholz, K.; Wolburg, H.; Mack, A.; Nasdala, I.; August, B.; Westermann, J.; Rathjen, F.G. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J. Cell Sci. 2005, 118, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lee, K.M.; KiM, Y.H.; SHiM, H.M.; Bae, Y.K.; Hwang, J.H.; ParK, H. Pregnancy Loss Following Coxsackievirus B3 Infection in Mice during Early Gestation Due toHigh Expression of Coxsackievirus-Adenovirus Receptor (CAR) in Uterus and Embryo. Exp. Anim. 2014, 63, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.; Davis, J.; Holzman, I.; Nuovo, G.J. Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstet. Gynecol. 2001, 98, 1019–1026. [Google Scholar] [PubMed]
- Johansson, M.A.; Mier-y-Teran-Romero, L.; Reefhuis, J.; Gilboa, S.M.; Hills, S.L. Zika and the risk of microcephaly. New Engl. J. Med. 2016, 375, 1–4. [Google Scholar] [CrossRef]
- Lee, J.K.; Shin, O.S. Advances in Zika virus–host cell interaction: Current knowledge and future perspectives. Int. J. Mol. Sci. 2019, 20, 1101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.; Malinger, G.; Ximenes, R.; Szejnfeld, P.; Alves Sampaio, S.; Bispo de Filippis, A. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.; Sampaio, S.A.; De Filippis, I.; Fabri, A.; Araujo, E.S.; de Sequeira, P.C.; de Mendonça, M.C.; de Oliveira, L. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T.D.A.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch. Gynecol. Obstet. 2017, 295, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.Z.; Yu, W.; Hill, D.A.; Reyes, C.A.; Schwartz, D.A. Placental pathology of Zika virus: Viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch. Pathol. Lab. Med. 2016, 141, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.M.; Lahon, A.; Suter, M.A.; Arya, R.P.; Seferovic, M.D.; Vogt, M.B.; Hu, M.; Stossi, F.; Mancini, M.A.; Harris, R.A. Primary human placental trophoblasts are permissive for Zika virus (ZIKV) replication. Sci. Rep. 2017, 7, 41389. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C.; Repic, M.; Knoblich, J.A. Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 2015, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.L.; Rossi, S.L.; Gao, J.; Widen, S.G.; Grant, A.C.; Dunn, T.J.; Azar, S.R.; Roundy, C.M.; Xiong, Y.; Prusak, D.J. Differential responses of human fetal brain neural stem cells to Zika virus infection. Stem Cell Rep. 2017, 8, 715–727. [Google Scholar] [CrossRef]
- Pouliot, S.H.; Xiong, X.; Harville, E.; Paz-Soldan, V.; Tomashek, K.M.; Breart, G.; Buekens, P. Maternal dengue and pregnancy outcomes: A systematic review. Obstet. Gynecol. Surv. 2010, 65, 107–118. [Google Scholar]
- Bebell, L.M.; Oduyebo, T.; Riley, L.E. Ebola virus disease and pregnancy: A review of the current knowledge of Ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 2017, 109, 353–362. [Google Scholar] [CrossRef]
- Johnson, E.L.; Chakraborty, R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012, 9, 101. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Schaefer, T.M.; Fahey, J.V.; Visintin, I.; Wright, J.A.; Aldo, P.B.; Romero, R.; Wira, C.R.; Mor, G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly (I: C). Hum. Reprod. 2006, 21, 2432–2439. [Google Scholar] [CrossRef]
- Dalsgaard, A.; Aboagye-Mathiesen, G.; Justesen, J.; Zdravkovic, M.; Ebbesen, P. Basal and interferon-induced 2′, 5′-oligoadepylate synthetase activity in human placental trophoblast and trophoblast-derived malignant cell lines. Placenta 1995, 16, 137–146. [Google Scholar] [CrossRef]
- Bebell, L.M.; Riley, L.E. Ebola virus disease and Marburg disease in pregnancy: A review and management considerations for filovirus infection. Obstet. Gynecol. 2015, 125, 1293. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.B.; Marzi, A.; Safronetz, D.; Robertson, S.J.; Feldmann, H.; Best, S.M. Immunobiology of Ebola and Lassa virus infections. Nat. Rev. Immunol. 2017, 17, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Agboeze, J.; Nwali, M.I.; Nwakpakpa, E.; Ogah, O.E.; Onoh, R.; Eze, J.; Ukaegbe, C.; Ajayi, N.; Nnadozie, U.U.; Orji, M.-L. Lassa fever in pregnancy with a positive maternal and fetal outcome: A case report. Int. J. Infect. Dis. 2019, 89, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; DeLoia, J. Globoside expression within the human placenta. Placenta 1999, 20, 103–108. [Google Scholar] [CrossRef]
- Pasquinelli, G.; Bonvicini, F.; Foroni, L.; Salfi, N.; Gallinella, G. Placental endothelial cells can be productively infected by Parvovirus B19. J. Clin. Virol. 2009, 44, 33–38. [Google Scholar] [CrossRef]
- De Jong, E.P.; de Haan, T.R.; Kroes, A.C.; Beersma, M.F.; Oepkes, D.; Walther, F.J. Parvovirus B19 infection in pregnancy. J. Clin. Virol. 2006, 36, 1–7. [Google Scholar] [CrossRef]
- Mendelson, E.; Aboudy, Y.; Smetana, Z.; Tepperberg, M.; Grossman, Z. Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV). Reprod. Toxicol. 2006, 21, 350–382. [Google Scholar] [CrossRef]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Enders, A.; Carter, A. Comparative placentation: Some interesting modifications for histotrophic nutrition–a review. Placenta 2006, 27, 11–16. [Google Scholar] [CrossRef]
- Carter, A.M. Animal models of human placentation–a review. Placenta 2007, 28, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Georgiades, P.; Ferguson-Smith, A.; Burton, G. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002, 23, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538. [Google Scholar] [CrossRef]
- Perez-Garcia, V.; Fineberg, E.; Wilson, R.; Murray, A.; Mazzeo, C.I.; Tudor, C.; Sienerth, A.; White, J.K.; Tuck, E.; Ryder, E.J. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 2018, 555, 463. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Persson, E. Placental transfer of glucose and amino acids in intrauterine growth retardation: Studies with substrate analogs in the awake guinea pig. Pediatric Res. 1990, 28, 203. [Google Scholar] [CrossRef] [PubMed]
- Dyson, R.M.; Palliser, H.K.; Kelleher, M.A.; Hirst, J.J.; Wright, I.M. The guinea pig as an animal model for studying perinatal changes in microvascular function. Pediatric Res. 2012, 71, 20. [Google Scholar] [CrossRef]
- Bia, F.J.; Griffith, B.P.; Fong, C.K.; Hsiung, G. Cytomegaloviral infections in the guinea pig: Experimental models for human disease. Rev. Infect. Dis. 1983, 5, 177–195. [Google Scholar] [CrossRef]
- Choi, Y.; Hsiung, G. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J. Infect. Dis. 1978, 138, 197–202. [Google Scholar] [CrossRef]
- Kumar, M.L.; Nankervis, G.A. Experimental congenital infection with cytomegalovirus: A guinea pig model. J. Infect. Dis. 1978, 138, 650–654. [Google Scholar] [CrossRef]
- Griffith, B.; McCormick, S.; Fong, C.; Lavallee, J.; Lucia, H.; Goff, E. The placenta as a site of cytomegalovirus infection in guinea pigs. J. Virol. 1985, 55, 402–409. [Google Scholar]
- Kumar, M.; Krause, K.K.; Azouz, F.; Nakano, E.; Nerurkar, V.R. A guinea pig model of Zika virus infection. Virol. J. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.K.; Azouz, F.; Shin, O.S.; Kumar, M. Understanding the pathogenesis of Zika virus infection using animal models. Immune Netw. 2017, 17, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.; Chapeau, C. Evaluation of the pregnancy immunotrophism hypothesis by assessment of the reproductive performance of young adult mice of genotype. Reproduction 1990, 88, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Malassine, A.; Frendo, J.L.; Evain--Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 2003, 9, 531–539. [Google Scholar] [CrossRef]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Froehlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.; Jones, C.; Mess, A.; Pfarrer, C.; Pijnenborg, R.; Soma, H. Comparative placentation and animal models: Patterns of trophoblast invasion—A workshop report. Placenta 2006, 27 (Suppl. A), S30–S33. [Google Scholar] [CrossRef]
- Kaufmann, P. Guinea pig Cavia porcellus. Comp. Placentation 2004. [Google Scholar]
- Mess, A. The guinea pig placenta: Model of placental growth dynamics. Placenta 2007, 28, 812–815. [Google Scholar] [CrossRef]
- Noronha, L.E.; Antczak, D.F. Maternal immune responses to trophoblast: The contribution of the horse to pregnancy immunology. Am. J. Reprod. Immunol. 2010, 64, 231–244. [Google Scholar] [CrossRef]
- De Mestre, A.M.; Miller, D.; Roberson, M.S.; Liford, J.; Chizmar, L.C.; McLaughlin, K.E.; Antczak, D.F. Glial cells missing homologue 1 is induced in differentiating equine chorionic girdle trophoblast cells. Biol. Reprod. 2009, 80, 227–234. [Google Scholar] [CrossRef][Green Version]
- Adams, A.; Antczak, D. Ectopic transplantation of equine invasive trophoblast. Biol. Reprod. 2001, 64, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction 2011, 141, 391. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.L. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy, Seminars in reproductive medicine, 2016; Thieme Medical Publishers: New York, NY, USA, 2016; pp. 11–16. [Google Scholar]
- Enders, A.C.; Lantz, K.C.; Peterson, P.E.; Hendrickx, A.G. From blastocyst to placenta: The morphology of implantation in the baboon. Hum. Reprod. Update 1997, 3, 561–573. [Google Scholar]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.J.; Smith, A.M.; Arora, N.; Diamond, M.S.; Coyne, C.B.; Miner, J.J. Zika virus–related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 2018, 10, eaao7090. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Wang, R.; Lu, X.; Dang, Y.-L.; Wang, H.; Lin, H.-Y.; Zhu, C.; Ge, H.; Cross, J.C. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 2018, 28, 819. [Google Scholar] [CrossRef]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B. Modeling host-pathogen interactions in the context of the microenvironment: Three-dimensional cell culture comes of age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef]
- Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A. Organotypic 3D cell culture models: Using the rotating wall vessel to study host–pathogen interactions. Nat. Rev. Microbiol. 2010, 8, 791. [Google Scholar] [CrossRef]
- McConkey, C.A.; Delorme-Axford, E.; Nickerson, C.A.; Kim, K.S.; Sadovsky, Y.; Boyle, J.P.; Coyne, C.B. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci. Adv. 2016, 2, e1501462. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018, 564, 263. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Gilmore, C.; Sood, A.; Matsusaki, M.; Collett, G.; Tannetta, D.; Sargent, I.L.; McGarvey, J.; Halemani, N.D.; Hanley, J. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials 2019, 192, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicology 2019, 54, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.S.; Chen, W.; Chan, Y.; Bowman, J.W.; Chang, L.C.; Choi, Y.; Yoo, J.S.; Ge, J.; Cheng, G.; Bonnin, A.; et al. Asian Zika virus strains target CD14(+) blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat. Microbiol. 2017, 2, 1558–1570. [Google Scholar] [CrossRef]
- Casazza, R.L.; Lazear, H.M.; Miner, J.J. Protective and pathogenic effects of interferon signaling during pregnancy. Viral Immunol. 2019, 10, 768–775. [Google Scholar] [CrossRef]
- Yockey, L.J.; Jurado, K.A.; Arora, N.; Millet, A.; Rakib, T.; Milano, K.M.; Hastings, A.K.; Fikrig, E.; Kong, Y.; Horvath, T.L.J. Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 2018, 3, 19. [Google Scholar] [CrossRef]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat. Commun. 2019, 10, 280. [Google Scholar] [CrossRef]
- Luo, S.-S.; Ishibashi, O.; Ishikawa, G.; Ishikawa, T.; Katayama, A.; Mishima, T.; Takizawa, T.; Shigihara, T.; Goto, T.; Izumi, A. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol. Reprod. 2009, 81, 717–729. [Google Scholar] [CrossRef]
- Donker, R.; Mouillet, J.; Chu, T.; Hubel, C.; Stolz, D.; Morelli, A.; Sadovsky, Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012, 18, 417–424. [Google Scholar] [CrossRef]
- Bortolin-Cavaillé, M.-L.; Dance, M.; Weber, M.; Cavaillé, J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009, 37, 3464–3473. [Google Scholar] [CrossRef]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766. [Google Scholar] [CrossRef] [PubMed]
- Noguer-Dance, M.; Abu-Amero, S.; Al-Khtib, M.; Lefevre, A.; Coullin, P.; Moore, G.E.; Cavaillé, J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum. Mol. Genet. 2010, 19, 3566–3582. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.-F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef]
- Steel, J.H.; Malatos, S.; Kennea, N.; Edwards, A.D.; Miles, L.; Duggan, P.; Reynolds, P.R.; Feldman, R.G.; Sullivan, M.H. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatric Res. 2005, 57, 404. [Google Scholar] [CrossRef]
- Fortner, K.B.; Grotegut, C.A.; Ransom, C.E.; Bentley, R.C.; Feng, L.; Lan, L.; Heine, R.P.; Seed, P.C.; Murtha, A.P. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PLoS ONE 2014, 9, e83338. [Google Scholar] [CrossRef]
- Prince, A.L.; Ma, J.; Kannan, P.S.; Alvarez, M.; Gisslen, T.; Harris, R.A.; Sweeney, E.L.; Knox, C.L.; Lambers, D.S.; Jobe, A.H. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 2016, 217, 627.e1–627.e16. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microb. 2018, 24, 133–145.E5. [Google Scholar] [CrossRef] [PubMed]
- De Goffau, M.C.; Lager, S.; Sovio, U.; Gaccioli, F.; Cook, E.; Peacock, S.J.; Parkhill, J.; Charnock-Jones, D.S.; Smith, G.C.S. Human placenta has no microbiome but can contain potential pathogens. Nature 2019, 572, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Darland, N.W. Infertility associated with luteal phase defect. J. Obstet. Gynecol. Neonatal Nurs. 1985, 14, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Ditroi, F.; Lampe, G. Serum progesterone levels in early imminent abortion. Acta Physiol. Hung. 1985, 65, 275–279. [Google Scholar]
- Tai, P.; Wang, J.; Jin, H.; Song, X.; Yan, J.; Kang, Y.; Zhao, L.; An, X.; Du, X.; Chen, X. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2008, 214, 456–464. [Google Scholar] [CrossRef]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Skountzou, I. Hormonal regulation of physiology, innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Front. Immunol. 2018, 9, 2455. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Esser, E.S.; Antao, O.Q.; Vassilieva, E.V.; Compans, R.W.; Skountzou, I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. Plos Pathog. 2017, 13, e1006757. [Google Scholar] [CrossRef]
- Hall, O.J.; Nachbagauer, R.; Vermillion, M.S.; Fink, A.L.; Phuong, V.; Krammer, F.; Klein, S.L. Progesterone-based contraceptives reduce adaptive immune responses and protection against sequential influenza A virus infections. J. Virol. 2017, 91, e02160-16. [Google Scholar] [CrossRef]

| Virus | Family | Host | Typical transmission route | Pregnancy outcomes |
|---|---|---|---|---|
| Rubella virus | Togaviridae | Humans | Aerosols, secretions | Miscarriage, cognenital rubella syndromes (hearing loss, cataract, congenital heart disease, microcephaly etc.) |
| Herpes simplex virus | α-herpesviridae | Humans | Oral or sexual contact | Miscarriage, FGR, stillbirth in rare cases |
| Varicella zoster virus | α-herpesviridae | Humans | Aerosols, vesicles | Miscarriage, FGR, congenital varicella syndromes(skin and limb malformation, cataracts, microcephaly, hydrocephalus etc.) |
| Cytomegalovirus | β-herpesviridae | Humans, monkeys | Direct contact (bodily fluids, blood, saliva, urine and breastmilk) | Premature birth, FGR, congenital disorders (microcephaly, hearing loss, vision loss, seisure, intellectual disability etc.) |
| Coxsackievirus B | Picornaviridae | Humans | Aerosols, fecal-oral route | Miscarriage, stillbirth, fetal sepsis |
| Zika virus | Flaviviridae | Humans, monkeys | Mosquito, sexual | Miscarriage, microcephaly |
| Dengue virus | Flaviviridae | Humans | Mosquito, breast milk | Miscarriage, premature birth, stillbirth |
| Ebola virus | Filoviridae | Humans, bats, primates | Blood, bodily fluids | Miscarriage, stillbirth |
| Marburg virus | Filoviridae | Human, bats | Blood, bodily fluids | Miscarriage, stillbirth |
| Human immunodeficiency virus | Retroviridae | Humans | Blood, bodily fluids | Miscarriage, stillbirth |
| Parvovirus B19 | Parvoviridae | Humans | Aerosols, saliva, blood | Miscarriage, fetal anemia, nonimmune hydrops fetalis |
| Lassa virus | Arenaviridae | Humans, rodents | Aerosols, contact with infected rodent hosts | Perinatal mortality |
| Animal | Similarities | Differences | References |
|---|---|---|---|
| Mice |
|
| [100,101,102] |
| Guinea pigs |
|
| [103,104,105] |
| Horses |
|
| [86,106,107,108] |
| Non-human primates |
|
| [109,110,111] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Oh, S.-J.; Park, H.; Shin, O.S. Recent Updates on Research Models and Tools to Study Virus–Host Interactions at the Placenta. Viruses 2020, 12, 5. https://doi.org/10.3390/v12010005
Lee JK, Oh S-J, Park H, Shin OS. Recent Updates on Research Models and Tools to Study Virus–Host Interactions at the Placenta. Viruses. 2020; 12(1):5. https://doi.org/10.3390/v12010005
Chicago/Turabian StyleLee, Jae Kyung, Soo-Jin Oh, Hosun Park, and Ok Sarah Shin. 2020. "Recent Updates on Research Models and Tools to Study Virus–Host Interactions at the Placenta" Viruses 12, no. 1: 5. https://doi.org/10.3390/v12010005
APA StyleLee, J. K., Oh, S.-J., Park, H., & Shin, O. S. (2020). Recent Updates on Research Models and Tools to Study Virus–Host Interactions at the Placenta. Viruses, 12(1), 5. https://doi.org/10.3390/v12010005





