Abstract
Forests play a pivotal role in the global carbon cycle, yet accurately simulating forest soil carbon dynamics remains a significant challenge for process-based models. This review systematically compares the mechanistic foundations of traditional models (e.g., Century, CLM5) with emerging microbial-explicit models (e.g., MEND), highlighting key differences in mathematical formulation (first-order kinetics vs. Michaelis–Menten kinetics), carbon pools partitioning (measurable vs. non-measurable experimentally), and the representation of soil carbon stabilization mechanisms (inherent recalcitrance, physical protection, and chemical protection). Despite advances in process-based models in predicting forest soil organic carbon (SOC), improving prediction accuracy, and assessing SOC response to climate change, current research still faces several challenges. These include difficulties in capturing depth-dependent variations in critical microbial parameters such as microbial carbon use efficiency (CUE), limited capacity to distinguish the relative contributions of aboveground and belowground litter inputs to SOC formation, and a general lack of long-term observational data across soil profiles. To address these limitations, this study emphasizes the importance of integrating remote sensing data and refining cross-scale simulation approaches. Such improvements are essential for enhancing model predictive accuracy and establishing a more robust theoretical basis for forest carbon management and climate change mitigation.
1. Introduction
Forest soils represent the largest organic carbon reservoir in terrestrial ecosystems [] and play an irreplaceable role in regulating atmospheric carbon dioxide (CO) concentrations and mitigating climate change [,,,]. Meanwhile, global change significantly affects the stock, stability, and saturation capacity of SOC in forests []. Climate warming and land-use changes often lead to SOC loss, although the extent of this loss varies across forest types and soil depths []. For instance, boreal and mountain forests have been identified as potential global hotspots for substantial long-term carbon loss following natural disturbances and logging activities []. Furthermore, the spatial distribution of global forest SOC stocks differs significantly among forest types []. Therefore, accurately predicting the spatiotemporal dynamics of forest SOC stocks under climate change scenarios is crucial to determining whether forests will function as a net carbon source or a carbon sink.
Process-based models are essential for simulating SOC dynamics, as they explicitly represent key carbon pools and stabilization mechanisms [,,,,,]. A major strength of these models is their ability to partition SOC into conceptually and functionally distinct fractions, such as particulate organic carbon (POC) and mineral-associated organic carbon (MAOC) [,,,]. These simulate the transformation of litter and other organic inputs into SOC and track the subsequent allocation of carbon into these measurable pools [,,]. Empirical studies have shown that the distribution of litter-derived carbon between POC and MAOC varies considerably across forest ecosystems [], directly affecting both the magnitude and persistence of SOC stocks []. The representation of stabilization mechanisms in process-based models has advanced significantly over time []. Early multi-pool models primarily emphasized chemical recalcitrance [,,], but the current scientific consensus emphasizes physical and chemical protection mechanisms—a shift that is now embedded in modern modeling frameworks [,,,,,]. Contemporary microbial-explicit models incorporate detailed microbial-enzymatic pathways [,] and account for the dual role of microorganisms in both decomposition organic matter and contributing to carbon stabilization [,,]. This enables more accurately predictions of carbon fate—whether it is stabilized in long-term storage or released as CO2 through heterotrophic respiration (Figure 1). By integrating mechanistic representations of specific carbon pools and microbial processes, process-based models offer a powerful and dynamic tool for projecting how forest SOC responds to global environmental changes [], thereby addressing a central challenge in contemporary carbon cycle science.
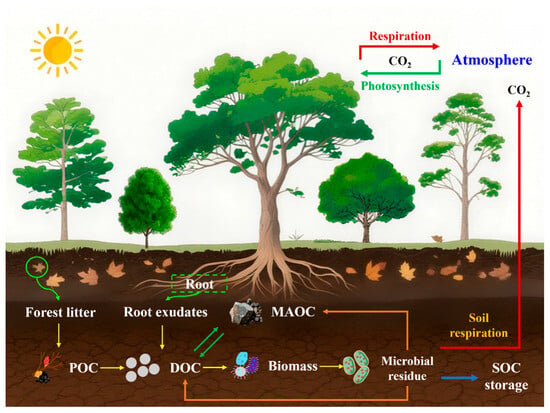
Figure 1.
Forest soil organic carbon cycle. POC: Particulate Organic Carbon; DOC: Dissolved Organic Carbon; MAOC: Mineral-Associated Organic Carbon; CO2: Carbon Dioxide.
Despite their advanced capabilities, process-based models continue to encounter substantial challenges in accurately predicting forest SOCstocks and dynamics []. A prominent example is microbial carbon use efficiency (CUE) [], a critical parameter in microbial-explicit models that represents the fraction of assimilated carbon allocated to microbial growth [,]. However, empirical studies report inconsistent depth-dependent patterns of CUE across forest soils—showing increases, decreases, or no clear trend with depth—as well as considerable spatial variability in its response to environmental drivers [,]. This combination of importance and uncertainty complicates reliable parameterization and hinders regional-scale extrapolation. To systematically address these and related challenges in projecting forest SOC dynamics, this review synthesizes key controlling factors, traces the historical evolution and current state of process-based models, and evaluates their specific applications in forest ecosystems. We conclude by proposing targeted strategies for future model development.
2. Factors Influencing Forest SOC
2.1. Climate
The dynamics and spatial distribution of forest SOC are governed by the interactive effects of multiple environmental factors []. Temperature influences SOC content primarily through changes in plant productivity, litterfall rates inputs, and microbial activity, all of which regulate the rate of SOC decomposition []. Precipitation also plays a crucial role in SOC stability, as variations in precipitation alter soil moisture and aeration conditions, thereby affecting processes that govern SOC stabilization []. Peplau et al. [] conducted a soil warming experiment in a Canadian forest to investigate its long-term impact on SOC in both the topsoil and subsoil. Their findings revealed that temperature affected the dynamics of both soil layers, which exhibited a uniform response to the warming treatment. Furthermore, Wang et al. [] demonstrated that carbon cycle processes in forest ecosystems exhibit strong feedback response to climate change, with SOC responses varying regionally and according to warming intensity. Specifically, under warming scenarios of 1 °C and 5 °C, topsoil SOC stocks in temperate forests declined by 10% and 34%, respectively, while tropical and subtropical forests showed smaller reductions of 10% and 12% under the same conditions. In a separate study, Wang et al. [] analyzed spatiotemporal changes and driving factors of SOC stocks in forest soils across Liaoning Province, China, over the past 25 years using the boosted regression trees (BRT) model. The findings revealed that mean annual precipitation (MAP) was the most influential factor shaping SOC stock dynamics.
2.2. Nitrogen Deposition
Nitrogen (N) is a critical factor influencing forest soil carbon cycles [], as it modulates plant diversity, above- and belowground litter inputs, soil microbial communities, and physicochemical properties, thereby regulating SOC decomposition [,]. For instance, Wu et al. [] demonstrated that SOC in Phyllostachys edulis forests responds differently to short-term versus long-term nitrogen addition, with this variation likely influenced by geographical conditions and inherent soil characteristics. Similarly, Duan et al. [] reported that the effects of N deposition on forest SOC are mediated by altitude, highlighting that the complexity of mechanisms through which N deposition influences SOC dynamics. As a result, the response of forest SOC stocks to N deposition varies considerably across regions. In a long-term nitrogen fertilization experiment conducted by Turner et al. [], in a nitrogen-saturated tropical rainforest in Panama, no significant changes in SOC were observed over a 10-year-period. In contrast, Chu et al. [] found that nitrogen application in Phyllostachys edulis forests increased POC but decreased SOC and MAOC contents. Conversely, studies by Janssens et al. [] showed that nitrogen addition enhances SOC accumulation in temperate forests where nitrogen availability is limited. Furthermore, Fu et al. [] observed that DOC and MBC in rural forests respond positively to nitrogen addition.
2.3. Wildfire
Wildfire is a major driver of forest SOC dynamics []. Globally, approximately 1% of forests are affected by wildfires annually [], with these events releasing about 3.9 Pg of carbon per year [], equivalent to 20% of annual fossil fuel emissions []. As a distinct environmental disturbance, wildfire alters both the quantity and quality of plant-derived carbon inputs to the soil by consuming vegetation and litter carbon pools during combustion, thereby influencing key processes such as SOC decomposition, transformation, and leaching [,,]. The magnitude and direction of wildfire impacts on SOC are influenced by factors including fire intensity (mild, moderate, severe), fire type, duration, and post-fire rainfall patterns. Most studies report a net decrease in SOC following wildfire events []. For instance, Wang et al. [], through a meta-analysis of over 200 studies on post-fire SOC changes, concluded that SOC generally declines after fire. In contrast, Jiang et al. [] found no significant effect of wildfire on SOC in tropical dry and moist forests, and Ran et al. [] observed minimal changes in SOC in subtropical forests of southwestern China across varying fire intensities. Granged et al. [] reported that in eucalyptus forests, low-intensity fires slightly increased SOC content, whereas moderate and high-intensity fires led to reductions. Cheng et al. [] found in Larix gmelinii forests of the Greater Khingan Range, MBC was significantly higher in burned areas than in unburned forests during intermediate recovery stages.
2.4. Other Factors
Vegetation and forest management practices also play a significantly role in influencing the stabilization and turnover of SOC in forests. Forest ecosystems contain diverse vegetation, and different vegetation types directly affect both the quantity and quality of litter inputs to the soil, thereby shaping distinct spatial patterns of SOC content and stocks across forest types []. Globally, coniferous forests have the highest SOC stocks, followed by broadleaf and mixed forests []. Furthermore, Li et al. [] reported that although natural forests store more than twice as much SOC as planted forests, the latter exhibit greater carbon storage in subsoil layers. Regional studies further support this variation: Bao et al. [] found that DOC concentrations in broadleaf forests were significantly higher than those in coniferous forests in the Hengduan Mountains.
Forest management practices constitute a significant component of human influence on SOC in forest ecosystems []. Although afforestation is widely considered an effective strategy for enhancing soil carbon stocks, its actual carbon sequestration potential remains uncertain and context-dependent. Hong et al. [], through a global analysis of afforestation hotspots, revealed that afforestation does not consistently lead to increase SOC, rather, the outcome largely depends on the initial SOC levels. Menichetti et al. [] investigated the effects of stand rotation on SOC dynamics in boreal forests using long-term observational data, finding that when carbon sequestration is a primary management objective, conventional rotation forestry systems exhibit limited effectiveness. Moreover, Yang et al. [] conducted a global synthesis of harvesting impacts on forest SOC, demonstrating that harvesting activities elevate soil respiration by 6.0%, with the most pronounced effects occurring in coniferous forests and subtropical regions.
3. Overview of the Process-Based Model
3.1. Historical Progression of Process-Based Models
Process-based models have undergone significant evolution over the past century, progressing from simple empirical relationships to advanced frameworks that integrate biogeochemical processes and their interactions with climate (Figure 2). In the 1930s, researchers began focusing on how SOC decomposition responds to environmental changes [], a process later formalized using first-order kinetic equations []. During the 1980s and 1990s, early-generation models such as Century and RothC introduced the concept of carbon pools differentiated by decomposition rates—commonly classified as active, slow, and passive pools—with the assumption that carbon turnover within these pools leads to CO2 emissions [,]. These models primarily emphasized the intrinsic chemical recalcitrance of SOC, while microbial processes were largely as a “black-box” mechanism subsumed within the decomposition rate constant k [].
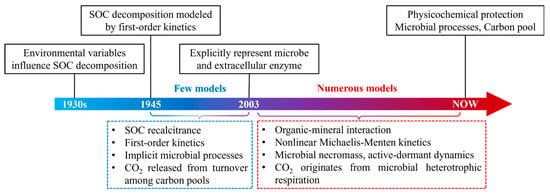
Figure 2.
Historical progression of process-based models.
Since the 21st century, advances in microbial ecology have enabled models to increasingly incorporate explicit representations of microbial processes [], leading to the development of numerous novel modeling frameworks [,,,,,,]. Concurrently, microbial necromass has been integrated into these models as a major contributor to stableSOC [], while stabilization mechanisms—such as the chemical bonding between soil minerals and organic matter—have been quantitatively characterized [,].
3.2. Traditional SOC Models
Classic soil carbon models include the Century model [], Roth C model [], and Community Land Model version5 (CLM5) [], among others. In this study, the Century and CLM5 models are selected as representative examples of traditional soil carbon models, with their structures presented in Figure 2. These conventional soil carbon models describe soil carbon turnover using first-order kinetic equations, as defined in Equation (1).
in Equation (1), X denotes the carbon pool size, t represents time, and k is the decomposition rate, assumed to be a constant. The functions f(W) and f(T) represent the moisture and temperature response function, respectively, which are used to modulate k. Commonly used formulations for these functions are presented in Table 1.

Table 1.
Soil moisture and temperature response function.
The Century model is a process-based biogeochemical model originally developed by Parton et al. [] in the 1980s, with its structure depicted in Figure 3a. Litter inputs are partitioned into structural carbon and metabolic carbon fractions based on the lignin-to-nitrogen (lignin/N) ratio, where a higher ratio results in a greater proportion of litter being allocated to structural carbon. SOC is compartmentalized into three fractions—active, slow, and passive carbon—according to their distinct decomposition rates. Active carbon decomposes rapidly, typically within five years, and consists primarily of soil microorganisms and their metabolic byproducts. Slow carbon decomposes over several decades and includes recalcitrant residues and stabilized microbial products. Passive carbon exhibits an extremely slow turnover rate, lasting up to thousands of years, and comprises chemically and physically protected components highly resistant to decomposition. Due to its mechanistic representation of carbon dynamics, the Century model’s SOC pool structure has been widely adopted in contemporary soil carbon modeling studies and is commonly referred to as the three-pool model [].
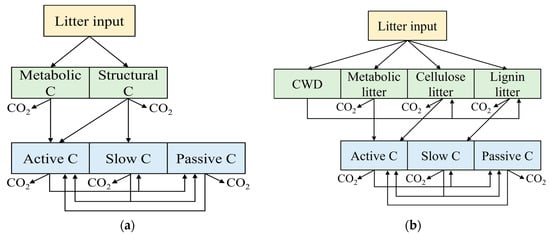
Figure 3.
Traditional SOC models. (a) Century model; (b) CLM5 model. CWD: Coarse Wood Debris.
CLM5 as the latest land model of the Community Earth System Model [], is designed to simulate energy, water, and carbon-nitrogen fluxes exchanges at the atmosphere-land interface. It comprises three core components: biophysical processes, hydrological cycles, and biogeochemical cycles. In the simulation litter and soil carbon dynamics, CLM5 defaults to a 20 layers configuration extending to a total depth of 8.4 m, with each layer containing 4 litter pools (coarse woody debris, metabolic litter, cellulose litter, and lignin litter) and 3 SOC pools (active, slow, and passive carbon pools). In this study, the original layered structure was simplified (Figure 3b). Carbon transfer process as follows: aboveground biomass is initially allocated to specific litter pools according to their decomposition characteristics. During decomposition, part of the carbon is released via respiration, while the remainder fraction is transferred to SOC pools or other litter pools. Importantly, carbon movement occurs not only within individual soil layer but also across adjacent layers through vertical transport mechanisms.
3.3. Microbial Models
Microbial models employ Michaelis–Menten kinetics to quantify carbon transfer among soil carbon pools, offering a mechanistic representation of SOC decomposition mediated by microorganisms and extracellular enzymes (Figure 4a) []. These models typically partition SOC into distinct and measurable fractions—such as POC, MAOC, and DOC (Figure 4b) [,,,,,]. In the following sections, we provide a systematic review of microbial models, with particular emphasis on microbial physiological processes and organo-mineral interactions.
in the equation, X represents the size of the carbon pool, t denotes time, ENZ refers to enzyme concentration, Vmax is the maximum decomposition rate, and Km stands for the half-saturation constant.
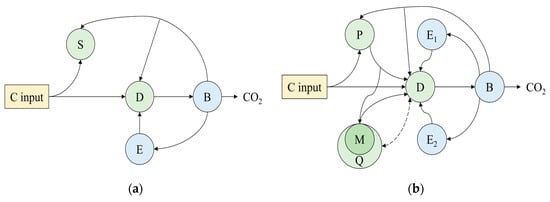
Figure 4.
Microbial models. (a) Classic microbial model. (b) Microbial model with measurable carbon pools. S: Soil Organic carbon; P: Particulate Organic Carbon; D: Dissolved Organic Carbon; M: Mineral-Associated Organic Carbon; B: Microbial Biomass carbon; E: extracellular enzyme; CO2: Carbon Dioxide.
Microorganisms play a dual role in the soil carbon cycle: on the one hand, they decompose exogenous organic carbon, such as plant residues, through heterotrophic respiration, thereby releasing CO2 into the atmosphere; on the other hand, microbial necromass constitutes a significant component of stable soil carbon pool. In recent years, advances in microbial ecology research have led to the development of a next-generation of process-based models that better represent the critical role of microorganisms in the carbon cycle by integrating microbial community traits, physiological regulation mechanisms, and necromass turnover processes [,,,,,,,,,]. For instance, the MIMICS model differentiates MBC into copiotrophic (MBCr) and oligotrophic (MBCk) functional groups based on life-history strategies [,]. The MEND model incorporates microbial physiological dynamics by partitioning MBC into active and dormant pools and simulating transitions between these states []. The ORCHIDEE model accounts for three microbial functional types, each capable of switching between active and dormant states in response to environmental constraints []. The MIND model innovatively introduces a dedicated microbial necromass carbon pool to more accurately quantify its contribution to long-term soil carbon stabilization []. Collectively, these models have substantially improved the mechanistic representation and predictive accuracy of microbial contributions to soil carbon dynamics.
Organic-mineral interactions are a key mechanism for stabilizing exogenous organic carbon []. This process primarily involves the adsorption of organic carbon onto soil mineral surfaces, which reduces its bioavailability and thereby limits the decomposition of SOC. Current microbial models represent this interaction through two main pathways: one simulates the adsorption-desorption dynamics between DOC and minerals, while the other adjusts organic carbon decomposition rates based on soil properties. The ORCHIDEE model establishes separate available and adsorbed carbon pools, using linear equations to describe carbon adsorption onto desorption from mineral surfaces []. The MEND model specifically identifies the fraction of organic carbon bound to DOC within MAOC as the Q pool, modeling adsorption-desorption process based on DOC concentration and maximum adsorption capacity (Qmax) []. Guo et al. [] further partitioned MAOC into a labile fraction (MAOCL) and a protected fraction (MAOCP), applying the Langmuir equation to simulate their adsorption behavior. The MIMICS model incorporates a clay content-dependent correction factor to quantify the chemical protection effect of soil minerals [,]. Furthermore, Liao et al.’s [] comparative analysis of the MiFe model versus non-Fe models demonstrated that non-Fe models fail to accurately capture CO2 release dynamics during the later stages of lignin decomposition in incubation experiments. The MiFe model emphasizes the critical regulatory role of Fe-microbe interactions in lignin degradation, highlighting the importance of iron minerals in soil carbon cycling processes.
4. Advances in Process-Based Model Applications for Forest Soils
4.1. Enhanced Parameterization and Calibration
Currently, over 108 process-based model structures have been developed [], yet significant substantial uncertainty persists in their simulations. For instance, Terrer et al. [] demonstrated that when conventional soil carbon models were applied to assess the response of SOC to elevated CO2 (eCO2), the simulated trends contradicted empirical observation. Furthermore, process-based models face significant challenges in accurately simulating global SOC stocks, with estimated uncertainties as high as 50% []. Todd-Brown et al. [] reported that simulations of global SOC stocks from 11 widely used soil carbon models exhibited considerable variation, ranging from 510 to 3040 Pg, reflecting large inter-model discrepancies. Notably, none of these models produced estimates that aligned well with the Harmonized World Soil Database (HWSD).
Currently, the Bayesian Markov Chain Monte Carlo (MCMC) algorithm has become a widely used method for data assimilation [,,,,], demonstrating substantial effectiveness in reducing model uncertainties. Xu et al. [] pioneered its application by integrating field observations from Duke Forest to optimize process-based model parameters. Subsequently, Zhou et al. [] developed an innovative two-step assimilation framework: first constraining model parameters using measured data and MCMC under steady-state conditions, then refining soil carbon sequestration parameters under non-steady-state conditions to improve the accuracy of regional forest SOC estimates. To improve the SOC simulation accuracy in the CASA model, Zhou and Williams [] introduced a hierarchical assimilation strategy consisting of three stages—initial parameter ranges estimation using aboveground biomass data, parameters updating through observational data, and iterative optimization for optimal model performance—thereby substantially reducing modeling uncertainties 0. Furthermore, He et al. [] constructed a comprehensive dataset on China’s forest soil carbon cycle by assimilating multi-source long-term monitoring data, providing valuable benchmarks for future research. Collectively, these advances have significantly improved the precision and standardization of forest carbon cycle research.
4.2. Forest SOC Decomposition Kinetics
Soil carbon decomposition kinetics are crucial in determining accuracy of predictions made by process-based models []. Traditional soil carbon models often assume constant decomposition rates; however, the decomposition rates of different soil carbon fractions exhibit significant spatial heterogeneity, and the dominant environmental controls vary accordingly [,,]. This variability introduces substantial uncertainty into current process-based models when estimating SOC decomposition rates []. For instance, Xiang et al. [] used the 3P model as a case study, demonstrated that soil texture exerts the strongest influence on the decomposition rate of the active carbon pool, while MAP is the primary driver for the slow carbon pool. In contrast, soil pH emerges as the key regulator of the passive carbon pool’s decomposition rate. Globally, the decomposition rate of the fast carbon pool ranges from 0.01 to 0.4 d−1, underscoring the importance of incorporating spatially variable and pool-specific decomposition rates into forest ecosystem SOC modeling to reduce predictive uncertainty in process-based frameworks. Wang et al. [] reported, in a study of forests in eastern China, that the turnover time of surface forest SOC is considerably shorter than that of deeper layers, with marked differences across forest ecosystems. Specifically, SOC turnover time in the forests of Changbai Mountain, Jilin reach approximately 3849 years, whereas in Dongling Mountain, Beijing, and Heishiding, Guangdong, they are around 400 years. Ren et al. [] also applied the 3P model and revealed substantial differences in intrinsic SOC turnover times across global forest ecosystems: tropical forests exhibit the longest intrinsic turnover times across global forest ecosystems. Tropical forests exhibit the longest intrinsic turnover times, with the fast, slow, and passive pools at 0.43 years, 9.41 years, and 601 years, respectively. Temperate forests rank second, with corresponding values of 0.33 years, 6.19 years, and 380 years. Boreal forests have the shortest turnover times, with the fast, slow, and passive pools at 0.32 years, 5.58 years, and 326 years, respectively. Notably, a multiplicative discrepancy exists between the intrinsic and actual turnover times.
4.3. Modeling Forest SOC Responses to Environmental Variability
Process-based models are widely used to investigate the impacts of climate change on SOC stocks. In a study by Chiti et al. [], the Sasso Fratino Forest in Italy was selected as a case, and the Century model was applied to simulate the effects of two future extreme climate change scenarios on SOC dynamics in undisturbed primary forests. The results indicated that soils in primary forests play a significant role in carbon sequestration. Golchin et al. [] employed the Century model to assess the combined effects of climate change and human activities on SOC stocks across different altitudinal gradients in forest ecosystems. They designed a scenario involving a decrease in precipitation by 2.15 mm per decade and an increase in temperature by 0.4 °C. The findings revealed that SOC stocks would decline by 28.36% to 36.35%, with the most pronounced losses occurring in high-altitude regions. Moreover, the study demonstrated that grazing activities, when combined with climate change, could further accelerate soil carbon loss, thereby emphasizing the importance of implementing appropriate forest management strategies under future climate scenarios to mitigate the risk of soil carbon depletion. Chen et al. [] incorporated extracellular enzyme activity into the TECO model and developed the Data-driven ENZYme (DENZY) model. Using this model, they found that under nitrogen deposition conditions, there is a negative correlation between SOC and ligninase activity in the Duke Forest, USA. Wang et al. [] applied the MEND model to simulate soil carbon cycling processes in broadleaf and pine forests under varying litter input, soil moisture, and soil temperature conditions. Their results indicated that increased soil moisture promotes SOC accumulation, while change in litter input and soil temperature also positively contribute to SOC stocks across the entire soil profile. Hu et al. [] integrated high-precision remote sensing data with the model to simulate SOC stock dynamics in Chinese coniferous forests under different fire scenarios. The findings revealed that under the RCP2.6, RCP4.5, RCP6.0, and RCP8.5 scenarios, SOC stocks decreased by 10.14%, 12.06%, 12.41%, and 15.70% respectively. Manusch et al. [] employed LPJ-GUESS model to investigate the response of forest vegetation carbon and soil carbon stocks to varying climatic conditions in Switzerland. Ito et al. [] conducted a comparative analysis of five models, including Biome-BGC, CASA, and LPJ, to evaluate their ability to predict soil respiration in forest-dominated regions of Japan. The predicted annual soil respiration ranged from 210 to 396 Tg C/year; further analysis showed that inter-model discrepancies were pronounced summer and winter, suggesting significant differences in how models simulate soil responses to temperature fluctuations. Additionally, Johnson et al. [] applied the Century model and Yasso model to systematically examine the mechanisms through which different harvesting scenarios affect SOC stocks in northern forests.
5. Challenges and Future Directions
5.1. Depth-Varying CUE in Forest Ecosystem
CUE is a pivotal regulator of SOC accumulation []. However, the precise relationship between CUE and SOC remains debated, necessitating accurate quantification of CUE to improve the predictive capability of carbon cycle process models [,]. Experimental evidence shows that CUE in both surface and deep soil layers across forest ecosystems in different regions exhibits distinct spatial distribution patterns. As CUE is not a static parameter, its variability elicits sensitive responses in process-based models, thereby directly influencing predictive accuracy. Nevertheless, intensive soil sampling for GUE determination is impractical at global or national scales. Therefore, leveraging big data integration techniques to accurately quantify CUE is essential. To this end, there is an urgent need to develop an environmentally constrained response equation for CUE that demonstrates universality across spatial dimensions and soil profiles.
By synthesizing these depth-dependent mechanisms, our study advances beyond conventional modeling approaches through an innovative framework that explicitly incorporating vertical CUE dynamics. This integration is critical for reducing uncertainties in predicting whole-soil carbon stocks and their feedback to climate change, thereby providing a robust foundation for the development of next-generation soil carbon models.
5.2. Aboveground vs. Belowground Vegetation Effects on Forest SOC Storage
Litter input is a critical factor influencing the stability of SOC []. For instance, Shen et al. [] conducted a Detritus Input and Removal Transfer (DIRT) experiment in a forest ecosystem in Hebei, China, implementing three aboveground litter treatments (removal, normal input, and addition) and two belowground treatments (with and without roots). Their findings demonstrated that increased aboveground litter input significantly enhanced soil CO2 emissions. Moreover, the presence of roots further significantly enhanced CO2 release [].
Forest SOC primarily originates from aboveground and belowground litter inputs, although their relative contributions to SOC accumulation differ significantly. While earlier studies often emphasized aboveground litter as the dominant source of forest SOC [], growing evidence indicates that belowground litter inputs contribute more substantially to SOC formation than aboveground sources []. Notably, most process-based models currently fail to explicitly distinguish between these two input pathways []. Moreover, different soil carbon pools respond distinctly to each sources of organic matter. Aboveground litter and root residues mainly contribute to POC, which is not protected by mineral associations []. In contrast, MAOC is predominantly derived from DOC released via leaching of aboveground litter and from microbial turnover products, including microbial necromass []. Additionally, both aboveground litter inputs and root exudates are highly sensitive to climate change, thereby influencing SOC turnover dynamics []. Therefore, accurately quantifying the differential contributions of aboveground and belowground litter inputs in process-based models is crucial, especially in vegetation-rich forest ecosystems.
5.3. Acquisition of Profile Data and Long-Term Monitoring Data
Data represents one of the primary sources of uncertainty in process-based models []. In forest ecosystems, large-scale soil profile sampling and long-term monitoring of SOC respiration dynamics face significant challenges, due to complex terrain, dense vegetation cover, and the unique characteristics of deep soil carbon pools. Evidence shows that deep soil carbon stocks are substantially larger than those in surface layers; however, their response to climate change, decomposition rates, and the contribution from plant-derived carbon inputs vary markedly []. Accurately quantifying the vertical distribution and translocation of carbon inputs across soil horizons is therefore critical for improving SOC modeling accuracy []. Nevertheless, in situ measurements of these processes are difficult to obtain, and sustained long-term monitoring remains logistically and technically challenge, contributing significantly to uncertainties in model parameter calibration.
The limitations of data availability in forest ecosystems are especially pronounced in wildfire-related research. Under global warming, increasing temperatures and prolonged droughts have intensified the risk of forest fires, posing significant threats to regional and even global ecosystems and leading to substantial economic losses. Although wildfires play a crucial role in modeling SOC dynamics, their infrequent and destructive nature results in a severe lack of empirical data for model validation.
6. Conclusions
Looking ahead, future research should prioritize three critical directions to bridge existing knowledge gaps: (1) integrating remote sensing data with process-based models to scale up predictions from site-level observations to regional and global forest systems; (2) improving model structure to explicitly represent vertical carbon transport processes and priming effects, which is essential for accurately quantifying the contribution of plant-derived carbon to different soil layers and its interaction with native SOC; and (3) establishing mechanistically robust functional relationships between key microbial parameters (e.g., CUE) and environmental variables to enhance the predictability of spatial variability of SOC in forests. Applying the process-based models discussed in this study to forest SOC dynamics will not only support model refinement and inform evidence-based forest management, but also offer valuable perspectives for soil carbon management in related fields, from sustainable agroforestry to agricultural soil stewardship.
Author Contributions
Conceptualization, S.W. and M.Z.; Methodology, S.W. and X.J.; Data curation, Z.Y. and C.G.; Writing—original draft preparation, M.Z. and S.W.; Writing—review and editing, Q.Z. Funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key R&D Program of China (Grant No. 2023YFD1501300).
Data Availability Statement
The data presented in this study are available in the paper.
Acknowledgments
The authors would like to thank the anonymous reviewers and editors for their valuable advices.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, S.; Roland, B.; Adhikari, K.; Zhuang, Q.L.; Jin, X.X.; Han, C.L.; Qian, F.K. Spatial-temporal variations and driving factors of soil organic carbon in forest ecosystems of Northeast China. For. Ecosyst. 2023, 10, 100101. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Forest soils and carbon sequestration. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Jonard, M.; Nicolas, M.; Coomes, D.A.; Caignet, I.; Saenger, A.; Ponette, Q. Forest soils in France are sequestering substantial amounts of carbon. Sci. Total Environ. 2017, 574, 616–628. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Warkentin, I.G. Global estimates of boreal forest carbon stocks and flux. Glob. Planet. Change 2015, 128, 24–30. [Google Scholar] [CrossRef]
- Wu, Y.T.; Peng, X.W.; Wang, X.; Huang, J.S.; Yang, L.; Liu, L.L. Warmer climate reduces the carbon storage, stability and saturation levels in forest soils. Earth’s Future 2025, 13, e2024EF004988. [Google Scholar] [CrossRef]
- Mayer, M.; Baltensweiler, A.; James, J.; Rigling, A.; Hagedorn, F. A global synthesis and conceptualization of the magnitude and duration of soil carbon losses in response to forest disturbances. Glob. Ecol. Biogeogr. 2023, 33, 141–150. [Google Scholar] [CrossRef]
- Wang, M.M.; Guo, X.W.; Zhang, S.; Xiao, L.J.; Mishra, U.; Yang, Y.H.; Zhu, B.; Wang, G.C.; Mao, X.L.; Qian, T.; et al. Global soil profiles indicate depth-dependent soil carbon losses under a warmer climate. Nat. Commun. 2022, 13, 5514. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Guo, X.W.; Chen, L.X.; Kuzyakov, Y.; Wang, R.Z.; Zhang, H.Y.; Han, X.G.; Jiang, Y.; Sun, J.X.O. Global pattern of organic carbon pools in forest soils. Glob. Change Biol. 2024, 30, e17386. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Gao, Q.; Yang, Y.F.; Hobbie, S.E.; Reich, P.B.; Zhou, J.Z. Soil enzymes as indicators of soil function: A step toward greater realism in microbial ecological modeling. Glob. Change Biol. 2021, 28, 1935–1950. [Google Scholar] [CrossRef]
- Guo, X.W.; Viscarra, R.R.A.; Wang, G.C.; Xiao, L.J.; Wang, M.M.; Zhang, S.; Luo, Z.K. Particulate and mineral-associated organic carbon turnover revealed by modelling their long-term dynamics. Soil. Biol. Biochem. 2022, 173, 108780. [Google Scholar] [CrossRef]
- Wang, G.S.; Jagadamma, S.; Mayes, M.A.; Schadt, C.W.; Steinweg, J.M.; Gu, L.H.; Post, W.M. Microbial dormancy improves development and experimental validation of ecosystem model. ISME J. 2015, 9, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Gao, D.C.; Zhao, C.H.; Wang, C.; Qu, Y.; Zhang, J.; Bai, E. Improved model simulation of soil carbon cycling by representing the microbially derived organic carbon pool. ISME J. 2021, 15, 2248–2263. [Google Scholar] [CrossRef]
- Wieder, W.R.; Grandy, A.S.; Kallenbach, C.M.; Bonan, G.B. Integrating microbial physiology and physio-chemical principles in soils with the Microbial- MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 2014, 11, 3899–3917. [Google Scholar] [CrossRef]
- Wieder, W.R.; Grandy, A.S.; Kallenbach, C.M.; Taylor, P.G.; Bonan, G.B. Representing life in the Earth system with soil microbial functional traits in the MIMICS model. Geosci. Model. Dev. Discuss. 2015, 8, 2011–2052. [Google Scholar] [CrossRef]
- Wang, G.S.; Post, W.M.; Mayes, M.A. Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecol. Appl. 2013, 23, 255–272. [Google Scholar] [CrossRef]
- Hu, J.L.; Ao, G.; Feng, J.G.; Chen, X.; Zhu, B. The patterns of forest soil particulate and mineral associated organic carbon characteristics with latitude and soil depth across eastern China. For. Ecosyst. 2025, 12, 360–367. [Google Scholar] [CrossRef]
- Chandel, A.K.; Jiang, L.F.; Luo, Y.Q. Microbial models for simulating soil carbon dynamics: A review. J. Geophys. Res. Biogeosci. 2023, 128, e2023JG007436. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factor controlling soil organic matter levels in Great Plains Grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Rayner, J.H. The turnover of soil organic matter in some of the Rothamsted classical experiments. Soil Sci. 1977, 123, 298–305. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Fisher, R.A.; Koven, C.D.; Oleson, K.W.; Swenson, S.C.; Bonan, G.; Collier, N.; Ghimire, B.; van Kampenhout, L.; Kennedy, D.; et al. The Community Land Model version 5: Description of new features, benchmarking, and impact of forcing uncertainty. Adv. Model. Earth Syst. 2019, 11, 4245–4287. [Google Scholar] [CrossRef]
- Liao, C.J.; Huang, W.J.; Wells, J.; Zhao, R.Y.; Allen, K.; Hou, E.Q.; Huang, X.; Qiu, H.; Tao, F.; Jiang, L.F.; et al. Microbe-iron interactions control lignin decomposition in soil. Soil Biol. Biochem. 2022, 173, 108803. [Google Scholar] [CrossRef]
- Schimel, P.J.; Weintraub, N.M. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Allison, D.S.; Wallenstein, D.M.; Bradford, A.M. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Liang, C.; Cheng, G.; Wixon, D.L.; Balser, T.C. An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry 2011, 106, 303–309. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Wieder, W.R.; Bonan, G.B.; Allison, S.D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Change 2013, 3, 909–912. [Google Scholar] [CrossRef]
- Todd-Brown, K.E.O.; Randerson, J.T.; Post, W.M.; Hoffman, F.M.; Tarnocai, C.; Schuur, E.A.G.; Allison, S.D. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 2013, 10, 1717–1736. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.F.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Pei, J.M.; Li, J.Q.; Luo, Y.Q.; Rillig, M.C.; Smith, P.; Gao, W.J.; Li, B.; Fang, C.M.; Nie, M. Patterns and drivers of soil microbial carbon use efficiency across soil depths in forest ecosystems. Nat. Commun. 2025, 16, 5218. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Su, T.Y.; Wang, H.F.; Yang, Q.; Lu, J.L.; Fu, Q.Y.; Mao, H.; Xu, W.X.; Luo, Y.Q.; Liu, W.J.; et al. Deep soil microbial carbon use efficiency responds stronger to nitrogen deposition than top soil in tropical forests, southern China. Plant Soil 2025, 500, 605–622. [Google Scholar] [CrossRef]
- Sun, F.; Fan, L.N.; Deng, G.Y.; Kuzyakov, Y.; Zhang, Y.; Wang, J.C.; Li, Y.W.; Wang, F.M.; Li, Z.A.; Tariq, A.; et al. Responses of tropical forest soil organic matter pools to shifts in precipitation patterns. Soil Biol. Biochem. 2024, 197, 109530. [Google Scholar] [CrossRef]
- Peplau, T.; Schroeder, J.; Gregorich, E.; Poeplau, C. Long-term geothermal warming reduced stocks of carbon but not nitrogen in a subarctic forest soil. Glob. Change Biol. 2021, 27, 5341–5355. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhuang, Q.L.; Yang, Z.J.; Yu, N.; Jin, X.X. Temporal and Spatial Changes of Soil Organic Carbon Stocks in the Forest Area of Northeastern China. Forests 2019, 10, 1023. [Google Scholar] [CrossRef]
- Wang, Q.K.; Zhao, X.C.; Liu, S.G.; Wang, Q.G.; Zhang, W.; Fontaine, S.; Zhu, B.; Tian, P. Contrasting responses of the priming effect to nitrogen deposition in temperate and subtropical forests. Catena 2024, 238, 107839. [Google Scholar] [CrossRef]
- Zhao, X.C.; Tian, P.; Zhang, W.; Wang, Q.G.; Guo, P.; Wang, Q.K. Nitrogen deposition caused higher increases in plant-derived organic carbon than microbial-derived organic carbon in forest soils. Sci. Total Environ. 2024, 925, 171752. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.Y.; Luo, X.; Liang, C.; Han, S.J.; Liu, G.C.; Yin, L.M.; Wang, X.C.; Zhang, Z.; Xu, L.J.; Xing, Y.J.; et al. Nitrogen deposition enhances soil organic carbon sequestration through plant-soil–microbe synergies. J. Ecol. 2025, 113, 2889–2904. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Q.X.; Su, X.C.; Zheng, W.; Zhang, Q.F.; Chen, Y.M. The different factors driving SOC stability under different N addition durations in a Phyllostachys edulis forest. Forests 2023, 14, 1890. [Google Scholar] [CrossRef]
- Duan, P.P.; Wang, K.L.; Li, D.J. Nitrogen addition effects on soil mineral-associated carbon differ between the valley and slope in a subtropical karst forest. Geoderma 2023, 430, 116357. [Google Scholar] [CrossRef]
- Turner, B.L.; Yavitt, J.B.; Harms, K.E.; Garcia, M.N.; Wright, S.J. Seasonal changes in soil organic matter aftera decade of nutrient addition in a lowland tropical forest. Biogeochemistry 2015, 123, 221–235. [Google Scholar] [CrossRef]
- Chu, H.Y.; Ni, H.J.; Su, W.H.; Fan, S.H.; Long, Y.M.; Sun, Y.T. Enhanced nitrogen fertilizer input alters soil carbon dynamics in Moso Bamboo Forests, impacting particulate organic and mineral-associated carbon pools. Forests 2023, 14, 2460. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Fu, R.X.; Yu, Y.C.; He, Y.; Xu, X.N.; Sun, X.; Yang, J.; Tao, X. The impacts of nitrogen addition on the active carbon pools in forest soils along an urban–rural gradient. Catena 2024, 236, 107769. [Google Scholar] [CrossRef]
- Fraser, R.; Li, Z. Estimating fire-related parameters in boreal forest using SPOT VEGETATION. Remote Sens. Environ. 2002, 82, 95–110. [Google Scholar] [CrossRef]
- Mouillot, F.; Field, C.B. Fire history and the global carbon budget: A 1 degrees × 1 degrees fire history reconstruction for the 20th. Glob. Change Biol. 2005, 11, 398–420. [Google Scholar] [CrossRef]
- Chinese Academy of Sciences. Blue Book on Forest Fire Carbon Emissions Research; Chinese Academy of Sciences Publisher: Beijing, China, 2023. [Google Scholar]
- Kucharik, C.J.; Foley, J.A.; Delire, C.; Fisher, V.A.; Coe, M.T.; Lenters, J.D.; Young-Molling, C.; Ramankutty, N.; Norman, J.M.; Gower, S.T. Testing the performance of a dynamic global ecosystem model: Water balance, carbon balance, and vegetation structure. Glob. Biogeochem. Cycles 2000, 14, 795–825. [Google Scholar] [CrossRef]
- Dalal, R.C.; Chan, K.Y. Soil organic matter in rainfed cropping systems of the Australian cereal belt. Aust. J. Soil. Res. 2001, 39, 435–464. [Google Scholar] [CrossRef]
- Hu, T.X.; Ding, H.L.; Sun, L. Research progress on effects of fire disturbance on nitrogen cycling and transformation in forest soil. Chin. J. Ecol. 2024, 43, 372–382. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Xu, Z.H.; Zhou, Q.X. Impact of fire on soil gross nitrogen transformations in forest ecosystems. J. Soil. Sediments 2014, 14, 1030–1040. [Google Scholar] [CrossRef]
- Jiang, D.L.; Xu, C.H.; Xu, X.; Luo, Y.Q.; Chen, C.; Ju, C.H.; Chen, H.Y.H.; Shi, Z.; Ruan, H.H. Carbon and nitrogen dynamics in tropical ecosystems following fire. Glob. Ecol. Biogeogr. 2021, 31, 378–391. [Google Scholar] [CrossRef]
- Ran, Y.Q.; Zhou, J.; Zhang, X.Y.; Fan, J.R.; Li, X.L.; Ma, Y.W. Effects of burning intensity on soil chemical and physical properties in southwests subtropical forests. Acta Agrestia Sin. 2023, 31, 2796–2804. [Google Scholar]
- Granged, A.J.P.; Jordán, A.; Zavala, L.M.; Muñoz-Rojas, M.; Mataix-Solera, J. Short-term effects of experimental fire for a soil under eucalyptus forest (SE Australia). Geoderma 2011, 167, 125–134. [Google Scholar] [CrossRef]
- Cheng, Z.C.; Yang, L.B.; Fu, X.Y.; Lu, X.M.; Liu, S.Y.; Wei, D. Soil microbial diversity in cold temperate forests recovering in the mid-term recovery after fire. China Environ. Sci. 2025, 1–13. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, G.Y.; Zhang, D.Q. Soil organic carbon accumulation modes between pioneer and old-growth forest ecosystems. J. Appl. Ecol. 2020, 57, 2419–2428. [Google Scholar] [CrossRef]
- Li, B.B.; Gao, G.Y.; Luo, Y.Q.; Xu, M.X.; Liu, G.B.; Fu, B.J. Carbon stock and sequestration of planted and natural forests along climate gradient in water-limited area: A synthesis in the China’s Loess plateau. Agric. For. Meteorol. 2023, 333, 109419. [Google Scholar] [CrossRef]
- Bao, Y.; Men, X.X.; Liao, C.; Zhai, D.; Li, J.S.; Wang, Y.Y.; Wang, C.; Cheng, X.L. Forest types predominantly regulate soil-dissolved organic matter dynamics along an elevational gradient in the Hengduan Mountains. J. Plant Ecol. 2025, 18, rtaf087. [Google Scholar] [CrossRef]
- Menichetti, L.; Lehtonen, A.; Lindroos, A.J.; Merilä, P.; Huuskonen, S.; Ukonmaanaho, L.; Mäkipää, R. Soil carbon dynamics during stand rotation in boreal forests. Eur. J. Soil Sci. 2025, 76, e70154. [Google Scholar] [CrossRef]
- Hong, S.B.; Yin, G.D.; Piao, S.L.; Dybzinski, R.; Cong, N.; Li, X.Y.; Wang, K.; Peñuelas, J.; Zeng, H.; Chen, A.P. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.R.; Qin, J.H.; Liu, X.Z.; Mayer, M. A global meta-analysis of forest harvesting effects on soil respiration, its components, and temperature sensitivity. Agric. For. Meteorol. 2024, 358, 110259. [Google Scholar] [CrossRef]
- Salter, R.M.; Green, T.C. Factors affecting the accumulation and loss of nitrogen and organic carbon in cropped soils. Agron. J. 1933, 25, 622–630. [Google Scholar] [CrossRef]
- Hénin, S.; Dupuis, M. Essai de bilan de la matière organique du sol. Dudod 1945, 15, 17–29. [Google Scholar]
- Zhou, J.; Chen, S.P.; Yan, L.M.; Wang, J.; Jiang, M.; Liang, J.Y.; Zhang, X.Z.; Xia, J.Y. A comparison of linear conventional and nonlinear microbial models for simulating pulse dynamics of soil heterotrophic respiration in a semi-arid grassland. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006120. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.M.; Xiao, L.J.; Guo, X.W.; Zheng, J.Y.; Zhu, B.; Luo, Z.K. Reconciling carbon quality with availability predicts temperature sensitivity of global soil carbon mineralization. Proc. Natl. Acad. Sci. USA 2024, 121, e2313842121. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Ren, C.J.; Wang, C.K.; Baquerizo, M.D.; Luo, Y.Q.; Luo, Z.K.; Du, Z.G.; Zhu, B.; Yang, Y.H.; Jiao, S.; et al. Global turnover of soil mineral-associated and particulate organic carbon. Nat. Commun. 2024, 15, 5329. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guenet, B.; Ciais, P.; Janssens, I.A.; Soong, J.L.; Wang, Y.; Goll, D.; Blagodatskaya, E.; Huang, Y.Y. ORCHIMIC (v1.0), a microbe-mediated model for soil organic matter decomposition. Geosci. Model. Dev. 2018, 11, 2111–2138. [Google Scholar] [CrossRef]
- Kang, J.; Qu, C.C.; Chen, W.L.; Cai, P.; Chen, C.R.; Huang, Q.Y. Organo–organic interactions dominantly drive soil organic carbon accrual. Glob. Change Biol. 2024, 30, e17147. [Google Scholar] [CrossRef]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Pett, R.J.; Craig, M.E.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Tao, F.; Houlton, B.Z.; Huang, Y.Y.; Wang, Y.P.; Manzoni, S.; Ahrens, B.; Mishra, U.; Jiang, L.F.; Huang, X.M.; Luo, Y.Q. Convergence in simulating global soil organic carbon by structurally different models after data assimilation. Glob. Change Biol. 2024, 30, e17297. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Jiang, J.; Jing, X.; Feng, W.T.; Luo, Z.K.; Wang, Y.G.; Xu, X.; Luo, Y.Q. Optimizing duration of incubation experiments for understanding soil carbon decomposition. Geoderma 2022, 428, 116225. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Zhang, W.X.; Duan, Z.; Chen, S.C.; Shi, Z. An improved estimate of soil carbon pool and carbon fluxes in the Qinghai-Tibetan grasslands using data assimilation with an ecosystem biogeochemical model. Geoderma 2023, 430, 116283. [Google Scholar] [CrossRef]
- Xu, T.; White, L.; Hui, D.F.; Luo, Y.Q. Probabilistic inversion of a terrestrial ecosystem model: Analysis of uncertainty in parameter estimation and model prediction. Glob. Biogeochem. Cycles 2006, 20, GB2007. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, P.J.; Jia, G.S.; Luo, Y.Q. Nonsteady state carbon sequestration in forest ecosystems of China estimated by data assimilation. J. Geophys. Res-Biogeo. 2023, 118, 1369–1384. [Google Scholar] [CrossRef]
- Zhou, T.; Williams, C.A. Forest carbon modeling improved through hierarchical assimilation of pool--based measurements. J. Adv. Model. Earth Syst. 2025, 17, e2024MS004622. [Google Scholar] [CrossRef]
- He, H.L.; Ge, R.; Ren, X.L.; Zhang, L.; Chang, Q.Q.; Xu, Q.; Zhou, G.Y.; Xie, Z.Q.; Wang, S.L.; Wang, H.M.; et al. Reference carbon cycle dataset for typical Chinese forests via colocated observations and data assimilation. Sci. Data 2021, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.F.; Wang, G.S.; Tian, J.; Li, W.Y. Global patterns and edaphic-climatic controls of soil carbon decomposition kinetics predicted from incubation experiments. Nat. Commun. 2023, 14, 2171. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.; Wei, N.; Liu, R.Q.; Zhang, B.W.; Chu, C.J.; Su, H.X.; Xu, Y.Z.; Cheng, Z.N.; Zhu, S.Y.; et al. Biotic and abiotic factors controlling spatial variation of mean carbon turnover time in forest soil. J. Geophys. Res. Biogeo. 2023, 128, e2023JG007438. [Google Scholar] [CrossRef]
- Wang, J.; Xia, J.Y.; Zhou, X.H.; Huang, K.; Zhou, J.; Huang, Y.Y.; Jiang, L.F.; Xu, X.; Liang, J.Y.; Wang, Y.P.; et al. Evaluating the simulated mean soil carbon transit times by Earth system models using observations. Biogeosciences 2019, 16, 917–926. [Google Scholar] [CrossRef]
- Ren, S.; Wang, T.; Guenet, B.; Liu, D.; Cao, Y.F.; Ding, J.Z.; Smith, P.; Piao, S.L. Projected soil carbon loss with warming in constrained Earth system models. Nat. Commun. 2024, 15, 102. [Google Scholar] [CrossRef]
- Chiti, T.; Benilli, N.; Mastrolonardo, G.; Certini, G. The potential for an old-growth forest to store carbon in the topsoil: A case study at Sasso Fratino, Italy. J. For. Res. 2023, 35, 10. [Google Scholar] [CrossRef]
- Golchin, A.; Misaghi, M. Investigating the effects of climate change and anthropogenic activities on SOC storage and cumulative CO2 emissions in forest soils across altitudinal gradients using the model. Sci. Total. Environ. 2024, 943, 173758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Chen, J.; Luo, Y.Q. Data-driven ENZYme (DENZY) model represents soil organic carbon dynamics in forests impacted by nitrogen deposition. Soil. Biol. Biochem. 2019, 138, 107575. [Google Scholar] [CrossRef]
- Wang, K.F.; Wang, G.S.; Qu, R.S.; Huang, W.J.; Zhou, G.Y.; Yue, M.; Peng, C.H. Differential responses of soil microbial and carbon--nitrogen processes to future environmental changes across soil depths and environmental factors. Earth’s Future 2024, 12, e2023EF004085. [Google Scholar] [CrossRef]
- Hu, T.X.; Yu, C.; Dou, X.; Zhang, Y.J.; Li, G.X.; Sun, L. Simulation of soil organic carbon dynamics in postfire boreal forests of China by incorporating high-resolution remote sensing data and field measurement. Fire 2023, 6, 414. [Google Scholar] [CrossRef]
- Manusch, C.; Bugmann, H.; Wolf, A. The impact of climate change and its uncertainty on carbon storage in Switzerland. Reg. Environ. Change 2014, 14, 1437–1450. [Google Scholar] [CrossRef]
- Ito, A.; Ichii, K.; Kato, T. Spatial and temporal patterns of soil respiration over the Japanese Archipelago: A model intercomparison study. Ecol. Res. 2010, 25, 1033–1044. [Google Scholar] [CrossRef]
- Johnson, K.; Scatena, N.F.; Pan, Y. Short- and long-term responses of total soil organic carbon to harvesting in a northern hardwood forest. Forest Ecol. Manag. 2009, 259, 1262–1267. [Google Scholar] [CrossRef]
- Yang, Y.; Gunina, A.; Cheng, H.; Liu, L.X.; Wang, B.R.; Dou, Y.X.; Wang, Y.Q.; Liang, C.; An, S.S.; Chang, S.X. Unlocking Mechanisms for Soil Organic Matter Accumulation: Carbon Use Efficiency and Microbial Necromass as the Keys. Glob. Change Biol. 2025, 31, e70033. [Google Scholar] [CrossRef]
- Tan, B.C.; Luo, S.M. Microbial carbon use efficiency and soil organic carbon: Which is the determinant? Innovation 2025, 6, 100984. [Google Scholar] [CrossRef]
- Feng, J.G.; He, K.Y.; Zhang, Q.F.; Han, M.G.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Shen, Y.W.; Feng, J.G.; Zhou, D.Y.; He, K.Y.; Zhu, B. Impacts of aboveground litter and belowground roots on soil greenhouse gas emissions: Evidence from a DIRT experiment in a pine plantation. Agric. For. Meteorol. 2023, 343, 109792. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecologyof soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tang, Z.X.; You, Y.M.; Guo, X.W.; Wu, C.J.; Liu, S.R.; Sun, O.J.X. Differential effects of forest-floor litter and roots on soil organic carbon formation in a temperate oak forest. Soil. Biol. Biochem. 2023, 180, 109017. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Liu, L.; Hou, L. Soil organic carbon stabilization and formation: Mechanism and model. J. Beijing For. Univ. 2022, 44, 11–22. [Google Scholar]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Ahlström, A.; Allison, S.; Batjes, N.H.; Brovkin, V.; Garvalhais, N.; Chappell, A.; Ciais, P.; Davidson, E.A.; Finzi, A.; et al. Toward more realistic projections of soil carbon dynamics by earth system models. Glob. Biogeochem. Cycles 2015, 30, 40–56. [Google Scholar] [CrossRef]
- Xiao, L.J.; Wang, G.C.; Chang, J.F.; Chen, Y.Y.; Guo, X.W.; Mao, X.L.; Wang, M.M.; Zhang, S.; Shi, Z.; Luo, Y.Q.; et al. Global depth distribution of belowground net primary productivity and its drivers. Glob. Ecol. Biogeogr. 2023, 32, 1435–1451. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhang, S.; Zeng, L.Z.; Luo, Z.K. Whole--profile soil carbon responses to climate change modulated by vertical carbon transport and priming effect gradients. Adv. Model. Earth Syst. 2025, 17, e2024MS004670. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).