Abstract
A metatranscriptomic study of RNA viruses in cold-blooded vertebrates identified two related viruses from frogfish (Antennarius striatus) that represent a new genus Antennavirus in the family Arenaviridae (Order: Bunyavirales). Computational analyses were used to identify features common to class I viral fusion proteins (VFPs) in antennavirus glycoproteins, including an N-terminal fusion peptide, two extended alpha-helices, an intrahelical loop, and a carboxyl terminal transmembrane domain. Like mammarenavirus and hartmanivirus glycoproteins, the antennavirus glycoproteins have an intracellular zinc-binding domain and a long virion-associated stable signal peptide (SSP). The glycoproteins of reptarenaviruses are also class I VFPs, but do not contain zinc-binding domains nor do they encode SSPs. Divergent evolution from a common progenitor potentially explains similarities of antennavirus, mammarenavirus, and hartmanivirus glycoproteins, with an ancient recombination event resulting in a divergent reptarenavirus glycoprotein.
1. Introduction
Until 2015, the Arenaviridae was comprised of a single genus of mammalian ambisense RNA viruses divided into two large monophyletic groups [1]. New World (NW) arenaviruses infect rodents of the family Cricetidae [2,3]. An exception is Tacaribe virus (TCRV) that infects Artibeus bats and may be vectored by insects [4]. NW arenaviruses from Northern America infect rodents from the subfamily Neotominae, whereas those from Latin American and the Caribbean infect rodents from the subfamiliy Sigmodontinae [5,6]. Old World (OW) arenaviruses are found in African rodents from the family Muridae subfamily Murinae [7,8,9,10]. Recently, OW arenaviruses have been isolated from mice and shrews in Asia [11,12,13]. Several arenaviruses spillover from their rodent hosts to humans where they can cause severe hemorrhagic fevers [14,15]. Lassa virus (LASV), an OW arenavirus, has been designated as a priority pathogen by the World Health Organization (WHO) [16] and the Coalition for Epidemic Preparedness and Innovations (CEPI) [17]. These rankings for LASV are based in part on the potential for further geographic expansion of its rodent reservoirs, the frequent importation to North America and Europe, and the possible emergence of novel strains in densely populated West Africa.
In 2012, DeRisi and colleagues reported the isolation of a novel arenavirus and demonstrated that it was representative of viruses that can cause a disease in pet snakes worldwide [18]. Inclusion body disease (IBD) spreads among boid snakes (boas and pythons) in captivity [18,19,20,21,22,23,24]. Bacterial infections, neurological pathology, anorexia, and withering that result in death of the snakes are characteristics of IBD. Further metagenomic analyses identified numerous genetically diverse arenaviruses from captive snakes [21,25,26]. Two genera, Reptarenavirus and Hartmanivirus, were established in the Arenaviridae to include the snake arenaviruses [1,27,28]. While sometimes present in healthy snakes, reptarenaviruses are the causative agent of IBD [20,29,30], whereas hartmaniviruses have not been shown to cause disease [25]. With the discovery of these new arenaviruses, NW and OW arenaviruses were placed taxonomically into the genus Mammarenavirus [1]. Further expansion of the Arenaviridae was necessitated based on results of an extraordinary large-scale metatranscriptomic study of RNA viruses in cold-blooded vertebrates that identified two arenaviruses from frogfish (Antennarius striatus) among hundreds of novel viruses [31]. Based on sequence similarity and phylogenetic analyses, Wēnlǐng frogfish arenavirus-1 (WlFAV-1, striated antennavirus) and Wēnlǐng frogfish arenavirus-2 (WlFAV-2, hairy antennavirus) represent a new genus, Antennavirus [32].
Genetic differences are apparent when comparing the mammalian, reptilian, and fish arenaviruses. Mammarenaviruses, reptarenaviruses, and hartmaniviruses have bi-segmented genomes [33]. The small segment is ambisense and encodes the glycoprotein complex (GPC) and the nucleocapsid protein (NP). The large segment encodes the RNA-dependent RNA polymerase (L) and, in mammarenaviruses and reptarenaviruses in which the segment is ambisense, a zinc-binding or matrix protein (Z). Sequencing of the genome of the prototype hartmanivirus, Haartman Institute snake virus (HISV), showed that this virus does not encode a Z protein [26]. This unexpected result was confirmed in subsequent isolates of related hartmaniviruses that also lack a gene encoding Z [25]. Z participates in several processes in the virus replication cycle, including the formation of infectious viral particles and viral budding [34], indicating that hartmaniviruses utilize alternative mechanisms. In contrast, the frogfish arenavirus contains three genome segments [31].
The LASV GPC is a trimer of heterodimers, each containing receptor-binding glycoprotein 1 (GP1) and a transmembrane fusion protein GP2 [35]. LASV also encodes an unusual long stable signal peptide (SSP) that is required for proper processing of GPC and is retained in the virion as part of the complex. The GPC precursor is trafficked from the endoplasmic reticulum to the Golgi, where it is heavily N-glycosidated and processed by cellular proteases (SPase, SKI1/SP1) into its mature form comprising non-covalently linked GP1, GP2, and SSP. Reptarenavirus L and NP proteins share 17%–26% pairwise amino acid identity with the L and NP proteins of OW and NW mammarenaviruses [18,33]. However, the reptarenavirus glycoproteins did not contain similarity to other arenavirus glycoproteins detectable by commonly used search programs. Instead, reptarenavirus glycoproteins were related to the glycoproteins of filoviruses (e.g., Ebola and Marburg viruses), avian retroviruses (e.g., avian leukosis virus), and cellular syncytin, the product of a repurposed endogenous retroviral gene [18]. No similarities with arenavirus or filovirus glycoproteins or other proteins could be detected for the putative glycoprotein sequence of the frogfish arenaviruses, whereas antennavirus L and NP proteins show homology to L and NP proteins of other arenaviruses [31,33]. Here, we examine the amino acid sequences of proteins encoded by the newly described arenaviruses using computational tools that have previously proven potent in identifying structural features of viral fusion proteins [36,37,38,39,40,41].
2. Materials and Methods
2.1. Sequences
GenBank accession numbers of the arenavirus and filovirus proteins used for sequence and structural analyses are provided in Table S1.
2.2. Proteomics Computational Methods
Pairwise alignments were performed using William Pearson’s Lalign program [42] that implements the algorithm of Huang and Miller [43]. To generate alignments between multiple sequences we used Clustal Omega, a part of the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) search and sequence analysis tool kit that uses seeded guide trees and hidden Markov model (HMM) profile–profile techniques [44]. Secondary structure, solvent accessibility, transmembrane helices, globular regions, coiled-coil regions, and other protein features were analyzed using PredictProtein [45]. Domains with significant propensity to form transmembrane helices were identified with TMpred v. 1.0 [46]. TMpred is based on a statistical analysis of TMbase, a database of naturally occurring transmembrane glycoproteins [47]. Topology and other features of membrane proteins were explored by means of hydropathy plots with the Membrane Protein Explorer from the Stephen White laboratory [48]. The presence of signal peptides and the location of their cleavage sites was analyzed using SignalP (v5.0), which is based on deep convolutional and recurrent neural network architecture including a conditional random field [49]. O-linked glycosylation sites were predicted by Net-O-Glyc v. 4.0, which produces neural network predictions of mucin-type GalNAc O-glycosylation sites in mammalian proteins [50].
3. Results
3.1. Antennavirus Glycoprotein 2 Has Features of a Class I Viral Fusion Protein
Shi and colleagues [31] used metatranscriptomic sequencing to identify two frogfish arenaviruses, WlFAV-1 and WlFAV-2, among 214 novel vertebrate-associated viruses of reptiles, amphibians, lungfish, ray-finned fish, cartilaginous fish, and jawless fish. Unlike other arenaviruses, the antennaviruses have three genome segments. The middle segment of both viruses is ambisense and encodes a glycoprotein precursor and a shorter protein of unknown function. No apparent similarity of the glycoprotein precursor to other arenavirus glycoproteins was detected [33]. The uncleaved glycoprotein precursor of WlFAV-1 is 728 amino acids and that of WlFAV-2 is 635 amino acids. The proteins share 26.5% identical amino acids (55.9% chemically similar amino acids) in a 626 amino acid overlap (Figure 1a). Features found in other arenaviruses are present in both glycoproteins. Following furin-like cleavage sites are N-terminal fusion peptides that contain hydrophobic amino acids. The fusion peptide of WlFAV-1 contains a dicysteine as previously described for mammarenavirus (LASV and lymphocytic choriomeningitis virus, LCMV) GP2 [38]. The N-terminal helices (helical region 1, HR1) in GP2 of WlFAV-1 and WlFAV-2 and hartmanivirus GP2 contain numerous leucine and isoleucine residues. The C-terminal helices (HR2) of mammarenavirus, hartmanivirus, and antennavirus GP2 have an abundance of charged residues [38]. Like the mammarenavirus and hartmanivirus GPCs [25,51,52], antennavirus GPC includes an intracellular zinc-binding domain that follows the transmembrane (TM) domain. At least seven cysteine or histidine residues that can coordinate with zinc are present. Signal prediction algorithms predict WlFAV-1 and WlFAV-2 encode extended virion-associated stable signal peptides (SSP), both with C-terminal cysteine residues that likely coordinate with the zinc-binding domains [52]. We also constructed two-dimensional models of the SSP and GP2 of LASV (Figure 1b), HISV (Figure 1c), and WlFAV-2 (Figure 1d) to compare the structure of antennavirus GPC to other arenavirus glycoproteins. These models suggest that the overall structure of antennavirus GPC is similar to the GPCs of mammarenaviruses and hartmaniviruses. Similar sequences or common structural/functional motifs are located similarly.
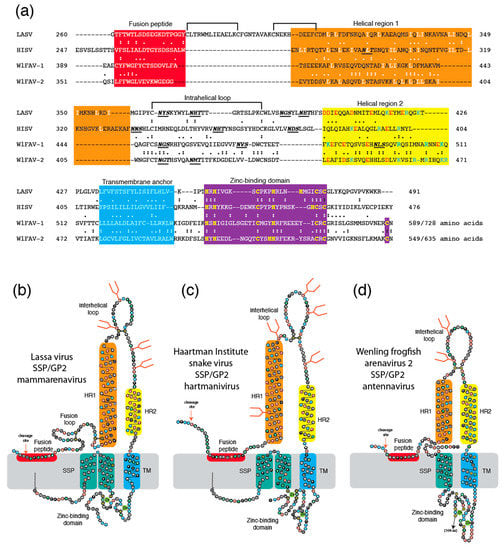
Figure 1.
Comparisons of glycoproteins from representative members of three arenavirus genera. Panel (a): Amino acid sequence alignment of the glycoprotein 2 from Lassa virus (LASV, mammarenavirus), Haartman Institute snake virus (HISV, hartmanivirus), Wēnlǐng frogfish arenavirus-1 (WlFAV-1, striated antennavirus), and Wēnlǐng frogfish arenavirus-2 (WlFAV-2, hairy antennavirus). Panel (b): Two-dimensional model of LASV. Panel (c): Two-dimensional model of HISV. Panel (d): Two-dimensional model of HISV. Fusion peptide red, helical region 1 (HR1) orange, helical region 2 (HR2, yellow), aromatic domain (AD) green, transmembrane domain (TM) blue. Zinc-binding domain violet in Panel (a). Stable signal peptide (SSP) teal in Panels (b–d). Black lines: Dicysteine linkages. N-glycosylation sites: Bold italics in Panel (a) and orange stick figures in Panels (b–d). Similar amino acids are considered as hydrophobic: I,L,V,A,M,F; aromatic: W,F,Y; positive charged: K,R,H; negatively charged or chemically related: E,D,Q,N; special: G,P; polar: S,T.
3.2. Reptarenavirus Glycoprotein 2 Is a Filovirus-Like Viral Fusion Protein
The reptarenavirus GP2 appears to contain all structural features that are common to class I viral fusion proteins, including an N-terminal fusion peptide, two extended α-helices, an intrahelical loop, and a carboxyl terminal transmembrane domain (Figure 2). However, as noted previously [18], the reptarenavirus glycoprotein bears greater sequence similarity to the glycoproteins of filoviruses than to arenaviruses. Comparison of the Golden Gate virus (GGV) GP2 with Ebola virus (EBOV) GP2 shows a 27.9% similarity (55.3% chemically similar) in a 179 amino acid overlap (Figure 2a). However, sequence similarity alone is insufficient to establish whether the GGV GP2 more closely resembles the GP2 equivalents of filoviruses, retroviruses, or the cellular α-penetrene syncytin. Analysis of protein structural features identifies reptarenavirus GP2 as a filovirus-like VFP. The reptarenavirus glycoprotein contains a C-7X-C-C intrahelical loop (X = any amino acid), which is similar to the C-6X-C-C intrahelical loop of filoviruses. GP2 of GGV and EBOV each contain two N-linked glycans, both in the same relative locations (Figure 2b,c). Furthermore, both the reptarenavirus glycoprotein and the filovirus glycoprotein (Figure 2) [53] contain a domain with several aromatic amino acids prior to the transmembrane anchor. A membrane proximal aromatic domain is contained in class I fusion proteins of certain retroviruses (including HIV and other lentiviruses) [54] and coronaviruses [55] but has not previously been described in arenaviruses.
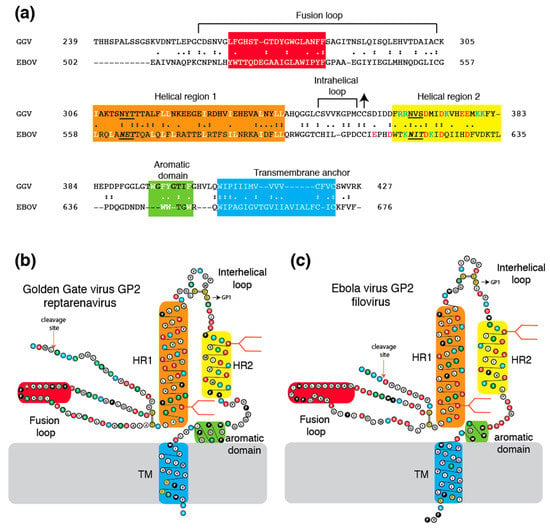
Figure 2.
Comparison of glycoproteins of a reptarenavirus and a filovirus. Panel (a): Amino acid sequence alignment of glycoprotein 2 of Golden Gate virus (GGV, reptarenavirus) and Ebola virus (EBOV, filovirus). Panel (b): Two-dimensional model of GGV. Panel (c): Two-dimensional model of EBOV. Coloring and abbreviations as in Figure 1. Arrows indicate cysteine linkage to glycoprotein 1 (GP1).
Linear models of glycoprotein 2 from members of the four arenavirus genera and a filovirus demonstrate the structural similarities and differences (Figure 3). Common features include N-terminal fusion domains, two extended α-helices, an intrahelical loop, and a carboxyl terminal transmembrane domain. However, the intrahelical loops of mammarenavirus, hartmanivirus, and antennavirus glycoproteins contain 18 or more amino acids between a pair of cysteines, which is longer than the intrahelical loop of the reptarenavirus glycoprotein. The third cysteine in the intrahelical loop of reptarenavirus and filovirus glycoproteins, which links the GP1 and GP2 subunits of GPC, is not present in mammarenaviruses, hartmaniviruses, and antennavirus glycoproteins. Neither reptarenavirus nor filovirus glycoproteins have an intracellular zinc-binding domain or an extended virion-associated SSP, which are present in the GPCs of mammarenaviruses, hartmaniviruses, and antennaviruses.
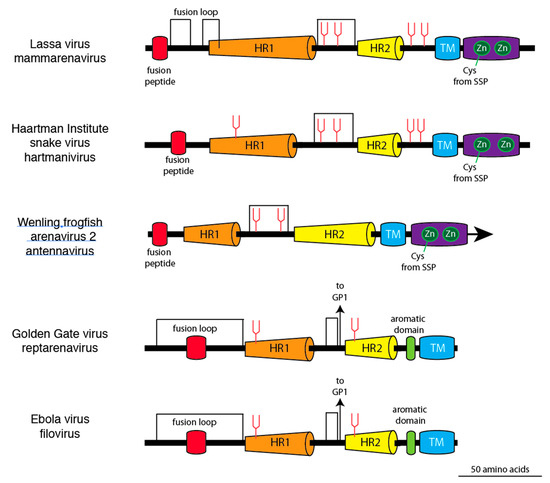
Figure 3.
Linear models of glycoprotein 2 from representative members of the four arenavirus genera and a filovirus. Coloring and abbreviations as in Figure 1. Zn in green circle represents zinc-binding domains. A cysteine (Cys) from the stable signal peptide (SSP) coordinates with the first zinc-binding domain in Lassa virus, Haartman Institute snake virus, and Wēnlǐng frogfish arenavirus-2. Arrows indicate cysteine linkages to glycoprotein 1 (GP1) of Golden Gate virus and Ebola virus.
3.3. Antennavirus Glycoprotein 1 Does Not Display Features Conserved in Glycoprotein 1 of Other Arenaviruses
GP1s of mammarenaviruses have a conserved structure, consisting of commonly placed α-helices and a β-sandwich that comprises the receptor-binding domain (Figure S1) [35,56,57]. There are two conserved cysteine linkages and two conserved N-linked glycans in both NW and OW arenavirus GP1. This scaffold accommodates binding to divergent receptors. OW arenaviruses and some NW arenaviruses use α-dystroglycan as a cellular receptor, while other NW arenaviruses use the transferrin receptor [58,59]. Reptarenavirus, hartmanivirus, or antennavirus GP1 do not appear to display any of the conserved features found in mammarenavirus GP1, nor do they have any discernable structural features in common with each other. Such features would manifest as a conserved pattern of dicysteine bonds or glycosylation sites (Figure 4). The distinct structures of the GP1 may be due to the requirement for binding to distinct fish or reptile cellular receptors.
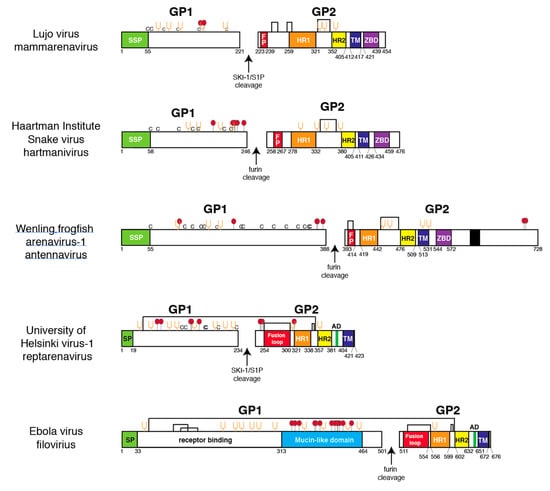
Figure 4.
Linear models of glycoprotein 1 and 2 from selected members of the four arenavirus genera and a filovirus. Viruses were selected for the presence of potential O-linked glycosylation sites. Coloring and abbreviations as in Figure 1, except arrows represent cleavage sites by the indicated cellular proteases. Cysteine (C), fusion peptide (FP), zinc-binding domain (ZBD, aromatic domain (AD). Red balls: O-linked glycosylation sites. Amino acids are numbered from the N-terminus.
EBOV GP1 contains a distinctive mucin-like domain with numerous O-linked glycans that may offer a shield against antibody recognition [60]. With the exception of Lujo virus (LUJV), no mammarenavirus GP1 is predicted to contain O-linked glycans (Figure 4). In contrast, GP1 of certain reptarenaviruses, such as University of Helsinki virus (UHV), contain several predicted O-linked glycosylation sites and numerous predicted N-linked glycosylation sites. Hartmanivirus and antennavirus GP1s also contain several O-linked glycosylation sites, but fewer predicted N-linked glycosylation sites. While computational models can predict potential glycosylation sites, confirmation by chemical analysis, crystallography, or other methods is required to establish that the sites contain glycans.
3.4. Antennavirus Nucleoprotein and Large Proteins Share Similarities to Hartmanivirus Nucleoprotein and Large Proteins
Previous analyses indicated that NP and L genes of WlFAV-1 and WlFAV -2 have similarities to NP and L genes of other arenaviruses and lead to the designation of a new genus Antennavirus within the Arenaviridae. Phylogenetic analyses suggested a closer relationship of antennaviruses to hartmaniviruses than to members of other arenavirus genera [33]. To further examine the relationship of the antennaviruses to other arenaviruses we performed alignments of the amino acid sequences of proteins from representative members of the four arenavirus genera. LASV NP has distinct N- and C-terminal domains connected by a flexible linker [61,62]. Hastie et al. [62] showed that the N-terminal domain binds viral RNA but not cellular mRNA and contains a deep basic groove that channels the single-stranded RNA. While numerous residues in the N-terminus of NP are highly conserved amongst all arenaviruses, antennaviruses and hartmaniviruses also share several similar sequences not present in NP of members from other genera (Figure 5). The C-terminus of the LASV NP functions as a double-stranded RNA-specific exonuclease [61]. Antiviral responses are initiated by dsRNA, and the NP endonuclease of NP could serve to evade the immune system. The C-terminal domain of antennavirus NP contains the active site of the endonuclease (DEDDh box) and a metal-binding domain both of which are conserved in the NP of arenaviruses of other genera (Figure S2). Antennavirus L proteins also show sequence similarities to other arenavirus L proteins (Figure S3). These analyses confirm the previously suggested phylogeny of the four arenavirus genera [33]. Like hartmaniviruses, the antennaviruses do not encode a zinc-binding protein (Z, matrix). Reptarenaviruses encode a Z protein with highly conserved residues for binding two metal ions (Figure S4). In contrast to Z of mammarenaviruses, Z of reptarenaviruses contains a hydrophobic transmembrane domain suggesting that it is membrane associated.
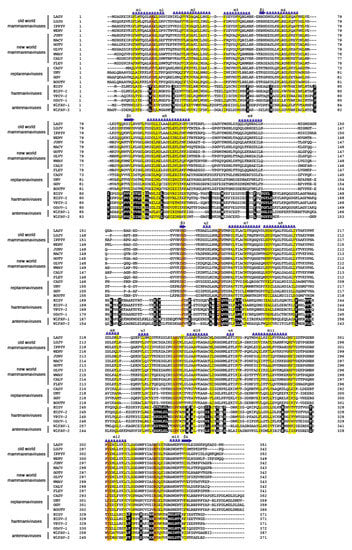
Figure 5.
Amino acid sequence alignment of the N-termini of nucleoproteins from members of the four arenavirus genera. Virus abbreviations: Lassa, LASV; Lujo, LUJV; Ippy, IPPYV; Wenzhou, WENV; Junin, JUNV; Machupo, MACV; Guanarito, GOTV; Olivaros, OLLV; Whitewater Arroyo, WWAV, California (Pichinde), CALV; University of Helsinki, UHV; Golden Gate, GGV; Haartman Institute snake, HISV and HISV-2; Old Schoolhouse, OScV; Wēnlǐng Frogfish arenavirus, WlFAV-1 and WlFAV-2. Residues that are conserved (identical or chemically similar) are highlighted in yellow. Conserved residues involved in RNA binding are highlighted in orange. Blue springs represent helical structures and blue arrows are beta sheets. Similar residue/sequences in antennaviruses and hartmaniviruses not present in NP of members from other genera are highlighted in black.
4. Discussion
Based on common features, such as an amino-terminal fusion peptide, and computer algorithms that predict protein configurations, Gallaher and colleagues suggested [37] that the ectodomain of the HIV-1 transmembrane protein (TM, gp41) and TM proteins of other retroviruses, all fit the scaffold of the postfusion structure of influenza virus hemagglutinin 2 (HA2) [63]. This was the first evidence that enveloped viruses from different families employ a common mechanism for cell entry. While initially considered speculative, our retroviral TM model was confirmed later by X-ray crystallographic structural determinations [64,65,66]. Subsequently, it was observed that common structural motifs are present not only in myxovirus HA2 and retrovirus TM, but also in the GP2 proteins of filoviruses [36] and arenaviruses [38], the spike proteins (S) of coronaviruses [67,68,69] and paramyxoviruses F proteins [70], which are now referred to collectively as class I VFPs. The envelope glycoproteins of flaviviruses, alphaviruses, and bunyaviruses form the structurally distinct class II VFPs [71,72], whereas rhabdoviruses and herpesviruses, have class III VFPs [73,74,75]. Here, we present proteomic computational analyses suggesting that antennavirus GPC includes a class I VFP with an internal zinc-binding domain and a SSP.
The discovery of arenaviruses in fish establishes a long evolutionary history for this virus family [31]. Hartmaniviruses and antennaviruses appear more closely related to each other than to either mammarenaviruses or reptarenaviruses. Mammarenaviruses and reptarenaviruses may also share a common progenitor. The lack of Z protein in both antennaviruses and hartmaniviruses is consistent with this evolutionary scenario. The sequence similarities detected between GPCs of mammarenaviruses, hartmaniviruses, and antennaviruses are consistent with divergent evolution from a common class I VFP progenitor (Figure 6). In this scenario, acquisition of the SSP and intracellular zinc-binding domain in GPC followed divergence of members of the Arenaviridae from a virus encoding the common progenitor of arenavirus and filovirus glycoproteins and other class I VFPs.
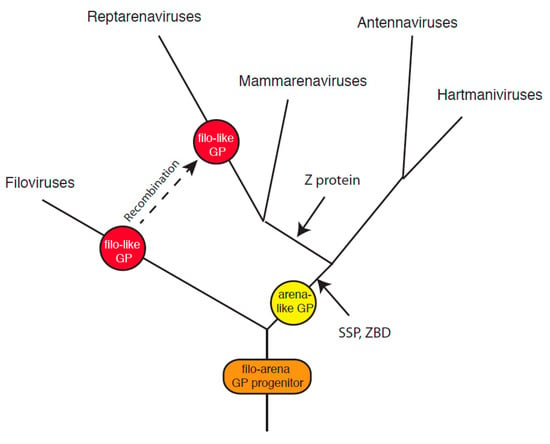
Figure 6.
Possible evolution of the Arenaviridae. Members of the Arenaviridae and Filoviridae may have a common ancestor encoding the progenitor of arenavirus and filovirus glycoproteins. Acquisition of the stable signal peptide (SSP) and intracellular zinc-binding domain (ZBD) in the arenavirus glycoprotein complex (GPC) followed divergence from the filoviruses. Mammarenaviruses and reptarenaviruses acquired a zinc-binding protein (Z) following divergence from antennaviruses and hartmaniviruses. Reptarenaviruses then replaced the arenavirus GPC or a progenitor with a filovirus-like class I glycoprotein via recombination.
The entry proteins of the DNA containing baculoviruses, including group II nucleopolyhedroviruses (NPV) and granuloviruses, are class I VFPs [76]. However, baculoviruses in group I NPV are class III VFPs [40]. Orthomyxoviruses such as influenza virus encode class I VFPs, but members of the Thogotovirus genus of the Orthomyxoviridae encode class III VFPs. The examples of baculoviruses and orthomyxoviruses suggest that viruses within a family may diverge by acquiring VFPs of distinct classes through recombination events. Recombination has been shown to occur in reptarenaviruses [77]. While other evolutional pathways are possible, reptarenaviruses may have replaced the arenavirus GPC or a progenitor with a filovirus-like class I glycoprotein via recombination (Figure 6). An unlikely alternative scenario involves a highly convergent pathway involving a common arenavirus and filovirus glycoprotein progenitor evolving to a reptarenavirus glycoprotein bearing both extensive structural and sequence similarity to filovirus GP2 as well as loss of or failure to acquire the SSP and zinc-binding domain plus independent acquisition or failure to lose the third cysteine in the intrahelical loop.
Hypotheses that rodent-borne viruses, such as hantaviruses and arenaviruses, co-evolved with their hosts [78] are proving to be overly simplistic [79]. There is no credible genetic evidence for co-evolution of hantaviruses and their rodent reservoirs [80]. In Africa, genetic signatures of co-evolution of arenaviruses, if any existed, have been eliminated by multiple host-switching and lineage extinction events [81]. The current studies suggest that evolution of arenaviruses, particularly with regard to their glycoproteins, may not be a simple linear process. Characterization of arenaviruses from other fish or additional vertebrates may illuminate complex pathways for evolution/acquisition of arenavirus glycoproteins.
5. Conclusions
Divergent evolution from a common progenitor potentially explains similarities of antennavirus, mammarenavirus, and hartmanivirus glycoproteins, with an ancient recombination event resulting in a divergent reptarenavirus glycoprotein. Arenaviruses infecting snakes appear to have followed distinct evolutionary pathways. Analyses of sequence and structural similarities and differences suggest that mammarenaviruses and reptarenaviruses split from antennaviruses and hartmaniviruses and then acquired a zinc-binding protein.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/11/8/750/s1, Figure S1: Conserved structure of mammarenaviruses glycoprotein 1, Figure S2: Amino acid sequence alignments of the C-termini of nucleoproteins from representative members of the four arenavirus genera, Figure S3: Amino acid sequence alignments of the L protein enzymatic domain from representative members of the four arenavirus genera, Figure S4: Amino acid sequence alignments of zinc-binding (matrix) proteins from representative members of the Mammarenavirus and Hartmanivirus genera, Table S1: GenBank accession numbers used for sequence and structural analyses.
Author Contributions
C.E.G. performed sequence alignments and assisted in the preparation of figures. R.F.G. supervised the work and wrote the manuscript. All authors read and approved the final manuscript.
Funding
Funding support came from NIH grants U19AI135995, AI132244, AI132223, U54 HG007480, U19 AI115589, U19AI142790, and contract HHSN272201400048C and grant INTU1901 from the Coalition for Epidemic Preparedness and Innovations.
Acknowledgments
We thank Kristian G. Andersen for advice on phylogenic analyses and William R. Gallaher for continued intellectual support and encouragement.
Conflicts of Interest
The Viral Hemorrhagic Fever Consortium (available at: http://www.vhfc.org) is a partnership of academic and industry scientists who are developing diagnostic tests, therapeutic agents, and vaccines for Lassa fever, Ebola, and other severe diseases. Tulane University and its various academic and industry partners have filed U.S. and foreign patent applications on behalf of the consortium for several of these technologies. Technical information may also be kept as trade secrets. If commercial products are developed, consortium members may receive royalties or profits. This does not alter our adherence to all policies of the NIH and Viruses on sharing data and materials. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- Radoshitzky, S.R.; Bao, Y.; Buchmeier, M.J.; Charrel, R.N.; Clawson, A.N.; Clegg, C.S.; DeRisi, J.L.; Emonet, S.; Gonzalez, J.P.; Kuhn, J.H.; et al. Past, present, and future of arenavirus taxonomy. Arch. Virol. 2015, 160, 1851–1874. [Google Scholar] [CrossRef] [PubMed]
- Cajimat, M.N.; Milazzo, M.L.; Haynie, M.L.; Hanson, J.D.; Bradley, R.D.; Fulhorst, C.F. Diversity and phylogenetic relationships among the north american tacaribe serocomplex viruses (family arenaviridae). Virology 2011, 421, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; Feldmann, H.; Fulhorst, C.F.; Khelifa, R.; de Chesse, R.; de Lamballerie, X. Phylogeny of new world arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem. Biophys. Res. Commun. 2002, 296, 1118–1124. [Google Scholar] [CrossRef]
- Downs, W.G.; Anderson, C.R.; Spence, L.; Aitken, T.H.; Greenhall, A.H. Tacaribe virus, a new agent isolated from artibeus bats and mosquitoes in trinidad, west indies. Am. J. Trop. Med. Hyg. 1963, 12, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.L.; Cajimat, M.N.; Mauldin, M.R.; Bennett, S.G.; Hess, B.D.; Rood, M.P.; Conlan, C.A.; Nguyen, K.; Wekesa, J.W.; Ramos, R.D.; et al. Epizootiology of tacaribe serocomplex viruses (arenaviridae) associated with neotomine rodents (cricetidae, neotominae) in southern california. Vector Borne Zoonotic Dis. 2015, 15, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fulhorst, C.F.; Bowen, M.D.; Salas, R.A.; Duno, G.; Utrera, A.; Ksiazek, T.G.; De Manzione, N.M.; De Miller, E.; Vasquez, C.; Peters, C.J.; et al. Natural rodent host associations of guanarito and pirital viruses (family arenaviridae) in central venezuela. Am. J. Trop. Med. Hyg. 1999, 61, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kronmann, K.C.; Nimo-Paintsil, S.; Guirguis, F.; Kronmann, L.C.; Bonney, K.; Obiri-Danso, K.; Ampofo, W.; Fichet-Calvet, E. Two novel arenaviruses detected in pygmy mice, ghana. Emerg. Infect. Dis. 2013, 19, 1832–1835. [Google Scholar] [CrossRef]
- Olayemi, A.; Cadar, D.; Magassouba, N.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New hosts of the lassa virus. Sci. Rep. 2016, 6, 25280. [Google Scholar] [CrossRef]
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa virus isolation from mastomys natalensis rodents during an epidemic in sierra leone. Science 1974, 185, 263–265. [Google Scholar] [CrossRef]
- Rowe, W.P.; Murphy, F.A.; Bergold, G.H.; Casals, J.; Hotchin, J.; Johnson, K.M.; Lehmann-Grube, F.; Mims, C.A.; Traub, E.; Webb, P.A. Arenoviruses: Proposed name for a newly defined virus group. J. Virol. 1970, 5, 651–652. [Google Scholar]
- Li, K.; Lin, X.D.; Li, M.H.; Wang, M.R.; Sun, X.Y.; Zhang, Y.Z. Genomic analysis of wenzhou virus in rodents from zhejiang province. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 384–387. [Google Scholar] [PubMed]
- Blasdell, K.R.; Becker, S.D.; Hurst, J.; Begon, M.; Bennett, M. Host range and genetic diversity of arenaviruses in rodents, united kingdom. Emerg. Infect. Dis. 2008, 14, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, X.D.; Wang, W.; Shi, M.; Guo, W.P.; Zhang, X.H.; Xing, J.G.; He, J.R.; Wang, K.; Li, M.H.; et al. Isolation and characterization of a novel arenavirus harbored by rodents and shrews in zhejiang province, China. Virology 2015, 476, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.G.; Grant, D.S.; Schieffelin, J.S.; Boisen, M.L.; Goba, A.; Hartnett, J.N.; Levy, D.C.; Yenni, R.E.; Moses, L.M.; Fullah, M.; et al. Lassa fever in post-conflict sierra leone. PLoS Negl. Trop. Dis. 2014, 8, e2748. [Google Scholar] [CrossRef] [PubMed]
- Okokhere, P.; Colubri, A.; Azubike, C.; Iruolagbe, C.; Osazuwa, O.; Tabrizi, S.; Chin, E.; Asad, S.; Ediale, E.; Rafiu, M.; et al. Clinical and laboratory predictors of lassa fever outcome in a dedicated treatment facility in nigeria: A retrospective, observational cohort study. Lancet. Infect. Dis. 2018, 18, 684–695. [Google Scholar] [CrossRef]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The who r&d blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res. 2018, 159, 63–67. [Google Scholar]
- Burki, T. Cepi: Preparing for the worst. Lancet. Infect. Dis. 2017, 17, 265–266. [Google Scholar] [CrossRef]
- Stenglein, M.D.; Sanders, C.; Kistler, A.L.; Ruby, J.G.; Franco, J.Y.; Reavill, D.R.; Dunker, F.; Derisi, J.L. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: Candidate etiological agents for snake inclusion body disease. MBio 2012, 3, e00180-12. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Ibrahim, O.E.; Ilyasu, Y.; Bande, F.; Mohd Lila, M.A.; Noordin, M.M. In vitro isolation and molecular identification of reptarenavirus in malaysia. Virus Genes 2016, 52, 640–650. [Google Scholar] [CrossRef]
- Keller, S.; Hetzel, U.; Sironen, T.; Korzyukov, Y.; Vapalahti, O.; Kipar, A.; Hepojoki, J. Co-infecting reptarenaviruses can be vertically transmitted in boa constrictor. PLoS Pathog 2017, 13, e1006179. [Google Scholar] [CrossRef]
- Bodewes, R.; Kik, M.J.; Raj, V.S.; Schapendonk, C.M.; Haagmans, B.L.; Smits, S.L.; Osterhaus, A.D. Detection of novel divergent arenaviruses in boid snakes with inclusion body disease in the netherlands. J. Gen. Virol. 2013, 94, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; Raj, V.S.; Kik, M.J.; Schapendonk, C.M.; Haagmans, B.L.; Smits, S.L.; Osterhaus, A.D. Updated phylogenetic analysis of arenaviruses detected in boid snakes. J. Virol. 2014, 88, 1399–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hetzel, U.; Sironen, T.; Laurinmaki, P.; Liljeroos, L.; Patjas, A.; Henttonen, H.; Vaheri, A.; Artelt, A.; Kipar, A.; Butcher, S.J.; et al. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J. Virol. 2013, 87, 10918–10935. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, U.; Sironen, T.; Laurinmaki, P.; Liljeroos, L.; Patjas, A.; Henttonen, H.; Vaheri, A.; Artelt, A.; Kipar, A.; Butcher, S.J.; et al. Reply to updated phylogenetic analysis of arenaviruses detected in boid snakes. J. Virol. 2014, 88, 1401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hepojoki, J.; Hepojoki, S.; Smura, T.; Szirovicza, L.; Dervas, E.; Prahauser, B.; Nufer, L.; Schraner, E.M.; Vapalahti, O.; Kipar, A.; et al. Characterization of haartman institute snake virus-1 (hisv-1) and hisv-like viruses-the representatives of genus hartmanivirus, family arenaviridae. PLoS Pathog. 2018, 14, e1007415. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, J.; Salmenpera, P.; Sironen, T.; Hetzel, U.; Korzyukov, Y.; Kipar, A.; Vapalahti, O. Arenavirus coinfections are common in snakes with boid inclusion body disease. J. Virol. 2015, 89, 8657–8660. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, E.; Blair, C.D.; Briese, T.; et al. Taxonomy of the order bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.; Alkhovsky, S.V.; Bao, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the family arenaviridae and the order bunyavirales: Update 2018. Arch. Virol. 2018, 163, 2295–2310. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, T.H.; Marschang, R.E.; Bruce, M.; Clark, P.; Vitali, S.D. Reptarenaviruses in apparently healthy snakes in an australian zoological collection. Aust. Vet. J. 2019, 97, 93–102. [Google Scholar] [CrossRef]
- Stenglein, M.D.; Sanchez-Migallon Guzman, D.; Garcia, V.E.; Layton, M.L.; Hoon-Hanks, L.L.; Boback, S.M.; Keel, M.K.; Drazenovich, T.; Hawkins, M.G.; DeRisi, J.L. Differential disease susceptibilities in experimentally reptarenavirus-infected boa constrictors and ball pythons. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Chen, X.; Tian, J.H.; Chen, L.J.; Li, K.; Wang, W.; Eden, J.S.; Shen, J.J.; Liu, L.; et al. The evolutionary history of vertebrate rna viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, E.; Blair, C.D.; et al. Taxonomy of the order bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, C.; Forni, D.; Sironi, M. Arenavirus genomics: Novel insights into viral diversity, origin, and evolution. Curr. Opin. Virol. 2019, 34, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Zandonatti, M.; Liu, T.; Li, S.; Woods, V.L., Jr.; Saphire, E.O. Crystal structure of the oligomeric form of lassa virus matrix protein z. J. Virol. 2016, 90, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Zandonatti, M.A.; Kleinfelter, L.M.; Heinrich, M.L.; Rowland, M.M.; Chandran, K.; Branco, L.M.; Robinson, J.E.; Garry, R.F.; Saphire, E.O. Structural basis for antibody-mediated neutralization of lassa virus. Science 2017, 356, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, W.R. Similar structural models of the transmembrane proteins of ebola and avian sarcoma viruses. Cell 1996, 85, 477–478. [Google Scholar] [CrossRef]
- Gallaher, W.R.; Ball, J.M.; Garry, R.F.; Griffin, M.C.; Montelaro, R.C. A general model for the transmembrane proteins of hiv and other retroviruses. AIDS Res. Hum. Retrovir. 1989, 5, 431–440. [Google Scholar] [CrossRef]
- Gallaher, W.R.; DiSimone, C.; Buchmeier, M.J. The viral transmembrane superfamily: Possible divergence of arenavirus and filovirus glycoproteins from a common rna virus ancestor. BMC Microbiol. 2001, 1, 1. [Google Scholar] [CrossRef]
- Garry, C.E.; Garry, R.F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of bunyaviruses are class ii viral fusion protein (beta-penetrenes). Theor. Biol. Med Model. 2004, 1, 10. [Google Scholar] [CrossRef]
- Garry, C.E.; Garry, R.F. Proteomics computational analyses suggest that baculovirus gp64 superfamily proteins are class iii penetrenes. Virol. J. 2008, 5, 28. [Google Scholar] [CrossRef]
- Garry, C.E.; Garry, R.F. Proteomics computational analyses suggest that the bornavirus glycoprotein is a class iii viral fusion protein (gamma penetrene). Virol. J. 2009, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W. Lalign. Available online: https://embnet.vital-it.ch/software/LALIGN_form.html (accessed on 7 July 2019).
- Huang, X.; Miller, W. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 1991, 12, 337–357. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The embl-ebi search and sequence analysis tools apis in 2019. Nucleic Acids Res. 2019, 47, w636–w641. [Google Scholar] [CrossRef] [PubMed]
- Yachdav, G.; Kloppmann, E.; Kajan, L.; Hecht, M.; Goldberg, T.; Hamp, T.; Honigschmid, P.; Schafferhans, A.; Roos, M.; Bernhofer, M.; et al. Predictprotein--an open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014, 42, w337–w343. [Google Scholar] [CrossRef]
- Hofmann, K.; Stoffel, W. Tmpred. Available online: https://embnet.vital-it.ch/software/TMPRED_form.html (accessed on 7 July 2019).
- Hofmann, K.; Stoffel, W. Tmbase—A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 1993, 374, 166. [Google Scholar]
- White, S.H.; Snider, C.; Jaysinghe, S.; Kim, J. Membrane Protein Explorer Version 2.2a. Available online: http://blanco.biomol.uci.edu/mpex/ 2003 (accessed on 7 July 2019).
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Signalp 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human o-galnac glycoproteome through simplecell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Briknarova, K.; Thomas, C.J.; York, J.; Nunberg, J.H. Structure of a zinc-binding domain in the junin virus envelope glycoprotein. J. Biol Chem 2011, 286, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- York, J.; Nunberg, J.H. A novel zinc-binding domain is essential for formation of the functional junin virus envelope glycoprotein complex. J. Virol. 2007, 81, 13385–13391. [Google Scholar] [CrossRef] [PubMed]
- Saez-Cirion, A.; Gomara, M.J.; Agirre, A.; Nieva, J.L. Pre-transmembrane sequence of ebola glycoprotein. Interfacial hydrophobicity distribution and interaction with membranes. FEBS Lett. 2003, 533, 47–53. [Google Scholar] [CrossRef]
- Suarez, T.; Gallaher, W.R.; Agirre, A.; Goni, F.M.; Nieva, J.L. Membrane interface-interacting sequences within the ectodomain of the human immunodeficiency virus type 1 envelope glycoprotein: Putative role during viral fusion. J. Virol. 2000, 74, 8038–8047. [Google Scholar] [CrossRef] [PubMed]
- Sainz, B., Jr.; Rausch, J.M.; Gallaher, W.R.; Garry, R.F.; Wimley, W.C. The aromatic domain of the coronavirus class i viral fusion protein induces membrane permeabilization: Putative role during viral entry. Biochemistry 2005, 44, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Corbett, K.D.; Farzan, M.; Choe, H.; Harrison, S.C. Structural basis for receptor recognition by new world hemorrhagic fever arenaviruses. Nat. Struct. Mol. Biol. 2010, 17, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Igonet, S.; Sullivan, B.M.; Legrand, P.; Zandonatti, M.A.; Robinson, J.E.; Garry, R.F.; Rey, F.A.; Oldsone, M.B.; Saphire, E.O. Crystal structure of the prefusion surface glycoprotein of the prototypic arenavirus lcmv. Nat. Struct. Mol. Biol. 2016, 6, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and lassa fever virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- Spiropoulou, C.F.; Kunz, S.; Rollin, P.E.; Campbell, K.P.; Oldstone, M.B. New world arenavirus clade c, but not clade a and b viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 2002, 76, 5140–5146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Fusco, M.L.; Hessell, A.J.; Oswald, W.B.; Burton, D.R.; Saphire, E.O. Structure of the ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008, 454, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Kimberlin, C.R.; Zandonatti, M.A.; MacRae, I.J.; Saphire, E.O. Structure of the lassa virus nucleoprotein reveals a dsrna-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc. Natl. Acad. Sci. USA 2011, 108, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Liu, T.; Li, S.; King, L.B.; Ngo, N.; Zandonatti, M.A.; Woods, V.L., Jr.; de la Torre, J.C.; Saphire, E.O. Crystal structure of the lassa virus nucleoprotein-rna complex reveals a gating mechanism for rna binding. Proc. Natl. Acad. Sci. USA 2011, 108, 19365–19370. [Google Scholar] [CrossRef]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 a resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef]
- Chan, D.C.; Fass, D.; Berger, J.M.; Kim, P.S. Core structure of gp41 from the hiv envelope glycoprotein. Cell 1997, 89, 263–273. [Google Scholar] [CrossRef]
- Weissenhorn, W.; Dessen, A.; Harrison, S.C.; Skehel, J.J.; Wiley, D.C. Atomic structure of the ectodomain from hiv-1 gp41. Nature 1997, 387, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Potz, J.; Basiripour, L.; Dorfman, T.; Haseltine, W.; Sodroski, J. Attenuation of hiv-1 cytopathic effect by mutation affecting the transmembrane glycoprotein. J. Virol. 1991, 65, 281–291. [Google Scholar] [PubMed]
- Gallaher, W.R.; Garry, R.F. Model of the Pre-Insertion Region of the Spike (s2) Fusion Glycoprotein of the Human Sars Coronavirus: Implications for Antiviral Therapeutics. Available online: http://www.virology.net/sars/s2model.html (accessed on 7 July 2019).
- Liu, S.; Xiao, G.; Chen, Y.; He, Y.; Niu, J.; Escalante, C.R.; Xiong, H.; Farmar, J.; Debnath, A.K.; Tien, P.; et al. Interaction between heptad repeat 1 and 2 regions in spike protein of sars-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 2004, 363, 938–947. [Google Scholar] [CrossRef]
- Tripet, B.; Howard, M.W.; Jobling, M.; Holmes, R.K.; Holmes, K.V.; Hodges, R.S. Structural characterization of the sars-coronavirus spike s fusion protein core. J. Biol. Chem. 2004, 279, 20836–20849. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.A.; Dutch, R.E.; Lamb, R.A.; Jardetzky, T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 1999, 3, 309–319. [Google Scholar] [CrossRef]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 a resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Lescar, J.; Roussel, A.; Wien, M.W.; Navaza, J.; Fuller, S.D.; Wengler, G.; Wengler, G.; Rey, F.A. The fusion glycoprotein shell of semliki forest virus: An icosahedral assembly primed for fusogenic activation at endosomal ph. Cell 2001, 105, 137–148. [Google Scholar] [CrossRef]
- Heldwein, E.E.; Lou, H.; Bender, F.C.; Cohen, G.H.; Eisenberg, R.J.; Harrison, S.C. Crystal structure of glycoprotein b from herpes simplex virus 1. Science 2006, 313, 217–220. [Google Scholar] [CrossRef]
- Roche, S.; Bressanelli, S.; Rey, F.A.; Gaudin, Y. Crystal structure of the low-ph form of the vesicular stomatitis virus glycoprotein g. Science 2006, 313, 187–191. [Google Scholar] [CrossRef]
- Roche, S.; Rey, F.A.; Gaudin, Y.; Bressanelli, S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein g. Science 2007, 315, 843–848. [Google Scholar] [CrossRef]
- Misseri, Y.; Labesse, G.; Bucheton, A.; Terzian, C. Comparative sequence analysis and predictions for the envelope glycoproteins of insect endogenous retroviruses. Trends Microbiol. 2003, 11, 253–256. [Google Scholar] [CrossRef]
- Stenglein, M.D.; Jacobson, E.R.; Chang, L.W.; Sanders, C.; Hawkins, M.G.; Guzman, D.S.; Drazenovich, T.; Dunker, F.; Kamaka, E.K.; Fisher, D.; et al. Widespread recombination, reassortment, and transmission of unbalanced compound viral genotypes in natural arenavirus infections. PLoS Pathog. 2015, 11, e1004900. [Google Scholar] [CrossRef]
- Hugot, J.P.; Gonzalez, J.P.; Denys, C. Evolution of the old world arenaviridae and their rodent hosts: Generalized host-transfer or association by descent? Infect. Genet. Evol. 2001, 1, 13–20. [Google Scholar] [CrossRef]
- Forni, D.; Pontremoli, C.; Pozzoli, U.; Clerici, M.; Cagliani, R.; Sironi, M. Ancient evolution of mammarenaviruses: Adaptation via changes in the l protein and no evidence for host-virus codivergence. Genome Biol. Evol. 2018, 10, 863–874. [Google Scholar] [CrossRef]
- Ramsden, C.; Holmes, E.C.; Charleston, M.A. Hantavirus evolution in relation to its rodent and insectivore hosts: No evidence for codivergence. Mol. Biol. Evol. 2009, 26, 143–153. [Google Scholar] [CrossRef]
- Coulibaly-N’Golo, D.; Allali, B.; Kouassi, S.K.; Fichet-Calvet, E.; Becker-Ziaja, B.; Rieger, T.; Olschlager, S.; Dosso, H.; Denys, C.; Ter Meulen, J.; et al. Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from cote d’ivoire: Implications for evolution of arenaviruses in africa. PLoS ONE 2011, 6, e20893. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).