Characterization of Pathogenic Bacteria Associated with Wetwood Disease in Populus deltoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Bacterial Isolation
2.3. Pathogen Inoculation and Pathogenicity Identification
2.3.1. Growth Curve
2.3.2. Pathogen Culture
2.3.3. Bacterial Inoculation and Pathogenicity Verification
- (1)
- Inoculation of pathogenic bacteria
- (2)
- Determination of plant growth parameters
- (3)
- Symptom observation and pathogen isolation of wetwood
2.3.4. Pathogenic Bacteria Identification
- (1)
- Morphological identification
- (2)
- Molecular identification
- (3)
- Physiological and biochemical identification
2.4. Data Processing
3. Results
3.1. Bacterial Isolation and Purification
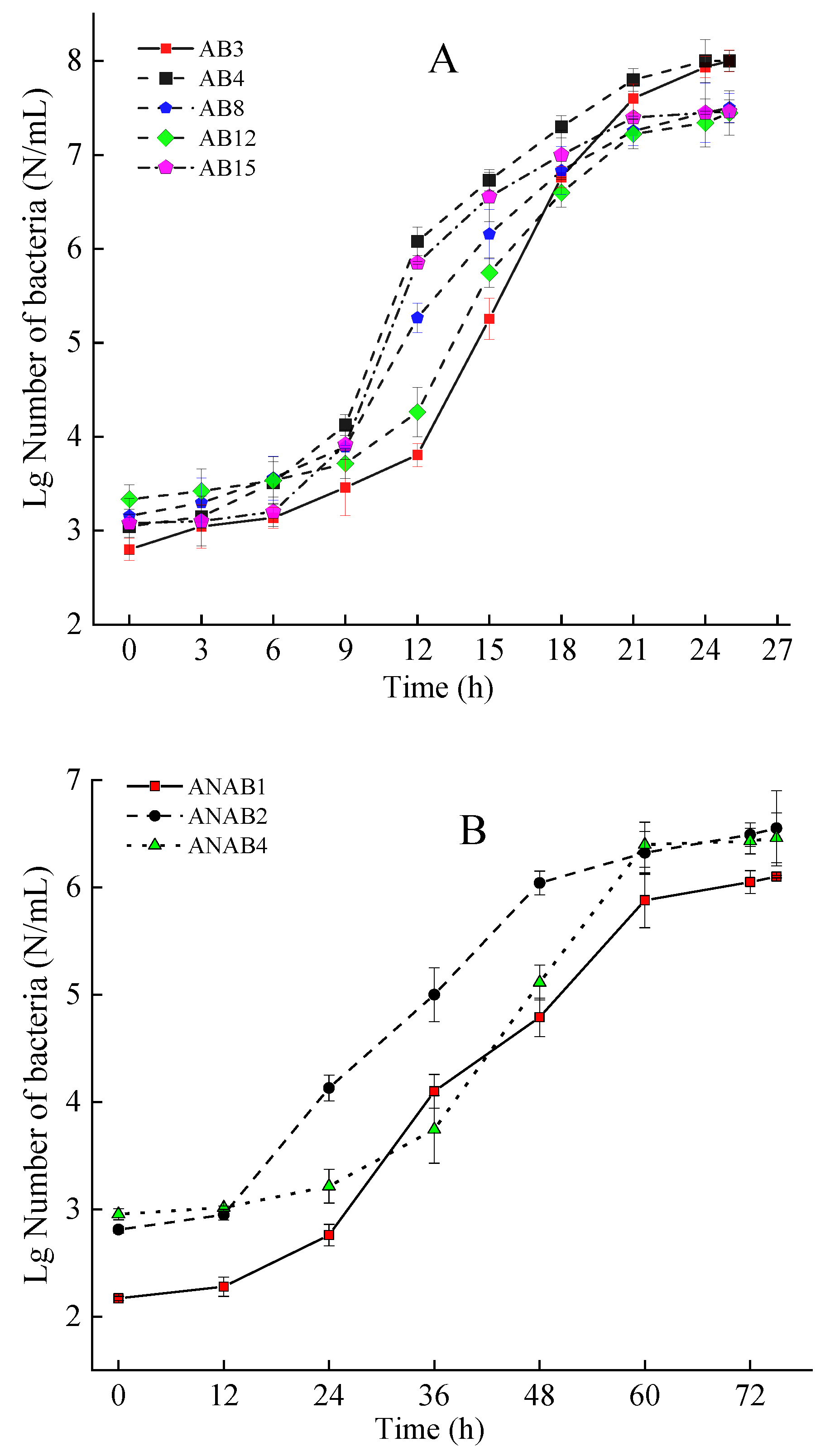
3.2. Logistic Growth Curve of Pathogenic Bacteria
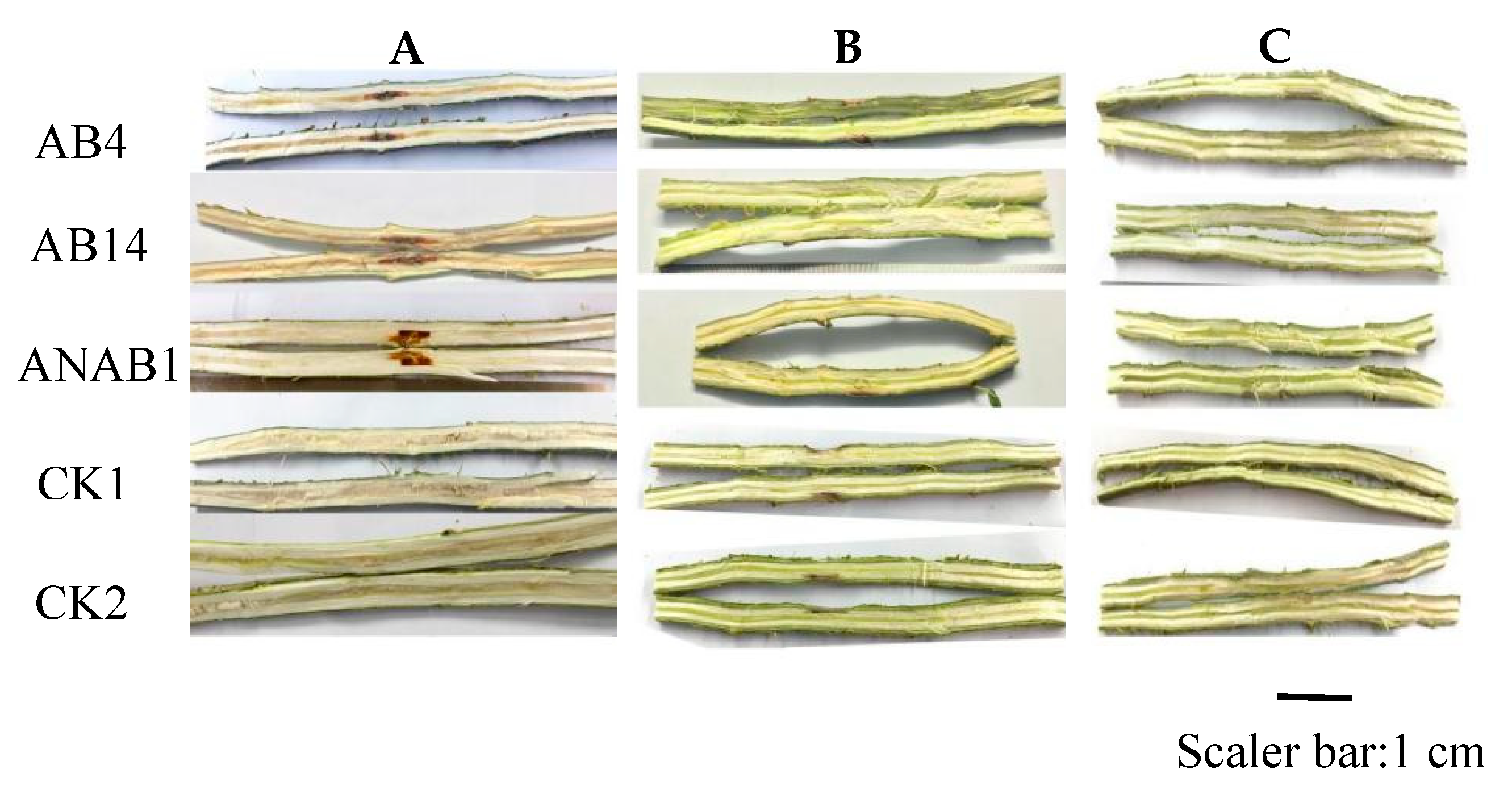
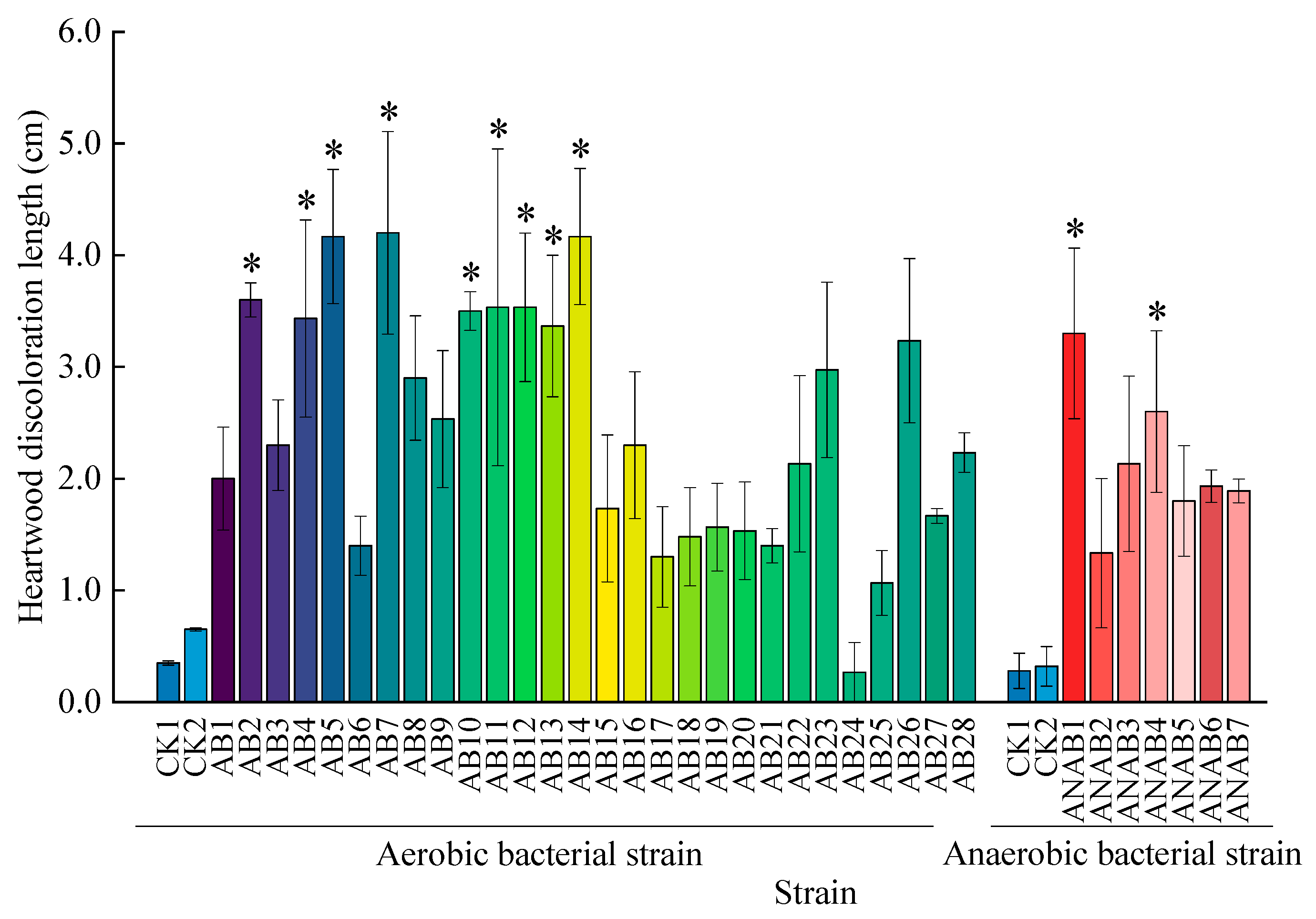
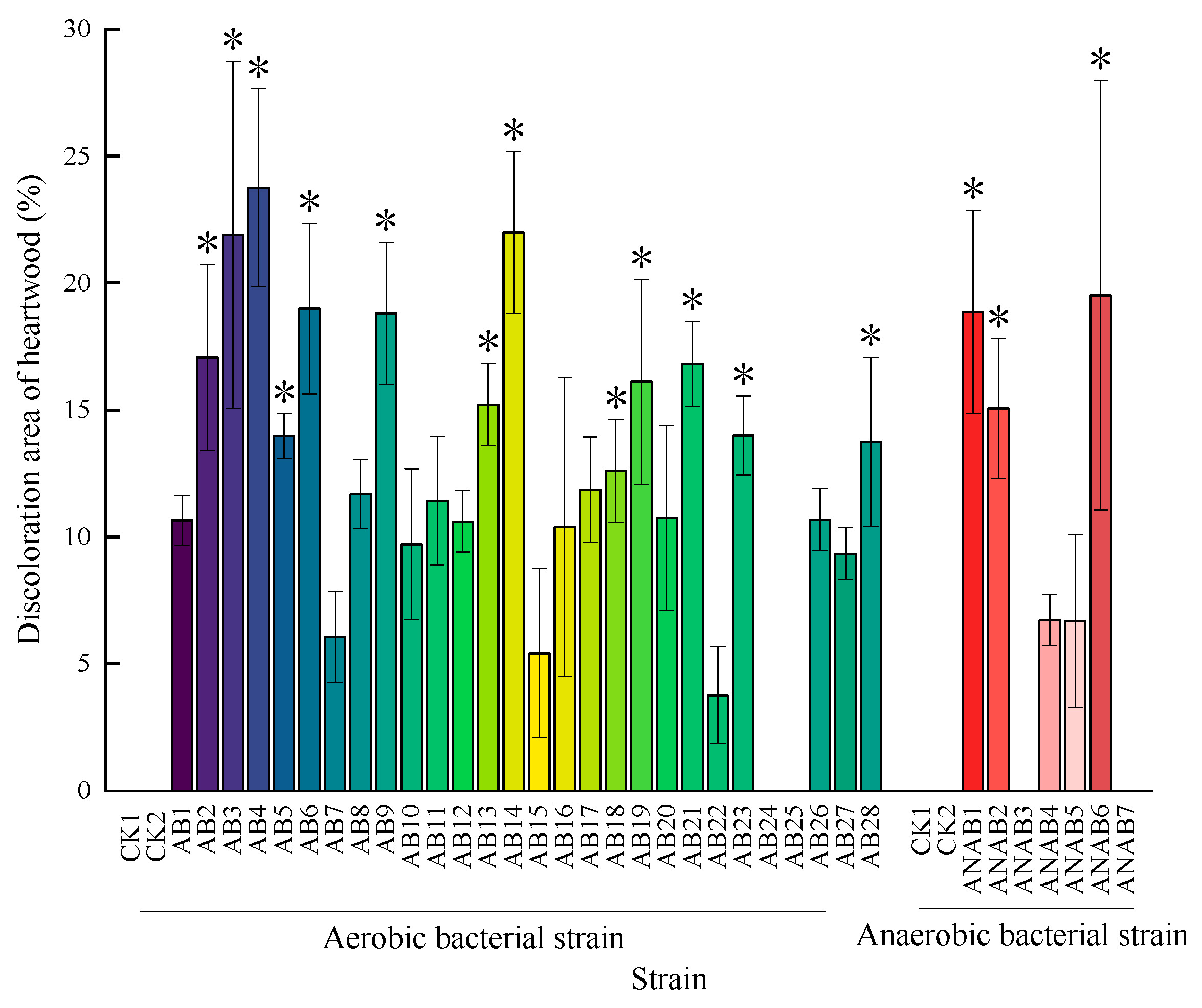
3.3. Pathogenicity Testing of Bacteria
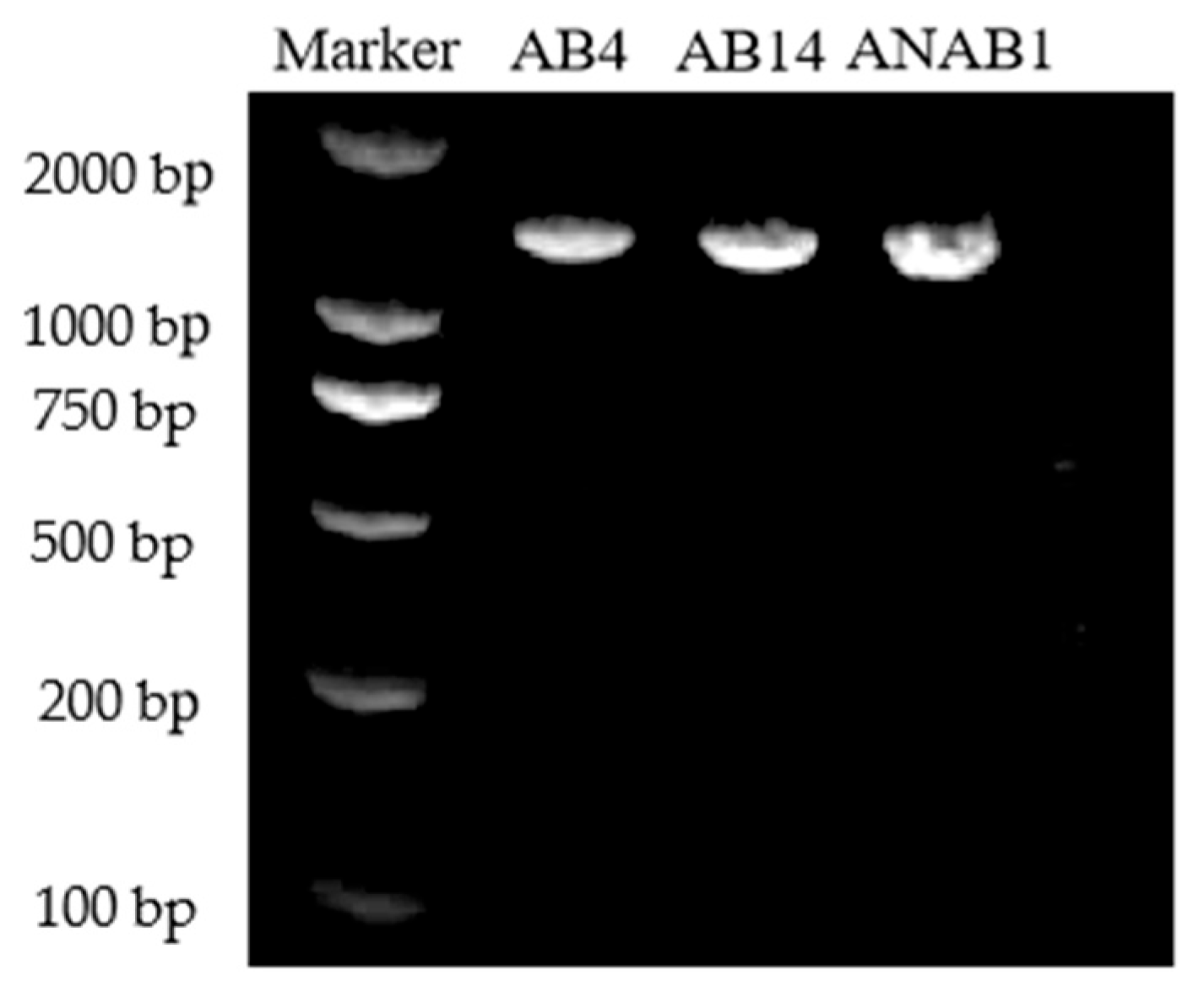
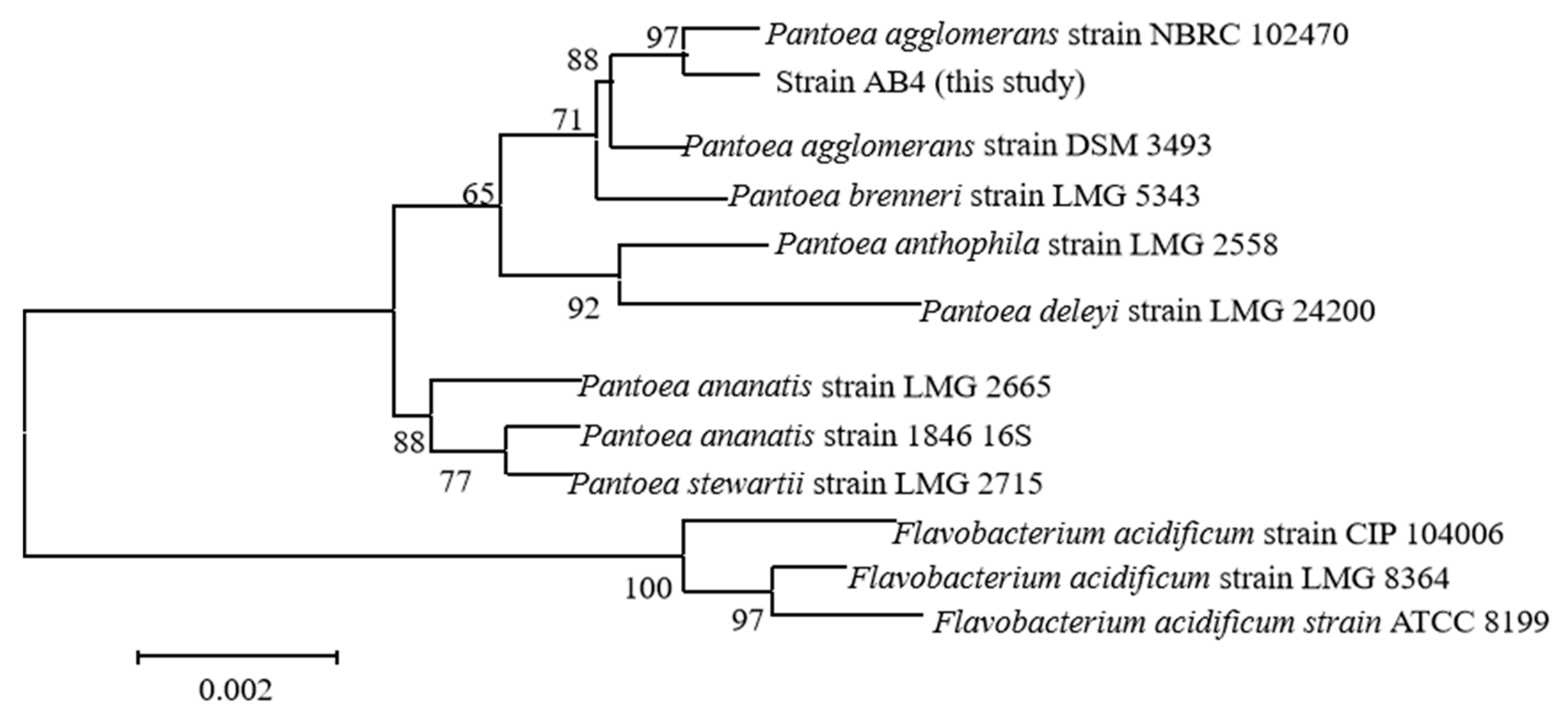
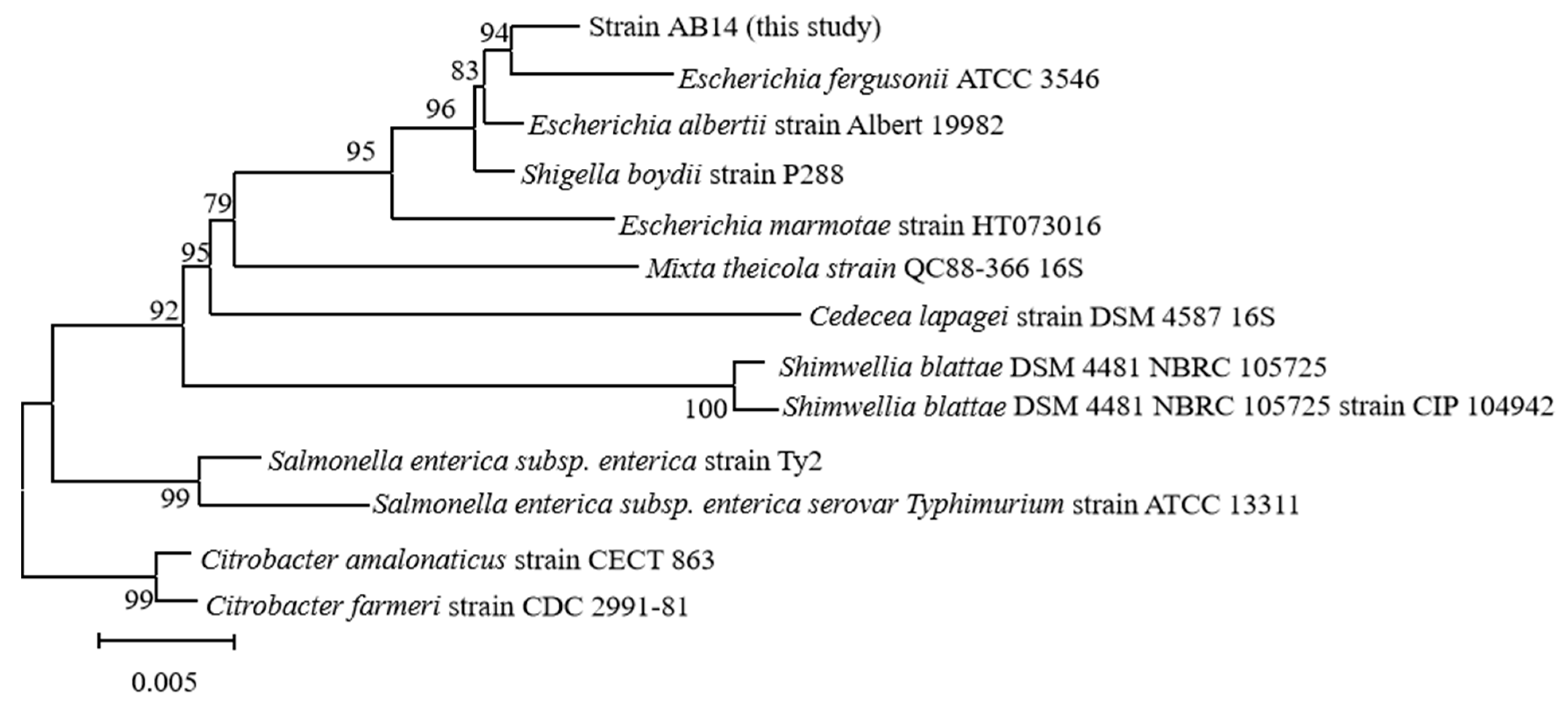
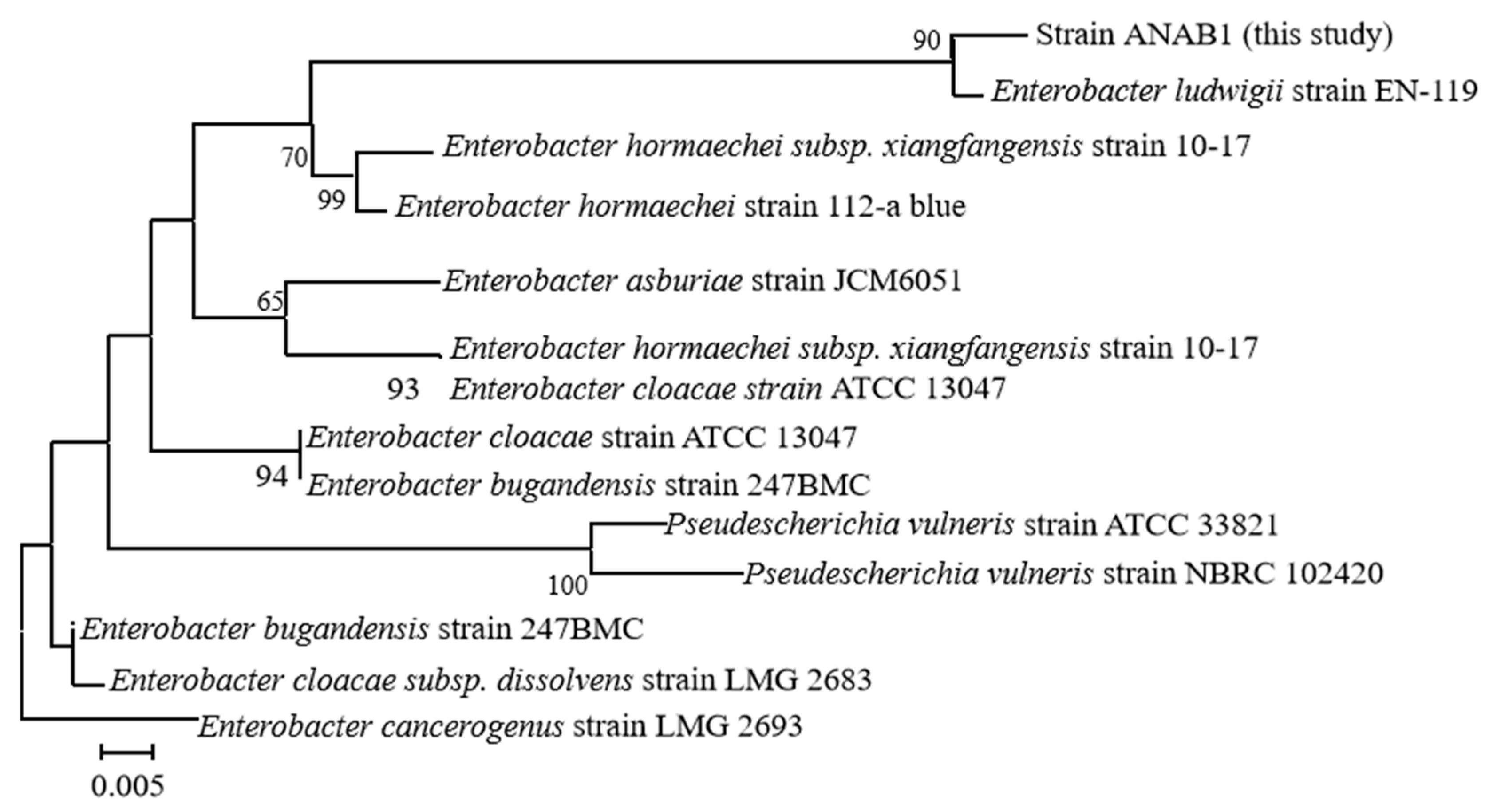
3.4. Pathogen Identification
- (1)
- Molecular identification
- (2)
- Physiological and biochemical identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, J.; Wang, S.; Ding, C.; Ma, C.; Chen, X.; Wang, J.; Yang, M.; Su, X. Correlation Analysis of the Bacterial Community and Wood Properties of Populus × Euramericana Cv. “74/76” Wet Heartwood. Front. Microbiol. 2022, 13, 868078. [Google Scholar] [CrossRef]
- Zu, B.S. Foreign studies on wet heart wood of poplars. Sci. Silvae Sin. 2000, 36, 85–91. [Google Scholar]
- Worrall, J.J.; Parmeter, J.R., Jr. Formation and Properties of Wetwood in White Fir. Phytopathology 1982, 72, 1209–1212. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.; Hu, Q.; Yang, J.; Zhao, T.; Du, K. Factors affecting poplar wetwood characteristics. J. For. Res. 2023, 34, 1615–1626. [Google Scholar] [CrossRef]
- Ward, J.; Schink, B. Microaerobic and anaerobic bacterial activities involved in formation of wetwood and discoloured wood. IAWA J. 1984, 5, 105–109. [Google Scholar] [CrossRef]
- Krause, C.; Gagnon, R. Wet heartwood distribution in the stem, stump, and root wood of black spruce in the Quebec boreal forest, Canada. North. J. Appl. For. 2005, 22, 12–18. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Xu, B.; Zheng, S. Study on the Occurrence, Distribution and Properties of Wet Heartwood in I-69 Poplar. For. Res. 1993, 6, 480–485. [Google Scholar]
- Xu, H.M.; Hu, X.Y.; Zheng, L.Y.; Fan, X.P. Research progress on wetwood. Hubei For. Sci. Technol. 2010, 4, 39–42. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Peng, Y.; Wu, Z.; Zhang, Q.; Li, Z.; Shi, C.; Du, K. Amplicon Sequencing Reveals Different Microbial Communities in Living Poplar Wetwood and Sapwood. Trees 2019, 33, 851–865. [Google Scholar] [CrossRef]
- Ward, J.C.; Pong, W.Y. Wetwood in Trees: A Timber Resource Problem; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1980. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Ward, J.C. Methane formation in living trees: A microbial origin. Science 1974, 184, 1181–1183. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Shu, D.Y.; Liu, S.Q. pH value, buffer content and the content of chemical components between heartwood and sapwood in poplar 72 and poplar 69. J. Anhui Agric. Univ. 2005, 32, 360–364. [Google Scholar] [CrossRef]
- Martin, L.; Brunel-Michac, N.; Conchon, P.; Cochard, H.; Badel, E. Mechanism of Wetwood Formation in Silver Fir (Abies alba Mill.). Trees 2024, 38, 1581–1592. [Google Scholar] [CrossRef]
- Shaw, D.; Edmonds, R.; Littke, W. Incidence of wetwood and decay in precommercially thinned western hemlock stands. Can. J. For. Res. 1995, 25, 1269–1277. [Google Scholar] [CrossRef]
- Murdoch, C. Stem and branch distribution of wetwood and relationship of wounding to bleeding in American elm trees. Plant Dis. 1984, 68, 890–892. [Google Scholar] [CrossRef]
- Guan, L.H.; Pan, H.X.; Huang, M.R.; Shi, J.S. Genetic variation of new clones of Populus deltoides (I-69) × Populus euramericana (I-45). J. Zhejiang For. Coll. 2004, 21, 376–381. [Google Scholar]
- Anguita-Maeso, M.; Olivares-García, C.; Haro, C.; Imperial, J.; Navas-Cortés, J.A.; Landa, B.B. Culture-Dependent and Culture-Independent Characterization of the Olive Xylem Microbiota: Effect of Sap Extraction Methods. Front. Plant Sci. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Warshaw, J.E.; Leschine, S.B.; Canale-Parola, E. Anaerobic cellulolytic bacteria from wetwood of living trees. Appl. Environ. Microbiol. 1985, 50, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Schink, B.; Ward, J.C.; Zeikus, J.G. Microbiology of wetwood: Role of anaerobic bacterial populations in living trees. Microbiology 1981, 123, 313–322. [Google Scholar] [CrossRef]
- Chen, C.W.; Sun, Q.G.; Zhu, J.; Wu, X.P.; Huang, H.Q.; Bao, S.X. Isolation and Screening for Endophyte with Antitumor Activities from Three Medicinal Plants. Microbiol. China 2010, 37, 1462–1466. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, K.; Sun, M.; Zhang, J.; Liu, L.; Niu, S. Isolation and Identification of Pathogens of Morchella sextelata Bacterial Disease. Front. Microbiol. 2023, 14, 1231353. [Google Scholar] [CrossRef]
- Dowarah, B.; Agarwal, H.; Krishnatreya, D.B.; Sharma, P.L.; Kalita, N.; Agarwala, N. Evaluation of seed-associated endophytic bacteria from tolerant chilli cv. Firingi Jolokia for their biocontrol potential against bacterial wilt disease. Microbiol. Res. 2021, 248, 126751. [Google Scholar] [CrossRef]
- Lin, L.-H.; Ntambo, M.S.; Rott, P.C.; Wang, Q.-N.; Lin, Y.-H.; Fu, H.-Y.; Gao, S.-J. Molecular detection and prevalence of Xanthomonas albilineans, the causal agent of sugarcane leaf scald, in China. Crop Prot. 2018, 109, 17–23. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Bergey, D.H.; Holt, J.G. Bergey’s Manual of Determinative Bacteriology, 8th ed.; Williams & Wilkins: Baltimore, MD, USA, 1993. [Google Scholar]
- Chi, M. The Endophyte Diversity and the Screening of Antagonistic Bacteria in Poplar Stems; Chinese Academy of Forestry: Beijing, China, 2014; pp. 1–19. [Google Scholar]
- An, X.Y.; Ma, Y.M.; Liu, X.X.; Zhou, T.N.; Yan, H.; Jia, L.H. Identification of a rare actinomycetes and screening of natural products biosynthesis genes from valerian rhizosphere soil. Acta Agric. Boreali-Occident. Sin. 2023, 7, 1080–1086. [Google Scholar]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef]
- O’Mahony, F.C.; Papkovsky, D.B. Rapid high-throughput assessment of aerobic bacteria in complex samples by fluorescence-based oxygen respirometry. Appl. Environ. Microbiol. 2006, 72, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.J. Fundamentals of Microbiology; Hohai University Press: Nanjing, China, 2000. [Google Scholar]
- Cao, H.; Pan, J.; Zhang, Y.; Li, Y. A Method for Anaerobic Bacterial Isolation and Cultivation. China Patent CN101270334B, 20 April 2011. [Google Scholar]
- Tuttle, A.R.; Trahan, N.D.; Son, M.S. Growth and Maintenance of Escherichia coli Laboratory Strains. Curr. Protoc. 2021, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Sherald, J.L. Pathogenicity of Xylella fastidiosa in American elm and failure of reciprocal transmission between strains from elm and sycamore. Plant Dis. 1993, 77, 190–193. [Google Scholar] [CrossRef]
- Fang, Z.D. Methods of Plant Pathology Research; Agricultural Press: Beijing, China, 1979. [Google Scholar]
- Suo, Y.T.; Zhang, Y.N.; Li, Z.; Zhao, M.Z.; Shen, W.N.; Fu, Y.C.; Wang, D.L.; Pan, L.; Su, X.H.; Zhao, J.P. In vivo leaf inoculation: An alternative method to assess the disease resistance of hybrid clones in poplar breeding of stem canker disease. J. Vis. Exp. 2024, 211, e67290. [Google Scholar] [CrossRef]
- Ainsworth, G.C.; Jones, B.L. Plant Pathology, 5th ed.; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Song, C.C.; Wang, K.; Mei, L.; Fan, X.P.; Zhang, S.; Zhu, Y.J. Pathogen isolation, identification and infection characteristics of wet-heartwood disease in poplar. J. Huazhong Agric. Univ. 2017, 36, 15–21. [Google Scholar] [CrossRef]
- Minas, K.; McEwan, N.R.; Newbold, C.J.; Scott, K.P. Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol. Lett. 2011, 325, 162–169. [Google Scholar] [CrossRef]
- Yarza, P.; Richter, M.; Peplies, J.; Euzeby, J.; Amann, R.; Schleifer, K.-H.; Ludwig, W.; Glöckner, F.O.; Rosselló-Móra, R. The all-species living tree project: A 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 2008, 31, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Song, C.C.; Mei, L.; Cai, L.; Zhang, S.; Fan, X.P. Identification of pathogen bacteria and antagonistic bacteria of wet-heart wood disease in Populus. J. Southwest For. Univ. 2018, 38, 142–152. [Google Scholar]
- She, X.M.; Yu, L.; Lan, G.B.; Tang, Y.F.; Li, Z.G.; Deng, M.G.; He, Z.F. Identification of the pathogen causing Ziziphus jujuba Mill. cv. Dongzao leaf necrotic disease in Guangdong Province. Acta Phytopathol. Sin. 2021, 51, 317–324. [Google Scholar] [CrossRef]
- Ke, H.J.; Wang, Y.; Wei, T.; Gu, C.; Liu, H.X.; Guo, J.H. Suppression of tomato yellow leaf curl virus disease by the Pantoea agglomerans strain ljb-2 in the field. Acta Hortic. Sin. 2014, 41, 985–993. [Google Scholar] [CrossRef]
- Cruz, A.T.; Cazacu, A.C.; Allen, C.H. Pantoea agglomerans, a plant pathogen causing human disease. J. Clin. Microbiol. 2007, 45, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Wang, F.F.; Yan, J.; Ye, J.B.; Ma, K.; Mao, D.B. Optimization of medium for bacterial strain of carotenoids degradation. Sci. Technol. Food Ind. 2016, 5, 210–214+219. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Steigerwalt, A.G.; Hill, B.C.; Farmer, J.J., 3rd. Enterobacter hormaechei, a new species of the family Enterobacteriaceae formerly known as Enteric group 75. J. Clin. Microbiol. 1989, 27, 2046–2049. [Google Scholar] [CrossRef]
- Srinivas, K.; Ghatak, S.; Pyngrope, D.A.; Angappan, M.; Milton, A.A.P.; Das, S.; Lyngdoh, V.; Lamare, J.P.; Prasad, M.C.B.; Sen, A. Avian strains of emerging pathogen Escherichia fergusonii are phylogenetically diverse and harbor the greatest AMR dissemination potential among different sources: Comparative genomic evidence. Front. Microbiol. 2023, 13, 1080677. [Google Scholar] [CrossRef]
- Wang, K. The Infection Way of Wetwood Disease Pathogens of Poplar and Their Effects on the Growth of Poplar; Huazhong Agricultural University: Wuhan, China, 2015. [Google Scholar]
- Zheng, H.Y.; Dai, Y.S.; Xie, C.X.; Guo, T.B.; Wang, Z.Y. Studies on the cause of formation of poplar red core wood. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2006, 30, 82–84. [Google Scholar]
- Eckert, C.; Wildhagen, H.; Paulo, M.J.; Scalabrin, S.; Ballauff, J.; Schnabel, S.K.; Vendramin, V.; Keurentjes, J.J.B.; Bogeat-Triboulot, M.-B.; Taylor, G.; et al. Genotypic and tissue-specific variation of Populus nigra transcriptome profiles in response to drought. Sci. Data 2022, 9, 297. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. Bacterial identification for publication: When is enough enough? J. Clin. Microbiol. 2002, 40, 1887–1891. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Glaeser, S.P.; Kämpfer, P. Multilocus sequence analysis (MLSA) in prokaryotic taxonomy. Syst. Appl. Microbiol. 2015, 38, 237–245. [Google Scholar] [CrossRef]
- Adesina, T.; Nwinyi, O.; De, N.; Akinnola, O.; Omonigbehin, E. First Detection of Carbapenem-Resistant Escherichia fergusonii Strains Harbouring Beta-Lactamase Genes from Clinical Samples. Pathogens 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- De Maayer, P.; Green, T.; Jordan, S.; Smits, T.H.M.; Coutinho, T.A. Pan-Genome Analysis of the Enterobacter hormaechei Complex Highlights Its Genomic Flexibility and Pertinence as a Multidrug Resistant Pathogen. BMC Genom. 2025, 26, 11590. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, B.; Bachani, P.; Singh, A.; Mishra, S. Enterobacter hormaechei as Plant Growth-Promoting Bacteria for Improvement in Lycopersicum esculentum. Curr. Microbiol. 2021, 78, 1208–1217. [Google Scholar] [CrossRef] [PubMed]

| Dilution Factor | Replication | CFU(/g) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Aerobic bacteria | 10−2× (CFUAN-1) | >300 | >300 | >300 | Incalculable |
| 10−3× (CFUAN-2) | 211 | 206 | 208 | 2.08 × 106 | |
| 10−4× (CFUAN-3) | 22 | 20 | 43 | 2.83 × 106 | |
| 10−5× (CFUAN-4) | 3 | 5 | 21 | 2.1 × 106 | |
| 10−6× (CFUAN-5) | 0 | 0 | 0 | Incalculable | |
| 10−7× (CFUAN-6) | 0 | 0 | 0 | Incalculable | |
| Anaerobic bacteria | 10−2× (CFUANAB-1) | 175 | 130 | 151 | 1.52 × 105 |
| 10−3× (CFUANAB-2) | 41 | 46 | 53 | 4.67 × 105 | |
| 10−4× (CFUANAB-3) | 6 | 1 | 18 | Incalculable | |
| 10−5× (CFUANAB-4) | 0 | 0 | 0 | Incalculable | |
| 10−6× (CFUANAB-5) | 0 | 0 | 0 | Incalculable | |
| 10−7× (CFUANAB-6) | 0 | 0 | 0 | Incalculable | |
| Strains | Color | Colony Morphology | Strains | Color | Colony Morphology | |
|---|---|---|---|---|---|---|
| Aerobic bacteria (AB) | AB1 | White | Small, smooth edges, flat, round | AB15 | Off-white | Medium, transparent center, eye-like, dry |
| AB2 | Yellow | Small, punctate, elevated | AB16 | Transparent | Large, smooth edges, smooth surface | |
| AB3 | Transparent | Medium-sized, smooth surface | AB17 | White | Small, punctate | |
| AB4 | Yellow | Punctate, round, elevated | AB18 | Transparent | Small dots, smooth and even edges | |
| AB5 | Off-white | Small, punctate, smooth, elevated | AB19 | Transparent | Medium, irregular | |
| AB6 | Transparent | Round | AB20 | Off-white | Off-white, irregular, elevated, wrinkled | |
| AB7 | Round | Smooth, elevated | AB21 | Transparent | Large, flat | |
| AB8 | White | Large, irregular, wet, elevated | AB22 | Off-white | Large, irregular, wrinkled and elevated | |
| AB9 | Transparent | Small, smooth, round | AB23 | Transparent | Small, round, flat | |
| AB10 | White | Wrinkled, elevated | AB24 | Yellow | Small, smooth edges | |
| AB11 | Transparent | Smooth, elevated | AB25 | Transparent | Small, smooth surface, elevated | |
| AB12 | White | Large, irregular, wrinkled and elevated | AB26 | Off-white | Large, rapid growth, irregular edges | |
| AB13 | Yellow | Smooth, elevated | AB27 | White | Large, smooth edges | |
| AB14 | White | Small, smooth | AB28 | Off-white | Medium, irregular halo at colony edge | |
| Anaerobic bacteria (ANAB) | ANAB1 | Grayish-white | Medium, round, even edges | ANAB5 | White | Large, elevated, moist |
| ANAB2 | Yellow | Small, round, smooth edges | ANAB6 | Off-white center, transparent edges | Medium, deeper color in the center than the edge, eye-like | |
| ANAB3 | White | Round, smooth, elevated | ANAB7 | Yellow | Small, punctate | |
| ANAB4 | Transparent | Medium, thin, extremely irregular serrated edges |
| Inoculation Method | Treatments | Height Growth (cm) | Root-Collar Diameter Growth (cm) | Inoculation-Point Diameter Growth (mm) | Heartwood Discoloration Rate (%) |
|---|---|---|---|---|---|
| Stem injection | CK1 | 29.33 ± 1.47 | 0.77 ± 0.03 | 6.33 ± 2.20 | 0.00 ± 0.00 |
| CK2 | 28.88 ± 0.77 | 0.65 ± 0.11 | 7.43 ± 1.20 | 0.00 ± 0.00 | |
| Bacterial suspension | 27.15 ± 4.47 | 0.82 ± 0.27 | 15.73 ± 4.10 * | 100.00 ± 0.00 * | |
| Root infection | CK1 | 26.21 ± 1.95 | 0.69 ± 0.06 | N/A | 0.00 ± 0.00 |
| CK2 | 25.45 ± 0.37 | 0.77 ± 0.35 | N/A | 0.00 ± 0.00 | |
| Bacterial suspension | 24.59 ± 3.97 | 0.73 ± 0.22 | N/A | 0.00 ± 0.00 | |
| Leaf infection | CK1 | 25.28 ± 1.84 | 0.87 ± 0.35 | N/A | 0.00 ± 0.00 |
| CK2 | 26.34 ± 0.56 | 0.46 ± 0.03 | N/A | 0.00 ± 0.00 | |
| Bacterial suspension | 24.18 ± 4.55 | 0.58 ± 0.10 | N/A | 0.00 ± 0.00 |
| Item | Result | |||||
|---|---|---|---|---|---|---|
| AB4 | Pantoea agglomerans | AB14 | Escherichia fergusonii | ANAB1 | Enterobacter hormaechei | |
| Semi-solid Culture Medium | + | + | + | + | + | + |
| Glucose Fermentation | + | + | − | − | − | − |
| Ornithine Decarboxylase Medium | − | − | + | + | + | + |
| Lysine Decarboxylase Medium | − | − | + | + | + | + |
| Amino Acid Decarboxylase Medium | − | − | − | − | − | − |
| Simon’s Citrate | − | − | + | (+) | + | + |
| Hydrogen Sulfide | − | − | − | − | − | − |
| Urease | − | − | − | + | − | − |
| Protein Hydrolysate | − | − | + | + | − | − |
| MR | − | + | + | + | − | − |
| VP | − | − | − | − | + | + |
| Phenylalanine | − | (+) | − | − | − | − |
| Mannitol | + | + | + | + | + | + |
| Inositol | − | − | − | − | − | − |
| Sorbitol | + | + | − | − | + | + |
| Maltose | + | + | + | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, Q.; Hu, X.; Ren, Z.; Tang, H.; Du, K. Characterization of Pathogenic Bacteria Associated with Wetwood Disease in Populus deltoides. Forests 2025, 16, 1414. https://doi.org/10.3390/f16091414
Jiang Y, Zhang Q, Hu X, Ren Z, Tang H, Du K. Characterization of Pathogenic Bacteria Associated with Wetwood Disease in Populus deltoides. Forests. 2025; 16(9):1414. https://doi.org/10.3390/f16091414
Chicago/Turabian StyleJiang, Yilei, Qilin Zhang, Xingyi Hu, Zekai Ren, Haiyan Tang, and Kebing Du. 2025. "Characterization of Pathogenic Bacteria Associated with Wetwood Disease in Populus deltoides" Forests 16, no. 9: 1414. https://doi.org/10.3390/f16091414
APA StyleJiang, Y., Zhang, Q., Hu, X., Ren, Z., Tang, H., & Du, K. (2025). Characterization of Pathogenic Bacteria Associated with Wetwood Disease in Populus deltoides. Forests, 16(9), 1414. https://doi.org/10.3390/f16091414






