Abstract
When natural regeneration of Quercus robur stands is hampered by an insufficient acorn yield, human assisted sowing of acorns collected in non-affected stands and stored for some period of time is performed. To inhibit the development of fungi and acorn deterioration during storage, thermotherapy is usually applied by submerging acorns for 2.5 h in water heated to 41 °C. This research aimed to test the effect of four thermotherapy treatments of different durations and/or applied temperatures as well as short-term storage at −1 °C or 3 °C on acorn internal mycobiota and germination. Fungal presence in cotyledons was analyzed in 450 acorns by isolation of mycelia on artificial media, followed by a DNA-based identification. Germination of 2000 acorns was monitored in an open field trial. Thermotherapy significantly decreased fungal diversity, while storage at 3 °C increased the isolation frequency of several fungi, mainly Penicillium spp. The most frequently isolated fungi did not show a negative impact on acorn germination after short-term storage. The study confirmed the efficiency of thermotherapy in the eradication of a part of acorn internal mycobiota, but also its effect on the proliferation of fast-colonizing fungi during storage. However, the latter showed to be more stimulated by storage conditions, specifically by storage at 3 °C.
1. Introduction
Quercus robur L. (English oak, pedunculate oak) is one of the most important European tree species both economically and ecologically. It is widespread throughout most of the continent, predominantly in forest stands, where it provides multiple benefits including wood highly valued for construction, fuel or furniture manufacturing, or as a habitat and food source for various animal and fungal species [1,2,3,4]. In the last several decades a number of European countries have reported a decline of Q. robur caused by a synergy of different abiotic and biotic factors [5]. The usual symptoms include increased crown defoliation and tree mortality, which are likely associated with an insufficient acorn yield and the suppression of grown seedlings by more competitive species in unfavorable site conditions. This often leads to impeded natural regeneration [5,6]. Given the more prominent pressure of newly emerging invasive pathogens and pests, such as the oak lace bug (Corythuca arcuata (Say, 1832)), and spread of the already established ones ensuing from global trade and climate change, there is justified concern for the health status and regeneration of natural Q. robur forest stands both in the present and future [7,8].
The difficulties related to impeded natural regeneration caused by an insufficient acorn yield are overcome by collecting seeds from other stands, storing them, and introducing them in the regeneration area by seeding or sowing, or using them for the production of seedlings which can be utilized for the same purpose [9]. The success of such human assisted regeneration and the progress of a future forest stand highly depend on seed quality [10,11]. Important factors that determine the quality of acorns are their size, weight, viability, moisture content, mineral nutrient reserves, levels of insect and fungus damage, and germination [12,13,14,15,16]. Since Q. robur acorns belong to a group of recalcitrant seeds, which are characterized by a high moisture content and sensitivity to desiccation, they are prone to viability loss during storage [17,18]. Several authors agree that Q. robur acorns cannot be stored for longer than one to three years, depending on storage conditions [19,20,21]. The recommended storage temperatures vary between −3 °C and 4 °C and are usually related to other factors, such as oxygen concentrations, type of storage containers, medium used (e.g., dry peat, sawdust, or no medium), seed pretreatment, etc. [18,22,23,24]. The main factor preserving acorn viability during storage is the moisture content, which should be at least 40% [23,25,26].
However, humid conditions suitable for the preservation of viability are conducive to fungal growth, which can again negatively affect acorn viability and the overall quality [19,27]. In general, fungal presence in seeds of forest tree species is known to have adverse effects on seed viability during storage, germination success, and health condition of the related plants in successive developmental stages. However, some fungi act only as endophytes or saprotrophs with no detrimental impact on the seeds or seedlings [28,29,30]. Oak acorns also host diverse mycobiota externally on the shell and internally on the cotyledons, which is shaped by the maternal (mother tree) and environmental effects and interactions with other seed-inhabiting organisms (like insects) [11,31,32,33,34,35]. Some of the fungi which invade oak acorns can act as antagonists of fungal pathogens or as harmless endophytes or saprotrophs [11,27,31]. On the other hand, some of these fungi are reported to initiate premature acorn shedding from a tree, cause acorn decay, and reduce germination ability [11,27,34,35,36,37,38]. The most detrimental fungal pathogen infecting oak acorns in Europe is considered to be Ciboria batschiana, as it causes black rot and mumification, especially during storage [39,40]. Other common pathogenic fungi found in acorns are Apiognomonia quercina, Botrytis cinerea, Schizophyllum commune, Stereum hirsutum, Diaporthe insularis, Phomopsis spp., Fusarium spp., and Ophiostoma spp [11,32,33].
One of the most effective methods to control Ciboria batschiana prior to acorn storage is thermotherapy treatment by hot water. It is usually conducted for 2.5 h at 41 °C [21,27,41,42]. These conditions do not have a negative impact on acorn germination or subsequent seedling growth, but at the same time they are destructive for the fungus [27,42,43]. Although this treatment can affect other pathogens as well, it does not have an eradicative effect on all seed-borne fungi and can potentially increase acorn susceptibility to new fungal invasions during storage, mostly by various Penicillium and Mucor species [44,45,46]. It is even suggested that changes in acorn mycobiota, followed by thermotherapy treatment, may reduce their storability [44]. Higher treatment temperatures or longer duration times may be more effective for a broader range of fungal species, but it is essential to find the optimal conditions that will eradicate or inhibit target organism(s) without reducing acorn quality [45,47]. However, there are only a few available studies on the use of higher temperatures to treat acorns. They deal, for example, with their effect on insect presence and moisture content [48,49]; however, little is known about their impact on general mycobiota.
Given the importance of acorn quality for the success of regeneration of oak forest stands and the great impact of the applied seed pretreatments, the aim of this research was to test the effect of different thermotherapy treatments and short-term storage conditions on the chosen attributes of Quercus robur acorns. The objectives were to: (1) test the impact of different thermotherapy temperatures on acorn germination in controlled conditions; (2) explore the effect of thermotherapy treatments of different temperatures and durations, followed by short-term storage at two different temperatures, on the mycobiota of acorn cotyledons; and (3) determine if the applied thermotherapy and storage conditions influence acorn germination in an open field trial.
2. Materials and Methods
Acorns were collected in October 2017 from the ground in Quercus robur forest stands. The stands are located in Croatia around the Training and Research Forest Centre Lipovljani, which is managed by the University of Zagreb, Faculty of Forestry and Wood Technology (45.3744° N, 16.8208° E). Empty and insect-damaged acorns were excluded from further analyses by water flotation and visual inspection. The acorns were stored at 3 °C in polyvinyl chloride (PVC) bags until analysis.
2.1. Testing of Acorn Germination at Different Temperatures
Temperature impact on germination was tested in controlled conditions on a sample of 300 acorns separated in 10 groups of 30. Every group was treated by thermotherapy at a different temperature, ranging from 42 to 60 °C, raising 2 °C per each group. For this purpose, groups of acorns were placed in separate PVC containers filled with 350 mL of water preheated to the target temperature and incubated for 2.5 h at the same temperature in a growth chamber (Kambič RK-980 CHCO2, Semič, Slovenia).
Germination was expressed as a proportion of acorns (%) in each group which developed into a normal seedling in the monitoring period, which was tested in accordance with ISTA (International Seed Testing Association) rules [50]. A PVC container filled with 1400 mL of heat-sterilized sand moistened with 518 mL of water was prepared for each group of 30 acorns. The pericarp and testa (seed coat) were removed, and acorns were cut-off two-thirds at the side opposite to the radicle, firmly placed on the sand surface and covered with a 1 cm layer of the same sand. The containers were then covered with PVC foil and lid and placed in a growth chamber (Kambič RK-980 CHCO2, Semič, Slovenia) set to the following conditions: constant temperature 20 °C and constant humidity 80%, with 12 h exposure to daylight (13,400–14,000 lux) and 12 h in the dark. Grown seedlings were inspected weekly for a total period of 42 days.
2.2. Thermotherapy Treatment and Storage Conditions
Considering the standardized acorn thermotherapy method applied in most accredited ISTA laboratories in the world, which entails placing the acorns in a water bath at 41 °C for 2.5 h [21] and based on the results of the previously described acorn germination testing, the chosen thermotherapy conditions in this study were 41 and 45 °C, for 2.5 and 5 h at each temperature. For this purpose, 1960 acorns were separated in four groups of 490, which were exposed to four thermotherapy treatments differing in applied temperature and/or duration. Each group was placed in a separate PVC container in 3 L of water preheated to 41 or 45 °C and then incubated in a growth chamber (Kambič RK-980 CHCO2, Semič, Slovenia) at the same temperature for 2.5 or 5 h.
After treatment, acorns were surface dried at room temperature for several hours and then stored in different conditions. Out of the 490 acorns used for each thermotherapy treatment, 90 were separated for the analysis of fungal presence and 400 for the nursery trial. Those planned for the analysis of fungi were further divided in three groups of 30. The first group was analyzed within 24 h from the treatment (no storage), the second one was stored for one month in a laboratory freezer at −1 °C (Pol-Eko-Aparatura ZLN-T 300, Wodzisław Śląski, Poland), and the third group was stored for the same period in a laboratory refrigerator at 3 °C (Kirsch Super-720, Offenburg, Germany). Acorns designated for the nursery trial were separated in two groups of 200, which were stored for a period of two months at −1 °C and at 3 °C (Figure 1). All groups were stored separately in sealed PVC bags.
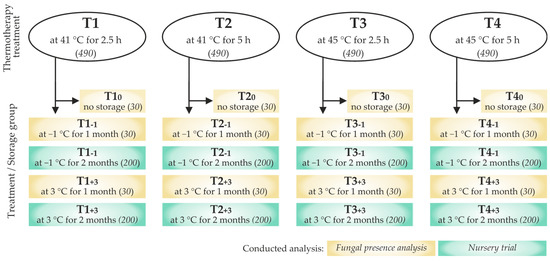
Figure 1.
Scheme of Quercus robur acorn groups exposed to different thermotherapy treatment and storage conditions, used for the analysis of fungal presence, and for the nursery trial (group labels are given in bold, numbers of used acorns are given in brackets in italic).
2.3. Isolation and Identification of Fungi
Fungal mycelia were isolated from 360 acorns treated by thermotherapy and stored at different conditions (Figure 1) and from an additional 90 non-treated acorns that served as a control, of which 30 were processed immediately upon field sampling and quality inspection, 30 were stored at −1 °C, and 30 at 3 °C for a period of one month.
Acorns were surface sterilized in a sodium hypochlorite (NaOCl) solution (1% v/v) for five minutes and rinsed three times in sterile distilled water. The pericarp was then removed and small pieces (5 × 5 mm) of cotyledons taken from the edges of advancing discolorations or necroses if present, or from visually healthy tissue. The pieces were then plated in 90 mm Petri dishes on potato dextrose agar (PDA, Oxoid, Basingstoke, UK) amended with streptomycin sulphate (200 mg/L, Sigma-Aldrich, St. Louis, MO, USA). Petri dishes were incubated in the dark at 20 °C for four weeks and checked daily for fungal growth. The emerging mycelia were subcultured to the PDA medium. Pure cultures were grouped into morphotypes based on mycelium color, structure and growth speed, coloration of agar, and sporulation. At least one isolate of each morphotype group was used for molecular identification.
DNA extraction was performed according to Ježić et al. with modifications [51,52] and PCR amplification was conducted with primers ITS1-F [53] and ITS 4 [54] in 65 µL reactions containing 200 µM deoxyribonucleoside triphosphates, 0.4 µM of each primer, 0.5 U of Taq DNA polymerase (Sigma-Aldrich, St. Louis, MO, USA), 1.5 mM MgCl2, 1x reaction buffer, and 1 µL of DNA template. Cycling conditions were as follows: an initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 45 s, extension at 72 °C for 90 s, and a final extension step at 72 °C for 5 min. The resulting PCR products were sequenced using the same primers as in the PCR, at the DNA sequencing facility of Macrogen Europe (Amsterdam, Netherlands). After processing raw data using the BioEdit Sequence Alignment Editor v.7.2.5 software [55], sequences were identified by comparison with reference sequences in the National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST) [56]. Sequences with 99.0–100% similarity were identified to the species level and those with 95.0–98.99% of similarity to the genus level. In cases of ambiguous results for some morphotypes belonging to the genus Penicillium, additional DNA regions were amplified in PCR reactions, depending on each individual morphotype. Translation elongation factor 1-α (TEF1-α) was amplified using primers EF1-1018F and EF1-1620R [57] following the protocol of Rehner and Buckley [58] and the β-tubulin (TUB2) locus was amplified with a primer set Bt2a and Bt2b [59] according to the protocol of Braun et al. [60].
The impact of thermotherapy treatment and storage conditions on the number of isolated mycelia and different fungal taxa per acorn was analyzed using the Kruskal–Wallis test and multiple comparison of mean ranks for all groups post hoc test. Associations of the ten most frequent fungal taxa with treatment and storage conditions were explored using correspondence analysis (CA). Tests were performed in the TIBCO Statistica 13.5.0 software package (TIBCO Software Inc., Palo Alto, CA, USA).
2.4. Nursery Trial
Nursery germination of acorns was monitored in an open field trial conducted in the nursery “Šumski vrt i arboretum” located at the University of Zagreb, Faculty of Forestry and Wood Technology (45.8202° N, 16.0228° E). A total of 2000 acorns were used in the trial; 1600 treated by thermotherapy (Figure 1) and 400 non-treated control ones, half of which were stored at −1 °C and another half at 3 °C. Acorns were sown on 3 May 2018 in 10 blocks corresponding to 10 groups exposed to different thermotherapy and storage conditions (eight treated groups and two control ones) (Figure 2). In each block acorns were sown in 4 rows per 50 at the depth of 5 cm. Prior to sowing, the acorns were treated with contact fungicide (35% copper + 2% zinc).
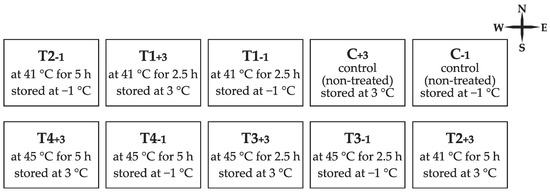
Figure 2.
Block distribution of Quercus robur acorn groups exposed to different thermotherapy treatment and storage conditions sown in the nursery trial.
Grown seedlings were counted after their first emergence, 21 days after sowing (24 May 2018), and again after an additional 42 days of growth (5 July 2018). Nursery germination (%) was expressed as a share of grown seedlings in a total of 200 sown acorns per each block.
Correlation between the number of obtained isolates of the eight most frequent fungal species (which occurred in at least two acorn groups) and the germination proportion of each acorn group was tested in the TIBCO Statistica 13.5.0 software package (TIBCO Software Inc., Palo Alto, CA, USA).
3. Results
3.1. Impact of Temperature on Acorn Germination in Controlled Conditions
Germination of Quercus robur acorns exposed to a thermotherapy treatment at temperatures ranging from 42 to 60 °C was evaluated based on the number of grown seedlings in the monitoring period. On the 7th day of monitoring, there was no seedling growth in any of the 10 groups of acorns treated at different temperatures. The first seedlings were observed on the 14th day of monitoring only in groups treated at 46 and 48 °C. Generally, most of the seedlings emerged in the third week of monitoring (between the 14th and 21st days) in the groups treated at lower temperatures (42, 44, 46, and 48 °C), while in the groups treated at higher temperatures (54, 56, 58, and 60 °C) there was no observed growth in the entire monitoring period (Figure 3). Germination was the highest in the group treated at 46 °C.
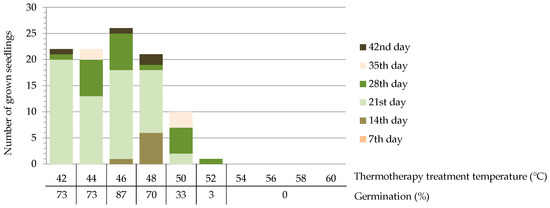
Figure 3.
Germination success of Quercus robur acorns treated by thermotherapy for 2.5 h at different temperatures.
3.2. Impact of Thermotherapy and Storage on Acorn Mycobiota
Mycobiota was analyzed in a total of 450 acorns distributed in 15 groups, of which 12 were exposed to different thermotherapy and storage conditions (Figure 1), and three were control groups stored under different conditions (no storage, at −1 °C, and at 3 °C). The analysis resulted in the growth of 1149 mycelia belonging to 46 different fungal taxa. There was not a single acorn without fungal growth in the group treated at 41 °C for 5 h and stored at −1 °C or in any of the groups stored at 3 °C, regardless of the thermotherapy treatment. In all other groups 10 to 53% of acorns did not reveal fungal presence (Figure 4). The highest number of mycelia was isolated from acorns in groups stored at 3 °C, while the highest number of different fungal taxa was found in the control groups (Figure 4).
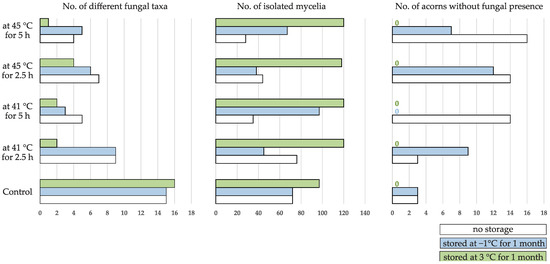
Figure 4.
Number of obtained fungal taxa, isolated mycelia, and acorns without fungal growth in Quercus robur acorn groups exposed to different thermotherapy treatment and storage conditions.
The Kruskal–Wallis test revealed that thermotherapy treatment conditions had a significant impact on the number of different fungal taxa found per acorn (H (4) = 66.39, p < 0.001) and that storage conditions significantly affected both the number of isolated mycelia (H (2) = 151.93, p < 0.001) and different fungal taxa per acorn (H (2) = 18.85, p < 0.001). Multiple comparison of mean ranks for all groups showed that the number of fungal taxa per acorn was significantly higher in control groups compared to all treated groups (Table 1). According to the same test, a significantly higher number of mycelia per acorn developed in groups stored at 3 °C in comparison with non-stored ones (p < 0.001) and with those stored at −1 °C (p = < 0.001). However, the groups stored at 3 °C exhibited significantly less different fungal taxa per acorn in comparison with the latter two groups (p = 0.001 and p = 0.031, respectively).

Table 1.
Impact of thermotherapy treatment conditions on the number of obtained fungal taxa per acorn tested with multiple comparison of mean ranks for all groups post hoc test (significant p values among treatments are highlighted in red).
The most frequently isolated species were Penicillium glandicola, encompassing 67% of all isolated mycelia and occurring in all 15 acorn groups, and Penicillium glabrum, represented in 15% of all isolates and found in 13 acorn groups. Among the ten most frequently found fungal taxa were also Truncatella angustata, Tubakia dryina, Fusarium solani, Trichoderma citrinoviride, Alternaria alternata, Talaromyces sp., and Fusarium avenaceum, encompassing together 11% of all isolates obtained in the research. The remainder of 36 fungal taxa was present in five or less isolates and occurred in only one or two acorn groups (Appendix A, Table A1).
The correspondence analysis revealed that association of fungal taxa was statistically significant with both acorn storage (X2 = 306.62, df = 18, p < 0.001) and treatment conditions (X2 = 381.93, df = 36, p < 0.001). P. glandicola, F. solani, and Talaromyces sp. were most associated with acorns stored at 3 °C, while P. glabrum, T. citrinoviride, and F. avenaceum were mostly found in non-stored acorns (Figure 5). In regards to thermotherapy treatments, P. glandicola, T. angustata, and T. citrinoviride were more associated with treated acorns; while T. dryina, F. solani, A. alternata, and F. avenaceum with non-treated acorns (Figure 6).
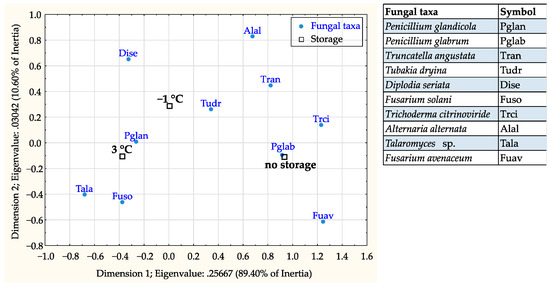
Figure 5.
Correspondence analysis map showing association of the 10 most frequent fungal taxa with acorn storage categories (no storage, storage at −1 °C, and storage at 3 °C).
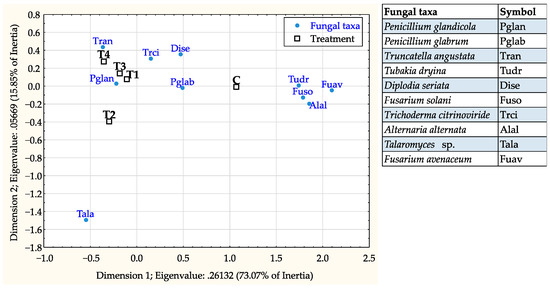
Figure 6.
Correspondence analysis map showing association of the 10 most frequent fungal taxa with acorn thermotherapy treatment categories (C = control, T1 = at 41 °C for 2.5 h, T2 = at 41 °C for 5 h, T3 = at 45 °C for 2.5 h, and T4 = at 45 °C for 5 h).
3.3. Impact of Thermotherapy and Storage on Acorn Germination in A Nursery
Nursery germination of thermotherapy treated and control acorns stored under different conditions (at −1 °C or at 3 °C) was monitored on the 21st and 63rd day after sowing. Acorns stored at 3 °C revealed equal or higher germination success in comparison with the ones stored at −1 °C, except in the control. The highest nursery germination (79%) was recorded in a group treated at 41 °C for 5 h and stored at 3 °C. It was generally higher in groups treated at 41 °C in comparison with groups treated at 45 °C (Figure 7).
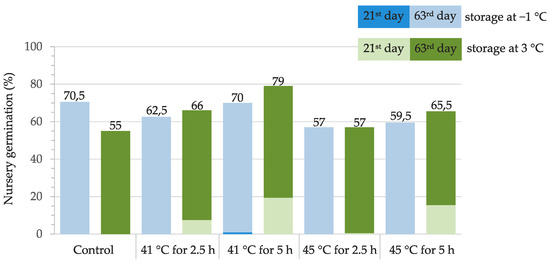
Figure 7.
Nursery germination of thermotherapy treated and non-treated (control) acorns stored under different conditions, monitored 21 and 63 days after sowing.
For the eight most frequent fungal taxa, which occurred in more than two treatment/storage acorn groups, there was no correlation between the number of their isolates and acorn germination in either of the groups sown in a nursery trial (Table 2).

Table 2.
Pearson correlation coefficients and related p values reveal no significant correlation between the number of obtained isolates of the eight most frequent fungal species and acorn germination in groups sown in a nursery trial.
4. Discussion
Thermotherapy is a recommended acorn treatment for the efficient control or eradication of pathogens and pests [41,48,61] and is usually applied before acorn storage. However, it is suggested by some authors that it can have adverse effects on the stored acorns, in terms of inflicting injuries, increasing their susceptibility to ubiquitous fungi, and reducing their viability [39,44,45]. Therefore, this study aimed to test the impact of different thermotherapy treatments followed by a short-term storage on the Quercus robur acorn mycobiota and germination.
The first experiment, which entailed exposing the acorns to different temperatures for 2.5 h, revealed a high or complete lack of germination in groups treated at temperatures above 48 °C. In those treated at 42 to 48 °C, germination varied between 70 and 87%, which is in accordance with other research reporting scarified acorn germination [62,63] and indicates that these treatment temperatures did not adversely affect germination in laboratory conditions. The temperature of 48 °C could thus be determined as the highest applicable temperature for acorn thermotherapy (among the ones used in this research) with no harmful effect on germination. In the groups treated at 46 and 48 °C, the first seedlings emerged 14 days after sowing, one week earlier than in the groups treated at 42 and 44 °C. This suggests the possible positive effects of the higher temperature on the time of germination, although more elaborative studies including acorn quality attributes are required for such a conclusion. Some of the possible explanations include the widely applicable Van’t Hoff’s rule, which states that metabolic activities rise with temperature increase [64], or the potential stimulating effect of heat damage on the faster development of radicle, as was the case with rodent-damaged acorns in the study conducted by Drvodelić and Oršanić [65].
Since the commonly performed thermotherapy treatment (at 41 °C for 2.5 h) can lead to the proliferation of ubiquitous fungal species during acorn storage [39,41,44,45], the second part of this study focused on the impact of different thermotherapy and short-term storage conditions on the internal acorn mycobiota. Generally, among the ten most frequently isolated species were Penicillim glandicola, P. glabrum, Fusarium solani, F. avenaceum, and Alternaria alternata, which was not surprising as members of these genera were reported to be the most common potentially harmful fungi inhabiting Quercus robur acorns in similar studies [11,27,32,33,66]. Several other frequently isolated species in this research were reported as plant pathogens by other authors, although not on oak acorns. Truncatella angustata is confirmed as the causative agent of leaf spots and fruit rot on several woody hosts [67,68,69,70], Tubakia dryina is described as pathogenic on Q. robur petioles and leaves [71], and Diplodia seriata is reported as a pathogen of Vitis vinifera L. and various pome and stone fruit trees and a xylem colonizer in declining oak trees [72,73,74,75]. As these most common fungi were mostly associated with cotyledon discoloration or necroses (personal observation, data not shown), they might play a role in acorn deterioration or viability loss during longer-term storage or storage under more unfavorable conditions. Some of the species, such as P. glandicola, T. angustata, and T. citrinoviride, were shown to be relatively more associated with treated acorns, indicating that thermotherapy favored their spread. The first one was the most numerous fungus isolated in this study, relatively more frequently found on acorns stored at 3 °C, thus confirming previous findings of Penicillium spp. dominance in treated and stored acorns [27,41]. However, the results indicate that all Penicillium spp. do not share such abilities, since the second most frequent species, P. glabrum, did not reveal a notable association with treated acorns in comparison to control ones and was mostly found on non-stored acorns.
The most detrimental fungal pathogen on European oak acorns, Ciboria batschiana, was not identified in this research. Although this could be predicted for the thermotherapy treated acorns, since this procedure successfully eliminates this pathogen [27,41], its absence from control acorns was not expected. The same results were also reported in Turkey [33]. There are several possible explanations for this, such as the fact that infections depend on forest sites and host conditions [11,33], so they might not have been prominent in the sampling area chosen for this research. Although infections can occur in the crown, most of the acorns are invaded while on the ground [32,76], and as the acorns in this research were collected very shortly after shedding, there might not have been enough time for the noticeable infection to occur. With regard to the fact that damaged and empty acorns, with possible advanced progression of the infection were discarded before analysis, that C. batschiana might not have prevailed in this particular sampling location, and that there might not have been enough opportunity for ground infection, it could be that there was no sufficient inoculum for the spread of the fungus inside and among acorns, especially since storage time was relatively short.
Thermotherapy treatment had a significant impact on the acorn mycobiota diversity in this research. Non-treated control acorns were invaded by a significantly higher number of different fungal species in comparison to the treated ones, regardless of the conditions they were stored in (Figure 4, Table 1), thus confirming the efficiency of thermotherapy in the eradication of a part of acorn mycobiota as stated in research by Knudsen et al. [27]. CA also indicated that some species, namely T. dryina, F. solani, A. alternata, and F. avenaceum, were relatively more associated with control acorns (Figure 6). However, the last was the only one completely eradicated already at 41 °C and 2.5 h exposure (Appendix A, Table A1). There were no significant differences in fungal diversity among the treatment groups, suggesting that an increase in the thermotherapy temperature (from 41 to 45 °C) and duration (from 2.5 to 5 h) did not noticeably affect the survival of more persistent fungal species, although there were on average more various species found in acorns treated at 41 °C for 2.5 h in comparison with the other three treatment groups (Figure 4). For example, Fusarium solani, Alternaria alternata, and Talaromyces sp. were not found in acorns treated at 45 °C, while Tubakia dryina was not present in acorns treated at 45 °C and those treated at 41 °C for 5 h (Appendix A, Table A1).
Acorn storage at 3 °C proved to be more favorable for fungal proliferation compared to non-stored acorns and those stored at −1 °C, as it resulted in zero acorns without fungal growth and a significantly higher number of obtained isolates per acorn than in the latter groups. Thermotherapy treatment did not impact the reduction of isolated mycelia, whose number in all groups stored at 3 °C regardless of the treatment conditions, exceeded the number of isolates obtained in the control groups (Figure 4). These results confirm higher susceptibility of thermotherapy treated acorns to the spread of persistent and ubiquitous fungi during storage as reported [27,44,45,46]. However, they also indicate that storage temperature also plays a significant role, as stated by Schröder [77]. This was corroborated by the smaller number of obtained mycelia per acorn in most of the treated groups which were non-stored or stored at −1 °C, compared to control groups stored in the same conditions. Exceptionally, in the group treated at 41 °C for 5 h and stored at −1 °C and in the non-stored group treated at 41 °C for 2.5 h, these numbers were bigger than for related groups of non-treated acorns, because of the higher occurrence of two ubiquitous species, P. glandicola in the former and both P. glandicola and P. glabrum in the latter.
The number of different fungal taxa colonizing thermotherapy treated acorns stored at 3 °C was significantly smaller compared to acorns stored under other conditions, contrary to the control groups where it was somewhat bigger (Figure 4). This indicates that a few eurivalent species that survived the treatment spread more successfully during storage at this temperature and thus suppressed the potential isolation of other species or maybe even their growth in acorns. This was corroborated by the fact that by far the most numerous fungus in this research, P. glandicola, was mostly associated with acorns stored at 3 °C.
The lowest nursery germination of the analyzed acorns was observed in the control group stored at 3 °C (55%), while the highest in the group treated at 41 °C for 5 h (79%). Generally, germination was somewhat higher in the groups treated at 41 °C compared to those treated at 45 °C, especially when comparing treatments conducted for 5 h. Shorter treatment times should thus be considered when higher temperatures are applied, since such conditions did not seem to have an adverse effect on acorn germination in the study by Belletti et al. [48].
In all thermotherapy treated groups, germination was higher than in the control group stored at 3 °C, which revealed the highest fungal diversity in this research. This supports previous findings that thermotherapy increases germination by eradicating some of the pathogenic fungi present in acorns that could potentially spread during storage, especially at temperatures above zero [27,41,44,77]. At the same time, there was no significant correlation between the occurrence of the most frequently isolated fungi and acorn germination proportion, indicating that the presence and spread of the ubiquitous fungi after treatment did not adversely affect germination after short-term storage. Except in the control group, germination was comparable or was somewhat higher in groups stored at 3 °C than in equally treated groups stored at −1 °C, which confirms that the dominantly present fungus P. glandicola, most frequently associated with treated acorns stored at 3 °C, did not have a detrimental impact on acorn germination after short-term storage. However, these findings do not exclude the possibility of a different outcome after storage for a longer time period, as reported for some fungal species in other studies [27,78,79].
Non-treated acorns stored at −1 °C revealed unexpectedly high nursery germination, which could be explained by the impact of other factors which were not taken into consideration in this study. It was noticed that the position of the group in the open-field layout, which was directly associated with the exposition of soil and planted acorns to the sun, had an effect on germination. Four groups which were positioned at the eastern and western edge of the layout (Figure 2), including the control one stored at −1 °C, resulted in higher acorn germination.
5. Conclusions
The first experiment revealed that in order to ensure the normally expected acorn germination, the thermotherapy temperature applied for 2.5 h should not exceed 48 °C.
The treatment temperatures chosen for further analysis were lower, 41 and 45 °C, and half of the treatments were performed for a longer period of time (5 h). The obtained results confirmed a significant impact of hot-water thermotherapy on the reduction of fungal diversity in treated acorns compared to the non-treated ones. However, there was no significant difference among the applied treatments, indicating that the tested increase of temperature and duration did not contribute materially to the elimination of persistent fungi, although a few potentially harmful species, like F. solani, A. alternata, and T. dryina were eradicated only at the 45 °C or during the 5 h treatment duration at 41 °C.
The study confirmed that thermotherapy treatment can bring about the proliferation of a few fast-colonizing fungi during storage, as two of the three most frequently isolated species were shown to be more associated with the treated acorns. However, the results suggest that storage temperatures play a more important role in their spread, as the most dominant species, P. glandicola, occurred more frequently in acorns stored at 3 °C in comparison with other storage conditions. Storage at this temperature was generally more conducive to the spread of the most persistent fungal colonizers, regardless of the thermotherapy treatment applied, as acorn groups stored at 3 °C revealed significantly more isolated mycelia compared to others.
Given the dominant occurrence of P. glandicola, the treated acorns stored at 3 °C harbored the least number of other fungal taxa, which shows the aggressiveness and adaptability of the fungus during colonization of these tissues. Despite its colonizing abilities, P. glandicola did not seem to have a detrimental effect on acorn germination in the nursery, and neither did the other frequently isolated fungi; however, this does not exclude the possibility of their detrimental impacts after a longer-term storage or storage at higher temperatures.
Acorn nursery germination was affected by factors other than thermotherapy and storage conditions; therefore, the obtained results cannot be considered conclusive. Nevertheless, all treated acorn groups showed higher germination compared to the control group stored at 3 °C, thus justifying the use of thermotherapy as acorn pretreatment, especially when storage at higher temperatures is planned. Somewhat lower germination in groups treated at 45 °C compared to those treated at 41 °C suggests that shorter treatment times should be considered when higher temperatures are applied.
This study has confirmed some of the known benefits of the application of thermotherapy, but also some of its drawbacks. Although thermotherapy can indirectly affect acorn storability and germination, storage temperatures and duration also play a significant role.
Author Contributions
Conceptualization, J.K.O. and D.D. (Damir Drvodelić); Data curation, J.K.O. and D.D. (Damir Drvodelić); Formal analysis, J.K.O., M.V., and M.R.; Funding acquisition, M.O.; Methodology, J.K.O. and D.D. (Damir Drvodelić); Project administration, D.D. (Danko Diminić) and M.O.; Supervision, D.D. (Danko Diminić) and M.O.; Validation, D.D. (Danko Diminić) and M.O.; Writing—original draft, J.K.O.; Writing—review and editing, J.K.O. and D.D. (Damir Drvodelić). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors are grateful to the reviewers for their constructive comments which improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Fungal taxa and related number of isolated mycelia from Quercus robur acorns exposed to different thermotherapy (C, T1, T2, T3, and T4) and storage conditions (no storage, at −1 °C, and at 3 °C).
Table A1.
Fungal taxa and related number of isolated mycelia from Quercus robur acorns exposed to different thermotherapy (C, T1, T2, T3, and T4) and storage conditions (no storage, at −1 °C, and at 3 °C).
| Closest BLAST Match | GenBank Accession No. | Control C | 41 °C, 2.5 h T1 | 41 °C, 5 h T2 | 45 °C, 2.5 h T3 | 45 °C, 5 h T4 | No. Isol 1 | No. Gro 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | −1 °C | 3 °C | No | −1 °C | 3 °C | No | −1 °C | 3 °C | No | −1 °C | 3 °C | No | −1 °C | 3 °C | ||||
| Penicillium glandicola | MW540446MW540447 | 26 | 20 | 28 | 34 | 27 | 117 | 2 | 92 | 99 | 14 | 19 | 112 | 6 | 50 | 120 | 766 | 14 |
| Penicillium glabrum | MW540448MW531879 | 10 | 25 | 29 | 32 | 7 | 3 | 25 | 4 | 25 | 2 | 3 | 11 | 3 | 179 | 13 | ||
| Truncatella angustata | MW540450 | 1 | 2 | 1 | 6 | 1 | 2 | 7 | 12 | 32 | 8 | |||||||
| Tubakia dryina | MW540456 | 3 | 4 | 4 | 1 | 1 | 13 | 5 | ||||||||||
| Diplodia seriata | MW540462 | 1 | 2 | 3 | 2 | 1 | 9 | 5 | ||||||||||
| Fusarium solani | MW540452 MW540453 | 1 | 13 | 1 | 1 | 16 | 4 | |||||||||||
| Trichoderma citrinoviride | MW540463 | 1 | 2 | 2 | 1 | 6 | 4 | |||||||||||
| Alternaria alternata | MW540457 | 3 | 7 | 1 | 11 | 3 | ||||||||||||
| Talaromyces sp. | MW540451 | 2 | 21 | 23 | 2 | |||||||||||||
| Fusarium avenaceum | MW540454 MW540455 | 10 | 3 | 13 | 2 | |||||||||||||
| Talaromyces marneffei | MW540464 | 1 | 4 | 5 | 2 | |||||||||||||
| Cucurbitariaceae sp. | MW540466 | 2 | 1 | 3 | 2 | |||||||||||||
| Epicoccum nigrum | MW540468 | 1 | 2 | 3 | 2 | |||||||||||||
| Stereum hirsutum | MW540470 | 2 | 1 | 3 | 2 | |||||||||||||
| Gnomoniopsis sp. | MW540473 | 1 | 1 | 2 | 2 | |||||||||||||
| Penicillium vulpinum | MW540477 MW531882 | 1 | 1 | 2 | 2 | |||||||||||||
| Unidentified mycelium 1 | 12 | 12 | 1 | |||||||||||||||
| Alternaria sp. 1 | MW540459 | 4 | 4 | 1 | ||||||||||||||
| Didymellaceae sp. | MW540465 | 4 | 4 | 1 | ||||||||||||||
| Alternaria sp. 2 | MW540458 | 3 | 3 | 1 | ||||||||||||||
| Cladosporium sp. 1 | MW540469 | 3 | 3 | 1 | ||||||||||||||
| Diaporthe sp. | MW540471 | 3 | 3 | 1 | ||||||||||||||
| Fusarium graminearum | MW540467 | 3 | 3 | 1 | ||||||||||||||
| Unidentified mycelium 2 | 3 | 3 | 1 | |||||||||||||||
| Apiognomonia sp. | MW540476 | 2 | 2 | 1 | ||||||||||||||
| Penicillium concentricum | MW540478 MW531881 | 2 | 2 | 1 | ||||||||||||||
| Penicillium polonicum | MW540475 MW531880 | 2 | 2 | 1 | ||||||||||||||
| Pleurostoma sp. 1 | MW540474 | 2 | 2 | 1 | ||||||||||||||
| Tubakia iowensis | MW540472 | 2 | 2 | 1 | ||||||||||||||
| Unidentified mycelium 3 | 2 | 2 | 1 | |||||||||||||||
| Alternaria sp. 3 | MW540460 | 1 | 1 | 1 | ||||||||||||||
| Alternaria sp. 4 | MW540461 | 1 | 1 | 1 | ||||||||||||||
| Cladosporium sp. 2 | MW540486 | 1 | 1 | 1 | ||||||||||||||
| Clonostachys rosea | MW540489 | 1 | 1 | 1 | ||||||||||||||
| Coryneliaceae sp. | MW540485 | 1 | 1 | 1 | ||||||||||||||
| Pestalotiopsis sp. | MW540479 | 1 | 1 | 1 | ||||||||||||||
| Phaeoacremonium hungaricum | MW540480 | 1 | 1 | 1 | ||||||||||||||
| Phaeoacremonium tuscanicum | MW540482 | 1 | 1 | 1 | ||||||||||||||
| Pleosporales sp. 1 | MW540488 | 1 | 1 | 1 | ||||||||||||||
| Pleosporales sp. 2 | MW540484 | 1 | 1 | 1 | ||||||||||||||
| Pleurostoma sp. 2 | MW540481 | 1 | 1 | 1 | ||||||||||||||
| Psathyrellaceae sp. | MW540487 | 1 | 1 | 1 | ||||||||||||||
| Talaromyces minioluteus | MW540483 | 1 | 1 | 1 | ||||||||||||||
| Unidentified mycelium 4 | 1 | 1 | 1 | |||||||||||||||
| Unidentified mycelium 5 | 1 | 1 | 1 | |||||||||||||||
| Unidentified mycelium 6 | 1 | 1 | 1 | |||||||||||||||
| TOTAL | 72 | 72 | 97 | 76 | 45 | 120 | 35 | 97 | 120 | 44 | 38 | 118 | 28 | 67 | 120 | 1149 | ||
1 Total number of obtained isolates. 2 Number of treatment/storage and control groups in which fungal taxon occurred.
References
- Eaton, E.; Caudullo, G.; Oliveira, S.; De Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the EU: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Mölder, A.; Meyer, P.; Nagel, R.-V. Integrative Management to Sustain Biodiversity and Ecological Continuity in Central European Temperate Oak (Quercus robur, Q. petraea) Forests: An Overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M.; Milanović, S. Pedunculate Oak Leaf Miners’ Community: Urban vs. Rural Habitat. Forests 2020, 11, 1300. [Google Scholar] [CrossRef]
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Nowakowska, J.A.; Oszako, T.; Urban, A.; Hilszczańska, D. Ectomycorrhizal Colonisation in Declining Oak Stands on the Krotoszyn Plateau, Poland. Forests 2019, 10, 30. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and Biotic Factors and Their Interactions as Causes of Oak Decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Delb, H. Folgewirkungen der Schwammspinner-Kalamität von 1992 Bis 1995 (Lymantria dispar L.) in Einem Mitteleuropäischen Eichenwaldgebiet Am Beispiel des Bienwaldes in Rheinland-Pfalz. Ph.D Thesis, Faculty of Forest Sciences, University Göttingen, Göttingen, Germany, 1999. [Google Scholar]
- Demeter, L.; Molnár, Á.P.; Öllerer, K.; Csóka, G.; Kiš, A.; Vadász, C.; Horváth, F.; Molnár, Z. Rethinking the Natural Regeneration Failure of Pedunculate Oak: The Pathogen Mildew Hypothesis. Biol. Conserv. 2021, 253, 108928. [Google Scholar] [CrossRef]
- Csóka, G.; Hirka, A.; Mutun, S.; Glavendekić, M.; Mikó, Á.; Szőcs, L.; Paulin, M.; Eötvös, C.B.; Gáspár, C.; Csepelényi, M. Spread and Potential Host Range of the Invasive Oak Lace Bug [Corythucha arcuata (Say, 1832)–Heteroptera: Tingidae] in Eurasia. Agric. For. Entomol. 2020, 22, 61–74. [Google Scholar] [CrossRef]
- Oršanić, M.; Drvodelić, D. Natural Regeneration of Pedunculate Oak. In Proceedings of the Forest Management Systems and Regeneration of Floodplain Forest Sites, Mendel University of Agriculture and Forestry, Brno, Czech Republic, 9 October 2007; pp. 99–106. [Google Scholar]
- Vasić, V.; Konstantinović, B.; Orlović, S. Application of Post-Emergence Herbicides in the Regeneration of Pedunculate Oak (Quercus robur L.) Forests. For. Int. J. For. Res. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Novak Agbaba, S. Mycoflora of Pedunculate Oak (Quercus robur L.) in Croatia. Ph.D. Thesis, Faculty of Forestry, University of Zagreb, Zagreb, Croatia, 2006. [Google Scholar]
- Devetaković, J.R.; Nonić, M.; Prokić, B.; Šijačić-Nikolić, M.; Popović, V. Acorn Size Influence on the Quality of Pedunculate Oak (Quercus robur L.) One-Year Old Seedlings. Reforesta 2019, 17–24. [Google Scholar] [CrossRef]
- Nikolić, N.; Orlović, S.; Krstić, B.; Kevrešan, Ž. Variability of Acorn Nutrient Concentrations in Pedunculate Oak (Quercus robur L.) Genotypes. J. For. Sci. 2006, 52, 51–60. [Google Scholar] [CrossRef]
- Welander, N.T.; Ottosson, B. The Influence of Shading on Growth and Morphology in Seedlings of Quercus robur L. and Fagus sylvatica L. For. Ecol. Manag. 1998, 107, 117–126. [Google Scholar] [CrossRef]
- Nilsson, U.; Gemmel, P.; Löf, M.; Welander, T. Germination and Early Growth of Sown Quercus robur L. in Relation to Soil Preparation, Sowing Depths and Prevention against Predation. New For. 1996, 12, 69–86. [Google Scholar] [CrossRef]
- Phillips, R.L. Environmental Factors Contribute to Acorn Quality: Elevation, on- or off-Tree Collection Influence the Viability of Blue Oak Acorns. Calif. Agric. 1992, 46, 30–32. [Google Scholar] [CrossRef]
- Hendry, G.A.; Finch-Savage, W.E.; Thorpe, P.C.; Atherton, N.M.; Buckland, S.M.; Nilsson, K.A.; Seel, W.E. Free Radical Processes and Loss of Seed Viability during Desiccation in the Recalcitrant Species Quercus Robur L. New Phytol. 1992, 122, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A. Seed Dormancy, Seed Treatment and Seed Sowing. Bulletin 1992, 83, 116–121. [Google Scholar]
- Aniszewska, M.; Błuszkowska, U.; Zychowicz, W.; Brzózko, J. Impact of Mechanical Treatment of Pedunculate Oak (Quercus robur L.) Seeds on Germination Time and Seedling Quality. J. For. Res. 2020, 25, 420–425. [Google Scholar] [CrossRef]
- Connor, K.F.; Sowa, S. Recalcitrant Behavior of Temperate Forest Tree Seeds: Storage, Biochemistry, and Physiology. In Proceedings of the 11th Biennial Southern Silvicultural Research Conference, Department of Agriculture, Forest Service, Southern Research Station, Asheville, NC, USA, 20–22 March 2002; pp. 47–50. [Google Scholar]
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M. Seeds of Forest Broadleaves: From Harvest to Sowing; INRA: Paris, France, 1996; ISBN 2-7380-0659-0. [Google Scholar]
- Özbingöl, N.; O’reilly, C. Increasing Acorn Moisture Content Followed by Freezing-Storage Enhances Germination in Pedunculate Oak. Forestry 2005, 78, 73–81. [Google Scholar] [CrossRef]
- Gosling, P.G. The Effect of Drying Quercus robur Acorns to Different Moisture Contents, Followed by Storage, Either with or without Imbibition. For. Int. J. For. Res. 1989, 62, 41–50. [Google Scholar] [CrossRef]
- Doody, C.N.; O’Reilly, C. Drying and Soaking Pretreatments Affect Germination in Pedunculate Oak. Ann. For. Sci. 2008, 65, 1. [Google Scholar] [CrossRef]
- Poulsen, K.M. Seed Storage Physiology of Recalcitrant Acorns from the Pedunculate Oak (Quercus robur L.) and Orthodox Nuts from the European Beech (Fagus sylvatica L.). Ph.D. Thesis, Royal Vetrinary and Agricultural University, Copenhagen, Denmark, 1992. [Google Scholar]
- Suszka, B.; Tylkowski, T. Storage of Acorns of the English Oak (Quercus robur L.) over 1-5 Winters. Arbor. Kórn. 1980, 25, 199–229. [Google Scholar]
- Knudsen, I.; Thomsen, K.; Jensen, B.; Poulsen, K. Effects of Hot Water Treatment, Biocontrol Agents, Disinfectants and a Fungicide on Storability of English Oak Acorns and Control of the Pathogen, Ciboria Batschiana. For. Pathol. 2004, 34, 47–64. [Google Scholar] [CrossRef]
- Sutterland, J.; Diekmann, M.; Berjak, P. Forest Tree Seed Health for Germplasm Conservation. IPGRI Tech. Bull. Rome 2002, 6, 1–85. [Google Scholar]
- Cram, M.; Fraedrich, S. Seed Diseases and Seedborne Pathogens of North America. Tree Plant. Notes 2010, 53, 35–44. [Google Scholar]
- Mittal, R.; Wang, B. Fungi Associated with Seeds of Eastern White Pine and White Spruce during Cone Processing and Seed Extraction. Can. J. For. Res. 1987, 17, 1026–1034. [Google Scholar] [CrossRef]
- Fort, T.; Pauvert, C.; Zanne, A.; Ovaskainen, O.; Caignard, T.; Barret, M.; Compant, S.; Hampe, A.; Delzon, S.; Vacher, C. Maternal Effects Shape the Seed Mycobiome in Quercus petraea. New Phytol. 2020. [Google Scholar] [CrossRef]
- Jankowiak, R. Fungi Occurring in Acorn of Quercus robur L. Infested by Insects. Acta Sci. Pol. Silvarum Cal. Ratio Ind. Lignaria 2008, 7, 19–29. [Google Scholar]
- Oskay, F.; Bora İmal, F.O.; Meşe, Ö. Preliminary Results on The Fungi Damaging Pedunculate Oak (Quercus robur L.) Acorns. In Proceedings of the Diseases and Insects in Forest Nurseries, IUFRO Working Party 7.03.04, Kuşadası, Turkey, 21 October 2018; Volume 7, pp. 17–22. [Google Scholar]
- Swiecki, T.J.; Bernhardt, E.A.; Arnold, R.A. Insect and Disease Impacts on Blue Oak Acorns and Seedlings. In Proceedings of the Symposium on Oak Woodlands and Hardwood Rangeland Management, US Department of Agriculture, Pacific Southwest Research Station, Davis, CA, USA, 31 October–2 November 1991; Volume 126, pp. 149–155. [Google Scholar]
- Washington, D.M. Fungi Associated with Northern Red Oak (Quercus rubra) Acorns. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2003. [Google Scholar]
- Winston, P.W. The Acorn Microsphere, with Special Reference to Arthropods. Ecology 1956, 37, 120–132. [Google Scholar] [CrossRef]
- Szynkiewicz, A.; Kwaśna, H. The Effects of Fungi from Acorns with Symptoms of Black Rot and Necrotic Twigs of Oak on Quercus Seedlings. Phytopathol. Pol. 2004, 32, 49–59. [Google Scholar]
- Andersson, C. The Effect of Weevil and Fungal Attacks on the Germination of Quercus robur Acorns. For. Ecol. Manag. 1992, 50, 247–251. [Google Scholar] [CrossRef]
- Schröder, T.; Kehr, R.; Procházková, Z.; Sutherland, J. Practical Methods for Estimating the Infection Rate of Quercus robur Acorn Seedlots by Ciboria batschiana. For. Pathol. 2004, 34, 187–196. [Google Scholar] [CrossRef]
- Delatour, C.; Muller, C.; Bonnet-Masimbert, M. Progress in Acorns Treatment in a Long Term Storage Prospect. In Proceedings of the International Symposium on Forest Tree Storage, IUFRO Working Party on Seed Problems, Chalk River, ON, Canada, 23–27 September 1980; pp. 126–133. [Google Scholar]
- Delatour, C. A curative control method for Ciboria batschiana on acorns. Eur. J. For. Pathol. 1978, 8, 193–200. [Google Scholar] [CrossRef]
- Finch-Savage, W.; Clay, H.; Budge, S.; Dent, K.; Clarkson, J.; Whipps, J. Biological Control of Sclerotinia pseudotuberosa and Other Fungi during Moist Storage of Quercus Robur Seeds. Eur. J. Plant Pathol. 2003, 109, 615–624. [Google Scholar] [CrossRef]
- Tylkowski, T. Height Increment of 1-Year Shoots of the English Oak (Quercus Robur L.) and the Northern Red Oak (Q. borealis Michx. = Q. rubra L.) on 4-Year-Old Roots of Seedlings Raised from Acorns Stored over 1–5 Winters. Arbor. Korn. 1982, 27, 357–365. [Google Scholar]
- Kehr, R.D.; Schroeder, T. Long-Term Storage of Oak Seeds-New Methods and Mycological Aspects. In Proceedings of the Tree Seed Pathology Meeting, Opocno, Czech Republic, 9–11 October 1996; pp. 50–61. [Google Scholar]
- Grondeau, C.; Samson, R.; Sands, D. A Review of Thermotherapy to Free Plant Materials from Pathogens, Especially Seeds from Bacteria. Crit. Rev. Plant Sci. 1994, 13, 57–75. [Google Scholar] [CrossRef]
- Schröder, T. Über Die Eignung Verschiedener Physikalisch-Technischer Verfahren Zur Phytosanitären Behandlung Und Zur Lagerung von Forstsaatgut Unter Besonderer Berücksichtigung Der Stiel- Und Traubeneiche; Parey Buchverlag: Berlin, Germany, 1999; ISBN 3-8263-3244-X. [Google Scholar]
- Baker, R. Thermotherapy of Planting Material. Phytopathology 1962, 52, 1244–1255. [Google Scholar]
- Belletti, P.; Monteleone, I.; Cartarasa, M. Optimisation of Stone-Oak Seed Management Aimed at Nursery Production of High Quality Seedlings for Reforestation. In Proceedings of the International Conference “Forest Research: A challenge for an Integrated European Approach”, NAGEF-Forest Research Institute, Thessaloniki, Greece, 27 August–1 September 2001; pp. 413–416. [Google Scholar]
- Gradečki–Poštenjak, M.; Liović, B.; Agbaba, S.N. Investigation of Conditions for Processing and Storage of Pedunculate Oak Acorns (Quercus Robur L.) and Acorn Quality During Storage. In Proceedings of the International Conference on Natural Resources, Green Technology & Sustainable Development, University of Zagreb Faculty of Food Technology and Biotechnology, Zagreb, Croatia, 26–28 November 2014; p. 135. [Google Scholar]
- ISTA (International Seed Testing Association). The Germination Test. In International Rules for Seed Testing, January 2006 ed.; ISTA: Bassersdorf, Switzerland, 2011. [Google Scholar]
- Ježić, M.; Krstin, L.; Rigling, D.; Ćurković-Perica, M. High Diversity in Populations of the Introduced Plant Pathogen, Cryphonectria parasitica, due to Encounters between Genetically Divergent Genotypes. Mol. Ecol. 2012, 21, 87–99. [Google Scholar] [CrossRef]
- Kranjec, J.; Milotić, M.; Hegol, M.; Diminić, D. Gljivama Slični Organizmi u Tlu Odumirućih Sastojina Poljskog Jasena (Fraxinus angustifolia Vahl). Šumar. List 2017, 141, 115–122. [Google Scholar] [CrossRef][Green Version]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes-application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Elsevier BV: Amsterdam, The Netherlands, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Stielow, J.B.; Levesque, C.A.; Seifert, K.A.; Meyer, W.; Iriny, L.; Smits, D.; Renfurm, R.; Verkley, G.; Groenewald, M.; Chaduli, D. One Fungus, Which Genes? Development and Assessment of Universal Primers for Potential Secondary Fungal DNA Barcodes. Pers. Mol. Phylogeny Evol. Fungi. 2015, 35, 242. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Buckley, E. A Beauveria Phylogeny Inferred from Nuclear ITS and EF1-α Sequences: Evidence for Cryptic Diversification and Links to Cordyceps Teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Nakashima, C.; Crous, P.; Groenewald, J.; Moreno-Rico, O.; Rooney-Latham, S.; Blomquist, C.; Haas, J.; Marmolejo, J. Phylogeny and Taxonomy of the Genus Tubakia s. Lat. Fungal Syst. Evol. 2018, 1, 41. [Google Scholar] [CrossRef]
- Branco, M.; Branco, C.; Merouani, H.; Almeida, M.H. Germination Success, Survival and Seedling Vigour of Quercus suber Acorns in Relation to Insect Damage. For. Ecol. Manag. 2002, 166, 159–164. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Tylek, P. Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.). Forests 2018, 9, 100. [Google Scholar] [CrossRef]
- Giertych, M.J.; Suszka, J. Consequences of Cutting off Distal Ends of Cotyledons of Quercus robur Acorns before Sowing. Ann. For. Sci. 2011, 68, 433–442. [Google Scholar] [CrossRef]
- van’t Hoff, J.H. A Suggestion Looking to the Extension into Space of the Structural Formulas at Present Used in Chemistry, and a Note upon the Relation between the Optical Activity and the Chemical Constitution of Organic Compounds. Arch. Neerl. Sci. Exact Nat. 1874, 9, 445–454. [Google Scholar]
- Drvodelić, D.; Oršanić, M. Problematika sjemenarstva i rasadničarske proizvodnje sadnica poljskog jasena (Fraxinus angustifolia Vahl) i hrasta lužnjaka (Quercus robur L.). In Ekologija, obnova i zaštita poplavnih šuma Posavine; University of Zagreb Faculty of Forestry: Zagreb, Croatia, 2020; ISBN 978-953-292-061-1. [Google Scholar]
- Vasinauskienė, R.; Silingiene, G.; Sinkevičienė, J. Surface Sterilization of English Oak (Quercus Robur L.) Acorns Using Wet Water Steam. Balt. For. 2020, 26. [Google Scholar] [CrossRef]
- Alidadi, A.; Kowsari, M.; Javan-Nikkhah, M.; Karami, S. First Report of Leaf Spot Caused by Truncatella angustata on Persian Oak (Quercus brantii) in Iran. Plant Dis. 2018, 102, 1173. [Google Scholar] [CrossRef]
- Wenneker, M.; Pham, K.; Boekhoudt, L.; de Boer, F.; van Leeuwen, P.; Hollinger, T.; Thomma, B. First Report of Truncatella angustata Causing Postharvest Rot on ‘Topaz’Apples in the Netherlands. Plant Dis. 2017, 101, 508. [Google Scholar] [CrossRef]
- Eken, C.; Spanbayev, A.; Tulegenova, Z.; Abiev, S. First Report of Truncatella angustata Causing Leaf Spot on Rosa canina in Kazakhstan. Australas. Plant Dis. Notes 2009, 4, 44–45. [Google Scholar]
- Arzanlou, M.; Torbati, M.; Jafary, H. Fruit Rot of Olive (Olea europaea) Caused by Truncatella angustata. Plant Pathol. Quar. 2012, 2, 117–123. [Google Scholar] [CrossRef]
- Kowalski, T. Tubakia dryina, Symptoms and Pathogenicity to Quercus robur. Acta Mycol. 2006, 41, 299–304. [Google Scholar] [CrossRef][Green Version]
- Elena, G.; Garcia-Figueres, F.; Reigada, S.; Luque, J. Intraspecific Variation in Diplodia seriata Isolates Occurring on Grapevines in Spain. Plant Pathol. 2015, 64, 680–689. [Google Scholar] [CrossRef]
- Kaliterna, J.; Miličević, T.; Ivić, D.; Benčić, D.; Mešić, A. First Report of Diplodia seriata as Causal Agent of Olive Dieback in Croatia. Plant Dis. 2012, 96, 290. [Google Scholar] [CrossRef]
- Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Ginetti, B.; Ragazzi, A.; Tiberi, R.; Moricca, S. Dispersal and Propagule Pressure of Botryosphaeriaceae Species in a Declining Oak Stand is Affected by Insect Vectors. Forests 2017, 8, 228. [Google Scholar] [CrossRef]
- Slippers, B.; Smit, W.; Crous, P.W.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Taxonomy, Phylogeny and Identification of Botryosphaeriaceae Associated with Pome and Stone Fruit Trees in South Africa and Other Regions of the World. Plant Pathol. 2007, 56, 128–139. [Google Scholar] [CrossRef]
- Prochazkova, Z.; Sikorova, A.; Peskova, V. Preliminary Observations on the Occurrence of Ciboria batschiana (Zopf) Buchwald in the Czech Republic. Work Pap. Finn. Res. Inst. 2005, 11, 13–18. [Google Scholar]
- Schröder, T. On the Geographic Variation of Ciboria batschiana (Zopf) Buchwald, the Main Pathogenic Fungus on Acorns of Quercus Robur and Q. petraea in Europe. Dendrobiology 2002, 47, 13–19. [Google Scholar]
- Guthke, J.; Spethmann, W. Physiological and Pathological Aspects of Long-Term Storage of Acorns. Ann. des Sci. For. 1993, 50, 384s–387s. [Google Scholar] [CrossRef]
- Noland, T.L.; Morneault, A.E.; Dey, D.C.; Deugo, D. The Effect of Storage Temperature and Duration on Northern Red Oak Acorn Viability and Vigour. For. Chron. 2013, 89, 769–776. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).