Reference Gene Identification and RNAi-Induced Gene Silencing in the Redbay Ambrosia Beetle (Xyleborus glabratus), Vector of Laurel Wilt Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Candidate Gene Selection and Primer Design
2.2. dsRNA Synthesis
2.3. Insects and dsRNA Treatments
2.4. Gene Expression
3. Results
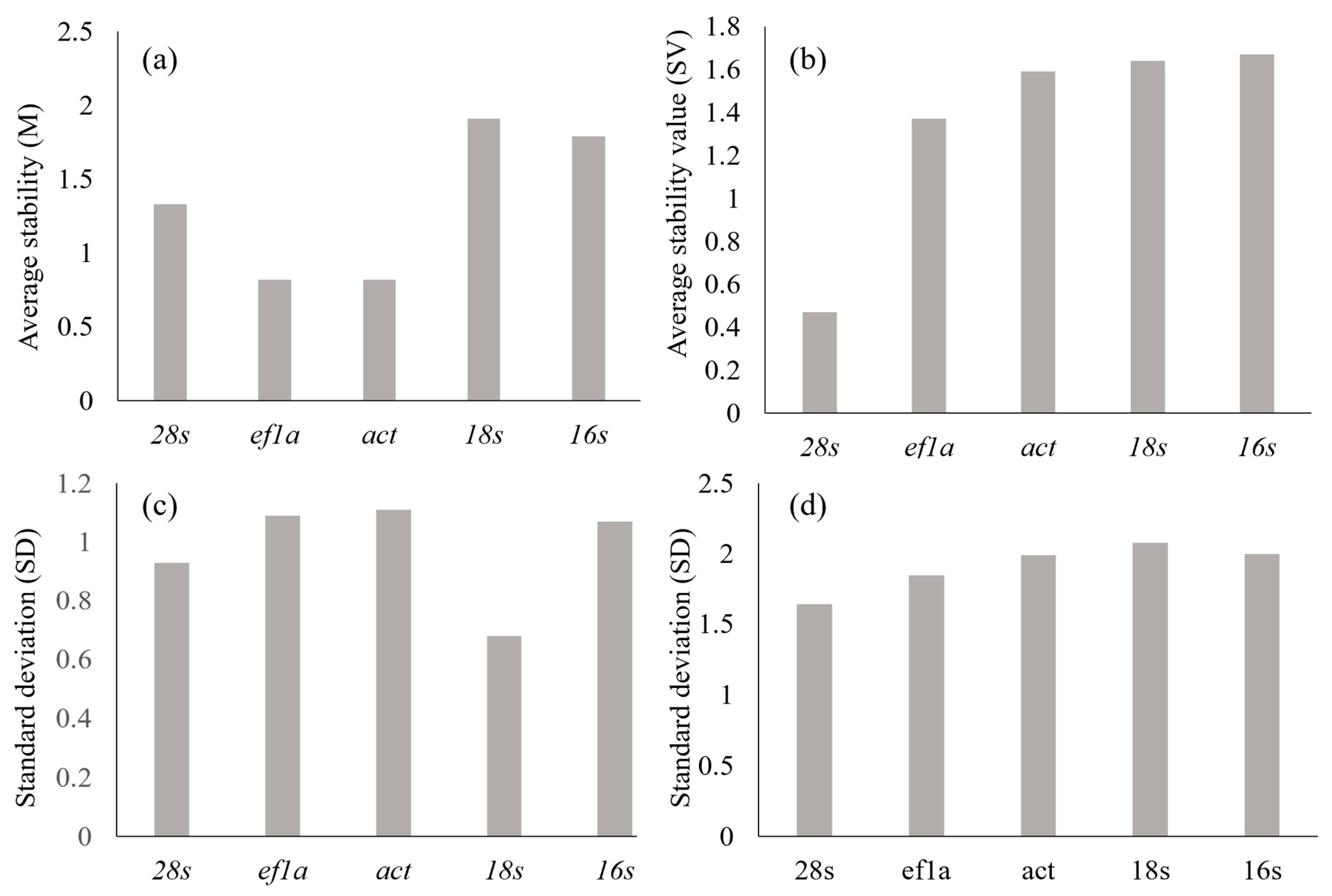
3.1. Reference Gene Stability
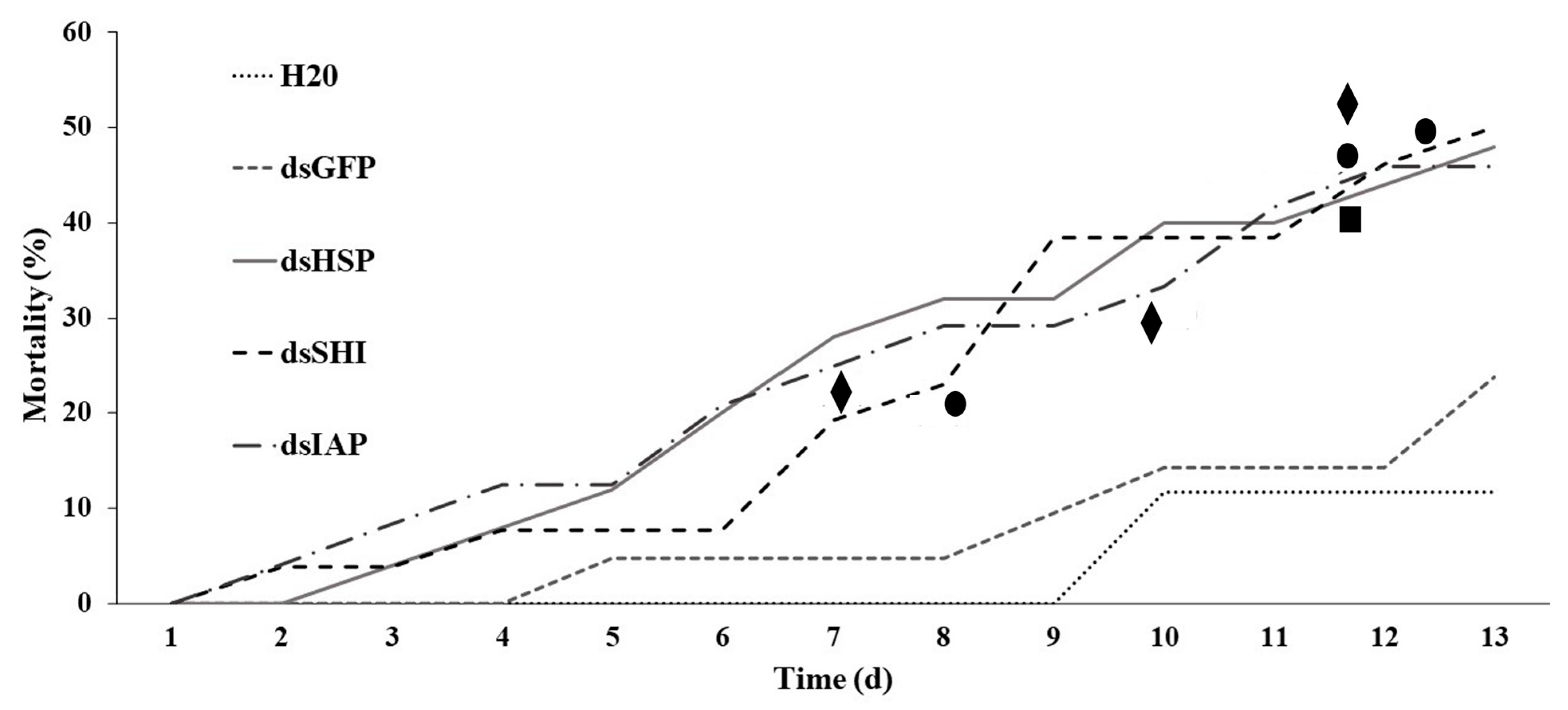
3.2. RAB Mortality
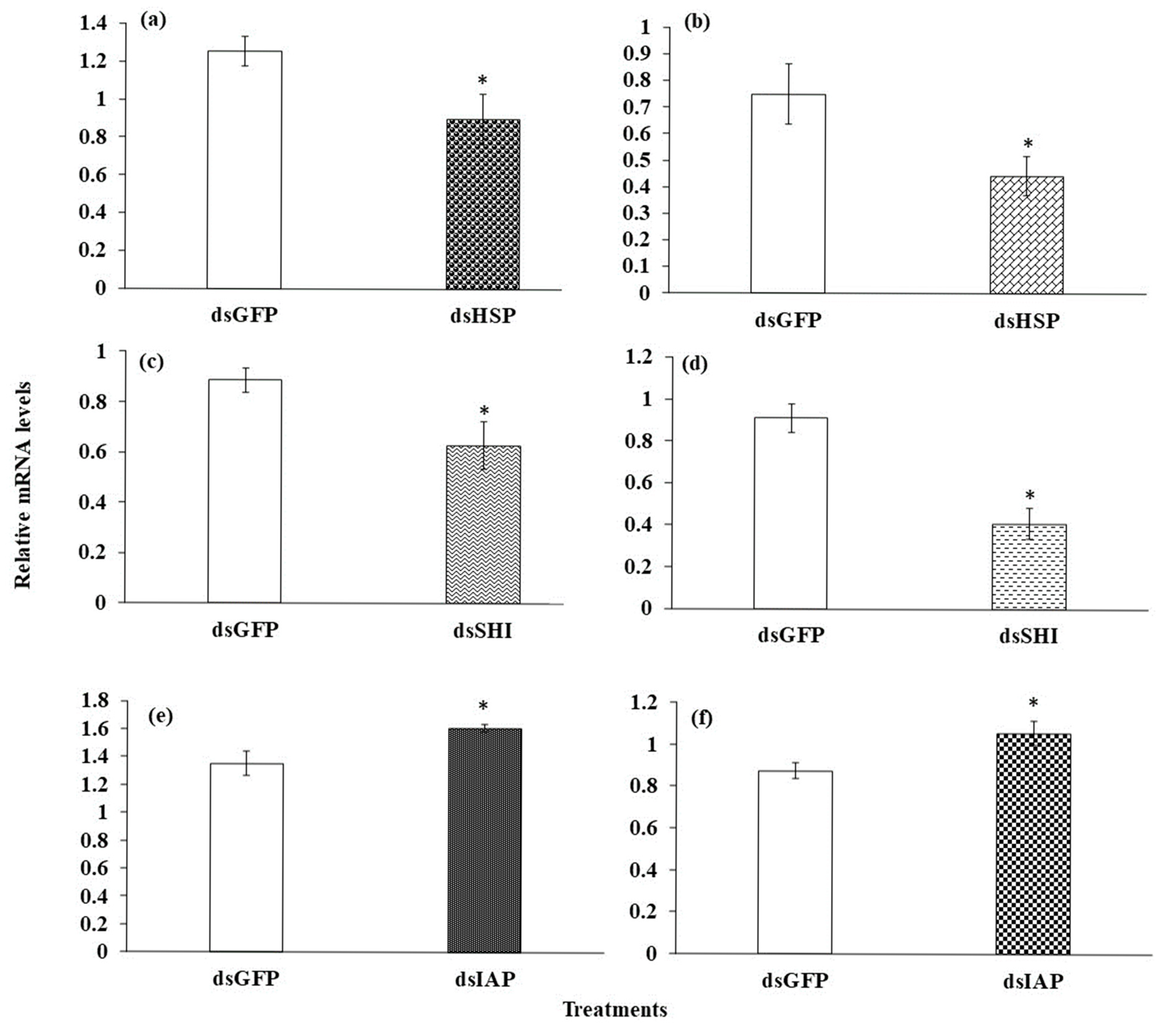
3.3. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meurisse, N.; Rassati, D.; Hurley, B.P.; Brockerhoff, E.G.; Haack, R.A. Common pathways by which non-native forest insects move internationally and domestically. J. Pest Sci. 2019, 92, 13–27. [Google Scholar] [CrossRef]
- Williamson, M.; Fitter, A. The Varying Success of Invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- McNeely, J.A.; Mooney, H.; Neville, L.E.; Schei, P.; Waage, J.K. A Global Strategy on Invasive Alien Species; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Haack, R.A. Intercepted Scolytidae (Coleoptera) at U.S. ports of entry: 1985–2000. J. Integr. Pest Manag. 2001, 6, 253–282. [Google Scholar] [CrossRef]
- Crooks, J.A. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Écoscience 2005, 12, 316–329. [Google Scholar] [CrossRef]
- Inch, S.A.; Ploetz, R.C. Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado, Persea americana. For. Pathol. 2012, 42, 239–245. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Biedermann, P.W.; Jordal, B.H. Chapter 3—Evolution and Diversity of Bark and Ambrosia Beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 85–156. [Google Scholar] [CrossRef]
- Hanula, J.L.; Sullivan, B. Manuka Oil and Phoebe Oil are Attractive Baits for Xyleborus glabratus (Coleoptera: Scolytinae), the Vector of Laurel Wilt. Environ. Entomol. 2008, 37, 1403–1409. [Google Scholar] [CrossRef]
- USDA Forest Service. Laurel Wilt Public Dashboard. 2022. Available online: https://www.arcgis.com/apps/dashboards/d43391c8fdb741b597e6ccf1236d2a02 (accessed on 16 July 2021).
- Shearman, T.M.; Wang, G.G.; Mayfield, A.E. The Silvics of Persea borbonia (L.) Spreng., Red Bay, and Persea palustris (Raf.) Sarg., Swamp Bay, Lauraceae (Laurel Family). In Gen. Tech. Rep.; US Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Riggins, J.J.; Chupp, A.D.; Formby, J.P.; Dearing, N.A.; Bares, H.M.; Brown, R.L.; Oten, K.F. Impacts of laurel wilt disease on arthropod herbivores of North American Lauraceae. Biol. Invasions 2019, 21, 493–503. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E., III.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A Fungal Symbiont of the Redbay Ambrosia Beetle Causes a Lethal Wilt in Redbay and Other Lauraceae in the Southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Bates, C.A.; Johnson, J.; Reid, L.S.; Best, G.S.; Leininger, T.D.; Hawkins, T.S. Susceptibility to Laurel Wilt and Disease Incidence in Two Rare Plant Species, Pondberry and Pondspice. Plant Dis. 2011, 95, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.A.; Shin, K.; Eickwort, J.; Smith, J.A. First Report of Laurel Wilt Disease Caused by Raffaelea lauricola on Silk Bay in Florida. Plant Dis. 2012, 96, 910. [Google Scholar] [CrossRef]
- Mayfield, A.E.; MacKenzie, M.; Cannon, P.G.; Oak, S.W.; Horn, S.; Hwang, J.; Kendra, P.E. Suitability of California bay laurel and other species as hosts for the non-native redbay ambrosia beetle and granulate ambrosia beetle. Agri. For. Entomol. 2013, 15, 227–235. [Google Scholar] [CrossRef]
- Evans, J.P.; Scheffers, B.R.; Hess, M. Effect of laurel wilt invasion on redbay populations in a maritime forest community. Biol. Invasions 2014, 16, 1581–1588. [Google Scholar] [CrossRef]
- Spiegel, K.S.; Leege, L.M. Impacts of laurel wilt disease on redbay (Persea borbonia (L.) Spreng.) population structure and forest communities in the coastal plain of Georgia, USA. Biol. Invasions 2013, 15, 2467–2487. [Google Scholar] [CrossRef]
- Nielsen, A.; Rieske, L.K. Potential host and range expansion of an exotic insect-pathogen complex: Simulating effects of sassafras mortality from laurel wilt disease invasion in the central hardwoods region. J. Torrey Bot. 2015, 142, 292–301. [Google Scholar] [CrossRef]
- Formby, J.P.; Rodgers, J.C.; Koch, F.H.; Krishnan, N.; Duerr, D.A.; Riggins, J.J. Cold tolerance and invasive potential of the redbay ambrosia beetle (Xyleborus glabratus) in the eastern United States. Biol. Invasions 2018, 20, 995–1007. [Google Scholar] [CrossRef]
- Kendra, P.E.; Guillén, L.; Tabanca, N.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Cloonan, K.R. Risk assessment of Hass avocado and Mexican Lauraceae for attack by redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). Agri. For. Entomol. 2023, 25, 285–302. [Google Scholar] [CrossRef]
- Spence, D.J.; Smith, J.A.; Ploetz, R.; Hulcr, J.; Stelinski, L.L. Effect of chipping on emergence of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) and recovery of the laurel wilt pathogen from infested wood chips. J. Econ. Entomol. 2013, 106, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.; Pena, J.; Osborne, J. Redbay Ambrosia Beetle-Laurel Wilt Pathogen: A Major Potential Problem for the Florida Avocado Industry. EDIS 2008, 2008. [Google Scholar] [CrossRef]
- Mayfield, A.E., III.; Barnard, E.L.; Smith, J.A.; Bernick, S.C.; Eickwort, J.M.; Dreaden, T.J. Effect of propiconazole on laurel wilt development in redbay trees and on the pathogen in vitro. Arboric. For. 2008, 34, 317–324. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Pérez-Martínez, J.M.; Fernandez, R. Management of laurel wilt of avocado, caused by Raffaelea lauricola. Eur. J. Plant Pathol. 2017, 149, 133–143. [Google Scholar] [CrossRef]
- Johnson, C.; Olatinwo, R.; Hwang, J.; Brownie, C. Efficacy of Propiconazole for Prevention of Sassafras Mortality from Laurel Wilt Disease Using a Tree Micro-Injection and Micro-Infusion Delivery System. Arboric. Urb. For. 2023, 49, 2–15. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA Treatment in Trees and Grapevines for Insect Pest Suppression. Southwest. Entomol. 2012, 37, 85–97. [Google Scholar] [CrossRef]
- Kyre, B.R.; Bentz, B.J.; Rieske, L.K. Susceptibility of mountain pine beetle (Dendroctonus ponderosae Hopkins) to gene silencing through RNAi provides potential as a novel management tool. For. Ecol. Manag. 2020, 473, 118322. [Google Scholar] [CrossRef]
- Kyre, B.R.; Rodrigues, T.B.; Rieske, L.K. RNA interference and validation of reference genes for gene expression analyses using qPCR in southern pine beetle, Dendroctonus frontalis. Sci. Rep. 2019, 9, 5640. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Duan, J.J.; Palli, S.R.; Rieske, L.K. Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci. Rep. 2018, 8, 5020. [Google Scholar] [CrossRef]
- Wallace, M.; Rieske, L.K. Ingestion of Species-Specific dsRNA Alters Gene Expression and Can Cause Mortality in the Forest Pest, Ips calligraphus. Forests 2023, 14, 422. [Google Scholar] [CrossRef]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, X.; Zhu, Q.; Wu, D.; Zhong, J.; Wen, L.; Yu, X. The dsRNA Delivery, Targeting and Application in Pest Control. Agronomy 2023, 13, 714. [Google Scholar] [CrossRef]
- Zotti, M.J.; Smagghe, G. RNAi technology for insect management and protection of beneficial insects from diseases: Lessons, challenges and risk assessments. Neotrop. Entomol. 2015, 44, 197–213. [Google Scholar] [CrossRef]
- Palli, S. RNA interference in Colorado potato beetle: Steps toward development of dsRNA as a commercial insecticide. Curr. Res. Insect Sci. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Smagghe, G.; Swevers, L. Editorial overview: Pests and resistance—RNAi research in insects. Curr. Opin. Insect Sci. 2014, 6, 4–5. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Changjian, J.; Uffman, J.; Levine, S.; Carson, D. Environmental fate of double-stradned RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Zapata, F.; Dubrlman, S.; Mueller, G.; Uffman, J.; Jiang, C.; Jensen, P.; Levine, S. Aquatic facte of a double-stranded RNA in a sediment—Water system following an over-water application. Environ. Toxicol. Chem. 2017, 36, 727–734. [Google Scholar] [CrossRef]
- Ai, X.; Wei, Y.; Huang, L.; Zhao, J.; Wang, Y.; Liu, X. Developmental control of Helicoverpa armigera by ingestion of bacteria expressing dsRNA targeting an arginine kinase gene. Biocontrol Sci. Technol. 2018, 28, 253–267. [Google Scholar] [CrossRef]
- Yang, J.; Tian, Y.; Lie, H.; Kan, Y.; Zhou, Y.; Luo, Y. Harnessing the endogenous 2μ Plasmid of Saccharomyces cerevisiae for Pathway Construction. Front. Microbiol. 2021, 12, 679665. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Dhandapani, R.K.; Duan, J.J.; Palli, S.R. RNA interference in the Asian longhorned beetle: Identification of key RNAi genes and reference genes for RT-qPCR. Sci. Rep. 2017, 7, 8913. [Google Scholar] [CrossRef]
- Lü, J.C.; Guo, M.; Ye, C.; Qiu, B.; Wu, J.; Yang, C.; Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insect: A systematic review. Front. Physiol. 2018, 9, 1560. [Google Scholar] [CrossRef]
- Rajarapu, S.; Mamidala, P.; Mittapalli, O. Validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar]
- Wallace, M.; Rieske, L.K. Validation of reference genes for quantitative PCR in the forest pest, Ips calligraphus. Sci. Rep. 2021, 11, 23523. [Google Scholar] [CrossRef]
- Lü, J.; Guo, S.; Ye, M.; Qiu, C.; Wu, B.; Yang, C.; Pan, H. Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Song, L.; Florea, L. Rcorrector: Efficient and accurate error correction for Illumina RNA-seq reads. Gigascience 2015, 4, 48. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Regev, A. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Molec. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 0034. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hruz, T.; Wyss, M.; Docquier, M.; Pfaffl, M.W.; Masanetz, S.; Borghi, L.; Verbrugghe, P.; Kalaydjieva, L.; Bleuler, S.; Laule, O.; et al. RefGenes: Identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genom. 2011, 12, 156. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Shi, X.Q.; Guo, W.C.; Wan, P.J.; Zhou, L.T.; Ren, X.L.; Ahmat, T.; Fu, K.Y.; Li, G.Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res. Notes 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Khajuria, C.; Wang, H.; Matz, N.; Cunha Cardoso, D.; Valicente, F.H.; Zhou, X.; Siegfried, B. Validation of reference housekeeping genes for gene expression studies in western corn rootworm (Diabrotica virgifera virgifera). PLoS ONE 2014, 9, e109825. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Zhang, H. RNA interference of four genes in adult Bactrocera dorsalis by feeding their dsRNAs. PLoS ONE 2011, 6, e17788. [Google Scholar] [CrossRef]
- Portnoy, V.; Huang, V.; Place, R.F.; Li, L.C. Small RNA and transcriptional upregulation. Wiley Interdiscip. Rev. RNA 2011, 2, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Schwartz, J.C.; Chu, Y.; Younger, S.T.; Gagnon, K.T.; Elbashir, S.; Janowski, B.A.; Corey, D.R. Transcriptional regulation by small RNAs at sequences downstream from 3’ gene termini. Nat. Chem. Biol. 2010, 6, 621–629. [Google Scholar] [CrossRef]
- Karpala, A.; Doran, T.; Bean, A. Immune responses to dsRNA: Implications for gene silencing technologies. Immunol. Cell Biol. 2005, 83, 211–216. [Google Scholar] [CrossRef]
- Persengiev, S.P.; Zhu, X.; Green, M.R. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA 2004, 10, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Carmichael, G. Effects of length and location on the cellular response to double-stranded RNA. Microbiol. Mol. Biol. Rev. 2004, 68, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.L.; Kelly, D.; Bader, M.F.; Brockerhoff, E.G. Olfactory Cues, Visual Cues, and Semiochemical Diversity Interact During Host Location by Invasive Forest Beetles. J. Chem. Ecol. 2017, 43, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kyre, B.R. Gene Silencing Provides Insights into Bark Beetle Biology and Creates Potential for Broad Scale Forest Protection. Doctoral Dissertation, Department of Entomology, University of Kentucky, Lexington, KY, USA, 2022. [Google Scholar]
- Kemppainen, M.J.; Pardo, A.G. Gene knockdown by ihpRNA-triggering in the ectomycorrhizal basidiomycete fungus Laccaria bicolor. Bioeng. Bugs 2010, 1, 354–358. [Google Scholar] [CrossRef]
- Nakayashiki, H.; Hanada, S.; Nguyen, B.Q.; Kadotani, N.; Tosa, Y.; Mayama, S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 2005, 42, 275–283. [Google Scholar] [CrossRef]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, R.C.; Ploetz, R.C.; Pena, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. J. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Cruz, L.F.; Menocal, O.; Kendra, P.E.; Carrillo, D. Phoretic and internal transport of Raffaelea lauricola by different species of ambrosia beetle associated with avocado trees. Symbiosis 2021, 84, 151–161. [Google Scholar] [CrossRef]
- Knutsen, M.C.; Rieske, L.K. Presence of the causal agent of laurel wilt disease in sassafras-associated insects. Environ. Entomol. 2023, 52, 1042–1047. [Google Scholar] [CrossRef]
- Hollowell, H.; Rieske, L. Southern pine beetle-specific RNA interference exhibits no effect on model nontarget insects. J. Pest Sci. 2022, 95, 1429–1441. [Google Scholar] [CrossRef]
- Hollowell, H.; Rieske, L. Southern Pine Beetle-Specific RNA Interference Demonstrates no Effects on Non-Target Organisms. Msater’s Thesis, Department of Entomology, Univeristy of Kentucky, Lexington, KY, USA, 2021. [Google Scholar] [CrossRef]
- Pampolini, F.; Rieske, L. Emerald ash borer specific gene silencing has no effect on non-target organisms. Front. Agron. 2020, 2, 608827. [Google Scholar] [CrossRef]
- Kyre, B.R.; Rieske, L.K. Using RNAi to silence heat shock protein has congeneric effects in North America’s Dendroctonus bark beetles. For. Ecol. Manag. 2022, 520, 120367. [Google Scholar] [CrossRef]
- Poreddy, S.; Li, J.; Baldwin, I.T. Plant-mediated RNAi silences midgut-expressed genes in congeneric lepidopteran insects in nature. BMC Plant Biol. 2017, 17, 199. [Google Scholar] [CrossRef]
- Bragg, Z.; Rieske, L.K. Spatial Distribution and Retention in Loblolly Pine Seedlings of Exogenous dsRNAs Applied through Roots. Int. J. Mol. Sci. 2022, 23, 9167. [Google Scholar] [CrossRef] [PubMed]
- Bragg, Z.; Rieske, L.K. Feasibility of systemically applied dsRNAs for pest-specific RNAi-induced gene silencing in white oak. Front. Plant Sci. 2022, 13, 830226. [Google Scholar] [CrossRef] [PubMed]
- Pampolini, F.; Rodrigues, T.; Leelesh, R.; Kawashima, T.; Rieske, L.K. Confocal microscopy provides visual evidence and confirms the feasibility of dsRNA delivery to emerald ash borer through plant tissues. J. Pest Sci. 2020, 93, 1143–1153. [Google Scholar] [CrossRef]
- Pampolini, F.; Rieske, L.K. Root uptake, translocation and persistence of EAB-specific dsRNA in ash seedlings. Sci. Rep. 2025, 15, 6378. [Google Scholar] [CrossRef]
- Pampolini, F.; Rieske, L. Foliar Application of dsRNA to Induce Gene Silencing in Emerald Ash Borer: Systemic Distribution, Persistence, and Bioactivity. Forests 2023, 14, 1853. [Google Scholar] [CrossRef]
- Ghosh, S.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double strand RNA delivery system for plant-sap-feeding insects. PLoS ONE 2017, 12, e0171861. [Google Scholar] [CrossRef]
- Pampolini, F. RNA Interference for Emerald Ash Borer Supression: Ecotoxicological Assessment and Delivery Methods. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2022. [Google Scholar]
- Tull, A.R.; Gladfelter, H.; Pampolini, F.; Rieske, L.K.; Nelson, C.D.; Merkle, S. Development of a New Genetic Transformation System for White and Green Ash Using Embryogenic Cultures. Forest 2022, 13, 671. [Google Scholar] [CrossRef]
- Olatinwo, R.; Fraedrich, S.; Mayfield, A. Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management. Forest 2021, 12, 181. [Google Scholar] [CrossRef]


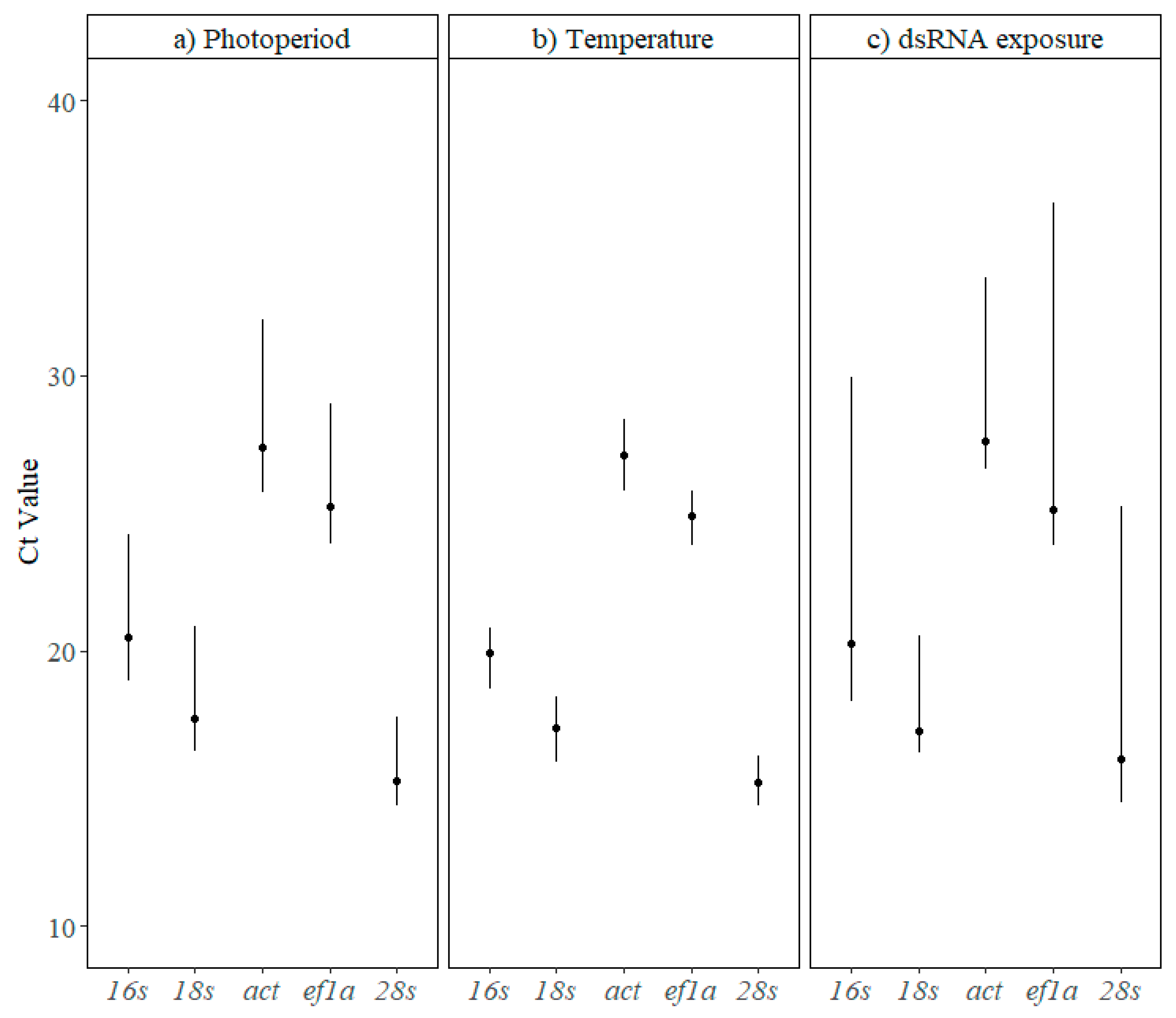
| Gene Name | Primers | Amplicon (bp) | R2 | E(%) |
|---|---|---|---|---|
| 28s ribosomal RNA (28s) | F - CGTCTCGTCATTCGTACGTG R - ACGAGTCGAACGCTCCTAAA | 107 | 0.997 | 109 |
| Elongation factor-1 alpha (ef1a) | F - CTCGGCCTTCAATTTATCCA R - CATCGACAAACGTACCATCG | 100 | 0.990 | 105 |
| Actin (act) | F - CCAGACGCGTATAAGGACAAA R - CCCAAGGCCAACAGAGAAA | 104 | 0.994 | 104 |
| 18s ribosomal RNA (18s) | F - GTTTTCGGAACACCGAGGTA | 102 | 0.992 | 93 |
| R - GCTTCTGTCCGTCTTTCGAC | ||||
| 16s ribosomal RNA (16s) | F - GCGACCTCGATGTTGGATTA | 100 | 0.998 | 97 |
| R - GCCGGTCTAAACTCAGATCAT | ||||
| Heat shock protein (hsp) | F - CCAAGGATGGAGAACGATTAG | 100 | 0.997 | 95 |
| R - CGTCTTCCAATCAACCTCTT | ||||
| Shibire (shi) | F - AGATGGGTACTGTGGGATCT | 107 | 0.992 | 94 |
| R - TCCTGGTCTGTTGGGAATTG | ||||
| Inhibitor of apoptosis (iap) | F - GTGATGGCTCGGCATAGAAA | 100 | 0.996 | 91 |
| R - CAGGGCTGGTGGATGTTATT |
| Gene | Primer Sequences | Amplicon |
|---|---|---|
| hsp | F:TAATACGACTCACTATAGGGGTGATTGCAGGGTTGAATGTG | 419 |
| R:TAATACGACTCACTATAGGGCTGGCTCTAGTGATCTTGGAATAG | ||
| shi | F:TAATACGACTCACTATAGGGGACATGGCGTTTGAAGCAATAG | 420 |
| R:TAATACGACTCACTATAGGGGGAAGTGAGGACAAACCAGTAG | ||
| iap | F:TAATACGACTCACTATAGGGTGGTCAAAGGGCAGGATTT | 399 |
| R:TAATACGACTCACTATAGGGGTTTGGAAGCGTGGTCAATG | ||
| gfp | F:TAATACGACTCACTATAGGGCGATGCCACCTACGGCAA | 288 |
| R:TAATACGACTCACTATAGGGTGTCGCCCTCGAACTTCA |
| GeNorm | NormFinder | BestKeeper | delta-CT | Comprehensive | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | M | R | SV | R | SD | R | SD | R | GM | R |
| 28s | 1.33 | 2 | 0.47 | 1 | 0.93 | 2 | 1.64 | 1 | 1.57 | 1 |
| ef1a | 0.82 | 1 | 1.37 | 2 | 1.09 | 4 | 1.85 | 2 | 2 | 2 |
| act | 0.82 | 1 | 1.59 | 4 | 1.11 | 5 | 1.99 | 3 | 2.78 | 3 |
| 18s | 1.91 | 4 | 1.64 | 5 | 0.68 | 1 | 2.08 | 5 | 3.34 | 4 |
| 16s | 1.79 | 3 | 1.67 | 3 | 1.07 | 3 | 2 | 4 | 3.46 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knutsen, M.C.; Rieske, L.K. Reference Gene Identification and RNAi-Induced Gene Silencing in the Redbay Ambrosia Beetle (Xyleborus glabratus), Vector of Laurel Wilt Disease. Forests 2025, 16, 1577. https://doi.org/10.3390/f16101577
Knutsen MC, Rieske LK. Reference Gene Identification and RNAi-Induced Gene Silencing in the Redbay Ambrosia Beetle (Xyleborus glabratus), Vector of Laurel Wilt Disease. Forests. 2025; 16(10):1577. https://doi.org/10.3390/f16101577
Chicago/Turabian StyleKnutsen, Morgan C., and Lynne K. Rieske. 2025. "Reference Gene Identification and RNAi-Induced Gene Silencing in the Redbay Ambrosia Beetle (Xyleborus glabratus), Vector of Laurel Wilt Disease" Forests 16, no. 10: 1577. https://doi.org/10.3390/f16101577
APA StyleKnutsen, M. C., & Rieske, L. K. (2025). Reference Gene Identification and RNAi-Induced Gene Silencing in the Redbay Ambrosia Beetle (Xyleborus glabratus), Vector of Laurel Wilt Disease. Forests, 16(10), 1577. https://doi.org/10.3390/f16101577





