Entomopathogenic Fungi from Minnesota Are Virulent Against Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), Adults in a Laboratory Autodissemination Device Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Entomopathogen Identification
2.3. Inoculum Pouches
2.4. Autodissemination Device-Mediated Inoculation of Beetles
2.5. Recovery of Inoculum from Infected Beetle Cadavers
2.6. Statistical Analysis
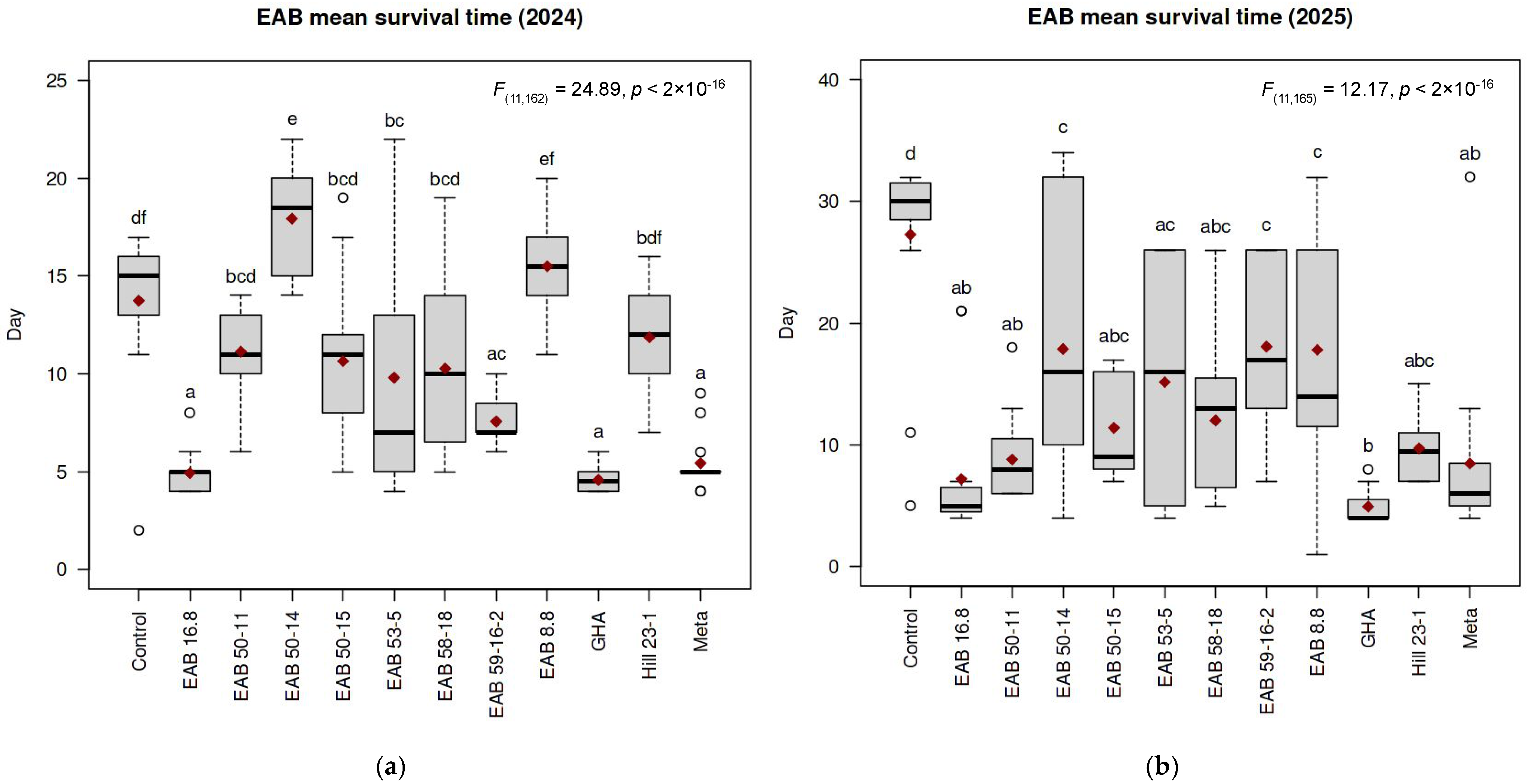
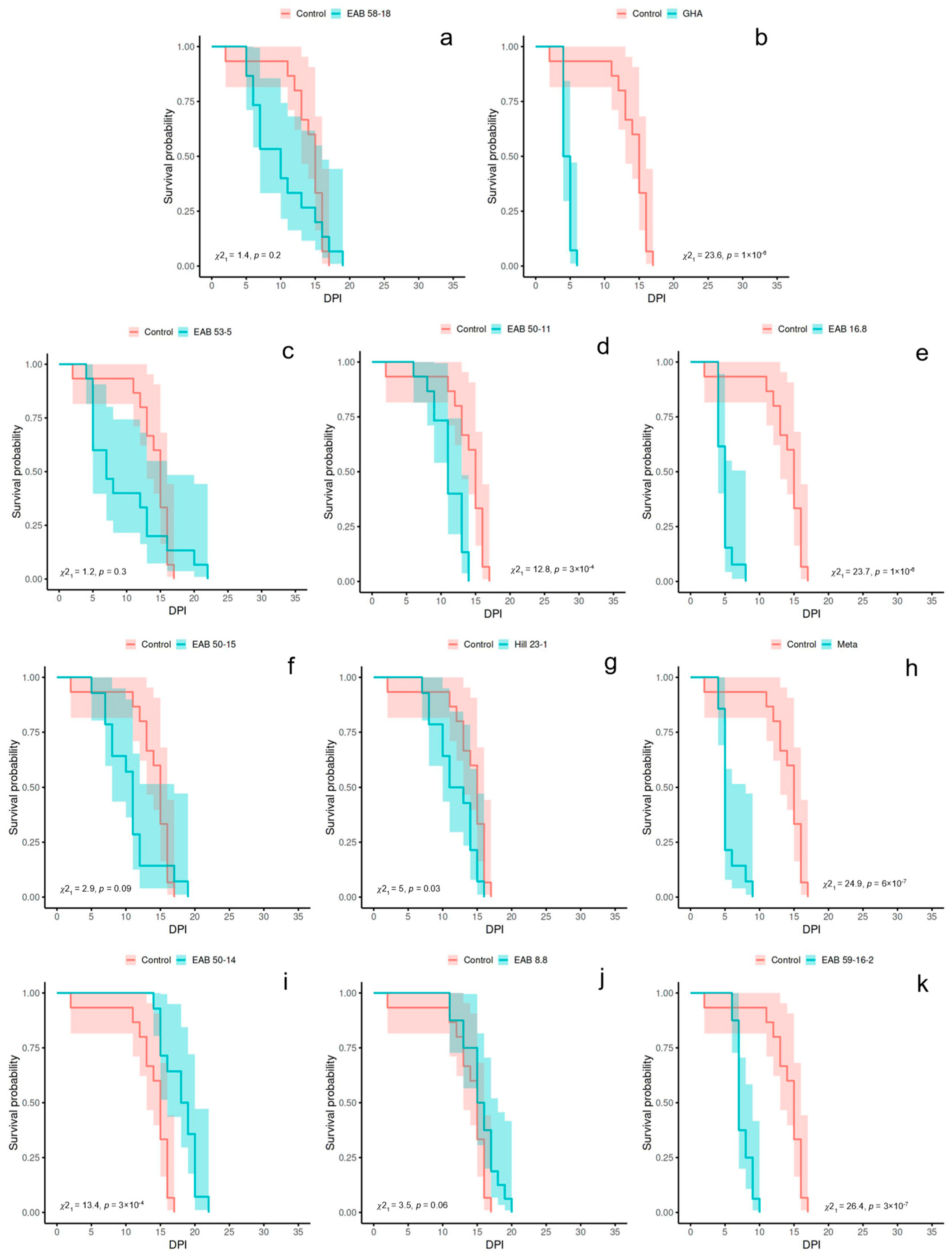
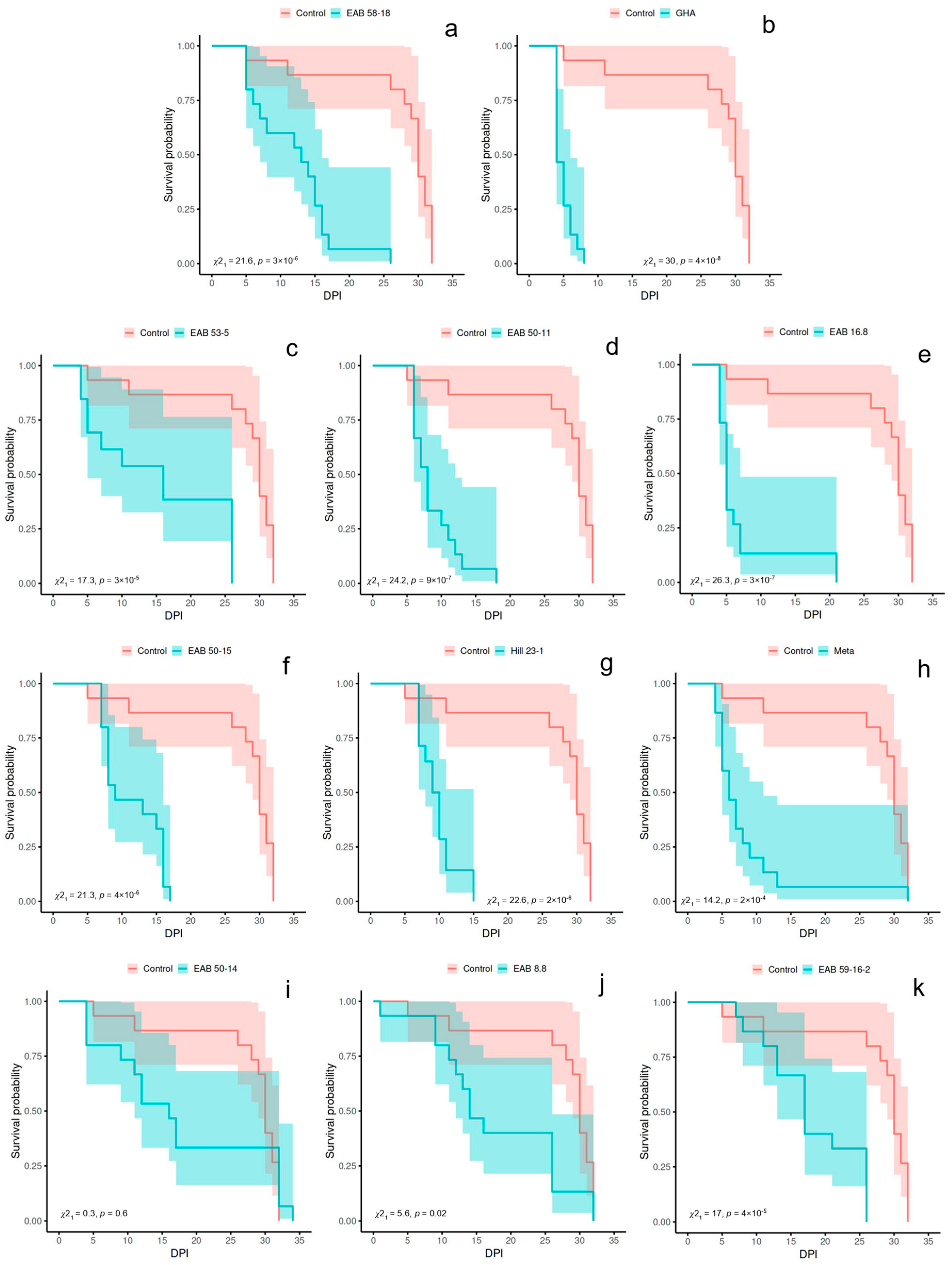
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EAB | Emerald ash borer |
| EPF | Entomopathogenic fungi |
| ADD | Autodissemination device |
| IPM | Integrated pest management |
| MST | Mean survival time |
| GDD | Growing degree days |
| DPI | Days post-inoculation |
References
- Sun, J.; Koski, T.M.; Wickham, J.D.; Baranchikov, Y.N.; Bushley, K.E. Emerald Ash Borer Management and Research: Decades of Damage and Still Expanding. Annu. Rev. Entomol. 2024, 69, 239–258. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendak, E.; Houping, L.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- EAB Information Network. Available online: https://www.emeraldashborer.info/ (accessed on 29 October 2025).
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef] [PubMed]
- McCullough, D.G. Challenges, tactics and integrated management of emerald ash borer in North America. Forests 2019, 93, 197–211. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.B. What’s killing the green menace: Mortality factors affecting the emerald ash borer (Coleoptera: Buprestidae) in North America? Can. Entomol. 2015, 147, 263–276. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; van Driesche, R.G.; Gould, J.R. Progress and Challenges of Protecting North American Ash Trees from the Emerald Ash Borer Using Biological Control. Forests 2018, 9, 142. [Google Scholar] [CrossRef]
- Minnesota Department of Agriculture EAB Status Map. Available online: https://mnag.maps.arcgis.com/apps/webappviewer/index.html?id=63ebb977e2924d27b9ef0787ecedf6e9 (accessed on 29 October 2025).
- Bauer, L.S.; Liu, H.; Haack, R.A.; Petrice, T.R.; Miller, D.L. Natural enemies of emerald ash borer in southeastern Michigan. In Proceedings of the Emerald Ash Borer Research and Technology Development Meeting, Port Huron, MI, USA, 30 September–1 October 2003; U.S. Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2004. [Google Scholar]
- Liu, H.; Bauer, L.S. Susceptibility of Agrilus planipennis (Coleoptera: Buprestidae) to Beauveria bassiana and Metarhizium anisopliae. J. Econ. Entomol. 2006, 99, 1096–1103. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Bauer, L.S.; Liu, H.; Griggs, M.H.; Vandenberg, J.D. Characterization of Beauveria bassiana (Ascomycota: Hypocreales) isolates associated with Agrilus planipennis (Coleoptera: Buprestidae) populations in Michigan. Biol. Control 2010, 54, 135–140. [Google Scholar] [CrossRef]
- Johny, S.; Kyei-Poku, G.; Gauthier, D.; van Frankenhuyzen, K.; Krell, P.J. Characterization and virulence of Beauveria spp. recovered from emerald ash borer in southwestern Ontario, Canada. J. Invertebr. Pathol. 2012, 111, 41–49. [Google Scholar] [CrossRef]
- Johny, S.; Kyei-Poku, G.; Gauthier, D.; van Frankenhuyzen, K. Isolation and characterization of Isaria farinosa and Purpureocillium lilacinum associated with emerald ash borer, Agrilus planipennis in Canada. Biocontrol Sci. Technol. 2012, 22, 723–732. [Google Scholar] [CrossRef]
- Lyons, D.B.; Lavallée, R.; Kyei-Poku, G.; van Frankenhuyzen, K.; Johny, S.; Guertin, C.; Francese, J.A.; Jones, G.C.; Blais, M. Towards the Development of an Autocontamination Trap System to Manage Populations of Emerald Ash Borer (Coleoptera: Buprestidae) With the Native Entomopathogenic Fungus, Beauveria bassiana. J. Econ. Entomol. 2012, 105, 1929–1939. [Google Scholar] [CrossRef]
- Pope, T.W.; Hough, G.; Arbona, C.; Roberts, H.; Bennison, J.; Buxton, J.; Prince, G.; Chandler, D. Investigating the potential of an autodissemination system for managing populations of vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae) with entomopathogenic fungi. J. Invertebr. Pathol. 2018, 154, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, C.; Barzanti, G.P.; Marianelli, L.; Peverieri, G.S.; Paoli, F.; Bosio, G.; Venanzio, D.; Giacometto, E.; Roversi, P.F. A new device for auto-disseminating entomopathogenic fungi against Popillia japonica: A study case. Bull. Insectol. 2019, 72, 219–225. [Google Scholar]
- Srei, N.; Guertin, C.; Lavallée, R.; Lajoie, M.E.; Brousseau, C.; Bergevin, R.; Miller, F.; McMillin, K.; Trudel, R. Microbial Control of the Emerald Ash Borer (Coleoptera: Buprestidae) Using Beauveria bassiana (Hypocreales: Cordycipitaceae) by the Means of an Autodissemination Device. J. Econ. Entomol. 2020, 113, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Unlu, I.; Rochlin, I.; Suman, D.S.; Wang, Y.; Chandel, K.; Gaugler, R. Large-Scale Operational Pyriproxyfen Autodissemination Deployment to Suppress the Immature Asian Tiger Mosquito (Diptera: Culicidae) Populations. J. Med. Entomol. 2020, 57, 1120–1130. [Google Scholar] [CrossRef]
- Wey, M.; Neuenschwander, H.; Hoesli, E.; Maurhofer, M.; Grabenweger, G. Autodissemination of Metarhizium brunneum: A strategy for biological control of adult Japanese beetles. J. Pest Sci. 2025, 98, 1745–1758. [Google Scholar] [CrossRef]
- Srei, N.; Lavallée, R.; Guertin, C. Horizontal Transmission of the Entomopathogenic Fungal Isolate INRS-242 of Beauveria bassiana (Hypocreales: Cordycipitaceae) in Emerald Ash Borer, Agrilus planipennis. J. Econ. Entomol. 2019, 113, 543–545. [Google Scholar] [CrossRef]
- Cherry, A.J.; Abalo, P.; Hell, K. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Simeto, S.; Held, B.W.; Showalter, D.N.; Bushley, K.E.; Blanchette, R.A. Ovicidal Effect of Entomopathogenic Fungi on Emerald Ash Borer, Agrilus planipennis Fairmaire, Eggs. Forests 2024, 15, 2170. [Google Scholar] [CrossRef]
- Batalla-Carrera, L.; Morton, A.; Santamaria, S.; García-del-Pino, F. Isolation and virulence of entomopathogenic fungi against larvae of hazelnut weevil Curculio nucum (Coleoptera, Curculionidae) and the effects of combining Metarhizium anisopliae with entomopathogenic nematodes in the laboratory. Biocontrol Sci. Technol. 2013, 23, 101–125. [Google Scholar] [CrossRef]
- Islam, S.M.N.; Chowdhury, Z.H.; Mim, M.F.; Momtaz, M.B.; Islam, T. Biocontrol potential of native isolates of Beauveria bassiana against cotton leafworm Spodoptera litura (Fabricius). Sci. Rep. 2023, 13, 8331. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Held, B.W.; Simeto, S.; Rajtar, N.N.; Cotton, A.J.; Showalter, D.N.; Bushley, K.E.; Blanchette, R.A. Fungi associated with galleries of the emerald ash borer. Fungal Biol. 2021, 125, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Rajtar, N.N.; Held, B.W.; Blanchette, R.A. Fungi from Galleries of the Emerald Ash Borer Produce Cankers in Ash Trees. Forests 2021, 12, 1509. [Google Scholar] [CrossRef]
- Simeto, S.; Held, B.W.; Blanchette, R.A. Wood Decay Fungi Associated with Galleries of the Emerald Ash Borer. Forests 2023, 14, 576. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S. Microbial control of Agrilus planipennis (Coleoptera: Buprestidae) with Beauveria bassiana strain GHA: Field applications. Biocontrol Sci. Technol. 2008, 18, 557–571. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S. Microbial control of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae) with Beauveria bassiana strain GHA: Greenhouse and field trials. Biol. Control 2008, 45, 124–132. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Liu, H.; Bauer, L.S.; Vandenberg, J.D. Assessing deposition and persistence of Beauveria bassiana GHA (Ascomycota: Hypocreales) applied for control of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), in a commercial tree nursery. Biol. Control 2010, 54, 61–67. [Google Scholar] [CrossRef]
- Francese, J.A.; Fraser, I.; Lance, D.R.; Mastro, V.C. Efficacy of Multifunnel Traps for Capturing Emerald Ash Borer (Coleoptera: Buprestidae): Effect of Color, Glue, and Other Trap Coatings. J. Econ. Entomol. 2011, 104, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Crippen, T.L.; Sheffield, C. External Surface Disinfection of the Lesser Mealworm (Coleoptera: Tenebrionidae). J. Med. Entomol. 2006, 43, 916–923. [Google Scholar] [CrossRef]
- Luangsa-ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Oliva, M.; Leiva, S.; Mendoza, J.E.; Bobadilla, L.; Angulo, G.; Calderon, M.S. Phylogeny and species delimitations in the entomopathogenic genus Beauveria (Hypocreales, Ascomycota), including the description of B. peruviensis sp. nov. MycoKeys 2019, 58, 47–68. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.Q.; Thanarut, C.; Dao, V.M.; Wang, Y.B.; Yu, H. Phylogeny and species delimitations in the economically, medically, and ecologically important genus Samsoniella (Cordycipitaceae, Hypocreales). MycoKeys 2023, 99, 227–250. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, D.X.; Luo, R.; Wang, Y.B.; Thanarut, C.; Dao, V.M.; Yu, H. Phylogeny and systematics of the genus Clonostachys. Front. Microbiol. 2023, 14, 1117753. [Google Scholar] [CrossRef]
- Nishi, O. Phylogenetic classification and physiological and ecological traits of Metarhizium spp. Mycoscience 2024, 65, 235–243. [Google Scholar] [CrossRef]
- Lins, J.C.; Bueno, V.H.P.; Sidney, L.A.; Silva, D.B.; Sampaio, M.V.; Pereira, J.M.; Nomelini, Q.S.S.; van Lenteren, J.C. Cold storage affects mortality, body mass, lifespan, reproduction and flight capacity of Praon volucre (Hymenoptera: Braconidae). Eur. J. Entomol. 2013, 110, 263–270. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, T.; Zhang, G.; Wang, M.; Liu, C.; Wang, M. The Impact of Cold Storage on the Survival and Viability of Parasitoid Bee Pupae and Whole Insects. Int. J. Vet. Res. Allied Sci. 2024, 4, 20–26. [Google Scholar] [CrossRef]
- Fick, W.E.; MacQuarrie, C.J.K. An artificial delay in emergence influences the number but not the fitness of adult emerald ash borer emerging from infested ash wood. Entomol. Exp. Appl. 2018, 166, 171–182. [Google Scholar] [CrossRef]
- Smith, D.N. Prolonged Larval Development in Buprestis aurulenta L. (Coleoptera: Buprestidae). A Review with New Cases. Can. Entomol. 1962, 94, 586–593. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Clissold, F.J.; Simpson, S.J. Temperature, food quality and life history traits of herbivorous insects. Curr. Opin. Insect Sci. 2015, 11, 63–70. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Bazzaz, F.A. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 1995, 101, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Bai, J.; Xu, Z.; Li, L.; Zhang, Y.; Diao, J.; Cao, J.; Xu, L.; Ma, L. Gut bacterial microbiota of Lymantria dispar asiatica and its involvement in Beauveria bassiana infection. J. Invertebr. Pathol. 2023, 197, 107897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, H.; Tu, C.; Han, R.; Luo, J.; Xu, L. Enhanced capacity of a leaf beetle to combat dual stress from entomopathogens and herbicides mediated by associated microbiota. Integr. Zool. 2024, 19, 1092–1104. [Google Scholar] [CrossRef]
- Rodrigues, J.; Bergamini, C.; Montalva, C.; Humber, R.A.; Luz, C. Simple method to detect and to isolate entomopathogenic fungi (Hypocreales) from mosquito larvae. J. Invertebr. Pathol. 2021, 182, 107581. [Google Scholar] [CrossRef]
- Toledo, A.V.; Virla, E.; Humber, R.A.; Paradell, S.L.; López Lastra, C.C. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. J. Invertebr. Pathol. 2006, 92, 7–10. [Google Scholar] [CrossRef]
- Mahmoud, F.M.; Bendebbah, R.; Benssaci, B.; Toudji, F.; Tafifet, L.; Krimi, Z. Entomopathogenic efficacy of the endophytic fungi: Clonostachys sp. and Beauveria bassiana on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) larvae under laboratory and greenhouse conditions. Egypt. J. Biol. Pest Control 2021, 31, 43. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Siddiqui, J.A.; Akutse, K.S.; Aguila, L.C.R.; Xu, Y. General Limitations to Endophytic Entomopathogenic Fungi Use as Plant Growth Promoters, Pests and Pathogens Biocontrol Agents. Plants 2021, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, J.C.; Fatoretto, M.B.; Demétrio, C.G.B.; Meyling, N.V.; Delalibera, I. UV-B Radiation Tolerance and Temperature-Dependent Activity Within the Entomopathogenic Fungal Genus Metarhizium in Brazil. Front. Fungal Biol. 2021, 2, 645737. [Google Scholar] [CrossRef] [PubMed]
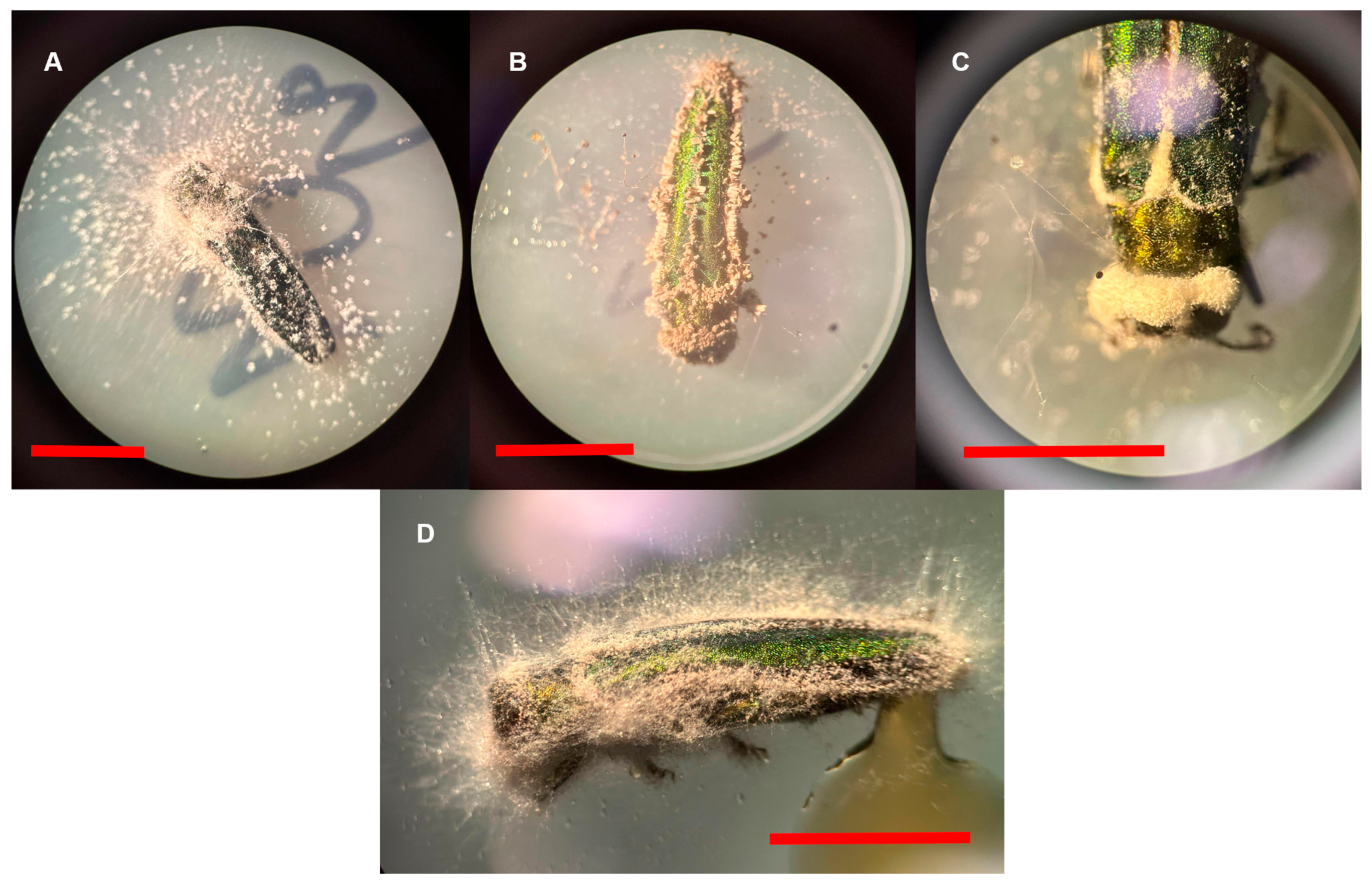
| Isolate | Organism | GenBank | Location |
|---|---|---|---|
| EAB 8.8 | Clonostachys sp. Corda | PX459604 | MN, USA |
| EAB 50-14 | Clonostachys sp. Corda | PX459605 | MN, USA |
| GHA | Beauveria bassiana (Bals.-Criv) Vuill. | PX459606 | MT, USA |
| EAB 16.8 | Beauveria pseudobassiana S.A. Rehner and Humber | PX459607 | MN, USA |
| EAB 50-11 | Purpureocillium sp. Luangsa-ard, Hywel-Jones, Houbraken & Samson | PX459608 | MN, USA |
| EAB 50-15 | Purpureocillium sp. Luangsa-ard, Hywel-Jones, Houbraken & Samson | PX459609 | MN, USA |
| EAB 53-5 | Beauveria pseudobassiana S.A. Rehner and Humber | PX459610 | MN, USA |
| EAB 58-18 | Samsoniella sp. Mongkols., Noisrip., Thanakitp., Spatafora & Luangsa-ard | PX459611 | MN, USA |
| EAB 59-16-2 | Purpureocillium sp. Luangsa-ard, Hywel-Jones, Houbraken & Samson | PX459612 | MN, USA |
| Hill 23-1 | Purpureocillium sp. Luangsa-ard, Hywel-Jones, Houbraken & Samson | PX459613 | MN, USA |
| Meta | Metarhizium sp. Sorokin | PX459614 | MN, USA |
| Isolate | Species | MST (Days) | Log-Rank p Value | Fungal Recovery | |||
|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | ||
| Control | - | 13.73 | 27.27 | 1.0 | 1.0 | 0 | 0 |
| GHA | Beauveria bassiana | 4.57 | 4.93 | 1 × 10−6 ** | 4 × 10−8 ** | 60% | 86.67% |
| EAB 16.8 | Beauveria pseudobassiana | 4.92 | 7.20 | 1 × 10−6 ** | 3 × 10−7 ** | 30% | 86.67% |
| EAB 53-5 | Beauveria pseudobassiana | 9.80 | 15.15 | 0.3 | 3 × 10−5 ** | 13.3% | 46.15% |
| EAB 50-15 | Purpureocillium sp. | 10.64 | 11.40 | 0.09 | 4 × 10−6 ** | 57.1% | 100% |
| EAB 50-11 | Purpureocillium sp. | 11.13 | 8.80 | 3 × 10−4 ** | 9 × 10−7 ** | 20% | 100% |
| Hill 23-1 | Purpureocillium sp. | 11.86 | 9.71 | 0.03 | 2 × 10−6 ** | 42.8% | 100% |
| EAB 59-16-2 | Purpureocillium sp. | 7.56 | 18.07 | 3 × 10−7 ** | 4 × 10−5 ** | 62.5% | 73.33% |
| EAB 58-18 | Samsoniella sp. | 10.27 | 12.00 | 0.2 | 3 × 10−6 ** | 26.6% | 100% |
| EAB 50-14 | Clonostachys sp. | 17.93 | 17.87 | 3 × 10−4 ** | 0.6 | 42.8% | 93.33% |
| EAB 8.8 | Clonostachys sp. | 15.50 | 17.80 | 0.06 | 0.02 | 33.3% | 100% |
| Meta | Metarhizium sp. | 5.43 | 8.47 | 6 × 10−7 ** | 2 × 10−4 ** | 53% | 93.33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, C.J.; Rajtar, N.N.; Blanchette, R.A. Entomopathogenic Fungi from Minnesota Are Virulent Against Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), Adults in a Laboratory Autodissemination Device Assay. Forests 2025, 16, 1742. https://doi.org/10.3390/f16111742
Peters CJ, Rajtar NN, Blanchette RA. Entomopathogenic Fungi from Minnesota Are Virulent Against Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), Adults in a Laboratory Autodissemination Device Assay. Forests. 2025; 16(11):1742. https://doi.org/10.3390/f16111742
Chicago/Turabian StylePeters, Colin J., Nickolas N. Rajtar, and Robert A. Blanchette. 2025. "Entomopathogenic Fungi from Minnesota Are Virulent Against Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), Adults in a Laboratory Autodissemination Device Assay" Forests 16, no. 11: 1742. https://doi.org/10.3390/f16111742
APA StylePeters, C. J., Rajtar, N. N., & Blanchette, R. A. (2025). Entomopathogenic Fungi from Minnesota Are Virulent Against Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), Adults in a Laboratory Autodissemination Device Assay. Forests, 16(11), 1742. https://doi.org/10.3390/f16111742







