Abstract
The carbon cycle within a terrestrial ecosystem is a pivotal functional process that drives ecosystem evolution, and the precipitation pattern variations exert a profound influence on it. To comprehensively assess the response of carbon release in the global terrestrial ecosystem to water variation, we performed a global meta-analysis by extracting data from 144 publications. Additionally, we incorporated various moderators to elucidate the heterogeneity observed in the data. The results showed that soil carbon release was highly sensitive to water variation, with drying and moisturizing treatments responding differently to water variability. Specifically, drought inhibited the soil carbon release of terrestrial ecosystems (24% reduction in effect size), but precipitation promoted it (11% increase in effect size). Moreover, this sensitivity could be affected by other ambient factors, depending on water manipulation (drying or moisturizing treatment). In moisturizing treatment cases, ambient precipitation, altitude, and vegetation type more or less affected the sensitivity of soil carbon release to a water increase. However, in drying treatment cases, these factors had no significant influence on the water sensitivity of soil carbon release. Unlike the above ambient factors, a temperature increase strengthened this sensitivity in both of the treatments. In addition, our study also showed that the response of carbon release to water variation did not depend on the substrate type or the carbon–nitrogen ratio (C/N) of the substrates, revealing that these effect factors on carbon release on the local scale could be overshadowed by water conditions. Overall, water variation positively affected soil carbon release on the global scale. Particularly, drought had a strong controlling effect on carbon release over the other environmental factors. Therefore, the impact of soil water loss on carbon release should be of great concern for the management of ecosystems and the prediction of carbon release models, especially when high temperatures and drought have been occurring more and more frequently on the planet in recent years.
1. Introduction
In recent decades, the global pattern of precipitation has undergone substantial changes, resulting in the increased variation of precipitation distribution on both spatial and temporal scales [1,2,3]. Extreme precipitation events can directly cause insufficient soil moisture or oversaturation, leading to either soil drought or waterlogging, respectively [4]. Notably, several atmospheric circulation models also predict that the future precipitation variation regime will continue to strengthen globally [5,6]. These variations can sensitively affect terrestrial carbon cycling, which may ultimately impact the ecosystem structure and function [7,8]. As one of the largest fluxes in the global carbon cycle, soil carbon release plays a vitally important role in regulating the atmospheric CO2 concentration and supporting the carbon balance of soil ecosystems [9]. Consequently, investigating the consequences of altered precipitation patterns on soil carbon release will significantly advance our comprehension of the potential impact of global changes on the structure and function of ecosystems.
Changes in precipitation patterns impact not only abiotic soil processes, such as soil water availability, infiltration, and leaching [9,10,11], but also biotic processes, including soil microbial communities, plant growth, and soil enzyme activities. Both abiotic and biotic factors are highly important to soil carbon release [12,13]. Specifically, altered precipitation regimes can noticeably change the effects of the water supply on soil or litter substrate leaching and the activities of microbes and plant roots, as well as indirectly, through effects on the chemical components of the soil or litter [14,15,16]. Though the effect of water content on soil respiration is not always linear, and sometimes is logarithmic, quadratic, or parabolic, it is generally believed that soil respiration is significantly higher in moist soil than in dry soil, and can decrease from wet to dry seasons, because of the water demand of microbe or plant metabolism [17,18]. For example, after precipitation, soil water can stimulate microbes to quickly decompose organic substrates for metabolism, and, thus, lots of carbon dioxide is released from the soil to the air [19,20]. Generally, sufficient soil moisture can accelerate the metabolism rate of microbes and plants, but drought can inhibit this process [4,21,22]. Also, climatic factors can dominate many ecological processes on a global scale [23]. Therefore, we propose our first hypothesis that, despite the frequent occurrence of extreme precipitation events, moisturizing can still positively affect soil carbon release globally, i.e., drought can inhibit carbon release while moisturizing can improve the process. Furthermore, in comparison with moisturizing, drought can generally bring about a stronger lethal effect and stay for longer. Moreover, the latest research highlights that drought stress can induce autophagy and programmed cell death [24]. This impact will permanently and irreversibly shift the succession trend of the microbiome as drought stress is prolonged [25,26,27]. As a result, drought can exert stronger stress on soil carbon release than moisturizing.
Moisture manipulations provide a powerful approach for understanding how water availability affects soil carbon release under in situ field conditions. Up to now, numerous studies have attempted to explain the response of carbon release to water variation from various aspects, such as soil respiration, litter decomposition, and soil organic matter decomposition [28,29,30]. Nevertheless, individual moisture manipulations have shown conflicting responses to carbon release, either for increasing or reducing water treatments. This may be due to ecological heterogeneity that refers to soil texture, microbial composition, vegetation, substrate, climate, etc. [31,32,33]. Hence, it is of utmost importance to conduct a comprehensive and large-scale synthesis of soil carbon cycles to an altered precipitation pattern. Recent research has suggested that the response of soil organic carbon decomposition to intensified water variability is co-determined by the microbial communities and aggregate changes [34], and the biotic and abiotic factors can affect the responses of carbon release to water availability in different ecosystems or at different scales [35]. Therefore, we put forward our second hypothesis that some ambient factors can affect the sensitivity of soil carbon release to water availability on a global scale.
In this study, we addressed the following questions using meta-analysis techniques to integrate the currently available global data on the above information: (1) Is soil carbon release sensitive to drought or moisturizing on the global scale? and (2) How do ambient factors affect the sensitivity of carbon release to water variation? This work will provide strong data support for evaluating the effect of water variation on the carbon cycle of terrestrial ecosystems and will also provide valuable information for improving the predictions of carbon cycle models.
2. Materials and Methods
2.1. Data Collection and Selection
We searched published articles before May 2023 using China National Knowledge Infrastructure (CNKI, https://www.cnki.net/, accessed on 12 May 2023), Web of Science (https://www.webofscience.com/, accessed on 12 May 2023), Google Scholar (https://scholar.google.com/, accessed on 12 May 2023), Springer (https://www.springer.com/cn, accessed on 12 May 2023), Wiley (https://onlinelibrary.wiley.com/, accessed on 12 May 2023), Elsevier (https://service.elsevier.com/, accessed on 12 May 2023), and other Chinese or English databases. The keywords and phrases for searching the literature were as follows: rainfall/water/moisture changes, drying, watering, moisturizing, enhance/exclude/reduce rainfall (precipitation), alter/change rainfall (precipitation), litter decomposition, soil respiration, and soil carbon release/flux/efflux/emission. Finally, we retrieved 2304 related studies and identified 144 published studies. The study selection process (flow diagram) is shown in Figure S1. All of the articles were selected based on the following criteria: (1) the experiments included the data of a treatment group (simulated drying/moisturizing) and control group; (2) the experiments were carried out only in field sites; (3) the target variables were measured under a drying or moisturizing treatment and compared to the same control; and (4) the means, standard deviations, and sample sizes were reported or could be determined.
Then, we extracted the means, standard deviations, and sample sizes for each target variable from the selected articles. The data presented in figures were digitized using Web Plot Digitizer [36]. We also recorded the longitude, latitude, altitude, and mean annual precipitation (MAP) and mean annual temperature (MAT) of each experiment site, vegetation type, and carbon–nitrogen ratio (C/N) of substrates. When the information on climate data of the experimental site were not provided in a study case, we retrieved them through the global climate database (https://www.worldclim.org/, accessed on 29 May 2023) according to the longitude and latitude coordinate information of the experimental sites.
According to the experimental design, we grouped the studies into drying or moisturizing treatments, and a meta-analysis was conducted for them, respectively. Among them, there are 379 cases for drying treatment and 328 cases for moisturizing treatment (Supplementary Information Table S1). The distribution of selected field sites is shown in Figure 1.
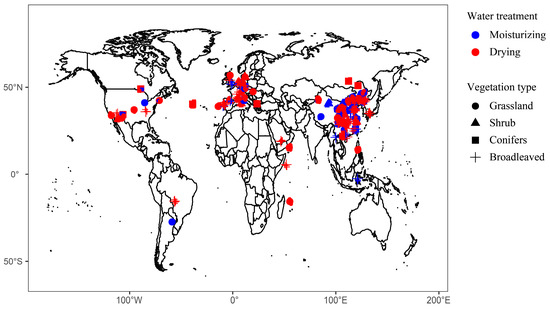
Figure 1.
Geographical location of the 707 study cases included in this meta-analysis.
At the same time, for analyzing the difference in effect size among rainbands, the arid, semi-arid, semi-humid, and humid zones were distinguished from the study cases according to the mean annual precipitation (Table S2) [37,38].
2.2. Statistical Analyses
We used a meta-analysis to determine the effect of precipitation on the carbon release from terrestrial ecosystems [39,40,41]. A response ratio (RR) was used to estimate the effect magnitude for each individual observation, in which RR and its variance (Vi) were calculated as follows:
where and represent the mean value of the experimental group and the control group, respectively. and represent the standard deviation of the experimental group and the control group, respectively. and represent the sample size of the experimental group and the control group, respectively.
The random effects model was used to analyze the data, and the total variance of a single study case () was calculated as follows:
where is the inter-study variance caused by different study cases.
The weight of a single study case () was calculated as follows:
The cumulative effect size () was calculated as follows:
The overall standard error (SE) was calculated as follows:
The confidence interval (CI, 95%) of the cumulative effect size was calculated as follows:
The effect of a treatment was deemed significant if the 95% CI did not overlap with zero. It showed a positive effect when it was greater than zero.
We used the R 4.0.2 with “metafor” [42], “ggplot2” [43], “glmulti” [44], and “piecewiseSEM” [45] packages to perform the statistical analyses and graphical presentation. First, we calculated the cumulative effect size by response ratio and identified the overall heterogeneity of the effect size by the random effect model with 95% CI. If the heterogeneity was very strong (Q is high, and p < 0.05), moderators were introduced into the model to explain it. We used the mixed-effect model to analyze the influence of the other variables on the effect size. For the categorical variables, the manipulation type (drying vs. moisturizing), rainbands (arid, semi-arid, semi-humid, and humid zones were zoned by the precipitation of study site), vegetation type (grassland, shrub, conifers, and broadleaves were categorized based on their morphological characteristics, and represent different stages of ecosystem succession), and substrate type (soil vs. litter were divided according to the source of soil carbon release) were taken into account. For the continuous variables, the C/N of the substrate and the environmental factors (MAP, MAT, and altitude) were introduced into the analyses. We used the piecewise structural equation model to analyze the causal relationship between the variables, in which we treated cases as random effects. Lastly, we carried out the analysis of publication bias using a fail safe number [46].
3. Results
We finally identified 144 published studies that utilized rain-out shelters to reduce or exclude rainfall or simulate increased precipitation. These studies covered 4 distinct rainbands, 4 different vegetation types, and 2 substrate types, from which we extracted 707 independent observations. The analysis of publication bias showed that the Rosenthal fail safe number was 78,717, which was larger than 3545 (the critical value of the fail safe number calculated by 5K + 10, where K is the observation case number). This result suggested that there was no publication bias in the data.
3.1. The Influence of Categorical Variables on Effect Size
The result of the random effect model showed that drying treatment significantly reduced the effect size by an average of 24.0%, while moisturizing treatment significantly increased it by an average of 11.0% (Figure 2), indicating a clear pattern of carbon release along the direction of water variation. The random effect model also showed a strong heterogeneity among the study cases for both the drying treatment group (Q(df=378) = 15,194.99, p < 0.0001) and the moisturizing treatment group (Q(df=327) = 21,507.01, p < 0.0001). Thus, to further explain this heterogeneity, it was necessary to introduce other moderators into the random effect model.
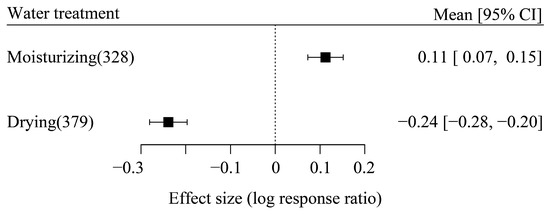
Figure 2.
The influence of water variation on effect size.
Climate is believed to be one of the dominant factors controlling the ecological process at the global scale; therefore, ambient precipitation was used as categorical data (named as rainbands) to check the difference in the effect size among local ranges of precipitation. The effect sizes of semi-arid, semi-humid, and humid areas in the drying treatment were all significantly different from 0 (p < 0.05, Figure 3a), however, the drying treatment did not significantly affect the carbon release of the arid area (p > 0.05, Figure 3a). In the moisturizing treatment, the effect sizes of arid, semi-arid, and semi-humid areas showed a significant difference from 0 (p < 0.05, Figure 3b), however, moisturizing had no significant effect on the carbon release in humid regions (p > 0.05, Figure 3b). Figure 3 also shows that only the arid area had a significantly higher effect size than the other rainbands for the drying treatment group; however, an increasing pattern in the effect sizes from the humid regions (−0.01 of average) to the arid area (0.32 of average) was found in the moisturizing treatment group.
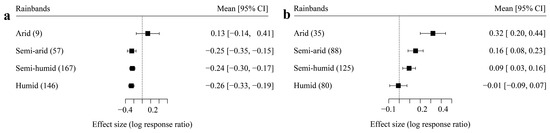
Figure 3.
The influence of rainbands on effect size ((a) drying treatment and (b) moisturizing treatment).
The vegetation type is also considered to drive the variation of ecological function. Figure 4 shows that there was no significant difference among the four vegetation types in drying treatment. However, moisturizing treatment amplified the variation of the effect size across the vegetation types, especially for the shrub, which presented a significantly higher effect size than the other vegetation types.
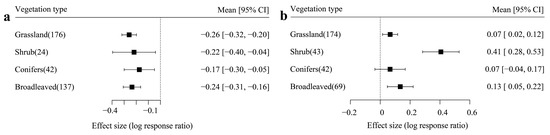
Figure 4.
The influence of different vegetation types on effect size ((a) drying treatment and (b) moisturizing treatment).
Litter and soil have different organic chemical components and are an important source of carbon release from forest floors. Figure 5 shows that soil had a marginally lower mean effect size than litter, but no statistical significance of difference, either in drying or moisturizing treatments.

Figure 5.
The influence of different substrate types on effect size ((a) drying treatment and (b) moisturizing treatment).
3.2. The Influence of Continuous Variables on Effect Size
The ambient precipitation of study case sites for moisturizing treatment showed an overall negative correlation with the effect sizes, in which precipitation of less than about 1428.2 mm exerted a positive effect on carbon release, while, when precipitation was more than 1428.2 mm, the opposite was true (Figure 6a). This negative correlation was partly from the contribution of study cases from the humid area of the rainbands that showed a slight inhibiting effect on carbon release (see Figure 3b). However, for the drying treatment, there was no significant correlation between the ambient precipitation and the effect size (Figure 6a).
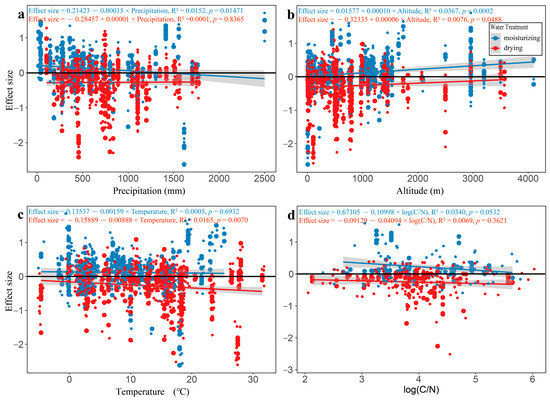
Figure 6.
Relationships between the effect size of soil carbon release and different factors ((a) precipitation; (b) altitude; (c) temperature; and (d) logarithm of C/N based on 10).
The altitude of the study sites exhibited a significantly positive correlation with the effect size for both moisturizing and drying treatments (Figure 6b).
Contrary to the altitude, the temperature of the study sites showed a negative correlation with the effect size for both treatments, of which temperature had a significant effect on carbon release only in drying treatment (Figure 6c).
Substrate C/N was found to have a negative correlation with effect size, but no significant statistics for either moisturizing or drying treatments (Figure 6d).
3.3. Structural Equation Model Analysis
In order to further explore the causal relationship between variables and the relative importance of effect size, structural equation models were fitted for drying and moisturizing treatments, respectively (Figure 7). Given that the number of cases with C/N data in this study was only 205, which was about 29% of the total cases, this variable was not included in the structural equation model.
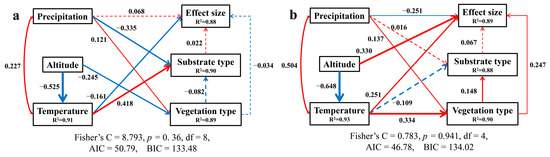
Figure 7.
Structural equation model of effect size interactions with altitude, temperature (mean annual temperature), precipitation (mean annual precipitation), and vegetation type (Solid arrows indicate notable effect sizes, p < 0.05, dashed lines p > 0.05, where the thickness of the arrow represents the strength of the relationship. Red and blue lines indicate positive and negative relationships, respectively ((a) drying treatment and (b) moisturizing treatment).
For the drying treatment, among all of the predictive variables, only the ambient temperature of study cases showed a significant negative effect, which was consistent with the single factor effect result mentioned above. There was no significant causal relationship between the ambient precipitation, altitude, and vegetation type with the effect size (Figure 7a). For the moisturizing treatment, contrary to the single factor effect, temperature showed a significant positive contribution to the effect size. Both altitude and vegetation type also exhibited similar contributions to the effect size as temperature. However, precipitation presented a significant negative influence on the effect size (Figure 7b). In both drying and moisturizing treatments, the substrate type had no significant influence on the effect size in structural equation models like the single factor effect described above.
4. Discussion
Our results from this meta-analysis clearly showed an overall positive sensitivity of soil carbon release to water variation. In simpler terms, our findings support the notion that drought tends to inhibit soil ecosystem carbon release while moisture promotes it, which is line with previous views [47,48,49]. Carbon release is typically represented by soil respiration through the soil surface from autotrophic root respiration and heterotrophic microbial respiration, which is associated with the decomposition of litter, roots, and soil organic matter [41,50,51,52]. Numerous studies have clearly demonstrated the importance of water content as a factor influencing soil respiration [9,53]. As the primary gatekeepers for carbon loss, the adaptation of heterotrophic microbes to changes in water content is crucial for soil organic matter decomposition [54]. A few studies have shown that an increase in precipitation can improve the content of lignin, which is the recalcitrant of litter decomposition [18,19]; however, more studies have shown that an increase in water can facilitate the leaching of the soluble matter of litter [55,56,57], improve the activity of microbial decomposers [58,59], and activate the lignin-degrading enzyme, which contributes to litter decomposition [49]. Conversely, as the environment becomes drier, microbes tend to devote more resources to maintaining cellular penetration, resulting in a decrease in microbial carbon use efficiency. This also implies that, under drought conditions, microbes become less active and less efficient in the soil, which may result in a potential decrease in soil carbon storage [54,60]. Taken as whole, the increase in water stimulates heterotrophic respiration in the soil, and drought can suppress it. Plant root autotrophic respiration also presents the same response to water shift as heterotrophic respiration [61,62,63], although it is generally believed that the response of soil microbes to precipitation is more sensitive than that of plants [38,64,65], given that the metabolism is different between plants and microbes [9]. Moreover, due to the difficulty in differentiating the components of soil respiration, autotrophic and heterotrophic respiration have not been separately measured in most study cases. Based on the above references, we believe that the differences in soil respiration components do not significantly affect the response of soil respiration to water variation in this meta-analysis.
The sensitivity of soil carbon release to water variation can often be affected by ambient factors, such as precipitation, temperature, and elevation. In this meta-analysis, ambient precipitation did not significantly influence the overall inhibitory effect of soil carbon release by drying; however, the promotion effect of carbon release by moisturizing was dependent on ambient precipitation. Wang et al. [66] also proposed that the original MAP partially determined the responses of the soil carbon cycles to altered precipitation. These results indicated that drought had a stronger controlling effect on the ecological process over other ecological factors, relative to precipitation, and the tolerance of an ecosystem to drought was lower than that of moisturizing [67]. However, despite these findings, soil carbon release in the arid rainband was less sensitive to drought. Similarly, carbon release in the humid rainband was also less sensitive to precipitation. This was probably due to the fact that the microbial decomposers and plants had already adapted to the local extreme drought or humid environmental conditions by altering their physiological properties, and, thus, a further strengthening of increasing or reducing precipitation did not significantly alter the local ecological processes [68,69,70]. Conversely, soil carbon release in the humid ecosystems was more sensitive to drought, while the arid ecosystems were more sensitive to precipitation [56]. The underlying mechanism is believed to be that both water manipulations make the living conditions of microbes and plants close to their optimum niche [66].
Similar to ambient precipitation, elevation also did not show a significant correlation with the sensitivity of carbon release to drought, suggesting that drought had a strong controlling effect on carbon release over altitude. However, unlike drought, the sensitivity of carbon release to precipitation was increased with elevation. This contradicts previous finding that indicate soil respiration can be limited due to low temperature with the rising of elevation. In fact, the stress induced by low temperatures was mainly due to the lack of water availability, which further limits root respiration and microbial decomposition [71]. In our meta-analysis, at the sites at an elevation of 3000~4000 m, the temperature was lower than 2 °C, or even below 0 °C. Öquist et al. [72] believed that the CO2 production response of frozen soils was attributed to water restriction induced by a partial phase transition of water, rather than a biochemical temperature response. Other studies have also reported that soil respiration was primarily regulated by soil moisture or precipitation rather than soil temperature in alpine or arctic ecosystems [73,74]. However, an increase in water can effectively relieve this stress, and even greatly activate soil respiration (also named “Birch effect”) [75,76]. Meisner et al. [77] also reported that soil subjected to greater drought-induced stress exhibited a higher respiration rate upon rewetting.
In addition, like ambient precipitation, temperature is another crucial climatic factor affecting soil carbon release [78,79]. Elevated temperature increased the inhibition of carbon release by drought in this meta-analysis, because that elevated temperature strengthened the drought, which inhibited the activities of the microbes and plants [80,81]. However, the elevated temperature, along with water addition, accelerated the soil carbon release. Many studies have reported that the temperature sensitivity of ecosystems was dependent on soil moisture levels [9,72,82]. As with most primarily biological reactions, soil respiration greatly depends on temperature and adequate water availability, and, thus, increasing temperature and suitable moisture content can be in favor of soil carbon release [83,84,85,86,87].
At the ecosystem scale, soil carbon and hydrological processes are closely interconnected [88]. Under different vegetation types, the soil texture, litter quality, plant species, microbial community, and microhabitat factors may exhibit significant variations due to their management or rainfed regimes [38,89,90,91,92,93,94]. Consequently, analyzing the influence of vegetation types is vitally important in the context of vegetation regeneration and increasingly severe climate change effects. Consistent with the other environmental factors mentioned above, the response of soil carbon release to drought showed no obvious variation across the vegetation types, which further verified that drought had a more potent controlling effect on carbon release over the other environmental factors. However, the promotion of carbon release by precipitation contingent on the vegetation type; and notably, the response of carbon release to precipitation was more pronounced in the shrub group compared to the other vegetation types. Generally, it is believed that grass litter is less recalcitrant relative to shrub or tree litter, resulting in a faster decomposition rate because woody plants store more carbon as lignin [95]. However, soil respiration is usually found to be lower in grasslands than in shrublands or forests [96,97]. Relative to grasslands, the ground cover and overstory vegetation from shrub ecosystems can affect soil respiration through changes in rhizosphere conditions, soil temperature, soil moisture, and substrate quantity and quality [48]. Ground cover, such as lichens and mosses, tends to adjust soil temperatures to a suitable value for microbial respiration, regardless of seasonal and diurnal changes [97,98,99]. The increase in aboveground vegetation can also improve root respiration through changes in root biomass, productivity, and turnover [100]. In addition, the clustered microhabitat resulting from the scattered distribution of shrubs can easily become a “fertile island,” which can effectively keep microbial and plant root respiration stable [101,102]. Precipitation can reinforce the advantages of the shrub ecosystem in terms of soil respiration. In contrast to coniferous and broadleaf forests, shrubland is characterized by fast-growth plant species, and, thus, the plant litter tends to be formed from readily-decomposable organic components [95]. In addition, due to the rapid growth of the plants in the shrubland, the metabolic rate of the roots is also high. Therefore, soil environments with suitable water conditions can favor soil respiration under shrubs [103].
Substrate type and quality are believed to be important factors in controlling carbon release, particularly for soil microbial respiration [104]. Soil and litter are the two important substrate types of forest ground. The carbon release from the litter layer is driven by microbes, while the soil layer includes respiration from the microbes and plant roots. The driving force for carbon release is different between the two substrate types, but there is no significant difference in carbon release between them. Although the dependency on substrate availability of soil respiration is noticed in field studies, when averaged on the global scale, heterotrophic respiration accounts for 54% of soil respiration in forests [105,106]. Therefore, the above observed result is possible. In addition, C/N, one of the traits characterizing the substrate quality, is still believed to be an important affecting factor controlling microbial respiration by changing substrate availability and enzymatic activities or root respiration by changing belowground carbon allocation [107,108,109,110,111,112]. However, this meta-analysis also showed that C/N had no significant effect on the carbon release from soil. These results have indicated that the effects of substrate type and quality on soil respiration could be overshadowed by precipitation. Many studies have also shown that, on a global scale, climatic factors had an important controlling effect on soil respiration over other factors [113,114], because climatic factors can not only immediately affect the activities of microbes and plant roots, but can also chronically alter the community composition of the vegetation and the chemical components of individual plants [28].
5. Conclusions
Overall, our global meta-analysis showed the following: (1) drought inhibited soil carbon release, while moisturizing accelerated carbon release; (2) the stress of the ecological process caused by drought was barely relieved by other environmental or ecological factors, compared to moisturizing; (3) shrubland soil carbon release is particularly pronounced with moisturizing; and (4) soil carbon release in arid areas is more sensitive to moisturizing, while humid areas are more sensitive to drought. These findings should be incorporated into terrestrial ecosystem carbon cycling models in order to reasonably predict the global climate-change-induced soil carbon emissions.
Based on the findings of this global meta-analysis, the relative role of drought and the corresponding factors that can strengthen drought should be of particular concern when estimating the effects of climate change on carbon balance for policy makers. Furthermore, it is crucial to prioritize regions with a high sensitivity of soil carbon emissions to changes in moisture levels, both in arid and humid zones, when assessing regional soil carbon dynamics. Additionally, considering that some environmental factors in our meta-analysis exhibited significant buffering effects on water stress, it is advisable to incorporate these influencing effects of drought or waterlogging on ecosystems caused by precipitation stress. Last but not least, while we have already considered factors such as vegetation type, substrate type, and ambient conditions, it is important to acknowledge the existence of other variables that may impact carbon release, beyond those mentioned above. These factors, such as soil microbes, soil texture, land use, and others, should be integrated into predictive models in order to facilitate the formulation of more effective policies and remedial measures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14101957/s1. Table S1: Information list of the literature selected in this study. Table S2: Classification of rainbands. Figure S1: Flow diagram of the literature search and study selection process.
Author Contributions
Conceptualization, X.H. and J.X.; software, J.X. and Y.L.; formal analysis, X.H., J.X. and Y.L.; data curation, X.H., J.X. and Y.L.; writing—original draft preparation, X.H. and J.X.; writing—review and editing, X.H., Z.H. and X.K.; visualization, J.X.; supervision, X.H. and Y.L.; projection administration, X.H. and Y.L.; funding acquisition, X.H., Y.L., Z.H. and X.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers 32060332, 31670624, and 31560205), the Key Program of Scientific Research Project of Hunan Provincial Education Department (21AO334), and the General Program of Scientific Research Project of Hunan Provincial Education Department (22CO278).
Data Availability Statement
The datasets in this study are available in Supplementary Materials Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, R.; Zhang, J.Q.; Guo, E.L.; Zhao, C.L.; Cao, T.H. Spatial and temporal variations of precipitation concentration and their relationships with large-scale atmospheric circulations across Northeast China. Atmos. Res. 2019, 222, 62–73. [Google Scholar] [CrossRef]
- Fu, S.J.; Zhang, H.L.; Zhong, Q.; Chen, Q.G.; Liu, A.; Yang, J.; Pang, J.Z. Spatiotemporal variations of precipitation concentration influenced by large-scale climatic factors and potential links to flood-drought events across China 1958–2019. Atmos. Res. 2023, 282, 106507. [Google Scholar] [CrossRef]
- Rahman, M.S.; Senkbeil, J.C.; Keellings, D.J. Spatial and temporal variability of extreme precipitation events in the Southeastern United States. Atmosphere 2023, 14, 1301. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.H.; Kim, D.G.; Li, J.W.; Liu, Y.L.; Hai, X.Y.; Liu, Q.Y.; Huang, C.B.; Shangguan, Z.P.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Allan, R.P.; Soden, B.J. Atmospheric warming and the amplification of precipitation extremes. Science 2008, 321, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Working Group I Contribution of to the IPCC Fifth Assessment Report, Climate Change in 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.F.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Marschner, P.; Zheng, B. Direction and magnitude of the change in water content between two periods influence soil respiration, microbial biomass and nutrient availability which can be modified by intermittent air-drying. Soil Biol. Biochem. 2022, 166, 108559. [Google Scholar] [CrossRef]
- Gan, H.J.; Roper, W.R.; Groffman, P.M.; Morris, T.F.; Guillard, K. Automated sensor-based quantification of soil water retention and microbial respiration across drying conditions. Soil Biol. Biochem. 2023, 180, 108987. [Google Scholar] [CrossRef]
- Luo, Z.K.; Feng, W.T.; Luo, Y.Q.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.L.; Griffin, R.W.; Fares, A.; Elhassan, A.; Awal, R.; Woldesenbet, S.; Risch, E. Soil CO2 emission in response to organic amendments, temperature, and rainfall. Sci. Rep. 2020, 10, 5849. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Vitousek, P.M. Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J. Ecol. 2000, 88, 129–138. [Google Scholar] [CrossRef]
- Li, X.F.; Han, S.J.; Zhang, Y. Indirect effects of precipitation on litter decomposition of Quercus mongolica. Chin. J. Appl. Ecol. 2007, 18, 261–266. Available online: http://www.cjae.net/CN/abstract/abstract166.shtml (accessed on 9 July 2023).
- Huo, L.X.; Hong, M.; Zhao, B.; Gao, H.Y.; Ye, H. Effects of increased nitrogen deposition and changing rainfall patterns on litter decomposition in a desert grassland. Acta Ecol. Sin. 2019, 39, 2139–2146. [Google Scholar] [CrossRef]
- Cavelier, J.; Peñuela, M.C. Soil respiration in the cloud forest and dry deciduous forest of Serrania de Macuira, Colombia. Biotropica 1990, 22, 346–352. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Cattânio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef]
- Bian, H.F.; Li, C.; Zhu, J.X.; Xu, L.; Li, M.X.; Zheng, S.; He, N.P. Soil moisture affects the rapid response of microbes to labile organic C addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Rodríguez, A.; Durán, J.; Yuste, J.C.; Valladares, F.; Rey, A. The effect of tree decline over soil water content largely controls soil respiration dynamics in a Mediterranean woodland. Agric. For. Meteorol. 2023, 333, 109398. [Google Scholar] [CrossRef]
- Li, Y.B.; Cui, D.Z.; Sui, X.X.; Huang, C.; Huang, C.Y.; Fan, Q.Q.; Chu, X.S. Autophagic survival precedes programmed cell death in wheat seedlings exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 5777. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medellín, C.; Liechty, Z.; Edwards, J.; Nguyen, B.; Huang, B.; Weimer, B.C.; Sundaresan, V. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat. Plants 2021, 7, 1065–1077. [Google Scholar] [CrossRef]
- Li, W.T.; Pacheco-Labrador, J.; Migliavacca, M.; Miralles, D.; van Dijke, A.H.; Reichstein, M.; Forkel, M.; Zhang, W.; Frankenberg, C.; Panwar, A.; et al. Widespread and complex drought effects on vegetation physiology inferred from space. Nat. Commun. 2023, 14, 4640. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef]
- Almagro, M.; López, J.; Querejeta, J.I.; Martínez-Mena, M. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol. Biochem. 2009, 41, 594–605. [Google Scholar] [CrossRef]
- Yu, S.Q.; Mo, Q.F.; Li, Y.W.; Li, Y.X.; Zou, B.; Xia, H.P.; Li, Z.A.; Wang, F.M. Changes in seasonal precipitation distribution but not annual amount affect litter decomposition in a secondary tropical forest. Ecol. Evol. 2019, 9, 11344–11352. [Google Scholar] [CrossRef]
- Nahdia, Y.T.; Paembonan, S.A. Cacao leaf litter decomposition under different moisture and pH: Characteristic of soil C mineralization (NH4+ and NO3–) and greenhouse gas CO2, CH4, N2O flux emission. IOP Conf. Ser. Earth Environ. Sci. 2020, 473, 012096. [Google Scholar] [CrossRef]
- Patel, K.F.; Fansler, S.J.; Campbell, T.P.; Bond-Lamberty, B.; Smith, A.P.; RoyChowdhury, T.; McCue, L.A.; Varga, T.; Bailey, V.L. Soil texture and environmental conditions influence the biogeochemical responses of soils to drought and flooding. Commun. Earth Environ. 2021, 2, 127. [Google Scholar] [CrossRef]
- Heidrich, L.; Bae, S.; Levick, S.; Seibold, S.; Weisser, W.; Krzystek, P.; Magdon, P.; Nauss, T.; Schall, P.; Serebryanyk, A.; et al. Heterogeneity–diversity relationships differ between and within trophic levels in temperate forests. Nat. Ecol. Evol. 2020, 4, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Deák, B.; Kovács, B.; Rádai, Z.; Apostolova, I.; Kelemen, A.; Kiss, R.; Lukács, K.; Palpurina, S.; Sopotlieva, D.; Báthori, F.; et al. Linking environmental heterogeneity and plant diversity: The ecological role of small natural features in homogeneous landscapes. Sci. Total Environ. 2021, 763, 144199. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Huo, T.C.; Zhang, Y.W.; Guo, T.T.; Liang, J.Y. Response of soil organic carbon decomposition to intensified water variability co-determined by the microbial community and aggregate changes in a temperate grassland soil of northern China. Soil Biol. Biochem. 2023, 176, 108875. [Google Scholar] [CrossRef]
- Ladau, J.; Eloe-Fadrosh, E.A. Spatial, temporal, and phylogenetic scales of microbial ecology. Trends Microbiol. 2019, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating data from figures with a web-based program: Considerations for a systematic review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Bian, J.J.; Ge, Q.S.; Yin, Y.H. The climate regionalization in China for 1951-1980 and 1981-2010. Geogr. Res. 2013, 32, 987–997. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, W.J.; Chen, S.P.; Tan, X.R.; Wang, S.S.; Chen, M.L.; Ren, T.T.; Xia, J.Y.; Huang, J.H.; Han, X.G. Changing precipitation exerts greater influence on soil heterotrophic than autotrophic respiration in a semiarid steppe. Agric. For. Meteorol. 2019, 271, 413–421. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.G.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Liao, C.Z.; Peng, R.H.; Luo, Y.Q.; Zhou, X.H.; Wu, X.W.; Fang, C.M.; Chen, J.K.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. N. Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Feng, J.F.; Wang, J.S.; Ding, L.B.; Yao, P.P.; Qiao, M.P.; Yao, S.C. Meta-analyses of the effects of major global change drivers on soil respiration across China. Atmos. Environ. 2017, 150, 181–186. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Calcagno, V.; Mazancourt, C.D. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Lin, L.F.; Chu, H.T.; Murad, M.H.; Hong, C.; Qu, Z.Y.; Cole, S.R.; Chen, Y. Empirical comparison of publication bias tests in meta-analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.; Táncsics, A.; Kriszt, B.; Kröel-Dulay, G.; Ónodi, G.; Hornung, E. Extreme effects of drought on composition of the soil bacterial community and decomposition of plant tissue. Eur. J. Soil Sci. 2017, 68, 504–513. [Google Scholar] [CrossRef]
- Ge, X.G.; Tong, R.; Cao, Y.H.; Zhou, B.Z.; Xiao, W.F.; Wang, X.M.; Lu, R.F. Effect of litterfall input on soil respiration and its temperature sensitivity in moso bamboo forest under simulated drought. Chin. J. Appl. Ecol. 2018, 29, 2233–2242. [Google Scholar] [CrossRef]
- Gao, H.Y.; Hong, M.; Huo, L.X.; Ye, H.; Zhao, B.Y.; De, H.S. Effects of exogenous nitrogen input and water change on litter decomposition in a desert grassland. Chin. J. Appl. Ecol. 2018, 29, 3167–3174. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Jandl, R. Carbon losses due to soil warming: Do autotrophic and heterotrophic soil respiration respond equally? Glob. Chang. Biol. 2009, 15, 901–913. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.F.; Yin, L.; Ma, L.; Xu, C.; Chen, H.L.; Ma, T.; Su, Y.H.; Zhu, Y.T.; He, L.Y.; et al. Optimal soil water content and temperature sensitivity differ among heterotrophic and autotrophic respiration from oasis agroecosystems. Geoderma 2022, 425, 116071. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Dong, X.J.; Xu, B.X.; Chen, Y.L.; Zhao, Y.; Gao, Y.H.; Hu, Y.G.; Huang, L. Soil respiration sensitivities to water and temperature in a revegetated desert. J. Geophys. Res. Biogeosci. 2015, 120, 773–787. [Google Scholar] [CrossRef]
- Allison, S.D. Microbial drought resistance may destabilize soil carbon. Trends Microbiol. 2023, 8, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Vitousek, P.M. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Shang, Q.; Wang, L.; Tian, Y.; Ju, Y.X.; Gan, J.B. 2016. Responses of soil respiration to changing precipitation regimes in an oak forest at a climate transitional zone. Acta Ecol. Sin. 2016, 36, 8054–8061. [Google Scholar] [CrossRef][Green Version]
- Zhou, S.X.; Huang, C.D.; Xiang, Y.B.; Han, B.H.; Xiao, Y.X.; Tang, J.D. Effects of nitrogen deposition and precipitation change on soil respiration in natural evergreen broadleaved forest in the rainy area of western China. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2017, 45, 94–101+110. Available online: http://www.xnxbz.net/xbnlkjdxzr/ch/reader/view_abstract.aspx?file_no=20170414&flag=1 (accessed on 12 July 2023).
- Osono, T.; Takeda, H. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 2006, 98, 172–179. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Chen, L.Q. The role of endophytic fungal individuals and communities in the decomposition of Pinus massionana needle litter. PLoS ONE 2014, 9, 105911. [Google Scholar] [CrossRef]
- Chen, Y.L.; Du, Z.L.; Weng, Z.; Sun, K.; Zhang, Y.Q.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.B.; Luo, Y.; et al. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Chang. Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Palta, J.A.; Nobel, P.S. Root respiration for Agave deserti: Influence of temperature, water status and root age on daily patterns. J. Exp. Bot. 1989, 40, 181–186. [Google Scholar] [CrossRef]
- Shi, W.Y.; Tateno, R.; Zhang, J.G.; Wang, Y.L.; Yamanaka, N.; Du, S. Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest-grassland transition zone of the Loess Plateau. Agric. For. Meteorol. 2011, 151, 854–863. [Google Scholar] [CrossRef]
- Illeris, L.; Michelsen, A.; Jonasson, S. Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 2003, 65, 15–29. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, Y.J.; Yan, G.Y.; Wang, Q.G. Response of fine roots to precipitation change: A meta-analysis. Chin. J. Plant Ecol. 2018, 42, 164–172. Available online: https://www.plant-ecology.com/CN/10.17521/cjpe.2017.0203 (accessed on 16 July 2023).
- Yu, C.L.; Hui, D.F.; Deng, Q.; Dzantor, E.K.; Fay, P.A.; Shen, W.J.; Luo, Y.Q. Responses of switchgrass soil respiration and its components to precipitation gradient in a mesocosm study. Plant Soil 2017, 420, 105–117. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.L.; Li, Y.; Zhang, H.; Yue, K.; Wang, X.C.; Ma, Y.D.; Chen, J.; Sun, M.; Chen, Z.; et al. Differential effects of altered precipitation regimes on soil carbon cycles in arid versus humid terrestrial ecosystems. Glob. Chang. Biol. 2021, 27, 6348–6362. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Y.X.; Wang, Y.J.; Fu, B.J. Greater increases in China’s dryland ecosystem vulnerability in drier conditions than in wetter conditions. J. Environ. Manage. 2021, 291, 112689. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Zhou, X.H.; Zhou, L.Y.; Nie, Y.Y.; Fu, Y.L.; Du, Z.G.; Shao, J.J.; Zheng, Z.M.; Wang, X.H. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016, 228, 70–81. [Google Scholar] [CrossRef]
- Ren, C.J.; Zhao, F.Z.; Shi, Z.; Chen, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Öquist, M.G.; Sparrman, T.; Klemedtsson, L.; Drotz, S.H.; Grip, H.; Schleucher, J.; Nilsson, M. Water availability controls microbial temperature responses in frozen soil CO2 production. Glob. Chang. Biol. 2009, 15, 2715–2722. [Google Scholar] [CrossRef]
- Li, C.B.; Peng, Y.F.; Nie, X.Q.; Yang, Y.H.; Yang, L.C.; Li, F.; Fang, K.; Xiao, Y.M.; Zhou, G.Y. Differential responses of heterotrophic and autotrophic respiration to nitrogen addition and precipitation changes in a Tibetan alpine steppe. Sci. Rep. 2018, 8, 16546. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Peng, Q.; Dong, Y.S.; He, Y.L.; Yan, Z.Q.; Guo, Y.; Qin, S.Q.; Qi, Y.C. Response of soil respiration to water and nitrogen addition and its influencing factors: A four-year field experiment in a temperate steppe. Plant Soil 2022, 471, 427–442. [Google Scholar] [CrossRef]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Norton, U.; Mosier, A.R.; Morgan, J.A.; Derner, J.D.; Ingram, L.J.; Stahl, P.D. Moisture pulses, trace gas emissions and soil C and N in cheatgrass and native grass-dominated sagebrush-steppe in Wyoming, USA. Soil Biol. Biochem. 2008, 40, 1421–1431. [Google Scholar] [CrossRef]
- Meisner, A.; Bååth, E.; Rousk, J. Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol. Biochem. 2013, 66, 188–192. [Google Scholar] [CrossRef]
- Zhu, E.X.; Cao, Z.J.; Jia, J.; Liu, C.Z.; Zhang, Z.H.; Wang, H.; Dai, G.H.; He, J.S.; Feng, X.J. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Chang. Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.C.; Ma, R.A.; Lin, J.J.; Kurganova, I.; Wang, X.G.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Priemé, A.; Nielsen, C.S.; Elberling, B.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2016, 23, 406–420. [Google Scholar] [CrossRef]
- Illeris, L.; Christensen, T.R.; Mastepanov, M. Moisture effects on temperature sensitivity of CO2 exchange in a subarctic heath ecosystem. Biogeochemistry 2004, 70, 315–330. [Google Scholar] [CrossRef]
- Welker, J.M.; Fahnestock, J.T.; Jones, M.H. Annual CO2 flux in dry and moist arctic tundra: Field responses to increases in summer temperatures and winter snow depth. Clim. Chang. 2000, 44, 139–150. [Google Scholar] [CrossRef]
- Atarashi-Andoh, M.; Koarashi, J.; Ishizuka, S.; Hirai, K. Seasonal patterns and control factors of CO2 effluxes from surface litter, soil organic carbon, and root-derived carbon estimated using radiocarbon signatures. Agric. For. Meteorol. 2012, 152, 149–158. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-term warming alters the composition of arctic soil microbial communities. FEMS Microbiol. Ecol. 2012, 82, 303–315. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, K.M.; Pold, G.; Topçuoğlu, B.D.; van Diepen, L.T.A.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Zang, Z.H.; Xie, Z.Q.; Chen, Q.S.; Xu, W.T.; Zhao, C.M.; Shen, G.Z. Soil respiration of four forests along elevation gradient in northern subtropical China. Ecol. Evol. 2019, 9, 12846–12857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, W.Q.; Feng, Y.J.; Mo, Q.F.; Su, Y.Q.; Njoroge, B.; Qu, C.; Gan, X.H.; Liu, X.D. Soil organic carbon primarily control the soil moisture characteristic during forest restoration in subtropical China. Front. Ecol. Evol. 2022, 10, 1003532. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Grogan, P. Cold season respiration across a low arctic landscape: The influence of vegetation type, snow depth, and interannual climatic variation. Arct. Antarct. Alp. Res. 2012, 44, 446–456. [Google Scholar] [CrossRef]
- Han, G.X.; Xing, Q.H.; Luo, Y.Q.; Rafique, R.; Yu, J.B.; Mikle, N. Vegetation types alter soil respiration and its temperature sensitivity at the field scale in an estuary wetland. PLoS ONE 2014, 9, 91182. [Google Scholar] [CrossRef]
- Grand, S.; Rubin, A.; Verrecchia, E.P.; Vittoz, P. Variation in soil respiration across soil and vegetation types in an Alpine valley. PLoS ONE 2016, 11, 0163968. [Google Scholar] [CrossRef]
- Shedayi, A.A.; Xu, M.; Naseer, I.; Khan, B. Altitudinal gradients of soil and vegetation carbon and nitrogen in a high altitude nature reserve of Karakoram ranges. SpringerPlus 2016, 5, 320. [Google Scholar] [CrossRef]
- Huang, N.; Wang, L.; Song, X.P.; Andrew Black, T.; Jassal, R.S.; Myneni, R.B.; Wu, C.Y.; Wang, L.; Song, W.J.; Ji, D.B.; et al. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 2020, 6, 8508. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Moyes, A.B.; Bowling, D.R. Plant community composition and phenological stage drive soil carbon cycling along a tree-meadow ecotone. Plant Soil 2016, 401, 231–242. [Google Scholar] [CrossRef]
- Thomas, A.D.; Elliott, D.R.; Dougill, A.J.; Stringer, L.C.; Hoon, S.R.; Sen, R. The influence of trees, shrubs, and grasses on microclimate, soil carbon, nitrogen, and CO2 efflux: Potential implications of shrub encroachment for Kalahari rangelands. Land Degrad. Dev. 2018, 29, 1306–1316. [Google Scholar] [CrossRef]
- Bewley, D.; Essery, R.; Pomeroy, J.; Ménard, C. Measurements and modelling of snowmelt and turbulent heat fluxes over shrub tundra. Hydrol. Earth Syst. Sci. 2010, 14, 1331–1340. [Google Scholar] [CrossRef]
- Blok, D.; Heijmans, M.M.P.D.; Schaepman-Strub, G.; Konono, A.V.; Maximov, T.C.; Berendse, F. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Glob. Chang. Biol. 2010, 16, 1296–1305. [Google Scholar] [CrossRef]
- Parker, T.C.; Subke, J.; Wookey, P.A. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob. Chang. Biol. 2015, 21, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Rajot, J.L.; Herbrig, C. Influence of shrubs on soil characteristics and their function in sahelian agro-ecosystems in semi-arid Niger. J. Arid Environ. 2000, 44, 383–398. [Google Scholar] [CrossRef]
- Qu, W.L.; Yang, X.P.; Zhang, C.T.; Wei, B. Shrub-mediated “fertile island” effects in arid and semi-arid grassland. Acta Prataculturae Sin. 2015, 24, 201–207. [Google Scholar] [CrossRef]
- Sun, Q.; Meyer, W.S.; Koerber, G.R.; Marschner, P. Response of respiration and nutrient availability to drying and rewetting in soil from a semi-arid woodland depends on vegetation patch and a recent wildfire. Biogeosciences 2015, 12, 5093–5101. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, W.P.; You, Y.; Ye, C.; Shu, X.; Zhang, Q.F.; Zhang, K.R. Soil properties and substrate quality determine the priming of soil organic carbon during vegetation succession. Plant Soil 2022, 471, 559–575. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Wei, H.; Chen, X.M.; Xiao, G.L.; Guenet, B.; Vicca, S.; Shen, W.J. Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Sci. Rep. 2015, 5, 18370. [Google Scholar] [CrossRef]
- Wang, H.; Button, T.W.; Xu, W.H.; Hu, G.Q.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef]
- Jing, H.; Liu, Y.; Wang, G.L.; Liu, G.B. Effects of nitrogen addition on root respiration of trees and understory herbs at different temperatures in Pinus tabulaeformis forest. Plant Soil 2021, 463, 447–459. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.X.; Hu, Y.; Zhang, M.Q.; Wang, G.D.; Shen, W.B. Soil microbial community and its interaction with soil carbon dynamics following a wetland drying process in Mu Us sandy land. Int. J. Environ. Res. Public Health 2020, 17, 4199–4217. [Google Scholar] [CrossRef]
- Huang, X.L.; Chen, J.Z.; Wang, D.; Deng, M.M.; Wu, M.Y.; Tong, B.L.; Liu, J.M. Simulated atmospheric nitrogen deposition inhibited the leaf litter decomposition of Cinnamomum migao H.W. Li in southwest China. Sci. Rep. 2021, 11, 1748. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.L.; Li, X.L.; Wang, S.L.; Liu, W.H.; Shi, S.L.; Cao, W.X. Nitrogen fertilizer regulates soil respiration by altering the organic carbon storage in root and topsoil in alpine meadow of the north-eastern Qinghai-Tibet Plateau. Sci. Rep. 2019, 9, 13735. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B. 1992, 44, 81–89. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).