Abstract
Functional traits provide key insights into plant ecological strategies and responses to environmental heterogeneity, yet the role of intraspecific trait variability (ITV) in tropical rainforests remains underexplored. We examined ITV in six leaf traits—leaf thickness (LT), leaf area (LA), specific leaf area (SLA), leaf dry matter content (LDMC), leaf nitrogen content (LNC), and stomatal density (SD)—in saplings of 15 dominant tree species across ridge and valley habitats in a Sri Lankan tropical lowland rainforest. We compared interspecific and intraspecific variation and quantified trait plasticity using the plasticity index. Significant ITV was observed for LT, LA, and SD, with ridge individuals showing smaller, thicker leaves with lower SD. SLA, LDMC, and LNC exhibited no overall habitat-level differences, though species-specific divergent responses were detected. Interspecific variation exceeded ITV for most traits, except for LNC, where ITV accounted for 55% of total variation. Trait plasticity varied among traits, with LNC showing the highest plasticity. These results indicate that individuals adjust leaf traits in response to fine-scale habitat heterogeneity, reflecting shifts in resource-use strategies. Overall, ITV is ecologically meaningful and should be incorporated into community-level studies and ecosystem models to improve predictions of plant community dynamics and ecosystem functioning under environmental change.
1. Introduction
Functional traits are fundamental for plant ecology, offering critical insights into how plants interact with their environment and adapt to varying ecological conditions [1,2,3,4,5]. Leaf traits such as specific leaf area, leaf dry matter content, and leaf nitrogen concentration are widely used to describe key aspects of plant resource-use strategies, photosynthetic capacity [6], and growth rates [7]. These traits serve as proxies for broader physiological processes and help elucidate species’ ecological strategies and performance across environmental gradients [8]. The leaf economic spectrum [6] reflects plant strategies from fast-growing, resource-acquisitive species to slow-growing, resource-conservative species. Thus, the study of leaf functional traits has become central to trait-based ecology, contributing to predictions of community assembly [4] and ecosystem responses to environmental change [5,9].
Examining variation in leaf traits across species-rich communities spanning environmental gradients can enhance our understanding of the abiotic factors driving plant performance [10]. Such quantitative insights support the development of predictive models for how diverse forest ecosystems respond to environmental change. Yet, most trait-based studies have emphasized interspecific variation, relying on species-mean trait values as representative of ecological strategies [4,6]. This focus is grounded in the assumption that interspecific differences are larger and more ecologically informative than within-species variation [1]. While this approach has revealed broad-scale patterns [4,6,7], it risks oversimplifying plant–environment interactions by neglecting trait variability expressed among individuals of the same species [11].
A growing body of evidence indicates that intraspecific trait variability (ITV) can be substantial and ecologically meaningful [11,12,13,14,15]. For instance, Messier et al. [12] demonstrated that the within-species variation in leaf dry matter content and leaf mass per area is roughly equal to interspecific variation in old-growth tropical forest in Panama. Zuleta et al. [14] further reported that in an Amazon terra firme forest, 10 out of 18 examined traits exhibited greater intraspecific variation than interspecific variation. A global meta-analysis of 629 plant communities and 36 plant functional traits revealed that ITV contributes, on average, 25% of trait variation within communities and 32% among communities [16]. Collectively, these findings highlight that ITV represents a critical component of functional diversity shaping community dynamics and ecosystem functioning.
Building on this evidence, understanding the drivers of ITV has become central to explaining how plants respond to environmental heterogeneity. Abiotic factors play a dominant role through both genetic differentiation and phenotypic plasticity [17,18]. In particular, local topographic variation generates spatial heterogeneity in key resources, such as soil nutrients, soil moisture, and light availability [19,20,21,22,23], thereby driving ITV in plants as they adjust to these fine-scale environmental differences [14,24]. For example, species in a Neotropical forest displayed ITV in leaf traits across topographic habitats: individuals on ridge tops developed smaller leaves with higher leaf mass per area and dry matter content, adaptations that confer tolerance to reduced water availability and soil fertility. In contrast, trees in bottomland habitats, where water availability and soil fertility are high, produced larger leaves with lower leaf mass per area and dry matter content [24]. Similarly, species in an Amazonian forest have shown intraspecific variation in specific leaf area and leaf density along topography, with specific leaf area decreasing and leaf density increasing toward drier ridge habitats, reflecting adjustments to variation in water limitation [14]. These trait shifts indicate a transition from acquisitive to conservative resource-use strategies under unfavorable conditions, demonstrating how ITV enables plants to optimize performance across environmental gradients [14,24]. Consequently, ITV broadens species’ ecological niches, enhancing their ability to persist across heterogeneous environments and maintain community stability [11,13,24,25,26].
Given that ITV arises largely from how individual traits respond to environmental gradients, identifying which traits are most plastic is essential for understanding plant adaptive strategies. Leaves, as the primary organs for resource acquisition and metabolism, are particularly sensitive to fluctuations in resource availability and frequently exhibit strong phenotypic plasticity [27,28], thereby contributing substantially to ITV. However, not all traits respond equally to environmental variation. For instance, leaf nutrient traits such as leaf nitrogen and phosphorus concentrations, and biomass allocation traits, generally display greater intraspecific variability than morphological traits [16,29,30,31]. In contrast, morphological traits related to leaf size, including leaf area, length, and leaf thickness, tend to exhibit comparatively low levels of ITV [16,30]. These patterns suggest that physiological and chemical traits are more labile than structural ones, reflecting differential constraints on plasticity and adaptive potential across functional trait dimensions.
Incorporating ITV into trait-environment analyses is essential for advancing predictions of plant responses to global environmental change [14,32]. Understanding how tropical trees respond to shifting environmental conditions is particularly critical, as tropical forests harbor a substantial proportion of the world’s biodiversity and play pivotal roles in regulating global carbon and water cycles [33,34]. Within these forests, topographically driven environmental heterogeneity offers a natural framework for examining plant functional responses, as topography strongly influences resource availability, micro-climate, and, consequently, the functional composition and structure of tropical communities [19,22,35,36]. Despite this, only a limited number of studies have explicitly incorporated ITV into trait–environment analyses when evaluating responses to topographically induced environmental gradients in tropical rainforests, the majority conducted in the Neotropics [14,24]. The magnitude, drivers, and ecological consequences of ITV in the wetter regions of South Asia and Southeast Asian mixed dipterocarp rainforests remain poorly understood. These forests are critically important, as they harbor exceptional tree diversity and play a key role in global biogeochemical cycles. The scarcity of empirical evidence constrains our understanding of how individual plants and populations in these forests adjust to fine-scale environmental heterogeneity, limiting predictions of community assembly processes and ecosystem functioning under changing environmental conditions.
Therefore, we investigated within-species variation in six leaf traits across contrasting topographic habitats in a mixed dipterocarp rainforest in Sri Lanka. By integrating ITV into trait-environment analyses, we aimed to elucidate how fine-scale environmental heterogeneity influences functional trait expression within species. Specifically, we addressed the following key questions and hypotheses:
- (1)
- Do leaf traits show significant intraspecific variability across different topographic habitats? We hypothesize that there is a conservative tendency of leaf traits in resource-limited habitats (e.g., ridges) compared with resource-rich areas (e.g., valleys).
- (2)
- How does the magnitude of intraspecific variability compare to interspecific variability? We expect that interspecific variation will generally exceed intraspecific variation. However, we also anticipate substantial ITV in certain traits, depending on their functional roles and sensitivity to environmental variation.
2. Materials and Methods
2.1. Study Site
This study was conducted within the 25-ha Sinharaja Forest Dynamics Plot (SFDP) (SFDP, 6°21–26′ N, 80°21–34′ E), located in the lowland rainforest of Sinharaja, in the southwestern region of Sri Lanka. Sinharaja is characterized as a mixed-dipterocarp rainforest and receives a mean annual rainfall of approximately 5000 mm, with annual temperatures varying between 23–29 °C. Topographically, the SFDP spans an elevational range from 424 m to 575 m above sea level (asl) and encompasses a central valley flanked by two distinct slopes: a steeper, higher slope oriented to the southwest, and a less-steep slope oriented to the northeast. The landscape is further characterized by the presence of seepage ways, spurs, small hillocks, at least two perennial streams, and several seasonal streamlets [37]. The SFDP is part of the Forest Global Earth Observatory (ForestGEO) plot network [38], and six comprehensive censuses have been conducted during the period from 1994 to 2025, adhering to the ForestGEO protocols, where all free-standing woody stems with diameter at breast height (1.30 m, DBH) ≥ 1 cm were tagged, measured, mapped, and identified to species.
2.2. Topographic Habitats
Three topographically defined habitats- ridge, slope, and valley- were identified within the SFDP based on the elevation classes as described by Gunatilleke et al. [37]. Strong habitat associations are known for nearly 79% of the 125 species examined within the SFDP, underscoring the significant role of local topography in shaping species distributions in this tropical rainforest [20]. The valley habitat covers the area below 460 m elevation, including both the relatively flat valley and the permanent stream, and comprises 49.8% of the total plot area. The ridge habitat includes an area above 520 m elevation, representing 15.7% of the plot. The intervening elevation band, between 460 m and 520 m, was identified as the slope habitat, which includes both mid-slope and upper slope areas and accounts for 34.5% of the plot. Each 20 m × 20 m quadrat within the plot was assigned to one of these three habitat types based on its mean elevation value, to delineate the area of each habitat. For the present study, only individuals in ridge and valley habitats, representing extremes in resource availability, were used.
2.3. Targeted Species and Individual Tree Selection
Tree species for this study were selected based on their Importance Value Index (IVI) within the SFDP, calculated from the 5th census data of the SFDP. The IVI quantifies a species’ overall significance in a community by integrating its relative frequency, density, and dominance [39]. The selection followed three criteria: (1) an IVI greater than 1%; (2) belongs to the canopy, sub-canopy, or understory strata; and (3) a minimum local abundance of more than 30 individuals (DBH 1–5 cm) in both ridge and valley habitats. Based on these criteria, fifteen species representing eight families were selected for the collection of trait data (Table S1). Within each habitat, individuals of each species were randomly selected across the habitat. For each species in each habitat, 25–30 individuals within the 1–5 cm DBH size class (saplings) were considered for leaf trait measurements.
2.4. Leaf Traits
Five fully expanded, mature, and shaded leaves were sampled from each selected individual to measure six leaf functional trait representing a range of plant resource acquisition strategies: leaf thickness (LT, mm), leaf area (LA, cm2), specific leaf area (SLA, cm2 g−1), leaf dry matter content (LDMC, %), leaf nitrogen content (LNC, %), and stomatal density (SD, mm−2). Sampling and trait measurements were completed between December 2021 to March 2023. All measurements followed the protocols described by Pérez-Harguindeguy et al. [40]. Leaf thickness was measured on fresh leaves using a digital micrometer (Mitutoyo, Roissy-en-France, France). For LA and SLA, fresh leaves were scanned using a flatbed scanner (Canon Scanner LiDE 300, Melville, NY, USA), and scanned images were analyzed with ImageJ software (Version 1.53e, NIH, Bethesda, MD, USA). Leaves were then oven-dried at 60 °C for 72 h to obtain dry weight. Both leaf fresh weight and dry weight were measured with an electronic balance (Denver Instruments, Denver, CO, USA) for LDMC calculation. To determine leaf nitrogen content, the five dried leaves were pooled from each of the 15 individuals per species in each habitat. The pooled samples were ground into a fine powder and sieved to obtain a homogeneous mixture. Approximately 2 mg of the powdered sample was then analyzed using a CHNS/O elemental analyzer (PerkinElmer® 2400 Series II, Shelton, CT, USA). LNC data were available for only six of the species analyzed in this study. For Stomatal density, three fully expanded, mature, and shaded leaves were sampled from each selected individual. SD was measured by creating lower epidermal impressions using clear nail varnish. Once dried, the impressions were mounted on glass slides and examined under a compound microscope (Olympus BX53, Hachioji, Japan) at 400× magnification. Stomata were counted in three randomly selected fields of view of a known area per leaf to calculate stomatal density.
2.5. Data Analysis
For each individual, trait values for each of the five leaves were first averaged to obtain a single value per trait for that individual. Since the distribution of trait values between individuals deviated from normality, trait data were log-transformed before hypothesis testing. To test whether leaf traits exhibit intraspecific variability between the two habitats we fit separate linear mixed-effects models (LMMs) for each trait. In these models, the log-transformed trait values were modeled as a function of habitat type, with species identity included as a random intercept and random slope to account for interspecific variation in mean trait values as well as variation among species in the effect of habitat. The models were implemented using the function “lmer” from the package “lmerTest” in R (version 4.4.2), which also provides p-values for fixed effects using Satterthwaite’s approximation for degrees of freedom. In addition, we extracted marginal and conditional R2 values using the package “performance” to quantify the proportion of variance explained by the fixed effect alone (habitat) and by the combination of fixed and random effects, including habitat, species identity (random intercept), and species-specific habitat responses (random slope), respectively.
After fitting LMMs, we conducted separate linear models for each species and trait (Table S2), with log-transformed trait values modeled as a function of habitat type. These species-level models allowed us to assess species-specific ITV patterns with habitat type.
To compare the magnitude of interspecific and intraspecific trait variability, first, we calculated the ‘population mean traits’, the average value of each trait for a given species within each habitat, and ‘species mean traits’, the average value of each trait for a given species across two habitats. ITV was then quantified for each species and trait as the coefficient of variation (CV) of the population mean traits across two habitats. We then averaged the ITV value across species for each trait to have an overall estimate of ITV for each trait [15]. To keep the sample size used to calculate CVs consistent for both ITV and interspecific trait variability (BTV), we calculated BTV for each trait by randomly pulling the species mean trait value for two different species, and calculated the CV. We repeated that 1000 times and then calculated the mean CV of the random draws as the bootstrapped species mean CV, which serves as our estimate of BTV. Then, we compared these values of BTV to the respective ITV for each trait to assess the relative magnitude of trait variation among and within species.
Furthermore, to compare the trait plasticity, the plasticity index (PI) was calculated for each species and each trait between two habitats following the method described by Liu et al. [27], using the formula:
where x denotes the lower value and X denotes the higher value of the population mean traits across the two habitats. This index quantifies the degree of phenotypic plasticity expressed by each species in response to habitat variation. Subsequently, the average PI was computed for each trait by taking the average of species-level PI values.
PI = 1 − x/X
3. Results
3.1. Intraspecific Trait Variation Among Topographic Habitats
Results from LMMs showed significant ITV in certain leaf traits between ridge and valley habitats (Table 1). Habitat type had a significant effect on leaf thickness, leaf area, and stomatal density. Specifically, and compared to the valley, LT was slightly higher in ridge individuals (Figure 1a), with an estimated increase of 0.019 units (p = 0.005). LA was significantly lower in the ridge (Figure 1b), compared to the valley, with an estimated reduction of 0.179 units (p < 0.001). Similarly, SD was lower in ridge individuals (Figure 1f; estimate = −0.145, p < 0.001), compared to the valley. No significant differences between ridge and valley were detected for SLA (Figure 1c; estimate = 0.001, p = 0.961), LDMC (Figure 1d; estimate = −0.006, p = 0.692), and LNC (Figure 1e; estimate = −0.066, p = 0.674).

Table 1.
Summary of LMMs testing the effect of topographic habitat (valley vs. ridge) on log-transformed leaf trait values. Estimates represent the mean trait values for individuals in the valley habitat and the estimated effect of the ridge habitat. Marginal R2 reflects variance explained by the fixed effect, and conditional R2 reflects variance explained by the fixed and random effects.
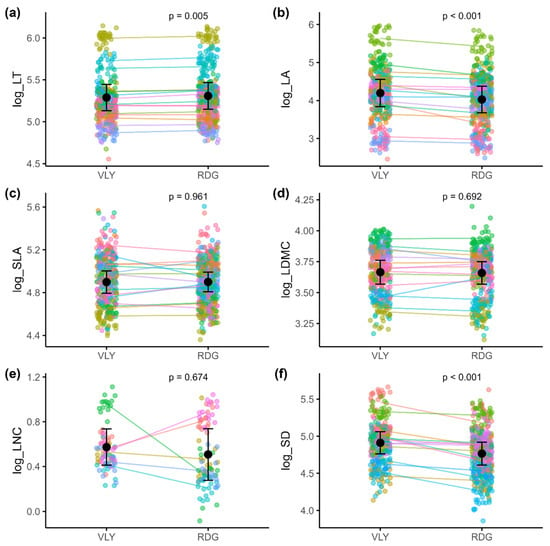
Figure 1.
Variation in log-transformed leaf traits between valley (VLY) and ridge (RDG) habitats, illustrating intraspecific trait variability (ITV) across multiple species: (a) leaf thickness (n = 878); (b) leaf area (n = 878); (c) specific leaf area (n = 878); (d) leaf dry matter content (n = 878); (e) leaf nitrogen content (n = 90); (f) stomatal density (n = 840). Black points with error bars represent the estimated mean trait values (±2 standard errors) for each habitat, based on linear mixed-effects models. Faded colored points show individual trait values for each species in each habitat, with each color corresponding to a different species. Colored lines connect conspecific mean trait values across habitats, allowing visualization of species-level trait shifts. Sample sizes (n) indicate the total number of individuals used for the analysis of each trait.
Species accounted for most of the variation in all traits (high conditional R2 values), reflecting both differences in species’ average trait values (random intercepts) and species-specific responses to habitat (random slope). The marginal R2 values were consistently low across all six models, ranging from <0.001 to 0.049 (Table 1). Among the traits, SD exhibited the highest marginal R2 value (0.049), indicating that habitat had a relatively greater influence on within-species variation in SD compared to the other traits. Conversely, traits such as LT, SLA, and LDMC showed minimal habitat effects, with marginal R2 values of 0.001 or less. LA and LNC demonstrated modest habitat contribution to trait variation, with marginal R2 values of 0.013 and 0.015, respectively.
The random-effect variances indicate interspecific differences in mean trait values (random intercepts) for all traits. For all traits, random intercept variance ranges from 0.003 to 0.230. The variance in random slopes, representing species-specific responses to habitat type, also varied among traits. For LT, the random slope variance was negligible, indicating that all species responded similarly to habitat differences (Figure 1a). The random slope variances for SLA (0.006) and LDMC (0.003) were also minimal, implying that the effect of habitat on these traits was largely consistent among species (Figure 1c,d). However, species-specific linear models revealed contrasting ITV patterns for certain species for SLA and LDMC. For SLA, three species (Palaquium canaliculatum, Shorea affinis, and Shorea stipularis) showed significantly higher SLA in the ridges, and three species (Palaquium petiolare, Shorea cordifolia, and Xylopia championii) showed significantly lower SLA in the ridges. Similarly, for LDMC, four species (Cullenia rosayroana, Garcinia hermonii, Mesua nagassarium, and Shorea affinis) showed significantly lower LDMC in the ridge, while two species (Palaquium petiolare and Shorea disticha) showed significantly higher LDMC in the ridge. For LA and SD, the moderate slope variances (0.019 and 0.012, respectively) suggest modest variation in how species adjusted their leaf size and stomatal density between ridge and valley habitats (Figure 1b,f). Significant species-specific patterns were also observed for both LA and SD; however, none of the species showed an opposite trend. In contrast, LNC displayed considerably higher slope variance (0.129), indicating pronounced species-specific responses to habitat type. Consistent with this, species-specific linear models for LNC revealed contrasting ITV patterns among species (Figure 1e). Two species (Anisophyllea cinnamomoides and Palaquium petiolare) showed significantly higher LNC values in the ridge compared to the valley, whereas three species (Humboldtia laurifolia, Mesua ferrea, and Mesua nagassarium) exhibited the opposite trend, with higher LNC values in the valley habitats.
3.2. Magnitude of Interspecific and Intraspecific Variability
The comparison of interspecific and intraspecific variation across six leaf traits revealed that interspecific variability exceeded intraspecific variability for five traits—LT, LA, SLA, LDMC, and SD. This pattern is reflected in the BTV/ITV ratios in Table 2, with the highest ratio observed for LT. Similarly, higher BTV/ITV ratios were found for LDMC, LA, SLA, and SD, indicating that trait differences among species were greater than the within-species variation of these traits. In contrast, leaf nitrogen content displayed a BTV/ITV ratio below 1 (0.813), indicating that intraspecific variation exceeds interspecific variation for this trait. These results are further represented by the proportional contributions of interspecific and intraspecific variation to total trait variability shown in Figure 2. For the five traits with greater interspecific variability (LT, LA, SLA, LDMC, and SD), ITV accounted for approximately 6% to 31% of the total trait variation. Conversely, LNC demonstrated a greater intraspecific contribution, comprising 55% of the total variability.

Table 2.
CV for interspecific and intraspecific variation for each leaf trait. BTV represents the CV of species mean traits across all species, while ITV represents the average CVs of population mean traits across species. The ratio BTV/ITV indicates the relative contribution of interspecific to intraspecific variation.
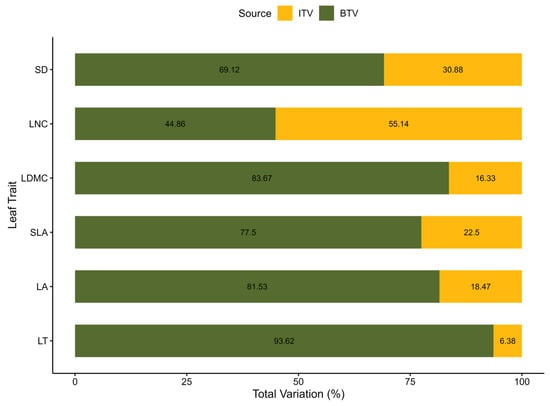
Figure 2.
Proportional contribution of interspecific (BTV; green) and intraspecific (ITV; yellow) variation to total trait variability for six leaf traits between ridge and valley habitats. Percentages were calculated based on the coefficients of variation (CV) for each trait at the interspecific and intraspecific levels using the formula: (ITV or BTV)/(ITV + BTV) × 100.
The comparison of plasticity indices further highlighted considerable phenotypic plasticity across the six leaf traits (Figure 3). LNC exhibited the highest plasticity (PI = 0.368) in response to habitat variation. Among the other traits, LA and SD showed moderate plasticity, with PI values of 0.04 and 0.03, respectively, reflecting the ability of individuals to adjust leaf size and number of stomata according to environmental conditions. In contrast, LDMC and SLA exhibited very low plasticity, with PI values of 0.011 and 0.013, respectively. The lowest plasticity was observed in LT, with a PI of 0.005.
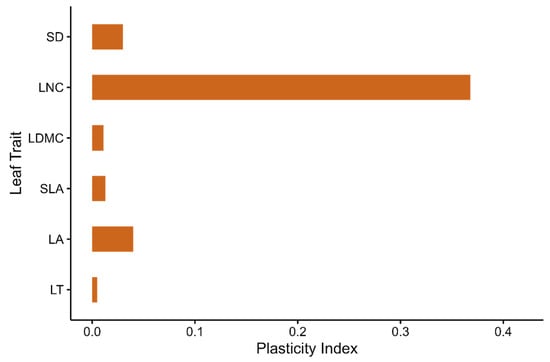
Figure 3.
Plasticity indices for each leaf trait.
4. Discussion
4.1. Intraspecific Trait Variability Driven by Habitat Heterogeneity
Our study demonstrates that ITV contributes significantly to plant functional responses across topographically distinct habitats in a tropical rainforest, emphasizing the role of environmental heterogeneity in shaping trait expression within species. In terms of intraspecific trait variation driven by habitat heterogeneity, we found that multiple leaf traits, specifically leaf thickness, leaf area, and stomatal density, exhibit ITV across the two habitats (Table 1). Our findings highlight topography as a significant driver of intraspecific trait variability in leaf traits. Within this forest, topography strongly structures habitat characteristics, where wetter soils and rich nutrient availability characterize valley habitats, in contrast to ridge habitats with water scarcity and limited soil nutrients for tree growth [20,21].
Leaf thickness was found to be slightly greater in individuals from ridge habitats (Figure 1a), indicating a significant effect of habitat. This finding aligns with previous studies from tropical forests that documented an increase in leaf thickness with elevation as an adaptive response to limited water availability [10,25], higher radiation levels, and lower CO2 concentrations [26] encountered at higher elevations. However, since the elevation range in our study is relatively narrow (424–575 m) compared to those in other studies [26], the effects of elevation on temperature and CO2 levels are likely negligible. Instead, factors like soil water availability appear to have a more pronounced influence. This is further confirmed by the finding that leaf thickening is primarily driven by reduced soil water availability rather than direct atmospheric changes [41]. These structural adaptations in leaves enhance water storage capacity and promote more efficient water utilization under adverse environmental conditions [27].
We also observed a decline in leaf area from the valley to the ridge habitat (Figure 1b). As a commonly used measure of leaf size, leaf area is known to be linked to various environmental stresses, including nutrient availability, drought, high levels of solar radiation, and wind exposure [27,40]. A similar response of leaf area to topographic habitats has been reported at the species level in the Yasuni Forest Dynamics Plot [4], where ridges were composed of species with smaller leaves compared to those in valley communities. In contrast, Umana & Swenson [26] found no clear within-species variation in LA across a broader elevational gradient in a subtropical forest. Even within the narrow elevational range of our study, the smaller leaf area observed on the ridge may be due to reduced soil resource availability at the ridges [20,21,24], which limits plant growth. Smaller leaf areas decrease solar energy absorption [28], and so the smaller leaves in the ridge habitat might result in lower photosynthetic rates.
The lower stomatal density observed in the ridge habitat compared to the valley (Figure 1f) represents an important drought-adaptive trait commonly found in species thriving under water-limited conditions. This aligns with previous findings from the Sinharaja rainforest, where plants in the ridge habitat exhibited reduced stomatal frequency to cope with drier soils that retain less moisture [42]. Stomata regulate carbon uptake through photosynthesis and water loss via transpiration, so reduced stomatal density helps minimize water loss, providing a crucial advantage in dry environments. In contrast, the significantly higher stomatal density in the valley habitat suggests that plants there maintain photosynthetic rates by increasing stomatal numbers in response to greater soil nutrient and water availability. This adaptation enhances stomatal conductance, carbon assimilation, and water-use efficiency under more favorable conditions [43].
Although LNC showed no significant overall difference between ridge and valley habitats, the pronounced species-specific ITV patterns (Figure 1e) indicate that, while habitat-driven differences in LNC are not consistent across all species, individual species adopt divergent strategies in nitrogen utilization. The higher LNC observed in valley individuals, as initially expected, likely reflects greater soil nutrient availability in these habitats compared to the more nutrient-poor ridge tops in Sinharaja [20,36,44]. Plants inhabiting nutrient-rich environments generally maintain higher LNC than those in nutrient-limited soil [31]. Conversely, the higher LNC observed in ridge individuals of certain species may reflect distinct species-specific nutrient-use and allocation strategies. This interpretation aligns with previous evidence that foliar traits such as LNC are strongly constrained by taxonomic identity [45]. Therefore, the observed ITV in LNC likely arises from the combined influence of local edaphic heterogeneity and intrinsic species-specific physiological adaptations, rather than from a uniform topographic effect acting similarly across all species.
Likewise, SLA and LDMC showed no significant overall differences between ridge and valley habitats, yet the species-specific analyses revealed contrasting ITV patterns (Figure 1c,d), suggesting that species differ in their strategies for resource acquisition and conservation across habitats. In general, higher SLA values are associated with resource-acquisitive strategies that facilitate rapid growth and nutrient uptake, whereas higher LDMC reflects a more conservative strategy, promoting tissue longevity and resistance to stress through denser, tougher leaves [14,24,25,26,31]. Accordingly, the lower SLA observed in ridge individuals of some species likely represents an adaptive adjustment to drier and more nutrient-poor ridge conditions, achieved resource conservation, while species with higher LDMC in the ridges similarly adopt conservative strategies to cope with resource limitation. In contrast, the higher SLA in some ridge species and lower LDMC in others likely reflect species-specific adaptations shaped by both taxonomic identity and local environmental conditions [45].
4.2. Interspecific vs. Intraspecific Trait Variability
We found that except for LNC, all traits (LT, LA, SLA, LDMC, and SD) exhibited greater interspecific variation than intraspecific variation across the two habitats, supporting the widely accepted view that trait values tend to differ more among species than within species [1,46]. Intraspecific variation of LNC (55%) exceeded the variation observed between species (45%). Even though the interspecific variation is generally greater, other leaf traits, specifically SD, SLA, LA, and LDMC, still exhibited considerable ITV (16%~31%) in the trait-habitat relationship. Substantial ITV has also been documented in other tropical regions. For example, Xu et al. [25] reported that, across a narrow elevational gradient, the ITV of LA, SLA, and LT within a single dominant tree species accounted for 22%–34% of the interspecific range in tropical seasonal rainforest in China. Similarly, a study in an aseasonal Amazonian forest found that 10 out of the 18 traits examined showed greater ITV than interspecific variation along the local topographic gradient [14]. Ignoring such intraspecific trait variability by relying solely on species mean traits can bias interpretations of trait–environment relationships, potentially underestimating the influence of habitat filtering and the predictive power of community structure and ecosystem functioning under environmental change [11]. For example, analyses in French Guiana demonstrated that incorporating within-species trait variation increased the sensitivity of predictions regarding habitat filtering and niche differentiation, whereas species mean analyses underestimated the role of filtering in community assembly [47]. Moreover, individual-level trait data provide more accurate predictions of growth performance across varying habitats compared with species-level averages [48,49].
Our results further emphasize that microenvironmental variation between the two habitats influences leaf traits with varying intensity, as evidenced by the different plasticity indices exhibited by each trait. Comparative studies have found that leaf nutrient concentrations (e.g., LNC) are highly labile within species, displaying strong plastic responses to resource availability, whereas leaf morphology (e.g., LT, LA, SLA, and LDMC) tends to be more stable [16,31]. This aligns with our findings, where LNC demonstrated greater ITV (53%) and phenotypic plasticity (0.368) compared to the examined morphological traits. The ability of plants to store nutrients, dependent on environmental element availability, likely explains the relatively high N content in leaf chemical composition [46]. Moreover, evidence shows that ITV is relatively high within communities for both chemical and morphological traits associated with the leaf economics spectrum (e.g., leaf N and Phosphorus, SLA, LDMC) [16], which further supports our observation of greater ITV in LNC. In contrast, traits related to leaf size, including area, length, width, and thickness, are typically considered independent of the leaf economics spectrum and generally exhibit low ITV [16,30]. Consistent with this, we observed minimal ITV (5.36%) and low plasticity (0.005) in leaf thickness across the two habitats.
Our study demonstrates that topography and associated microenvironmental heterogeneity drive significant intraspecific variation in plant traits in tropical rainforests, with one trait exhibiting greater variation within species than between species. As initially expected, plants tend to shift their resource-use strategy from acquisitive to conservative under resource-limited habitat conditions. However, these resource-use and allocation strategies are also species-specific. Trait-specific plasticity highlights the differential sensitivity of traits to environmental heterogeneity.
5. Conclusions
This study demonstrates that intraspecific trait variation plays a critical role in shaping plant responses to habitat heterogeneity in tropical rainforests and is essential for accurately predicting community dynamics and ecosystem functioning under environmental change. Integrating intraspecific trait variability into trait-based ecological frameworks, including functional diversity indices and ecosystem models, enhances the mechanistic understanding of how environmental shifts affect biodiversity and ecosystem processes, thereby improving predictions of forest resilience and function under climate change. These findings underscore the need for future research to prioritize the inclusion of ITV in community-level trait analyses to strengthen the predictive power and robustness of ecological forecasts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f16111711/s1, Table S1: List of selected species with their corresponding families, vegetation strata, and IVI values recorded in the study. Table S2. Species-specific linear model results for six leaf traits across ridge and valley habitats. For each species, the estimates represent the mean trait values in the valley habitat and the corresponding effect of the ridge habitat. Significant species-specific patterns of intraspecific trait variation across habitats are highlighted.
Author Contributions
Conceptualization, S.M., L.S.C. and S.E.; Methodology, S.M., A.S., L.S.C. and S.E.; Software, S.M.; Formal Analysis, S.M.; Investigation, S.M., M.L. and A.S.; Resources, A.S. and S.E.; Data Curation, S.M. and M.L.; Writing—original draft preparation, S.M.; Writing—Review & Editing, S.M., A.S., L.S.C. and S.E.; Supervision, S.E. and L.S.C.; Project Administration, S.E.; Funding Acquisition, S.M., A.S. and S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Plant–Soil Feedback Project, ForestGEO Sinharaja Forest Dynamics Plot Project, and the J&J Ruinen Fellowship in Tropical Forestry (2023) awarded by the ForestGEO–Smithsonian Tropical Research Institute.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to sisira@uwu.ac.lk.
Acknowledgments
We thank Chathuranga Jayathilaka, Upul Shantha, Harsha Sampath, Janith Piumal, Ravindu Dushmantha, Dedula Madhushanka, Harsha Perera, and Lasantha Sanjeewa for their assistance in the field and laboratory experiments. We also thank Uva Wellassa University for logistical support and the Forest Department of Sri Lanka for allowing us access to the plots.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mcgill, B.; Enquist, B.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Ackerly, D.D. Assembly of Plant Communities. In Ecology and the Environment; Springer: New York, NY, USA, 2014; pp. 67–88. ISBN 978-1-4614-7501-9. [Google Scholar]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Arnillas, C.A.; Livingstone, S.W.; Yasui, S.L.E. Predicting Communities from Functional Traits. Trends Ecol. Evol. 2015, 30, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornellssen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; DíAz, S.; et al. Functional Traits and the Growth-Mortality Trade-off in Tropical Trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.-L.; Grigulis, K. Plant Functional Diversity; Oxford University Press: Oxford, UK, 2016; ISBN 2-01-594435-4. [Google Scholar]
- Diaz, S.; Cabido, M. Plant Functional Types and Ecosystem Function in Relation to Global Change. J. Veg. Sci. 1997, 8, 463–474. [Google Scholar] [CrossRef]
- Homeier, J.; Seeler, T.; Pierick, K.; Leuschner, C. Leaf Trait Variation in Species-Rich Tropical Andean Forests. Sci. Rep. 2021, 11, 9993. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The Return of the Variance: Intraspecific Variability in Community Ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How Do Traits Vary across Ecological Scales? A Case for Trait-Based Ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef]
- Alex Fajardo, F.I.P. Intraspecific Trait Variation and Covariation in a Widespread Tree Species (Nothofagus pumilio) in Southern Chile. New Phytol. 2011, 189, 259–271. [Google Scholar] [CrossRef]
- Zuleta, D.; Muller-, H.C.; Alvaro, L.; Natali, D.; Cardenas, D.; Castaño, N.; Diego, J.; Feeley, P.K.J. Interspecific and Intraspecific Variation of Tree Branch, Leaf and Stomatal Traits in Relation to Topography in an Aseasonal Amazon Forest. Funct. Ecol. 2022, 36, 2955–2968. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S. Intraspecific Variability and Trait-Based Community Assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A Global Meta-Analysis of the Relative Extent of Intraspecific Trait Variation in Plant Communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and How Should Intraspecific Variability Be Considered in Trait-Based Plant Ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific Trait Variation in Plants: A Renewed Focus on Its Role in Ecological Processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Zuleta, D.; Russo, S.E.; Barona, A.; Barreto-Silva, J.S.; Cardenas, D.; Castaño, N.; Davies, S.J.; Detto, M.; Sua, S.; Turner, B.L.; et al. Importance of Topography for Tree Species Habitat Distributions in a Terra Firme Forest in the Colombian Amazon. Plant Soil 2020, 450, 133–149. [Google Scholar] [CrossRef]
- Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Esufali, S.; Harms, K.E.; Ashton, P.M.S.; Burslem, D.F.R.P.; Ashton, P.S. Species-Habitat Associations in a Sri Lankan Dipterocarp Forest. J. Trop. Ecol. 2006, 22, 371–384. [Google Scholar] [CrossRef]
- Ediriweera, S.; Singhakumara, B.M.P.; Ashton, M.S. Variation in Canopy Structure, Light and Soil Nutrition across Elevation of a Sri Lankan Tropical Rain Forest. For. Ecol. Manag. 2008, 256, 1339–1349. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.F.R.P.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography Shapes the Structure, Composition and Function of Tropical Forest Landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Punchi-Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Savitri Gunatilleke, C.V.; Nimal Gunatilleke, I.A.U.; Wiegand, K.; Huth, A. Effects of Topography on Structuring Local Species Assemblages in a Sri Lankan Mixed Dipterocarp Forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- Schmitt, S.; Hérault, B.; Ducouret, É.; Baranger, A.; Tysklind, N.; Heuertz, M.; Marcon, É.; Cazal, S.O.; Derroire, G. Topography Consistently Drives Intra- and Inter-Specific Leaf Trait Variation within Tree Species Complexes in a Neotropical Forest. Oikos 2020, 129, 1521–1530. [Google Scholar] [CrossRef]
- Xu, W.; Tomlinson, K.W.; Li, J. Strong Intraspecific Trait Variation in a Tropical Dominant Tree Species along an Elevational Gradient. Plant Divers. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Umaña, M.N.; Swenson, N.G. Intraspecific Variation in Traits and Tree Growth along an Elevational Gradient in a Subtropical Forest. Oecologia 2019, 191, 153–164. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in Leaf Traits at Different Altitudes Reflects the Adaptive Strategy of Plants to Environmental Changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf Morphological and Anatomical Traits from Tropical to Temperate Coniferous Forests: Mechanisms and Influencing Factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef]
- Umaña, M.N.; Zhang, C.; Cao, M.; Lin, L.; Swenson, N.G. Quantifying the Role of Intra-specific Trait Variation for Allocation and Organ-level Traits in Tropical Seedling Communities. J. Veg. Sci. 2018, 29, 276–284. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in Leaf Traits of 38 Tropical Tree Species in Response to Light; Relationships with Light Demand and Adult Stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, J.; Ji, Y.; Liu, W.; Gao, J. Leaf Nutrient Traits Exhibit Greater Environmental Plasticity Compared to Resource Utilization Traits along an Elevational Gradient. Front. Plant Sci. 2024, 15, 1484744. [Google Scholar] [CrossRef]
- Da, R.; Hao, M.; Qiao, X.; Zhang, C.; Zhao, X. Unravelling Trait–Environment Relationships at Local and Regional Scales in Temperate Forests. Front. Plant Sci. 2022, 13, 907839. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. Plant Diversity in Tropical Forests: A Review of Mechanisms of Species Coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Sullivan, M.J.P.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and Carbon Storage across the Tropical Forest Biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D.; et al. Soil Resources and Topography Shape Local Tree Community Structure in Tropical Forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122532. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, G.J.M.S.R.; Pushpakanthie, W.; Lakkana, T.; Gunatilleke, C.V.S.; Ediriweera, S.; Wiegand, T. Species–Habitat Associations in a Sri Lankan Dipterocarp Forest. J. Veg. Sci. 2025, 36, e70049. [Google Scholar] [CrossRef]
- Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Ethugala, A.U.K.; Esufali, S. Ecology of Sinharaja Rain Forest and the Forest Dynamics Plot in Sri Lanka’s Natural World Heritage Site; WHT Publications (Pvt.) Ltd.: Colombo, Sri Lanka, 2004; ISBN 955-9114-31-6. [Google Scholar]
- Davies, S.J.; Abiem, I.; Abu Salim, K.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.; Andrade, A.; Arellano, G.; Ashton, P.S.; et al. ForestGEO: Understanding Forest Diversity and Dynamics through a Global Observatory Network. Biol. Conserv. 2021, 253, 108907. [Google Scholar] [CrossRef]
- Nguyen, H.; Lamb, D.; Herbohn, J.; Firn, J. Designing Mixed Species Tree Plantations for the Tropics: Balancing Ecological Attributes of Species with Landholder Preferences in the Philippines. PLoS ONE 2014, 9, e95267. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Schmitt, S.; Trueba, S.; Coste, S.; Ducouret, É.; Tysklind, N.; Heuertz, M.; Bonal, D.; Burban, B.; Hérault, B.; Derroire, G. Seasonal Variation of Leaf Thickness: An Overlooked Component of Functional Trait Variability. Plant Biol. 2022, 24, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.M.S.; Berlyn, G.P. Leaf Adaptations of Some Shorea Species to Sun and Shade. New Phytol. 1992, 121, 587–596. [Google Scholar] [CrossRef]
- Lin, Y.; Kuang, L.; Tang, S.; Mou, Z.; Phillips, O.L.; Lambers, H.; Liu, Z.; Sardans, J.; Peñuelas, J.; Lai, Y.; et al. Leaf Traits from Stomata to Morphology Are Associated with Climatic and Edaphic Variables for Dominant Tropical Forest Evergreen Oaks. J. Plant Ecol. 2021, 14, 1115–1127. [Google Scholar] [CrossRef]
- Ashton, P.M.; Gunatilleke, C.V.; Gunatilleke, G. Seedling Survival and Growth of Four Shorea Species in a Sri Lankan Rainforest. J. Trop. Ecol. 1995, 11, 263–279. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Patiño, S.; Baker, T.R.; Bielefeld Nardoto, G.; Martinelli, L.A.; Quesada, C.A.; Paiva, R.; Schwarz, M.; Horna, V.; Mercado, L.M.; et al. Basin-Wide Variations in Foliar Properties of Amazonian Forest: Phylogeny, Soils and Climate. Biogeosciences 2009, 6, 2677–2708. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Navas, M.; Vile, D.; Kattge, J.; Garnier, E. Are Trait-based Species Rankings Consistent across Data Sets and Spatial Scales? J. Veg. Sci. 2014, 25, 235–247. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Baraloto, C.; Chave, J.; Hérault, B. Functional Traits of Individual Trees Reveal Ecological Constraints on Community Assembly in Tropical Rain Forests. Oikos 2011, 120, 720–727. [Google Scholar] [CrossRef]
- Xu, S.; Su, H.; Ren, S.; Hou, J.; Zhu, Y. Functional Traits and Habitat Heterogeneity Explain Tree Growth in a Warm Temperate Forest. Oecologia 2023, 203, 371–381. [Google Scholar] [CrossRef]
- Liu, X.; Swenson, N.G.; Lin, D.; Mi, X.; Umaña, M.N.; Schmid, B.; Ma, K. Linking Individual-Level Functional Traits to Tree Growth in a Subtropical Forest. Ecology 2016, 97, 2396–2405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).