Abstract
Nest predation is a major factor limiting avian reproductive success. It depends on factors such as bird community, land use, vegetation structure and landscape. Anthropogenic disturbances in native forests, such as logging and livestock grazing, alter forest structure and understory, potentially affecting nest predation rates. In this study, we analysed the susceptibility of open-cup nests to predation in Nothofagus antarctica forests in Tierra del Fuego (Argentina), comparing 15–50 years ago thinned—T and unthinned forests, the latter classified as open—O, closed—C or very closed—VC. We also identified nest predators through camera traps and the main variables influencing predation using a Generalized Lineal Model. Data were collected from 32 sites representing the four studied categories of canopy cover across two years (256 artificial nests per year). Artificial nest predation rates varied between year (9.4% in 2018 and 40.2% in 2022) and among forest types. In 2018, the O forests had the highest predation rate (50%, 12 in total), whereas in 2022, VC forests showed the greatest predation (38%, 39 in total). Camera traps identified three nest predators: Milvago chimango, Campephilus magellanicus and Xolmis pyrope. In 2018, canopy cover was the only variable that influenced artificial nest predation, while in 2022, tree sapling cover, patch shape, open-cup nester density and tree basal area were the most influential (in that order). We found annual variations driven by different ecological factors in N. antarctica forest of southern Patagonia. Although thinning showed no significant long-term effects on artificial nest predation on this study, more research is needed to understand the influence of low impact forest management in austral bird communities.
1. Introduction
Nest predation is a major factor influencing nest survival and, consequently, breeding success [1,2,3,4]. This is especially true for open-cup nesters, for which predation often causes the greatest nest losses [5]. However, nest predation rates vary greatly among populations and sites [6,7], and numerous factors may account for this variation. These factors may be related to predators themselves [8,9], the bird community [10,11], and site characteristics [12,13]. For instance, several studies have found that higher predator abundance is associated with lower daily nest survival rate [9]. Skutch [14] proposed that increased parental activity at the nest heightens the risk of predation; for example, during years of food scarcity, greater parental foraging activity may provide more visual cues to predators regarding nest locations [15,16]. Additionally, site characteristics at different spatial scales play a crucial role. The extent and spatial arrangement of habitat types can influence local predator abundances and predation pressure [17,18], while certain habitat features—such as vegetation cover and tree or shrub density—may offer better concealment and protection from predators [13,19].
Vegetation structure and cover influence bird community composition, species diversity [20,21], and nest predation through the ability of predators to locate the nests [22]. Martin [5] proposed the total foliage hypothesis, which posits that increased vegetation density provides concealment and reduces predation risk by inhibiting olfactory, auditory, and visual cues predators use to detect nests. This hypothesis has been supported by several studies [12,16]. Consequently, habitat modifications that simplify vegetation structure may increase nests’ exposure and, in turn, elevate predation risk [23,24]. In managed forests, early-stage interventions, such as thinning, often lead to structural simplification of the canopy; however, in later successional stages, the development of understory vegetation tends to provide more concealed sites for species that breed in this stratum [25,26,27].
Thinning is a silvicultural technique used to reduce the number of trees in a forest stand, with the goal of redirecting resources toward selected individuals. By removing smaller or less vigorous trees, such as advanced regeneration with small diameters, managers can accelerate the growth of the remaining trees and improve their marketability. Additionally, thinning increases light penetration to the lower strata of the forest, promoting the growth of understory plants such as grasses. For this reason, it is also applied as a silvopastoral technique to enhance forage availability for livestock in South Patagonia [28]. Livestock activity in Tierra del Fuego has shown a gradual increase in stocking rates over time and has recently shifted toward cattle or mixed cattle–sheep production systems [29]. Over the past two decades, however, there has been a growing interest in reorienting management practices toward more sustainable forestry and silvopastoral strategies like low thinnings. In this sense, assessing and understanding the impacts of thinning within native forests become relevant.
In previous studies, Benitez [30] reported significant differences in mean bird species richness, composition, density, and some functional traits between thinned and unthinned forests in Tierra del Fuego. Furthermore, the density of open-cup nesters—one of the groups with the greatest number of species—was significantly higher in thinned forests compared to unthinned (closed) forests. However, some research regarding thinning indicates that it could affect the birds’ reproductive success [31], which has not been yet examined in Tierra del Fuego.
In Patagonia, some studies have identified native and exotic nest predators and examined nest survival rates in coastal areas [32], undisturbed forests [33,34,35,36], and wetlands [37]. Additionally, Vazquez et al. [38] investigated the effects of habitat attributes and predator identity across different forest stand types (Nothofagus betuloides, N. pumilio, and N. antarctica) within a protected area in northern Patagonia. Although previous research has analysed the long-term effect of thinning and landscape context on bird assemblage structure and functional traits of Nothofagus antarctica forests in Tierra del Fuego, Argentina, by comparing thinned and unthinned stands [21,39], no studies have yet evaluated the influence of silvicultural practices on nest predation in Nothofagus forest of Patagonia.
In this study, we examine the susceptibility of open-cup nests to predation in N. antarctica forests of Tierra del Fuego (Argentina), comparing thinned and unthinned forests. We also aim to identify the main nest predators and the key variables influencing artificial nest predation in these austral forests. We expect higher rates of nest predation in forests with greater canopy opening (such as thinned and naturally open forests) due to increased nest visibility. Furthermore, we hypothesize that forest structure variables will be more strongly associated with nest predation than variables related to bird community (predator and open-cup nesters) or landscape variables.
2. Materials and Methods
2.1. Study Site
This study was conducted in the central region of Isla Grande of Tierra del Fuego, Argentina (54° S), within the Andean-Patagonian forests. The area is dominated by deciduous N. antarctica forests (Figure 1). The growing season lasts approximately five months, and frost-free conditions occur for only about three months each year, as air temperatures frequently drop below 0 °C. Mean wind speed outside forested areas is around 8 km h−1, with frequent summer windstorms reaching up to 100 km h−1 [40]. Annual precipitation is evenly distributed throughout the year, averaging 400–600 mm yr−1 [41]. A more detailed description of the study area is provided in [21].
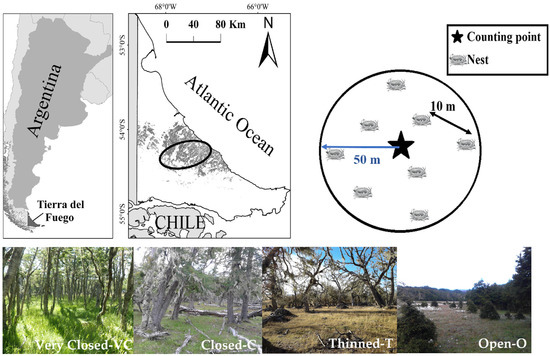
Figure 1.
Top left: Location of study area (circle) in Tierra del Fuego (Argentina), with Nothofagus antarctica forests in grey colour. Top right: Diagram with location of the counting point and the artificial nests at each sampling site (the black arrow indicates the minimum distance between nests and the blue arrow indicates the approximate radius of the site). Bottom: Studied forest types.
In these forests, passerines are the most abundant group in the bird community, with approximately half of the species being migratory. Both migratory and resident birds breed during spring and early summer season (from October to January), usually laying eggs in only one clutch. Most species construct open-cup nests, while others nest in tree cavities.
The study area is situated near the center of the distribution range of N. antarctica forests, which occur predominantly as patches interspersed with grasslands, shrublands and peatlands (Figure 1). The elevation of the study sites ranges from 100 to 250 m a.s.l., and the terrain is undulated, with gentle slopes that become more pronounced near foothills. Livestock production, primarily cattle breeding (Hereford), has been practiced in the area for approximately 50 years. This activity involves round-year, extensive grazing within large paddocks (approximately 1000 ha each), where cattle move freely across the landscape.
Mature N. antarctica stands were thinned during a single season between 15 and 50 years ago, reducing approximately 50% of the original canopy cover [42]. Thinning operations in the region generally target unhealthy or suppressed-intermediate crown class individuals, resulting in a relatively homogeneous residual stand with reduced crown cover that enhances understory forage production. This management practice promotes increased growth of remaining trees (i.e., higher basal area in the long term) and, in the short term, improves forage availability and provides shelter for livestock during winter. Harvested wood is typically used locally for firewood, poles and fence materials.
In contrast, unthinned N. antarctica forests within the study area display a wide gradient of canopy cover, shaped by natural disturbances such as wind and anthropogenic disturbances such as fire. Open canopies are associated with early stage expanding into grassland, whereas closed canopies characterize mature forests, and very closed canopies in young stands correspond to initial and final growth phases of tree development, with high tree density [30].
2.2. Sampling Design
Within the study area, four forest types were selected (Figure 1), corresponding to distinct categories of canopy cover: one forest type resulting from 15–50 years ago thinned forests—T (35%–65% canopy cover) and three unthinned forests types classified as open-O (<35% canopy cover), closed—C (65%–85% canopy cover) and very closed-CV (>85% canopy cover). For each canopy cover category, eight replicates (forest patches averaging 96 ha each) were randomly selected, with a minimum distance of 100 m between patches (N = 4 canopy cover categories × 8 replicates = 32 plots).
2.3. Artificial Nest Experiment
We used artificial nests to overcome the extreme difficulty of finding and monitoring real nests without disturbing them, thus obtaining sufficiently large sample sizes to test ecological hypotheses. Although artificial nests may provide inaccurate estimates of the actual failure risk of real nests [43,44], they are generally considered appropriate for identifying factors that influence the spatial and temporal variation in nest survival [45,46,47,48].
During two breeding seasons (October 2018 and 2022), we installed eight artificial nests, spaced 10 m apart, within each of the 32 plots (Figure 1). Artificial nests were constructed with an external diameter of 15 cm using a hemispherical plastic base covered by branches, grasses, and lichens (Usnea spp.), one of the main materials used by forest birds for nest construction in this region ([49], personal observation). Each nest was positioned 1.0–1.5 m above the ground, corresponding to the average nesting height of the five most common forest passerine species in the area [49,50].
Three white clay eggs were placed in each nest. The eggs were oval in shape, measuring approximately 13 mm in width and 26 mm in length. Artificial nests were left active for a period of 16 days, which approximates the mean incubation period of forest passerines in the region [50]. All nests were inspected every four days to record their status. The number of predated nests per plot was determined as the total number of nests in which at least one egg was either marked or removed. Additionally, we calculated the number of nests predated per day after installation, forest type, and year to assess how quickly predators located the nests.
Because predator identification based solely on eggs/nest remains can be difficult [51], we supplemented nest checks with camera traps. Cameras were placed 1–2 m from 16 nests (two per forest type), set to trigger by motion and programmed to take three consecutive photographs per activation. The potential bird species acting as nest predators were identified following the bibliography [32,33,34,37,38,49,50,52,53,54].
2.4. Bird Counts
Bird community structure (richness and density of open-cup nesters and potential nest predators) was assessed using the point count method. Other potential predators (as foxes) were not evaluated, because birds are considered the primary nest predators in the region [35,38,50,52]. Bird surveys were conducted during the 2018 and 2022 breeding seasons, within a four-hour period after sunrise, corresponding to the peak of avian social and foraging activity [55]. Sampling was carried out under favourable weather conditions, avoiding foggy, windy, or rainy days.
We employed a point count protocol with unlimited detection distance, previously developed and validated for studies in Tierra del Fuego [41,56]. One observation point was established at each plot, and two observers conducted the surveys. To minimize temporal bias, the order of sampling points was rotated among visits [57]. Each point was revisited two or three times per month, resulting in a total of 256 counts across the four forest types during the two breeding seasons.
Each count consisted of a two-minute acclimation period followed by an eight-minute effective counting period. Observations included direct visual detections with binoculars, while vocalizations were used solely to locate individuals visually. All birds were identified to species following the South American Classification Committee (SACC) taxonomy [58]. For each detection, observers recorded the species, number of individuals, and estimated distance to each individual or group.
The richness of open-cup nesters and nest predators (number of species) of each forest type and year was estimated by summing the total number of bird species recorded across all surveys and replicates (plots) within each forest type. In addition, species richness per plot and year was calculated adding the total number of species detected in that plot during the breeding season. The total density of open-cup nesters, large open-cup nesters (species with larger eggs: Curaeus curaeus, Turdus falcklandii, and Xolmis pyrope [59]), and nest predators (individuals ha−1) was estimated for each forest type (mean of eight replicates) and for each plot following the methodology of Lencinas et al. [56]. This method estimates bird density based on the number of individuals observed within a circular sampling area, where the radius varies among forest types to account for reduced detectability with increasing distance. In this study, the radii used for density calculations were 25 m in VC, 32 m in C, 35 m in T and 40 m in O forests.
2.5. Forest Structure and Landscape Variables
Based on previous studies, we evaluated the influence of a selected group of forest structure and landscape variables, potentially related to nest predation susceptibility. For forest structure, the selected variables included canopy cover (%), dominant height (m), mean diameter (cm), tree density (ind ha−1), basal area (m2 ha−1), tree saplings cover (%) and total understory cover (%). Forest types (VC, C, T and O) were also included in this group of variables. For landscape attributes, we considered the area (ha) and shape of the forest patch where each plot was located, the total forest area (ha) and forest connectivity (%), and the total open habitat area (ha) and open habitat connectivity (%). Detailed descriptions of the procedures used to obtain these variables were provided in Benitez et al. [21,39].
2.6. Data Analysis
To assess whether bird community structure, forest structure and landscape variables influenced the susceptibility of artificial nests to predation, we performed Generalized Linear Models (GLM). Artificial nest predation susceptibility was estimated as the number of predated artificial nests (nests in which at least one egg was either marked or removed) per plot and year. We first examined the effects of forest type, year, and their interaction. Because the interaction had a highly significant effect (p = 0.01), subsequent analyses were conducted separately for each year.
For each year, we fitted a single explanatory variable (including bird community structure, forest structure and landscape variables) to identify those with a significant influence on response variable. Species richness and densities data were modelled using a Poisson error distribution, and overdispersion was assessed by dividing the residual deviance by the residual degrees of freedom. Continuous variables were transformed into categorical variables to facilitate interpretation of the results. Depending on the data distribution, variables were classified into two (high and low) or three (high, medium and low/null) categories, ensuring both equitable and coherent representation (see Appendix A).
LSD Fisher a posteriori tests were performed to evaluate differences among categories of each explanatory variable [60,61]. Model performance was assessed using the Akaike Information Criterion (AIC) to compare goodness of fit among candidate models; AIC was also used to evaluate the importance of the analysed variables [62]. Variables were considered influential when their models presented the lowest AIC value and a difference of less than two units relative to the best-fitting model. All GLM and LSD analyses were conducted using Infostat software version 2020 [63]. We used Bonferroni multiple comparison test to adjust the significance level for each comparison [64]. A significance level is used that avoids an excessive risk of Type I errors (false positives) when performing multiple tests. This method involves dividing the original significance level by the number of tests, making each individual test more rigorous.
3. Results
We identified eight open-cup nesters species belonging to five families (Emberizidae, Fringillidae, Icteridae, Turdidae and Tyrannidae), and seven potential nest predators from six families (Falconidae, Icteridae, Picidae, Threskiornithidae, Troglodytidae and Tyrannidae) (Table 1). Species densities varied among canopy cover categories and between years (Table 1), with higher richness of open-cup nesters recorded in O forests during 2018 and in C forests during 2022. Overall, nesters reached higher densities in T forests across both years. Predator richness was also greater in T forests in 2018 and 2022, although predator density varied between years being higher in T during 2018 and in VC forests during 2022. The notably high density of Milvago chimango in VC forests during 2022 (3.0 ind ha−1) may be related to the proximity of nesting sites of this species.

Table 1.
Bird species list of open-cup nesters and potential nest predators recorded in Nothofagus antarctica forests of Tierra del Fuego (Argentina), according to literature. The table shows species densities (individuals ha−1) by forest type and sampling year, along with total density and species richness (number of species) for each category.
Predation susceptibility in the study area was 9.4% in 2018 and 40.2% in 2022. In 2018, O forests showed the highest number of predated nests (50%, n = 12, Figure 2), whereas C, T and VC forests exhibited low predation rates (≤5 predated nests each). In 2022, predation susceptibility was the highest in VC forests (38%, n = 39), followed by T (n = 27) and C (n = 26). The number of predated nests also varied among revisits (Figure 2): during 2018, most predation occurred during the first revisits, whereas in 2022, predation susceptibility increased progressively over time.
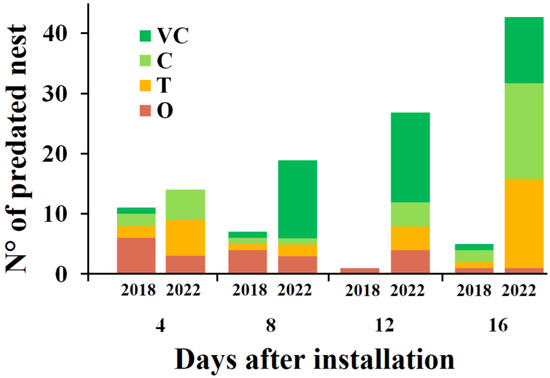
Figure 2.
Predated artificial nests by days after installation across forest types in 2018 and 2022, in Nothofagus antarctica forests of Tierra del Fuego (Argentina).
We identified three nest predators using camera traps: Milvago chimango, Campephilus magellanicus, and Xolmis pyrope (Figure 3). M. chimango accounted for the highest number of predation events (eight records), whereas C. magellanicus and X. pyrope (the latter being the only predator detected in 2018) were each recorded once (Table 2). In addition, Aphrastura spinicauda, Curaeus curaeus, Lycalopex griseus, Pygarrhichas albogularis, Theristicus melanopis, Troglodytes aedon and Turdus falklandii were photographed near some artificial nests in 2022, but no evidence of predation was found at those nests (Figure 3, Table 2). Due to technical issues, we were unable to identify species approaching but not preying upon nests during 2018. That year, only one camera-monitored nest was predated, and X. pyrope was identified as the predator. In contrast, in 2022, 13 of the 16 camera-monitored nests were predated, and the predator identity was identified in nine of those cases.

Figure 3.
Top (first line): bird predators on artificial nest identified with camera traps: (A) Milvago chimango, (B) Xolmis pyrope, and (C) Campephilus magellanicus. Bottom (second and third lines): other fauna (circles) observed near artificial nests (arrows) without exhibiting predatory behaviour (no evidence of egg predation): Aphrastura spinicauda, Lycalopex griseus, Theristicus melanopis, Pygarrhichas albogularis, Troglodytes aedon and Turdus falklandii. Note that L. griseus is an introduced fox species in Tierra del Fuego (Family: Canidae).

Table 2.
Bird species recorded with camera traps, showing evidence of predatory behaviour on artificial nests (or not) in Nothofagus antarctica forests of Tierra del Fuego (Argentina). Note that only one record was obtained in 2018.
In 2018, one of the variables studied significantly influenced nest predation susceptibility (canopy cover, Table 3). However, when we adjust the p-value, this variable ceases to be significant. In 2022, 4 variables were significantly associated with nest predation when we adjust the p-value (Table 3). The best-fitting models included tree sapling cover and patch shape as the explanatory variables (F = 12.3 and F = 26.7, respectively; p < 0.01). Forests with no sapling cover showed the highest number of predated nests (mean = 5.2), followed by forests with low (2.7) and medium sapling cover (0.9; Figure 4). Regarding patch shape, predation was higher in forests located within uniform patches (Figure 4).

Table 3.
Effect of bird community structure, forest structure and landscape variables (as explanatory variables) on the quantity of predated artificial nests (response variable) during 2018 and 2022 in Nothofagus antarctica forests of Tierra del Fuego (Argentina), analysed by Generalized Linear Models (GLM). The red font for p-values shows a significant effect of the explanatory variable (p < 0.05). Bold font for p-values and AICs highlights the best models.
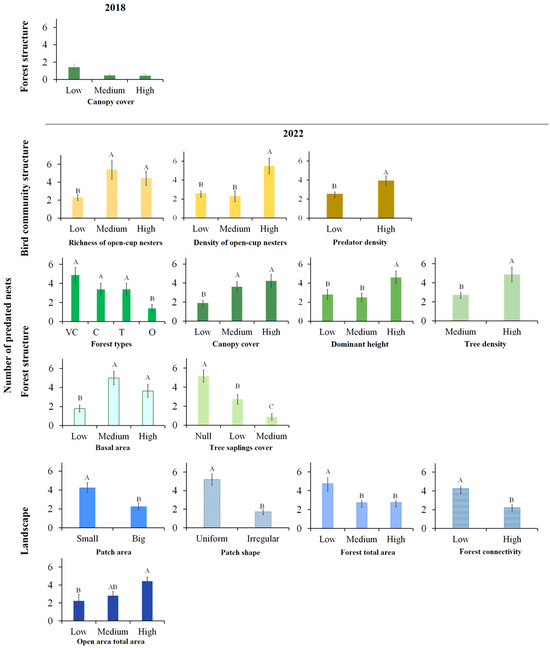
Figure 4.
Average values of predated artificial nests (Y Axes) in Nothofagus antarctica forests of Tierra del Fuego (Argentina) in relation to different explanatory variables (X Axes), according to the significant models detected by GLM (Table 2). The analysed categories for each explanatory variable are detailed in the Appendix A. Different letters indicate differences by LSD test (p < 0.05).
One variable describing bird community structure (density of open-cup nesters) were also significantly related to nest predation (Table 3). Forests with high bird density had greater numbers of predated nests (Figure 4). Among forest structure variables, basal area also showed significant effects (Table 3). Only O forests had lower nest predation rates (Figure 4), whereas forests with medium to high basal area exhibited higher predation levels.
4. Discussion
The use of artificial nests presents some limitations that should be considered when interpreting the results. The absence of parental behavior and the lack of olfactory or visual cues associated with adults may underestimate actual predation rates, as some predators locate nests based on parental activity [43,44]. In addition, artificial nests are usually uniform in size, shape, and placement, and do not reflect the natural nest-site selection performed by birds, which may introduce biases in the estimation of predation risk [46]. Therefore, results obtained from artificial nests should be interpreted as comparative indicators of spatial or temporal variation in susceptibility to predation. However, the use of artificial nests also offers several advantages, such as allowing for the deployment of sufficient sample sizes for statistical analyses, ensuring comparable distribution across forest types, and avoiding disturbance to real nests. These advantages make it possible to better understand the ecological processes involved.
This study provides the first assessment of the effects of forest intervention on artificial nest predation in Nothofagus antarctica forests of Patagonia. We detected differences in artificial nest predation between years and among forest types. Additionally, we identified the main nest predators and the key variables influencing nest predation at this austral latitude. Considering all forest types together, the overall nest predation rates observed in this study were low compared to those reported for other regions (e.g., [8,16,65,66,67,68]) and in northern Patagonia [34,38]. However, our results were similar to those recorded in nearby sites, such as Navarino island [33,35]. Artificial nest predation varied markedly between years (9.4% in 2018 and 40.2% in 2022), a pattern also observed in other studies (e.g., [4,16,68]). This interannual variability may be related to climatic conditions or to fluctuations in food availability for predators. For instance, rainfall during October 2022 was lower than in October 2018 in the study sites [69], which could have reduced the availability of alternative prey pressure such as insects, as observed in other forests [70], thereby increasing predator pressure on artificial nests. This could be considered since predators found consumed a large number of arthropods, so the availability of this other food resource could affect the search for and predation of nests. It highlights the importance of conducting multi-year studies to obtain more accurate estimates of nest predation susceptibility. Furthermore, the year with higher nest predation (2022) coincided with greater overall predator density, suggesting this may have been one of the main drivers of increased nest losses [9]. Camera traps identified three nest predators, with M. chimango emerging as the primary species, consistent with findings from other studies in the region [33,34,35,36]. M. chimango is an abundant raptor inhabiting a wide range of environments, from forest to open areas such as grasslands, wetlands, coastal zones, and areas heavily influenced by human activity [71,72]. Although originally described as a scavenger species [73], it is now recognized as a generalist and opportunistic predator [72]. This species is gregarious and typically places its nests near the central part of the trees, beneath dense canopy cover [74]; therefore, our study sites, particularly the VC forests, offer suitable nesting conditions. This was confirmed in 2022, when several active nests of M. chimango were observed in VC forests.
When comparing forest types, during 2018, O forests showed the highest number of predated nests, as we expected, however, this variable had little influence in the GLM (Table 3). This low canopy cover (<35%) in O forests likely facilitated the transmission of visual cues, increasing the probability of nest detection by predators. This finding is consistent with observations in protected N. antarctica forest in northwestern Patagonia, where the likelihood of predation decreased with increasing tree cover [38]. Although T forests exhibited medium canopy cover (35%–65%) and the highest predator richness with intermediate predator density during 2018 (Table 1), this treatment showed low susceptibility to nest predation. This may be due these forests were thinned only once, 15 to 50 years ago, allowing the understory to recover over time; together with the remaining trees, this likely enhanced nest concealment. Consequently, thinning did not show significant long-term effects on nest predation. This aligns with finding by Hagar et al. [31] in thinned forests of the Pacific Northwest USA, where a decade after thinning, observation rates of nest predators did not differ between thinned and unthinned stands. Nevertheless, high interannual variation could mask differences among forest types. Therefore, the continuity of this kind of research could give more solid information about the effect of old thinnings and significant explanatory variables, as well as further research is needed to better understand the effects of recent thinning on nest predation dynamics at these southern latitudes.
Unexpectedly, in 2022 we found higher predation susceptibility in the most closed forest (VC), although this was not statistically different from forest with intermediate canopy covers (T and C). This pattern coincided with the very high predator density recorded in VC during that year, particularly of M. chimango (Table 1). This raptor nests in the canopy and frequently uses branches as perches to search for prey [36]; therefore, its greater abundance and hunting behaviour in this forest type may explain the increased nest predation observed. According to the GLM results, the best explanatory variable for nest predation in 2022 was tree sapling cover (tree < 1.3 m in height; Table 3). This variable was null in VC forests, and null or low in most C and T plots. Tree saplings provide refuge, feeding sites, and nesting substrates for birds in these forests [30]; thus, low sapling cover (<5%) may increase nest visibility and detection probability by predators. This result supports the “total foliage” hypothesis proposed by Martin [5] and is consistent with findings from other parts of the world [12,67]. As expected, forests with higher densities of open-cup nesters also exhibit greater numbers of predated nests (Figure 4). A higher abundance of nesting birds may reflect greater overall avian activity, which can signal to predators an increased likelihood of finding nests, as hypothesized by Skutch [14]. Landscape at different scales also influence susceptibility to nest predation. We found higher nest predation in plots located in small and uniform patches of forests (local scale). In addition, at landscape with low total forest cover, greater open areas and low forest connectivity presented greater nest predation. These results are consistent with Maag et al. [75], who reported in Europe forests that nest predation was higher in landscapes with low forest cover. It is possible that such habitat generalist predators prefer to forage or spend more time in this type of landscape, where they also nest, while taking advantage of adjacent open areas that facilitate prey detection and movement.
5. Conclusions
We found a low and medium susceptibility to artificial nest predation in Nothofagus antarctica forests of southern Patagonia, with notable annual variation which could be associated with other larger-scale factors such as the climate (precipitation) and food resources availability (insects). According to this, our hypotheses were partially supported by our results, because only in 2018 were higher rates of nest predation observed in forests with greater canopy opening (naturally open forests). Regarding the influence of forest structure, canopy cover had some influence that affected artificial nest predation in 2018, while sapling cover was one of the two most influential factors affecting artificial nest predation in 2022. However, in 2022, other factors related to the bird community and landscape played important roles. This highlights the importance of conducting multi-year studies to more accurately estimate susceptibility to nest predation, including not only forest structure variables but also bird community and landscape variables. Our results indicate that bird community, forest structure and landscape features all affect the vulnerability of open-cup nests to predation in Nothofagus antarctica forests of Tierra del Fuego. Although we did not find significant effects of forest thinning on nest predation in this study, more studies would be necessary to comprehend the effect of thinning performed a long time ago (15–50 years before). Moreover, further research is needed to understand the effects of recently applied thinning on nest predation dynamics at these southern latitudes.
Author Contributions
Conceptualization, J.B. and M.V.L.; methodology, J.B., G.J.M.P. and M.V.L.; formal analysis, J.B.; investigation, J.B., M.D.B. and M.V.L.; resources, P.L.P. and G.J.M.P.; data curation, J.B.; writing—original draft preparation, J.B.; writing—review and editing, M.V.L.; visualization, J.B.; supervision, P.L.P., G.J.M.P. and M.V.L.; project administration, J.B.; funding acquisition, J.B., P.L.P., M.D.B. and M.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONCyT (Argentina), grant number PICT-2016-1968.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research is part of the doctoral thesis of JB developed in the Faculty of Forest and Agricultural Science (Facultad de Ciencias Agrarias y Forestales) in the UNLP (Universidad Nacional de la Plata). We especially thank Carlos Henninger and Luna Family for allowing our entry into and sampling in their establishments and Inés Ramos Vértiz, Francisco Sola and Jimena E. Chaves for helping during sampling.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| T | Thinned forests |
| O | Open unthinned forests |
| C | Closed unthinned forests |
| VC | Very Closed unthinned forests |
| AIC | Akaike Information Criterion |
| GLM | Generalized Linear Models |
Appendix A

Table A1.
Range of values of the explanatory variable categories used in GLM.
Table A1.
Range of values of the explanatory variable categories used in GLM.
| Variable | Null | Low | Medium | High |
|---|---|---|---|---|
| Bird community structure | ||||
| Richness of open-cup nesters | 0–1 | 2 | 3–5 | |
| Density of open-cup nesters (ind ha−1) | 0–2.0 | 2.1–4.0 | >4.1 | |
| Density of large open-cup nests (ind ha−1) | 0 | 0.1–1.3 | >1.3 | |
| Predator richness | 0 | 1.0 | >1.0 | |
| Predator density (ind ha−1) | 0 | 0.1–1.0 | >1.0 | |
| Forest structure | ||||
| Canopy cover (%) | 10–59 | 60–79 | 80–100 | |
| Dominant height (m) | 5.7–9.0 | 9.1–12.0 | 12.1–15.3 | |
| Mean diameter (cm) | <20 | 20–40 | >40 | |
| Tree density (ind ha−1) | <1000 | >1000 | ||
| Basal area (m2 ha−1) | <25 | 25–40 | >40 | |
| Tree saplings (%) | 0 | 0.1–5.0 | >5.0 | |
| Total understory plants (%) | <70 | 70–85 | >85 | |
| Landscape | ||||
| Patch area (ha) | <100 | >100 | ||
| Patch shape | <4 | >4 | ||
| Forest total area (ha) | <70 | 70–180 | >180 | |
| Forest connectivity (%) | <10 | >10 | ||
| Open area total area (ha) | <120 | 120–200 | >200 | |
| Open area connectivity (%) | <15 | >15 |
References
- Martin, T.E. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998. [Google Scholar]
- Rosoni, J.R.R.; Fontana, C.S.; Carlos, C.J. Nest predation of the Chestnut Seedeater Sporophila cinnamomea (Passeriformes: Thraupidae) by the Patagonian Green Racer Pseudablabes patagoniensis (Serpentes: Dipsadidae) in the Brazilian Pampas. Herpetol. Notes 2023, 16, 539–541. [Google Scholar]
- Rao, X.; Li, J.; He, B.; Wang, H.; Wu, G.; Teng, T.; Ling, Q. Nesting success and potential nest predators of the red Junglefowl (Gallus gallus jabouillei) based on camera traps and artificial nest experiments. Front. Ecol. Evol. 2023, 11, 1127139. [Google Scholar] [CrossRef]
- Martin, T.E. Nest predation among vegetation layers and habitat types: Revising the dogmas. Am. Nat. 1993, 141, 897–913. [Google Scholar] [CrossRef]
- Thompson, F.R., III. Factors affecting nest predation on forest songbirds in North America. Ibis 2007, 149, 98–109. [Google Scholar] [CrossRef]
- Matysioková, B.; Remeš, V. Nest predation decreases with increasing nest height in forest songbirds: A comparative study. J. Ornithol. 2024, 165, 257–261. [Google Scholar] [CrossRef]
- Remeš, V.; Matysioková, B.; Cockburn, A. Long-term and largescale analyses of nest predation patterns in Australian songbirds and a global comparison of nest predation rates. J. Avian Biol. 2012, 43, 435–444. [Google Scholar] [CrossRef]
- Sherry, T.W.; Wilson, S.; Hunter, S.; Holmes, R.T. Impacts of nest predators and weather on reproductive success and population limitation in a long-distance migratory songbird. J. Avian Biol. 2015, 46, 559–569. [Google Scholar] [CrossRef]
- Matysioková, B.; Remeš, V. Evolution of parental activity at the nest is shaped by the risk of nest predation and ambient temperature across bird species. Evolution 2018, 72, 2214–2224. [Google Scholar] [CrossRef]
- Unzeta, M.; Martin, T.E.; Sol, D. Daily nest predation rates decrease with body size in passerine birds. Am. Nat. 2020, 196, 743–754. [Google Scholar] [CrossRef]
- Bellamy, P.E.; Burgess, M.D.; Mallord, J.W.; Cristinacce, A.; Orsman, C.J.; Davis, T.; Grice, P.V.; Charman, E.C. Nest predation and the influence of habitat structure on nest predation of Wood Warbler Phylloscopus sibilatrix, a ground-nesting forest passerine. J. Ornithol. 2018, 159, 493–506. [Google Scholar] [CrossRef]
- Tallei, E.; Rivera, L.; Schaaf, A.; Scheffer, M.; Politi, N. Post-logging effects on nest predation and avian predator assemblages in a subtropical forest. For. Ecol. Manag. 2022, 505, 119858. [Google Scholar] [CrossRef]
- Skutch, A.F. Do tropical birds rear as many young as they can nourish? Ibis 1949, 91, 430–455. [Google Scholar] [CrossRef]
- Rastogi, A.D.; Zanette, L.; Clinchy, M. Food availability affects diurnal nest predation and adult antipredator behaviour in song sparrows, Melospiza melodia. Anim. Behav. 2006, 72, 933–940. [Google Scholar] [CrossRef]
- Rangel-Salazar, J.L.; Martin, K.; Marshall, P.; Elner, R.W. Influence of habitat variation, nest-site selection, and parental behavior on breeding success of Ruddy-capped Nightingale Thrushes (Catharus frantzii) in Chiapas, Mexico. Auk 2008, 125, 358–367. [Google Scholar] [CrossRef]
- Chalfoun, A.D.; Thompson III, F.R.; Ratnaswamy, M.J. Nest predators and fragmentation: A review and meta-analysis. Conserv. Boil. 2002, 16, 306–318. [Google Scholar] [CrossRef]
- Hoset, K.S.; Husby, M. Are predation rates comparable between natural and artificial open-cup tree nests in boreal forest landscapes? PLoS ONE 2019, 14, e0210151. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.P.; Brittingham, M.C. Nest-site selection and nesting success of Wood Thrushes. Wilson Bull. 1998, 110, 375–383. [Google Scholar]
- Wang, Y.; Liu, J.; Gao, S.; Tong, S.; Wang, Z.; Li, N. Foraging Guilds of Birds in Continuous and Fragmented Forests of Southeast China. Forests 2025, 16, 861. [Google Scholar] [CrossRef]
- Benitez, J.; Barrera, M.D.; Sola, F.J.; Blazina, A.P.; Martínez Pastur, G.J.; Peri, P.L.; Lencinas, M.V. Effects of long-term low intensity silviculture and habitat on birds in Nothofagus antarctica forests of south Patagonia. For. Ecol. Manag. 2022, 516, 120254. [Google Scholar] [CrossRef]
- Dagan, U.; Izhaki, I. Vegetation structure governs nest predation in three types of conifer forest habitats. Eur. J. For. Res. 2020, 139, 721–729. [Google Scholar] [CrossRef]
- Evans, K.L. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 2004, 146, 1–13. [Google Scholar] [CrossRef]
- Eggers, S.; Griesser, M.; Andersson, T.; Ekman, J. Nest predation and habitat change interact to influence Siberian jay numbers. Oikos 2005, 111, 150–158. [Google Scholar] [CrossRef]
- Valladares, F. La disponibilidad de luz bajo el dosel de los bosques y matorrales ibéricos estimada mediante fotografía hemisférica. Ecología 2006, 20, 11–30. [Google Scholar]
- Visco, D.M.; Michel, N.L.; Boyle, W.A.; Sigel, B.J.; Woltmann, S.; Sherry, T.W. Patterns and causes of understory bird declines in human-disturbed tropical forest landscapes: A case study from Central America. Biol. Conserv. 2015, 191, 117–129. [Google Scholar] [CrossRef]
- Martínez Pastur, G.J.; Cellini, J.M.; Chaves, J.E.; Rodríguez-Souilla, J.; Benitez, J.; Rosas, Y.M.; Soler, R.M.; Lencinas, M.V.; Peri, P.L. Changes in forest structure modify understory and livestock occurrence along the natural cycle and different management strategies in Nothofagus antarctica forests. Agrofor. Syst. 2022, 96, 1039–1052. [Google Scholar] [CrossRef]
- Peri, P.L.; Martínez Pastur, G.; Monelos, L.; Livraghi, E.; Allogia, M.; Christiansen, R.; Sturzenbaum, M.V. Sistemas silvopastoriles en bosques nativos de ñire: Una estrategia para el desarrollo sustentable en la Patagonia Sur. In Dinámicas Mundiales, Integración Regional y Patrimonio en Espacios Periféricos, 1st ed.; Zárate, R., Artesi, L., Eds.; Universidad Nacional de la Patagonia Austral: Río Gallegos, Argentina, 2005; pp. 251–259. [Google Scholar]
- Ormaechea, S.; Peri, P.L. Landscape heterogeneity influences on sheep habits under extensive grazing management in Southern Patagonia. Liv. Res. Rural Dev. 2015, 27, e105. [Google Scholar]
- Benitez, J. Las Comunidades de Aves Terrestres Como Indicadores de Impacto en Bosques de Nothofagus antarctica de Tierra del Fuego. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2021. [Google Scholar]
- Hagar, J.C.; Owen, T.; Stevens, T.K.; Waianuhea, L.K. Response of corvid nest predators to thinning: Implications for balancing short-and long-term goals for restoration of forest habitat. Avian Conserv. Ecol. 2024, 19, 3. [Google Scholar] [CrossRef]
- Liljesthröm, M.; Fasola, L.; Valenzuela, A.; Raya Rey, A.; Schiavini, A. Nest predators of flightless steamer-ducks (Tachyeres pteneres) and flying steamer-ducks (Tachyeres patachonicus). Waterbirds 2014, 37, 210–214. [Google Scholar] [CrossRef]
- Maley, B.M.; Anderson, C.B.; Stodola, K.; Rosemond, A.D. Identifying native and exotic predators of ground-nesting songbirds in subantartic forests in southern Chile. Ann. Inst. Patagon. 2011, 39, 51–57. [Google Scholar] [CrossRef][Green Version]
- Vazquez, M.S.; Rodríguez-Cabal, M.A.; Gonzalez, D.V.; Pacheco, G.S.; Amico, G.C. Different nest predator guild associated with egg size in the Patagonian temperate forest. Bird Study 2018, 65, 478–483. [Google Scholar] [CrossRef]
- Crego, R.D.; Jara, R.F.; Rozzi, R.; Jiménez, J.E. Unexpected lack of effect of the invasive American mink on nesting survival of forest birds. Ornitol. Neotrop. 2020, 31, 88–97. [Google Scholar] [CrossRef]
- Jara, R.F.; Crego, R.D.; Samuel, M.D.; Rozzi, R.; Jiménez, J.E. Nest-site selection and breeding success of passerines in the world’s southernmost forests. PeerJ 2020, 8, e9892. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.S.; Amico, G.C. Nest predation in Patagonian wetlands: Predator assemblage and microhabitat characteristics. Emu 2023, 123, 24–34. [Google Scholar] [CrossRef]
- Vazquez, M.S.; Zamora-Nasca, L.B.; Rodriguez-Cabal, M.A.; Amico, G.C. Interactive effects of habitat attributes and predator identity explain avian nest predation patterns. Emu 2021, 121, 250–260. [Google Scholar] [CrossRef]
- Benitez, J.; Barrera, M.D.; Rosas, Y.M.; Martínez Pastur, G.J.; Lencinas, M.V. Landscape and stand characteristics influence on the bird assemblage in Nothofagus antarctica Forests of Tierra del Fuego. Land 2022, 11, 1332. [Google Scholar] [CrossRef]
- Martínez Pastur, G.J.; Cellini, J.M.; Lencinas, M.V.; Barrera, M.; Peri, P.L. Environmental variables influencing regeneration of Nothofagus pumilio in a system with combined aggregated and dispersed retention. For. Ecol. Manag. 2011, 261, 178–186. [Google Scholar] [CrossRef]
- Lencinas, M.V.; Martínez Pastur, G.; Medina, M.; Busso, C. Richness and density of birds in timber Nothofagus pumilio forests and their unproductive associated environments. Biodivers. Conserv. 2005, 14, 2299–2320. [Google Scholar] [CrossRef]
- Soler, R.M. Regeneración Natural de Nothofagus antarctica en Bosques Primarios, Secundarios y Bajo Uso Silvopastoril. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2012. [Google Scholar]
- Major, R.E.; Kendal, C.E. The contribution of artificial nest experiments to understanding avian reproductive success: A review of methods and conclusions. Ibis 1996, 138, 298–307. [Google Scholar] [CrossRef]
- Villard, M.A.; Pärt, T. Don’t put all your eggs in real nests: A sequel to Faaborg. Conserv. Boil. 2004, 18, 371–372. [Google Scholar] [CrossRef]
- Roos, S. Functional response, seasonal decline and landscape differences in nest predation risk. Oecologia 2002, 133, 608–615. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A. Factors affecting the survival of real and artificial great reed warbler’s nests. Biologia 2005, 60, 215–219. [Google Scholar]
- Ludwig, M.; Schlinkert, H.; Holzschuh, A.; Fischer, C.; Scherber, C.; Trnka, A.; Tscharntke, T.; Batáry, P. Land-scape-moderated bird nest predation in hedges and forest edges. Acta Oecol. 2012, 45, 50–56. [Google Scholar] [CrossRef]
- Mandema, F.S.; Tinbergen, J.M.; Ens, B.J.; Bakker, J.P. Livestock grazing and trampling of birds’ nests: An experiment using artificial nests. J. Coast. Conserv. 2013, 17, 409–416. [Google Scholar] [CrossRef]
- Altamirano, T.A.; Ibarra, J.T.; Hernández, F.; Rojas, I.; Laker, J.; Bonacic, C. Hábitos de Nidificación de las Aves del Bosque Templado Andino de Chile; Pontificia Universidad Católica de Chile: Santiago de Chile, Chile, 2012; p. 113. [Google Scholar]
- Jara, R.F.; Crego, R.D.; Arellano, F.J.; Altamirano, T.A.; Ibarra, J.T.; Rozzi, R.; Jiménez, J.E. Breeding strategies of open-cup-nesting birds in sub-Antarctic forests of Navarino Island, Chile. Rev. Chil. Hist. Nat. 2019, 92, 2. [Google Scholar] [CrossRef]
- Larivière, S. Reasons why predators cannot be inferred from nest remains. Condor 1999, 101, 718–721. [Google Scholar] [CrossRef]
- Gorosito, C.A.; Tuero, D.T.; Cueto, V.R. More is better: Predator dilution effect increases Chilean Elaenia (Elaenia chilensis) nest survival. J. Ornithol. 2024, 165, 147–155. [Google Scholar] [CrossRef]
- Cerda-Pena, C.; Rau, J.R. Potential predators of nests of aquatic birds of the wetlands of Caulín, Chiloé, south of Chile and evaluation of methods of detection. Gayana 2018, 82, 171–176. [Google Scholar]
- Liljesthröm, M.; Schiavini, A.; Sáenz Samaniego, R.A.; Fasola, L.; Raya Rey, A. Kelp Geese (Chloephaga hybrida) and Flightless Steamer-Ducks (Tachyeres pteneres) in the Beagle Channel: The importance of islands in providing nesting habitat. Wilson J. Ornithol. 2013, 125, 583–591. [Google Scholar] [CrossRef]
- Reyes-Arriagada, R.; Jiménez, J.E.; Rozzi, R. Daily patterns of activity of passerine birds in a Magellanic sub-Antarctic forest at Omora Park (55 S), Cape Horn Biosphere Reserve, Chile. Polar Biol. 2015, 38, 401–411. [Google Scholar] [CrossRef]
- Lencinas, M.V.; Martínez Pastur, G.; Gallo, E.; Cellini, J.M. Alternative silvicultural practices with variable retention improve bird conservation in managed South Patagonian forests. For. Ecol. Manag. 2009, 258, 472–480. [Google Scholar] [CrossRef]
- Shields, W.M. The effect of time of day on avian census results. Auk 1977, 94, 380–383. [Google Scholar] [CrossRef]
- A Classification of the Bird Species of South America. American Ornithological Society. Available online: www.museum.lsu.edu/~Remsen/SACCBaseline.htm (accessed on 8 April 2020).
- De la Peña, M.R. Aves Argentinas: Huevos y Nidos; Eudeba-Ediciones UNL: Buenos Aires, Argentina, 2015; p. 82. [Google Scholar]
- Di Rienzo, J.A.; del Carmen, R.M. Un paquete R para el cálculo de medias ajustadas para modelos lineales y lineales generalizados. In Proceedings of the Décimo Congreso Latinoamericano de Sociedades de Estadística, Córdoba, Argentina, 16–19 October 2012. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; p. 621. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar] [CrossRef]
- InfoStat Software Estadístico. Available online: http://www.infostat.com.ar (accessed on 29 September 2020).
- Dunn, O.J. An empirical study of the Bonferroni procedure for multiple t-tests. Commun. Stat. 1961, 1, 1–13. [Google Scholar]
- Liebezeit, J.R.; George, T.L. Nest predators, nest-site selection, and nesting success of the Dusky Flycatcher in a managed ponderosa pine forest. Condor 2002, 104, 507–517. [Google Scholar] [CrossRef]
- Colombelli-Négrel, D.; Kleindorfer, S. Nest height, nest concealment, and predator type predict nest predation in superb fairy-wrens (Malurus cyaneus). Ecol. Res. 2009, 24, 921–928. [Google Scholar] [CrossRef]
- Segura, L.N.; Masson, D.A.; Gantchoff, M.G. Microhabitat nest cover effect on nest survival of the Red-crested Cardinal. Wilson J. Ornithol. 2012, 124, 506–512. [Google Scholar] [CrossRef]
- Malzer, I.; Helm, B. The seasonal dynamics of artificial nest predation rates along edges in a mosaic managed reedbed. PLoS ONE 2015, 10, e0140247. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horanyi, A.; Munoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Gorban, I.; Trakimas, G.; Stasiukynas, L.; Lekoveckaitė, A.; Samas, A.; Podėnienė, V. Precipitation as the primary environmental driver of saproxylic fly diversity (diptera: Bibionomorpha and Tipulomorpha) in forest ecosystems. Insects 2025, 16, 1109. [Google Scholar] [CrossRef]
- Couve, E.; Vidal, C. Aves de Patagonia, Tierra del Fuego y Península Antártica; Fantástico Sur Birding Ltda.: Punta Arenas, Chile, 2003; p. 656. [Google Scholar]
- Biondi, L.M.; Bó, M.S.; Favero, M. Dieta del chimango (Milvago chimango) durante el periodo reproductivo en el sudeste de la provincia de Buenos Aires, Argentina. Ornitol. Neotrop. 2005, 16, 3. [Google Scholar]
- Humphrey, P.; Bridge, D.; Reynolds, P.; Peterson, R. Birds of Isla Grande (Tierra del Fuego); Smithsonian Institution: Washington, DC, USA, 1970; p. 411. [Google Scholar]
- Morrison, J.L.; Phillips, L.M. Nesting habitat and success of the Chimango Caracara in southern Chile. Wilson Bull. 2000, 112, 225–232. [Google Scholar] [CrossRef]
- Maag, N.; Mallord, J.W.; Burgess, M.D.; Lüpold, S.; Cristinacce, A.; Arlettaz, R.; Carlotti, S.; Davis, T.M.; Grendelmeier, A.; Orsman, C.J.; et al. Accounting for predator species identity reveals variable relationships between nest predation rate and habitat in a temperate forest songbird. Ecol. Evol. 2022, 12, e7411. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).