Abstract
Insect herbivory is one of the most important ecological processes affecting plant–soil feedbacks and overall forest ecosystem health. In this study, we assess how elevated carbon dioxide (eCO2) impacts (i) leaf level insect herbivory and (ii) the stand-level herbivore-mediated transfer of carbon (C) and nitrogen (N) from the canopy to the ground in a natural mature oak temperate forest community in central England at the Birmingham Institute of Forest Research Free Air CO2 Enrichment (BIFoR FACE) site. Recently abscised leaves were collected every two weeks through the growing season in August to December from 2017–2019, with the identification of four dominant species: Quercus robur (pedunculate oak), Acer pseudoplatanus (sycamore), Crataegus monogyna (common hawthorn) and Corylus avellana (hazel). The selected leaves were scanned and visually analyzed to quantify the leaf area loss from folivory monthly. Additionally, the herbivore-mediated transfer of C and N fluxes from the dominant tree species Q. robur was calculated from these leaf-level folivory estimates, the total foliar production and the foliar C and N contents. This study finds that the leaf-level herbivory at the BIFoR FACE has not changed significantly across the first 3 years of eCO2 treatment when assessed across all dominant tree species, although we detected significant changes under the eCO2 treatment for individual tree species and years. Despite the lack of any strong leaf-level herbivory response, the estimated stand-level foliar C and N transferred to the ground via herbivory was substantially higher under eCO2, mainly because there was a ~50% increase in the foliar production of Q. robur under eCO2. This result cautions against concluding much from either the presence or absence of leaf-level herbivory responses to any environmental effect, because their actual ecosystem effects are filtered through so many (usually unmeasured) factors.
1. Introduction
1.1. Forests, Climate and CO2
Atmospheric carbon dioxide (CO2) concentrations are controlled by the exchange of CO2 between the atmosphere, terrestrial biosphere and oceans, which impact the global climate and overall carbon (C) budget [1]. Increasing atmospheric CO2 levels will likely alter multiple important system properties through higher temperatures [2], precipitation acidity [3], altered organic matter turnover [4], reduced soil moisture [5] and an overall influence on plant and animal biodiversity, composition and competition [6,7]. Plants grown under elevated CO2 have enhanced global photosynthesis by 11.85–13.98 PgC of carbon and are thus contributing a large proportion to the current terrestrial carbon sink [8]. Currently, CO2 fertilization offsets 20%–30% of the CO2 released by human activities [9], predominantly due to carbon sequestration in mature forest ecosystems [10,11]. Both experimental data and global climate models, however, predict that the CO2 fertilization effect may subside over time, but at varying magnitudes, which could accelerate the rate of climate change [12,13]. In order to moderate the progression of climate change, it is important, therefore, to understand the uncertainty, pressures and responses of increasing atmospheric CO2 concentrations on plants [14] and the associated impacts of this on the terrestrial and C cycle. Temperate forests are particularly important, covering just 8% of the global land area but accounting for 40% of the total C stored terrestrially [15]. These forests are not only important for sequestering C from the atmosphere [16], but temperate forest canopies promote insect species’ richness and abundance [17].
1.2. FACE Experiments
Forest-based free-air CO2 enrichment (FACE) experiments were established to help understand forest ecosystem responses to a future atmosphere with CO2 enrichment. Extensive knowledge has already been gained with a decade of FACE studies showing an increase in net primary productivity, leaf area index (LAI), nutrient cycling and soil microbial activity [18]. However, because most of these historic FACE experiments (AspenFACE and DukeFACE) were conducted on young mono-species forest plantations and concentrated around North American and European ecosystems [19], the conclusions from these experiments may not easily translate to diverse and mature ecosystems which are more representative of the natural forests around the world [20]. Currently, there are only two ongoing forest FACE experiments in natural, mature forest ecosystems: EucFACE in the eucalyptus forests of Australia and BIFoR in a mature northern temperate forest in the United Kingdom [20].
1.3. Herbivory and CO2
One potentially critical process which remains understudied in FACE experiments is the impacts of eCO2 on herbivory and the consequences for herbivore-mediated ecosystem processes [21]. Insect herbivory is one of the most important ecological processes affecting plant–soil feedbacks [22,23], forest ecosystem health [24,25], resource availability [26], function and structure and the composition and productivity of plant communities [27]. Research has been conducted on the effects of eCO2 treatments on insect herbivory under controlled situations [28], but studies based in mature and diverse forest ecosystems are still rare [21,29]. Interactions between eCO2 and insect herbivory appear to be predominantly regulated by plant chemistry and metabolism [30,31]. The responses of plants to eCO2 show that foliar nitrogen (N) decreases and leaf toughness, leaf biomass and the carbon-to-nitrogen ratio increase [32,33,34]. This altered foliar chemistry then negatively impacts herbivore performance by significantly decreasing the relative growth rate and pupal weight [35]. It can also result in compensatory feeding due to a reduction in food quality, causing increased defoliation, but still with a cost to the early stages of larval development and the overall herbivore abundance [31]. However, the limited evidence for eCO2 impacts on herbivore leaf damage from FACE studies suggests mixed, relatively weak responses. Some studies show a decline in herbivory under eCO2, apparently related to leaf chemistry, but this varies among species and years of measurement [36,37]. Another study found no clear effect [29], while others found an increase in insect herbivory under eCO2 dependent on the feeding guild and host plant type [21,38].
In this study, we assess how eCO2 impacts insect herbivory in a natural mature oak temperate forest community [39]. Further, for the dominant oak species, we estimate the net impact of CO2-induced shifts in canopy productivity, foliar chemistry and leaf-level herbivory on ecosystem-level herbivore-mediated C and N fluxes from the canopy to the ground. Specifically, we ask three questions:
- How does eCO2 affect leaf-level insect herbivory?
- Do eCO2 effects on leaf-level herbivory vary among tree species and years?
- How and why does eCO2 affect the herbivore-mediated transfer of C and N from the forest canopy to the ground?
2. Materials and Methods
2.1. Study Site
The BIFoR FACE experiment is located in a mature, temperate oak forest on Orthic Luvisol dominant soil [40] with a mul-moder humus classification in central England (52.801° N, 2.301° W), United Kingdom. The forest is characterized as a deciduous forest with a broadleaf tree species, with a dominant old oak (Quercus robur) overstory (~25 m tall, 175 years old) and a mid-understory of sycamore (Acer pseudoplatanus) and hazel (Corylus avellana).
The BIFoR FACE site consists of six structural arrays (eCO2, n = 3; Control, n = 3) and three non-infrastructural arrays (Figure 1). Their location and pairings were determined by testing key forest characteristics such as soil analysis, species distribution and biomass densities [41]. Each array consists of 16 peripheral towers and one central tower ~1 m above the canopy with a 15 m radius ground space for research. A highly consistent +150 ppm above ambient eCO2 treatment has been applied within FACE ‘treatment’ arrays since April 2017, running in parallel with ‘control’ arrays supplying ambient air via an identical infrastructure [39]. The plot tree species composition and basal area were recorded for all trees greater than 10 cm at diameter breast height (dbh) in all the arrays.
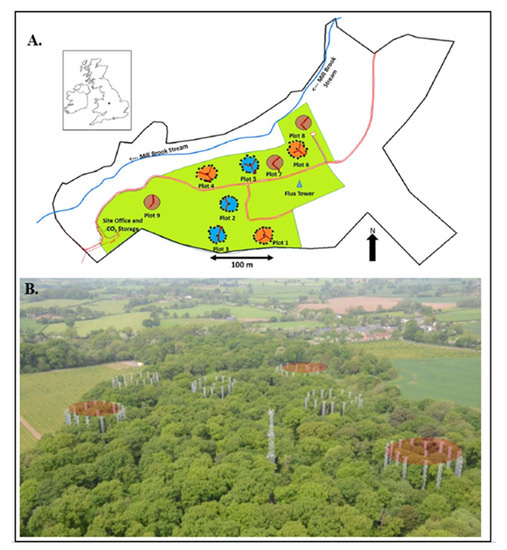
Figure 1.
(A). Map of the 19.1ha BIFoR FACE facility. Three enriched CO2 arrays are shown in orange; three experimental control arrays are shown in blue. The red line indicates elevated walkways to access plots, reducing forest floor disturbance. The 40 m high flux tower (blue triangle) collects environmental conditions at the site. (B). An aerial view of the experimental design, illustrating the treatment rings in relation to the ecosystem.
2.2. Leaf Herbivory
Two 1 m2 leaf litter traps have been in place within each of the three control and treatment arrays since 2017. Leaf litter was collected every two weeks through the growing season in August to December from 2017–2019, with the identification of four dominant species (S1): Quercus robur (pedunculate oak), Acer pseudoplatanus (sycamore), Crataegus monogyna (common hawthorn) and Corylus avellana (hazel).
After sorting by species, 10 leaves per species per month from each trap were randomly selected for scanning (with no overlap) and scanned at a 300 dpi tiff. format. The insect herbivory was visually quantified as the proportion of the leaf area missing (H) from the scanned images using a six-class leaf area loss system for calibration [42] (Figure 2). Additionally, Digimizer v.5.4.7 ® (MedCalc Software Ltd., Ostend, Belgium, 2020) was used to measure the individual leaf area for all leaves collected only in 2019 (N = 435 leaves). Skeletonizing, leaf mining and leaf rolling were negligible for the leaf tissue lost in the leaves sampled by this study and therefore were not included in the analysis. All the material collected in each trap was then dried at 60 °C for 24 h and weighed.
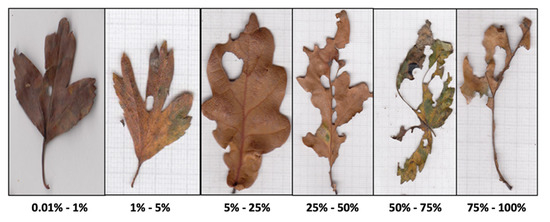
Figure 2.
Exemplar images for the visual quantification of herbivory and subsequent categorization of samples into six leaf area loss classes [42].
2.3. Leaf nutrients and Production
Oak leaves were collected from the top of the canopy in each month from 2017–2019 and stored immediately at −25 °C. Two upper canopy leaves from one tree per plot were selected for elemental analyses. Dried leaves were ground and analyzed for total C and N using an elemental analyzer interfaced with an isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK; [43]).
2.4. Calculations of Herbivore-Mediated Nutrient Fluxes
To estimate the canopy biomass production per plot, all oak leaves collected within the litter traps from our sampling years of 2017–2019 were dried at 60 °C for 2–3 days until constant mass and weighed. We calculated the mean oak basal area (BA, m2) across all six plots; then, we divided the individual plot BA by the mean BA. This value (the fraction of individual plot BA: mean plot BA) was then multiplied by the individual plot oak raw dry litterfall mass per unit ground area (Lraw, g m−2 year−1) to derive the abundance-corrected dry litterfall mass (Lcorr g m−2 year−1). The total peak oak live dry foliage mass per unit ground area (Q, g m−2) was calculated as Q = Lcorr + (Lcorr × H). The herbivore-mediated transfer of C and N fluxes per unit ground area from oak (Fx, g m−2 year−1) at the study site was then calculated following the basic approach in [44], as follows:
where subscript x is substituted with C or N for reference Fx and for leaf C and N content expressed as a proportion of dry biomass (Nx).
Fx = Q × H × Nx
2.5. Statistical Analysis
The response variable of the leaf-level insect herbivory was evaluated using repeated measured MANOVA with fixed effects of the eCO2 treatment and tree species. All statistical analyses were performed using R Statistical Software 1.1442 [45,46]. Before analysis, data normality was checked and the data were transformed where necessary, with a Box Cox transformation of the suggested 0.501 lambda. The values are reported as the mean ± SE or the percentage (%).
3. Results
3.1. Leaf-Level Insect Herbivory
Overall, the leaf-level herbivory showed no significant response to the eCO2 treatment across sampling years and tree species (Table 1), though there were some apparent effects within species and years (Table 1, Figure 3). Specifically, there was no significant difference in herbivory between the eCO2 treatment and control plots across all years for oak, while hawthorn and hazel experienced a significant reduction in leaf-level herbivory under eCO2 compared to the control in 2019 and 2018, respectively (Table 1, Figure 3). Sycamore experienced a significant increase in leaf-level herbivory for both treatments in 2019 (Figure 3).

Table 1.
F Ratio for MANOVA Wilk’s Lambda test of insect herbivory responses across different dominant tree species (oak, sycamore, hawthorn, hazel) and between eCO2 and controlled treatments measured over 2017, 2018 and 2019. Significant main effects and interactions are indicated with asterisks: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
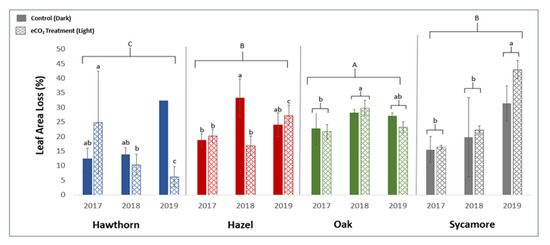
Figure 3.
The mean ± S.E. leaf area lost by insect herbivory per leaf for three years on hawthorn, hazel, oak and sycamore tree leaves. n = 3. Significant main effects and interactions (p ≤ 0.05) are indicated with letters between species, years and treatments (ABC, abc). For the 2019 control hawthorn, there was only one litter trap of material, and, thus, no S.E could be calculated.
3.2. Estimated Carbon and Nitrogen Fluxes from Insect Herbivory
Herbivore-induced C and N fluxes from oak to the ground were the net product of the leaf-level herbivory rate, the litterfall rate and the leaf carbon and nitrogen contents, all of which were influenced in different ways by the CO2 treatment. Across all scaled-up years, eCO2 was associated with a slight decrease in leaf-level insect herbivory and slightly increased foliar C and N but substantially increased tree abundance-corrected litter fall (Figure 4). Thus, even though leaf-level insect herbivory on oak was apparently slightly suppressed by eCO2, the estimated C and N nutrient fluxes from the canopy to the ground via herbivory were ~60–70% greater on the eCO2 treatment compared to those on the control.
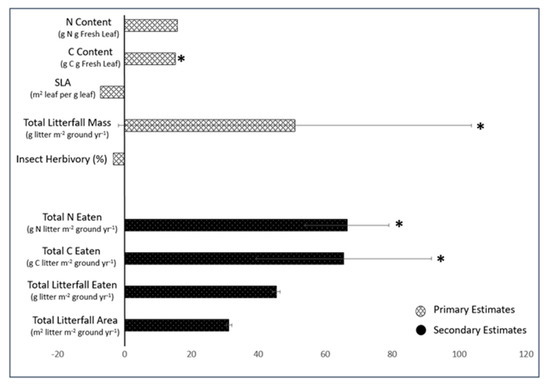
Figure 4.
Percent change from control treatment compared to that from eCO2 for total N content, C content, Specific Leaf Area and Total Litterfall Mass and Insect Herbivory for oak leaves over three sampling years (2017–2019). Bars represent mean total % change on the eCO2 plots relative to the control ± standard error, n = 3. Primary estimates are defined as parameters that were all directly measured in the field/lab; secondary estimates are parameters calculated from primary parameters. Significant percent change from control treatment compared to that from eCO2 are indicated with an asterisks *, p ≤ 0.05.
4. Discussion
4.1. Leaf-Level Insect Herbivory
The current study has shown that the total leaf level herbivory at the BIFoR FACE has not changed significantly over the first 3 years of eCO2 treatment (2017–2019) when assessed across all dominant tree species combined (Table 1; Figure 3). This agrees with the results from the EucFACE experiment in Australia, where the leaf-level herbivory observed on living leaves in the upper canopy was not affected by CO2 enrichment for the first two years since the initiation of the experiment [29]. There were, however, significant increases in herbivory noted in our study for hazel and sycamore in 2019, suggesting a potential rise in herbivory for certain tree species under eCO2. The overall weak herbivory response to eCO2 at the BIFoR may be due to the limited biochemical response of dominant and more mature oak trees, with no significant change in the foliar N of oak from 2015–2019 [43]. While our own calculations indicate an increase in the oak foliar N concentrations within eCO2 plots between 2017–2019 (Figure 4), no significant change in oak herbivory was noted for any year.
4.2. Plant Nutrients and Insect Herbivory
Field studies on the plant-mediated effects of eCO2 on insects are rare, but the consequences are potentially substantial at both an individual and ecosystem level [47]. Negative consequences on plant chemistry, such as reduced N and increased defensive compounds, e.g., tannins, can affect overall herbivore development [48] and potentially impact herbivore abundance and biodiversity. It is worth noting, however, that no significant changes in insect community composition were recorded between 2017 and 2019 at the BIFoR [38]. In line with our findings, varying plant species responses to eCO2 are common [47]. The current study reinforces the need to investigate biochemical changes in multiple tree species, as well as the impacts on several different insect herbivores. For example, studying the effects of leaf miners provides a localized perspective on the exposure to eCO2 or controlled conditions, as they typically spend their life on a single leaf from egg to adulthood. A detailed study of several leaf miner species has been undertaken at the BIFoR on both oak and hazel, which indicate differing responses across different tree and miner species combinations [38]. Our study observed a decrease in insect herbivory for oak, a canopy dominating species under eCO2 conditions [49].
4.3. Ecosystem Responses to Insect Herbivory
Beyond just understanding the direct interactions between herbivores and plants, it is also important to quantify whole ecosystem responses to insect herbivory, particularly the herbivore-mediated transfer of carbon and nutrients to the soil [50]. Inferring the ecosystem impacts of insect herbivory from leaf-level observations is not straightforward [51,52] because the leaf-level impacts are filtered through a range of other factors which are also affected by the environment such as the light intensity, nutrient availability and leaf composition [53]. For example, in our study, while there is lower leaf-level herbivory under elevated CO2, this is offset by the large increase in litterfall [54] on the eCO2 plots compared to the control, even after correcting for pre-existing plot differences in oak abundance and size. Increased litterfall from our diverse temperate species system tends to follow the deceleration model in response to herbivory [54] by inducing defenses, decreasing litter quality and increasing the C/N ratio [36,55]. In other words, herbivores on the eCO2 plots are able to maintain roughly similar levels of leaf-level damage despite a ~60% increase in foliar resources, indicating that the activity and/or abundance of the herbivore population is in fact higher on the eCO2 plots despite the lack of any strong leaf-level herbivory treatment difference. This result cautions against concluding much from either the presence or absence of leaf-level herbivory responses to any environmental effect, because their actual ecosystem effects are filtered through so many (usually unmeasured) factors.
5. Conclusions
We present a three-year study from a mature temperate forest, experimentally measuring the effects of eCO2 on insect herbivory rates and the stand-level herbivore-mediated transfer of C and N from the canopy to the ground. We found no overall effect of eCO2 on leaf-level herbivory within the first 3 years of the BIFoR FACE experiment, though some significant effects among individual tree species and measurement years were found, along with potential trajectories of increasing herbivory on some species in 2019. We showed that the herbivore-mediated transfer of C and N fluxes from the dominant tree species oak was 60%–70% higher under eCO2 even though the leaf level consumption was lower. This was because the total oak foliage mass was ~50% higher in the eCO2 treatments, even after correcting for the pre-existing plot variation in oak abundance. As such, our findings suggest that herbivores’ activity and/or abundance in eCO2 plots was enhanced.
Author Contributions
Conceptualization, A.J.R., J.P.S., D.B.M., S.A.L.H. and A.M.G.; methodology, A.J.R. and A.M.G.; software, A.J.R.; validation, A.J.R., D.B.M. and S.A.L.H.; formal analysis, A.J.R.; investigation, A.J.R. and T.T.T.N.; A.M.G.; resources, A.J.R., D.B.M. and S.A.L.H.; data curation, A.J.R. and T.T.T.N.; writing—original draft preparation, A.J.R., D.B.M., S.A.L.H. and L.M.C.; writing—review and editing, A.J.R., D.B.M., S.A.L.H., L.M.C. and J.P.S.; visualization, A.J.R.; supervision, D.B.M. and S.A.L.H.; project administration, D.B.M. and S.A.L.H.; funding acquisition, D.B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Research Council (ERC) under the European Union’s ECOHERB-682707.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all our collaborators for making this research possible, especially the BIFoR technical team for enabling and supporting research at the facility as well as Rob Mackenzie, Giulio Curioni and Deanne Brettle for all the data collection and advice on the site work. We thank Anna Gardner for the oak nutrient data and analysis. This research utilized BIFoR FACE data supported by the JABBS foundation and the University of Birmingham.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Candell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B.; et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature Lett. 2016, 531, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Handel, D.; Risbey, J.S. Reflection on more than a century of climate change research. Clim. Change 1992, 21, 91–96. [Google Scholar] [CrossRef]
- Berner, R.A. Weathering, plants, and the long-term carbon cycle. Geochimica et Cosmochimica Acta 1992, 56, 3225–3232. [Google Scholar] [CrossRef]
- McGuire, A.D.; Melillo, J.M.; Kicklighter, D.W.; Joyce, L.A. Equilibrium responses of soil carbon to climate change: Empirical and process-based estimates. J. Biogeog. 1995, 22, 785–796. [Google Scholar] [CrossRef]
- Manabe, S.; Spelman, M.J.; Stouffer, R.J. Transient Responses of a Coupled Ocean-Atmosphere Model to Gradual Changes of Atmospheric CO2. Part II: Seasonal Response. J. Clim. 1992, 5, 105–126. [Google Scholar] [CrossRef]
- Rochefort, L.; Woodward, F.I. Effects of climate change and doubling of CO2 on vegetation diversity. J. Exp. Bot. 1992, 43, 1169–1180. [Google Scholar] [CrossRef]
- Besford, R.T. The greenhouse effect: Acclimation of tomato plants growing in high CO2, relative changes in Calvin Cycle enzymes. J. Plant Physiol. 1990, 136, 458–463. [Google Scholar] [CrossRef]
- Keenan, T.F.; Luo, X.; De Kauwe, M.G.; Medlyn, B.E.; Prentice, I.C.; Stocker, B.D.; Smith, N.G.; Terrer, C.; Wang, H.; Zhang, Y.; et al. A constraint on historic growth in global photosynthesis due to increasing CO2. Nature 2021, 600, 253–258. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Gaubert, B.; Stephens, B.B.; Basu, S.; Chevallier, F.; Deng, F.; Kort, E.A.; Patra, P.K.; Peters, W.; Rödenbeck, C.; Saeki, T.; et al. Global atmospheric CO2 inverse models converging on neutral tropical land exchange, but disagreeing on fossil fuel and atmospheric growth rate. Biogeosciences 2019, 16, 117–134. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zeng, H.; Myneni, R.B.; Chen, C.; Ahao, Q.; Zha, J.; Zhan, S.; MacLachlan, I. Comment on “Recent global decline of CO2 fertilization effects on vegetation photosynthesis.” Science. Technical Comments. 2021. Available online: https://www.science.org/doi/epdf/10.1126/science.abg5673 (accessed on 1 December 2021).
- Arora, V.K.; Boer, G.J.; Friedlingstein, P.; Eby, M.; Jones, C.D.; Christian, J.R.; Bonan, G.; Bopp, L.; Brovkin, V.; Cadule, P.; et al. Carbon–Concentration and Carbon–Climate Feedbacks in CMIP5 Earth System Models. J. Clim. 2013, 26, 5289–5314. Available online: https://journals.ametsoc.org/view/journals/clim/26/15/jcli-d-12-00494.1.xml (accessed on 10 September 2021).
- Martin, P.H.; Nabuurs, G.-J.; Aubinet, M.; Karjalainen, T.; Vine, E.L.; Kinsman, J.; Heath, L.S. Carbon sinks in temperate forests. Annu. Rev. Ecol. Evol. Syst. 2001, 26, 435–465. [Google Scholar] [CrossRef]
- Basset, Y.; Lamarre, G.P.A. Toward a world that values insects. Science 2019, 364, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Brandl, R.; Brändle, M.; Förster, B.; De Araujo, B.C.; Gossner, M.M.; Ladas, A.; Wagner, M.; Maraun, M.; Schall, P.; et al. LiDAR-derived canopy structure supports the more-individuals hypothesis for arthropod diversity in temperate forests. Oikos 2018, 127, 814–824. [Google Scholar] [CrossRef]
- Norby, R.J.; Zak, D.R. Ecological lessons from Free-Air CO2 Enrichment (FACE) experiments. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 181–203. [Google Scholar] [CrossRef]
- Jones, A.G.; Scullion, J.; Ostle, N.; Levy, P.E.; Gwynn-Jones, D. Completing the FACE of elevated CO2 research. Environ. Int. 2014, 73, 252–258. [Google Scholar] [CrossRef]
- Norby, R.J.; De Kauwe, M.G.; Domingues, T.F.; Duursma, R.A.; Ellsworth, D.S.; Goll, D.S.; Lapola, D.M.; Luus, K.A.; MacKenzie, A.R.; Medlyn, B.E.; et al. Model-data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytol. 2015, 209, 17–28. [Google Scholar] [CrossRef]
- Couture, J.J.; Meehan, T.D.; Kruger, E.L.; Lindroth, R.L. Insect herbivory alters impact of atmospheric change on northern temperate forests. Nat. Plants 2015, 1, 15016. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 2003, 85, 2258–2268. [Google Scholar] [CrossRef]
- Hartley, S.E.; Jones, T.H. Insect herbivores, nutrient cycling and plant productivity. Book-Insects Ecosyst. Funct. 2004, 173, 27–52. [Google Scholar] [CrossRef]
- Lill, J.T.; Marquis, R.J. The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 2001, 126, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Stone, C. Reducing the impact of insect herbivory in eucalypt plantations through management of extrinsic influences on tree vigour. Austral Ecol. 2001, 26, 482–488. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S., III. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Klemola, T. Hemispheric asymmetries in herbivory: Do they exist? J. Ecol. 2017, 105, 1571–1574. [Google Scholar] [CrossRef]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An Emerging Understanding of Mechanisms Governing Insect Herbivory Under Elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef]
- Gherlenda, A.N.; Moore, B.D.; Haigh, A.M.; Johnson, S.N.; Riegler, M. Insect herbivory in a mature Eucalyptus woodland canopy depends on leaf phenology but not CO2 enrichment. BMC Ecol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Roth, S.K.; Lindroth, R.L. Elevated atmospheric CO2: Effects on phytochemistry, insect performance and insect-parasitoid interactions. Glob. Change Biol. 1995, 1, 173–182. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Jones, T.H. Plant-insect herbivore interactions in elevated atmospheric CO2: Quantitative analyses and guild effects. Oikos 1998, 82, 212–222. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Bowes, G. Facing the inevitable: Plants and increasing atmosphere CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 309–332. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The Interaction between Elevated Carbon Dioxide and Nitrogen Nutrition: The Physiological and Molecular Background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef]
- Knepp, R.G.; Hamilton, J.G.; Mohan, J.E.; Zangerl, A.R.; Berenbaum, M.R.; DeLucia, E.H. Elevated CO2 reduces leaf damage by insect herbivores in a forest community. New Phytol. 2005, 167, 2017–2218. [Google Scholar] [CrossRef]
- Stiling, P.; Cattell, M.; Moon, D.C.; Rossi, A.; Hungate, B.A.; Hymuss, G.; Drakes, B. Elevated atmospheric CO2 lowers herbivore abundance, but increases leaf abscission rates. Glob. Change Biol. 2002, 8, 658–667. [Google Scholar] [CrossRef]
- Crowley, L.M.; Sadler, J.P.; Pritchard, J.; Hayward, S.A.L. Elevated CO2 Impacts on Plant–Pollinator Interactions: A Systematic Review and Free Air Carbon Enrichment Field Study. Insects 2021, 12, 512. [Google Scholar] [CrossRef]
- Hart, K.M.; Curioni, G.; Blaen, P.; Harper, N.J.; Miles, P.; Lewin, K.F.; Nagy, J.; Bannister, E.J.; Cai, X.M.; Thomas, R.M.; et al. Characteristics of free air carbon dioxide enrichment of a northern temperate mature forest. Glob. Change Biol. 2019, 26, 1023–1037. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106. FAO: Rome, Italy, 2015. [Google Scholar]
- MacKenzie, A.R. Characteristics of a mature northern temperate broadleaf forest and its conjectured response to free-air CO2 enrichment. 2019; Unpublished work. [Google Scholar]
- Kozlov, M.V.; Lanta, V.; Zverev, V.; Zvereva, E.L. Background losses of woody plant foliage to insects show variable relationships with plant functional traits across the globe. J. Ecol. 2015, 103, 1519–1528. [Google Scholar] [CrossRef]
- Gardner, A.; Ellsworth, D.S.; Crous, K.Y.; Pritchard, J.; MacKenzie, A.R. Is photosynthetic enhancement sustained through three years of elevated CO2 exposure in 175-year-old Quercus robur? Tree Physiol. 2022, 42, 130–144. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Crutsinger, G.M.; Kumordzi, B.B.; Wardle, D.A. Nutrient fluxes from insect herbivory increase during ecosystem retrogression in boreal forest. Ecology 2016, 97, 124–132. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 23 September 2021).
- Friedrich, S.; Konietschke, F.; Pauly, M. MANOVA.RM. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 7 January 2021).
- Ode, P.J.; Johnson, S.N.; Moore, B.D. Atmospheric change and induced plant secondary metabolites–are we reshaping the building blocks of multi-trophic interactions? Curr. Opin. Insect Sci. 2014, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stiling, P.; Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob. Change Biol. 2007, 13, 1823–1842. [Google Scholar] [CrossRef]
- Kuchenbecker, J.; Macedo-Reis, L.E.; Fagundes, M.; Neves, F.S. Spatiotemporal Distribution of Herbivorous Insects Along Always-Green Mountaintop Forest Islands. Front. For. Glob. Ch. 2021, 4, 709403. [Google Scholar] [CrossRef]
- Kristensen, J.A.; Rousk, J.; Metcalfe, D.B. Below-ground responses to insect herbivory in ecosystem with woody plant canopies: A meta-analysis. J. Ecol. 2019, 108, 917–930. [Google Scholar] [CrossRef]
- Shaw, D.C.; Ernest, K.A.; Rinker, H.B.; Lowman, M.D. Stand-level herbivory in an old-growth conifer forest canopy. West. North Am. Nat. 2006, 66, 473–481. [Google Scholar] [CrossRef][Green Version]
- Brown, B.J.; Allen, T.F.H. The importance of scale in evaluating herbivory impacts. Oikos 1989, 54, 189–194. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, Z.; Li, X. Influences of environmental factors of leaf morphology. PLoS ONE 2015, 10, e0127825. [Google Scholar]
- Chapman, S.; Schweitzer, J.A.; Whitham, T.G. Herbivory differently alters plant litter dynamics of evergreen and deciduous trees. Oikos 2006, 114, 3. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Zhao, K.; Niklaus, P.A.; Schmid, B.; He, J.S. Positive effects of tree species diversity on litterfall quantity and quality along a secondary successional chronosequence in a subtropical forest. J. Plant Ecol. 2017, 11, 28–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).