Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Trapping Activity of the Forest Managers
2.2. Study Design and Sampling
2.3. Morphological Identification
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Predators
4.2. Parasitoids
4.3. Monitoring vs. Mass-Trapping Setup
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pettenella, D.; Marchetti, M.; Motta, R.; Vacchiano, G. Vaia storm facing the unbearable lightness of forest reporting. Forest@ 2021, 18, 1–4. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Fettig, C.J.; Hilszczański, J. Management Strategies for Bark Beetles in Conifer Forests. In Bark Beetles; Elsevier: Oxford, UK, 2015; pp. 555–584. [Google Scholar] [CrossRef]
- Pietzsch, B.W.; Peter, F.J.; Berger, U. The Effect of Sanitation Felling on the Spread of the European Spruce Bark Beetle—An Individual-Based Modeling Approach. Front. For. Glob. Change 2021, 4, 704930. [Google Scholar] [CrossRef]
- Kuhn, A.; Hautier, L.; San Martin, G. Do pheromone traps help to reduce new attacks of Ips typographus at the local scale after a sanitary cut? PeerJ 2022, 10, e14093. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera: Curculionidae: Scolytinae). Ann. For. Sci. 2008, 65, 309. [Google Scholar] [CrossRef]
- Weslien, J.; Lindelöw, Å. Recapture of marked spruce bark beetles (Ips typographus) in pheromone traps using area-wide mass trapping. Can. J. For. Res. 2011, 41, 1786–1790. [Google Scholar] [CrossRef]
- Šramel, N.; Kavčič, A.; Kolšek, M.; de Groot, M. A cost-benefit analysis of different traps for monitoring European spruce bark beetle (Ips typographus). Aust. J. For. Sci. 2022, 139, 137–168. [Google Scholar]
- Bakke, A.; Kvamme, T. Kairomone response in Thanasimus predators to pheromone components of Ips typographus. J. Chem. Ecol. 1981, 7, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Zumr, V. Effect of synthetic pheromones Pheroprax on the coleopterous predators of the spruce bark beetle Ips typographus (L.). Z. Angew. Entomol. 1983, 95, 47–50. [Google Scholar] [CrossRef]
- Valkama, H.; Räty, M.; Niemelä, P. Catches of Ips duplicatus and other non-target Coleoptera by Ips typographus pheromone trapping. Entomol. Fennica 1997, 8, 153–159. [Google Scholar] [CrossRef]
- Hulcr, J.; Ubik, K.; Vrkoc, J. The role of semiochemicals in tritrophic interactions between the spruce bark beetle Ips typographus, its predators, and infested spruce. J. Appl. Entomol. 2006, 130, 275–283. [Google Scholar] [CrossRef]
- Heber, T.; Helbig, C.E.; Osmers, S.; Müller, M.G. Evaluation of attractant composition, application rate, and trap type for potential mass trapping of Ips typographus (L.). Forests 2021, 12, 1727. [Google Scholar] [CrossRef]
- Šramel, N.; Kavčič, A.; Kolšek, M. Estimating the most effective and economical pheromone for monitoring the European spruce bark beetle. J. Appl. Entomol. 2021, 145, 312–325. [Google Scholar] [CrossRef]
- Sousa, M.; Andersson, A.; Englund, J.E.; Flöhr, A.; Pollet, M.; Karlsson Green, K.; Birgersson, G.; Becher, P.G. Behavioral responses of predatory flies of the genus Medetera Fischer von Waldheim (Diptera: Dolichopodidae) and the tree-killing beetle Ips typographus L. (Coleoptera: Scolytinae) to odor compound blends. Ann. For. Sci. 2024, 81, 42. [Google Scholar] [CrossRef]
- Faccoli, M. Bioecologia di coleotteri scolitidi: Ips typographus (Linnaeus) e specie di recente interesse per la selvicoltura italiana. II Contributo: Fattori naturali di contenimento di Ips typographus con particolare riferimento ai parassitoidi. Boll. Ist. Ent. “G. Grandi” Univ. Bologna 2000, 54, 35–54. [Google Scholar]
- Hellrigl, K.; Schwenke, W. Begleitinsekten in Buchdrucker-Pheromonfallen in Südtirol. Anz. Schädlingskunde Pflanzenschutz Umweltschutz 1985, 58, 47–50. [Google Scholar] [CrossRef]
- Thomaes, A.; Drumont, A.; Warzée, N.; Grégoire, J.-C.; Stassen, E.; Crèvecoeur, L.; Berckvens, N.; Casteels, H.; Van de Vijver, D.; Raemdonck, H. Ecology and Distribution of Thanasimus formicarius (Linnaeus, 1758) and the Newly Discovered Thanasimus femoralis (Zetterstedt, 1828) in Belgium (Coleoptera: Cleridae). Bull. Soc. R. Belg. Entomol. 2017, 153, 206–214. [Google Scholar]
- Öztürk, N.; Yüksel, B. First Report of Thanasimus femoralis (Zetterstedt, 1828) (Coleoptera: Cleridae) in a Forest Nursery in the Western Black Sea Region of Türkiye. J. For. Sci. 2023, 69, 360–365. [Google Scholar] [CrossRef]
- Peacock, E.R. Coleoptera: Rhizophagidae. In Handbooks for the Identification of British Insects; Royal Entomological Society: London, UK, 1977; Volume 5, pp. 1–16. [Google Scholar]
- Balachowsky, A. Coléoptères Scolytides. In Faune de France, 50; Librairie de la Faculté des Sciences: Paris, France, 1949; 320p. [Google Scholar]
- Noblecourt, T. Cryphalus intermedius Ferrari, 1867 and Cryphalus saltuarius Weise, 1891, Species New for the Fauna of France (Coleoptera: Scolytidae). Ann. Soc. Linnéenne Lyon 2004, 73, 290–292. [Google Scholar] [CrossRef]
- Bouček, Z.; Pulpan, J.; Sedivý, J. Remarks on Hymenopterous Parasites of Ips typographus in Czechoslovakia. Zool. Entomol. Listy 1953, 2, 145–158. [Google Scholar]
- Oosterbroek, P. The European Families of the Diptera: Identification, Diagnosis, Biology; KNNV Publishing: Utrecht, The Netherlands, 2006; 205p. [Google Scholar]
- Robinson, H.; Vockeroth, J.R. Dolichopodidae. In Manual of Nearctic Diptera; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Wood, D.M., Eds.; Agriculture Canada Monograph No. 27; Agriculture Canada: Ottawa, ON, Canada, 1981; Volume 1, pp. 625–639. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/inext-online/ (accessed on 23 August 2025).
- Sun, X.L.; Yang, Q.Y.; Sweeney, J.D.; Gao, C.Q. A review: Chemical ecology of Ips typographus (Coleoptera, Scolytidae). J. For. Res. 2006, 17, 65–70. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Wermelinger, B.; Herrmann, M. Natural Enemies of Bark Beetles: Predators, Parasitoids, Pathogens, and Nematodes. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F., Hofstetter, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 247–304. [Google Scholar]
- Feicht, E. Parasitoids of Ips typographus (Col., Scolytidae), their frequency and composition in uncontrolled and controlled infested spruce forest in Bavaria. J. Pest Sci. 2004, 77, 165–172. [Google Scholar] [CrossRef]
- Bracalini, M.; Florenzano, G.T.; Panzavolta, T. Verbenone Affects the Behavior of Insect Predators and Other Saproxylic Beetles Differently: Trials Using Pheromone-Baited Bark Beetle Traps. Insects 2024, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, K. Zur Wirkung von Aasgeruch auf die Fangleistung von Buchdruckerfallen. Anz. Schadlingskde Pflanzenschutz Umweltschutz 1990, 63, 46–48. [Google Scholar] [CrossRef]
- Zhang, H.; Jakuš, R.; Schlyter, F.; Birgersson, G. Can Ips typographus (L.) (Col., Scolytidae) smell the carrion odours of the dead beetles in pheromone traps? Electrophysiological analysis. J. Appl. Entomol. 2003, 127, 185–188. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Räty, L.; Drumont, A.; De Windt, N.; De Proft, M. Ips typographus: Natural Enemies and the Forester. In Behavior, Population Dynamics and Control of Forest Insects, Proceedings of the Joint IUFRO Working Party Conference, Maui, Hawaii, 6–11 February 1994; Ohio State University, Ohio Agricultural Research and Development Center: Columbus, OH, USA, 1995; pp. 202–207. [Google Scholar]
- Kasumović, L.; Hrašovec, B.; Jazbec, A. Efficiency of dry and wet flight barrier Theysohn® pheromone traps in catching the spruce bark beetles Ips typographus L. and Pityogenes chalcographus L. Šumar. List 2016, 140, 477–483. [Google Scholar]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a “benign neglect strategy” in a national park: Response of saproxylic beetles to dead wood accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Bässler, C.; Brandl, R.; Burton, P.J.; Müller, J. Conservation value of forests attacked by bark beetles: Highest number of indicator species is found in early successional stages. J. Nat. Conserv. 2013, 21, 97–104. [Google Scholar] [CrossRef]
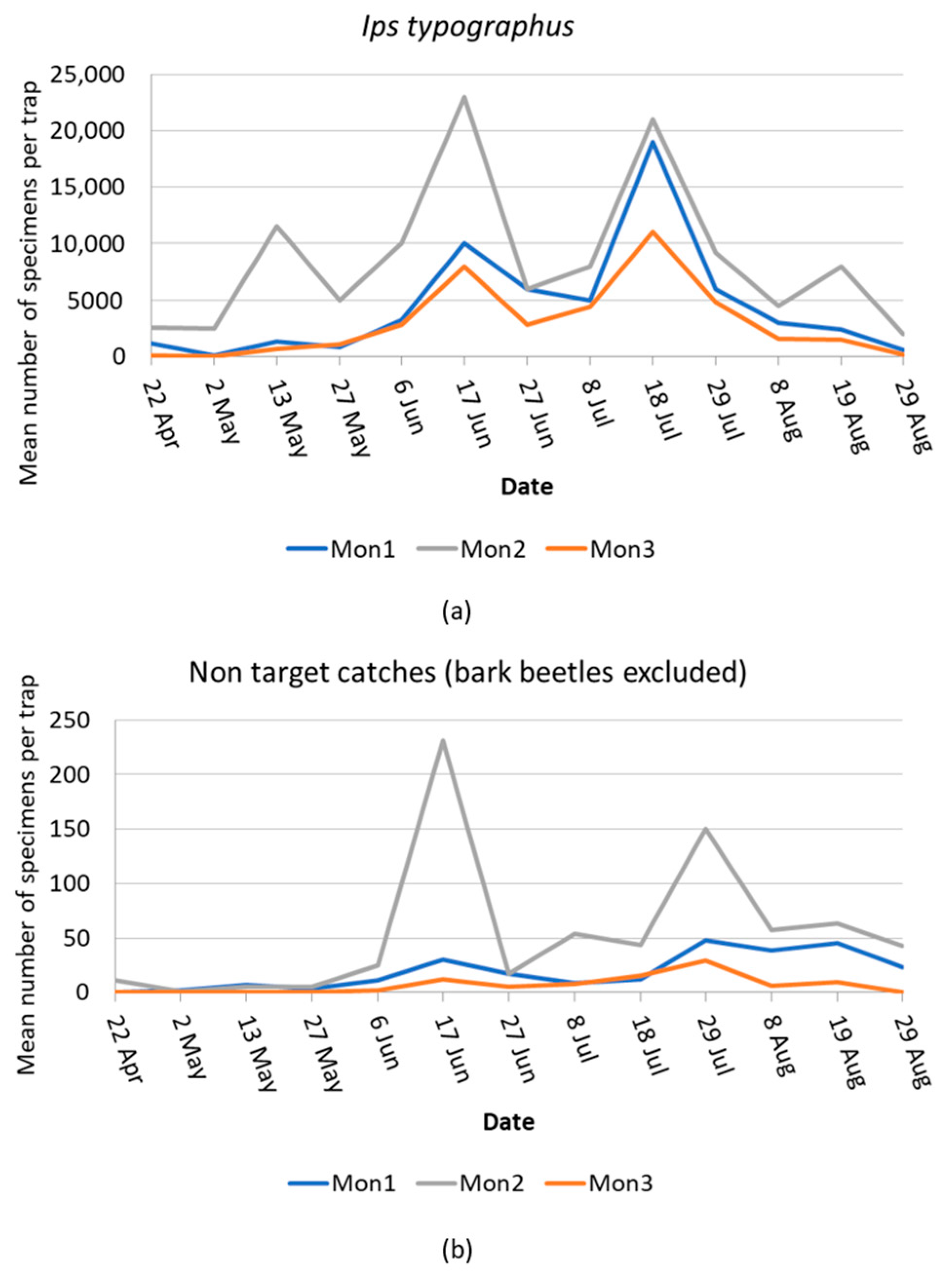
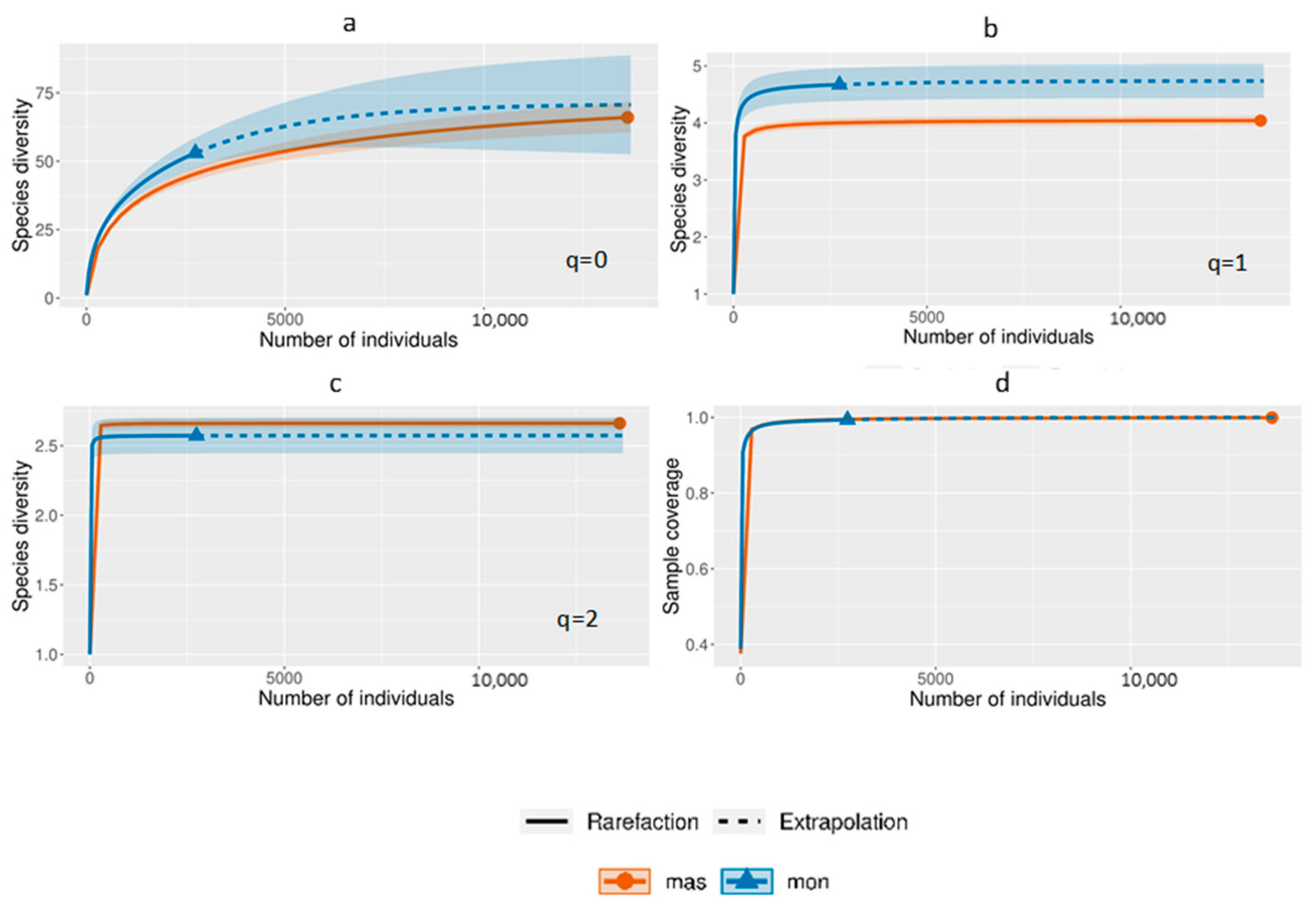
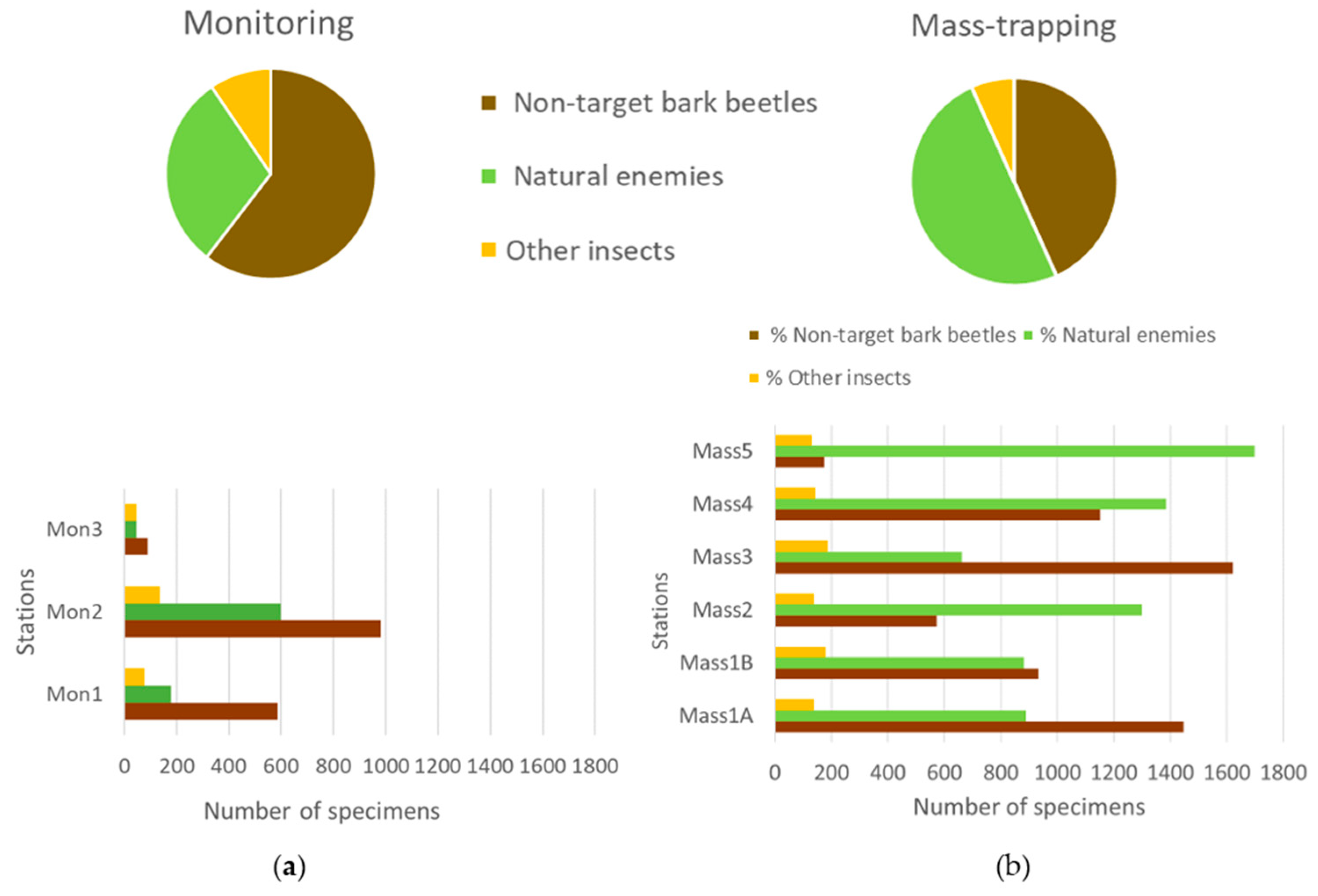
| Sites | Coordinates | Mean Altitude m a.s.l. | Monitoring Trapping Stations | Mass-Trapping Stations |
|---|---|---|---|---|
| Site 1 | N46.578903 E11.910383 | 1600 | Mon1 | Mass1A Mass1B |
| Site 2 | N46.628673 E11.898967 | 1300 | Mon2 | Mass2 |
| Site 3 | N46.548217 E11.968640 | 1700 | Mon3 | Mass3 |
| Site 4 | N46.568657 E11.892362 | 1500 | Mass4 | |
| Site 5 | N46.528091 E11.883775 | 1800 | Mass5 |
| Order | Family/Subfamily | Species | Monitoring Setup | Mass-Trapping Setup |
|---|---|---|---|---|
| Target species | ||||
| Coleoptera | Curculionidae Scolytinae | Ips typographus (Linnaeus) | 106,444 | 180,000 |
| Other bark beetles | ||||
| Coleoptera | Curculionidae Scolytinae | Cryphalus asperatus Gyllenhal | 1 | 9 |
| Cryphalus intermedius Ferrari | 0 | 2 | ||
| Crypturgus cinereus (Herbst) | 1 | 24 | ||
| Crypturgus pusillus (Gyllenhal) | 1 | 3 | ||
| Dryocoetes autographus (Ratzeburg) | 1 | 23 | ||
| Hylastes cunicularius Erichson | 31 | 115 | ||
| Hylurgops palliatus (Gyllenhal) | 1 | 1 | ||
| Orthotomicus erosus (Wollaston) | 0 | 6 | ||
| Phthorophloeus spinulosus Rey | 5 | 13 | ||
| Pityogenes bistridentatus (Eichhoff) | 12 | 62 | ||
| Pityogenes chalcographus (Linnaeus) | 1594 | 5556 | ||
| Polygraphus poligraphus (Linnaeus) | 1 | 40 | ||
| Trypodendron lineatum (Olivier) | 8 | 42 | ||
| Other Scolytinae | 0 | 2 | ||
| Natural enemies | ||||
| Coleoptera | Carabidae | Ocydromus tibialis (Duftschmid) | 1 | 0 |
| Carabidae | Ocydromus deletus deletus (Audinet-Serville) | 0 | 1 | |
| Cleridae | Thanasimus femoralis (Zetterstedt) | 15 | 11 | |
| Cleridae | Thanasimus formicarius (Linnaeus) | 1 | 24 | |
| Cleridae | Trichodes sp. | 0 | 1 | |
| Elateridae | Athous (Haplathous) subfuscus (Müller) | 0 | 12 | |
| Elateridae | Melanotus castanipes (Paykull) | 0 | 2 | |
| Histeridae | Plegaderus vulneratus (Panzer) | 2 | 3 | |
| Histeridae spp. | 7 | 6 | ||
| Monotomidae | Rhizophagus ferrugineus (Paykull) | 5 | 15 | |
| Monotomidae | Rhizophagus nitidulus (Fabricius) | 0 | 4 | |
| Nitidulidae | Pityophagus ferrugineus (Linnaeus) | 1 | 4 | |
| Nitidulidae | Epuraea sp. | 1 | 18 | |
| Staphylinidae | Nudobius lentus (Gravenhorst) | 11 | 39 | |
| Staphylinidae | Placusa sp. | 5 | 146 | |
| Staphylinidae | Aleochara sp. | 186 | 22 | |
| Tenebrionidae | Corticeus sp. | 2 | 7 | |
| Diptera | Dolichopodidae | Medetera sp. | 1 | 18 |
| Hymenoptera | Braconidae | Ropalophorus clavicornis (Wesmael) | 8 | 291 |
| Pteromalidae | Tomicobia seitneri (Ruschka) | 578 | 6206 | |
| Other insects | ||||
| Coleoptera | Anobiidae | Ernobius sp. | 0 | 1 |
| Anobiidae | Hadrobregmus pertinax (Linnaeus) | 1 | 3 | |
| Buprestidae sp. | 0 | 1 | ||
| Cerambycidae | Acanthocinus griseus (Fabricius) | 2 | 6 | |
| Cerambycidae | Callidium violaceum (Linnaeus) | 0 | 1 | |
| Cerambycidae | Clytus lama Mulsant | 0 | 1 | |
| Ciidae spp. | 1 | 3 | ||
| Curculionidae spp. | 2 | 2 | ||
| Dermestidae spp. | 2 | 2 | ||
| Elateridae | Ampedus scrofa (Germar) | 2 | 0 | |
| Ampedus nigrinus (Herbst) | 0 | 1 | ||
| Athous (Athous) vittatus (Fabricius) | 1 | 1 | ||
| Cardiophorus ruficollis (Linnaeus) | 14 | 1 | ||
| Dalopius marginatus (Linnaeus) | 0 | 1 | ||
| Elateridae spp. | 2 | 7 | ||
| Lycidae | Platycis minutus (Fabricius) | 1 | 0 | |
| Melyridae spp. | 3 | 0 | ||
| Mordellidae spp. | 27 | 10 | ||
| Nitidulidae spp. | 2 | 31 | ||
| Scarabaeidae | Hoplia argentea (Poda) | 1 | 0 | |
| Scarabaeidae | Aphodius sp. | 2 | 2 | |
| Silphidae | Necrodes littoralis (Linnaeus) | 0 | 1 | |
| Silphidae | Nicrophorus vespillo (Linnaeus) | 3 | 1 | |
| Silphidae | Nicrophorus vespilloides Herbst | 35 | 25 | |
| Silphidae | Oiceoptoma thoracicum (Linnaeus) | 33 | 8 | |
| Silphidae | Thanatophilus rugosus (Linnaeus) | 2 | 3 | |
| Silphidae | Thanatophilus sinuatus (Fabricius) | 11 | 6 | |
| Silvanidae spp. | 0 | 3 | ||
| Staphylinidae spp. | 60 | 110 | ||
| Trogossitidae spp. | 0 | 3 | ||
| Coleoptera spp. | 19 | 15 | ||
| Blattodea spp. | 1 | 1 | ||
| Dermaptera spp. | 0 | 17 | ||
| Diptera spp. | 18 | 392 | ||
| Embioptera spp. | 0 | 4 | ||
| Hemiptera spp. | 2 | 40 | ||
| Hymenoptera | Apidae spp. | 3 | 5 | |
| Chrysididae spp. | 3 | 2 | ||
| Formicidae spp. | 3 | 165 | ||
| Siricidae spp. | 1 | 2 | ||
| Sphecidae spp. | 0 | 5 | ||
| Vespidae | Vespula vulgaris (Linnaeus) | 0 | 4 | |
| Hymenoptera spp. | 0 | 5 | ||
| Lepidoptera spp. | 3 | 0 | ||
| Mecoptera spp. | 0 | 2 | ||
| Raphidioptera spp. | 0 | 1 | ||
| Total | 2740 | 13,622 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracalini, M.; Martini, A.; Tagliaferri, L.; Panzavolta, T. Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects. Forests 2025, 16, 1510. https://doi.org/10.3390/f16101510
Bracalini M, Martini A, Tagliaferri L, Panzavolta T. Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects. Forests. 2025; 16(10):1510. https://doi.org/10.3390/f16101510
Chicago/Turabian StyleBracalini, Matteo, Andrea Martini, Lorenzo Tagliaferri, and Tiziana Panzavolta. 2025. "Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects" Forests 16, no. 10: 1510. https://doi.org/10.3390/f16101510
APA StyleBracalini, M., Martini, A., Tagliaferri, L., & Panzavolta, T. (2025). Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects. Forests, 16(10), 1510. https://doi.org/10.3390/f16101510







