Evaluation of Diagnostic Performance of Three Indirect Enzyme-Linked Immunosorbent Assays for the Detection of IgG Antibodies to Ebola Virus in Human Sera
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Ethics Clearance
2.2. ELISA Serum Controls and Internal Quality Control (IQC)
2.3. Enzyme-Linked Immunosorbent Assays
2.3.1. Production of Ebola Virus Whole Antigen
2.3.2. Prodution of Recombinant Ebola Virus Nucleoprotein
2.3.3. Recombinant Ebola Virus Glycoprotein
2.3.4. Indirect Enzyme-Linked Immunosorbent Assay Procedures
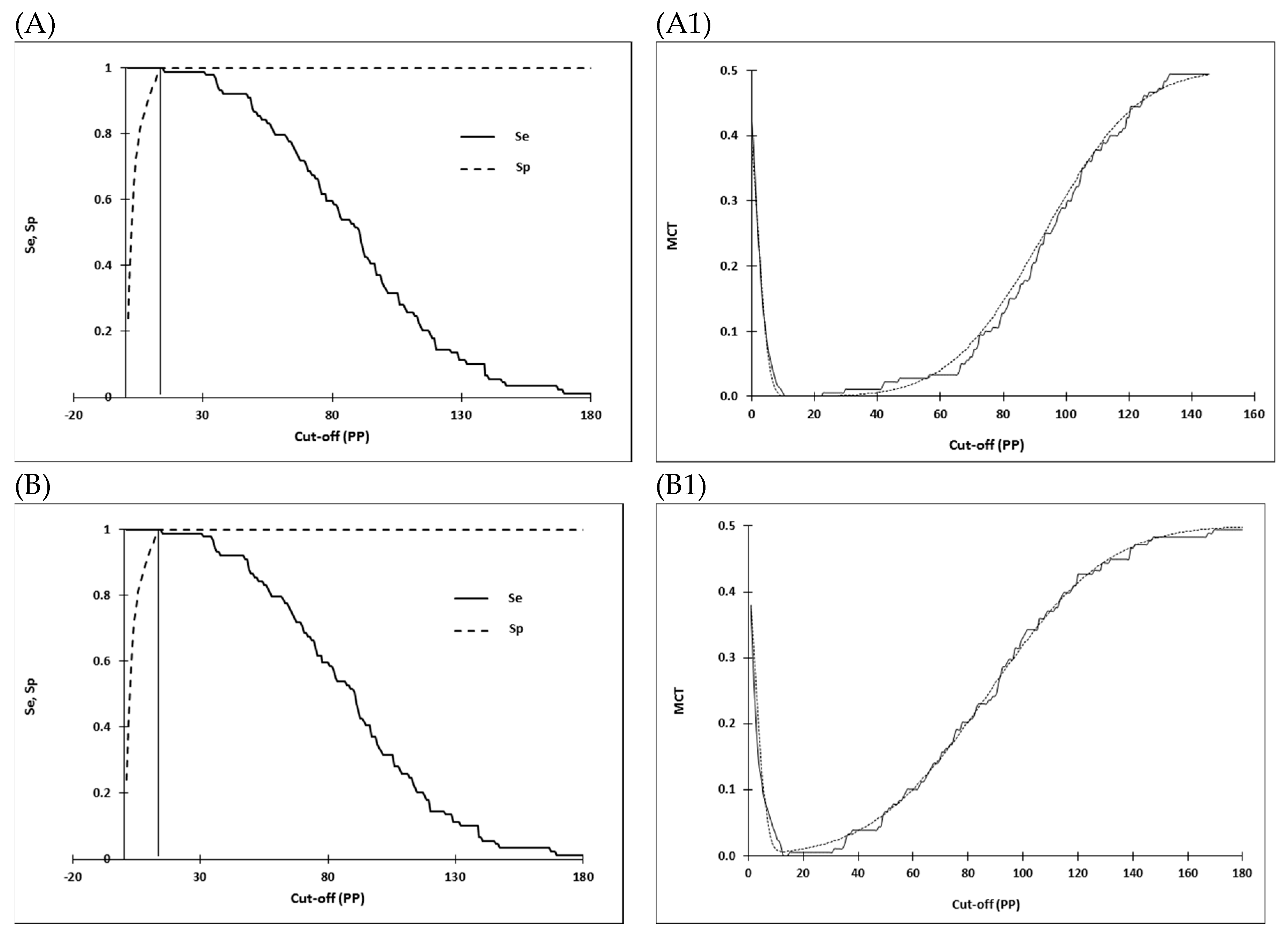
2.4. Selection of Cut-off Values and Statistical Analysis
2.5. Serum Inactivation
3. Results
3.1. IQC and Repeatability
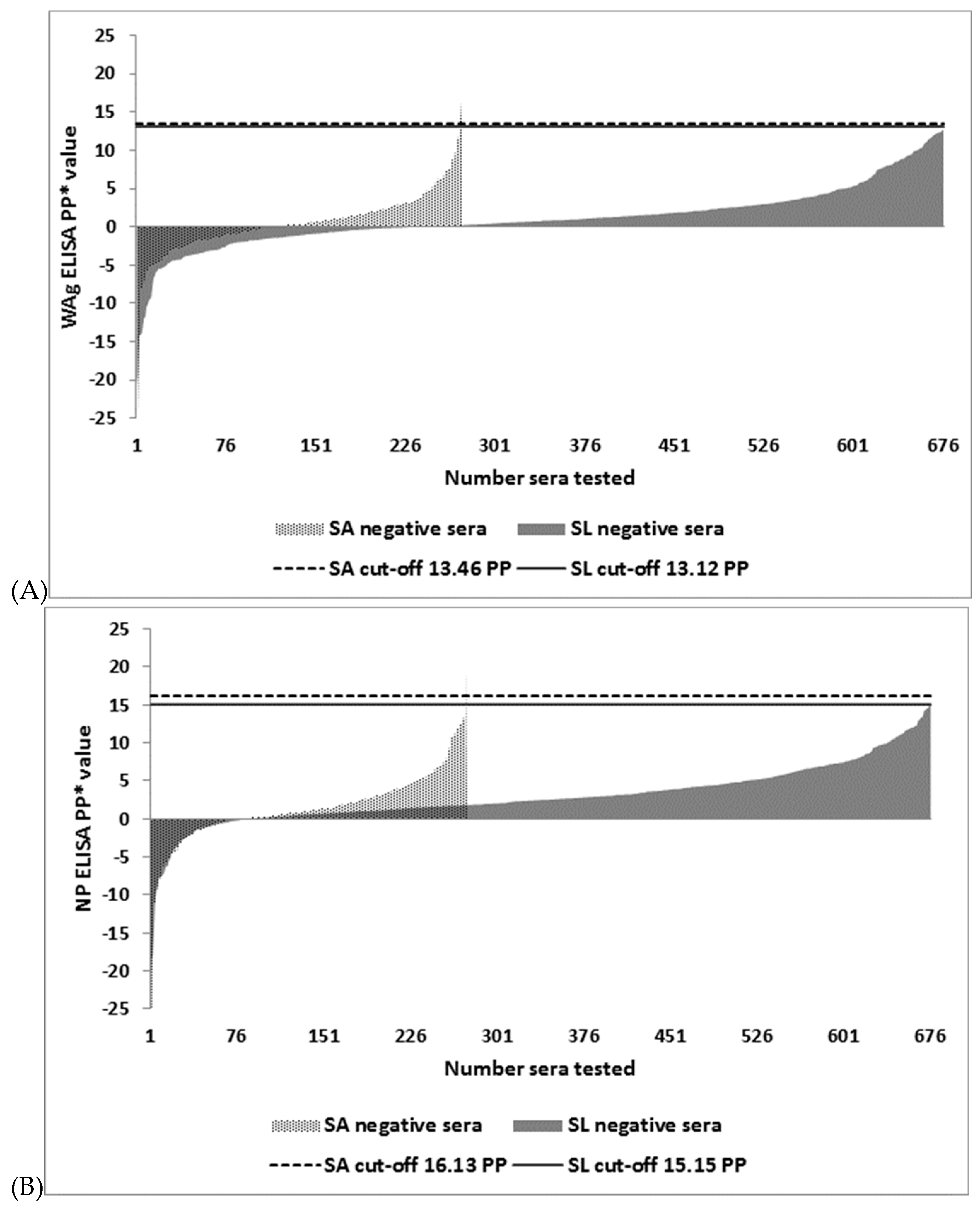
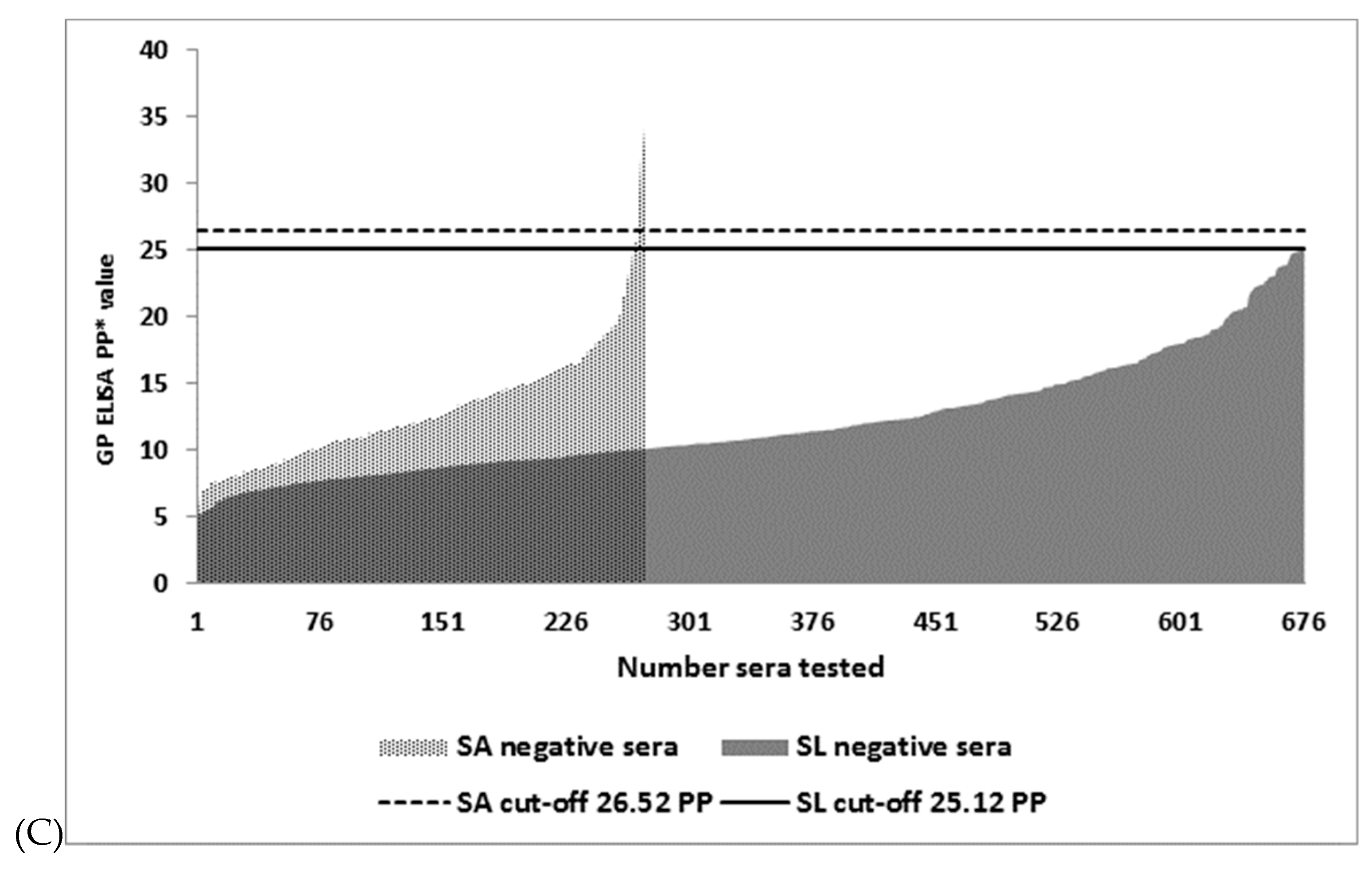
3.2. Distribution of I-ELISA PP Values in EBOV IgG Negative Sera and the Selection of Cut-off Values
3.3. Comparison of D-Sp and D-Se
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuhn, J.H.; Adachi, T.; Adhikari, N.K.J.; Arribas, J.R.; Bah, I.E.; Bausch, D.G.; Bhadelia, N.; Borchert, M.; Broch Brantsaeter, A.; Brett-Major, D.M.; et al. New filovirus disease classification and nomenclature. Nat. Rev. Microbiol. 2019, 17, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Muyembe-Tamfum, J.J.; Mulangu, S.; Masumu, J.; Kayembe, J.M.; Kemp, A.; Paweska, J.T. Ebola virus outbreaks in Africa: Past and present. Onderstepoort J. Vet. Res. 2012, 79, 451. [Google Scholar] [CrossRef] [PubMed]
- Baize, S.; Pannetier, D.; Oestereich, L.; Rieger, T.; Koivogui, L.; Magassouba, N.; Soropogui, B.; Sow, M.S.; Keita, S.; de Clerck, H.; et al. Emergence of Zaire Ebola virus disease in Guinea. N. Engl. J. Med. 2014, 371, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Briand, S.; Bertherat, E.; Cox, P.; Formenty, P.; Kieny, M.P.; Myhre, J.K.; Roth, C.; Shindo, N.; Dye, C. The international Ebola emergency. N. Engl. J. Med. 2014, 371, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N. Engl. J. Med. 2014, 371, 1481–1495. [Google Scholar] [CrossRef]
- World Health Organization—Ebola virus Disease—Democratic Republic of the Congo. Available online: https://www.who.int/csr/don/07-february-2019-ebola-drc/en/ (accessed on 11 February 2019).
- Médecins Sans Frontières. DRC Ebola outbreak crisis update—April 2019. Available online: https://www.msf.org/drc-2018-ebola-outbreak-crisis-update (accessed on 18 April 2018).
- Epstein, H. 11 313 unnecessary Ebola-related deaths: Building citizen trust in health systems. Brenthurst Foundation Special Report 1/2016. Sheaf Publishing: Benoni, South Africa. Available online: www.thebrenthurstfoundation.org/Brenthurst-special-report-201601.p (accessed on 20 May 2019).
- World Bank Group. The economic impact of the 2014 Ebola epidemic: Short- and medium-term estimates for West Africa. Available online: https://openknowlege.worldbank.org/handle/10986/20592. (accessed on 20 May 2019).
- Evans, D.K.; Goldstein, M.; Popova, A. Health-care worker mortality and the legacy of the Ebola epidemic. Lancet Glob. Health 2015, 3, e439–e440. [Google Scholar] [CrossRef]
- Gostin, L.O.; Fridman, A.A. A retrospective and prospective analysis of the West African Ebola virus disease epidemic: Robust national health systems at the foundation and an empowered WHO at the apex. Lancet 2015, 385, 1902–1909. [Google Scholar] [CrossRef]
- Shoman, H.; Karafillakis, E.; Rawaf, S. The link between the West African Ebola outbreak and health systems in Guinea, Liberia and Sierra Leone: A systematic review. Glob. Health 2017, 13, 1. [Google Scholar] [CrossRef]
- Vinck, P.; Pham, P.N.; Bindu, K.K.; Bedford, J.; Nilles, E. Institutional trust and misinformation in the response to the 2018-19 Ebola outbreak in North Kivu, DR Congo; a population-based survey. Lancet Infect. Dis. 2019, 19, 529–536. [Google Scholar] [CrossRef]
- Gostin, L.; Phelan, A.; Coutinho, A.G.; Eccleston-Turner, M.; Erondu, N.; Filani, O.; Inglesby, T.; Katz, R.; Maleche, A.; Nuzzo, B.; et al. Ebola in the Democratic Republic of the Congo; time to sound a global alert? Lancet Infect. Dis. 2019, 393, 617–620. [Google Scholar] [CrossRef]
- Jansen van Vuren, P.; Grobbelaar, A.; Storm, N.; Conteh, O.; Konneh, K.; Kamara, A.; Sanne, I.; Paweska, J.T. Comparative evaluation of the diagnostic performance of the prototype Cepheid GeneXpert Ebola assay. J. Clin. Microbiol. 2016, 54, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Raftery, P.; Condell, O.; Wasunna, C.; Kpaka, J.; Zwizwai, R.; Nuha, M.; Fallah, M.; Freemen, M.; Harris, V.; Miller, M.; et al. Establishing Ebola virus disease (EVD) diagnostics using GeneXpert technology at a mobile laboratory in Liberia: Impact on outbreak response, case management and laboratory systems strengthening. PLoS Negl. Trop. Dis. 2018, 12, e0006135. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.C.; Pettitt, J.; George, J.S.; Fakoli, L.S., III; Taweh, F.M.; Bateman, S.L.; Bennett, R.S.; Norris, S.L.; Spinnler, D.A.; Pimentel, G.; et al. Lateral flow immunoassays for Ebola virus disease detection in Liberia. J. Infect. Dis. 2016, 214, S222–S228. [Google Scholar] [CrossRef] [PubMed]
- Wonderly, B.; Jones, S.; Gatton, M.L.; Barber, J.; Killip, M.; Hudson, C.; Carter, L.; Sipson, A.J.H.; Urassa, W.; Chua, A.; et al. Comparative performance of four rapid Ebola antigen-detection lateral flow immunoassays during the 2014-2016 Ebola epidemic in West Africa. PLoS ONE 2019, 7, e0212113. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, A.; Reed, Z.D.; Rollin, P.E. Serologic cross-reactivity of human IgM and IgG antibodies to five species of Ebola virus. PLoS Negl. Trop. Dis. 2011, 5, e1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Wu, W.; Li, A.; Yang, X.; Zhang, S.; Li, C.; Su, Q.; Cai, S.; Sun, D.; et al. Serological investigation of laboratory-confirmed and suspected Ebola virus disease patients during the late phase of the Ebola outbreak in Sierra Leone. Virol. Sin. 2018, 33, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Ksiazek, T.G. Production of antigens for ELISA. In Ebolaviruses. Methods in Molecular Biology; Hoenen, T., Groseth, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1628, pp. 353–362. [Google Scholar]
- Niikura, M.; Ikegami, T.; Saijo, M.; Kurata, T.; Kurane, I.; Morikawa, S. Analysis of linear B-cell epitopes of the nucleoprotein of Ebola virus that distinguish Ebola virus subtypes. Clin. Diagn. Lab. Immunol. 2003, 10, 83–87. [Google Scholar] [CrossRef]
- Sanchez, A.; Kiley, M.P.; Holloway, B.P.; Auperin, D.D. Sequence analysis of the Ebola virus genome: Organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993, 29, 215–240. [Google Scholar] [CrossRef]
- Saijo, M.; Niikura, M.; Morikawa, S.; Ksiazek, T.G.; Meyer, R.F.; Peters, C.J.; Kurane, I. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J. Clin. Microbiol. 2001, 39, 1–7. [Google Scholar] [CrossRef]
- Natesan, M.; Jensen, S.M.; Keasey, S.L.; Kamata, T.; Kuehne, I.E.; Stonier, S.W.; Lutwama, J.J.; Lobel, L.; Dye, J.M.; Ulrich, R.G. Human survivors of disease outbreaks caused by Ebola or Marburg virus exhibit cross-reactive and long-lived antibody responses. Clin. Vaccine Immunol. 2016, 23, 717–724. [Google Scholar] [CrossRef]
- Nakayama, E.; Yokoyama, A.; Miyamoto, H.; Igarashi, M.; Kishida, N.; Matsuno, K.; Marzi, A.; Feldmann, H.; Ito, K.; Saijo, M.; et al. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 2010, 17, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Ebihara, H.; Jones, S.; Feldmann, H.; Kawaoka, Y. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine 2007, 25, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Prehaud, C.; Hellebrand, E.; Coudrier, D.; Volchkov, V.E.; Volchkova, V.A.; Feldmann, H.; Le Guenno, B.; Bouloy, M. Recombinant Ebola virus nucleoprotein and glycoprotein (Gabon 94 strain) provide new tools for the detection of human infections. J. Gen. Virol. 1998, 79, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; van den Hoogen, B.G.; Burghoorn-Maas, C.P.; Fooks, A.R.; Burton, J.; Clegg, C.J.; Zeller, H.; Osterhaus, A.D. Serological reactivity of baculovirus-expressed Ebola virus VP35 and nucleoproteins. Microbes Infect. 2003, 5, 379–385. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Yang, M.; Zhang, Z.; Song, D.; Yuan, Z. Nucleoprotein-based indirect enzyme-linked immunosorbent assay (indirect ELISA) for detecting antibodies specific to Ebola virus and Marburg virus. Virol. Sin. 2014, 29, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.; Shulenin, S.; Grolla, A.; Audet, J.; He, S.; Kobinger, G.; Unfer, R.C.; Warfield, K.L.; Aman, M.J.; Holtsberg, F.W. Quantitative serology assays for determination of antibody responses to Ebola virus glycoprotein and matrix protein in nonhuman primates and humans. Antiviral Res. 2016, 126, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Jarvis, D.L.; Kawar, Z.S.; Hollister, J.R. Engineering N-glycosylation pathways in the baculovirus-insect cell system. Curr. Opin. Biotechnol. 1998, 9, 528–533. [Google Scholar] [CrossRef]
- Glynn, J.R.; Bower, H.; Johnson, S.; Houlihan, C.F.; Montesano, C.; Scott, J.T.; Semple, M.G.; Bangura, M.S.; Kamara, A.J.; Kamara, O.; et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: A cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect. Dis. 2017, 17, 645–653. [Google Scholar] [CrossRef]
- Shurtleff, A.C.; Biggins, J.E.; Keeney, A.E.; Zumbrun, E.E.; Bloomfield, H.A.; Kuehne, A.; Audet, J.L.; Alfson, K.J.; Griffiths, A.; Olinger, G.G.; et al. Standardization of the filovirus plaque assay for use in preclinical studies. Viruses 2012, 4, 3511–3530. [Google Scholar] [CrossRef]
- Paweska, J.T.; Jansen van Vuren, P.; Meier, G.H.; Le Roux, C.; Conteh, O.S.; Kemp, A.; Fourie, C.; Naidoo, P.; Naicker, S.; Ohaebosim, P.; et al. South African Ebola diagnostic response in Sierra Leone: A modular high biosafety field laboratory. PLoS Negl. Trop. Dis. 2017, 11, e0005665. [Google Scholar] [CrossRef] [PubMed]
- Panning, M.; Laue, T.; Olschlager, S.; Eickmann, M.; Becker, S.; Raith, S.; Georges Courbot, M.C.; Nilsson, M.; Gopal, R.; Lundkvist, A.; et al. Diagnostic reverse-transcription polymerase chain reaction kit for filoviruses based on the strain collections of all European biosafety level 4 laboratories. J. Infect. Dis. 2007, 196, S199–S204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ebola hemorrhagic fever—South Africa. Wkly. Epidemiol. Rec. 1996, 71, 359. [Google Scholar]
- Richards, G.A.; Murphy, S.; Jobson, R.; Mer, M.; Zinman, C.; Taylor, R.; Swanepoel, R.; Duse, A.; Sharp, G.; de la Rey, I.C.J.; et al. Unexpected Ebola virus in a tertiary setting: Clinical and epidemiological aspects. Crit. Care Med. 2000, 28, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; West, C.P.; Rollin, P.E.; Jahrling, P.B.; Peters, C.J. ELISA for the detection of antibodies to Ebola viruses. J. Infect. Dis. 1999, 179, S192–S198. [Google Scholar] [CrossRef]
- Paweska, J.T.; Burt, F.J.; Swanepoel, R. Validation of IgG-sandwich and IgM-capture ELISA for the detection of antibody to Rift Valley fever virus in humans. J. Virol. Methods 2005, 124, 173–181. [Google Scholar] [CrossRef]
- Crowther, R. Elisa Theory and Practice, in Methods in Molecular Biology; Crowther, R., Ed.; Humana Press Inc.: Tatowa, NJ, USA, 1995. [Google Scholar]
- Greiner, M. Two-graph receiver operating characteristics (TG-ROC)—A Microsoft-Excel template for the selection of cut-off values in diagnostic tests. J. Immunol. Methods 1995, 185, 123–132. [Google Scholar] [CrossRef]
- Greiner, M. Two-graph receiver operating characteristics (TG-ROC)—A Microsoft-Excel template for the selection of cut-off values that minimize overall misclassification costs. J. Immunol. Methods 1996, 191, 93–94. [Google Scholar] [CrossRef]
- Greiner, M.; Gardner, I.A. Application of diagnostic tests in veterinary epidemiological studies. Prev. Vet. Med. 2000, 45, 43–59. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Mitchell, S.W.; McCormick, J.B. Physicochemical inactivation of Lassa, Ebola and Marburg viruses and effect on clinical laboratory analyses. J. Clin. Microbiol. 1984, 20, 486–489. [Google Scholar] [PubMed]
- Haddock, E.; Feldmann, F.; Feldmann, H. Effective chemical inactivation of Ebola virus. Emerg. Infect. Dis. 2016, 22, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.H. Validation of serological assays for diagnosis infectious diseases. Rev. Sci. Tech. Off. Int. Epiz. 1998, 17, 469–486. [Google Scholar] [CrossRef]
- Jacobson, R.H. Principles of validation of diagnostic assays for infectious diseases. In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th ed.; Cambridge University Press: Cambridge, UK, 2004; Volume 1, pp. 21–29. [Google Scholar]
- Vizard, A.L.; Anderson, G.A.; Gasser, R.B. Determination of the optimum cut-off value of a diagnostic test. Prev. Vet. Med. 1990, 10, 137–143. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Rollin, P.E.; Williams, A.J.; Bressler, D.S.; Martin, M.L.; Swanepoel, R.; Burt, F.J.; Leman, P.A.; Khan, A.S.; Rowe, A.K.; et al. Clinical virology of Ebola hemorrhagic fever (EHF): Virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kiwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999, 179, S177–S187. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Nilsson, E.; van Rooij, E.M.A.; Lelenta, M.; Jeggo, M.H. Standarization and validation of enzyme-linked immunosorbent assay techniques for the detection of antibody in infectious disease diagnosis. Rev. Sci. Tech. Off. Int. Epiz. 1993, 12, 435–450. [Google Scholar] [CrossRef]
- Krähling, V.; Becker, D.; Rohde, C.; Eickmann, M.; Eroglu, Y.; Herwig, A.; Kerber, R.; Kowalski, K.; Vergara-Alert, J.; Becker, S.; et al. Development of an antibody capture ELISA using inactivated Ebola Zaire Makona virus. Med. Microbiol. Immunol. 2016, 205, 173–183. [Google Scholar] [CrossRef]
- Sobarzo, A.; Perelman, E.; Groseth, A.; Dolnik, O.; Becker, S.; Lutwama, J.J.; Dye, J.M.; Yavelsky, V.; Lobel, L.; Marks, R.S. Profiling the native specific human humoral immune response to Sudan ebolavirus strain Gulu by chemiluminescence enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 2012, 19, 1844–1852. [Google Scholar] [CrossRef]
- Lopez, L.; Venteo, A.; Garcia, M.; Camuñas, A.; Ranz, A.; Garcia, J.; Sarraseca, J.; Anaya, C.; Rueda, P. Antigen-capture blocking enzyme-linked immunosorbent assay based on a baculovirus recombinant antigen to differentiate transmissable gastroenteritis virus from porcine respiratory coronavirus antibodies. J. Vet. Diagn. Investig. 2009, 21, 598–608. [Google Scholar] [CrossRef]
- Rowe, A.K.; Bertolli, J.; Khan, A.S.; Mukunu, R.; Muyembe-Tamfum, J.J.; Bressler, D.; Williams, A.J.; Peters, C.J.; Rodriguez, L.; Feldmann, H.; et al. Clinical, virologic and immunologic follow-up of convalescent Ebola hemorrhagic fever patientsand their household contacts, Kikwit, Democratic Republic of the Congo. J. Infect. Dis. 1999, 179, S28–S35. [Google Scholar] [CrossRef]
- Wauquier, N.; Becquart, P.; Gasquet, C.; Leroy, E.M. Immunoglobulin G in Ebola outbreak survivors, Gabon. Emerg. Infect. Dis. 2009, 15, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Rudge, T.L., Jr.; Sankovich, K.A.; Niemuth, N.A.; Anderson, M.S.; Badorrek, C.S.; Skomrock, N.D.; Cirimotich, C.M.; Carol, L.; Sabourin, C.L. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS ONE 2019, 14, e0215457. [Google Scholar] [CrossRef] [PubMed]
| C++ 1 | OD 2 IQC 3 | Mean OD ± SD 4 | Mean CV 5 ± SD (%) | |||
|---|---|---|---|---|---|---|
| LCL 6 | UCL 7 | Intra-Plate Variation | Inter-Plate Variation | Intra-Plate Variation | Inter-Plate Variation | |
| WAg | 0.81 | 1.29 | 1.00 ± 0.09 | 1.00 ± 0.08 | 4.26 ± 2.67 | 4.41 ± 1.85 |
| NP | 1.14 | 1.79 | 1.45 ± 0.18 | 1.50 ± 0.10 | 3.54 ± 1.99 | 3.11 ± 1.05 |
| GP | 0.9 | 1.39 | 1.19 ± 0.09 | 1.2 ± 0.04 | 4.76 ± 2.16 | 4.8 ± 1.56 |
| Test | Cut-off PP 1 | SA Negative Serum Panel (n = 273) | SL Negative Serum Panel (n = 676) | ||
|---|---|---|---|---|---|
| FP 2/TN 3 | DSp 4 (95% CI 5) | FP/TN | DSp (95% CI) | ||
| WAg I-ELISA | 13.46 6 | 2/271 | 99.3 (97.4–99.9) | 0/676 | 100 (99.5–100) |
| WAg I-ELISA | 13.12 7 | 2/271 | 99.3 (97.4–99.9) | 0/676 | 100 (99.5–100) |
| WAg I-ELISA | 13.53 8 | 2/271 | 99.3 (97.4–99.9) | 0/676 | 100 (99.5–100) |
| NP I-ELISA | 16.13 6 | 1/272 | 99.6 (98–100) | 0/676 | 100 (99.5–100) |
| NP I-ELISA | 15.15 7 | 1/272 | 99.6 (98–100) | 2/674 | 99.7 (98.9–100) |
| NP I-ELISA | 16.44 8 | 1/272 | 99.6 (98–100) | 0/676 | 100 (99.5–100) |
| GP I-ELISA | 26.52 6 | 5/268 | 98.2 (95.8–99.4) | 0/676 | 100 (99.5–100) |
| GP I-ELISA | 25.12 7 | 7/268 | 97.4 (94.8–99) | 2/674 | 99.7 (98.9–100) |
| GP I-ELISA | 26.28 8 | 5/268 | 98.2 (95.8–99.4) | 0/676 | 100 (995–100) |
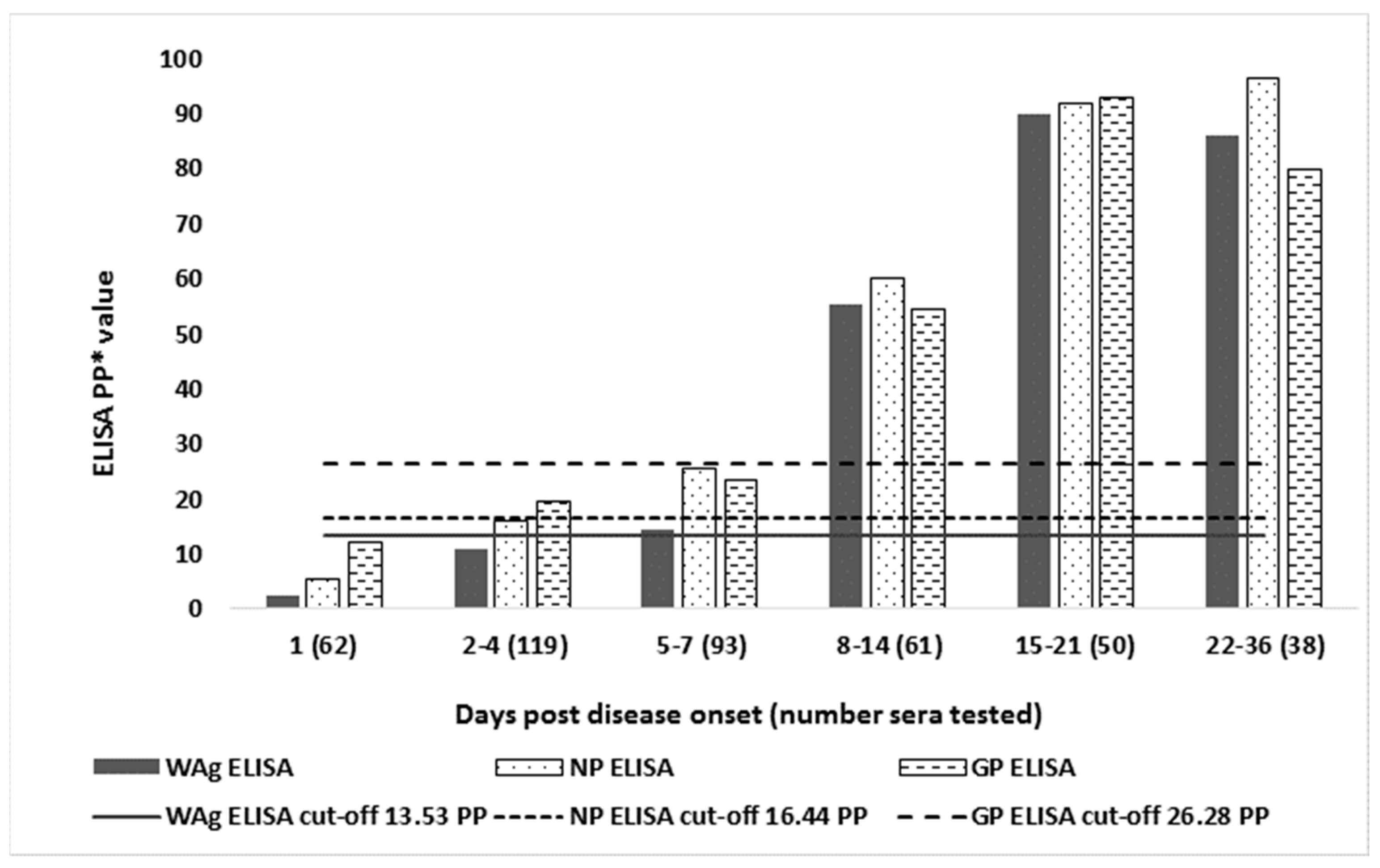
| Days Post Disease Onset (Number Individuals Tested) | WAg No.pos. 1 (% pos.) | NP No. pos.(% pos.) | GP No. pos.(% pos.) | Only WAg pos. | Wag and NP pos. | Wag and GP pos. | Only NP pos. | NP and GP pos. | Only GP pos. |
|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 62) | 3 2 (4.8) | 4 (6.5) | 1 (1.6) | 0 | 3 | 0 | 0 | 1 | 0 |
| 3 3 (4.8) | 5 (8.1) | 2 (3.2) | 0 | 3 | 0 | 1 | 1 | 1 | |
| 3 4 (4.8) | 4 (6.5) | 1 (1.6) | 0 | 3 | 0 | 0 | 1 | 0 | |
| 2–4 (n = 119) | 20 (16.8) | 27 (22.7) | 13 (11.0) | 1 | 6 | 0 | 8 | 0 | 0 |
| 20 (16.8) | 28(23.5) | 13 (11.0) | 1 | 6 | 0 | 9 | 0 | 0 | |
| 20 (16.8) | 27 (22.7) | 13 (11.0) | 1 | 6 | 0 | 8 | 0 | 0 | |
| 5–7 (n = 93) | 27 (29.0) | 34 (36.6) | 24 (25.8) | 1 | 7 | 0 | 6 | 2 | 3 |
| 27 (29.0) | 34 (36.6) | 26 28.0) | 1 | 7 | 0 | 5 | 3 | 4 | |
| 27 (29.0) | 34 (36.6) | 24 (25.8) | 1 | 7 | 0 | 6 | 2 | 3 | |
| 8–14 (n = 61) | 42 (68.9) | 49 (80.3) | 37 (60.7) | 1 | 8 | 0 | 5 | 4 | 0 |
| 43 (70.5) | 50 (82.0) | 39 (64.0) | 1 | 9 | 0 | 3 | 6 | 0 | |
| 42 (68.9) | 49 (80.3) | 37 (60.7) | 1 | 8 | 0 | 5 | 4 | 0 | |
| 15–21 (n = 50) | 50 (100) | 50 (100) | 49 (98.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 (100) | 50 (100) | 49 (98.0) | 0 | 0 | 0 | 0 | 0 | 0 | |
| 50 (100) | 50(100) | 49 (98.0) | 0 | 0 | 0 | 0 | 0 | 0 | |
| 22–36 (n = 38) | 38 (100) | 38 (100) | 37 (97.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| 38 (100) | 38(100) | 38 (100) | 0 | 0 | 0 | 0 | 0 | 0 | |
| 38 (100) | 38 (100) | 37 (97.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Test | Cut-off PP 1 | SL EBOV RT-PCR Positive Cases Days 8–36 Post Onset (n = 149) | SL EBOV RT-PCR Positive Cases Days 15–36 Post Onset (n = 88) | ||
|---|---|---|---|---|---|
| FN 2/TP 3 | DSe 4 (95% CI 5) | FN/TP | DSe (95% CI) | ||
| WAg I-ELISA | 13.46 6 | 19/130 | 87.2 (80.8–92.1) | 0/88 | 100 (95.9–100) |
| WAg I-ELISA | 13.12 7 | 18/131 | 87.9 (81.6–92.7) | 0/88 | 100 (95.5–100) |
| WAg I-ELISA | 13.53 8 | 19/130 | 87.2 (80.8–92.1) | 0/88 | 100 (95.5–100) |
| NP I-ELISA | 16.13 6 | 12/137 | 91.9 (86.4–95.8) | 0/88 | 100 (95.5–100) |
| NP I-ELISA | 15.15 7 | 11/138 | 92.6 (87.2–96.3) | 0/88 | 100 (95.9–100) |
| NP I-ELISA | 16.44 8 | 12/137 | 91.9 (86.4–95.8) | 0/88 | 100 (95.5–100) |
| GP I-ELISA | 26.52 6 | 26/123 | 82.6 (75.5–88.3) | 2/86 | 97.7 (92–99.7) |
| GP I-ELISA | 25.12 7 | 23/126 | 84.6 (77.7–90) | 1/87 | 98.9 (93.8–100) |
| GP I-ELISA | 26.28 8 | 26/123 | 82.6 (75.5–88.3) | 2/86 | 97.7 (92–99.7) |
| Assay (Cut-off PP Value) 1 | Mean log10 Serum Titre 2/Dose Response Curve R Square 3 | |||
|---|---|---|---|---|
| Untreated | 60° 1 h | 0.5%Tween 20 15 min 60° | 0.5%Triton X-100 15 min 60° | |
| WAg I-ELISA (13.53) | 3.5/0.945 | 3.5/0.950 | 3.5/0.942 | 3.5/0.952 |
| NP I-ELISA (16.44 PP) | 3.8/0.959 | 3.5/0.961 | 3.5/0.956 | 3.5/0.957 |
| GP I-ELISA (26.28) | 3.2/0.932 | 3.2/0.942 | 3.2/0.924 | 3.2/0.928 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paweska, J.T.; Moolla, N.; Storm, N.; Msimang, V.; Conteh, O.; Weyer, J.; Vuren, P.J.v. Evaluation of Diagnostic Performance of Three Indirect Enzyme-Linked Immunosorbent Assays for the Detection of IgG Antibodies to Ebola Virus in Human Sera. Viruses 2019, 11, 678. https://doi.org/10.3390/v11080678
Paweska JT, Moolla N, Storm N, Msimang V, Conteh O, Weyer J, Vuren PJv. Evaluation of Diagnostic Performance of Three Indirect Enzyme-Linked Immunosorbent Assays for the Detection of IgG Antibodies to Ebola Virus in Human Sera. Viruses. 2019; 11(8):678. https://doi.org/10.3390/v11080678
Chicago/Turabian StylePaweska, Janusz T., Naazneen Moolla, Nadia Storm, Veerle Msimang, Ousman Conteh, Jacqueline Weyer, and Petrus Jansen van Vuren. 2019. "Evaluation of Diagnostic Performance of Three Indirect Enzyme-Linked Immunosorbent Assays for the Detection of IgG Antibodies to Ebola Virus in Human Sera" Viruses 11, no. 8: 678. https://doi.org/10.3390/v11080678
APA StylePaweska, J. T., Moolla, N., Storm, N., Msimang, V., Conteh, O., Weyer, J., & Vuren, P. J. v. (2019). Evaluation of Diagnostic Performance of Three Indirect Enzyme-Linked Immunosorbent Assays for the Detection of IgG Antibodies to Ebola Virus in Human Sera. Viruses, 11(8), 678. https://doi.org/10.3390/v11080678






