Abstract
Ozone (O3) pollution is accompanied by drought stress, especially at high temperatures. Tree species in cities often face dual stresses from O3 and drought. In this study, Acer rubrum ‘Autumn Blaze’ and A. pictum were used as test plants in open-top-chambers (OTCs) to investigate the trees most tolerant to increasing O3 and drought stresses in urban gardens. The results showed that the dual stresses induced a change in A. rubrum’s leaf coloration from green to red. The leaf representation in A. rubrum was more variable than that of A. pictum. The leaf pigment content affected the plant leaf color difference, and the Chl and Car contents of both species were negatively correlated with L*. Under the dual stresses of O3 and drought, the changes in the net photosynthetic rate (Pn) and transpiration rate (Tr) were less variable in A. rubrum than A. pictum. The stomatal conductance (Gs) was more sensitive to higher O3 stress, the effect of which was enhanced by moderate drought (MD) conditions on Gs. The Tr decreased more significantly under drought stress, which mitigated the effect of O3 stress on the stomatal limit value (Ls). A. rubrum displayed differential color changes, resulting in greater structural heterogeneity within the garden landscape. The saplings adjusted their photosynthetic parameters under the dual stresses, whereas the dual stresses played an antagonistic role in protecting A. rubrum, suggesting that A. rubrum can resist O3 and drought. Our study suggests that A. rubrum is an alternative tree species for inclusion in urban gardens exposed to increasing O3 and drought stresses.
1. Introduction
Tropospheric ozone (O3) is the primary component of atmospheric photochemical smog and greenhouse gases, which is a specific term for ”surface O3” [], and has strong oxidizing properties. Rapid development during the industrial age significantly increased the emissions of O3 precursors, such as nitrogen oxides (NOx = NO + NO2) and volatile organic compounds (VOCs), thereby elevating the concentration of near-surface O3 []. Notably, the O3 concentration in China has been increasing at an annual rate of 0.3%–2.0%, and it is estimated to double by 2100. The north-central regions of North and East China are the most O3-polluted areas of China [], with O3 concentrations considerably above the damage threshold of 40 ppb for sensitive plants [].
Near-surface O3 seriously hinders the growth of natural plants [,] and reduces the quality and yield of crops [,]. Furthermore, O3 can cause yellow and green color loss, spots, dryness, wilting, and early aging in leaves. Tropospheric O3 can reduce the photosynthesis rate, cause stomatal closure in plants, hinder photosynthetic electron transport, inhibit plant photosynthesis [,,], cause the stomata on the leaf surface to lag or fail [,], and increase water consumption by transpiration [,].
Drought stress is the most common challenge for trees. The primary contributors to drought stress are a high-temperature climate and lack of irrigation conditions caused by limited water resources and the fragility of the ecological environment. Currently, the lack of water resources and the fragility of the ecological environment remains challenging in urban landscape construction []. In drought conditions [,], plants’ response to physiological factors, such as net photosynthetic rate, transpiration rate, stomatal conductance, and hydraulic conductivity, is limited []; therefore, drought can affect plant photosynthesis []. When plants are subjected to drought stress, the stomata close as stress levels increase; this reduces water loss and increases plant resistance to drought []. It also affects the absorption of carbon dioxide, ultimately inhibiting photosynthesis. For the whole plant, the damage caused by drought stress is more severe than that of O3 stress alone []. Water scarcity and drought remain long-standing and challenging problems worldwide [].
The primary manifestation of the compounding effect of O3 and drought is the synergistic aggravation of plant damage, while an antagonistic effect reduces plant damage. The response depends on the plant and species, the magnitude and duration of the drought, and high O3 conditions [,]. The impact of multiple stresses on plants is complex, and the interaction between multiple factors at the same time needs to be further investigated. For example, moderate water restriction helps to slow down the harmful damage caused by O3 to poplars (Populus deltoides cv. ‘55/56’ × P. deltoides cv. ‘Imperial’) []. However, Hao et al. [] and Ye et al. [] found that the seedlings of Alstonia scholaris and Syzygium hainanense were more affected by the dual stresses of O3 and drought than by their individual stress effects and that S. hainanense seedlings showed stronger anti-O3 and drought resistant responses than A. scholaris. Another study has shown that plants are unaffected by dual stresses [], suggesting a lack of consensus regarding the effects of these dual stresses on different plants.
Acer rubrum L. (American red maple) and A. pictum Thunb. ex Murray (mono maple) are important broad-leaved landscaping tree species in North China [,]. The urban landscape provides an important ecosystem for the city’s inhabitants; therefore, the woody plants play an essential role in fine-scale monitoring through unmanned aerial systems (UAS) imagery of the urban environment and the effect of O3 []. Research on the effects of O3 and drought stress on plant photosynthetic physiology is mainly focused on grass crops, fruits, and vegetables. However, the photosynthetic physiology of landscaping tree species has yet to be investigated, especially for A. rubrum and A. pictum, the two main species of Aceraceae used in urban landscapes in China. So far, previous studies have primarily investigated drought as a single stress and drought resistance when evaluating this tree species [,]; hence, data on the dual stresses of this tree species still need to be provided.
In this study, we considered the seedlings of two tree species as research objects and explored their response to variable environmental conditions, such as an O3 fumigation gradient and drought stress. In addition, changes in the appearance and physiological indicators were explored to provide a reference for the selection and configuration of landscaping tree species. For example, these species could exhibit physiological and ecological changes that reduce the ornamental effect of a garden landscape; alternatively, environmental stress could have little impact on the physiological indicators.
2. Materials and Methods
2.1. Test Area
This study was performed at an experimental field station in Yanqing District, Beijing (40°48′ N, 115°60′ E). The station experiences a temperate and semi-humid continental climate; the average annual temperature and precipitation are 9.9 °C and 467 mm, respectively; the warmest month is July, with an average temperature of 24.5 °C [].
2.2. Test Materials and Design
The experimental materials were 1–2-year-old seedlings of Acer rubrum ‘Autumn Blaze’ and A. pictum, which are widely used as landscaping plants in northern Chinese cities. Polyethylene plastic flowerpots with a height of 20 cm and diameter of 25 cm were uniformly used for potting. Each pot was filled with a uniform mixture of local sandy loam and substrate at a 3:1 ratio, with the pH ranging from 5.5 to 6.5.
The fumigation facilities included nine open-top air chambers (OTCs, 2.2 m high, and 11.34 m2), with three O3 and three H2O levels, in a 3 × 3 treatment design. The three O3 levels were non-filtered ambient air (NF), NF with an O3 addition of 40 nmol/mol (NF40), and NF with an O3 addition of 60 nmol/mol (NF60), with each O3 level having three replicates (OTCs). Each chamber had three pots for each of the two varieties of seedlings [,]. Three H2O levels, including well-watered conditions (WW, approximately 75%–80% field capacity), moderate drought stress (MD, approximately 50%–55% field capacity), and severe drought stress (SD, approximately 30%–35% field capacity), were applied to each O3 treatment. Experimental and control fields were randomly distributed.
2.3. Test Methods
2.3.1. Determination of Plant Leaf Color Parameters
A colorimeter is an instrument suitable for nondestructive testing of plant color. It can accurately measure the leaf color of plants, directly output the leaf color parameters L*, a*, and b*, and quantitatively describe its brightness and color. Leaf color was determined by using a CR-400 (Konica Minolta, Inc., Tokyo, Japan) type fully automatic colorimeter. The measured light source was the built-in D65 standard light source, the window diameter was 8 mm, and CIE L a b color space was selected. Three leaves were selected for each treatment, and each leaf was repeatedly determined three times. The brightness value (L*), color value (a*), and color value (b*) are directly measured by the colorimeter. In this space, L* indicates the brightness of the measured sample color, where 0 indicates black, 100 indicates white, and the greater the L* value, the higher the brightness. The a* value indicates the red and green concentration of the measured sample color, which is positive for red and negative for green; the larger the a* value, the deeper the red, the smaller the a* value, the darker the green. The b* value indicates the yellow-blue concentration of the measured sample color, positive for yellow and negative for blue; the larger the b* value, the darker yellow, and the smaller the b* value, the darker blue. All indicators were determined repeatedly in triplicate.
Photosynthetic pigments were sampled using a 0.7 cm diameter punch, 2 leaf discs per leaf, and extracted with 2 mL of 95% ethanol solution in 4 °C shading until completely faded to white. Subsequently, the absorbance values of the extracts were determined at 664, 649, and 470 nm, and carotenoids (Car), chlorophyll a (Chl a), and chlorophyll b (Chl b) were calculated. The sum of Chl a and Chl b was calculated as the total chlorophyll content. The calculation formula for the photosynthetic pigment concentration is as follows:
Chl a = 13.95 × OD664 − 6.88 × OD649
Chl b = 24.96 × OD649 − 7.32 × OD664
Chl = Chl a + Chl b = 6.63 × OD664 + 18.08 × OD649
Car = (1000 × OD470 − 2.05 × Chl a − 114.8 × Chl b)/245
The unit in the formula is mg·L−1.
Finally, the chlorophyll content in the plant leaves was obtained:
Chlorophyll content = (sample chlorophyll concentration × dilution multiple × extract volume)/leaf area
The unit in the formula is mg·L−1.
2.3.2. Determination of the Photosynthetic Indices in Plants
Sunny and calm weather conditions were selected to sample and measure photosynthetic indicators. From 09:00 to 12:00 every day, healthy, uniformly sized, and uniformly colored leaves were randomly selected from mature leaves in the middle and upper parts of the plants. A Li-6400 photosynthetic analyzer (Li-cor Portable, Lincoln, OR, USA) was used. The test conditions were as follows: the leaf chamber temperature was 30 °C, the light intensity was 1000 μmol/m2/s, the CO2 concentration was 400 μmol/mol, and the flow rate was 500 μmol/s. Photosynthetic gas parameters were determined under the open gas path. Three leaves were selected for each treatment, and each leaf was repeatedly determined three times. Photosynthetic gas parameters include the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr). Water use efficiency (WUEg) was calculated as Pn/Tr, and the stomatal limit value (Ls) was calculated using 1 − Ci/Ca.
2.3.3. Determination of the O3 and Soil Moisture Content
O3 was produced by an O3 generator (HY003, Jinan Chuangcheng Co., Jinan, China). Pure oxygen was mixed with natural air by a 1.1 kW Fengda air-blower (Shanghai, China), and the outlet cylinder at the inner center of the hood rotated 360° to fill the O3 gas and maintain the target O3 concentration of the plant canopy; natural air was fed into the NF treatments. O3 fumigation (08:00–18:00 every day, except rainy days) lasted from 15 July to 23 September 2021. A Teflon solenoid valve system was used to sample the gas at the canopy height of the OTC plants. Subsequently, the system was measured using an ultraviolet (UV) absorption O3 analyzer (Model 49i; Thermo Scientific, Franklin, MA, USA) and periodically calibrated using a 49i-PS calibrator (Thermo Scientific). The average concentration range of NF over the entire period was 41.71 ± 0.29 nmol/mol. The soil water content was quantified using a field soil moisture meter (TRIME-HD, Chicago, IL, USA). There were three NF treatments corresponding to the three drought levels.
2.4. Data Analysis
WPS Office Excel was used for statistical data processing, and SPSS software (version 26.0) was used to conduct the analyses. The effects of O3 and drought on the dependent variables and the dual stress were analyzed using variance analysis, and the mean and standard deviation were calculated using Duncan’s multiple comparison test. Origin 2021 software was used to draw graphs. There were three replicates of each stress and three subsamples. In each replicate, three test plants (3 pots) were collected from the middle leaves of a single plant, and at least 3–4 leaves were collected on a single seedling for determination.
3. Results
3.1. Morphological Changes in the Leaves of A. rubrum and A. pictum
As seen in Figure 1 and Table 1, the leaves of A. rubrum and A. pictum showed changes in appearance following exposure to the dual stresses of O3 and drought. A. rubrum had the highest brightness value, L*, at NF40 + SD, and A. pictum had the highest L* at NF60 + SD. Under drought single stress, A. rubrum had the highest saturation, a*, under WW, and the lowest a* at NF60. A. pictum’s a* was not significantly different under the compound stress of O3 and drought. A. rubrum had the highest yellow and blue saturation, b*, under NF40 + SD, and the lowest b* under NF + MD. Among them, A. rubrum appeared to have significant differential changes visually with the dual stress of O3 × drought. The difference in the leaf color of A. rubrum was the most pronounced (Figure 1). The leaf color change in NF60 was more affected by increasing drought compared to that of NF. While the leaf color changed from green to bright red in NF+WW, A. rubrum remained in the green leaf stage in NF60+SD. At the same time, the leaf size of A. rubrum decreased with drought stress. Changes in the appearance and morphology of A. pictum leaves were primarily reflected in the yellowing and chlorosis of leaves, from the leaf edge to the interior, and the degree of leaf cracking. Under O3 single stress, the degree of leaf cracking of A. pictum showed three palmate lobes in NF and NF40, but the trilobate trend disappeared at NF60. The number of serrations on the leaf edge of A. pictum increased in NF60. The leaf size of A. pictum did not change significantly under single stress yet showed a shrinking trend under the dual stresses, suggesting that they affected the leaf appearance compared to trees without environmental stress exposure.
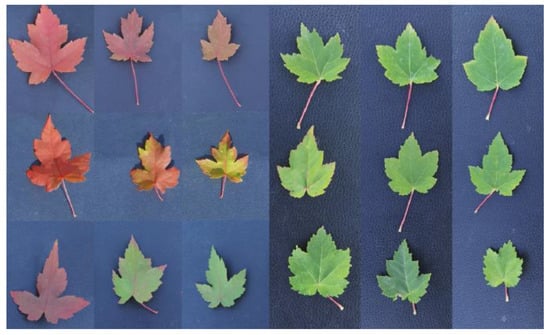
Figure 1.
Effects of O3 and drought stresses on leaf appearance of Acer rubrum and A. pictum. The left and right sets of nine photographs (3 × 3) show the processed A. rubrum and A. pictum, respectively. The treatments of the two sets in the top rows from left to right are NF + WW, NF + MD, and NF + SD, respectively; the two sets in the second row are NF40 + WW, NF40 + MD, and NF40 + SD, respectively; the two sets in the third row are NF60 + WW, NF60 + MD, and NF60 + SD, respectively.

Table 1.
Comparison of leaf color parameters between Acer rubrum and A. pictum under nine different treatments.
3.2. Effects of O3 and Drought Stresses on Photosynthetic Pigments in Leaves
The total chlorophyll (Chl a + b) and chlorophyll a (Chl a) contents of A. rubrum and A. pictum decreased significantly with an increase in stress degree under O3 single stress, drought single stress, and dual stress (Figure 2, Table 2), while the carotenoid (Car) and chlorophyll b (Chl b) contents of both species decreased significantly with an increase in stress degree under single and dual stresses (p < 0.001). A. rubrum had the lowest Car at NF40 + SD, and A. pictum had the lowest Car at NF60 + SD. The Chl a/b of A. rubrum did not change significantly under each stress, first increasing and then decreasing under O3 single stress, with the overall Chl a/b ratio of each stress treatment remaining at levels between 2:1–3:1.
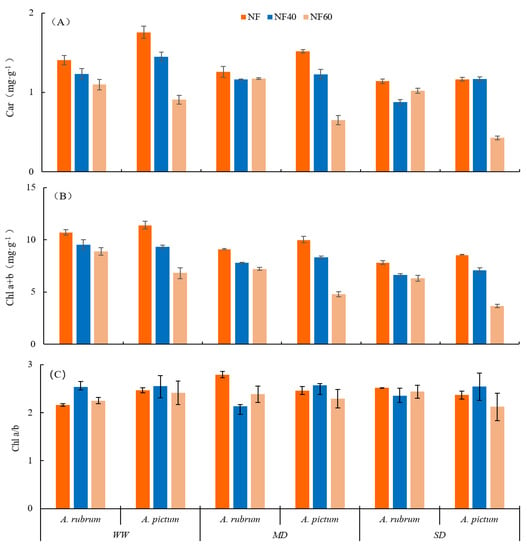
Figure 2.
Effects of O3 and drought stresses on photosynthetic pigments in leaves of Acer rubrum and A. pictum. Abbreviations: (A) carotenoids (Car), (B) total chlorophyll (Chl a + b), and (C) proportion of chlorophyll a:b (Chl a/b) in leaves of A. rubrum and A. pictum.

Table 2.
Multi-way ANOVA of photosynthetic parameters of Acer rubrum and A. pictum under O3 and drought stress.
3.3. Effects of O3 and Drought Stresses on Photosynthetic Parameters
Compared to A. rubrum, the difference in the leaf net photosynthetic rate (Pn) of A. pictum was more significant under the dual stress of O3 and water. Compared with WW, water deficiency (MD and SD) significantly decreased Pn by 57.6% and 78.8% under the NF treatment (p < 0.05), 41.3% and 51.6% under NF40 treatment, and 3.2% and 42.9% under NF60 treatment, respectively. Under the O3 single stress, the Pn of A. rubrum first decreased and then increased slightly, but the overall change was not significant. Meanwhile, in A. pictum, the Pn decreased first and then increased slightly with an increase in O3 concentration under the WW and MD treatments. Among the A. pictum samples, the Pn of the O3 stress treatments (NF40 and NF60) under the WW condition was significantly lower than that of the NF treatment by 41.1% (NF40 treatment) and 28.4% (NF60 treatment) (p < 0.05). However, the Pn of A. rubrum increased with O3 enrichment under SD treatment, and the Pn of the O3 stress increased by 65.9% (NF40 treatment) and 31.4% (NF60 treatment) compared to NF (Figure 3A).
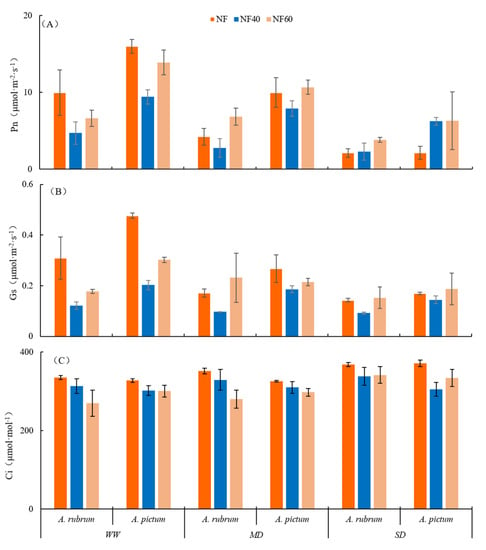
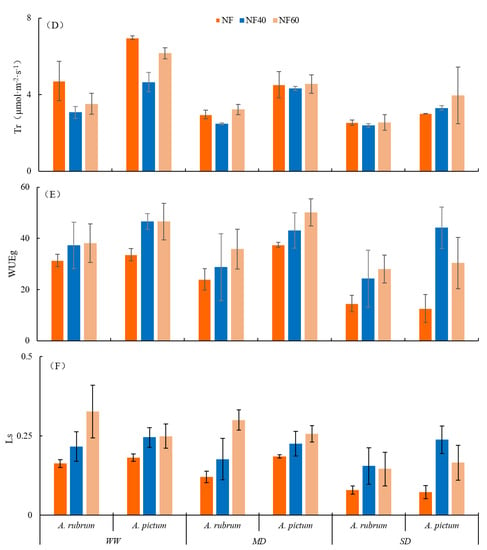
Figure 3.
Effects of O3 and drought stresses on leaf photosynthesis gas exchange parameters in Acer rubrum and A. pictum. Abbreviations: (A) net photosynthetic rate (Pn), (B) stomatal conductance (Gs), (C) intercellular CO2 concentration (Ci), (D) transpiration rate (Tr), (E) water use efficiency (WUEg), and (F) stomatal limit values (Ls) in leaves of A. rubrum and A. pictum.
Under the dual stresses, the stomatal conductance (Gs) of A. rubrum decreased significantly (p < 0.01), and the stress treatment groups showed a declining range of more than 50% with NF + MD treatment (60.5%) < NF60 + SD treatment (63.5%) < NF40 + MD treatment (68.7%) < NF60 + MD treatment (70.3%). Furthermore, the Gs of A. pictum leaves also decreased under each stress treatment, which showed that NF + MD treatment (57.4%) < NF60 + SD treatment (63.5%) < NF40 + MD treatment (60.9%) < NF60 + WW treatment (64.3%) < NF60 + MD treatment (69.7%) (Figure 3B).
Compared to NF, the intercellular CO2 concentration (Ci) of A. rubrum decreased slightly, by 2.9% and 2.4%, under NF40 and NF60, respectively. Acer pictum also showed a downward trend, while both A. rubrum and A. pictum increased slightly with the degree of drought stress, but the changes were not significant under drought single stress (Figure 3C). This shows that, on the whole, the Ci of A. rubrum and A. pictum is more affected by O3 than by drought as a single stress.
Under the dual stresses, the transpiration rate (Tr) of the leaves of A. rubrum and A. pictum decreased (p < 0.01), with the most significant changes in A. rubrum observed for NF60 + SD (43.8%) < NF + SD (45.9%) < NF40 + MD (47.5%) < NF40 + SD (49.3%); the most significant change in Tr of A. pictum was NF60 + SD (43.2%) < NF40 + SD (52.7%) < NF + SD (56.6%) (Figure 3D). The change in the Tr range for Tr in A. rubrum was smaller than that of A. pictum under stress, i.e., the change in Tr was more stable in the former than the latter. The Tr of A. rubrum and A. pictum showed different degrees of significant decline under SD and, hence, were more sensitive to drought stress.
The water use efficiency (WUEg) of A. rubrum increased with single-factor O3 stress, whereas that of A. pictum increased and then decreased significantly (p < 0.05). In the case of drought stress, the WUEg of A. rubrum decreased with a decreasing water gradient, but the difference among the treatments was not significant. However, the SD treatment of A. pictum under NF and NF60 was significantly lower than that of WW by 62.4% and 53.9%, respectively, and the difference between MD + NF40, MD + NF, and MD + NF60 treatments under MD was the smallest (Figure 3E).
In the case of O3 single stress, the stomatal limit values (Ls) of A. rubrum and A. pictum showed an overall increasing trend with increasing O3 concentration. The Ls of A. rubrum under the NF40 and NF60 treatments increased by 24.7% and 50.1% (WW treatment), 31.7% and 48.9% (MD treatment), and 48.7% and 45.5% (SD treatment) compared to NF. Under the NF40 and NF60 treatments, the Ls of A. pictum increased by 25.9% and 27.0% (WW treatment), 17.8% and 27.7% (MD treatment), and 69.5% and 56.1% (SD treatment), respectively, compared to NF (p < 0.05). Under drought stress, the Ls of A. rubrum decreased under MD and SD treatments by 25.9% and 51.2% (NF treatment) and 18.3% and 28.4% (NF40 treatment), respectively, and 55.3% under SD treatment compared to WW (NF60 treatment). Under the NF and NF60 treatments, the Ls of A. pictum showed a small fluctuation and then decreased significantly with water deficiency and fluctuated only slightly with the water gradient under the NF40 treatment. In contrast, Ls under SD treatment decreased by 60.1% (NF treatment) and 33.7% (NF60 treatment), and Ls under MD and SD treatments decreased by 8.1% and 3.1% (NF40 treatment), respectively. There was no significant difference between the treatments of A. rubrum under dual stress, but the difference between those of A. pictum was significant (p < 0.05) (Figure 3F; Table 3).

Table 3.
Multi-way ANOVA of photosynthetic parameters of Acer rubrum and A. pictum under O3 and drought stress.
The impact of A. rubrum on Ls under O3 stress was greater than that of A. pictum. During SD, the antagonism between drought and O3 slowed down the damage to the stomata of A. rubrum, whereas A. pictum did not show self-preservation mechanisms under SD.
4. Discussion
4.1. Stress Changes in Leaf Color and Appearance
As a color-changing leaf tree species, A. rubrum is one of the most important landscaping tree species globally. Following the reproductive and vegetative growth of flowers and leaves in the growing season, its leaf color gradually changes from emerald green to fiery red in late summer and early autumn as temperatures drop []. This study showed that high concentrations of O3 and drought conditions interfered with the discoloration of the leaves of A. rubrum, delaying or even changing the color of the leaves [,]. At the same time, the leaf area of A. rubrum decreased to different degrees with stress levels, whereas the leaf area and leaf crack degree of A. pictum were most affected. These results are similar to recent studies on Acer truncatum Bunge [,]. These results show that O3 and drought stresses have a particular impact on the appearance of A. rubrum and A. pictum leaves, affecting the garden landscape. Furthermore, our results show that under O3 stress, the degree of leaf cracking in A. pictum tends to slow down. The dual stresses of O3 and drought tended to cause the leaf area of A. pictum to shrink compared to the control leaves.
4.2. Effect of Stress on Photosynthetic Pigments
The contents of Chl and Car in the leaves of both species decreased with a single stress increase, and the variation in the leaf pigment of A. rubrum in the stress group under O3 and drought conditions was smaller than that of A. pictum; this shows that A. rubrum can cope with O3 and drought stress better than A. pictum. Furthermore, Car changed in response to O3, consistent with previous findings []. The net photosynthetic rate of plants is an important indicator of plant productivity. Previous studies have shown that Chl and Car are important photosynthetic pigments in energy transfer in the photosynthetic system, and changes in their content directly affect the intensity of plant photosynthesis [,]. In A. rubrum, the Chl and Car were the lowest at NF40 + SD, and the leaves were yellow-green, while b* and L* were the highest. In A. pictum, they were the lowest at NF60 + SD, the leaves were green, and L* was the highest. We concluded that the leaf pigment content influenced the leaf color difference, and the Chl and Car contents of both species were negatively correlated with L*. This corresponds to the overall change being more stable in the Pn of A. rubrum under the dual stress, compared to the relative results of A. pictum. The Chl of A. rubrum and A. pictum showed significant differences under the single stresses of O3 and drought, with no significant differences under the dual stresses, showing that the dual stresses could help to alleviate the differential effect of a single stress on the leaf pigment content. Significant differences between the different tree species indicated that the two Acer species are different. Significant differences were also observed in the O3-stress × tree species, while the three-factor stress of the O3 × drought × tree species was not significant, indicating that drought stress affected the leaf pigment content’s differential changes in breaking the dual stress of the O3 × tree species. There was no significant difference in the value of Chl a/b for each treatment factor before or after O3 and drought stress, and the two species maintained the biological characteristics of photosynthesis in heliophytic plants in terms of leaf pigment.
4.3. Effect of Stress on Photosynthetic Physiological Indicators
In this study, the Pn of A. pictum under O3 single stress first decreased, then increased slightly, Ci decreased sequentially, and Ls increased sequentially. Studies have shown that O3 can cause plant stomatal closure, hinder photosynthetic electron transfer, and inhibit plant photosynthesis. Changes in plant photosynthetic efficiency are generally divided into two categories: stomatal limitation caused by stomatal closure and non-stomatal limitation caused by impaired photosynthetic cells and decreased photosynthetic activity [,,]. Therefore, the decrease in Pn is caused by the limitation of the stomata of A. pictum. Stomata are the gateway for gas exchange between plants and the environment and regulate important physiological processes, such as transpiration, photosynthesis, and respiration, by controlling CO2 and water, the main channels through which gaseous pollutants, such as O3, enter the plant. There was no such change during a severe drought.
The Ls of A. rubrum changed more under NF60 than under NF40; that is, the correlation of stomatal restriction was greater in NF60. Under these conditions, the degree of stomatal restriction of A. rubrum was positively correlated with the degree of O3 stress, indicating that A. rubrum can cope with this O3 stress.
The Ls of A. rubrum under the NF, NF40, and NF60 treatments decreased with increased drought stress, and changes in Ls were the largest in the SD treatment. A. rubrum could cope with O3 and drought stress during the entire stress period through stomatal self-regulation. The dual stress forms an antagonism to protect the plants. With increasing drought stress, the increase in O3 concentration was negatively correlated with the change range of Pn, i.e., the dual stress of O3 and drought played an antagonistic role in protecting the Pn of A. rubrum. The ability of this plant to purify polluted air highlights its application value for inclusion in urban landscaping, ecological environment planning, and construction.
The MD condition led to a smaller effect of O3 stress on the Pn and Gs of A. pictum. Therefore, MD can reduce the effects of O3 stress on the Pn decline []. Under SD conditions, the Pn of A. rubrum and A. pictum increased under O3 stress, and Gs increased slightly or remained stable, indicating that drought can alleviate the damage caused by O3 to the Pn of plant leaves, which is consistent with previous studies []. Drought induces the closure of stomata, limits the O3 dose entering the leaves, and plays an antagonistic role in protecting plants, thus stimulating the adaptation and protection mechanisms of plants against adversity and reducing the damage caused by O3 to leaves [].
Among the stress treatment groups with a declining range of >50%, the change rate of NF60+MD treatment on the Gs of A. rubrum and A. pictum was the largest, more prone to a significantly high decline rate in the NF60 treatment group; therefore, we speculate that the impact of O3 stress on the Gs of A. rubrum and A. pictum was greater than that of drought stress. In addition, this suggests that Gs is more sensitive to a higher O3 concentration stress and that the decrease in Gs under MD was positively correlated with O3 stress, indicating that the MD condition can enhance the effect of O3 stress on stomatal conductance. At this time, the dual stresses played an antagonistic role in protecting stomatal conductance, making the maximum Gs decline rate of the two tree species appear in MD but not SD at NF60.
Under the dual stresses, the Tr of the stress treatment group decreased in both A. rubrum and A. pictum, indicating that these two tree species can reduce their leaf transpiration rate in a stressful environment to improve their response to adverse conditions. The WUEg of A. rubrum tended to increase with an increasing O3 concentration and was highest under the NF60 treatment. We speculate that A. rubrum can adapt to environmental pollution caused by increasing atmospheric O3 concentrations by adjusting its water use efficiency. In contrast, the change in the WUEg of A. pictum indicates a lack of stability or regularity under high O3 concentrations and drought stress. As a result, O3 entering the plant through the stomata and cuticle harms plants, and the landscaping and beautification effects are negatively impacted []. At the same time, to a certain extent, the O3 concentration in the air is reduced.
5. Conclusions
According to a comprehensive analysis of leaf appearance, photosynthetic pigment content, and photosynthetic gas exchange parameters, the difference in leaf color of A. rubrum under stress is more prevalent. Leaf pigment content affected plant leaf color difference, and the Chl and Car contents of both species were negatively correlated with the L*. Although stressful environmental conditions have adverse effects on the photosynthetic physiology of A. rubrum, these plants can still adapt to environmental changes through self-regulation and resist them. In addition, the dual stresses resulted in an antagonistic response by the plants. Therefore, A. rubrum is recommended as a candidate tree species in urban environments exposed to high levels of O3 and drought and can be used as an indicator plant for O3 and drought in urban greening construction. However, in this study, the leaf area was not scanned in situ with a leaf area meter, nor was the leaf color compared with a color card or measured using spectrophotometry. Therefore, future studies should record the leaf area and color in the field.
Author Contributions
Conceptualization, resources, project administration, funding acquisition, H.L. and L.Q.; methodology, L.Q.; software, L.W.; validation, H.L., L.Q. and X.Y.; formal analysis, L.W.; investigation, L.W. and X.H.; data curation, L.Q. and L.W.; writing—original draft preparation, L.W.; writing—review and editing, X.Y. and T.W.; visualization, X.G.; supervision, T.W. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D program (2017YFE0127700), the National Science Foundation of China (31971718), and the National Natural Science Foundation of China (No. 32271588).
Data Availability Statement
The data supporting the findings of this study are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoshika, Y.; Watanabe, M.; Inada, N.; Koike, T. Ozone-induced stomatal sluggishness develops progressively in Siebold’s beech (Fagus crenata). Environ. Pollut. 2012, 166, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Sitch, S.; Cox, P.M.; Collins, W.J.; Huntingford, C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Li, S.J.; Liu, P.F. Spatial and Temporal Changes of Ozone Concentration in Chinese Cities in 2016. J. Environ. Sci. 2018, 38, 1263–1274. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Sun, J.S.; Wan, W.X.; Hu, E.Z.; Calatayud, V. Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ. Pollut. 2014, 193, 296–301. [Google Scholar] [CrossRef]
- Wan, W.X.; Manning, W.J.; Wang, X.K.; Zhang, H.X.; Sun, X.; Zhang, Q.Q. Ozone and ozone injury on plants in and around Beijing, China. Environ. Pollut. 2014, 191, 215–222. [Google Scholar] [CrossRef]
- Gao, F.; Calatayud, V.; García-Breijo, F.; Reig-Armiñana, J.; Feng, Z.Z. Effects of elevated ozone on physiological, anatomical, and ultrastructural characteristics of four common urban tree species in China. Ecol. Indic. 2016, 67, 367–379. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.K.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Zeng, H.Q.; Wang, X.K.; Zheng, Q.W.; Feng, Z.W. Sensitivity of Metasequoia glyptostroboides to ozone stress. Photosynthetica 2008, 46, 463–465. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Change Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Nikula, S.; Percy, K.; Oksanen, E.; Holopainen, T.; Manninen, S. Effects of elevated ozone on growth and foliar traits of European and hybrid aspen. Boreal Environ. Res. 2009, 14, 29–47. [Google Scholar]
- Sun, G.E.; McLaughlin, S.B.; Porter, J.H.; Uddling, J.; Mulholland, P.J.; Adams, M.B.; Pederson, N. Interactive influences of ozone and climate on stream flow of forested watersheds. Glob. Change Biol. 2012, 18, 3395–3409. [Google Scholar] [CrossRef]
- Uddling, J.; Teclaw, R.M.; Pregitzer, K.S.; Ellsworth, D.S. Leaf and canopy conductance in aspen and aspen-birch forests under free-air enrichment of carbon dioxide and ozone. Tree Physiol. 2009, 29, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Bai, T.Y.; Li, Q.F. Growth and physiological responses of four garden plant seedlings to drought stress. Resour. Environ. Arid Areas. 2021, 35, 173–179. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.J.; Sun, J.B. Heat tolerance of five evergreen plants for roof greening. J. N. For. Univ. 2021, 36, 233–237. [Google Scholar] [CrossRef]
- Yin, H.X.; Hao, G.Y. The differentiation of structural features of xylem ring holes and holes in the species leads to significant differences in their hydraulic traits. J. Appl. Ecol. 2018, 29, 352–360. [Google Scholar] [CrossRef]
- Eghdami, H.; Werner, W.; De Marco, A.; Sicard, P. Influence of ozone and drought on tree growth under field conditions in a 22 year time series. Forests 2022, 13, 1215. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Aliyeva, D.R.; Aliyev, J.A. Subcellular localization and responses of superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 2014, 81, 54–60. [Google Scholar] [CrossRef]
- Li, L.; Niu, J.F.; Wen, Z.; Cui, J.; Wang, X.K. The effects of elevated ozone and chronic drought on leaf pigments and abscisic acid contents in early and late-flush leaves of Shantung maple (Acer truncatum Bunge). J. Ecol. 2016, 36, 6804–6811. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Guan, J.L.; Liang, Q.; Zhang, X.Y.; Hu, H.L.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Li, P.; Yuan, X.Y.; Gao, F.; Jiang, L.J.; Dai, L.L. Progress in ecological and environmental effects of ground-level O3 in China. Acta Ecol. Sin. 2018, 38, 1530–1541, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Wu, R.J. Research progress on the interaction between surface ozone and soil water deficit on plants. Chin. J. Ecol. 2017, 36, 846–853. [Google Scholar] [CrossRef]
- Zhou, H.M.; Li, P.; Feng, Z.Z.; Zhang, Y.B. Short-term effects of combined elevated ozone and limited irrigation on accumulation and allocation of non-structural carbohydrates in leaves and roots of poplar sapling. Chin. J. Plant Ecol. 2019, 43, 296–304. [Google Scholar] [CrossRef]
- Hao, Y.T.; Lin, M.; Xue, L.; Wang, Z.Y.; Lin, J.T.; Liang, Z.Y.; Sun, B.C.; Tian, M.T. Effects of ozone stress and drought stress on photosynthesis characteristics of Syzygium hainanense and Alstonia scholaris seedlings. J. Anhui Agric. Univ. 2014, 41, 193–197, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Ye, L.H.; Yi, L.S.; Huang, W.L.; Lai, M.T.; Mo, Y.B.; Zhang, C.H. Effect of ozone and drought stress on fluorescence physiology of Alstonia scholaris and Syzygium hainane. For. Environ. Sci. 2019, 35, 60–64. [Google Scholar] [CrossRef]
- Gao, F.; Li, P.; Feng, Z.Z. Interactive effects of ozone and drought stress on plants: A review. Chin. J. Plant Ecol. 2017, 41, 252–268, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Wang, L.M. Introduction to ornamental plant resources of Acer aceae in Tianshui City, Gansu Province. Spec. Econ. Anim. Plants. 2020, 23, 11–13+15. [Google Scholar] [CrossRef]
- Wei, X.F.; Chen, H.J.; Hyun, S.H. Research on the improvement of environmental pollution by garden landscape design using different vegetation configurations. J. Inst. Eng. 2022, Series A, 81–88. [Google Scholar] [CrossRef]
- Kašpar, V.; Zapletal, M.; Samec, P.; Komárek, J.; Bílek, J.; Juráň, S. Unmanned aerial systems for modelling air pollution removal by urban greenery. Urban For. Urban Green. 2022, 78, 127757. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, T.T.; Li, J.; Wang, Y.T.; Liu, G.L. Effect of water stress on seedling growth and leaf color changes in Acer rubrum seedlings. Jiangsu Agric. Sci. 2016, 44, 224–227, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Du, Q.S.; Gao, C.R.; Wang, R.X.; Zhou, X.P.; Zhang, Y.T. Comparison of evaluation methods of 3 tree species to cope with drought. For. Sci. Technol. Commun. 2022, 7, 48–54, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Xu, Y.; Feng, Z.; Shang, B.; Dai, L.; Uddling, J.; Tarvainen, L. Mesophyll conductance limitation of photosynthesis in poplar under elevated ozone. Sci. Total Environ. 2019, 657, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.B.; Hao, Z.P.; Qu, L.Y.; Wu, H.; Du, X.; Yuan, X.Y.; Zhang, X.; Chen, B.D. Mycorrhizal symbiosis and water condition affect ozone sensitivity of Medicago sativa L. by mediating stomatal conductance. Environ. Exp. Bot. 2022, 202, 105037. [Google Scholar] [CrossRef]
- Xu, Y.S.; Feng, Z.Z.; Shang, B.; Yuan, X.Y.; Lasse, T. Limited water availability did not protect poplar saplings from water use efficiency reduction under elevated ozone. For. Ecol. Manag. 2020, 462, 117999. [Google Scholar] [CrossRef]
- Chazdon, R.L. Forest landscape restoration: Integrated approaches to support effective implementation. Restor. Ecol. 2020, 28, 1654–1655. [Google Scholar] [CrossRef]
- Zhang, X. The Effect Study of Drought Stress on Growth and Leaf Color Change in Acer rubrum Seedlings. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Moura, B.B.; Paoletti, E.; Badea, O.; Ferrini, F.; Hoshika, Y. Visible foliar injury and ecophysiological responses to ozone and drought in oak seedlings. Plants 2022, 11, 1836. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, D.L. Effects of shading on the leaves and photosynthetic characteristics of Ligustrum robustum. Sheng Tai Xue Bao 2014, 34, 3538–3547, (In Chinese with English a abstract). [Google Scholar] [CrossRef]
- Li, L.; Manning, W.J.; Tong, L.; Wang, X.K. Chronic drought stress reduced but not protected Shantung maple (Acer truncatum Bunge) from adverse effects of ozone (O3) on growth and physiology in the suburb of Beijing, China. Environ. Pollut. 2015, 201, 34–41. [Google Scholar] [CrossRef]
- Zapletal, M.; Juráň, S.; Krpeš, V.; Michna, K.; Edwards, M.; Cudlín, P. Effect of Ozone Flux on Selected Structural and Antioxidant Characteristics of a Mountain Norway Spruce Forest. Balt. For. 2018, 24, 261–267. [Google Scholar]
- Peng, S.; Jin, Y.Z.; Chen, Y.Q.; Wu, C.M.; Wang, Y.J.; Wang, X.W.; Jin, Q.J.; Xu, Y.C. Growth response, enrichment effect, and physiological response of different garden plants under combined stress of polycyclic aromatic hydrocarbons and heavy metals. Coatings 2022, 12, 1054. [Google Scholar] [CrossRef]
- Xu, X.K.; Shen, R.X.; Mo, L.Q.; Yang, X.F.; Chen, X.; Wang, H.X.; Li, Y.D.; Hu, C.F.; Lei, B.F.; Zhang, X.J.; et al. Improving plant photosynthesis through light-harvesting upconversion nanoparticles. ACS Nano 2022, 16, 18027–18037. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.L. Physiological Responses of Populus alba and Syringa oblata to Ozone and Drought Stress. Ph.D. Thesis, Southwest Forestry University, Kun Ming, China, 2017. [Google Scholar]
- Tao, S.Q.; Zhang, Y.X.; Tian, C.M.; Duplessis, S.; Zhang, N.L. Elevated ozone concentration and nitrogen addition increase poplar rust severity by shifting the phyllosphere microbial community. J. Fungi 2022, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.C.; Xu, S.; Fu, W.; He, X.Y.; Chen, W.; Ma, C.L.; Li, Y.; Wu, X. Effect of elevated ozone concentration and drought on photosynthesis physiology of Lilac. Jiangsu. J. Agric. Sci. 2019, 47, 186–190. [Google Scholar] [CrossRef]
- Zhang, W.P. Design of urban garden landscape visualization system based on GIS and remote sensing technology. Comput. Intell. Neurosci. 2022, 2022, 9592376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).