Abstract
A Mediterranean holm oak forest was subjected to experimental partial rainfall exclusion during 21 consecutive years to study the effects of the expected decrease in water availability for Mediterranean vegetation in the coming decades. Allocation in woody structures and total aboveground allocation were correlated with annual rainfall, whereas canopy allocation and the ratio of wood/canopy allocation were not dependent on rainfall. Fruit productivity was also correlated with annual rainfall, but only in Quercus ilex. In the studied site, there were two types of forest structure: high canopy stand clearly dominated by Quercus ilex, and low canopy stand with more abundance of a tall shrub species, Phillyrea latifolia. In the tall canopy stand, the allocation to woody structures decreased in the experimental rainfall exclusion, but not the allocation to canopy. In the low canopy stand, wood allocation in Quercus ilex was very small in both control and plots with rainfall exclusion, but wood allocation in Phillyrea latifolia was even higher than that obtained in tall canopy plots, especially in the plots receiving the experimental rainfall exclusion. These results highlight likely future changes in the structure and functioning of this ecosystem induced by the decrease in water availability. A serious drop in the capacity to mitigate climate change for this Mediterranean forest can be expected, and the ability of Phillyrea latifolia to take advantage of the limited capacity to cope with drought conditions detected in Quercus ilex makes likely a forthcoming change in species dominance, especially in the low canopy stands.
1. Introduction
The net primary productivity (NPP) allocation in different tissues is one of the best descriptors of ecosystem functioning [1]. Every woody plant has a trade-off between resource allocation in canopy structures (leaves, flowers, and fruits) or in woody structures (branches and stem). The fraction invested in canopy structures will affect the production (and the consumption) of flowers and fruits, canopy leaf area, their photosynthetic capacity, litter through fall, and its decomposition by soil organisms [1]. The fraction invested in woody structures will be more related to the growth of vegetation and its capacity to ameliorate climate change due to atmospheric CO2 uptake [2]. Variations in wood/canopy allocation could drive important changes in ecosystem functioning and services. Several factors contribute to the variation in resource allocation in trees: tree age [3], stand structure, environment in which the tree develops [4,5,6], silvicultural treatments [7,8], ontogeny, and many other factors [6,9].
Water availability is the main limiting factor for plant productivity in some semiarid ecosystems such as Mediterranean forests, where dry season coincides with the hot summer. For these Mediterranean areas, higher rates of evapotranspiration are induced by higher air temperatures with no increase in precipitation [10]. Furthermore, an increase in the frequency of extreme droughts [11] and heat waves [12] is forecasted for these areas. The Mediterranean forest, seasonally exposed to water stress, is often particularly vulnerable to a decrease in water availability, which can induce reductions in stem growth [13,14], defoliation [15,16], tree mortality, and forest dieback [17,18,19,20,21,22].
The effect of climate change on ecosystem functioning is quite uncertain, but changes in woody and canopy allocation could be a key factor determining some variations of ecosystem structure and functioning because how carbon is allocated in different tissues determines how long carbon remains in plant biomass and thus remains a central challenge for understanding the global carbon cycle [23]. For example, if carbon allocation in tissues with long turnover time (such as stem and branches) decreases and carbon allocation in tissues with short turnover time (leaves, flowers, and fruits) proportionally increases, the function of atmospheric CO2 sinks and its capacity to ameliorate climate change will strongly decrease.
Climate manipulating experiments have the potential to verify ecosystem responses when it is subjected to continuous climate change [24,25,26]. Furthermore, long-term experiments could highlight the usual dampening effect size of treatments reported in global-change experiments [27]. As a result, the use of experiments simulating the environmental conditions projected by models of global change as long as possible is the best tool to study long-term climate change effects on natural ecosystems. The Mediterranean forest studied here has been subjected to rainfall exclusion since 1999, and is one of the longest ongoing climate manipulation experiments conducted in natural ecosystems in the world [26].
Plant development in Mediterranean forests is mainly limited by summer drought, and a future decrease in water availability is projected in these ecosystems (about 15%) [13,14,21]. For these reasons, we aimed to study the effects on carbon allocation in woody or canopy structures, derived from an experimental 15% decrease in soil moisture. We also aimed to discuss the possible variations in the structure and functioning of the Mediterranean forest as a consequence of these effects on carbon wood/canopy allocation.
2. Materials and Methods
2.1. Study Site
The study was carried out on a south-facing slope (25%) in the Prades holm oak forest, in Catalonia, NE Spain (41°21′ N, 1°2′ E; 930 m asl). This forest has not been perturbed during the last 70 years, but as a result of ancient coppicing, the vegetation is a dense multi-stem forest (15.433 stems ha−1) dominated by Quercus ilex L., with a high presence of evergreen tall shrub species such as Phillyrea latifolia L (Appendix A Figure A1). In the forest distribution, there are two types of forest structure: a tall canopy forest (8–10 m tall) clearly dominated by Q. ilex, and a low canopy forest (4–6 m tall) with more abundance of P. latifolia. Different soil depths determined different water and nutrient availability and this different forest structure [28]. Eight 15 × 10 m plots were delimited at the same altitude along the slope (four in the tall canopy area and another four in the low canopy area). In four of these plots (two in the tall canopy area and another two in the low canopy area) a partial rainfall exclusion system was installed, whereas the other four plots were control. The partial rainfall exclusion treatment consisted of transparent PVC strips installed 1 m above ground level and covering ca. 30% of the plot surface, as shown in Figure A1; these PVC strips also covered a buffer of 2 m above and below the plot area. Soil moisture in these drought plots was on average 15% smaller than in control plots during the overall studied period [21].
2.2. Carbon Allocation Measurements
Before the start of the experiment and outside the plots, different sizes of 10 Q. ilex and 10 P. latifolia trees were cut (covering the range of stem sizes of trees from the experimental plots). The circumference of each stem at 50 cm height was measured with a metric tape, and after that, each stem was cut from the base and all the aboveground biomass was dried in an oven at 105 °C for enough time to reach constant weight. For each species, allometric relationships between the stem circumference and the weight of dry leaves and total aboveground dry biomass were determined, and these allometric relationships were used to estimate total aboveground and leaf biomass of all stems from the plots (Table A1). Every winter, the circumference at 50 cm height of all stems from the experimental plots was measured, and the aboveground and leaf biomass of all species per plot was estimated using the allometric relationships. We estimated annual wood net primary productivity (Wood NPP) from the difference of the total aboveground biomass increment and the leaf biomass increment during the overall studied period (1999–2019).
In each plot, 20 baskets (27 cm diameter, and with a 1.5 mm mesh inside them) were randomly placed on the ground (Figure A1). The litter that fell in the baskets was collected every two months and separated per species and litter fraction (leaves, flowers, and fruits). Finally, the litter was weighed after drying in an oven at 70 °C. Litter collected in the baskets for one year was used to estimate total flower, fruit, and leaf production per species and plot; it was assumed that all fallen leaves were replaced by new ones. The canopy net primary productivity (canopy NPP) was estimated as the sum of all fallen leaves, fruits, and flowers, and the aboveground net primary productivity (ANPP) was estimated as the sum of annual wood NPP and canopy NPP.
2.3. Statistics
General linear models were performed to test the effect of climatic conditions and rainfall exclusion treatment on ANPP, wood NPP, canopy NPP (flower and fruit production and leaf replacement), and the proportion of wood allocation in relation to canopy allocation (wood/canopy allocation). For each type of canopy plot, several analyses of variance (ANOVAs) were conducted with the percentage of ANPP, wood NPP, canopy NPP (related to the initial biomass per species and plot at the start of the experiment), and wood/canopy allocation as dependent variables, and species (only the two dominant species, Q. ilex and P. latifolia, were considered separately) and rainfall exclusion treatment as independent factors. Other ANOVAs were conducted with each fraction of canopy NPP as a dependent variable, and species, rainfall exclusion, and type of canopy as independent factors. Analyses of covariance (ANCOVAs) were performed with ANPP, wood NPP, canopy NPP, and wood/canopy allocation as dependent variables; species, canopy type, and rainfall exclusion treatment as independent factors; and mean annual temperature and total annual rainfall as covariates. Finally, linear regressions with the same variables were conducted to determine the effect of annual climatic conditions on each dependent variable. The proportions of ANPP, wood NPP, and canopy NPP (p) were arcsin p0.5 transformed to reach the assumptions of a normal distribution. All statistical analyses were performed with the Statistica 12 software package (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Climate and Productivity
3.1.1. Climatic Data
Mean annual temperature was on average 12.38 °C and mean annual precipitation 619 mm during these 21 years of experiments at the studied site (Figure A1). Both temperature and rainfall fluctuated a lot depending on the year. For example in 2015, the highest mean temperature (13.25 °C) and also the lowest precipitation (355 mm) were recorded. On the contrary, the coldest year, 2010 (10.98 °C), coincided with the second wettest year (902 mm, just below the 1021 mm corresponding to year 2018) (Figure A2).
3.1.2. Relationships between Productivity and Climatic Conditions
Forest wood NPP and total ANPP were positively correlated with annual rainfall (p < 0.01 and p = 0.05 for wood NPP and ANPP, respectively), but there was no significant relationship between canopy NPP or wood/canopy allocation and annual rainfall (Figure 1), and none of the wood NPP, canopy NPP, wood/canopy, or ANPP variables were significantly correlated with mean annual temperature. There was a trend of wood NPP inversely correlating with temperature (p = 0.1) (Figure 1). When both dominant species were analyzed separately, the wood NPP of both species was correlated with annual rainfall (p < 0.05), and Q. ilex ANPP was positively correlated with annual rainfall (p < 0.05) (Figure 2) but not with mean annual temperature. However, neither the canopy NPP nor the wood/canopy allocation ratio were correlated with meteorological data. Finally, ANPP was correlated with annual rainfall (p < 0.05) in Q. ilex, but not in P. latifolia (Figure 2). The meteorological data also determined the fruit allocation, which was correlated with annual rainfall only in Q. ilex (p < 0.05) (Figure 3).
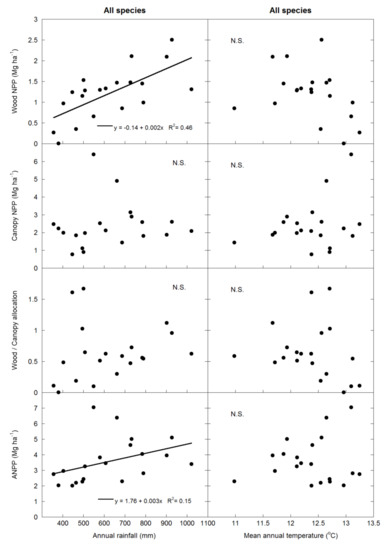
Figure 1.
Linear relationships between average net primary productivity (NPP) values (wood NPP, canopy NPP, aboveground net primary productivity (ANPP), and wood/canopy allocation) and meteorological data (annual rainfall and mean annual temperature) of all species analyzed together.
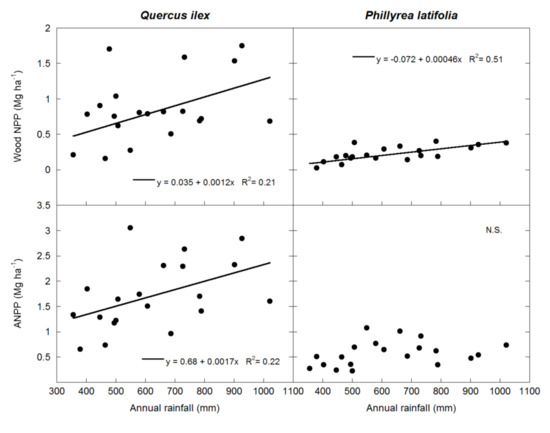
Figure 2.
Linear relationships between wood NPP and ANPP values, and annual rainfall in the two dominant species of the studied forest.
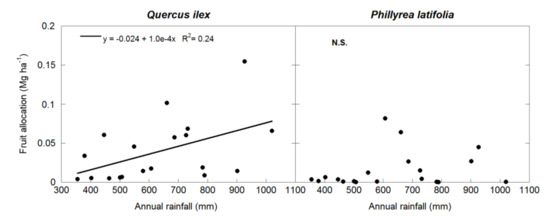
Figure 3.
Linear relationships between fruit allocation and annual rainfall in the two dominant species of the studied forest.
The effect of annual rainfall on the studied variables was similar in plots located in tall and low canopy areas, and also in control and plots with the partial rainfall exclusion system.
3.2. Productivity Allocation in Different Forest Stands
3.2.1. Net Primary Productivity Allocation
The average ANPP of the studied forest during the entire studied period was 3.6 Mg ha−1 y−1. About one third of this ANPP corresponded to wood NPP (1.2 Mg ha−1 y−1), and about two thirds of the total ANPP corresponded to canopy NPP (2.4 Mg ha−1 y−1), so wood/canopy allocation was on average 0.56 Mg ha−1 y−1. ANPP was higher in tall canopy plots than in low canopy plots (4.0 and 3.2 Mg ha−1 y−1, respectively) (p < 0.01), but the wood/canopy allocation ratio was similar in both canopy-type plots. When ANPP was examined in relation to the previous aboveground biomass in each plot, Q. ilex experienced a similar percentage of ANPP in both tall and low canopy plots, but ANPP in P. latifolia was relatively higher in low canopy plots (p < 0.05).
3.2.2. Net Primary Productivity Allocation in Tall Canopy Forest Stand
In tall canopy plots, the percentage of wood NPP was similar in both species, but the partial rainfall exclusion reduced wood NPP in both species (p < 0.05) (Figure 4). The percentage of canopy NPP was higher in P. latifolia than in Q. ilex (p < 0.01). As a result of this, the percentage of ANPP was also higher in P. latifolia (p < 0.05) (Figure 4). In these tall canopy plots, wood/canopy allocation was higher in Q. ilex than in P. latifolia (p < 0.01), and this wood/canopy allocation ratio decreased in both species when submitted to rainfall exclusion treatment (p = 0.01) (Figure 4).
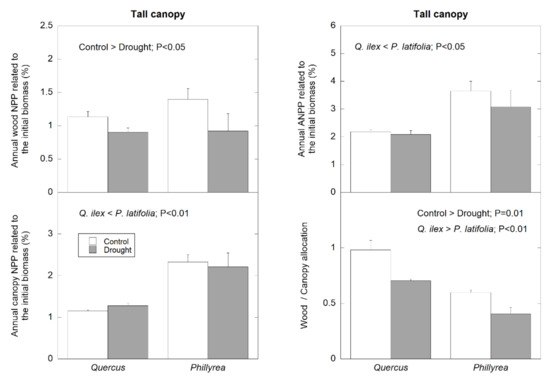
Figure 4.
Average percentage of wood NPP, canopy NPP, ANPP, and wood/canopy allocation of the two dominant species in control and plots with partial rainfall exclusion (drought plots), located in the tall canopy stand. Depicted data correspond to the NPP during the overall studied period (1999–2019).
3.2.3. Net Primary Productivity Allocation in Low Canopy Forest Stand
In low canopy plots, the percentage of wood NPP and the percentage of canopy NPP were higher in P. latifolia than in Q. ilex (p < 0.05 and p < 0.01 for wood NPP and canopy NPP, respectively), and as a result of this, the percentage of ANPP was also higher in P. latifolia than in Q. ilex (p < 0.01) (Figure 5). In low canopy plots, there was no significant difference between both species and plots with different treatment for the wood/canopy allocation (Figure 5). Surprisingly, the only significant effect of rainfall exclusion was an increase of ANPP in P. latifolia (Figure 5).
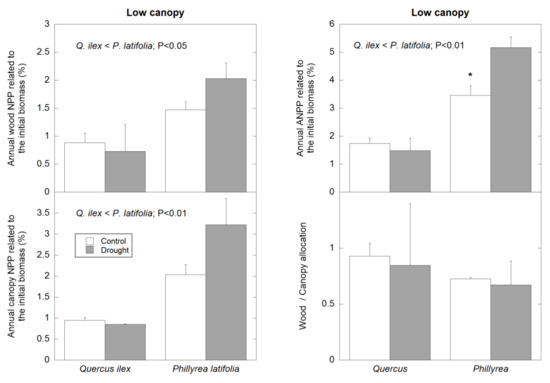
Figure 5.
Average percentage of wood NPP, canopy NPP, ANPP, and wood/canopy allocation of the two dominant species, in control and plots with partial rainfall exclusion (drought plots), located in the low canopy stand. Depicted data correspond to the NPP during the overall studied period (1999–2019). *: significant.
4. Discussion
A decrease in water availability usually drives lower carbon allocation in stem and branches compared to the allocation in roots and canopy [29]. In our experiment, wood allocation decreased during the drier years, especially in Q. ilex. In plots located in the tall forest stand, where water availability is not as limited as in low canopy stand, rainfall exclusion decreased wood allocation in both studied species, as expected, whereas no effect was observed in canopy allocation; as a result, wood/canopy allocation decreased in both species. In the low forest stand, wood and canopy allocation were very low in Q. ilex in both control and rainfall exclusion plots, showing that growth in this species is seriously constrained by the low water availability of this low canopy stand. In contrast, P. latifolia seemed to be more able to obtain an advantage from the decay of Q. ilex, because P. latifolia experienced larger wood and canopy allocation in the low canopy stand and even larger allocation in plots with rainfall exclusion as a result of the decrease in stem density induced by the tree mortality observed in Q. ilex [20,21]. As observed in previous studies conducted at the same experimental site, P. latifolia was better able to cope with drought conditions than Q. ilex [13,14,21].
Analyzing the fruit production, once again Q. ilex was more dependent than P. latifolia on water availability, because fruit allocation decreased when annual rainfall decreased in Q. ilex, but not in P. latifolia. Despite a slight decrease of Q. ilex fruit production in rainfall exclusion plots detected during the first years of study [30], this effect disappeared with time, and globally there was no effect of rainfall exclusion on fruit allocation during the overall studied period [31], in contrast with what was observed in another rainfall exclusion experiment where Q. ilex acorn production decreased in plots with rainfall exclusion [32]. However, rainfall exclusion exerted a decrease in wood growth of Q. ilex, but not in P. latifolia, so the more drought-sensitive species, Q. ilex, was able to maintain fecundity by shifting allocation of resources away from growth [31].
Our results highlight a strong decrease in the capacity of Mediterranean forest to mitigate climate change induced by future limitations in water availability, because of the high decrease in wood growth. However, this forest seems to be able to maintain leaf turnover and the production of flowers and fruits. Moreover, our estimation of wood allocation was provided from stem radial growth, but sometimes water scarcity could decrease wood density in tree rings instead of radial growth [33], as observed in Q. ilex [34]. If water scarcity also drives lower wood density, the decrease in wood resource allocation could be even greater than that detected in our experiment. The allocation to fine roots is another fraction of resource allocation competing with wood and canopy allocation, and fine root allocation could be more variable depending on water availability than wood and canopy allocation [1]. We reported the effects of rainfall exclusion in aboveground biomass allocation, but there is a lack of any estimation about the allocation to fine roots. Usually, water scarcity enhanced the growth of fine roots [1]. Another experiment conducted near the study site also reported shorter fine-root longevity in Q. ilex when water availability decreased [35], so more resource allocation is needed to restore dead roots. We did not measure how much is lost in the form of volatiles, root exudates, mycorrhizal associations, consumption by animals, and dissociated dead parts [36]. Previous measurements conducted at the same experimental site highlighted a decrease in volatile organic compound (VOC) emission under heavy drought in Q. ilex, whereas no VOC emission decrease was observed in P. latifolia [37]. It was also observed in Q. ilex, that 21 consecutive days of rainfall exclusion increased 21% root exudates compared to well-watered conditions [38]. Despite these changes in resource allocation described before, other authors reported higher plant ability to alter organ morphology, such as specific leaf area (SLA), to cope with climatic variations than to adjust allocation [6].
The small values of wood allocation described in Q. ilex trees from low canopy plots and the high wood and total aboveground allocation in P. latifolia in the same plots are in agreement with the defoliation and tree mortality observed in Q. ilex in the same study area when it is subjected to severe drought conditions [20,21], and highlight the ability of P. latifolia to take competitive advantage of Q. ilex decay. In the low canopy stand, Q. ilex cannot actually adjust the allocation of resources more to wood structures to cope with the drier conditions posed by climate change. The dominant tree species of this Mediterranean forest, Q. ilex, is currently starting to be replaced especially in the low canopy stand by P. latifolia, which is more adapted to the current and forthcoming drier and hotter conditions [13,14,21].
5. Conclusions
Increasing water deficits characterized by lower soil moisture and higher atmospheric aridity are leading to several changes in the ecosystem functioning of the Mediterranean forest, such as a dramatic decrease in the capacity to mitigate climate change, but it seems that this forest will be able to maintain leaf turnover and the production of reproductive structures. However, if current climate evolution leads to forest dieback, as is occurring in Q. ilex in the low canopy stand, the maintenance of the production and dispersion of seeds will be not enough to compensate for Q. ilex decay. A progressive substitution of the species most sensitive to water scarcity (Q. ilex) by other species more adapted to drought (such as P. latifolia) is expected, and it may transform the current Mediterranean forest to a tall shrubland, with different species composition and different ecosystem behavior and services [39].
Author Contributions
Conceptualization, design of the experiment, statistical analyses, and discussion of the results—R.O. and J.P.; writing—R.O.; supervision—J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the European Research Council Synergy grant ERC-2013-SyG-2013-610028 IMBALANCE-P, the Spanish government project PID2019-110521GB-I00 ELEMENTAL SHIFT, and the Catalan government grant SGR-2017–1005.
Acknowledgments
This study was undertaken with the approval of the staff of the PNIN de Poblet Natural Park.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1.
View of the study site and its localization, and view of the partial rainfall exclusion treatment installation and litter collectors. The PVC strips were only placed in plots subjected to partial rainfall exclusion, whereas litter collector baskets were placed in all plots.
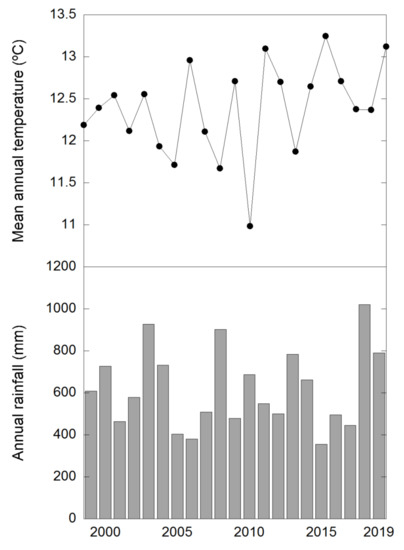
Figure A2.
Mean annual temperature and annual rainfall values of the study site during the overall duration of the experiment.

Table A1.
Allometric relationships between total aboveground biomass (AB) and total biomass of leaves (LB) from stem diameter at 50 cm height (D50), in Q. ilex and P. latifolia.
Table A1.
Allometric relationships between total aboveground biomass (AB) and total biomass of leaves (LB) from stem diameter at 50 cm height (D50), in Q. ilex and P. latifolia.
| Species | Total Aboveground Biomass | Total Biomass of Leaves |
|---|---|---|
| Quercus ilex | ln AB = 4.900 + 2.277 ln D50 | ln LB = 3.481 + 1.695 ln D50 |
| Phillyrea latifolia | ln AB = 4.251 + 2.463 ln D50 | ln LB = 1.433 + 2.426 ln D50 |
References
- Malhi, Y.; Doughty, C.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3225–3245. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.H. A model of dry matter partitioning in trees. Tree Physiol. 1998, 18, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Iivonen, S.; Kaakinen, S.; Jolkkonen, A.; Vapaavuori, E.; Linder, S. Influence of long-term nutrient optimization on biomass, carbon, and nitrogen acquisition and allocation in Norway spruce. Can. J. For. Res. 2006, 36, 1563–1571. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2011, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Lopez, B.C.; Sabaté, S.; Gracia, C.A. Thinning effects on carbon allocation to fine roots in a Quercus ilex forest. Tree Physiol. 2003, 23, 1217–1224. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Lehtonen, A.; Vacchiano, G.; Sever, M.Z.O.; Augustynczik, A.L.D.; Grote, R.; Kyselová, I.; Mäkelä, A.; Yousefpour, R.; et al. Forest carbon allocation modelling under climate change. Tree Physiol. 2019, 39, 1937–1960. [Google Scholar] [CrossRef]
- IPCC. “IPCC 2018: Summary of Policymakers” in Global warming of 1.5 °C. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008; p. 210. [Google Scholar]
- Fischer, E.M.; Schär, C. Consistent geographical patterns of changes in high-impact European heatwaves. Nat. Geosci. 2010, 3, 398–403. [Google Scholar] [CrossRef]
- Barbeta, A.; Ogaya, R.; Peñuelas, J. Dampening effects of long-term experimental drought on growth and mortality rates of a Holm oak forest. Glob. Chang. Biol. 2013, 19, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ogaya, R.; Barbeta, A.; Yang, X.; Peñuelas, J. Long-term experimental drought combined with natural extremes accelerate vegetation shift in a Mediterranean holm oak forest. Environ. Exp. Bot. 2018, 151, 1–11. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sanchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martinez-Vilalta, J.; Sabaté, S.; Lloret, F. Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean holm oak forest. Tree Physiol. 2012, 32, 478–489. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Ogaya, R.; Barbeta, A.; Başnou, C.; Peñuelas, J. Satellite data as indicators of tree biomass growth and forest dieback in a Mediterranean holm oak forest. Ann. For. Sci. 2014, 72, 135–144. [Google Scholar] [CrossRef]
- Ogaya, R.; Liu, D.; Barbeta, A.; Peñuelas, J. Stem Mortality and Forest Dieback in a 20-Years Experimental Drought in a Mediterranean Holm Oak Forest. Front. For. Glob. Chang. 2020, 2, 89. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2012, 3, 292–297. [Google Scholar] [CrossRef]
- Montané, F.; Fox, A.M.; Arellano, A.F.; MacBean, N.; Alexander, M.R.; Dye, A.W.; Bishop, D.A.; Trouet, V.; Babst, F.; Hessl, A.; et al. Evaluating the effect of alternative carbon allocation schemes in a land surface model (CLM4.5) on carbon fluxes, pools, and turnover in temperate forests. Geosci. Model Dev. 2017, 10, 3499–3517. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Peñuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Peñuelas, J.; Emmet, B.; Körner, C.; de Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation manipulation experiments—Challenges and recommendations for the future. Ecol. Lett. 2012, 15, 899–911. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Assessment of the impacts of climate change on Mediterranean terrestrial ecosystems based on data from field experiments and long-term monitored field gradients in Catalonia. Environ. Exp. Bot. 2018, 152, 49–59. [Google Scholar] [CrossRef]
- Leuzinger, S.; Luo, Y.; Beier, C.; Dieleman, W.; Vicca, S.; Körner, C. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol. Evol. 2011, 26, 236–241. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Gargallo-Garriga, A.; Sardans, J.; Oravec, M.; Mateu-Castell, L.; Pérez-Trujillo, M.; Parella, T.; Ogaya, R.; Urban, O.; Peñuelas, J. Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 2014, 202, 874–885. [Google Scholar] [CrossRef]
- Doughty, C.E.; Metcalfe, D.B.; Girardin, C.A.J.; Amezquita, F.F.; Durand, L.; Huasco, W.H.; Silva-Espejo, J.E.; Araujo-Murakami, A.; Da Costa, M.C.; Da Costa, A.C.L.; et al. Source and sink carbon dynamics and carbon allocation in the Amazon basin. Glob. Biogeochem. Cycles 2015, 29, 645–655. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Species-specific drought effects on flower and fruit production in a Mediterranean holm oak forest. Forestry 2007, 80, 351–357. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Fernández-Martínez, M.; Espelta, J.; Ogaya, R.; Peñuelas, J. Is forest fecundity resistant to drought? Results from an 18-yr rainfall-reduction experiment. New Phytol. 2020, 227, 1073–1080. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M.; Rambal, S. Mast seeding under increasing drought: Results from a long-term data set and from a rainfall exclusion experiment. Ecology 2010, 91, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; DesLauriers, A.; Giovannelli, A.; Beaulieu, M.; Delzon, S.; Rossi, S.; Rathgeber, C.B.K. How do drought and warming influence survival and wood traits of Picea mariana saplings? J. Exp. Bot. 2014, 66, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Zalloni, E.; Battipaglia, G.; Cherubini, P.; Saurer, M.; De Micco, V. Wood Growth in Pure and Mixed Quercus ilex L. Forests: Drought Influence Depends on Site Conditions. Front. Plant Sci. 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Sabaté, S.; Gracia, C. Fine-root longevity of Quercus ilex. New Phytol. 2001, 151, 437–441. [Google Scholar] [CrossRef]
- Reich, P.B. Root–shoot relations: Optimality in acclimation and adaptation or the “Emperor’s New Clothes”? In Plant Roots: The Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 205–220. [Google Scholar]
- Llusià, J.; Peñuelas, J.; Alessio, G.A.; Ogaya, R. Species-specific, seasonal, inter-annual, and historically-accumulated changes in foliar terpene emission rates in Phillyrea latifolia and Quercus ilex submitted to rain exclusion in the Prades Mountains (Catalonia). Russ. J. Plant Physiol. 2011, 58, 126–132. [Google Scholar] [CrossRef]
- Preece, C.; Farré-Armengol, G.; Llusià, J.; Peñuelas, J. Thirsty tree roots exude more carbon. Tree Physiol. 2018, 38, 690–695. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Impacts of Global Change on Mediterranean Forests and Their Services. Forestry 2017, 8, 463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).