Biotic Factors Drive Woody Plant Species Diversity across a Relative Density Gradient of Quercus aliena var. acuteserrata Maxim. in the Warm–Temperate Natural Oak Forest, Central China
Abstract
:1. Introduction
2. Materials and Methods
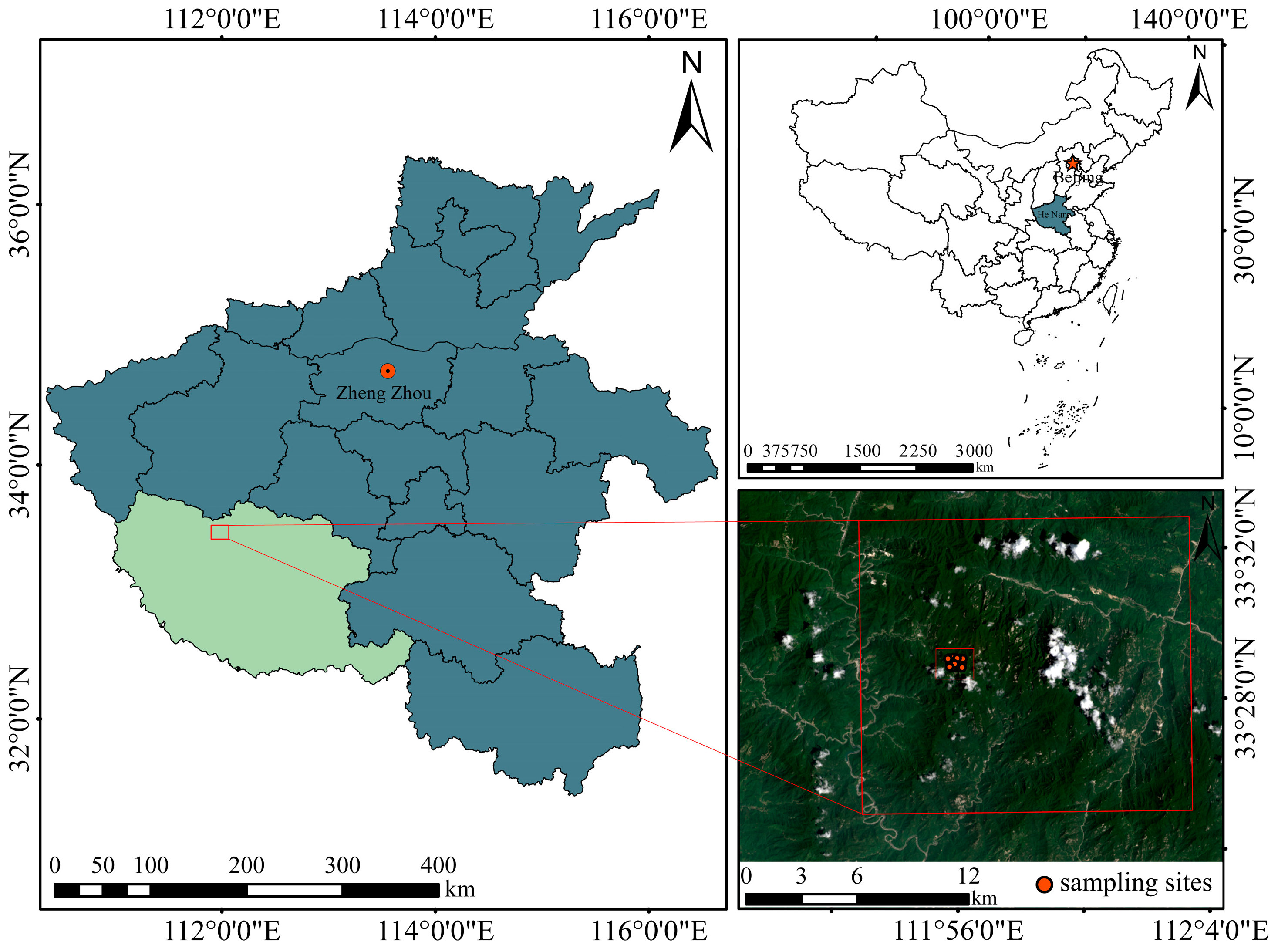
2.1. Study Area
2.2. Field Sampling and Vegetation Survey
2.3. Soil Sampling and Chemical Analyses
2.4. Diversity Index and Biotic Variables
2.5. Statistical Analysis
3. Results
3.1. Associations of Relative Density with Tree Diversity
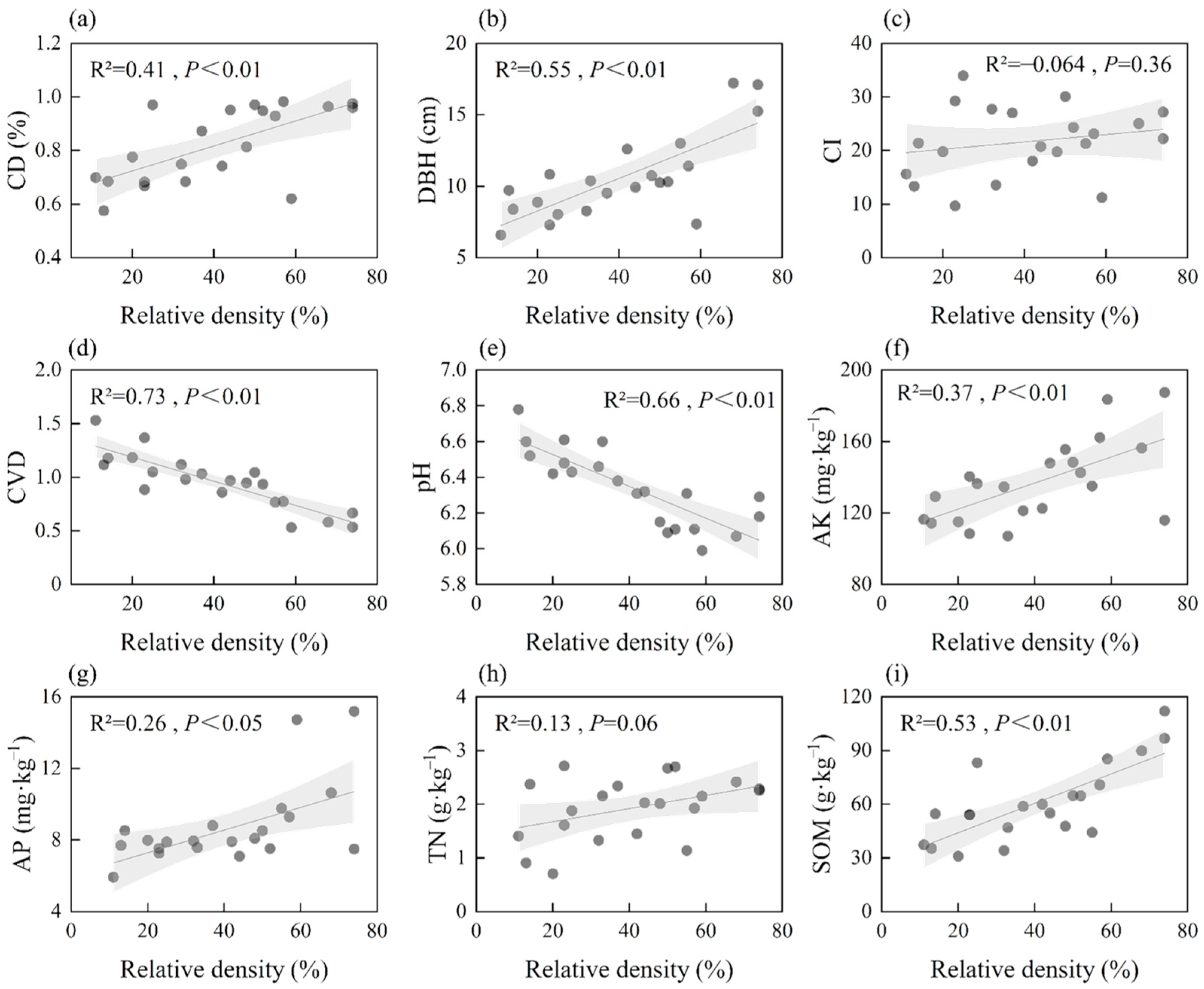
3.2. Associations of Relative Density with Biotic and Soil Factors
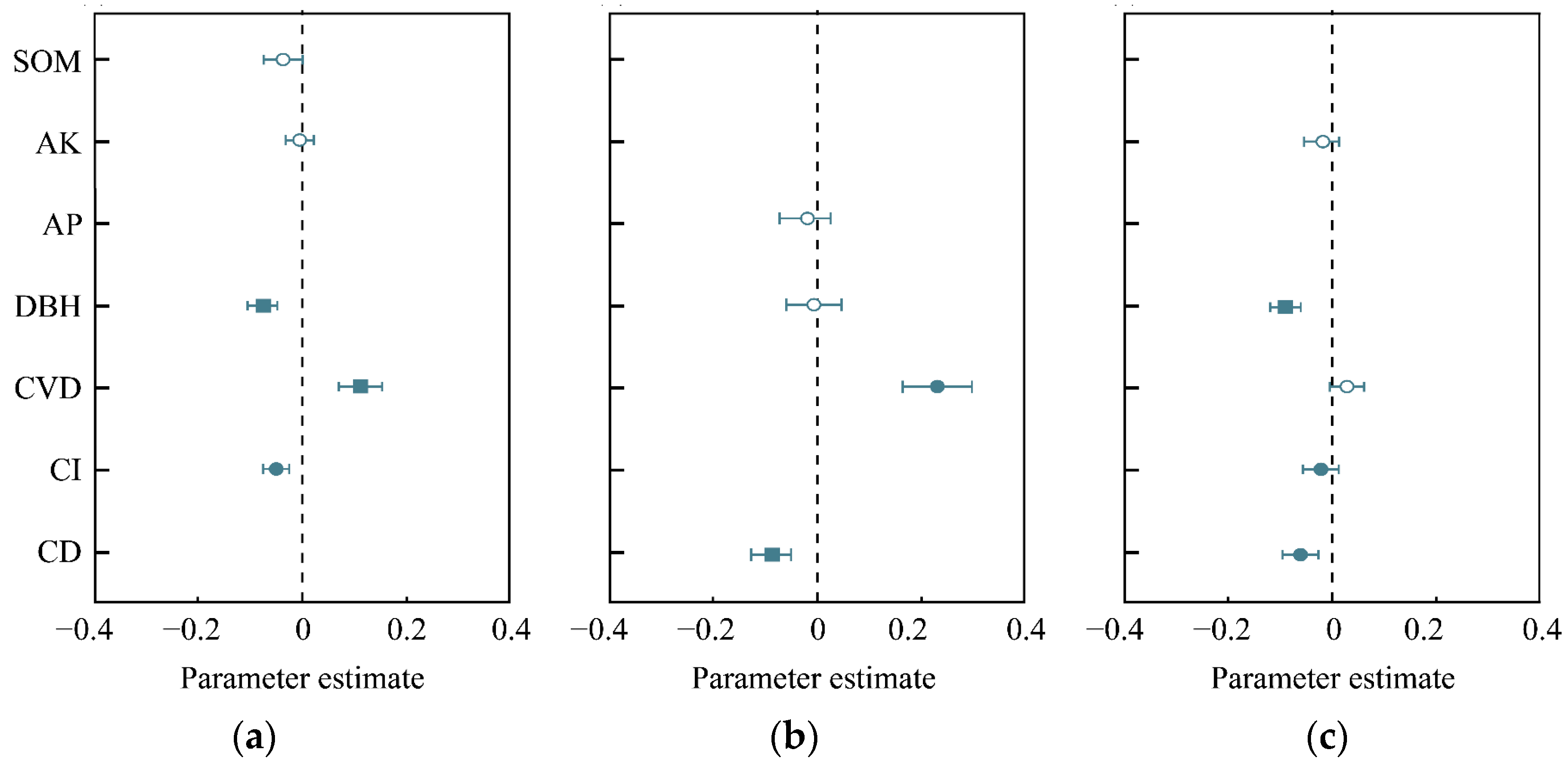
3.3. Dominant Determinants for Tree Species Diversity
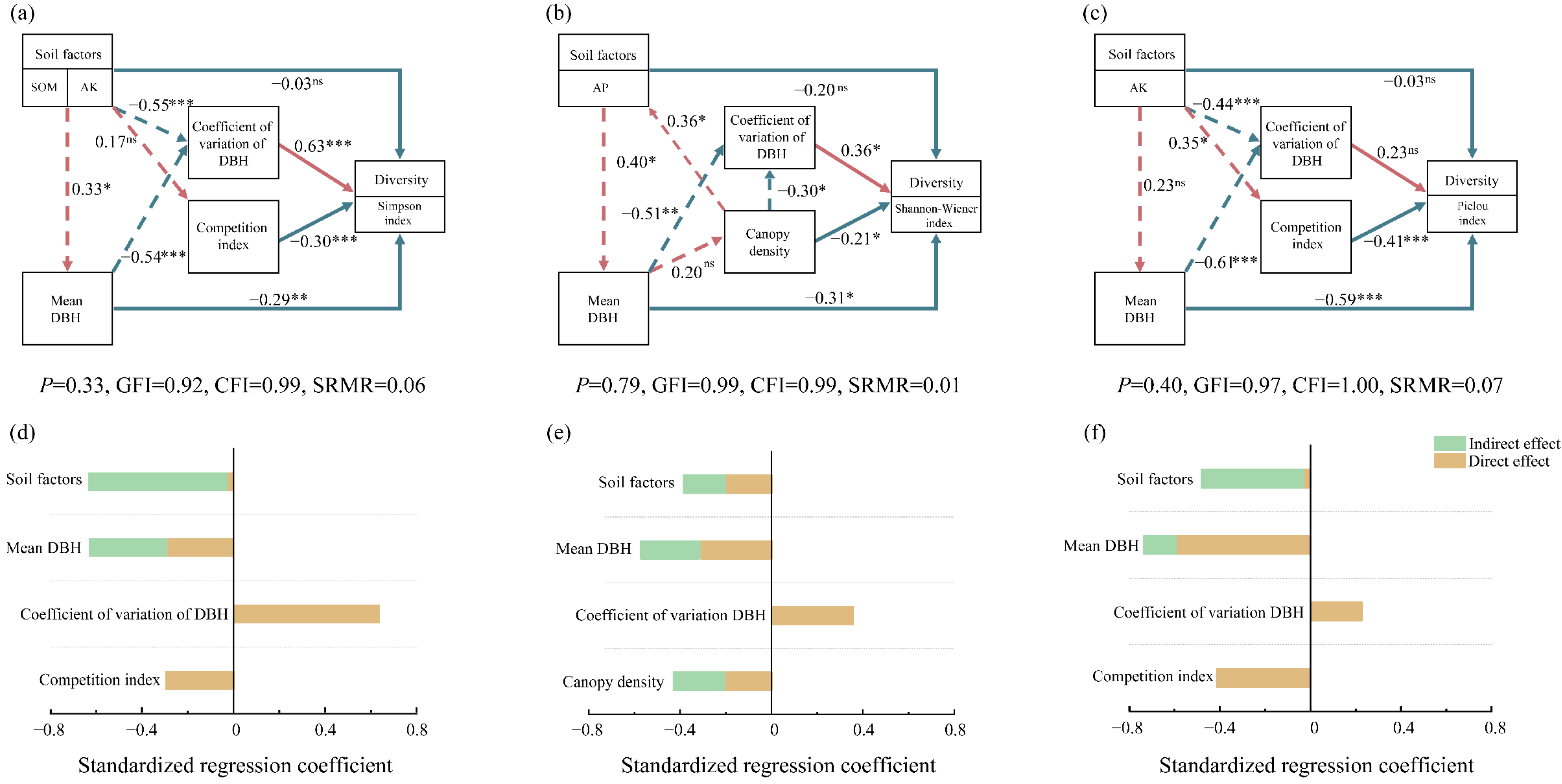
3.4. Direct and Indirect Effects on Tree Species Diversity
4. Discussion
4.1. Effects of Biotic Factors on Tree Species Diversity
4.2. Effects of Soil Factors on Species Diversity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Chave, J. The problem of pattern and scale in ecology: What have we learned in 20 years? Ecol. Lett. 2013, 16 (Suppl. S1), 4–16. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Munoz, F.; Ye, J.; Lin, F.; Yuan, Z.; Kuang, X.; Hao, Z.; Wang, X. Deterministic processes drive functional and phylogenetic temporal changes of woody species in temperate forests in Northeast China. Ann. For. Sci. 2019, 76, 42. [Google Scholar] [CrossRef]
- Shimadzu, H.; Dornelas, M.; Magurran, A.E. Measuring temporal turnover in ecological communities. Methods Ecol. Evol. 2015, 6, 1384–1394. [Google Scholar] [CrossRef]
- Wei, X.; Liang, W. Multifactor relationships between stand structure and soil and water conservation functions of Robinia pseudoacacia L. in the Loess Region. PLoS ONE 2019, 14, e0219499. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; Ouimet, R.; Lambert, M.C.; Logan, T. Aboveground carbon in Quebec forests: Stock quantification at the provincial scale and assessment of temperature, precipitation and edaphic properties effects on the potential stand-level stocking. PeerJ 2016, 4, e1767. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Bravo-Oviedo, A.; Hilmers, T.; Ruiz-Peinado, R.; Coll, L.; Löf, M.; Ahmed, S.; Aldea, J.; Ammer, C.; Avdagić, A. With increasing site quality asymmetric competition and mortality reduces Scots pine (Pinus sylvestris L.) stand structuring across Europe. For. Ecol. Manag. 2022, 520, 120365. [Google Scholar] [CrossRef]
- Binkley, D.; Kashian, D.M.; Boyden, S.; Kaye, M.W.; Bradford, J.B.; Arthur, M.A.; Fornwalt, P.J.; Ryan, M.G. Patterns of growth dominance in forests of the Rocky Mountains, USA. For. Ecol. Manag. 2006, 236, 193–201. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Nan, H. Abiotic and biotic drivers of species diversity in understory layers of cold temperate coniferous forests in North China. J. For. Res. 2019, 30, 2213–2225. [Google Scholar] [CrossRef]
- Linstädter, A.B.; Kemmerling, C.D.; Baumann, S.; Kleyer, M. Are trees of intermediate density more facilitative? Canopy effects of four East African legume trees. J. Veg. Sci. 2016, 27, 105–114. [Google Scholar] [CrossRef]
- Lett, C. Biodiversity faces its make-or-break. Nature 2022, 601, 298. [Google Scholar]
- Bartha, S.; Szentes, S.; Horváth, A.; Házi, J.; Zimmermann, Z.; Molnár, C.; Dancza, I.; Margóczi, K.; Pál, R.W.; Purger, D.; et al. Impact of mid-successional dominant species on the diversity and progress of succession in regenerating temperate grasslands. Appl. Veg. Sci. 2014, 17, 201–213. [Google Scholar] [CrossRef]
- Grubb, P.J. The Maintenance of Species-Richness in Plant Communities: The Importance of the Regeneration Niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Danyagri, G.; Baral, S.K.; Pelletier, G. Effects of disturbance and site factors on sapling dynamics and species diversity in northern hardwood stands. For. Ecol. Manag. 2019, 444, 225–234. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- Nadeau, M.B.; Sullivan, T.P. Relationships between Plant Biodiversity and Soil Fertility in a Mature Tropical Forest, Costa Rica. Int. J. For. Res. 2015, 2015, e732946. [Google Scholar] [CrossRef]
- Merunková, K.; Chytrý, M. Environmental control of species richness and composition in upland grasslands of the southern Czech Republic. Plant Ecol. 2012, 213, 591–602. [Google Scholar] [CrossRef]
- Putz, F.E. Biological Diversity: The Coexistence of Species on Changing Landscapes. Michael A. Huston. Q. Rev. Biol. 1996, 71, 141–142. [Google Scholar] [CrossRef]
- Bose, A.K.; Schelhaas, M.J.; Mazerolle, M.J.; Bongers, F. Temperate forest development during secondary succession: Effects of soil, dominant species and management. Eur. J. For. Res. 2014, 133, 511–523. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E.; Nissanka, S.P.; Mohotti, K.; Perera, K.A.R.S.; Wikramanayake, E.D.; Ashton, M.S. Topmost trees and foremost species underlie tropical forest structure, diversity and biomass through opposing mechanisms. For. Ecol. Manag. 2020, 473, 118299. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, B.; Chen, Y.; Jia, H.; Wei, Q.; Ye, Y. How do similarities in spatial distributions and interspecific associations affect the coexistence of Quercus species in the Baotianman National Nature Reserve, Henan, China. Ecol. Evol. 2018, 8, 2580–2593. [Google Scholar] [CrossRef]
- VanderSchaaf, C.L. Estimating understory vegetation response to multi-nutrient fertilization in Douglas-fir and ponderosa pine stands. J. For. Res. 2008, 13, 43–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Guo, J.; Tan, Z.; Dong, W.; Wang, H. Changes in the understory diversity of secondary Pinus tabulaeformis forests are the result of stand density and soil properties. Glob. Ecol. Conserv. 2021, 28, e01628. [Google Scholar] [CrossRef]
- Capers, R.S.; Chazdon, R.L.; Brenes, A.R.; Alvarado, B.V. Successional dynamics of woody seedling communities in wet tropical secondary forests. J. Ecol. 2005, 93, 1071–1084. [Google Scholar] [CrossRef]
- Woodall, C.W.; Perry, C.H.; Miles, P.D. The relative density of forests in the United States. For. Ecol. Manag. 2006, 226, 368–372. [Google Scholar] [CrossRef]
- He, Y.Y.; Srisombut, K.; Xing, D.-L.; Swenson, N.G.; Asefa, M.; Cao, M.; Song, X.-Y.; Wen, H.-D.; Yang, J. Ontogenetic trait variation and metacommunity effects influence species relative abundances during tree community assembly. Plant Divers. 2022, 44, 360–368. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Rahmad, Z.B. Environmental factors associated with liana community assemblages in a tropical forest reserve, Ghana. J. Trop. Ecol. 2015, 31, 69–79. [Google Scholar] [CrossRef]
- Wen, Y.; Tong, R.; Zhang, H.; Feng, K.; Song, R.; Wang, G.G.; Wu, T. N addition decreased stand structure diversity in young but increased in middle-aged Metasequoia glyptostroboides plantations. Glob. Ecol. Conserv. 2021, 30, e01803. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Ren, S.; Ali, A.; Liu, H.; Yuan, Z.; Yang, Q.; Shen, G.; Zhou, S.; Wang, X. Response of community diversity and productivity to canopy gap disturbance in subtropical forests. For. Ecol. Manag. 2021, 502, 119740. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, G.J.; Zhang, J.P.; Qi, Y.J. Stand, plot characteristics, and tree species diversity jointly dominate the recruitment biomass of subtropical forests. For. Ecol. Manag. 2023, 531, 120814. [Google Scholar] [CrossRef]
- Li, S.; Lang, X.; Liu, W.; Ou, G.; Xu, H.; Su, J. The relationship between species richness and aboveground biomass in a primary Pinus kesiya forest of Yunnan, southwestern China. PLoS ONE 2018, 13, e0191140. [Google Scholar] [CrossRef]
- Bartha, S.; Meiners, S.J.; Pickett, S.T.A.; Cadenasso, M.L. Plant colonization windows in a mesic old field succession. Appl. Veg. Sci. 2003, 6, 205–212. [Google Scholar] [CrossRef]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R. The mediation roles of intraspecific and interspecific functional trait diversity for linking the response of aboveground biomass to species richness across forest strata in a subtropical forest. Ecol. Indic. 2018, 85, 493–501. [Google Scholar] [CrossRef]
- Saito, V.S.; Laroche, F.; Siqueira, T.; Pavoine, S. Ecological versatility and the assembly of multiple competitors: Cautionary notes for assembly inferences. Ecology 2018, 99, 1173–1183. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Grace, J.B. A multivariate model of plant species richness in forested systems: Old-growth montane forests with a long history of fire. Oikos 2006, 114, 60–70. [Google Scholar] [CrossRef]
- Wu, A.; Zhou, G.; He, H.; Hautier, Y.; Tang, X.; Liu, J.; Zhang, Q.; Wang, S.; Wang, A.; Lin, L.; et al. Tree diversity depending on environmental gradients promotes biomass stability via species asynchrony in China’s forest ecosystems. Ecol. Indic. 2022, 140, 109021. [Google Scholar] [CrossRef]
- Klemmedson, J.O. Oak influence on nutrient availability in pine forests of central Arizona. Soil Sci. Soc. Am. J. 1991, 55, 248–253. [Google Scholar] [CrossRef]
- Bahru, T.; Ding, Y. Effect of stand density, canopy leaf area index and growth variables on Dendrocalamus brandisii (Munro) Kurz litter production at Simao District of Yunnan Province, southwestern China. Glob. Ecol. Conserv. 2020, 23, e01051. [Google Scholar] [CrossRef]
- Sayer, E.J.; Tanner, E.V.J. Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J. Ecol. 2010, 98, 1052–1062. [Google Scholar] [CrossRef]
| Relative Density (%) | Number of Species | Abundance | Sample Area (hm2) | Mean DBH (cm) |
|---|---|---|---|---|
| 10 | 35 | 214 | 0.12 | 8.85 ± 0.76 a |
| 20 | 31 | 252 | 0.12 | 9.13 ± 1.59 a |
| 30 | 30 | 287 | 0.12 | 8.90 ± 1.29 a |
| 40 | 24 | 228 | 0.12 | 10.96 ± 1.44 a |
| 50 | 25 | 271 | 0.12 | 10.42 ± 0.26 a |
| 60 | 19 | 212 | 0.12 | 10.60 ± 1.10 a |
| 70 | 13 | 266 | 0.12 | 16.53 ± 2.89 b |
| Relative Density (%) | Simpson Index (D) | Shannon–Wiener Index (N) | Pielou Index (J) |
|---|---|---|---|
| 10 | 0.85 ± 0.07 a | 2.34 ± 0.29 a | 0.83 ± 0.13 a |
| 20 | 0.82 ± 0.07 ab | 2.14 ± 0.31 a | 0.80 ± 0.08 a |
| 30 | 0.82 ± 0.02 ab | 2.14 ± 0.10 a | 0.76 ± 0.04 a |
| 40 | 0.76 ± 0.01 bc | 2.22 ± 0.50 a | 0.72 ± 0.02 a |
| 50 | 0.72 ± 0.02 c | 1.91 ± 0.12 a | 0.69 ± 0.02 a |
| 60 | 0.62 ± 0.04 d | 1.33 ± 0.32 b | 0.69 ± 0.10 a |
| 70 | 0.45 ± 0.04 e | 0.98 ± 0.15 b | 0.50 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Ren, S.; Huang, Y.; Wang, G.; Liu, S.; Li, Z.; Yuan, Y.; Huang, X.; Wang, T. Biotic Factors Drive Woody Plant Species Diversity across a Relative Density Gradient of Quercus aliena var. acuteserrata Maxim. in the Warm–Temperate Natural Oak Forest, Central China. Forests 2023, 14, 1956. https://doi.org/10.3390/f14101956
Yu C, Ren S, Huang Y, Wang G, Liu S, Li Z, Yuan Y, Huang X, Wang T. Biotic Factors Drive Woody Plant Species Diversity across a Relative Density Gradient of Quercus aliena var. acuteserrata Maxim. in the Warm–Temperate Natural Oak Forest, Central China. Forests. 2023; 14(10):1956. https://doi.org/10.3390/f14101956
Chicago/Turabian StyleYu, Chenyi, Siyuan Ren, Yudie Huang, Guanjie Wang, Shengyun Liu, Zhenjiang Li, Yabo Yuan, Xin Huang, and Ting Wang. 2023. "Biotic Factors Drive Woody Plant Species Diversity across a Relative Density Gradient of Quercus aliena var. acuteserrata Maxim. in the Warm–Temperate Natural Oak Forest, Central China" Forests 14, no. 10: 1956. https://doi.org/10.3390/f14101956
APA StyleYu, C., Ren, S., Huang, Y., Wang, G., Liu, S., Li, Z., Yuan, Y., Huang, X., & Wang, T. (2023). Biotic Factors Drive Woody Plant Species Diversity across a Relative Density Gradient of Quercus aliena var. acuteserrata Maxim. in the Warm–Temperate Natural Oak Forest, Central China. Forests, 14(10), 1956. https://doi.org/10.3390/f14101956




