Responses of Early Distribution and Developmental Traits of Male and Female Trees to Stand Density in Fraxinus mandshurica Rupr. Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
2.2. Distribution Pattern of Females and Males
2.3. Phenotype Traits Measurements
2.4. Data Analysis
3. Results
3.1. Number and Distribution of Males and Females Trees
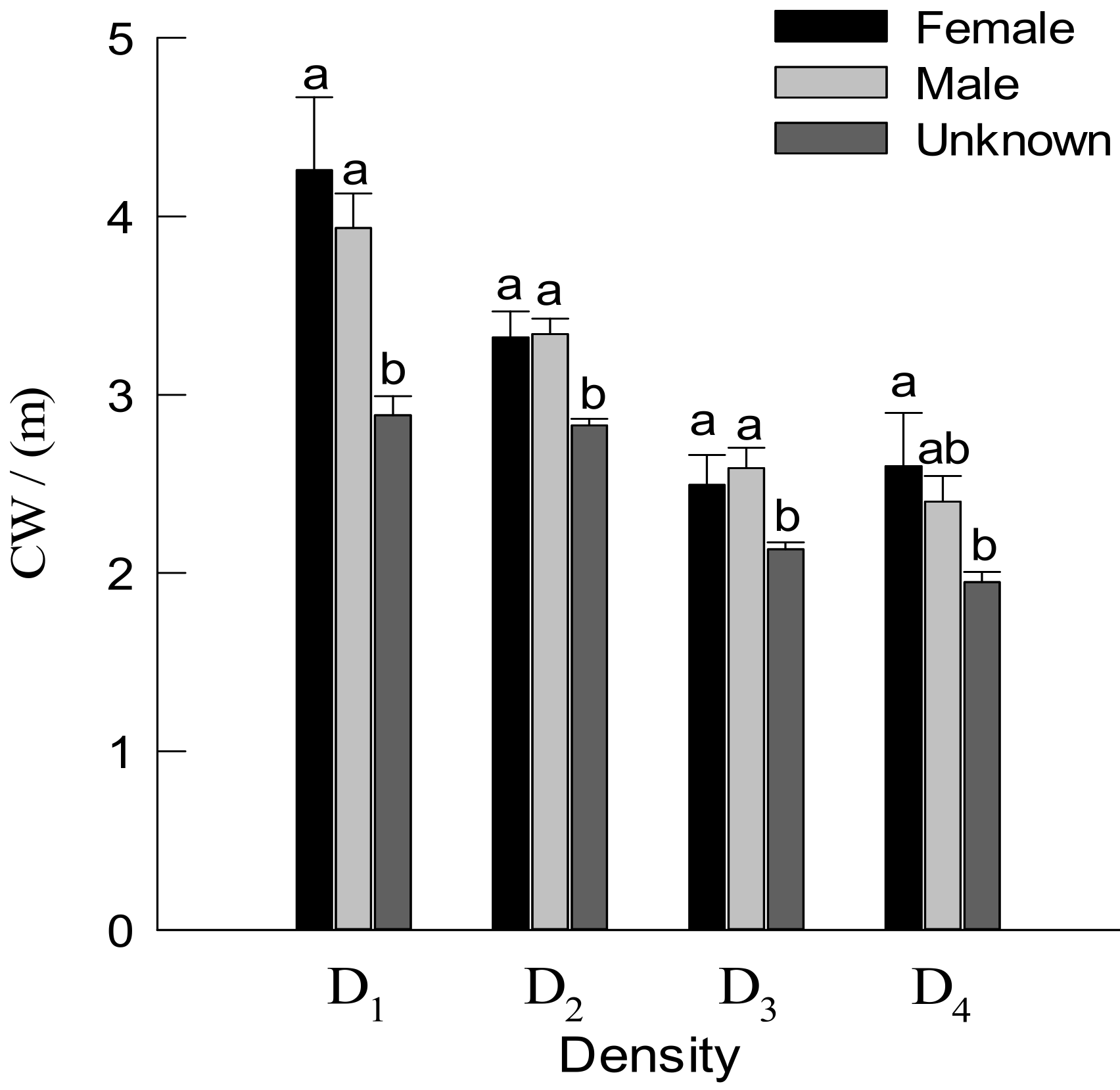
3.2. DBH, TH and Crown Traits of Males and Females Trees
3.3. Branch Traits of Males and Females Trees
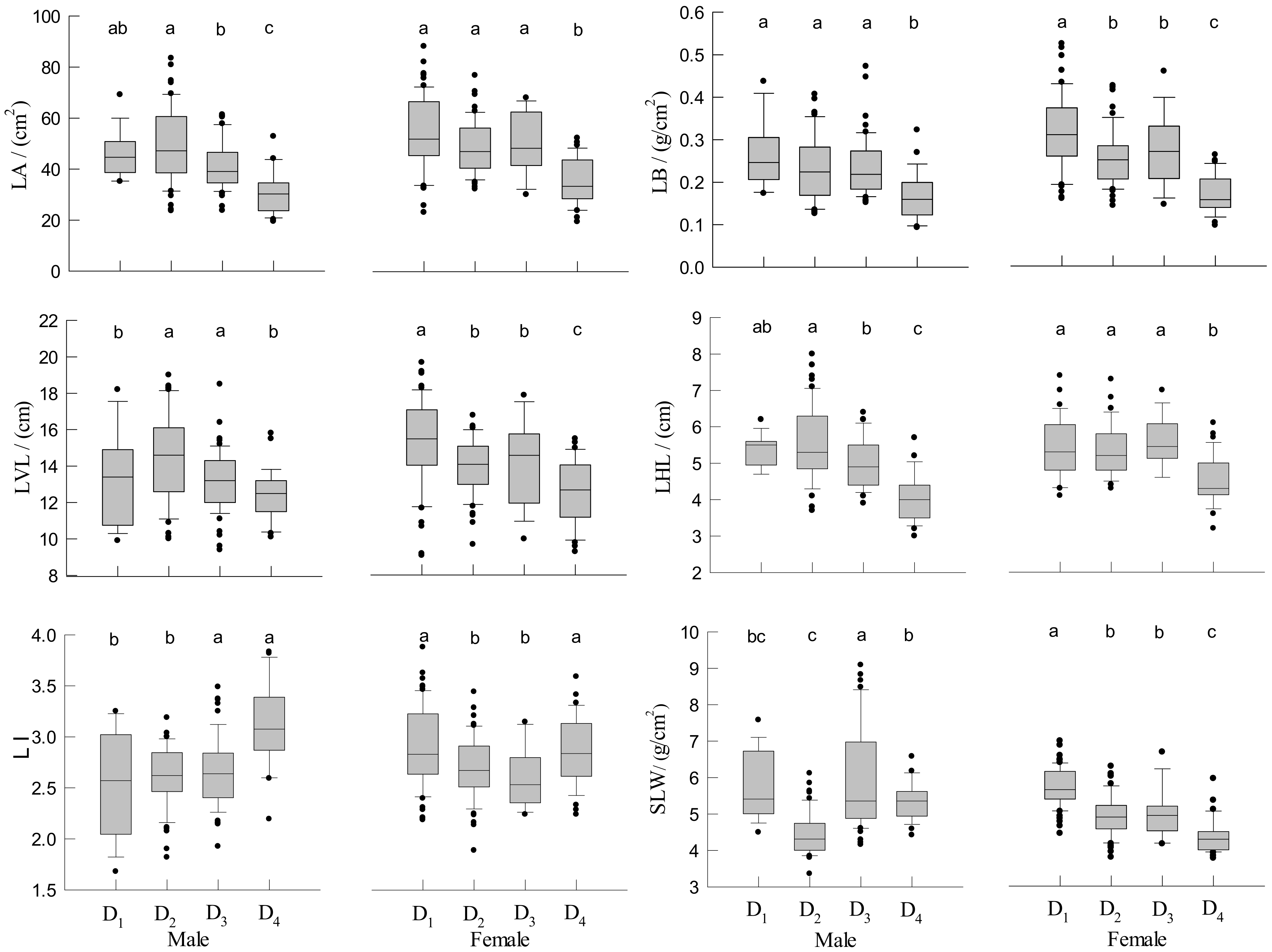
3.4. Leaf Traits of Male and Female Trees
3.5. The Influence of Density and Gender on Phenotypic Traits Variation
4. Discussion
4.1. The Number and Distribution of Males and Females for Different Densities
4.2. Effect of Density on Growth Traits
4.3. Effect of Density on Branch and Leaf Traits
4.4. Factors Affecting the Growth of Males and Females
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Andrews, C.M.; D’Amato, A.W.; Fraver, S.; Palik, B.; Battaglia, M.A.; Bradford, J.B. Low stand density moderates growth declines during hot droughts in semi-arid forests. J. Appl. Ecol. 2020, 57, 1089–1102. [Google Scholar] [CrossRef]
- Naji, H.R.; Nia, M.F.; Kiaei, M.; Abdul-Hmid, H.; Soltani, M.; Faghihi, A. Effect of intensive planting density on tree growth, wood density and fiber properties of maple (Acer velutinum Boiss.). Iforest-Biogeosciences For. 2016, 9, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Mead, D.J. Forests for energy and the we of named trees. Crit. Rev. Plant Sci. 2015, 24, 407–421. [Google Scholar] [CrossRef]
- McEwan, A.; Marchi, E.; Spinelli, R.; Brink, M. Past, present and future of industrial plantation forestry and implication on future timber harvesting technology. J. For. Res. 2020, 31, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Kerhoulas, L.P.; Kolb, T.E.; Koch, G.W. Tree size, stand density, and the source of water used across seasons by ponderosa pine in northern Arizona. For. Ecol. Manag. 2013, 289, 425–433. [Google Scholar] [CrossRef]
- Moore, J.R.; Dash, J.P.; Lee, J.R.; Russell, B.M.; Dungey, H.S. Quantifying the influence of seedlot and stand density on growth, wood properties and the economics of growing radiata pine. For. Int. J. For. Res. 2018, 91, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Thurm, E.A.; Pretzsch, H. Growth–density relationship in mixed stands—Results from long-term experimental plots. For. Ecol. Manag. 2021, 483, 118909. [Google Scholar] [CrossRef]
- Beniušienė, L.; Šilinskas, B.; Beniušis, R.; Aleinikovas, M.; Petrauskas, E.; Varnagirytė-Kabašinskienė, I. Scots Pine Stem Parameters in Sites with Different Stand Densities in Lithuania. Forests 2020, 11, 716. [Google Scholar] [CrossRef]
- Zhao, D.H.; Kane, M.; Borders, B.E. Growth responses to planting density and management intensity in loblolly pine plantations in the southeastern USA lower coastal plain. Ann. For. Sci. 2011, 68, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Trouvé, R.; Bontemps, J.D.; Seynave, I.; Collet, C.; Lebourgeois, F. Stand density, tree social status and water stress influence allocation in height and diameter growth of Quercus petraea (Liebl.). Tree Physiol. 2015, 35, 1035–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cregg, B.M.; Dougherty, P.M.; Hennessey, T.C. Growth and wood quality of young loblolly pine trees in relation to stand density and climatic factors. Can. J. For. Res. 1988, 18, 851–858. [Google Scholar] [CrossRef]
- Brunner, A.; Forrester, D.I. Tree species mixture effects on stem growth vary with stand density—An analysis based on individual tree responses. For. Ecol. Manag. 2020, 473, 118334. [Google Scholar] [CrossRef]
- Korgiopoulou, C.; Bresta, P.; Nikolopoulos, D.; Karabourniotis, G. Sex-specific structural and functional leaf traits and sun-shade acclimation in the dioecious tree Pistacia vera (Anacardiaceae). Funct. Plant Biol. 2019, 46, 649–659. [Google Scholar] [CrossRef]
- Renner, S.S.; Ricklefs, R.E. Dioecy and its correlates in the flowering plants. Am. J. Bot. 1995, 82, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; He, X.; Xu, X.; Yang, D.M. Scaling relationships among twig components are affected by sex in the dioecious tree Populus cathayana. Trees 2015, 29, 737–746. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhao, X. Biomass allocation patterns and allometric relationships between components of the androdioecious Acer tegmentosum. Ann. For. Sci. 2016, 73, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Kersten, B.; Pakull, B.; Fladung, M. Genomics of sex determination in dioecious trees and woody plants. Trees 2017, 31, 1113–1125. [Google Scholar] [CrossRef]
- Gao, L.S.; Zhang, C.Y.; Zhao, X.H.; Gadow, K.V. Gender-related climate response of radial growth in dioecious Fraxinum mandshurica trees. Tree-Ring Res. 2010, 66, 105–112. [Google Scholar] [CrossRef]
- Tanis, S.R.; McCullough, G.G.; Cregg, B.M. Effects of paclobutrazol and fertilizer on the physiology, growth and biomass allocation of three Fraxinus species. Urban For. Urban Green 2015, 14, 590–598. [Google Scholar] [CrossRef]
- Bochenek, G.M.; Eriksen, B. Annual growth of male and female individuals of the Common Ash (Fraxinus excelsior L.). Plant Ecol. Divers. 2010, 3, 47–57. [Google Scholar] [CrossRef]
- Salako, V.K.; Kenou, C.; Daainou, K.; Assogbadjo, A.E.; Kakai, R.G. Impacts of land use types on spatial patterns and neighbourhood distance of the agroforestry palm Borassus aethiopum Mart. in two climatic regions in Benin, West Africa. Agrofor. Syst. 2019, 93, 1057–1071. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zhang, C.Y.; Zhao, X.H. Effect of sex ratio, habitat factors and neighborhood competition on stem growth in the dioecious tree Fraxinus mandshurica. Ecol. Res. 2014, 29, 309–317. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhao, X.H.; Gao, L.S.; Klaus, V.G. Gender-related distributions of Fraxinus mandshurica in secondary and old-growth forests. Acta Oecologica 2010, 36, 55–62. [Google Scholar] [CrossRef]
- Allen, M.G.; Burkhart, H.E. Growth-density relationships in Loblolly Pine plantations. For. Sci. 2019, 65, 250–264. [Google Scholar] [CrossRef]
- Masinda, M.M.; Li, F.; Liu, Q.; Sun, L.; Hu, T.X. Prediction model of moisture content of dead fine fuel in forest plantations in Maoer Mountain, Northeast China. J. For. Res. 2021, 32, 2023–2035. [Google Scholar] [CrossRef]
- Kershaw, K.A. An Empirical Approach to the Estimation of Pattern Intensity from Density and Cover Data. Ecology 1970, 52, 729–734. [Google Scholar] [CrossRef]
- Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 2002, 80, 94–101. [Google Scholar] [CrossRef]
- Stehlik, I.; Friedman, J.; Barrett, S.C.H. Environment influence on primary sex ratio in a dioecious plant. Proc. Natl. Acad. Sci. USA 2008, 205, 10847–10852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, M.K.; Kane, M.; Zhao, D.H.; Teskey, R.O.; Daniels, R.F. Effect of planting density and cultural intensity on stand and crown attributes of mid-rotation loblolly pine plantations. For. Ecol. Manag. 2013, 310, 468–475. [Google Scholar] [CrossRef]
- Truax, B.; Fortier, J.; Gagnon, D.; Lambert, F. Planting density and site effects on stem dimensions, stand productivity, biomass partitioning, carbon stocks and soil nutrient supply in Hybrid poplar plantations. Forests 2018, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Litrico, I.; Maurice, S. Resources, competition and selfing: Their influence on reproductive system evolution. Evol. Ecol. 2013, 27, 923–936. [Google Scholar] [CrossRef]
- Nanami, S.; Kawaguchi, H.; Yamakura, T. Sex ratio and gender-dependent neighboring effects in Podocarpus nagi, a dioecious tree. Plant Ecol. 2005, 177, 209–222. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Borkhert, E.V.; Snezhkina, A.V.; Kudryavtseva, A.V.; Dmitriev, A.A. Sex-Specific response to stress in populus. Front. Plant Sci. 2017, 8, 1827. [Google Scholar] [CrossRef] [Green Version]
- Delph, C. The Effects of Gender and plant architecture on allocation to flowers in dioecious Silene latifolia (Caryophyllaceae). Int. J. Plant Sci. 1996, 157, 493–500. [Google Scholar]
- Garbarino, M.; Weisberg, P.J.; Bagnara, L.; Urbinati, C. Sex-related spatial segregation along environmental gradients in the dioecious conifer, Taxus baccata. For. Ecol. Manag. 2015, 358, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Iszkulo, G.; Broatynski, A. Initial period of sexual maturity determines the greater growth rate of male over female in the dioecious tree Juniperus communis subsp. Communis. Acta Oecologica-Int. J. Ecol. 2011, 37, 99–102. [Google Scholar] [CrossRef]
- Devaney, J.L.; Jansen, M.A.K.; Whelan, P.M. Spatial patterns of natural regeneration in stands of English yew (Taxus baccata L.); Negative neighbourhood effects. For. Ecol. Manag. 2014, 321, 52–60. [Google Scholar] [CrossRef]
- Walcroft, A.; Le Roux, X.; Diaz-Espejo, A.; Dones, N.; Sinoquet, H. Effects of crown development on leaf irradiance, leaf morphology and photosynthetic capacity in a peach tree. Tree Physiol. 2020, 22, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, G.; Hangartner, S.; Chapple, D.G.; Connallon, T. Quantifying maladaptation during the evolution of sexual dimorphism. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slate, M.L.; Rosenstiel, T.N.; Eppley, S.M. Sex-specific morphological and physiological differences in the moss Ceratodon purpureus (Dicranales). Ann. Bot. 2017, 120, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Jiang, X.-H. Size-dependent resource allocation and sex allocation in herbaceous perennial plants. J. Evol. 2002, 15, 74–83. [Google Scholar] [CrossRef]
- Chmura, D.J.; Rahman, M.S.; Tjoelker, M.G. Crown structure and biomass allocation patterns modulate aboveground productivity in young loblolly pine and slash pine. For. Ecol. Manag. 2007, 243, 219–230. [Google Scholar] [CrossRef]
- Pacey, E.K.; Maherali, H.; Husband, B.C. Endopolyploidy is associated with leaf functional traits and climate variation in Arabidopsis thaliana. Am. J. Bot. 2020, 107, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Oikawa, S.; Hirose, T. Leaf dynamics in growth and reproduction of Xanthium canadense as influenced by stand density. Ann. Bot. 2015, 116, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mckown, A.D.; Klápště, J.; Guy, R.D.; Soolanayakanahally, R.Y.; Mantia, J.L.; Porth, I.; Skyba, O.; Unda, F.; Douglas, C.J.; EI-Kassaby, Y.A.; et al. Sexual homomorphism in dioecious trees: Extensive tests fail to detect sexual dimorphism in Populus. Sci. Rep. 2018, 8, 1831. [Google Scholar] [CrossRef]
- Farooq, T.H.; Wu, W.J.; Tigabu, M.; Ma, X.Q.; He, Z.M.; Rashid, M.H.U.; Gilani, M.M.; Wu, P.F. Growth, Biomass Production and Root Development of Chinese fir in Relation to Initial Planting Density. Forests 2019, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.P.; Jiang, B.; Yuan, W.G.; Shen, A.H.; Yang, S.Z.; Yao, S.H.; Liu, J.J. On the Management of Large-Diameter Trees in China’s Forests. Forests 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Roessiger, J.; Ficko, A.; Clasen, C.; Griess, V.C.; Knoke, T. Variability in growth of trees in uneven-aged stands displays the need for optimizing diversified harvest diameters. Eur. J. For. Res. 2016, 135, 283–295. [Google Scholar] [CrossRef]
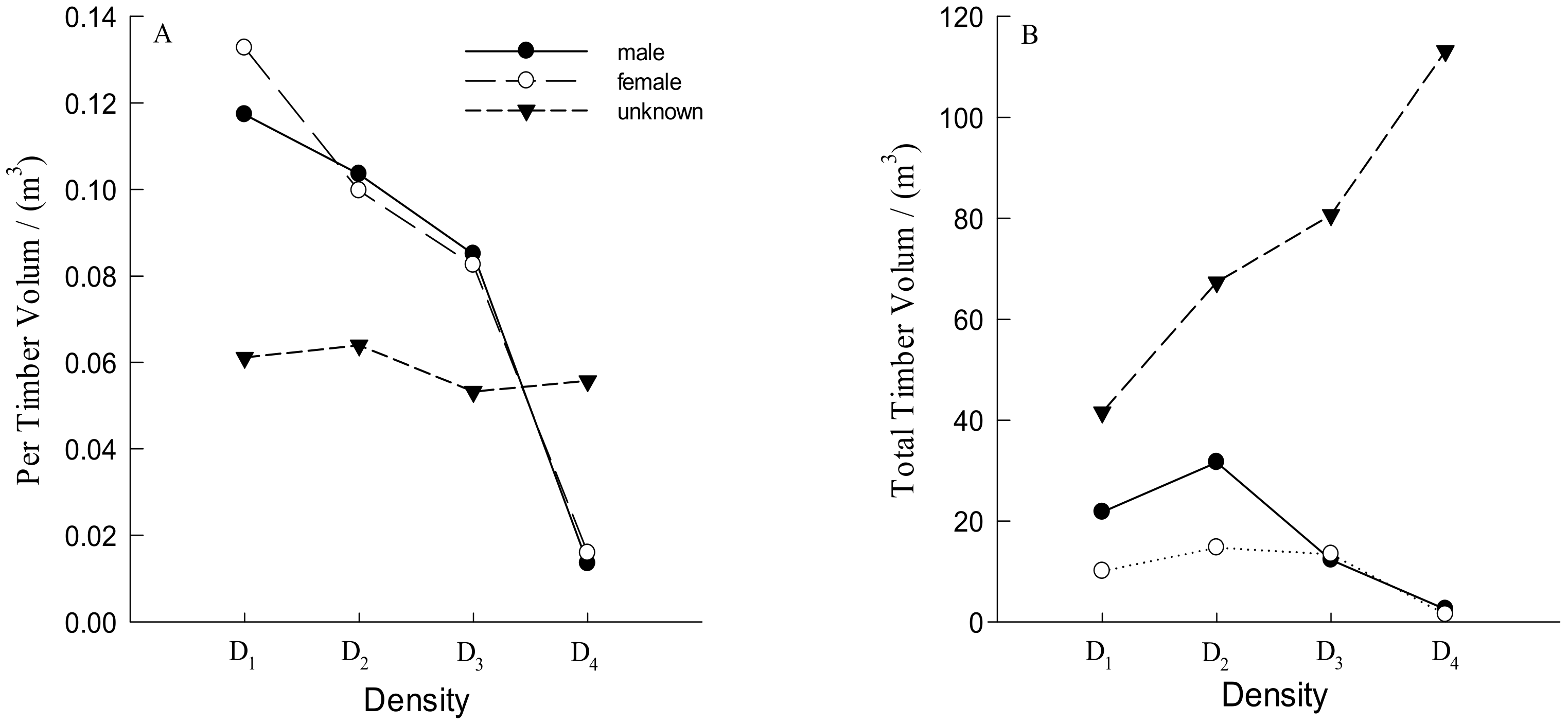
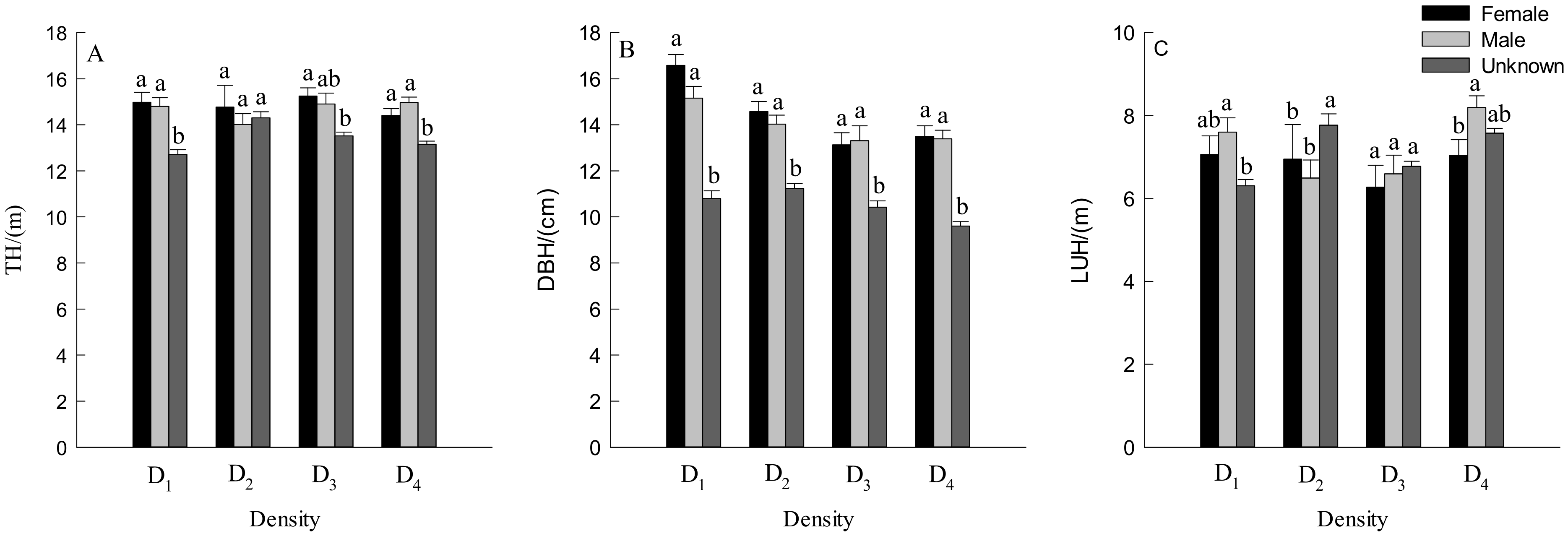
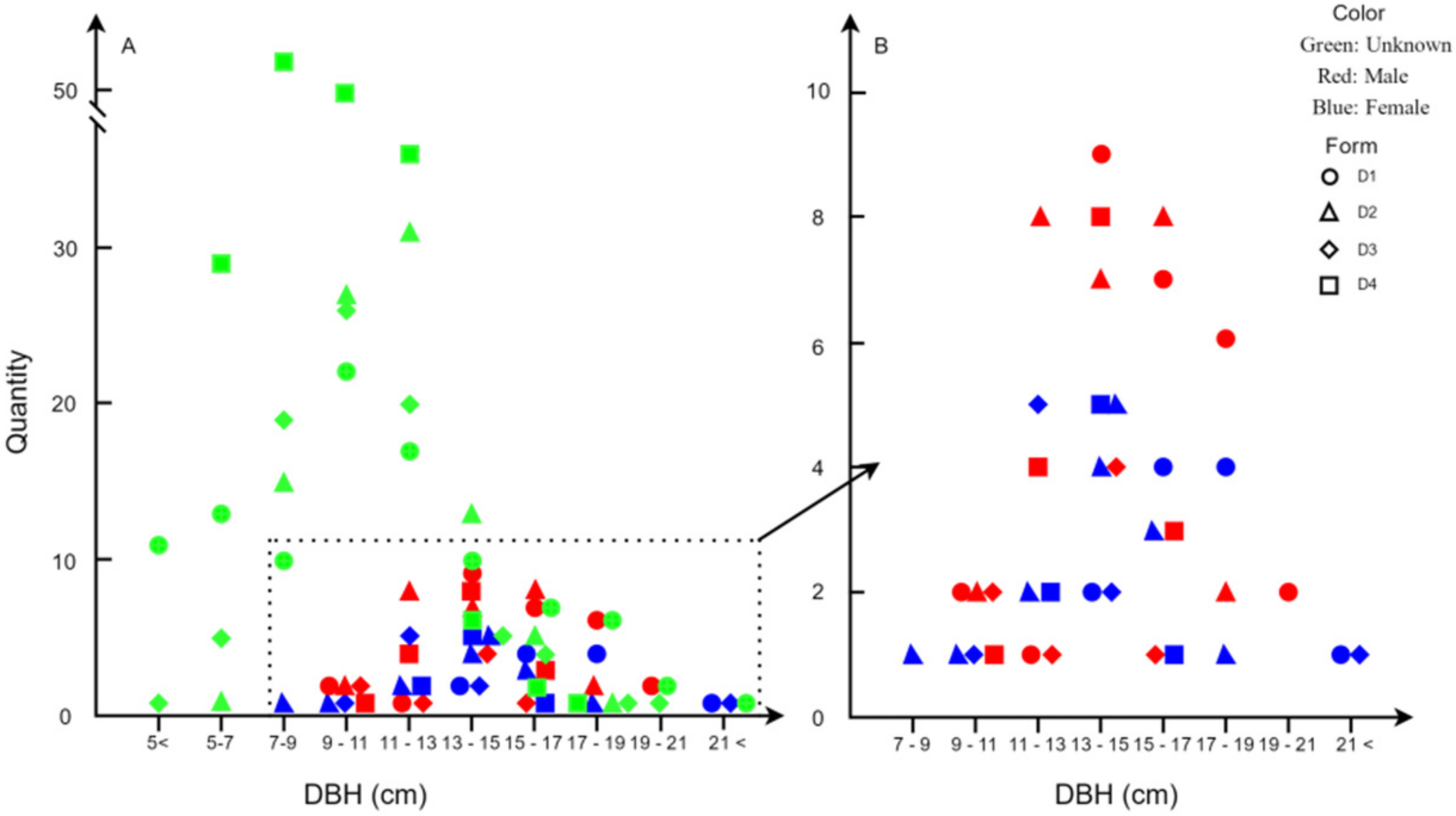
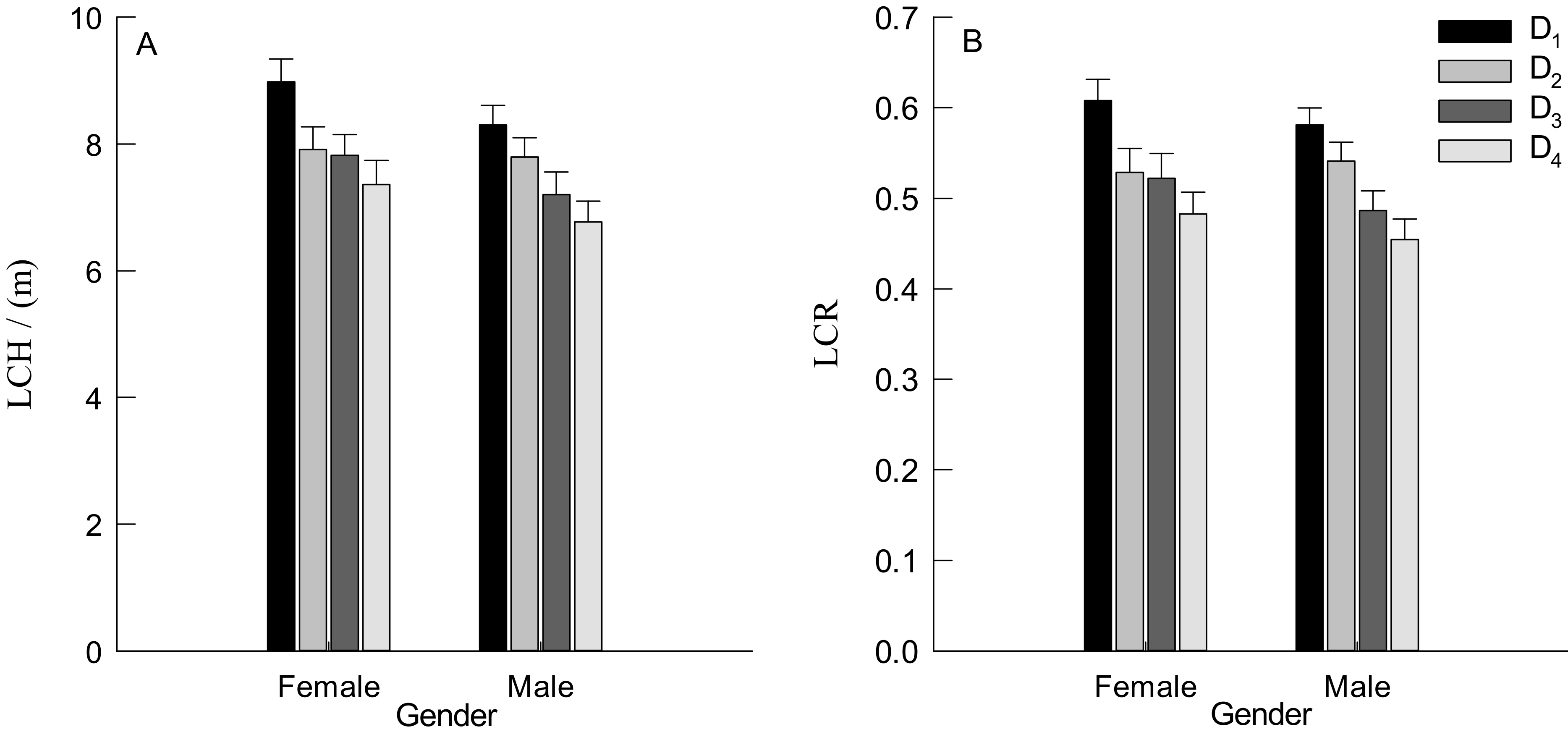

| Density Type | Plant Space × Row Space | Original Density (Quantity/hm2) | Current Density (Quantity/ha) | Survival Rate |
|---|---|---|---|---|
| D1 | 1.5 m × 3.0 m | 2200 | 941 ± 71 | 0.8 |
| D2 | 2.0 m × 2.0 m | 2500 | 1505 ± 101 | 0.9 |
| D3 | 1.5 m × 1.5 m | 4400 | 1823 ± 103 | 0.9 |
| D4 | 1.0 m × 1.0 m | 10,000 | 2308 ± 61 | 0.9 |
| Density | Males (%) | Females (%) | Unknown (%) |
|---|---|---|---|
| D1 | 19.71 | 8.03 | 72.26 |
| D2 | 20.30 | 9.77 | 69.92 |
| D3 | 7.92 | 8.91 | 83.16 |
| D4 | 8.00 | 4.00 | 88.00 |
| Density | Scale (m) | C | t | t0.05 | Type | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| D1 | 6 m × 6 m | 0.79 | 0.92 | −0.84 | −0.32 | 2.07 | 2.07 | Random | Random |
| 6 m × 12 m | 0.80 | 1.09 | −0.55 | 0.24 | 2.20 | 2.20 | Random | Random | |
| 12 m × 12 m | 0.79 | 1.10 | −0.40 | 0.19 | 2.57 | 2.57 | Random | Random | |
| 12 m × 24 m | 0.93 | 1.51 | −0.09 | 0.63 | 4.30 | 4.30 | Random | Random | |
| D2 | 6 m × 6 m | 0.73 | 0.92 | −0.91 | −0.28 | 2.07 | 2.07 | Random | Random |
| 6 m × 12 m | 0.53 | 0.75 | −1.11 | −0.60 | 2.20 | 2.20 | Random | Random | |
| 12 m × 12 m | 0.12 | 1.31 | −1.40 | 0.50 | 2.57 | 2.57 | Random | Random | |
| 12 m × 24 m | 0.25 | 1.46 | −0.75 | 0.46 | 4.30 | 4.30 | Random | Random | |
| D3 | 6 m × 6 m | 0.62 | 0.74 | −1.11 | −0.76 | 2.07 | 2.07 | Random | Random |
| 6 m × 12 m | 0.57 | 1.13 | −0.86 | 0.25 | 2.20 | 2.20 | Random | Random | |
| 12 m × 12 m | 0.50 | 0.70 | −0.79 | −0.47 | 2.57 | 2.57 | Random | Random | |
| 12 m × 24 m | 0.50 | 1.00 | −0.5 | 0 | 4.30 | 4.30 | Random | Random | |
| D4 | 6 m × 6 m | 1.12 | 0.64 | 0.42 | −1.22 | 2.07 | 2.07 | Random | Random |
| 6 m × 12 m | 1.14 | 0.52 | 0.32 | −1.14 | 2.20 | 2.20 | Random | Random | |
| 12 m × 12 m | 1.75 | 0.50 | 1.19 | −0.79 | 2.57 | 2.57 | Random | Random | |
| 12 m × 24 m | 1.80 | 0.13 | 0.8 | −0.88 | 4.30 | 4.30 | Random | Random | |
| Source | Df | P | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBH | TH | LUH | CW | LCH | LCR | ALT | LMD | LA | LB | LVL | LHL | LI | SLW | ||
| Density | 3 | 0.00 ** | 0.81 | 0.12 | 0.00 ** | 0.20 | 0.13 | 0.08 | 0.99 | 0.00 ** | 0.00 ** | 0.00 ** | 0.00 ** | 0.00 ** | 0.00 ** |
| Gender | 1 | 0.32 | 0.79 | 0.31 | 0.61 | 0.30 | 0.24 | 0.17 | 0.81 | 0.10 | 0.98 | 0.03 * | 0.31 | 0.75 | 0.04 * |
| D × G | 3 | 0.70 | 0.42 | 0.42 | 0.65 | 0.94 | 0.69 | 0.84 | 0.04 * | 0.04 * | 0.08 | 0.03 * | 0.08 | 0.00 ** | 0.00 ** |
| TH | DBH | LUH | LCH | LCR | CW | LMD | ALT | LA | LVL | LHL | LI | LB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBH | 0.22 (0.26) | ||||||||||||
| LUH | −0.06 (0.03) | 0.03 (0.29) | |||||||||||
| LCH | 0.64 ** (0.518 **) | 0.11 (−0.10) | −0.80 ** (−0.88 **) | ||||||||||
| LCR | 0.44 * (0.25) | 0.03 (−0.22) | −0.92 ** (−0.96 **) | 0.97 ** (0.96 **) | |||||||||
| CW | 0.03 (0.21) | 0.34 (0.55 **) | 0.16 (0.45 *) | −0.10 (−0.27) | −0.14 (−0.37) | ||||||||
| LMD | 0.27 (0.09) | 0.04 (0.33) | 0.02 (−0.17) | 0.15 (0.19) | 0.11 (0.18) | −0.08 (−0.09) | |||||||
| ALT | 0.20 (−0.16) | −0.20 (−0.29) | 0.03 (−0.23) | 0.08 (0.11) | 0.03 (0.18) | −0.43 * (−0.06) | −0.40 (−0.66 **) | ||||||
| LA | 0.17 (0.27) | −0.1575 | 0.12 (0.22) | 0.02 (−0.40) | −0.01 (−0.13) | −0.0126 | 0.06 (0.23) | 0.35 (−0.11) | |||||
| LVL | 0.03 (0.18) | −0.33 (0.18) | −0.12 (0.10) | 0.11 (0.01) | 0.13 (−0.50) | −0.30 (−0.12) | −0.21 (0.30) | 0.34 (−0.19) | 0.84 ** (0.84 **) | ||||
| LHL | 0.20 (0.30) | −0.36 (0.36) | 0.18 (0.12) | −0.02 (0.06) | −0.06 (−0.02) | −0.30 (0.10) | 0.39 * (0.20) | 0.10 (−0.01) | 0.68 ** (0.84 **) | 0.26 (0.55 **) | |||
| LI | −0.10 (−0.04) | −0.06 (−0.09) | −0.23 (0.04) | 0.12 (−0.06) | 0.16 (−0.07) | −0.07 (−0.23) | −0.072 | 0.27 (−0.20) | 0.35 (0.25) | 0.78 ** (0.68 **) | 0.096 | ||
| LB | 0.18 (0.29) | −0.18 (0.29) | −0.01 (0.21) | 0.11 (−0.12) | 0.08 (−0.12) | −0.45 (−0.01) | −0.07 (0.30) | 0.41 * (−0.19) | 0.75 ** (0.96 **) | 0.79 ** (0.90 **) | 0.31 (0.75 **) | 0.55 ** (0.38) | |
| SLW | 0.14 (0.04) | 0.22 (0.04) | −0.13 (−0.04) | 0.19 (0.05) | 0.16 (0.04) | −0.16 (−0.27) | −0.15 (0.28) | 0.28 (−0.27) | 0.02 (−0.01) | 0.25 (0.26) | −0.28 (−0.16) | 0.44 * (0.41 *) | 0.67 ** (0.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, Y.; Wei, X.; Wei, Q. Responses of Early Distribution and Developmental Traits of Male and Female Trees to Stand Density in Fraxinus mandshurica Rupr. Plantation. Forests 2022, 13, 472. https://doi.org/10.3390/f13030472
Li W, Zhang Y, Wei X, Wei Q. Responses of Early Distribution and Developmental Traits of Male and Female Trees to Stand Density in Fraxinus mandshurica Rupr. Plantation. Forests. 2022; 13(3):472. https://doi.org/10.3390/f13030472
Chicago/Turabian StyleLi, Wei, Yunlong Zhang, Xing Wei, and Qingyu Wei. 2022. "Responses of Early Distribution and Developmental Traits of Male and Female Trees to Stand Density in Fraxinus mandshurica Rupr. Plantation" Forests 13, no. 3: 472. https://doi.org/10.3390/f13030472
APA StyleLi, W., Zhang, Y., Wei, X., & Wei, Q. (2022). Responses of Early Distribution and Developmental Traits of Male and Female Trees to Stand Density in Fraxinus mandshurica Rupr. Plantation. Forests, 13(3), 472. https://doi.org/10.3390/f13030472





