Abstract
Interspecific hybridization in forest trees is common and can have important implications for ecology, evolution, and the conservation of forest habitats. Hybridization often results in greater genetic diversity and opportunities for backcrossing with one or both parents, which may introduce novel genotypes that influence biodiversity and ecosystem processes. However, the extent of hybridization, direction of backcrossing, and overall survival and performance of hybrids is often poorly understood, leading to inaccurate assessments of the role hybrids may play in forest ecology and conservation. Here, we investigate interspecific hybridization and the extent and direction of backcrossing between two species, Populus fremontii (S. Watson) and P. angustifolia (E. James ex Torr.), which are broadly distributed along riparian corridors in the riparian ecosystems of the southwestern United States. Using molecular assays of six putative hybrid zones and a common garden trial we test the following: (1) whether putative hybrids show evidence of genetic intermediacy relative to the parent species; (2) if confirmed hybrids exhibit higher genetic diversity than either parent species; (3) the extent and direction of backcrossing (uni- or bi-directional) within each site; and (4) whether hybrid derivatives show evidence of higher survival and performance in an experimental common garden consisting of both parents and hybrids that were propagated from the six sites. Our results confirm genetic intermediacy in all six sites, but with varying degrees of backcrossing, genetic diversity, and structure. All six locations reveal extensive bidirectional backcrossing to both parent species, a result that contrasts with previous findings, which suggest that backcrossing is predominantly unidirectional between the two species. Results from our common garden trial indicate that hybrids do not have higher survival or out-perform the parent species, suggesting that heterosis may be limited in this system, or that long-term assessments beyond the duration of our field experiment may be required. Results from this study improve our understanding of the frequency of hybridization, and the associated backcrossing in this system, and provide land managers with information on how hybrids may be employed for the long-term preservation of riparian habitats undergoing rapid environmental change.
1. Introduction
Hybridization has important implications for the ecology and evolution of many forest trees [,,]. In Populus L., investigations of hybridization have revealed unexpected patterns of reproductive isolation in European species [,], suggesting that post-zygotic selection, assortative mating, or selection against incompatible genotypes may serve to strengthen reproductive barriers between species, despite ongoing gene flow. A study of hybridization between two Populus species, Fremont (P. fremontii S. Watson) and narrowleaf (P. angustifolia E. James ex Torr.) cottonwood, in North America has also documented the movement of genes within and across hybrid zones between these two morphologically and genetically differentiated species, suggesting the formation of previously established reproductive barriers that are compromised in regions of overlap []. In this case, hybridization has been shown to affect ecological interactions that promote biodiversity and influence community structure [,,,], with important implications for the evolutionary trajectories of dependent communities [].
One consequence of hybridization in Populus and other forest trees is that F1 hybrids may persist as interfertile derivatives, or potentially backcross to one or both parental species []. If the F1 hybrid generation is both fertile and compatible, they may be self-sustaining in environments that neither parent species occupies, potentially leading to speciation and niche diversification []. Occupying novel environments is often facilitated by the fact that F1 hybrids combine the genomes of both parent species, and may exhibit traits that are either intermediate or novel with respect to the parents [,,]. Such novelty may produce hybrid genotypes that are more fit than either parent, depending on the environment they occupy, a phenomenon known as heterosis or “hybrid vigor” [,,,,]. For example, in spruce, a hybrid zone formed between Picea glauca (Moench) Voss and P. englemanii (Parry ex Engelm), F1 hybrids appear to exhibit hybrid vigor in an intermediate habitat relative to the parent species []. In Populus, F1 hybrids between P. fremontii and P. angustifolia yielded viable seed production rates that were similar to one of the parents (P. angustifolia), suggesting comparable fertility, but they also produced up to two- and four-times higher rate of clonal individuals than either parent species, respectively, suggesting significant vegetative vigor [].
In addition to exhibiting hybrid vigor, F1 derivatives may also backcross to one or both parent species, depending on mating compatibility and the extent to which viable F1 hybrids are produced. Backcrossing is well-documented in several forest trees [], including Populus [,,]. Backcrossing can have consequences for both hybrids and parental populations, potentially leading to the exchange of either deleterious or beneficial alleles, with the latter potentially facilitating adaptation to novel environments [,,,]. Repeated backcrossing to one or both parental species can also lead to a transfer of genetic information between species, a process known as introgression, which can be either unidirectional (toward one parent species) or bidirectional (toward both parent species). Introgression may also play an important role in the ecology and evolution of forest trees because backcrossed individuals may perform better in some environments due to the varying genetic contributions imparted by one parental source relative to the other [,]. Introgression has been documented in Populus, demonstrating widespread gene flow from P. fremontii into the genome of P. angustifolia []. Hybridization that involves backcrossing can also lead to adaptive introgression, creating novel genotypes that may rapidly respond to environmental change []. In the case of P. fremontii and P. angustifolia, introgression of P. fremontii alleles may promote adaptation to hotter, drier environments, as it is well-adapted to extremely hot, low elevation environments, as compared to P. angustifolia. Adaptive introgression between P. fremontii and P. angustifolia has been recently documented in a 30-year-old common garden trial, showing increased survival of P. angustifolia backcrosses that possess introgressed P. fremontii alleles across an elevational and environmental gradient [].
Finally, hybridization leading to adaptive introgression has been shown to enhance survival across environmental gradients by increasing genetic variation, as has been shown in Pinus [] and in Populus, where range expansion has been documented []. Together, these studies suggest that hybridization may be important for the conservation and management of forest trees where adaptive alleles may be introduced into threatened populations (e.g., through adaptive introgression) or where hybrid populations exhibit hybrid vigor [].
Previous studies have also shown that hybridization can have important consequences for associated communities. In Populus, for example, increased genetic variation due to hybridization has been shown to promote biodiversity and associated community structure [,,,], suggesting that it can play an important role in the evolutionary trajectory of these communities []. Such plant–herbivore interactions have also been investigated in the context of trait-mediated indirect genetic effects that drive community and ecosystem diversity [].
Although hybridization has been investigated extensively in many plant species, it is still unclear how widespread the phenomenon is within the ranges of the respective parent species, and whether it primarily produces F1 hybrid generations that persist solely as F1s (e.g, either by interbreeding or cloning), or whether backcrossing occurs with one or both parent species. This issue is particularly important in the context of ongoing climate change, where backcrossed individuals may be better suited to rapid environmental change. The issue is especially relevant in Populus where backcrossing between P. fremontii and P. angustifolia was initially determined to be primarily unidirectional toward the latter species []. Subsequent studies involving hybridization between P. deltoides, (Bartram ex Marshall) and P. angustifolia, however, revealed that bidirectional backcrossing is prevalent []. Such differences in hybrid zone dynamics, in different river systems and between different species, may have important implications for the ecology and evolution of hybridizing species, their derivatives, and their dependent communities [,,,,,].
To document the extent of hybridization and the incidence of backcrossing in Populus, we examined two species, Populus fremontii and P. angustifolia, which are widespread throughout riparian corridors in the southwestern USA [,]. Populations of these species frequently hybridize wherever their ranges overlap [], making them ideal for studying the effects of hybridization on genetic variation, population structure, and survival in environments currently experiencing rapid climate change [,]. Hybridization between these two species is typically homoploid, with documented unidirectional backcrossing to P. angustifolia [,], along with one example of bidirectional backcrossing between P. deltoides and P. angustifolia []. However, the extent and direction of backcrossing has yet to be examined across multiple hybrid zones. Furthermore, no studies have examined the degree to which hybrids may exhibit hybrid vigor in the context of an experimental common garden that includes both parents and their hybrids.
To investigate hybridization, backcrossing, and the potential for hybrid vigor, we conducted genetic investigations of several putative hybrid zones and their associated genetic structure across six different river systems. To investigate hybrid vigor, we assessed survival and performance in two growth traits, height and diameter of root crown (DRC), in a common garden setting containing parents and their hybrids propagated from the same six river system sites. Our specific objectives and associated hypotheses were as follows: (1) Profile patterns of hybridization and genetic structure across each of the six river systems, including the extent and direction of backcrossing to one (unidirectional) or both parent species (bidirectional). We expected that most of the sites would show evidence of unidirectional or bidirectional backcrossing, or at least show variation as revealed by molecular assessment of each putative hybrid zone; (2) Using classifications derived from our first objective, we then investigated the patterns of genetic diversity and structure of hybrids (F1s and backcrosses) relative to the parent species. Here, we expected genetic structuring consistent with our classification of hybrid and parental cross types with F1 hybrids showing genetic intermediacy, relative to the two parent species, and backcross hybrids exhibiting greater genetic similarity to one or the other parent (depending on the direction of backcrossing); and (3) Finally, we examined the potential for hybrid vigor (heterosis) on survival and performance in F1 or backcrossed individuals using a common garden trial. If hybridization promotes heterosis, we expected higher survival and performance in hybrids relative to the two parent species. Our over-arching goal was to examine hybridization to better understand its potential for facilitating adaptation to environmental change, promote biodiversity, and inform the conservation of riparian ecosystems in the southwestern USA.
2. Materials and Methods
2.1. Study Sites
Hybridization between Populus fremontii and P. angustifolia occurs in several river corridors in the southwest U.S., where their ranges intersect. We investigated six river systems dispersed across central Arizona and southern Utah (Table 1, Figure 1). Although we relied primarily on morphology to initially identify parental species and their hybrids, molecular assays using microsatellite markers were employed to confirm whether hybrids were F1s, based on genetic intermediacy, or backcrossed individuals whose genetic markers would tend to cluster with one or both parent species, which we consider to be ancestral backcrosses derived from several generations of mating to one or the other parent species. Because F1 hybrids can backcross with either species, resulting in a range of backcross genotypes [,,], we heretofore refer to the two species and their hybrids collectively as “cross types”.

Table 1.
Sampling location, collection dates, and GPS coordinates from which samples and cuttings for propagation were taken.
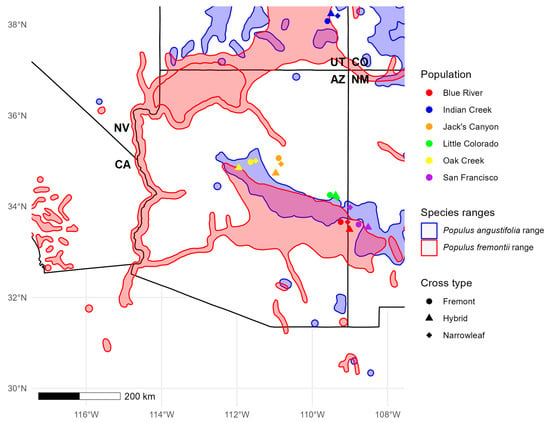
Figure 1.
Sampling locations of trees for each population and cross type sampled in Arizona (AZ) and Utah (UT). Red and blue outlines indicate the ranges of Fremont and narrowleaf cottonwood, respectively (modeled after Little, 1976).
2.2. Sampling
Leaves from 180 trees were gathered for genotyping from six sites in central Arizona and southern Utah, which we believed to contain hybrid zones between P. angustifolia and P. fremontii. Mature leaves were collected from lower branches that were still exposed to mid-day sunlight, closer to the terminal buds. Ten leaves were gathered from each tree, then dried and stored for up to 3 months using Dri-Rite© in a temperature regulated, shaded, and dry indoor environment until DNA extraction. The sites included the San Francisco River (SF), Little Colorado River (LC), Blue River (BR), Indian Creek (IC), Oak Creek (OC), and Jack’s Canyon River (JC) river systems. Each site consisted of all three cross types (P. fremontii, P. angustifolia, and hybrids) (Figure 1, Table 1). All trees were putatively identified as P. fremontii, P. angustifolia, or hybrids (either F1s or backcrosses) based on leaf morphology. The parent species show clear divergent morphologies in leaf shape with Fremont and narrowleaf cottonwood, exhibiting heart-shaped and narrow-shaped leaf structures, respectively. First generation hybrid genotypes are expected to exhibit an intermediate leaf morphology, but, because of backcrossing, leaves can appear morphologically like one or the other parent, depending on the direction of backcrossing []. Floate et al. [] argue that molecular markers in combination with morphology can assist in the identification of parent species and their hybrids. To this end, we initially sampled trees and assigned a cross type to each sample, sampling randomly until 10 trees from each putative cross type were sampled. “Pure” Fremont and narrowleaf were designated based on their divergent morphologies, while F1 hybrids and backcrosses were simply classified as “hybrids”, with the caveat that molecular genotyping and analysis would refine these classifications into F1 or backcrossed individuals (Figure 2).
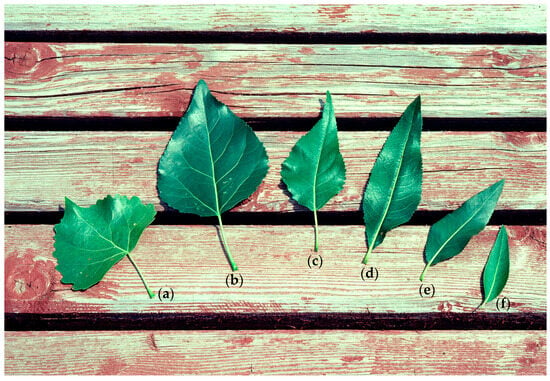
Figure 2.
Leaf morphologies in Fremont, narrowleaf, and hybrid individuals. Leaves are labeled as: (a) Fremont; (b) F1 hybrid; (c–e) complex backcrosses of F1 hybrids to narrowleaf; (f) narrowleaf leaves. Fremont leaves are cordate in shape with crenate margins. Narrowleaf leaves are lanceolate with fine-toothed margins. Hybrid individuals have an intermediate morphology dependent on relative ancestry to each parent. Photograph courtesy of Tom Whitham.
2.3. DNA Extraction and Microsatellite Genotyping
Genomic DNA from desiccated leaves was extracted using a modified high molecular weight protocol from Mayjonade et al. []. Twelve initial microsatellite primer combinations (Table 2) from Bothwell et al. [] were selected and amplified from genomic DNA using polymerase chain reaction (PCR). Each PCR was multiplexed to produce three non-overlapping amplicons per reaction, and reverse-direction microsatellite primers were fluorescently labeled with non-redundant fluorescent dyes. Each 10 μL reaction contained 10 ng genomic template DNA, 5 μL 1× Dreamtaq Taq DNA polymerase, 0.3 μL 2.8 mM MgCl2, and forward and reverse microsatellite primers at 5 mM. Reactions were denatured for 30 s, annealed for 60 s, and extended for 60 s 45 times, with a final extension step of 10 min.

Table 2.
Microsatellite primer names, repeat motifs, sequences, and fragment lengths as described in []. HWE values for these loci can be found in Table S1.
Each multiplexed amplicon product was diluted in 10 mM Tris pH 8.0 to a 1:20 ratio of product:buffer before fragment analysis. A total of 3 μL of GENESCAN-600 LIZ internal size standard (Applied Biosystems, Foster City, CA USA) was mixed with 1 mL of formamide HiDi, and 10 μL of the mixture was mixed with 1 μL amplicon product and used in fragment analysis. Multiplexed amplicons were analyzed in triplicate to verify consistent product sizes across samples. PCR products were verified with gel electrophoresis and then resolved using fragment analysis on an ABI 3730xl genetic analyzer (Applied Biosystems, Foster City, CA, USA).
Fragment analysis results were manually scored using GeneMapper v. 4.0 software []. Two loci (GCPM_2992 and GCPM_3592) showed inconsistent profiles across repeated samples and were omitted from the final analysis. The resulting 10 loci were used in subsequent analyses for individual genotyping (Table 2).
2.4. Parental and Hybrid Classification
We used Structure v.2.2 [,,] to determine a priori the most likely number of groups (K) for all samples. Structure uses allele frequencies at each locus to assign individuals to a given number of K groups and returns the percent ancestry of an individual to each group (Q-score). Structure runs were conducted for K 1–6 using admixed ancestry models assuming independent allele frequencies, with burn-in lengths of 1000 and Markov Chain Monte Carlo chain lengths of 10,000. To account for stochastic differences between different Structure simulations, runs for each K were repeated 3 times and the results of each run permuted to a single set of Q-scores using CLUMPP []. A best K value of 2 was selected based on the best Evanno’s delta-K (Table S2). Percent ancestry values to each group were plotted using ggplot2 [] in R version 2025.09.0-387 [] implemented in R Studio []. Individuals were re-assigned to five cross type classifications using ancestry values and associated Q-scores as follows: <0.1 for pure Fremont ancestry; 0.1–0.4 for backcrosses to Fremont; 0.4–0.6 for F1 hybrids; 0.6–0.9 for backcrosses to narrowleaf; and >0.9 for pure narrowleaf ancestry.
Hybrid classification and relative ancestry was also determined using hybrid index values [] and used to determine hybrid class and relative ancestry to each parent. Hybrid index is estimated by calculating allele frequencies for each locus in the reference (i.e., parent) species and using these to generate a maximum-likelihood confidence interval for a hybrid individual’s genotype from 0 to 1. For codominant markers like microsatellites, allele frequencies for the parent species can be calculated directly from parent genotype data. Hybrid index values were calculated using the package introgress in R version 1.2.3 []. These values were graphed using ggplot2. Similarly to reclassification using Structure Q-score, hybrid individuals were re-assigned to five cross type classifications using the same classification scheme as above. Using these hybrid classification scores, we also assessed observed heterozygosity as a function of percent ancestry using a polynomial regression analysis implemented in ggplot2.
2.5. Genetic Diversity Statistics and Analyses
Based on our categorical results obtained from Structure analysis (parents, hybrids, and backcrosses), we calculated standard genetic diversity and structure metrics including percent polymorphic loci (%P), average number of alleles per locus (NA), effective number of alleles per locus (NE), expected and observed heterozygosity (HE and HO), and FIS and FST, all of which were calculated per site and per cross type using GenAlEx v. 6.52 []. We also calculated pairwise FST values between sites using Analysis of Molecular Variance (AMOVA), as well as observed and expected heterozygosity (HE, HO) across river systems. We then calculated distance matrices using Nei’s genetic distance for all cross types and all the six sites, as well as for all cross types within each site. Nei’s genetic distance was then used to visualize genetic clustering using principal coordinate analysis (PCoA).
2.6. Common Garden and Growth Measurements
Cuttings of 180 trees from all six sites were propagated and grown in a greenhouse for one year in conical pots containing garden potting soil and rooting hormone applied to the cutting. They were then planted as a common garden trial at the Walnut Creek Education & Research Center at Walnut Creek, Arizona (Lat/Long: 34.9234956 N; −112.8432663 W) in 2020. Approximately 7 replicate cuttings of each tree were planted in February 2020, with genet and individual randomized across plots within the garden. Growth was measured in height and diameter of the root crown (DRC) in April–May of 2023 and December 2023–February 2024 to represent first- and second-year growth, respectively. The diameter of the root crown was measured three to ten times and averaged for each year of measurement. Trees were watered biweekly and allowed to grow for two years, prior to taking measurements.
2.7. Growth Measurement Comparisons to Hybrid Class, Relative Ancestry, and Source River
Growth metrics for height and diameter of root crown were collected in spring 2023. Percent ancestry, as determined by Structure, was related to growth metrics using cubic polynomial regression in R. Cubic terms were selected based on the observed hump-shaped relationship in the scatterplots and their appropriateness to the experimental design. Growth metrics were also compared among Structure-derived cross types using linear mixed models (LMMs). In these models, cross type was included as a fixed effect, with river system, tree identity, and sub-plot included as random intercepts to account for variation among rivers, non-independence of ramets from the same genet, and environmental differences among plots within gardens, respectively. Additional LMMs were used to test for river-specific effects of cross type, with both cross type and river system as fixed effects and the same random effects as above. Significant differences between hybrid classes and river systems were evaluated using pairwise Tukey tests, and all results were visualized with ggplot2.
3. Results
3.1. Parental and Hybrid Classification
Structure revealed varying patterns of admixture and associated hybridization across the different river systems (Figure 3). Hybrids within the Jack’s Canyon and Oak Creek populations are genetically more similar to narrowleaf cottonwood, whereas hybrids in Indian Creek are more genetically similar to Fremont cottonwood. These results indicate that hybridization and subsequent backcrossing can occur in the direction of either parent species. Structure also distinguished F1 hybrids with 50% ancestry relative to both parents in all river sites, except Jack’s Canyon, where most individuals were advanced backcrosses to narrowleaf cottonwood. Assessment of individuals that were confirmed to be of hybrid origin in Structure were confirmed by introgress as F1 or backcross hybrids (Figure 4). In contrast to Structure, several hybrids from Oak Creek were revealed to be of pure narrowleaf ancestry based on the criterion of having 90% genetic similarity to known narrowleaf cottonwood. Hybrids from Jack’s Canyon were also revealed to be advanced backcrosses to narrowleaf or pure narrowleaf using this same criterion. Hybrids from Indian Creek were found to be backcrosses to Fremont or of pure Fremont ancestry. The Blue River site consisted largely of advanced backcrosses to both parents, with no F1 hybrids. Little Colorado River and San Francisco River hybrids were a mix of F1 hybrids and backcrosses to either parent species. These results confirm that backcrossing occurs in all the six river systems and that, following the initial formation of F1 hybrids, hybridization is bidirectional with both parent species.
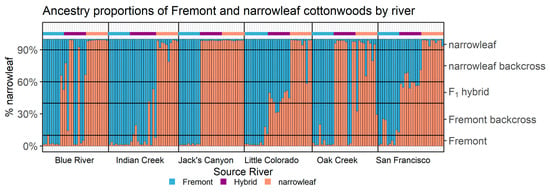
Figure 3.
Percent ancestry of individuals identified by Structure, grouped by sampling locations. Black gridlines are placed at 10%, 40%, 60%, and 90% narrowleaf-ancestry values to help visualize whether an individual is considered of “Pure” Fremont (<10%) or narrowleaf (>90%) ancestry, a F1 generation hybrid (between 40 and 60%), or some generation of backcross of hybrid to Fremont (between 10 and 40%) or narrowleaf (between 60 and 90%). Blue, purple, and red–orange marks above individual bars represent the original morphological-based classifications (Fremont, hybrid, and narrowleaf) given to each individual before genotyping. Population clusters (K = 2) show that individuals putatively assigned as either pure Fremont (blue) or pure narrowleaf (red–orange) largely show >90% ancestry to their assigned species, with notable exceptions in Indian Creek, Oak Creek, and San Francisco River Fremont individuals and Indian Creek, Little Colorado River, and Oak Creek narrowleaf individuals. Sampling locations vary in how many putative hybrid individuals were determined to be of F1, parental-backcross, or pure-parent ancestry.
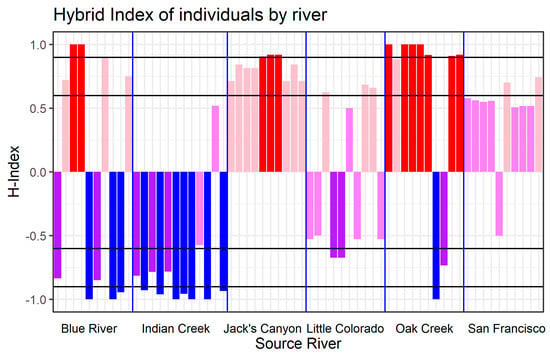
Figure 4.
Transformed Hybrid Index Score of putative hybrid individuals, grouped by sampling locations. Black gridlines are placed at 0.6, 0.9, −0.6, and −0.9 Transformed-H-index values to help visualize whether an individual is considered of “Pure” Fremont (<−0.9, blue) or narrowleaf (>0.9, red) ancestry, a F1 generation hybrid (between −0.6 and 0.6, violet), or some generation of backcross of hybrid to Fremont (between −0.6 and −0.9, purple) or narrowleaf (between −0.6 and −0.9%, pink). H-index calculations reveal that the putative hybrids of many sampling locations are of pure or hybrid-backcross ancestry to either Fremont (Indian Creek) or narrowleaf (Jack’s Canyon, Oak Creek, and San Francisco River) ancestry. Other sampling sites exhibit a mix of hybrid and hybrid-backcross indices. All samples were originally classified as hybrids based on morphology with intentions of further genotyping. Transformation of H-indices is performed by subtracting 1 from all H-index values below 0.5., e.g., an H-index value of 0.2 (classified as a hybrid backcross to Fremont) would have a Transformed-H-index value of −0.8.
3.2. Genetic Diversity and Structure of Hybrids
Population genetic summary statistics showed varying levels of genetic diversity and structure across different river systems and cross types (Table 3). Average percent polymorphic loci (%P) across all sites and cross types ranged from (59.5 to 92.6%), with backcrosses to narrowleaf and Fremont cottonwood showing the lowest and highest mean values, respectively. The average number of alleles per locus ranged from 1.6 to 1.93, with the highest in Fremont cottonwood. Observed and expected heterozygosity showed little variability among river systems. This pattern is maintained when observed heterozygosity is measured in individuals using Structure-derived precent ancestry, as well as hybrid index scores determined by introgress. Consistent with our expectation of greater genetic diversity in hybrids relative to the parent species, our regression analyses based on Structure showed that observed heterozygosity is significantly higher in hybrids (F1s and BCs combined) when compared to parental cross types (Figure 5). When comparing genetic structure across the six sites, FST values for cross types across all river systems are moderate (0.15–0.38), indicating low to moderate levels of genetic structure of cross types across the sample areas, consistent with bidirectional gene flow with both parent species. Pairwise FST values among individual river systems (0.01–0.26) indicate the greatest genetic differentiation between Jack’s Canyon populations and the other five populations (0.205–0.264), which is to be expected by Jack’s Canyon’s geographic distance from the other five sites. Pairwise differentiation between the remaining sites is low (0.01–0.15) (Table 4).

Table 3.
Summary metrics for genetic diversity and structure for each cross type per site. Abbreviations as follows: %P (percent polymorphic loci); NA (number of alleles); HE (expected heterozygosity); HO (expected homozygosity); FIS (inbreeding coefficient); and FST (genetic differentiation).
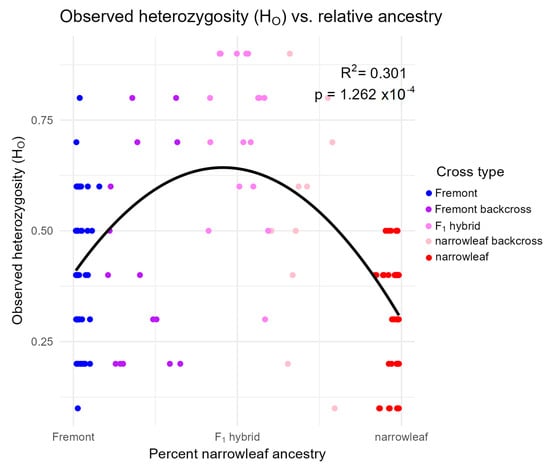
Figure 5.
Within-individual observed heterozygosity compared to the relative proportion of Fremont (left) or narrowleaf (right) ancestry, as measured by Structure-derived percent narrowleaf ancestry. Colors represent cross type classification.

Table 4.
Pairwise FST values between sampling sites.
Our hypothesis of genetic intermediacy of F1 hybrids and bidirectional introgression with both parent species is supported by principal coordinate analysis (PCoA) based on Nei’s genetic distance (Figure 6a). In addition, two distinct clusters of Fremont cottonwood are identified, and one of narrowleaf cottonwood. Hybrids (F1 and backcrosses), however, span the genetic range between the two parent species. The same cross type associations indicate that river sites are not genetically well-differentiated (consistent with estimates based on FST), except for Jack’s Canyon, where divergence, primarily driven by Fremont cottonwood, occurs relative to the other river sites (Figure 6b). Individual within-river analysis based on PCoA of all cross types per site reveals that some sites, such as Jack’s Canyon, the Little Colorado River, and the San Francisco River, have distinct differentiation of hybrid cottonwoods, while, in the other three river systems, hybrids are more closely associated with Fremont, narrowleaf, or both, again providing support for our hypothesis of bidirectional backcrossing (Figure 7).
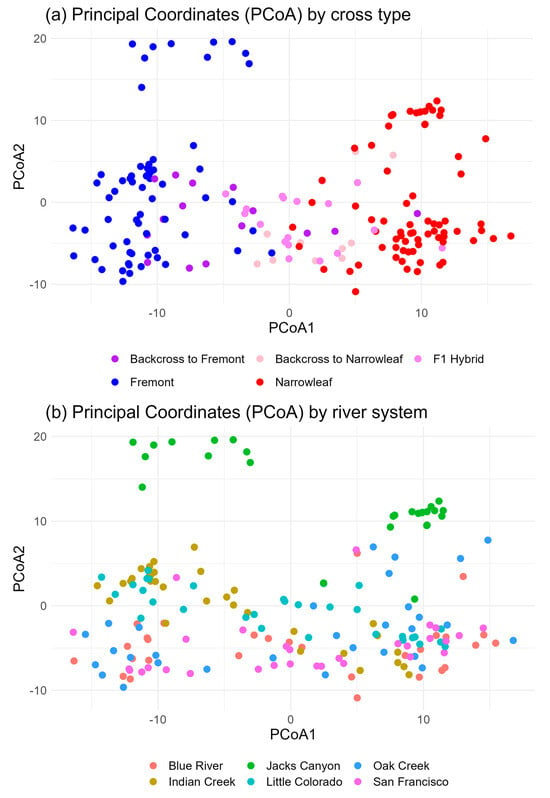
Figure 6.
(a,b). Principal Coordinate Analyses of 183 samples using genetic distance, organized by (a) cross type (Fremont, narrowleaf, F1, or backcross hybrids) and (b) sampling origin. Coordinate axis 1 explains 24.15% of variation, and coordinate axis 2 explains 4.95% of variation. Ordination reveals that putative hybrid clusters range from narrowleaf to Fremont clusters (a), the intermediate cluster being F1 hybrids along with backcrosses to each parent species. Fremont cottonwood is also identified as comprising two genetic clusters, with one of the clusters (Jack’s Canyon) forming a distinct group relative to the other cross types.
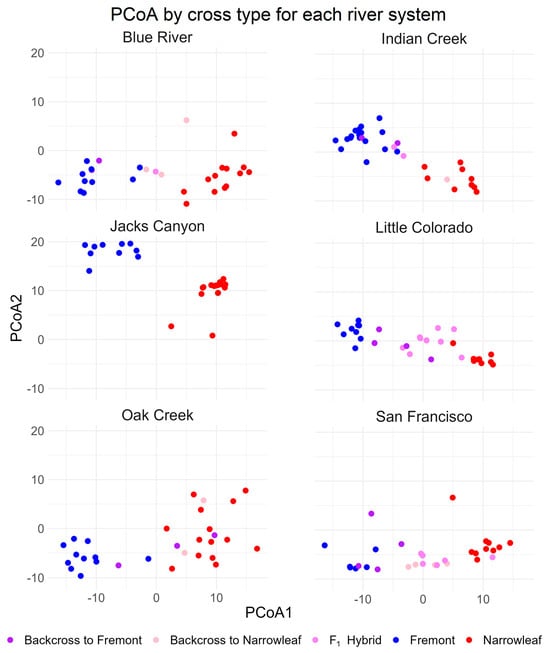
Figure 7.
Principal component analyses of the 6 river sites organized by cross type (Fremont, narrowleaf, F1, or backcross hybrid). Some locations (Little Colorado River, San Francisco River, and Jack’s Canyon) demonstrate strong clustering of hybrid trees distinct from Fremont or narrowleaf clusters, while others show hybrid trees strongly associated with either Fremont (Indian Creek) or both Fremont and narrowleaf trees (Oak Creek and Blue River).
3.3. Hybrid Vigor in the Common Garden
Our hypothesis, that hybrids would exhibit higher performance and survival than either parent species, was not well-supported over the course of our two-year common garden experiment. Comparison of two growth traits (height and DRC) showed that backcrosses to narrowleaf grew the tallest, followed by backcrosses to Fremont cottonwood, but these results were not statistically significant relative to either parent species (Figure 8a). The diameter of the root crown was significantly larger for Fremont-backcrosses and F1 hybrids compared to narrowleaf, but not significantly different from pure Fremont and narrowleaf-backcrosses (Figure 8b). These patterns for height were maintained when F1 hybrids were compared to the parent species, showing that F1 hybrids were not significantly larger than either parent (Figure 8c); however, Fremont and F1 hybrids had significantly larger DRC compared to narrowleaf cottonwood (Figure 8d).
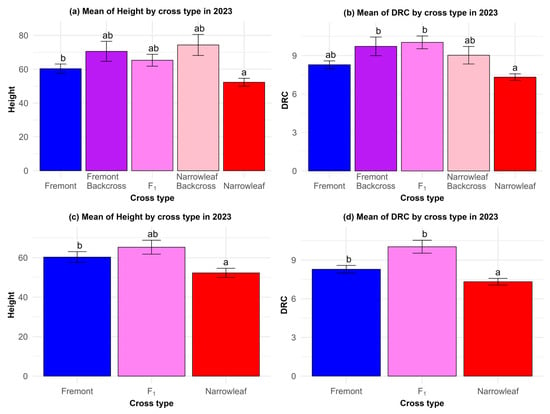
Figure 8.
(a–d) Common garden measurements show that growth in terms of (a) height and (b) diameter of root crown (DRC) in hybrids and backcrosses are not significantly different from the parent species. Comparison of height (c) in F1 hybrids also shows no significant difference relative to the parent species. Comparison of DRC for Fremont and F1 hybrids (d) is significantly different from narrowleaf. Letters indicate significant differences among cross types. Tukey tests of significant mean differences can be found in Table S3.
When comparing the three cross types within different river systems in the common garden, the relationships of growth between parents and their hybrids varied across sites (Figure 9a). For each cross type, height was significantly different between river systems compared to the same cross type in other river systems. For Blue River, Indian Creek, and the San Francisco River, hybrids’, either F1 or backcrosses, height was not significantly greater than in either parent. For Jack’s Canyon and Oak Creek, Fremont trees had greater height, while narrowleaf from the Little Colorado site were highest.
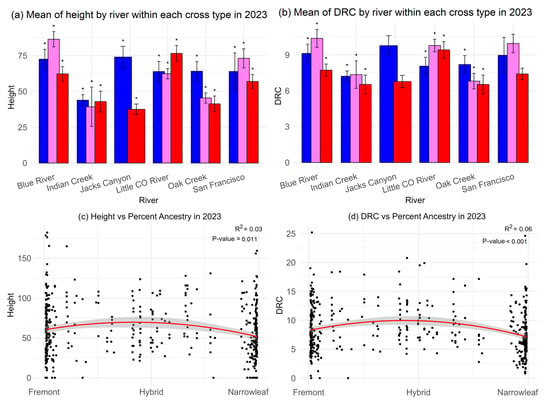
Figure 9.
(a–d). Growth in terms of (a) height and (b) diameter of root crown across different cross types and rivers; blue represents Fremont, violet is F1 hybrids, and red is narrowleaf. Not all cross types are represented within each river system due to mortality within the common garden. Polynomial (degree = 2) regressions of growth in terms of (c) height and (d) diameter of the root crown against relative ancestry to each parent species. Asterisks in (a,b) indicate significant differences among cross types within individual river systems. Tukey tests of significant mean differences can be found in Table S4. Curves in red in (c,d) indicate polynomonical regression fits, and the shadows represent the 95% confidence interval for each regression.
Comparison of the diameter of the root crown was significantly different for cross types from the Blue River, Indian Creek, and Oak Creek sites but not Jack’s Canyon, San Francisco River, and Little Colorado River (Figure 9b). F1 hybrids were significantly larger in the DRC from Indian Creek, followed by backcrosses to Fremont and narrowleaf from the Blue River. According to Structure-derived percent ancestry, hybrids show small, but significantly higher, values for both height and diameter of the root crown when compared to either parent species (Figure 9c,d).
In terms of mortality, the lowest percent mortality occurred in F1 and backcross hybrids to narrowleaf (47%), followed by higher mortality in Fremont (~60%) and narrowleaf, but ANOVA and Tukey test showed no statistically significant differences in mortality among the cross types (Figure 10), suggesting that hybrids do not necessarily survive better than either parent species in a common garden setting. Together, these results provide partial, but mixed, results suggesting that when hybrids are grown in a common environment, they are not necessarily more vigorous than either parent species.
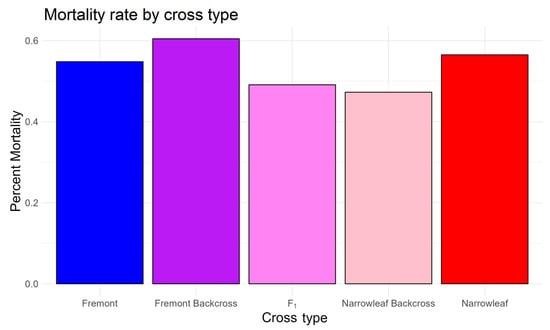
Figure 10.
Percent mortality by cross type. ANOVA and pairwise Tukey testing did not show significant differences among Structure-determined cross types.
4. Discussion
4.1. Overall Results and Findings
In this study, we found evidence for variable genetic structure and diversity across our six sites, along with strong genetic differentiation between P. fremontii and P. angustifolia, which accompanies clear morphological differentiation between the two species. Hybrids derived from the two species exhibited the expected genetic intermediacy as F1 progeny, but also showed extensive backcrossing to both parent species in subsequent generations (F2, etc.) at each site. This result contrasted with morphological expectations, especially for F1 hybrids, which, upon genetic analysis, appeared to be more advanced backcross progeny. This highlights the importance of genetic analysis to support assessments of hybrid progeny based on morphological observations []. Our results also confirm that backcrossing is bidirectional across all of our sites, suggesting that unidirectional backcrossing is uncommon [,]. Finally, assessments of hybrid vigor showed that hybrids do not necessarily out-perform the parent species, and instead exhibit only minor increases in performance. We address each of these major results below, with emphasis on their relevance to related studies and the conservation and management of riparian habitats in the southwestern USA.
4.2. Hybridization and Genetic Diversity and Structure
We found that the mean values for percent polymorphic loci and number of alleles were higher in the two parent species, compared to hybrids. These metrics also varied more between different river systems in narrowleaf and hybrid cottonwoods than in Fremont cottonwood, suggesting that Fremont cottonwoods are more genetically homogenous across different river systems, and that variation among hybrids may be driven more by variation in narrowleaf progenitors. This observation matches findings where narrowleaf cottonwoods exhibit high gene flow within river systems than across terrestrial uplands that separate them, which would encourage differing patterns of genetic diversity between different river systems []. The observed heterozygosity was higher in hybrid cottonwood (HO = 0.78) than in pure Fremont (HO = 0.45) or narrowleaf (HO = 0.33) cottonwood. This matches previous calculations of diversity metrics in a similar hybrid zone in southern Colorado, where the average number of alleles was roughly equal in broadleaf (4.8) and narrowleaf cottonwood (4.6) and greater in the hybrid zone (6.8), although the observed heterozygosity was roughly equal in broadleaf (0.38), hybrid (0.40), and narrowleaf cottonwood []. In this case, the average of the number of alleles was actually lowest in F1 hybrids (NA = 1.6). Expected heterozygosity was similar in Arizona–Utah and southwestern Colorado hybrid ranges, with Colorado hybrids having higher expected heterozygosity (0.68) than broadleaf or narrowleaf cottonwood (0.48). The inbreeding coefficients (FIS) in our analyses were lowest in F1 hybrids, narrowleaf, and narrowleaf backcross, indicating a greater heterozygosity in hybrids, as would be expected in hybrid swarms, perhaps with greater backcrossing toward narrowleaf.
Principal coordinate analyses showed clear differentiation between Fremont and narrowleaf cottonwood, and a lack of significant differentiation between most river systems, except Jack’s Canyon, which formed distinct clusters in the ordination. Jack’s Canyon is not significantly distant from most of the other populations; if geographic distance was a factor in genetic differentiation, one would expect Indian Creek, which is more distant, to form distinct genetic clusters in PCoA, not Jack’s Canyon. Jack’s Canyon has been known to significantly diverge from other river systems in other studies on hybridization between these species, such as in the case ofthe effects of hybridization on mite herbivore speciation [].
4.3. Directional Backcrossing
Initial studies of P. fremontii × P. angustifolia hybrid populations using restriction-length polymorphisms (RFLPs) for genotyping had indicated that backcrossing was primarily unidirectional, where fertile F1 hybrids were produced and then backcrossed with P. angustifolia, but not P. fremontii [,]. Later studies revealed that backcrossing in hybrid populations of P. angustifolia and P. deltoides, another broadleaf cottonwood species, was bi-directional []. Principal coordinate analysis in our study showed that hybridization across all river systems was bi-directional to varying degrees, with hybrids evenly spread across the narrowleaf and Fremont clusters, indicating backcrossing to both parents. Calculations of ancestry using Structure as well as hybrid index values were able to distinguish backcrossed individuals and reveal that different river systems had different profiles of hybridization, with some exhibiting more backcrossing to one or both parents than others. Percent ancestry and hybrid index also determined that many putative hybrids were of pure narrowleaf or pure Fremont ancestry, showing that morphology may not be a reliable indicator of true hybrid status. Percent ancestry derived from Structure analysis provided estimations of ancestry for parents as well as hybrids, giving it an advantage in genotyping compared to hybrid index, which calculated hybrid indices based on using assigned parents as representative genotypes.
4.4. Hybrid Vigor Hypothesis
Mean comparisons between different cross types, including backcrosses to each parent, showed that performance in the common garden was not significantly higher in hybrids compared to parent species. Different metrics for growth, height, and diameter of the root crown, differed in whether F1 hybrids or backcrosses performed best, making it unclear whether hybrid vigor is occurring in backcrosses to either parent or initial F1 generations. Polynomial regression, however, showed a slight increase in performance for hybrids, but these relationships were not robust, and were perhaps skewed by the high mortality that occurred across all cross types, or simply that hybrid vigor should not always be expected []. For example, investigation of hybrids formed between Eucalyptus risdonii Hook f. and E. amygdalina Labill. show that hybrids are not always more vigorous than the parent species []. Previous studies on productivity using both natural stands and common gardens, including Fremont–narrowleaf hybrids, have reported similar results to our findings, where productivity, measured as aboveground net primary productivity, was highest in Fremont cottonwood and lowest in narrowleaf, with hybrids being intermediate relative to the two parent species []. It should be noted, therefore, that studies of hybrids in other forest trees indicate that hybrid vigor is not universal, as in the case of pine, where heterosis has not been found to be exceptional [], or may be inferior or equivalent to the parent species, as in the case of spruce []. In some cases, hybrid vigor may only emerge under stressful conditions, as in hybrid Populus acuminata (Rydb.) where vigor is only observed under sub-optimal cooler temperatures [], suggesting that heterosis may be environment-specific.
4.5. Management Implications for Riparian Habitats in the Southwestern USA
Our study provides evidence for extensive hybridization and backcrossing in all six river systems investigated. The extent of backcrossing varies among river systems, suggesting that F1 hybrids repeatedly exchange genes with both parent species. These results have important implications for the management of riparian ecosystems. First, they suggest that hybridization likely occurs wherever the two species’ ranges overlap, a result consistent with previous molecular-based studies demonstrating the incidence of hybridization at a smaller scale []. Given that hybridization has been linked to a variety of ecosystem properties including the promotion of biodiversity [,,,], phylogenetic sorting of dependent communities [], and cryptic speciation [], it is all the more important to recognize the important role that hybrid zones play in the establishment and maintenance of ecosystem structure and diversity. Second, because backcrossing appears to readily occur with both parent species, this suggests that the opportunities for adaptive introgression are substantial. Adaptive introgression has been posited to have had an important influence for the ecology and evolution of many plant species and is linked to the potential for adaptation to climate change [,] and the occupation of novel environments in which neither parent species occurs, which is a concern for adaptive management of riparian ecosystems [,].
Third, adaptive management can also be facilitated through an understanding of how introgression influences species’ responses in the context of increased resilience to disease and drought brought about by climate change [,]. Our results suggest that it is important to assess hybridization using genetic-based approaches and that morphology alone is not the most reliable way to evaluate hybrid ancestry. We contend that morphology, combined with genetic markers, is a better approach to determine F1 hybrids and backcross progeny, a result consistent with Floate et al. []. This has important implications for management as well in that land managers would benefit from more accurate assessments of hybrid zones, which clearly vary in the composition of hybrid derivatives (F1s and backcrosses), which could affect the potential for hybrid vigor [,], adaptation to novel environments [,], and impacts they have on the composition and structure of dependent communities [,,,].
While previous studies [,] showed that backcrossing was primarily unidirectional in this hybridizing species pair, our study revealed clear evidence of bidirectional backcrossing to both parents in each of the six river systems. Thus, managers can take advantage of this fact, knowing that reproductive barriers between hybrids and their parents are minimal and may provide opportunities for introducing hybrid derivatives to areas where neither parent exists, potentially for restoration of riparian habitat. We note, however, that F1 hybrids are not interfertile [], so managers will need to include at least one parental species with any hybrid transplanting effort.
We observe that F1 hybrids show minor evidence for higher performance in growth metrics related to the potential for hybrid vigor occurring among these river systems. Management efforts wishing to pre-adapt populations of cottonwood in their current or novel environments to climate change may benefit by using hybrids, especially in the context of restoring riparian trees in areas affected by the impacts of climate change (e.g., drought or temperature changes). We also emphasize, however, that the common garden used in this study exposed clones from the six river systems to a single environment in Walnut Creek. Further common garden experiments in contrasting environments using a range of hybrid classes should be conducted to determine if hybrid vigor occurs across an environmental gradient.
Finally, results from this study can be applied to the management of hybrid-dependent communities, including communities of arthropods [,] and soil microbes [], which have been shown to be heritably associated with different genotypes, and hybrid zones between P. fremontii and P. angustifolia, many of which are expected to demonstrate similar community–genotype dynamics. Soil microbes, such as mycorrhizal fungi, can also, in turn, effect the performance of hybridizing species, and, in the case of P. fremontii × P. angustifolia hybrids, mycorrhizal fungi associated with F1 hybrids have been shown to increase performance in all cross types, pointing to the potential adaptive effects of hybrid vigor [].
5. Conclusions and Future Directions
Our results suggest that hybridization and associated backcrossing between P. fremontii and P. angustifolia is extensive across multiple sites encompassing a broad geographic range. We also note that genetic variation and structure vary considerably among sites, suggesting that gene flow and hybridization are dynamic throughout the area of investigation. It is also clear that morphology alone is not always a reliable indicator of hybrid status, and that genetic analysis should be conducted to verify the classification of hybrid derivatives (e.g., F1s vs advanced backcrosses). These results are especially important when considering the impacts that hybrids have on dependent communities, which appear to respond significantly to differences in genetic diversity and structure [,,,,] with implications for their evolutionary trajectories []. Finally, we note that additional experiments are needed to assess the degree to which hybrid vigor may occur, especially in the context of contrasting environments within common garden trials. Together, our results suggest that the identification of hybrid zones should be an important management goal for the maintenance and preservation of threatened riparian ecosystems throughout the southwestern USA, especially in the context of ongoing climate change [,].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16091491/s1, Table S1: Chi-squared test values of each microsatellite locus, per cross type; Table S2: Statistical values for pair-wise Tukey tests of cross type comparisons of (a) height and (b) diameter of root crown in 2023; Table S3: Statistical values for pair-wise Tukey tests of river-system comparisons of (a) height and (b) diameter of root crown in 2023; Table S4: Evanno’s delta-K testing.
Author Contributions
Conceptualization, G.J.A.; methodology, M.S., H.F.C. and A.R.K.; formal analysis, M.S. and H.F.C.; resources, M.S., H.F.C., A.R.K., C.A.G., T.G.W. and G.J.A.; data curation, M.S. and G.J.A.; writing—original draft preparation, M.S., G.J.A. and C.A.G.; writing—review and editing, all authors; supervision, G.J.A., H.F.C. and C.A.G.; funding acquisition, G.J.A., C.A.G., H.F.C. and T.G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the NSF Macrosystems grant DEB-2017877 (G.J.A., C.A.G., and T.G.W.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Genetic data are hosted on https://datadryad.org/submission/10.5061/dryad.v9s4mw789 (accessed 17 September 2025).
Acknowledgments
We thank Erica Sukovich for assistance with primer development, James Pierce for assistance with installing the common garden experiment, and the Walnut Creek Center for Education & Research for hosting the common garden.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arnold, M.L. Anderson’s and Stebbins’ Prophecy Comes True: Genetic Exchange in Fluctuating Environments. Syst. Bot. 2016, 41, 4–16. [Google Scholar] [CrossRef]
- Mallet, J. Hybrid Speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- Mallet, J.; Besansky, N.; Hahn, M.W. How Reticulated Are Species? BioEssays 2016, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lexer, C.; Joseph, J.A.; Van Loo, M.; Barbará, T.; Heinze, B.; Bartha, D.; Castiglione, S.; Fay, M.F.; Buerkle, C.A. Genomic Admixture Analysis in European Populus spp. Reveals Unexpected Patterns of Reproductive Isolation and Mating. Genetics 2010, 186, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lindtke, D.; Buerkle, C.A.; Barbará, T.; Heinze, B.; Castiglione, S.; Bartha, D.; Lexer, C. Recombinant Hybrids Retain Heterozygosity at Many Loci: New Insights into the Genomics of Reproductive Isolation in Populus. Mol. Ecol. 2012, 21, 5042–5058. [Google Scholar] [CrossRef]
- Martinsen, G.D.; Whitham, T.G.; Turek, R.J.; Keim, P. Hybrid Populations Selectively Filter Gene Introgression between Species. Evolution 2001, 55, 1325–1335. [Google Scholar] [CrossRef]
- Bangert, R.K.; Turek, R.J.; Martinsen, G.D.; Wimp, G.M.; Bailey, J.K.; Whitham, T.G. Benefits of Conservation of Plant Genetic Diversity to Arthropod Diversity. Conserv. Biol. 2005, 19, 379–390. [Google Scholar] [CrossRef]
- Wimp, G.M.; Young, W.P.; Woolbright, S.A.; Martinsen, G.D.; Keim, P.; Whitham, T.G. Conserving Plant Genetic Diversity for Dependent Animal Communities. Ecol. Lett. 2004, 7, 776–780. [Google Scholar] [CrossRef]
- Wimp, G.M.; Martinsen, G.D.; Floate, K.D.; Bangert, R.K.; Whitham, T.G. Plant Genetic Determinants of Arthropod Community Structure and Diversity. Evolution 2005, 59, 61–69. [Google Scholar]
- Whitham, T.G.; Martinsen, G.D.; Floate, K.D.; Dungey, H.S.; Potts, B.M.; Keim, P. Plant Hybrid Zones Affect Biodiversity: Tools for a Genetic-Based Understanding of Community Structure. Ecology 1999, 80, 416–428. [Google Scholar] [CrossRef]
- Jarvis, K.J.; Allan, G.J.; Craig, A.J.; Beresic-Perrins, R.K.; Wimp, G.; Gehring, C.A.; Whitham, T.G. Arthropod Communities on Hybrid and Parental Cottonwoods are Phylogenetically Structured by Tree Type: Implications for Conservation of Biodiversity in Plant Hybrid Zones. Ecol. Evol. 2017, 7, 5909–5921. [Google Scholar] [CrossRef]
- Eckenwalder, J.E. Taxonomic Signal and Noise in Multivariate Interpopulational Relationships in Populus mexicana (Salicaceae). Syst. Bot. 1996, 21, 261–271. [Google Scholar] [CrossRef]
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: Oxford, UK, 1997; ISBN 9780-1950-9975-1. [Google Scholar]
- Rieseberg, L.H.; Carney, S.E. Tansley Review No. 102: Plant Hybridization. New Phytol. 1998, 140, 599–624. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Stebbins, G.L. Hybridization as an Evolutionary Stimulus. Evolution 1954, 8, 378–388. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Willis, J.H. Plant Speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.L. The Role of Hybridization in Evolution. In Proceedings of the American Philosophical Society, Philadelphia, PA, USA, 23 April 1959; Volume 103, pp. 231–251. [Google Scholar]
- De La Torre, A.R.; Wang, T.; Jaquish, B.; Aitken, S.N. Adaptation and Exogenous Selection in a Picea glauca × Picea engelmannii Hybrid Zone: Implications for Forest Management under Climate Change. New Phytol. 2014, 201, 687–699. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Martinsen, G.D.; Whitham, T.G. Cottonwood Hybrids Gain Fitness Traits of Both Parents: A Mechanism for Their Long-Term Persistence? Am. J. Bot. 2002, 89, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Janes, J.K.; Hamilton, J.A. Mixing It Up: The Role of Hybridization in Forest Management and Conservation under Climate Change. Forests 2017, 8, 237. [Google Scholar] [CrossRef]
- Eckenwalder, J.E. Natural Intersectional Hybridization between North American Species of Populus (Salicaceae) in Sections Aigeiros and Tacamahaca. II: Taxonomy. Can. J. Bot. 1984, 62, 325–335. [Google Scholar] [CrossRef]
- Eckenwalder, J.E. Natural Intersectional Hybridization between North American Species of Populus (Salicaceae) in Sections Aigeiros and Tacamahaca. III: Paleobotany and Evolution. Can. J. Bot. 1984, 62, 336–342. [Google Scholar] [CrossRef]
- Keim, P.; Paige, K.N.; Whitham, T.G.; Lark, K.G. Genetic Analysis of an Interspecific Hybrid Swarm of Populus: Occurrence of Unidirectional Introgression. Genetics 1989, 123, 557–565. [Google Scholar] [CrossRef]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and Speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef]
- Arnold, M.L.; Martin, N.H. Adaptation by Introgression. J. Biol. 2009, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Archer, M.A.; Wayne, R.K. Transgressive Segregation, Adaptation and Speciation. Heredity 1999, 83, 363–373. [Google Scholar] [CrossRef]
- Hord, A.M.; Fischer, D.G.; Schweitzer, J.A.; LeRoy, C.J.; Whitham, T.G.; Bailey, J.K. Hybrid introgression as a mechanism of rapid evolution and resilience to climate change in a riparian tree species. Commun. Biol. 2025, 8, 1173. [Google Scholar] [CrossRef]
- Wang, X.R.; Szmidt, A.E.; Savolainen, O. Genetic Composition and Diploid Hybrid Speciation of a High Mountain Pine, Pinus densata, Native to the Tibetan Plateau. Genetics 2001, 159, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Gonzalez, A.; Hefer, C.A.; Lexer, C.; Douglas, C.J.; Cronk, Q.C.B. Introgression from Populus balsamifera Underlies Adaptively Significant Variation and Range Boundaries in P. trichocarpa. New Phytol. 2018, 217, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.J.; Shuster, S.M.; Woolbright, S.; Walker, F.; Meneses, N.; Keith, A.; Bailey, J.K.; Whitham, T.G. Perspective: Interspecific Indirect Genetic Effects (IIGEs). Linking Genetics and Genomics to Community Ecology and Ecosystem Processes. In Trait-Mediated Indirect Interactions: Ecological and Evolutionary Perspectives; Ohgushi, T., Schmitz, O.J., Holt, R.D., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 295–323. [Google Scholar]
- Hersch-Green, E.I.; Allan, G.J.; Whitham, T.G. Genetic Analysis of Admixture and Patterns of Introgression in Foundation Cottonwood Trees (Salicaceae) in Southwestern Colorado, USA. Tree Genet. Genomes 2014, 10, 527–539. [Google Scholar] [CrossRef]
- Kerbs, B.; Ressler, J.; Kelly, J.K.; Mort, M.E.; Santos-Guerra, A.; Gibson, M.J.S.; Caujapé-Castells, J.; Crawford, D.J. The Potential Role of Hybridization in Diversification and Speciation in an Insular Plant Lineage: Insights from Synthetic Interspecific Hybrids. AoB Plants 2017, 9, plx043. [Google Scholar] [CrossRef]
- García Verdugo, C.; Friar, E.; Santiago, L.S. Ecological Role of Hybridization in Adaptive Radiations: A Case Study in the Dubautia arborea–Dubautia ciliolata (Asteraceae) Complex. Int. J. Plant Sci. 2013, 174, 749–759. [Google Scholar] [CrossRef][Green Version]
- Edelman, N.B.; Mallet, J. Prevalence and Adaptive Impact of Introgression. Annu. Rev. Genet. 2021, 55, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Durphy, J.L.; Schwarzbach, A.E.; Donovan, L.A.; Lexer, C. Major Ecological Transitions in Wild Sunflowers Facilitated by Hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Whitham, T.G.; Bailey, J.K.; Schweitzer, J.A.; Shuster, S.M.; Bangert, R.K.; LeRoy, C.J.; Lonsdorf, E.V.; Allan, G.J.; DiFazio, S.P.; Potts, B.M.; et al. A Framework for Community and Ecosystem Genetics: From Genes to Ecosystems. Nat. Rev. Genet. 2006, 7, 510–523. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Miller, J.M. Adaptive Introgression as a Resource for Management and Genetic Conservation in a Changing Climate. Conserv. Biol. 2015, 30, 33–41. [Google Scholar] [CrossRef]
- Chan, W.Y.; Hoffmann, A.A.; van Oppen, M.J.H. Hybridization as a Conservation Management Tool. Conserv. Lett. 2019, 12, e12652. [Google Scholar] [CrossRef]
- Floate, K.D.; Whitham, T.G.; Keim, P. Morphological versus Genetic Markers in Classifying Hybrid Plants. Evolution 1994, 48, 929–941. [Google Scholar] [CrossRef]
- Mayjonade, B.; Gouzy, J.; Donnadieu, C.; Pouilly, N.; Marande, W.; Callot, C.; Langlade, N.; Muños, S. Extraction of High-Molecular-Weight Genomic DNA for Long-Read Sequencing of Single Molecules. BioTechniques 2016, 61, 203–205. [Google Scholar] [CrossRef]
- Bothwell, H.M.; Cushman, S.A.; Woolbright, S.A.; Hersch-Green, E.I.; Evans, L.M.; Whitham, T.G.; Allan, G.J. Conserving Threatened Riparian Ecosystems in the American West: Precipitation Gradients and River Networks Drive Genetic Connectivity and Diversity in a Foundation Riparian Tree (Populus angustifolia). Mol. Ecol. 2017, 26, 5114–5132. [Google Scholar] [CrossRef]
- Chatterji, S.; Pachter, L. Reference Based Annotation with GeneMapper. Genome Biol. 2006, 7, R29. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype Data: Linked Loci and Correlated Allele Frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype Data: Dominant Markers and Null Alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A Cluster Matching and Permutation Program for Dealing with Label Switching and Multimodality in Analysis of Population Structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Buerkle, C.A. Maximum-Likelihood Estimation of a Hybrid Index Based on Molecular Markers. Mol. Ecol. Notes 2005, 5, 684–687. [Google Scholar] [CrossRef]
- Gompert, Z.; Buerkle, C.A. Introgress: A Software Package for Mapping Components of Isolation in Hybrids. Mol. Ecol. Resour. 2010, 10, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Evans, L.M.; Allan, G.J.; Shuster, S.M.; Woolbright, S.A.; Whitham, T.G. Tree Hybridization and Genotypic Variation Drive Cryptic Speciation of a Specialist Mite Herbivore. Evolution 2008, 62, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Bush, D. Long-term research reveals potential role of hybrids in climate-change adaptation. A commentary on ‘Expansion of the rare Eucalyptus risdonii under climate change through hybridisation with a closely related species despite hybrid inferiority’. Ann. Bot. 2022, 129, i–iii. [Google Scholar] [CrossRef]
- Pfeilsticker, T.R.; Jones, R.C.; Steane, D.A.; Vaillancourt, R.E.; Harrison, P.A.; Potts, B.M. Expansion of the rare Eucalyptus risdonii under climate change through hybridization with a closely related species despite hybrid inferiority. Ann. Bot. 2022, 129, 1–14. [Google Scholar] [CrossRef]
- Lojewski, N.R.; Fischer, D.G.; Bailey, J.K.; Schweitzer, J.A.; Whitham, T.G.; Hart, S.C. Genetic Basis of Aboveground Productivity in Two Native Populus Species and Their Hybrids. Tree Physiol. 2009, 29, 1133–1142. [Google Scholar] [CrossRef]
- Little, S.; Somes, H.A. No Exceptional Vigor Found in Hybrid Pines Tested; Forest Research Note NE-10; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1951; 4p.
- Johnsen, K.H.; Major, J.E.; Loo, J.; McPhee, D. Negative heterosis not apparent in 22-year-old hybrids of Picea mariana and Picea rubens. Can. J. Bot. 1998, 76, 434–439. [Google Scholar] [PubMed]
- Zanewich, K.P.; Pearce, D.W.; Rood, S.B. Heterosis in poplar involves phenotypic stability: Cottonwood hybrids outperform their parental species at suboptimal temperatures. Tree Physiol. 2018, 38, 789–800. [Google Scholar] [CrossRef]
- Vallejo-Marín, M.; Hiscock, S.J. Hybridization and Hybrid Speciation under Global Change. New Phytol. 2016, 211, 1170–1187. [Google Scholar] [CrossRef]
- Whitney, K.D.; Randell, R.A.; Rieseberg, L.H. Adaptive Introgression of Abiotic Tolerance Traits in the Sunflower Helianthus annuus. New Phytol. 2010, 187, 230–239. [Google Scholar] [CrossRef]
- Gehring, C.A.; Ji, B.; Fong, S.; Whitham, T.G. Hybridization in Populus Alters the Species Composition and Interactions of Root-Colonizing Fungi: Consequences for Host Plant Performance. Botany 2014, 92, 287–293. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Bailey, J.K.; Fischer, D.G.; LeRoy, C.J.; Lonsdorf, E.V.; Whitham, T.G.; Hart, S.C. Plant–Soil–Microorganism Interactions: Heritable Relationship between Plant Genotype and Associated Soil Microorganisms. Ecology 2008, 89, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Larson, E.L.; Harrison, R.G. Hybrid Zones: Windows on Climate Change. Trends Ecol. Evol. 2015, 30, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Garfin, G.; Jardine, A.; Merideth, R.; Black, M.; LeRoy, S. (Eds.) Assessment of Climate Change in the Southwest United States: A Report Prepared for the National Climate Assessment; A Report by the Southwest Climate Alliance; Island Press: Washington, DC, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).