Abstract
Abies koreana E.H.Wilson (Korean fir), an endangered high-altitude conifer native to South Korea, is facing severe population decline due to climate change and low germination rates. While ecological factors have been studied, the genetic and epigenetic mechanisms underlying its seed development are still poorly understood. DNA methylation, regulated by MET1 and CMT2, plays a critical role in the stability of gene expression during seed development. This study investigates the expression patterns of MET1 and CMT2 across 12 developmental stages, from pre-germination to post-germination, with a focus on shoot and root tissues. RNA-seq data were analyzed to identify MET1 and CMT2, and expression patterns were validated using RT-qPCR. MET1 showed high sequence conservation with conifers such as Pinus sylvestris, indicating potential conservation of CG methylation mechanisms among conifer species. CMT2 showed lower sequence conservation across species, indicating reduced evolutionary conservation compared to MET1. Tissue-specific analysis showed MET1 being predominantly active in shoots during cotyledon development, while CMT2 was upregulated in roots at later stages. These findings highlight the dynamic and tissue-specific roles of DNA methylation in the seed development of A. koreana, contributing to a better understanding of the genetic and epigenetic mechanisms involved in its germination and early growth.
1. Introduction
Abies koreana E.H.Wilson (Korean fir) is native to high-altitude forests above 1000 m in South Korea, making it particularly susceptible to the impacts of climate change [1,2]. As a high-altitude species, A. koreana faces unique challenges, including increased vulnerability to rising temperatures and habitat loss, which have been linked to the species’ ongoing population decline [3]. Due to these threats, the species has been designated as endangered by the IUCN, underscoring the urgent need for conservation efforts [4].
Various studies have highlighted the connection between global warming and the decline of A. koreana populations, with reports of severe mortality rates across its native habitats [5]. While ecological research has focused on the external factors affecting A. koreana [6,7], relatively little is known about the genetic and epigenetic mechanisms controlling its response to such stressors. Understanding the genetic basis of A. koreana’s reproductive biology is critical, given its low seed germination rates and poor seedling survival, which further compound the challenges to population recovery [8]. In particular, DNA methylation, a key epigenetic modification, is essential in regulating gene expression during seed development. By enabling transcriptional reprogramming, DNA methylation facilitates plant growth and adaptation to environmental stress [9]. Thus, investigating the role of DNA methylation in A. koreana seed development is necessary to bridge the knowledge gap in its reproductive biology and contribute to conservation efforts.
Two key enzymes, MET1 (Methyltransferase 1) and CMT2 (Chromomethylase 2), are involved in maintaining DNA methylation at CG and non-CG sites, respectively, and are critical for stabilizing gene expression patterns during seed germination and development [10,11]. MET1 is responsible for maintaining methylation at CG sites and ensuring the faithful transmission of epigenetic marks during DNA replication [12]. CMT2, on the other hand, targets CHH sites, which are often associated with repetitive elements and transposable elements and are crucial in silencing potentially deleterious genomic sequences [13]. Since MET1 and CMT2 target distinct nucleotide sites, understanding the differential expression patterns of these two genes is critical for uncovering their roles in various stages of seed development.
While DNA methylation is known to be crucial for developmental transition in plants, its specific function in A. koreana remains unexamined. Investigating these genes across different developmental stages provides fundamental insights into how epigenetic regulation contributes to seed development. A. koreana faces challenges such as low germination rates and environmental stress. This study aims to investigate the expression patterns of MET1 and CMT2 in A. koreana across 12 developmental stages, from pre-germination to post-germination, and in shoot and root tissues. This research advances knowledge of the molecular processes governing seed development in A. koreana, as understanding the molecular mechanisms underlying its early growth can provide a foundation for future conservation research.
2. Materials and Methods
2.1. Plant Materials and Germination Test
Samples of cones were collected from the natural population of A. koreana in the Yeongsil area of Mt. Halla National Park, Jeju Island. Cones were collected from 20 individual trees spaced 15 m apart in September 2022, with the cooperation of the Baekdudaegan National Arboretum (Figure 1A). A total of 28 cones from 10 trees were selected for the experiment, with those affected by insect damage excluded.
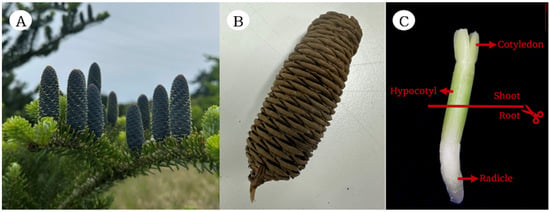
Figure 1.
Materials of A. koreana E.H.Wilson: (A) mature cone, (B) dried cone, and (C) embryo dissected from a seed for RNA extraction and gene expression analysis.
The cones were air-dried for a week to separate seeds and scales (Figure 1B). Only healthy and fully grown seeds were selected from the separated seeds. The seeds were then placed in a 90 mm Petri dish with two 90 mm filter papers moistened with sterilized water. Germination tests were carried out in a growth chamber at 20 °C, under 8 h light and 16 h dark conditions with 50% humidity [14]. Seed development was monitored daily for 28 days.
2.2. Germination Stages
Seeds with the highest germination potential were prioritized through a careful selection process. Damaged or underdeveloped seeds were excluded. Additional screening methods, including X-ray analysis and seed cutting, were employed to minimize errors in visual sorting. All cones from individuals with a high number of these low germination potential seeds were excluded from the experiment.
The pre-germination stage, Stage 0, involved imbibition in darkness at 4 °C for 12 h to synchronize biological clocks. The seeds were then exposed to different light conditions. Stage 1 involved a 12 h dark period followed by 1 h exposure to light. Stage 2 was exposed to light for 12 h after Stage 0, and Stage 3 was exposed to light for 24 h. A total of four pre-germination stages were defined based on continuous light exposure [15].
Post-germination stages were set based on morphological criteria. Stage 4 was defined as the point when the seed coat split, and Stage 5 was described as radicle emergence of at least 1 mm. Six additional stages (Stage 6 to Stage 11) were established by monitoring the daily development of 178 germinated seeds, focusing on the radicle, cotyledon, and hypocotyl.
2.3. Identification of DNA Methylation-Related Genes
This study used RNA-seq datasets (SRR6781816–SRR6781821) from the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) published in a prior study analyzing the transcriptome response of A. koreana in response to high-temperature stress. The dataset consists of six RNA-seq libraries, including three biological replicates for both control and heat-stressed samples [16]. The quality of the raw data was assessed through FastQC (v0.12.0, Babraham Institute), and low-quality reads were removed with Trimmomatic (v0.39, USADELLAB.org) using the following parameters: ILLUMINACLIP: TruSeq3-PE.fa:2:30:10, LEADING:3, TRAILING:3, SLIDINGWINDOW:4:15, MINLEN:36.
De novo transcriptome assembly was conducted using Trinity (v2.11.0, Broad Institute and Hebrew University) with the default k-mer size of 25. Reads with low-quality scores were removed before assembly, and the minimum contig length was set to 200 bp. The assembled transcriptome was analyzed using the ‘Trinity.Stats.pl’ script for calculating average contig length, median, and N50 values. ORFs exceeding 100 amino acids in length were extracted via TransDecoder. CD-HIT (v4.8.1) removed redundant isoforms, producing the final transcriptome.
A database comprising 3738 genes related to DNA methylation was constructed based on 371 plant and animal species. All available genes related to DNA methylation were downloaded from reference databases such as NCBI Gene, UniProt, and Ensembl, and the sequences were compared with the assembled A. koreana transcriptome. This database was used to compare sequences between the assembled A. koreana transcriptome and the existing gene database via NCBI BLASRN Ver.2.12.0+ (National Library of Medicine). To identify homologous genes across species, phylogenetic analysis was performed using MEGA (Molecular Evolutionary Genetics Analysis) (v11, Pennsylvania State University).
Genes were aligned using the default ALIGNMENT function, and a phylogenetic tree was constructed using the Neighbor-Joining method, with 2000 bootstrap replicates and ‘complete deletion’ for handling gaps in the data [17]. The selected genes were designed with primers using Primer3Plus (https://www.primer3plus.com/ (accessed on 26 September 2023)) (Table 1).

Table 1.
Primer information used for RT-qPCR in Abies koreana E.H.Wilson.
2.4. RT-qPCR Analysis
For RNA sample collection, embryos were dissected from seeds at each developmental stage. The embryos were divided into cotyledon (shoot) and radicle (root) tissues (Figure 1C). To ensure statistical robustness, RNA extraction was performed from three biological replicates collected for each stage and tissue, each of which included three embryos (combined) of each biological sample. RNA was extracted from a total of 72 samples, each including 12 stages, 2 tissues, and 3 biological replicates per condition. Samples were frozen in liquid nitrogen and stored at −80 °C prior to processing. Tissue disruption was performed using a TissueLyser (Qiagen, Hilden, Germany), and total RNA was extracted using the IQeasy™ Plus Plant Extraction Mini Kit (iNtRON, Seongnam-si, South Korea) according to protocol A. RNA purity and quantity were measured by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA).
All 72 RNA samples were synthesized into cDNA, each meeting the requirement of 100 ng. cDNA was synthesized using the ReverTra® Ace qPCR RT Master Mix (Toyobo, Osaka, Japan). The reaction mixture included 8 µL of diluted RNA template (total 100 ng) and 2 µL of RT Master Mix, resulting in a total reaction volume of 10 µL. The synthesis was performed under the following conditions: 15 min at 37 °C, 5 min at 50 °C, and 5 min at 98 °C. Synthesized cDNA was stored at −20 °C until further use.
Relative gene expression was assessed using RT-qPCR with ExcelTaqTM 2X Fast Q-PCR Master Mix (Sybr, no ROW) (SMOBIO, Hsinchu City, Taiwan). Each reaction contained 10 µL of Master Mix, 2 µL of cDNA template (total 100 fg), and 4 µL of forward and reverse primers (2 µM each), resulting in a total reaction volume of 20 µL. RT-qPCR was conducted using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Thermal cycling conditions included an initial denaturation step at 98 °C for 3 min, followed by 40 cycles of 98 °C for 15 s and 60 °C for 30 s. Fluorescence readings were collected after each cycle, and melting curve analysis was conducted from 65 to 95 °C at 0.5 °C increments with a 5 s hold. Relative gene expression was quantified using RT-qPCR, with Actin serving as a reference.
2.5. Statistical Methods
The relative gene expression (ΔCq, ΔΔCq, and fold change (FC)) was calculated using the following methods [18]. ΔCq values were determined using the difference between target and reference genes, and ΔΔCq values were calculated relative to the control group. Statistical analysis of FC values for gene expression across developmental stages was conducted using a one-way ANOVA and Duncan’s multiple range test in SPSS Ver. 28.0 (IBM, Armonk, NY, USA). To evaluate differences between tissues within the same developmental stage, a t-test was performed. Graphs were generated using Prism Ver. 10.1.0 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Developmental Stages of Seed Germination
To synchronize the developmental timing of A. koreana seeds, the embryos were analyzed across four pre-germination stages. No morphological differences were observed between Stage 0 (dark, 12 h imbibition) and Stage 1 (1 h light exposure), indicating that early light exposure did not yet trigger a notable developmental response. In contrast, by Stage 2 (12 h light exposure), the embryos exhibited discernible changes, such as firmer tissues and the development of chlorophyll, indicating the onset of radicle differentiation. The embryos in Stage 2 and Stage 3 (24 h light exposure) showed no significant morphological differences.
Clear morphological changes were observed once germination began. At Stage 4, characterized by the swelling of the micropyle and detachment of the seed coat, radicle emergence became visible. Further stages (Stages 5–11) exhibited a gradual increase in radicle elongation followed by cotyledon development. The most prominent changes were the length growth of the radicle at Stage 6 and the initiation of cotyledon expansion at Stage 7. By Stage 11, the cotyledons had developed into needle-like structures, marking a transition into a juvenile plant-like morphology.
3.2. Transcriptome Assembly of A. koreana
The transcriptomic analysis of A. koreana was performed using high-temperature stress RNA-seq data from six samples. A total of 347,361,434 100 bp reads were assembled using the de novo approach. The Trinity statistics revealed the assembly of 1,212,229 transcripts and 635,467 genes, with an average GC content of 41.00% (Table 2). Compared to the previous study that reported 42,056 unigenes, our analysis retained all transcript isoforms, rather than collapsing them into unigene representatives. This approach was chosen to maximize gene identification, ensuring that all potential isoforms related to DNA methylation genes were captured, rather than limiting the dataset to only a single representative sequence per gene.

Table 2.
Statistics of transcripts and genes from de novo assembly in A. koreana.
3.3. DNA Methylation Regulatory Genes
The BLAST search identified 96 candidate DNA methylation-related genes from 18 species. Of these, 24 were classified as MET1 or CMT2 genes, both of which are responsible for maintaining DNA methylation patterns. MET1, primarily from Arabidopsis thaliana, showed higher sequence similarity with conifers such as Pinus sylvestris (89.88% identity, E-value 0). CMT2, on the other hand, was less conserved and showed lower sequence similarity across species.
MET1 gene sequences were compared with those from related species. Among the best matches, the sequence from P. sylvestris showed the highest identity (89.88%) and formed a close phylogenetic relationship (Figure 2). Similarly, two candidate CMT2 sequences were identified with high identity (80.17%) to Jatropha curcas. These findings suggest that both MET1 and CMT2 in A. koreana share a close relationship with conifers and other plant species.
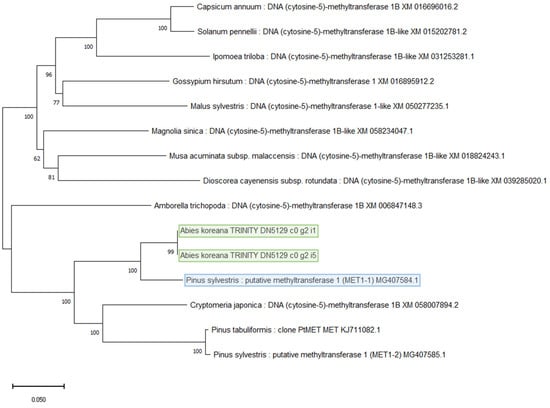
Figure 2.
Phylogenetic tree of MET1 gene in A. koreana.
3.4. Gene Expression Analysis
MET1 and CMT2 expressions were monitored in embryo tissues across the germination stages. MET1 expression was low during the pre-germination stages (Stages 0–3) but increased significantly at Stages 6 and 7, coinciding with rapid stem growth. CMT2 showed higher expression at Stage 4, with an additional peak at Stage 6, suggesting a role in cotyledon development (Figure 3).
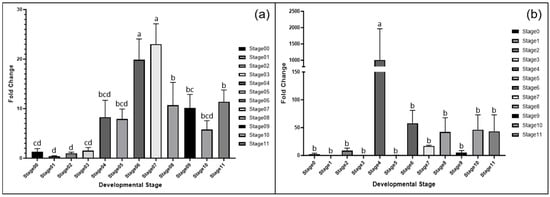
Figure 3.
Fold change in gene expression in the shoot tissue for each developmental stage determined via RT-qPCR analysis. (a) MET1 and (b) CMT2. Different lowercase letters indicate statistically significant differences among developmental stages, as determined by Duncan’s multiple range test (p ≤ 0.05).
In root tissues, MET1 expression was relatively low during the pre-germination stages and only increased significantly at Stage 5, peaking at Stage 6. CMT2 showed a similar pattern to MET1 but exhibited an additional increase during Stages 9 and 11, indicating a role in later root development (Figure 4).
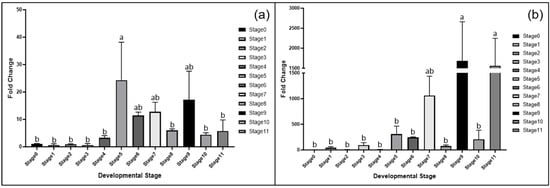
Figure 4.
Fold change in gene expression in the root tissue for each = developmental stage determined via RT-qPCR analysis. (a) MET1 and (b) CMT2. Different lowercase letters indicate statistically significant differences among developmental stages, as determined by Duncan’s multiple range test (p ≤ 0.05).
Statistical analysis identified developmental stages where gene expression differences between shoot and root tissues were statistically significant, and only these stages were presented in the graphs. MET1 was predominantly expressed in shoot tissues at Stage 8, while CMT2 showed higher expression in root tissues at multiple stages (Stages 3, 4, 5, 7, and 10) (Figure 5). Notably, at Stage 4, CMT2 expression in root tissues was approximately 1000 times higher than in shoot tissues, highlighting a significant tissue-specific role during this developmental stage (Figure 5C).
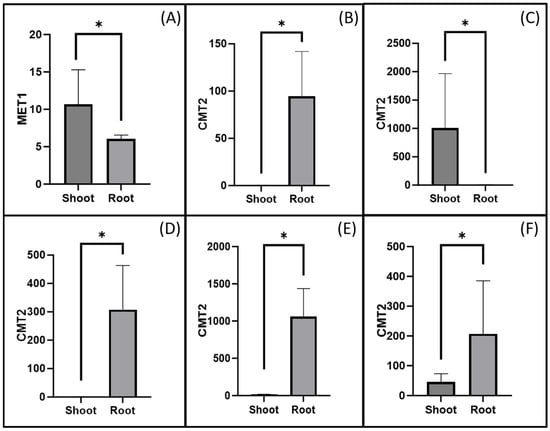
Figure 5.
Differences in fold change in the gene expression of MET1 and CMT2 by tissue within the same stage. (A) MET1 in Stage 8, (B) CMT2 in Stage 3, (C) CMT2 in Stage 4, (D) CMT2 in Stage 5, (E) CMT2 in Stage 7, and (F) CMT2 in Stage 10. Asterisks (*) indicate statistically significant differences between groups (p ≤ 0.05, t-test).
4. Discussion
4.1. Seed Germination and Development
The absence of significant morphological differences between Stages 0 and 1 in A. koreana suggests that early transcriptional changes require prolonged environmental stimuli, particularly light exposure. By Stage 2, the differentiation of the radicle becomes evident, highlighting light as a crucial factor in triggering early developmental processes. This aligns with prior research that identifies radicle elongation as the first critical step in germination, enabling the seedling to absorb water and nutrients essential for its development [19].
Water absorption during radicle elongation is also crucial for initiating metabolic activities necessary for seedling development [20]. This process marks the transition from dormancy to active growth and underscores the importance of environmental factors such as light and water in regulating these early stages. In addition to enabling nutrient uptake, radicle growth also triggers hormonal changes that further stimulate shoot development [21].
The coordination between radicle elongation and subsequent shoot emergence highlights the complex, stage-specific responses of seedlings to their environment, emphasizing the intricate balance between water uptake and photosynthetic capacity in driving early seedling development. This interaction aligns with broader research on conifer species, where both external environmental conditions and internal developmental signals are crucial for successful germination and growth [22,23]. Recent studies on NtCIPK23, a homolog of AtCIPK23 in Nicotiana tabacum, further support the critical role of specific regulatory genes in seed germination and early seedling development. NtCIPK23 was shown to enhance cotyledon greening, cotyledon expansion, and hypocotyl elongation under light and dark conditions, emphasizing its role in modulating seedling responses to environmental stimuli [24]. This highlights a potential intersection between molecular regulation and environmental cues, which may also apply to understanding the developmental processes in A. koreana.
4.2. DNA Methylation and Gene Regulation
The identification of MET1 and CMT2 genes, which regulate DNA methylation at CG and non-CG sites, respectively, underscores the conserved nature of methylation in controlling gene expression during plant development [11]. MET1’s high sequence conservation with other conifers, particularly P. sylvestris, suggests that its sequence is well preserved across conifer species [13]. In contrast, CMT2 exhibited lower sequence conservation, indicating greater variability in its sequence across different plant species [25].
The observed increase in MET1 and CMT2 expression in A. koreana shoot and root tissues suggests that these genes may be involved in regulating gene expression during seedling development. The upregulation of CMT2 during development points to its critical role in maintaining genome stability during rapid cell division, a finding consistent with other studies [13].
4.3. Tissue-Specific Methylation
The differential expression of MET1 and CMT2 between shoots and roots suggests that methylation demands vary between these tissues during seed germination. Higher MET1 expression in shoots during cotyledon development indicates a role for CG methylation in regulating genes related to photosynthetic capacity and shoot differentiation [26]. In contrast, the increased expression of CMT2 in roots points to its involvement in maintaining genome integrity in these rapidly growing tissues by suppressing transposable elements [10].
These tissue-specific methylation patterns are consistent with findings in other plant species, where distinct epigenetic mechanisms regulate root and shoot development [9]. The dynamic changes in methylation activity, as summarized in the graphical abstract, illustrate clear transitions in MET1 and CMT2 expression around germination. These patterns emphasize the essential function of DNA methylation in tissue-specific transcriptional reprogramming during the seed germination and early development of A. koreana.
The significant differences in gene expression between tissues highlight the complexity of methylation in seed development and support the importance of understanding these dynamics in the context of A. koreana’s adaptation to environmental stressors. As climate change continues to threaten this species, further research into epigenetic regulation could provide valuable insights into its resilience.
5. Conclusions
This study investigated the stage- and tissue- specific expression of MET1 and CMT2 during seed germination and early seedling development in A. koreana, an endangered conifer species. The findings indicate that MET1 is highly conserved among conifers, whereas CMT2 shows sequence variability compared to MET1. Given that MET1 and CMT2 target distinct nucleotide sites, the observed differences in their sequence variability warrant further investigation. Future studies are needed to investigate the potential impact of their genetic variation on their regulatory functions.
The differences in gene expression throughout developmental stages suggest that DNA methylation may play an important role in controlling seed germination. Further research integrating genome-wide methylation analysis and functional validation studies will be necessary to fully elucidate the role of DNA methylation in conifer seed germination. Additionally, understanding how environmental factors interact with epigenetic regulation could offer broader implications for plant adaptation under climate change.
Author Contributions
S.-c.H. and K.-s.K. conceived, designed, and performed the experiments and wrote the manuscript. K.J. contributed to the experiments and provided assistance in writing the manuscript. S.-c.H.: writing—review and editing, writing—original draft, visualization, methodology, investigation, formal analysis, data curation, conceptualization. K.J.: investigation, methodology, writing—review and editing. K.-s.K.: writing—review and editing, supervision, investigation, formal analysis, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of the ‘R&D Program for Forest Science Technology (Project No. RS-2024-00404133)’ provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Koo, K.A.; Kim, J.; Kong, W.S.; Jung, H.; Kim, G. Projecting the potential distribution of Abies koreana in Korea under the climate change based on RCP scenarios. Korea Soc. Environ. Restor. Technol. 2016, 19, 19–30. [Google Scholar] [CrossRef]
- Ahn, U.S.; Yun, Y.S. Causes of decline in the Korean fir based on spatial distribution in the Mt. Halla region in Korea: A meta-analysis. Forests 2020, 11, 391. [Google Scholar] [CrossRef]
- Choo, M.; Yo, C.; Im, J.; Cho, D.; Kang, Y.; Oh, H.; Lee, J. Trend analysis of vegetation changes of Korean fir (Abies koreana Wilson) in Hallasan and Jirisan using MODIS imagery. Korean Soc. Remote Sens. 2023, 39, 325–338. [Google Scholar]
- Song, J.H.; Han, S.H.; Lee, S.H.; Yun, C.W. Ecological characteristic of Abies koreana stand structure of Mt. Jirisan and Mt. Hallasan. J. Korean Soc. For. Sci. 2021, 110, 590–600. [Google Scholar]
- Kwak, M.; Hong, J.K.; Park, J.H.; Lee, B.Y.; Suh, M.H.; Kim, C.S. Genetic assessment of Abies koreana (Pinaceae), the endangered Korean fir, and conservation implications. Conserv. Genet. Resour. 2017, 18, 1165–1176. [Google Scholar] [CrossRef]
- Koo, K.A.; Kim, D.B. Review forty-year studies of Korean fir (Abies koreana Wilson). J. Ecol. Environ. 2020, 34, 358–371. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.; Lee, S.; Cho, Y.C. Abnormal winter drought-induced transient dieback of Korean fir in the montane forests of Mt. Jirisan, South Korea. J. Plant. Biol. 2024, 67, 123–136. [Google Scholar] [CrossRef]
- Song, J.H.; Jang, K.H.; Hur, S.D. Variation of seed and germination characteristics of natural populations of Abies koreana Wilson, a Korean endemic species. J. Korean Soc. For. Sci. 2010, 99, 849–854. [Google Scholar]
- Han, B.; Wu, D.; Zhang, Y.; Li, D.Z.; Xu, W.; Liu, A. Epigenetic regulation of seed-specific gene expression by DNA methylation valleys in castor bean. BMC Biol. 2022, 20, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.L.; Chen, H.; Ecker, J.R. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Nery, J.R.; Castanon, R.; Ecker, J.R. Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 2017, 18, 171. [Google Scholar] [CrossRef]
- Lister, R.; O’Malley, R.C.; Tonti-Filippini, J.; Gregory, B.D.; Berry, C.C.; Millar, A.H.; Ecker, J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 2008, 133, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell. Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Morar, I.M.; Dan, C.; Sestras, R.E.; Stoian-Dod, R.L.; Truta, A.M.; Sestras, A.F.; Sestras, P. Evaluation of different geographic provenances of Silver fir (Abies alba) as seed sources, based on seed traits and germination. Forests 2023, 14, 2186. [Google Scholar] [CrossRef]
- Narsai, R.; Gouil, Q.; Secco, D.; Srivastava, A.; Karpievitch, Y.V.; Liew, L.C.; Whelan, J. Extensive transcriptomic and epigenomic remodelling occurs during Arabidopsis thaliana germination. Genome Biol. 2017, 18, 172. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, Y.J.; Shin, M.H.; Hyun, H.J.; Bohnert, H.J.; Park, H.C. A comprehensive analysis of the Korean fir (Abies koreana) genes expressed under heat stress using transcriptome analysis. Sci. Rep. 2018, 8, 10233. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shearer, R.C. Proceedings—Conifer Tree Seed in the Inland Mountain West Symposium; Department of Agriculture, Forest Service, Intermountain Research Station: Missoula, MT, USA, 1985; Volume 85, p. 10. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed germination and dormancy: The classic story, new puzzles, and evolution. J. Integr. Plant Biol. 2019, 61, 541–563. [Google Scholar] [CrossRef]
- Kermode, A.R. Regulatory mechanisms involved in the transition from seed development to germination. CRC Crit. Rev. Plant Sci. 1990, 9, 155–195. [Google Scholar] [CrossRef]
- Lepiniec, L.; Devic, M.; Roscoe, T.J.; Bouyer, D.; Zhou, D.X.; Boulard, C.; Baud, S.; Dubreucq, B. Molecular and epigenetic regulations and functions of the LAFL transcriptional regulators that control seed development. Plant Reprod. 2018, 31, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; An, L.; Mao, J.; Aluko, O.O.; Ullah, Z.; Xu, F.; Liu, G.; Wang, Q. The CBL-interacting protein kinase NtCIPK23 positively regulates seed germination and early seedling development in tobacco (Nicotiana tabacum L.). Plants 2021, 10, 323. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef]
- Jullien, P.E.; Berger, F. DNA methylation reprogramming during plant sexual reproduction? Trends Genet. 2010, 26, 394–399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).